Abstract
Introduction:
The purpose of this study was to evaluate sweet-flavored electronic cigarette (EC) liquids for the presence of diacetyl (DA) and acetyl propionyl (AP), which are chemicals approved for food use but are associated with respiratory disease when inhaled.
Methods:
In total, 159 samples were purchased from 36 manufacturers and retailers in 7 countries. Additionally, 3 liquids were prepared by dissolving a concentrated flavor sample of known DA and AP levels at 5%, 10%, and 20% concentration in a mixture of propylene glycol and glycerol. Aerosol produced by an EC was analyzed to determine the concentration of DA and AP.
Results:
DA and AP were found in 74.2% of the samples, with more samples containing DA. Similar concentrations were found in liquid and aerosol for both chemicals. The median daily exposure levels were 56 μg/day (IQR: 26–278 μg/day) for DA and 91 μg/day (IQR: 20–432 μg/day) for AP. They were slightly lower than the strict NIOSH-defined safety limits for occupational exposure and 100 and 10 times lower compared with smoking respectively; however, 47.3% of DA and 41.5% of AP-containing samples exposed consumers to levels higher than the safety limits.
Conclusions:
DA and AP were found in a large proportion of sweet-flavored EC liquids, with many of them exposing users to higher than safety levels. Their presence in EC liquids represents an avoidable risk. Proper measures should be taken by EC liquid manufacturers and flavoring suppliers to eliminate these hazards from the products without necessarily limiting the availability of sweet flavors.
Introduction
Electronic cigarettes (ECs) are novel nicotine-delivery products which have gained popularity among smokers in recent years.1 They deliver nicotine in aerosol form through heating a nicotine-containing solution resulting in the production of visible “vapor.” Besides nicotine delivery, they address the whole smoking ritual and psycho-behavioral dependence through sensory stimulation and motor simulation.2
Sensory stimulation is perceived from EC use both by the “throat hit” induced during aerosol inhalation3 as well as by the use of flavored liquids. The use of flavorings has resulted in a large debate among public health professionals and regulators, suggesting that they can be attractive to youth. A recent survey of dedicated users (vapers) concluded that flavors variability contributes to both perceived pleasure and the effort to reduce cigarette consumption or quit smoking, and showed that dedicated vapers switch between flavors quite frequently.4 Although the majority of flavorings are “Generally Recognized As Safe” (GRAS) for food use, these substances have not been adequately tested for safety when inhaled. In fact, the Flavors and Extracts Manufacturers’ Association (FEMA) has issued an official statement mentioning that flavor ingredients are evaluated for exposure through ingestion only; thus, any results cannot be extrapolated to use through inhalation.5 Studies have shown that any cytotoxic properties of e-cigarette liquids and aerosol, although significantly lower than tobacco smoke, may be attributed to specific flavors,6–8 indicating that further research is certainly needed in this area.
Besides the lack of studies for the effects of flavoring substances when inhaled, there are some chemicals which, although approved for ingestion, have already established adverse health effects when inhaled. A characteristic example of this is diacetyl (DA, Figure 1). This substance, also known as 2,3-butanedione, is a member of a general class of organic compounds referred to as diketones, α-diketones or α-dicarbonyls. It is responsible for providing a characteristic buttery flavor, and is both naturally found in foods and used as a synthetic flavoring agent in food products such as butter, caramel, cocoa, coffee, dairy products, and alcoholic beverages.9 Although it is approved and safe when ingested (National Institute for