Abstract
Introduction:
We evaluated the accuracy of nicotine concentration labeling on electronic cigarette refill products.
Methods:
The nicotine concentration of 71 electronic cigarette refill fluid products and 1 related do-it-yourself (DIY) product was quantified using high-performance liquid chromatography. Quantified data were compared with manufacturers labeled concentrations. Duplicate refill fluid products purchased at different times were evaluated by visual comparison of fluid coloration and quantified nicotine concentration.
Results:
Thirty-five of the 54 nicotine-containing fluids had quantified nicotine concentrations that deviated by more than ±10% from the manufacturer labels, with 46 of 50 being in excess of labeled values. Refill fluids labeled as 0 nicotine had no detectable nicotine. Of the 5 products that were unlabeled for nicotine concentration, 3 contained no detectable nicotine, whereas the remaining 2 contained nicotine in excess of 100mg/ml and may have been intended for DIY use. Sixteen of the 18 duplicate bottles of refill fluid varied greatly in their nicotine concentrations. One of the 5 companies showed significant improvement in labeling accuracy among the most recently purchased products. Of the 23 total duplicate pairs, 15 of 23 varied in coloration from their mates.
Conclusions:
Nicotine concentration labeling on electronic cigarette refill products was often inaccurate but showed improvement recently in products from 1 company. To ensure the safety of refill fluids and DIY products, it is necessary to establish quality control guidelines for the manufacturing and labeling and to monitor products longitudinally.
Introduction
E-cigarettes (ECs) are composed of a battery that heats an atomizer, which aerosolizes a fluid that generally contains nicotine, a humectant(s), and flavorings (Trtchounian & Talbot, 2011; Trtchounian, Williams, & Talbot, 2010). EC cartridges, cartomizers, and tanks, which hold the fluid, can be refilled from drip bottles of refill fluid that are readily available over the Internet, in EC retail shops, and in malls. Although ECs per se are generally marketed in a limited number of flavors, refill fluids are available, often from third-party vendors, in hundreds of different flavors. Thus, they expand the flavor options and offer EC users a more cost-effective option by enabling EC cartridges to be reused. However, some refill fluids were cytotoxic when tested in vitro with different cell types, and cytotoxicity of several products was attributed to flavorings (Bahl et al., 2012; Behar et al., 2013).
Because nicotine contained in these fluids is both addictive and toxic (a dose of 6.5–13mg/kg is fatal to adult humans) (Mayer, 2013), it is important that the concentrations of nicotine on refill fluid and DIY product bottles be accurate. However, there are currently no federal regulations on the manufacturing of these products. Recently, the EC industry has begun some self-regulation with the creation of the American E-liquid Manufacturing Standards Association (AEMSA), which was formed to certify nicotine concentrations in EC products. According to the revised AEMSA guidelines (posted on the AEMSA Web site in February, 2014), nicotine concentrations should be ±10% of the label (AEMSA, 2014).
Previous studies have examined nicotine concentrations in a limited number of refill fluid products (Cameron et al., 2014; Etter, Zäther, & Svensson, 2013; Trehy et al., 2011) and in some cases found significant discrepancies between what was on the bottle and what was measured (Cameron et al., 2014; Trehy et al., 2011). The purpose of the current study was to quantify nicotine concentrations in a broad spectrum of refill fluids from different American manufacturers, to compare measured nicotine concentrations to those provided by the manufacturer, and to determine whether duplicate bottles of the same product purchased at different times had similar concentrations of nicotine. This study is the first to investigate nicotine concentrations in a broad range of American-made refill fluid products in longitudinal samples.
Materials and methods
Products Tested
Seventy-one EC refill fluids and one DIY (do-it-yourself) product were purchased from five different manufacturers using the Internet or a local vendor, and inventory numbers were assigned to each sample at the time of receipt (Table 1). Purchases were made on four different dates (inventory numbers 1–41 April 2011; numbers 49–68 summer 2011; numbers 70–92 February 2012, and numbers 93–96 May 2012). Johnson Creek (Johnson Creek) and Red Oak (a subsidiary of Johnson Creek) are two major manufacturers of EC refill fluid products, as shown by Google Trends, with sales in 101 countries. Recently, Johnson Creek partnered with Blu Cig, one of the most popular ECs (Time Magazine, 2013), thus increasing the potential distribution of their products. Freedom Smoke-USA and V2 Cigs were selected because the companies have consistently gained in popularity since their introduction to the refill fluid market in 2010, with their popularity still rising according to Google Trends. Global Smoke was selected because it is marketed and readily available in shopping malls in our area. Lastly, e-cigexpress.com was selected because, at the time of purchase, they were one of the few Internet vendors that sold flavorless nicotine in a propylene glycol base.
Table 1.
