Abstract
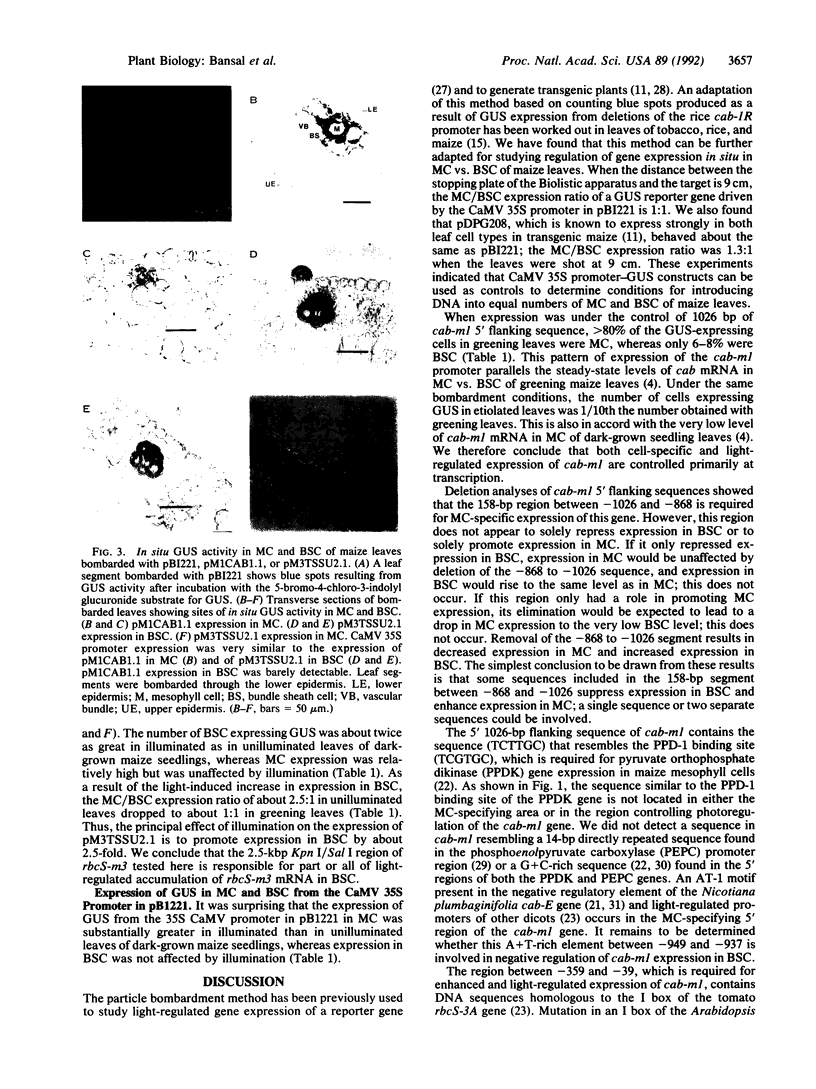
Cell-specific and light-regulated expression of the beta-glucuronidase (GUS) reporter gene from maize cab-m1 and rbcS-m3 promoter sequences was studied in maize leaf segments by using an in situ transient expression microprojectile bombardment assay. The cab-m1 gene is known to be strongly photoregulated and to be expressed almost exclusively in mesophyll cells (MC) but not in bundle sheath cells (BSC). Expression of GUS from a 1026-base-pair 5' promoter fragment of cab-m1 is very low in dark-grown leaves; GUS expression is increased about 10-fold upon illumination of dark-grown leaves. In illuminated leaves, the ratio of GUS expression in MC vs. BSC is about 10:1. The cab-m1 region between 868 and 1026 base pairs 5' to the translation start confers strong MC-preferred expression on the remainder of the chimeric gene in illuminated leaves, but a region between -39 and -359 from the translation start is required for photoregulated expression. Transcripts of rbcS-m3 are found in BSC but not in MC and are about double in BSC of greening dark-grown seedlings. In contrast to the behavior of the cab-m1-GUS construct, GUS expression driven by 2.1 kilobase pairs of the rbcS-m3 5' region was about twice as high in MC as in BSC of unilluminated dark-grown maize leaves. The number of BSC, but not MC, expressing GUS nearly doubled upon greening of bombarded etiolated leaves. These data suggest that the 5' region of rbcS-m3 used here could be responsible for most of the light-dependent increase in rbcS-m3 transcripts observed in BSC of greening leaves and that transcriptional or posttranscriptional mechanisms are responsible for the lack of rbcS-m3 transcripts in MC.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew D. M., Bartley G. E., Scolnik P. A. Abscisic Acid Control of rbcS and cab Transcription in Tomato Leaves. Plant Physiol. 1991 May;96(1):291–296. doi: 10.1104/pp.96.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M., Barnes W. M., Chilton M. D. Structure and transcription of the nopaline synthase gene region of T-DNA. Nucleic Acids Res. 1983 Jan 25;11(2):369–385. doi: 10.1093/nar/11.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana C., Garcia-Luque I., Alonso E., Malik V. S., Cashmore A. R. Both positive and negative regulatory elements mediate expression of a photoregulated CAB gene from Nicotiana plumbaginifolia. EMBO J. 1988 Jul;7(7):1929–1936. doi: 10.1002/j.1460-2075.1988.tb03030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Cashmore A. R. Binding of a pea nuclear protein to promoters of certain photoregulated genes is modulated by phosphorylation. Plant Cell. 1989 Nov;1(11):1069–1077. doi: 10.1105/tpc.1.11.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Favreau M., Bond-Nutter D., Bedbrook J., Dunsmuir P. Sequences downstream of translation start regulate quantitative expression of two petunia rbcS genes. Plant Cell. 1989 Feb;1(2):201–208. doi: 10.1105/tpc.1.