Occupational Safety and Health10; FEMA Nr 2370), it has been associated with decline in respiratory function, manifested as reduced Forced Expiratory Volume in 1s (FEV1), in subjects exposed to it through inhalation. Additionally it has been implicated in the development of bronchiolitis obliterans, an irreversible respiratory disease also called “popcorn lung disease” because it was initially observed in workers of popcorn factories.11–13 To the best of our knowledge, the issue of DA presence in EC liquids was first mentioned in 2008 in EC consumers’ forums (http://www.e-cigarette-forum.com/forum/health-safety-e-smoking/2666-inhaling-flavouring-chemicals.html). Subsequently, several companies released statements mentioning that DA was removed from their EC liquid products (e.g. http://clearstream.flavourart.it/site/?p=366&lang=en). Another chemical of concern is acetyl propionyl (AP), also called 2,3-pentanedione (Figure 1). This is also an α-diketone and is chemically and structurally very similar to DA. It has become a popular replacement for DA (Day et al.14; FEMA Nr 2841) since the negative press surrounding DA-induced bronchiolitis obliterans in popcorn workers, because it adds the desired flavor while claims of “diacetyl-free” can be made by the manufacturer. Unfortunately, the risks associated with inhalation of AP may well be as high as from DA, based on inhalation studies performed on rats.15 Due to the potential hazards associated with inhalation exposure to DA and AP, regulatory agencies have set specific Occupational Exposure Limits (OELs). For DA, the National Institute on Occupational Safety and Hazards (NIOSH) has proposed an upper limit of 5 ppb (18 µg/m3) for 8hr Time-Weighted Average exposure (TWA) and 25 ppb (88 µg/m3) as Short-Term Exposure Limit (STEL) for 15min, while the Scientific Committee on Occupational Exposure Limits (SCOEL) of the European Commission considered the NIOSH-defined limits for DA unnecessarily strict and has set upper limits of 20 ppb (70 µg/m3) and 100 ppb (360 µg/m3) respectively.10,16 For AP, NIOSH has set a TWA limit of 9.3 ppb (38 µg/m3) and an STEL of 31 ppb (127 µg/m3).10
Figure 1.
Chemical structures of diacetyl (DA) and acetyl propionyl (AP).
The purpose of this study was to examine the presence of DA and AP in a large sample of EC liquids obtain from European and US manufacturers and retailers. Additionally, we sought to measure the levels of these chemicals in aerosol produced from ECs, since this represents the realistic use of ECs and the relevant exposure route of vapers, and compare this with literature data evaluating exposure from smoking tobacco cigarettes.
Methods
Sample Selection
Samples of EC liquids were selected from European and US manufacturers and retailers. The selection was based on information from local or international EC consumers’ forums, in order to get samples from major or popular sources. Since the chemicals examined were more likely to be present in sweet flavorings, we chose samples with sweet flavors (butter, toffee, milky, cream, chocolate, coffee, caramel, etc). A total of 159 samples were selected from 36 manufacturers and retailers from six European countries (France, Germany, Greece, Italy, Poland, and United Kingdom, n = 78) and from the United States (n = 81). Both refill liquids (“ready to use,” n = 113) and concentrated flavors (n = 46), which are diluted by users in “base” liquids (mixtures of propylene glycol, glycerol, and nicotine), were obtained. Different number of samples per manufacturer was obtained, depending on the availability of sweet flavorings. In several cases, there were clear statements in the manufacturers’ websites that no DA was present in their liquids. All samples were bought anonymously from internet shops, without mentioning that the purpose of the purchase was to be analyzed for a scientific study. All bottles were received sealed, and were immediately sent to the laboratory for analysis.