Evaluated Refill Fluids/DIY Product
| Numbera | Manufacturerb | Flavor | Humc | Colord | [M]e (mg/ml) | [Q]f (mg/ml) | % Diff From [M] | ±10%g of label |
|---|---|---|---|---|---|---|---|---|
| Purchased April 2011 | ||||||||
| 1 | Red Oak | Domestic | G, V | Brown | 18 | 22.8±0.93 | +26.4 | No |
| 2 | Red Oak | Island | G, V | Lt Brown | 18 | 29.6±0.27 | +64.3 | No |
| 3 | Red Oak | Marcado | G, V | Lt Brown | 18 | NDh | n/ai | n/a |
| 4 | Red Oak | Swiss Dark | G, V | Dk Brown | 18 | 31.7±0.23 | +76.1 | No |
| 5 | Red Oak | Tennessee Cured | G, V | Lt Brown | 18 | 29.7±0.29 | +64.9 | No |
| 6 | Red Oak | Valencia | G, V | Cream | 18 | 24.2±0.34 | +34.2 | No |
| 7 | Red Oak | Wisconsin Frost | G, V | Brown | 18 | 23.8±0.23 | +32.2 | No |
| 8 | JCg | Arctic Menthol | P, V, G | Tan | 18 | 22.2±0.30 | +23.4 | No |
| 9 | JC | Black Cherry | P, V, G | Tan | 18 | 20.9±0.13 | +16.1 | No |
| 10 | JC | Chocolate Truffle | P, V, G | Med Brown | 18 | 19.7±0.56 | +9.7 | Yes |
| 11 | JC | Espresso | P, V, G | Lt Brown | 18 | 21.4±0.11 | +18.83 | No |
| 12 | JC | French Vanilla | P, V, G | Brown | 18 | 20.7±0.11 | +14.83 | No |
| 13 | JC | J.C. Original | P, V, G | Lt Brown | 18 | 24.9±0.45 | +38.5 | No |
| 14 | JC | Mint Chocolate | P, V, G | Tan | 18 | 24.3±0.47 | +35.1 | No |
| 15 | JC | Simply Strawberry | P, V, G | Tan | 18 | 17.0 ± 0.21 | −5.8 | Yes |
| 16 | JC | Summer Peach | P, V, G | Tan | 18 | 22.0±0.38 | +22.1 | No |
| 17 | JC | Tennessee Cured | P, V, G | Med Brown | 18 | 21.4±0.31 | +18.8 | No |
| 18 | FS-USA | Bubble Gum | U | Orange | 24 | 28.5±0.80 | +18.8 | No |
| 19 | FS-USA | Butterfinger | U | Dark Brown | 24 | 21.9±0.71 | −8.6 | Yes |
| 20 | FS-USA | Butterscotch FA | U | Yellow (Urine) | 0 | 0.0 | 0 | n/a |
| 21 | FS-USA | Caramel FA | U | Lt Brown | 0 | 0.0 | 0 | n/a |
| 23 | FS-USA | Menthol Arctic FA | U | Clear | 0 | 0.0 | 0 | n/a |
| 24 | FS-USA | PureNicLiquid | P | Clear (Lt Yw Tint) | U | 105.9±3.2 | n/a | n/a |
| 25 | FS-USA | Vanilla Tahity FA | U | Med Brown | U | 0.0 | n/a | n/a |
| 26 | FS-USA | Caramel | V | Clear | 0 | 0.0 | 0 | n/a |
| 27 | FS-USA | Caramel | U | Yellow-Orange | 6 | 10.2±0.27 | +69.5 | No |
| 28 | FS-USA | Caramel | V | Yellow-Orange | 6 | 10.5±0.72 | +75.0 | No |
| 29 | FS-USA | Butterscotch | V | Yellow-Orange | 6 | 5.6±0.03 | −6.0 | Yes |
| 30 | FS-USA | Butterscotch | V | Clear | 0 | 0.0 | 0 | n/a |
| 31 | JC | Tennessee Cured | P, V | Med Brown | 11 | 15.6±0.85 | +42.1 | No |
| 34 | JC | J.C. Original | P, V | Lt Brown | 11 | 17.5±0.55 | +58.6 | No |
| 35 | FS-USA | Chocolate Biscotti | U | Brown | 24 | 34.4±1.59 | +43.3 | No |
| 36 | FS-USA | Coconut | U | Clear (Lt Yw Tint) | 0 | 0.0 | 0 | n/a |
| 37 | FS-USA | Peanut Butter Cup | U | Dk Brown | 24 | 20.9±1.29 | −12.9 | No |
| 38 | FS-USA | Tiramisu | U | Dk Brown | 0 | 0.0 | 0 | n/a |
| 39 | Global Smoke | RY4 | U | Yellow | 18 | 26.6±0.63 | +47.9 | No |
| 40 | Global Smoke | Caramel | U | Yellow-Orange | 18 | 23.3±0.86 | +29.6 | No |
| 41 | FS-USA | Butterscotch FA | U | Yellow | 0 | 0.0 | 0 | n/a |
| Purchased June–August 2011 | ||||||||
| 49 | Red Oak | Marcado | V | Tan | 18 | ND | n/a | n/a |