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald R. G., Cashmore A. R. Mutation of either G box or I box sequences profoundly affects expression from the Arabidopsis rbcS-1A promoter. EMBO J. 1990 Jun;9(6):1717–1726. doi: 10.1002/j.1460-2075.1990.tb08295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M. E., Morrish F., Armstrong C., Williams R., Thomas J., Klein T. M. Inheritance and expression of chimeric genes in the progeny of transgenic maize plants. Biotechnology (N Y) 1990 Sep;8(9):833–839. doi: 10.1038/nbt0990-833. [DOI] [PubMed] [Google Scholar]
- Giuliano G., Pichersky E., Malik V. S., Timko M. P., Scolnik P. A., Cashmore A. R. An evolutionarily conserved protein binding sequence upstream of a plant light-regulated gene. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7089–7093. doi: 10.1073/pnas.85.19.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Kamm W. J., Spencer T. M., Mangano M. L., Adams T. R., Daines R. J., Start W. G., O'Brien J. V., Chambers S. A., Adams W. R., Jr, Willetts N. G. Transformation of Maize Cells and Regeneration of Fertile Transgenic Plants. Plant Cell. 1990 Jul;2(7):603–618. doi: 10.1105/tpc.2.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilley H., Dudley R. K., Jonard G., Balàzs E., Richards K. E. Transcription of Cauliflower mosaic virus DNA: detection of promoter sequences, and characterization of transcripts. Cell. 1982 Oct;30(3):763–773. doi: 10.1016/0092-8674(82)90281-1. [DOI] [PubMed] [Google Scholar]
- Guiltinan M. J., Marcotte W. R., Jr, Quatrano R. S. A plant leucine zipper protein that recognizes an abscisic acid response element. Science. 1990 Oct 12;250(4978):267–271. doi: 10.1126/science.2145628. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano-Murakami Y., Suzuki I., Sugiyama T., Matsuoka M. Sequence-specific interactions of a maize factor with a GC-rich repeat in the phosphoenolpyruvate carboxylase gene. Mol Gen Genet. 1991 Feb;225(2):203–208. doi: 10.1007/BF00269849. [DOI] [PubMed] [Google Scholar]
- Langdale J. A., Lane B., Freeling M., Nelson T. Cell lineage analysis of maize bundle sheath and mesophyll cells. Dev Biol. 1989 May;133(1):128–139. doi: 10.1016/0012-1606(89)90304-7. [DOI] [PubMed] [Google Scholar]
- Langdale J. A., Zelitch I., Miller E., Nelson T. Cell position and light influence C4 versus C3 patterns of photosynthetic gene expression in maize. EMBO J. 1988 Dec 1;7(12):3643–3651. doi: 10.1002/j.1460-2075.1988.tb03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun M., Waksman G., Freyssinet G. Nucleotide sequence of a gene encoding corn ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit (rbcs). Nucleic Acids Res. 1987 May 26;15(10):4360–4360. doi: 10.1093/nar/15.10.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link G., Coen D. M., Bogorad L. Differential expression of the gene for the large subunit of ribulose bisphosphate carboxylase in maize leaf cell types. Cell. 1978 Nov;15(3):725–731. doi: 10.1016/0092-8674(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Kano-Murakami Y., Yamamoto N. Nucleotide sequence of cDNA encoding the light-harvesting chlorophyll a/b binding protein from maize. Nucleic Acids Res. 1987 Aug 11;15(15):6302–6302. doi: 10.1093/nar/15.15.