Methods of Analysis
The samples were analyzed by High Performance Liquid Chromatography (HPLC). The procedure followed was a modified version of the HPLC carbonyl compound analysis method for mainstream cigarette smoke, by the Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA).17 This method was previous validated by the laboratory for the analysis of carbonyls in EC liquids and was expanded for the analysis of DA and AP. The performance of the method for diketones was evaluated for recovery from the sample matrix by addition of known amount of DA and AP before derivatization. In all cases the recovery of both compounds was greater than 80%. To prevent the formation of two carbonyl adducts, an aliquot of the sample for analysis was combined with 1ml of a standard 2,4-dinitrophenylhydrazine (DNPH) trapping solution and allowed to derivatize for 20min, then quenched with 0.050ml of pyridine. This ensures that only one of the two carbonyls is converted to its derivative. DA and AP standards were produced by adding known amounts of DA and AP to the DNPH trapping solution. Standards were treated in the same manner as samples, and were used to prepare a linear calibration curve which ranged from 0.4–30 μg/ml. All e-liquid samples were analyzed at an initial 22-fold dilution, while pure flavor samples were analyzed at an initial 43-fold dilution. At these dilutions, the maximum amount of propylene glycol and glycerol in the DNPH solution was less than 5% and had no effect on derivatization. The efficient derivatization of DA and AP requires excess DNPH, and all samples were evaluated for DNPH depletion by verifying that a large DNPH peak was observed by HPLC. Any samples that were found to have depleted DNPH were prepared and reanalyzed using a smaller sample aliquot (thus, DNPH trapping solution was used to dilute the samples). An Agilent Model 1100, High Performance Liquid Chromatograph was equipped with an Ultraviolet (UV) Detector operating at 365nm and a Waters Xterra MS C18, 3.0×250mm column. Two solutions, A and B, were used as mobile phases in varying relative concentrations over time. Mobile Phase A: 890ml water, 100ml of tetrahydrofuran and 10ml of isopropanol. Mobile Phase B: 890ml acetonitrile, 100ml of tetrahydrofuran, and 10ml of isopropanol. Separation was accomplished with the following linear gradient: 0.00min 65% A, 35% B; 11.00min 40.0% A, 60% B; 18min 0% A, 100% B. Flow rate was set to a constant 0.75ml/min.
The materials used for the HPLC analysis were: deionized water–Millipore; phosphoric acid (H3PO4), 85%, A.C.S Reagent, Sigma-Aldrich (P/N 438081); DNPH (50%), TCI America, P/N D0845; acetonitrile (CAS #75-05-8), HPLC grade; tetrahydrofuran (CAS #109-99-9), HPLC grade; isopropanol (CAS #67-63-0), distilled-in-glass; pyridine (CAS #110-86-1); diacetyl (97%) Sigma-Aldrich (P/N B85307) (CAS #431-03-8); 2,3-pentanedione (97%) Sigma-Aldrich (P/N 241962) (CAS # 600-14-6).
Aerosol Production and Analysis
To evaluate the amount of DA and AP that is transferred from liquid to aerosol, three liquids were prepared by diluting the sample of concentrated flavor with the highest level of diacetyl to 5%, 10%, and 20% in a mixture of 50% propylene glycol and 50% glycerol. These dilutions were chosen because they represent the most common dilutions of concentrated flavors used or recommended for EC use. The prepared liquids were analyzed by HPLC (with the method described above), to determine the concentration of DA and AP. Aerosol was produced by using a commonly used commercially-available EC device (eGo battery, Joyetech) with a bottom-coil clearomizer (EVOD, KangerTech). The device was fully charged before use and a new tank and atomizer was used for each sample. Approximately 2ml of the prepared liquid was added to the tank. The device was weighed before and after sample collection. A Cerulean SM 450 smoking machine was used to collect 50 puffs from all samples. The smoking machine was set to deliver a 55ml puff over 4 s every 30 s18 with a constant flow of 13.75ml/s. The EC device was automatically triggered at the beginning of the puff for 4 s, by using a custom air-piston mechanism to push the activation button. The aerosol was passed through an impinger containing 35ml of the DNPH trapping solution without the use of a filter pad. Once the aerosol collection was complete, 5ml of this solution was quenched with 250 µl of pyridine. The samples were then analyzed by HPLC monitoring at 365nm.
Interpreting NIOSH Safety Limits in the Context of EC Liquids
The TWA limits (8-hr exposure) defined by NIOSH (5 ppb, i.e. 18 μg/m3 for DA and 9.3 ppb, i.e. 38 μg/m3 for AP) were used as a guide to define potentially “acceptable” levels of DA and AP in EC liquids. The average resting respiratory rate for an adult is 15 breaths/min while the tidal volume is 0.5L.19 Within 8hr (480min), the total volume of air inhaled is 3.6m3 ([0.5L × 15 breaths/min × 480 min]/1,000L/m3). Thus, the total amount of DA that can be inhaled daily (according to NIOSH limits) is 65 μg (18 μg/m3 × 3.6 m3), while for AP it is 137 μg (38 μg/m3 × 3.6 m3).