| 50 | JC | J.C. Original | P, V | Tan | 18 | 19.9±0.26 | +10.4 | No |
| 51 | JC | Tennessee Cured | P, V | Lt Brown | 11 | 12.2±0.19 | +10.5 | No |
| 55 | FS-USA | PureNicotineLiquid | P | Clear (Yw Tint) | U | 134.7±4.0 | n/a | No |
| 56 | FS-USA | Butterscotch FA | U | Lt Brown | U | 0.0 | n/a | n/a |
| 57 | FS-USA | Wyatt Earp | U | Dk Brown | 24 | 31.3±2.55 | +30.4 | No |
| 68 | e-cigexpress | Unflavored PG Base | P | Clear | 60 | 72.9±3.14 | +21.5 | No |
| Purchased February 2012 | ||||||||
| 70 | Red Oak | Marcado | V | Tan | 18j | 27.8±1.36 | +54.3 | No |
| 71 | Red Oak | Domestic | G, V | Tan | 18 | 22.8±0.34 | +26.4 | No |
| 72 | Red Oak | Island | G, V | Tan | 18 | 23.5±0.40 | +30.6 | No |
| 73 | Red Oak | Marcado | G, V | Lt Yellow | 18 | ND | n/a | n/a |
| 74 | Red Oak | Swiss Dark | G, V | Lt Brown | 18 | 28.0±0.28 | +55.9 | No |
| 75 | Red Oak | Tennessee Cured | G, V | Lt Brown | 18 | 28.6±1.04 | +59.0 | No |
| 76 | Red Oak | Valencia | G, V | Clear | 18 | 19.5±0.53 | +8.4 | Yes |
| 77 | Red Oak | Wisconsin Frost | G, V | Lt Brown | 18 | 34.2±0.72 | +89.7 | No |
| 78 | JC | Arctic Menthol | P, V, G | Lt Brown | 18 | 19.7±0.23 | +9.2 | Yes |
| 79 | JC | Black Cherry | P, V, G | Tan | 18 | 19.0±0.42 | +5.6 | Yes |
| 80 | JC | Chocolate Truffle | P, V, G | Brown | 18 | 18.2±0.98 | +1.3 | Yes |
| 81 | JC | Espresso | P, V, G | Brown | 18 | 18.2±0.70 | +1.1 | Yes |
| 82 | JC | French Vanilla | P, V, G | Brown | 18 | 17.8±0.24 | −1.3 | Yes |
| 83 | JC | J.C. Original | P, V, G | Lt Brown | 18 | 18.4±0.33 | +2.3 | Yes |
| 84 | JC | Mint Chocolate | P, V, G | Brown | 18 | 19.4±0.44 | +9.3 | Yes |
| 85 | JC | Spiced Apple Cider | P, V, G | Light Cream | 18 | 16.8±0.52 | −6.6 | Yes |
| 86 | JC | Summer Peach | P, V, G | Lt Brown | 18 | 17.4±0.30 | −3.5 | Yes |
| 87 | JC | Tennessee Cured | P, V, G | Med Brown | 18 | 19.2±0.34 | +6.7 | Yes |
| 88 | JC | J.C. Original | P, V | Lt Brown | 18j | 17.1±0.24 | −5.2 | Yes |
| 89 | FS-USA | Menthol Arctic FA | U | Clear | U | 0.0 | n/a | n/a |
| 90 | FS-USA | Caramel | V | Clear | 0 | 0.0 | 0 | n/a |
| 91 | FS-USA | Butterscotch | V | Clear (Yw Tint) | 6 | 7.4±0.09 | +22.5 | No |
| 92 | FS-USA | Butterscotch | V | Clear (Lt Yw Tint) | 0 | 0.0 | 0 | n/a |
| Purchased May 2012 | ||||||||
| 93 | V2 Cigs | Peppermint | P, V | Lt Yellow | 18j | 20.2±0.09 | +12.1 | No |
| 94 | V2 Cigs | Menthol | P | Lt Yellow | 18 | 19.6±0.08 | +8.8 | Yes |
| 95 | V2 Cigs | Sahara | P | Yellow | 18 | 19.7±0.07 | +9.3 | Yes |
| 96 | V2 Cigs | V2 Red | P | Yellow (Urine) | 18 | 18.8±0.07 | +4.2 | Yes |
aInventory number (missing inventory numbers were assigned to refill fluids that we were unable to evaluate using this method or to authentic standards).
bJC = Johnson Creek, FS-USA = Freedom Smoke-USA.
cHum = humectant composition, G = glycerin, V = vegetable glycerin, P = propylene glycol, U = unknown.
dLt = light, Med = medium, Dk = dark, Yw = yellow.
e[M] = manufacturers concentration, U = unknown.
f[Q] = quantified concentration (±SD).
g±10% of labeled nicotine concentration = AEMSA tolerance level (version 1.8 posted February 2014).
hND = not determined.
in/a = not applicable.
jActual label read 1.8%.