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M., Minami E. Complete structure of the gene for phosphoenolpyruvate carboxylase from maize. Eur J Biochem. 1989 May 15;181(3):593–598. doi: 10.1111/j.1432-1033.1989.tb14765.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Numazawa T. Cis-acting elements in the pyruvate, orthophosphate dikinase gene from maize. Mol Gen Genet. 1991 Aug;228(1-2):143–152. doi: 10.1007/BF00282459. [DOI] [PubMed] [Google Scholar]
- Ngernprasirtsiri J., Chollet R., Kobayashi H., Sugiyama T., Akazawa T. DNA methylation and the differential expression of C4 photosynthesis genes in mesophyll and bundle sheath cells of greening maize leaves. J Biol Chem. 1989 May 15;264(14):8241–8248. [PubMed] [Google Scholar]
- Rolfe S. A., Tobin E. M. Deletion analysis of a phytochrome-regulated monocot rbcS promoter in a transient assay system. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2683–2686. doi: 10.1073/pnas.88.7.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster G., Ohad I., Martineau B., Taylor W. C. Differentiation and development of bundle sheath and mesophyll thylakoids in maize. Thylakoid polypeptide composition, phosphorylation, and organization of photosystem II. J Biol Chem. 1985 Sep 25;260(21):11866–11873. [PubMed] [Google Scholar]
- Schäffner A. R., Sheen J. Maize rbcS promoter activity depends on sequence elements not found in dicot rbcS promoters. Plant Cell. 1991 Sep;3(9):997–1012. doi: 10.1105/tpc.3.9.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Y., Bogorad L. Differential expression of six light-harvesting chlorophyll a/b binding protein genes in maize leaf cell types. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7811–7815. doi: 10.1073/pnas.83.20.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Y., Bogorad L. Expression of the ribulose-1,5-bisphosphate carboxylase large subunit gene and three small subunit genes in two cell types of maize leaves. EMBO J. 1986 Dec 20;5(13):3417–3422. doi: 10.1002/j.1460-2075.1986.tb04663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan T. D., Christensen A. H., Quail P. H. Isolation and characterization of a maize chlorophyll a/b binding protein gene that produces high levels of mRNA in the dark. Mol Gen Genet. 1989 Feb;215(3):431–440. doi: 10.1007/BF00427040. [DOI] [PubMed] [Google Scholar]
- Vainstein A., Ferreira P., Peterson C. C., Verbeke J. A., Thornber J. P. Expression of the Major Light-Harvesting Chlorophyll a/b-Protein and Its Import into Thylakoids of Mesophyll and Bundle Sheath Chloroplasts of Maize. Plant Physiol. 1989 Feb;89(2):602–609. doi: 10.1104/pp.89.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viret J. F., Schantz M. L., Schantz R. Nucleotide sequence of a maize cDNA coding for a light-harvesting chlorophyll a/b binding protein of photosystem II. Nucleic Acids Res. 1990 Dec 11;18(23):7179–7179. doi: 10.1093/nar/18.23.7179. [DOI] [PMC free article] [PubMed] [Google Scholar]