Statistical Analysis
Data were examined for distribution by Kolmogorov-Smirnov test. Continuous variables were expressed as median (interquartile range [IQR]) while categorical variables were expressed as number (%). For DA and AP levels, the medians were calculated from the samples which contained the chemicals only (samples with non-detectable DA and AP were excluded). To assess the difference in DA and AP levels between concentrated flavors and refill liquids, Mann-Whitney U test was used. To assess the realistic exposure to DA and AP from concentrated flavors, we multiplied the levels found in these samples with 0.2, assuming that they are diluted to 20% in order to prepare a refill liquid. Chi-square test was used to assess the differences between European countries and United States in the number of samples containing DA and AP. Pearson’s correlation coefficient was used to assess the correlation between expected and measured DA and AP levels in the aerosol analysis. To estimate the average daily exposure, consumption of EC liquid was assumed to be 3ml/day, based on the results of a large survey of vapers.20 To assess the difference in DA and AP daily exposure between smoking and EC use, Mann-Whitney U test was also used. A two-tailed P value of <.05 was considered statistically significant. Commercially-available statistical software was used for the analysis (SPSS v. 18).
Results
Analysis of Liquid Samples
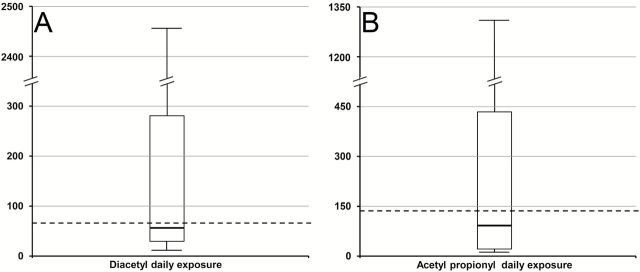
In 41 (25.8%) samples DA and AP was not detected, while in 73 (45.9%) samples one of the two chemicals was detected and in 45 (28.3%) samples both chemicals were detected. DA was found in 110 (69.2%) samples, containing a median concentration of 29 μg/ml (IQR: 10–170 μg/ml). Of those, 32 were concentrated flavors samples (69.6% of all concentrated flavors samples) and 78 were refill samples (69.0% of all refill samples). Concentrated flavors contained 3 times higher levels of DA compared to refill liquids (median: 68 μg/ml vs. 20 μg/ml, p = .001), with the highest levels being 32,115 μg/ml in the former and 10,620 μg/ml in the latter. DA was detected in the samples of 33 manufacturers (91.6%) from all seven countries (66.7% of European and 71.6% of US samples, chi-square p = .500). By converting the levels of DA found in concentrated flavors to represent realistic exposure (see Statistical analysis section), the median daily exposure level to DA from all DA-containing samples was calculated at 56 μg/day (IQR: 26–278 μg/day, Figure 2A). This is slightly lower than the NIOSH-defined safety limit (65 μg/day). However, 52 samples (47.3% of the positive samples) would expose consumers to levels higher than the NIOSH limits, with 26 of them (23.6%) having >5 times higher levels than the safety limit. The sample with the highest level of DA would result in 490 times higher daily intake compared to the NIOSH limit.
Figure 2.
Box-plots of the estimated daily exposure to diacetyl (A) and acetyl propionyl (B) from the liquid samples tested. The box represents the 25th and 75th percentiles, with the line inside the box showing the median value. The error bars represent the 10th and 90th percentiles. The dotted line represents the maximum acceptable levels of daily exposure estimated from the NIOSH limit for occupational exposure.