Of the 71 refill fluids/1 DIY products that were evaluated, 25 were purchased from Johnson Creek, and 20 of these were obtained from two sample kits. Kits were purchased at two different times, and 9 of the 10 products in each kit were exact duplicates (i.e., from the same manufacturer with the same label information). Of the additional five Johnson Creek refill fluids, two were Tennessee Cured (numbers 31, 51) with labeled nicotine content that differed from the refill fluids in the sample kits, and three were J.C. Original (numbers 34, 50, 88) of which two (numbers 50 and 88) were labeled with the same nicotine content as those in the sample kit and one (number 34) was labeled at a lower concentration. Sixteen refill fluids were from Red Oak, and 14 of these were also obtained in two sample kits. The Red Oak kits each contained seven refill fluids, and all seven were duplicate flavors. Two additional Red Oak Marcado (numbers 49, 70) refill fluids were purchased to further evaluate duplicates. For both Johnson Creek and Red Oak, all refill fluids contained within the kits had a different labeled humectant composition than individually purchased bottles. Twenty-four refill fluids were purchased from Freedom Smoke-USA. Two (number 24, number 55) of the 24 products were unflavored nicotine in 100% propylene glycol. At the time of purchase, neither the labels nor Web site indicated the nicotine concentrations of these products or that they were concentrates to be used in diluted form. Therefore, these two products were also considered refill fluids, not DIY products. Four of the flavors (eight refill bottles) were purchased as exact duplicates. Two refill fluids were purchased from Global Smoke, four were from V2 Cigs, and the one DIY product was from e-cigexpress.com. All products were stored in the dark at 4 °C, and all experiments were performed within 3 months of purchase.
Establishment of Nicotine Calibration Curve and Method Validation
Our high-performance liquid chromatography (HPLC) method for quantifying nicotine in refill fluids/DIY products was adapted from Trehy et al. (2011), which was shown previously to work well with EC refill fluids. Nicotine (≥99% purity) purchased from Sigma-Aldrich was used to establish a calibration curve. A stock solution of nicotine (10mg/ml) was prepared in nonbuffered mobile phase consisting of 77% water and 23% acetonitrile. Serial dilutions (1–1,500 μg/ml) were made, and the linear portion of instrumental response was determined. Three samples of five doses that spanned the linear range (100 µg to 1000 µg/ml) were used to create a calibration curve. The accuracy and precision of the calibration curve were validated by injecting four samples of nicotine, prepared as described above, at two concentrations (500 µg/ml and 637 µg/ml) and determining the percent error at each concentration. For each concentration, the error was < 1% (0.408% for 500 µg/ml and 0.843% for 637 µg/ml). The calibration curve was periodically validated to insure that no changes or drift were present.
HPLC Analysis of Nicotine Concentrations
HPLC-grade chemicals (triethylamine, water, and acetonitrile) and phosphoric acid (85%) were purchased from Fischer Scientific. Sodium hydroxide was purchased from EM Scientific. Samples were analyzed using a Hewlett Packard Series 1100 HPLC, consisting of a quaternary pump, degasser, column thermostat, and manual injector. A 200×4.6mm Thermo Scientific Hypersil ODS C18 column with a particle size of 5 μm was used at 35 °C with a flow rate of 0.8ml/min. The diode array detector signal was set to 260nm with a bandwidth of 40nm with a reference signal of 380nm and bandwidth of 10nm. An isocratic method was used with a buffered mobile phase consisting of 76.9% water, 23% acetonitrile, and 0.1% triethylamine. The pH of the mobile phase was adjusted daily to 7.6 using phosphoric acid and sodium hydroxide. Because no extraction procedure was necessary, 5% stock solutions of each fluid were prepared in a nonbuffered mobile phase. Care was taken to accurately pipette the fluids so as not to introduce air bubbles. The stock solutions were diluted down to the injection concentration of 0.5% by the further addition of nonbuffered mobile phase. The injection volume for all samples was 5 μl. The limit of quantification for nicotine was 10 μg/ml with a limit of detection of 50ng/ml. Each sample was injected and analyzed 5 times. The values reported in Tables 1 and 2 are the means and standard deviations of the five runs.
Table 2.
Comparison of Exact Duplicate Refill Fluids
| Numbersa | Manufacturerb | Flavor | Color comparisonc | [M]d (mg/ml) | ≤10% Difference from [M] (actual % diff) |
|---|---|---|---|---|---|
| 1/71 | Red Oak | Domestic | Brown/Tan | 18 | No (26.4)/No (26.4) |
| 2/72 | Red Oak | Island | Lt Brown/Tan | 18 | No (64.3)/No (30.6)e |
| 3/73 | Red Oak | Marcado | Lt Brown/Lt Yellow | 18 | ND/NDf |
| 4/74 | Red Oak | Swiss Dark | Dk Brown/Lt Yellow | 18 | No (76.1)/No (55.9)e |
| 5/75 | Red Oak | Tennessee Cured | Lt Brown/Lt Brown | 18 | No (64.9)/No (59.0) |
| 6/76 | Red Oak | Valencia | Cream/Clear | 18 | No (34.2)/Yes (8.4)e |
| 7/77 | Red Oak | Wisconsin Frost | Brown/Lt Brown | 18 | No (32.2)/No (89.7)e |
| 8/78 | JC | Arctic Menthol | Tan/Lt Brown | 18 | No (23.4)/Yes (9.2) |
| 9/79 | JC | Black Cherry | Tan/Tan | 18 | No (16.1)/Yes (5.6) |
| 10/80 | JC | Chocolate Truffle | Med Brown/Brown | 18 | Yes (9.7)/Yes (1.3) |
| 11/81 | JC | Espresso | Lt Brown/Brown | 18 | No (18.83)/Yes (1.1) |
| 12/82 | JC | French Vanilla | Brown/Brown | 18 | No (14.83)/Yes (1.3) |
| 13/83 | JC | J.C. Original | Lt Brown/Lt Brown | 18 | No (38.5)/Yes (2.3)e |
| 14/84 | JC | Mint Chocolate | Tan/Brown | 18 | No (35.1)/Yes (9.3)e |
| 16/86 | JC | Summer Peach | Tan/Lt Brown | 18 | No (22.1)/Yes (3.5) |
| 17/87 | JC | Tennessee Cured | Med Brown/Med Brown | 18 | No (18.8)/Yes (6.7) |
| 23/89 | FS-USA | Menthol Arctic FA | Clear/Clear | 0 | n/a/n/ag |
| 26/90 | FS-USA | Caramel | Clear/Clear | 0 | n/a/n/a |
| 29/91 | FS-USA | Butterscotch | Yellow-Orange/Clear (Yw Tint) | 6 | Yes (6)/No (22.5) |
| 30/92 | FS-USA | Butterscotch | Clear/Clear (Lt Yw Tint) | 0 | n/a/n/a |
| 31/51 | JC | Tennessee Cured | Med Brown/Lt Brown | 11 | No (42.1)/No (10.5)e |
| 49/70 | Red Oak | Marcado | Tan/Tan | 18 | ND/No (27.8) |
| 50/88 | JC | J.C. Original | Tan/Lt Brown | 18 | No (10.4)/Yes (5.2) |
aInventory numbers of duplicate pairs.
bJC = Johnson Creek, FS-USA = Freedom Smoke-USA.
cLt = light, Med = medium, Dk = dark, Yw = yellow.
d[M] = manufacturers concentration in mg/ml.
eDuplicate pairs that varied more than 20%.
fND = not determined.
gn/a = not applicable.
Results
Table 1 shows the results, organized by ascending inventory number, for the 72 products that were evaluated in this study. For each product, the date of purchase, inventory number, flavor, humectant, color, manufacturers’ nicotine concentration, quantified nicotine concentration, percent difference in nicotine concentrations, and whether the fluid was within ±10% of the concentration on the label. A broad range of flavors was included. In most cases, humectants were named on the label, and humectants varied among manufacturers and varied sometimes within a manufacturer. The color of the refill fluids also varied among products and ranged from clear to dark brown. Similarly flavored fluids produced by the same manufacturer sometimes varied in color. For example, Freedom Smoke Caramel (number 26) was clear, whereas Freedom Smoke Caramel (number 27) was orange-yellow.
Manufacturer-labeled nicotine concentrations were compared with the HPLC-quantified nicotine concentrations, and the percent differences were calculated (Table 1). Of the products tested, the 10 refill fluids that were labeled zero nicotine (numbers 20, 21, 23, 26, 30, 36, 38, 41, 90, and 92) contained no detectable nicotine. Eight of 72 samples had nicotine concentrations below labeled values. Five of these eight were from Johnson Creek (numbers 15, 82, 85, 86, and 88) and three were from Freedom Smoke-USA (numbers 19, 29, and 37). Of the remaining 54 fluids, 46 had nicotine concentrations that were higher than the labeled amount. Five bottles had no labeled nicotine concentration; three contained no detectable nicotine (numbers 25, 56, and 87) and two (numbers 24 and 55) had nicotine concentrations in excess of 100mg/ml. Three of the four Red Oak Marcado samples (numbers 3, 49, and 73) were not analyzable using this HPLC method. Of the analyzable nicotine-containing fluids, only 19 had concentrations within ±10% of the labeled concentration, which is the nicotine tolerance level set by AEMSA in their recently revised standard (AEMSA, 2014), as well as a standard that is acceptable for nicotine patches (US Pharmacopeia, 2011).
For some products purchased after February 2012, accuracy in labeling appeared to have improved. For example, of the 12 Johnson Creek products purchased in April 2011, only 2 of the 12 met the ±10% standard. For the 11 Johnson Creek products purchased in February 2012, all 11 were within ±10% of the labeled nicotine concentration, suggesting that manufacturing processes have improved at this company between 2011 and 2012. For Red Oak, a subsidiary of Johnson Creek, only 1 of the 7 analyzable recent purchases (February 2012) was within 10% of the labeled nicotine concentration, and 6 of the 7 were higher than the labeled concentrations by 26.6% to 89.7%. Only one sample of Freedom Smoke that was purchased in February 2012 had nicotine, and it deviated from the label by 22.5%, which was an increase from the April 2011 purchase that deviated by only 6%. In addition, of the four V2 products purchased in May 2012, three of the four products fell within ±10%, suggesting improved manufacturing practices.
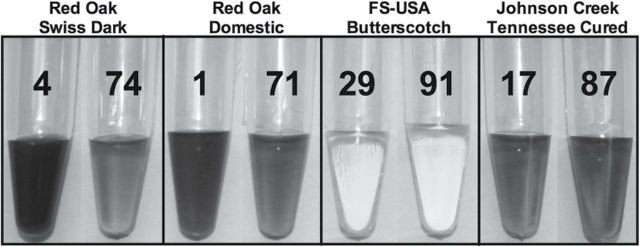
Table 2 presents longitudinal data comparing refill fluids that were considered exact duplicates, that is, produced by the same manufacturer and labeled with the same humectant composition, flavor, and nicotine concentration, but purchased on different dates. The table shows the inventory number of refill fluid duplicate pairs, manufacturer, flavor, coloration, comparisons between the exact duplicates, manufacturer’s labeled nicotine concentration, and whether the quantified nicotine concentration was ≤10% from the manufacturer’s concentration, as well as the actual percent difference (indicated in parenthesis). The color of the refill fluids, which was evaluated visually, varied within most of the 23 duplicate pairs. The most extreme example was observed between numbers 4 (dark brown) and 74 (light brown), which were duplicate bottles of Red Oak Swiss Dark (Figure 1). Some duplicate samples (e.g., Freedom Smoke numbers 29 and 91) had very slight differences in coloration (orange-yellow vs. clear with yellow tint), whereas other duplicates were similar in color (e.g., Johnson Creek numbers 17 and 87) (Figure 1).
Figure 1.
Examples of color variation among duplicate refill fluid pairs. Color variation between mates ranged from extreme, Red Oak Swiss Dark, to similar in coloration, Johnson Creek Tennessee Cured.
The 18 pairs of refill fluid that contained nicotine were compared to determine whether labeling accuracy improved between purchases. Table 2 shows those samples that had measured nicotine concentrations within 10% of the labeled value. Of the18 pairs, only 1 pair (Red Oak Domestic numbers 1, 71) had identical quantified nicotine concentrations and thus showed no change over time from the manufacture’s labeled concentration. Only one pair (numbers 10, 80) was within 10% of the labeled concentration. For five refill fluid pairs, all from Red Oak, both samples exceeded 10% of the labeled concentration, and of these, one (numbers 7, 77) showed an increase in the percent difference over time. Only one refill fluid pair (Freedom Smoke numbers 29, 91) showed a diminution in quality with the fluid purchased earlier meeting the criteria and the latter not. Ten pairs (which included 9 from Johnson Creek) showed significant improvements in labeling accuracy over time.
Discussion
The purpose of this study was to evaluate the accuracy of nicotine concentrations that appear on labels of EC refill fluids/DIY products and to test the fidelity of the manufacturing process by evaluating nicotine concentrations in duplicate products purchased on different dates. Of the 71 refill fluids/1 DIY product evaluated, 54 were labeled as containing nicotine and analyzable. Of these, 35 had nicotine concentrations that did not meet the revised AEMSA tolerance level of ±10%. Quantified nicotine concentrations in evaluated fluids varied from as little as 1.1% to as much as 89.7% from the labeled value, with the majority being higher than indicated on the label. Accuracy in labeling improved significantly in more recent samples purchased from one company. We also found significant variation in the color of fluids both between the same flavors from the same manufacturer and between duplicate bottles of the same product. Although color variation could be due to the use of different chemicals to create a particular flavor or to changes in color during storage, a dramatic color change would not be expected between products that are considered exact duplicates (i.e., the same product purchased at different times) as was seen with numbers 4 and 74. We have observed our products for over 2 years, and none have noticeably changed color during storage.
There have been relatively few studies on the accuracy of the labeled nicotine concentrations on EC products (Goniewicz, Knysak, et al., 2013; Goniewicz, Kuma, Gawron, Knysak, & Kosmider, 2013; Laugesen, Thornley, McRobbie, & Bullen, 2008; Trehy et al., 2011; Westenberger, 2009) with a subset of studies focusing specifically on refill fluids (Cameron et al., 2014; Etter et al., 2013; Goniewicz, Kuma, et al., 2013). In most of these studies, the quantified nicotine concentrations varied from the labeled concentrations, and the degree of variation was quite diverse. In two studies with relatively small sample sizes, the majority of EC refill fluids contained nicotine concentrations below that of the label (Cameron et al., 2014; Goniewicz, Kuma, et al., 2013). In a third study, measured nicotine levels tended to be higher than labeled concentrations; however, the differences between the labeled and measured concentrations for one manufacturer were minor (Trehy et al., 2011). A fourth recent study of mainly Western European products found the differences in the labeled and measured nicotine concentrations to be relatively small and suggested that manufacturing practices have improved and may be acceptable (Etter et al., 2013). In our study, which is the most comprehensive evaluation of American products to date, most nicotine concentrations were higher than the labeled values, with many being over 20% higher. For longitudinal samples from Johnson Creek, the products that were purchased last showed better accuracy in labeling than those purchased 10 months earlier, suggesting an improvement in manufacturing processes for this company. However, a similar improvement was not seen in Red Oak, the Johnson Creek subsidiary. Although the trend for at least one company appears to be toward better labeling, it will be important in the future to monitor progress in accuracy of nicotine labeling and to look at multiple products from a spectrum of companies, as there is still variability within and between companies, and there is currently no government regulation on these products.
The importance of evaluating longitudinal samples from a manufacturer is demonstrated in our study by the four samples of Johnson Creek J.C. Original (18mg of nicotine/ml) that were purchased at 3 different times and varied only in humectant composition. When calculated nicotine values were compared with labeled values, the deviations from the label were +38.5%, +10.4%, −5.2%, and +2.3%. Had only one of these products been evaluated, for example, the product that differed by +38.5%, the data generated would not be an accurate representation of the product line. Likewise, we have shown for the first time that duplicate samples of the same product can vary in their nicotine concentration. In 7 of 21 samples, nicotine concentration between duplicate bottles varied by more than 20% (as indicated by stars in the ≤10% Difference column of Table 2).
In October 2012, AEMSA was established as a volunteer organization to set responsible and sustainable standards for the safe manufacturing of EC refill fluid products, and their standards are quite stringent. Members, who pay a monthly membership fee, must agree to adhere to these standards and are allowed to display the AEMSA logo on their Web sites (AEMSA, 2012). Although none of the companies evaluated in this study are listed on the AEMSA Web site as members of this association, refill fluids purchased later in our study were more accurately labeled, with Johnson Creek being the most improved.
Accurate labeling of nicotine concentrations on EC products is important as nicotine is both addictive and toxic (Centers for Disease Control and Prevention, Office of Smoking and Health, 1988, 2010; Solarino, Rosenbaum, Rießelmann, Buschmann, & Tsokos, 2010; US Department of Health and Human Services, National Institute of Health, National Cancer Institute, 2001), and EC users should have reliable information on nicotine concentrations in these products. Moreover, some people use ECs as cessation devices (Odum, O’Dell, & Schepers, 2012; Polosa et al., 2011; Siegel, Tanwar, & Wood, 2011) and gradually wean themselves off higher doses of nicotine. For this group of EC users, accurately labeled products are important. Also, studies show that decreases in nicotine intake can lead to nicotine withdrawal symptoms and induce compensatory smoking (US Department of Health and Human Services, National Institute of Health, National Cancer Institute, 2001). Another concern with improper labeling is the potential for nicotine overexposure/overdose. Two refill fluids sold as unflavored nicotine in PG had no labeling indicating the nicotine content. Only through HPLC analysis were these products found to contain over 100mg of nicotine/ml. At these high concentrations, these products may have been intend as DIY products, but this was not stated on the manufacturer’s Web site at the time of purchase nor this was indicated on the bottles. A consumer would not know these products were DIY without proper labeling. Nicotine doses of 500–1000mg for adults (Mayer, 2013) and 10mg for children (Nicotine (PIM),” n.d.) can be lethal. Because neither of these bottles had the nicotine concentration or danger warnings printed on their labels, users of these products could be exposed to higher doses of nicotine than they intended. Moreover, bottles with such high concentrations of nicotine present a clear danger to children. When the concentration of a 10ml bottle of fluid is considered, the total nicotine content would exceed a lethal dose for both children and adults.
We previously showed that EC performance is highly variable both between and within brands of EC (Trtchounian et al., 2010; Williams & Talbot, 2011), that cytotoxicity of refill fluids varies among products (Bahl et al., 2012; Behar et al., 2013), and that puff duration varies among brands (Hua, Yip, & Talbot, 2013). Others have shown significant variability among products in the aerosolization of nicotine and in the concentration of tobacco-specific nitrosamines emitted in EC aerosols (Kim & Shin, 2013). Finally, EC users have sometimes reported symptoms consistent with nicotine overdoses (Hua, Alfi, & Talbot, 2013). Although this could occur for a number of reasons, accurate labeling would be important to prevent inadvertent overdosing. It is clear from these results that when evaluating the chemical components in EC fluid products and aerosols, multiple products and longitudinal duplicates of products should be tested before any determination can be made on the accuracy of a particular product line of refill fluids. These studies also demonstrate the importance of having regulations governing the accurate labeling of nicotine concentrations on EC products, as well as the need for guidelines to improve the integrity of manufacturing.
Funding
This work was supported by the Tobacco-Related Disease Research Program of California (grants number 20XT-0118 and 22RT-0127) and the UCR Academic Senate. BD was the recipient of a Cornelius Hopper Award and an NSF pre-doctoral fellowship.
Declaration of Interests
None declared.
Acknowledgments
We are grateful to RJ for his helpful suggestions on the manuscript.
References
- AEMSA. (2012). E-liquid manufacturing standards (Version 1.7, pp. 1–10). Author. [Google Scholar]
- AEMSA. (2014). E-Liquid manufacturing standards (Version 1.8, pp. 1–10). Author. [Google Scholar]
- Bahl V., Lin S., Xu N., Davis B., Wang Y. H., Talbot P. (2012). Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reproductive Toxicology (Elmsford, N.Y.), 34, 529–537. 10.1016/j.reprotox.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Behar R. Z., Davis B., Wang Y., Bahl V., Lin S., Talbot P. (2013). Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicology in Vitro, 28, 198–208. 10.1016/j.tiv.2013.10.006 [DOI] [PubMed] [Google Scholar]
- Cameron J. M., Howell D. N., White J. R., Andrenyak D. M., Layton M. E., Roll J. M. (2014). Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tobacco Control, 23, 77–78. 10.1136/tobaccocontrol-2012–050604 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, Office of Smoking and Health. (1988). The health consequences of smoking nicotine addiction. Rockville, MD: US Department of Health and Human Service, Public Health Service Office of the Surgeon General. [Google Scholar]
- Centers for Disease Control and Prevention, Office of Smoking and Health. (2010). How tobacco smoke causes disease: The biology and behavioral basis for smoking-attributable disease. Rockville, MD: US Department of Health and Human Service, Public Health Service Office of the Surgeon General. [PubMed] [Google Scholar]
- Etter J.-F., Zäther E., Svensson S. (2013). Analysis of refill liquids for electronic cigarettes. Addiction (Abingdon, England), 108, 1671–1679. 10.1111/add.12235 [DOI] [PubMed] [Google Scholar]
- Goniewicz M. L., Knysak J., Gawron M., Kosmider L., Sobczak A., Kurek J., … Benowitz N. (2013). Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco Control, 23, 133–139. 10.1136/tobaccocontrol-2012–050859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz M. L., Kuma T., Gawron M., Knysak J., Kosmider L. (2013). Nicotine levels in electronic cigarettes. Nicotine & Tobacco Research, 15, 158–166. 10.1093/ntr/nts103 [DOI] [PubMed] [Google Scholar]
- Hua M., Alfi M., Talbot P. (2013). Health-related effects reported by electronic cigarette users in online forums. Journal of Medical Internet Research, 15, e59. 10.2196/jmir.2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M., Yip H., Talbot P. (2013). Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tobacco Control, 22, 103–106. 10.1136/tobaccocontrol-2011–050226 [DOI] [PubMed] [Google Scholar]
- Kim H.-J., Shin H.-S. (2013). Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. Journal of Chromatography A, 1291, 48–55. 10.1016/j.chroma.2013.03.035 [DOI] [PubMed] [Google Scholar]
- Laugesen M., Thornley S., McRobbie H., Bullen C. (2008). How safe is an e-cigarette? (No. 67) (pp. 1–14), Christchurch, New Zealand: Health New Zealand Ltd; Retrieved from www.healthnz.co.nz [Google Scholar]
- Mayer B. (2013). How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Archives of Toxicology, 88, 5–7. 10.1007/s00204-013-1127-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotine (PIM). (n.d.). Nicotine (PIM) inchem.org Retrieved July 6, 2013, from www.inchem.org/documents/pims/chemical/nicotine.htm
- Odum L. E., O’Dell K. A., Schepers J. S. (2012). Electronic cigarettes: do they have a role in smoking cessation? Journal of Pharmacy Practice, 25, 611–614. 10.1177/0897190012451909 [DOI] [PubMed] [Google Scholar]
- Polosa R., Caponnetto P., Morjaria J. B., Papale G., Campagna D., Russo C. (2011). Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: A prospective 6-month pilot study. BMC Public Health, 11, 786. 10.1016/j.tips.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M. B., Tanwar K. L., Wood K. S. (2011). Electronic cigarettes as a smoking-cessation: Tool results from an online survey. American Journal of Preventive Medicine, 40, 472–475. 10.1016/j.amepre.2010.12.006 [DOI] [PubMed] [Google Scholar]
- Solarino B., Rosenbaum F., Rießelmann B., Buschmann C. T., Tsokos M. (2010). Death due to ingestion of nicotine-containing solution: Case report and review of the literature. Forensic Science International, 195, e19–e22. 10.1016/j.forsciint.2009.11.003 [DOI] [PubMed] [Google Scholar]
- Time Magazine. (2013). This is not a cigarette. Time Magazine, 39–46.
- Trehy M. L., Ye W., Hadwiger M. E., Moore T. W., Allgire J. F., Woodruff J. T., … Westenberger B. J. (2011). Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. Journal of Liquid Chromatography & Related Technologies, 34, 1442–1458. 10.1080/10826076.2011.572213 [Google Scholar]
- Trtchounian A., Talbot P. (2011). Electronic nicotine delivery systems: Is there a need for regulation? Tobacco Control, 20, 47–52. 10.1136/tc.2010.037259 [DOI] [PubMed] [Google Scholar]
- Trtchounian A., Williams M., Talbot P. (2010). Conventional and electronic cigarettes (e-cigarettes) have different smoking characteristics. Nicotine & Tobacco Research, 12, 905–912. 10.1093/ntr/ntq114 [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services, National Institute of Health, National Cancer Institute. (2001). Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. Bethesda: Maryland, Author. [Google Scholar]
- US Pharmacopeia 32/NF27. (2011). Nicotine transdermal system. 3, 3079–3081. [Google Scholar]
- Westenberger B. (2009). Evaluation of e-cigarettes (pp. 1–8). St Louis, MO: Department of Health & Human Services, Center for Drug Evaluation and Research, Food and Drug Administration, Division of Pharmaceutical Analysis. [Google Scholar]
- Williams M., Talbot P. (2011). Variability among electronic cigarettes in the pressure drop, airflow rate, and aerosol production. Nicotine & Tobacco Research, 13, 1276–1283. 10.1093/ntr/ntr164 [DOI] [PubMed] [Google Scholar]