AP was found in 53 (33.3%) samples, containing a median concentration of 44 μg/ml (IQR: 7–172 μg/ml). Of those, 10 were concentrated flavors samples (21.7% of all concentrated flavors samples) and 43 were refill samples (38.1% of all refill samples). Concentrated flavors contained 3 times higher levels of AP compared to refill liquids (median: 124 μg/ml vs. 37 μg/ml, p = .114). The difference was not statistically significant, probably due to the low number of concentrated flavors containing AP. The highest levels found were 3,082 μg/ml in concentrated flavors and 1,018 μg/ml in refills. AP was detected in the samples of 24 manufacturers (66.7%) from six countries (23.1% of European and 43.2% of US samples, chi-square p = .007). By converting the levels of AP found in concentrated flavors to represent realistic exposure (see Statistical analysis section), it was estimated that the median daily exposure level to AP from all AP-containing samples was 91 μg/day (IQR: 20–432 μg/day, Figure 2B). This is lower than the NIOSH-defined safety limit (137 μg/day). However, 22 samples (41.5% of the positive samples) would expose consumers to levels higher than the NIOSH limits, with 11 of them (20.8%) having >5 times higher levels than the safety limit. The sample with the highest level of AP would result in 22 times higher daily intake compared to the NIOSH limit.
Analysis of Aerosol
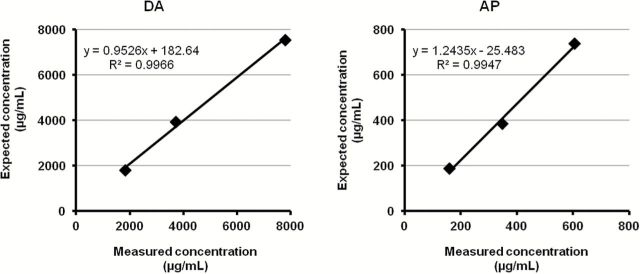
One concentrated flavor sample was diluted to 5%, 10%, and 20% into a mixture of 50% propylene glycol and 50% glycerol, in order to prepare the three liquids used for the aerosol analysis. The prepared liquids were analyzed by HPLC and were found to contain DA and AP at respective levels of 1,801 μg/ml and 160 μg/ml for the 5% sample, 3,921 μg/ml and 349 μg/ml for the 10% solution, and 7,546 μg/ml and 606 μg/ml for the 20% solution. Based on the weight-difference of the atomizer before and after the puffing session, we evaluated the volume of liquid consumed in each puffing session by dividing the amount (mg) of liquid consumed with the specific weight of the samples (which was determined to be 1.13). From that, the concentrations of DA and AP per ml of liquid consumed were determined. Similar concentrations of DA and AP were observed in the liquid and aerosol samples while a very strong correlation was observed between the expected (based on the liquid consumption) and the observed (measured) DA and AP concentrations (R 2 = 0.997 and 0.995 respectively, Figure 3). These results indicate that both DA and AP are readily delivered from the liquid to the aerosol.
Figure 3.
Correlation between the expected (based on liquid consumption during aerosol production) and the measured concentrations of diacetyl (DA) and acetyl propionyl (AP) in aerosol. A strong correlation was observed, while the expected and measured values were almost identical, verifying that DA and AP are readily delivered from the liquid to the aerosol and that no additional DA and AP are produced during the evaporation process.
Comparison With Exposure From Tobacco Cigarettes
To compare DA and AP exposure from EC use and smoking, the study by Pierce et al.21 was used. By using the ISO 3308 smoking regime, an average of 285 μg of DA and 43 μg of AP (average values) was emitted in the smoke of a single cigarette. Considering a daily consumption of 20 cigarettes, the median daily exposure would be 5,870 μg (4,970–6,195 μg) for DA and 894 μg (713–965 μg) for AP (we estimated the median values in order to be compared with the data from our study, which were not normally distributed). As mentioned previously, the median daily levels of DA and AP exposure from EC use were estimated to be 56 μg and 91 μg respectively, which are 100 and 10 times lower compared to smoking (Mann-Whitney p < .001 for DA and p = .020 for AP).
Discussion
Main Findings
This is the first study to analyze a large number of EC liquids with sweet flavors obtained from a variety of manufacturers and retailers from Europe and the United States for the presence of DA and AP. The main findings were that these substances were present in the majority of the samples tested, with a significant proportion containing both chemicals; they were detected even in samples coming from manufacturers who clearly stated that they were not present in their products. Additionally, it was determined that both DA and AP are readily delivered to the aerosol that the vaper inhales, an expected finding considering the volatility of these compounds. Although the median levels found were slightly lower than the strict NIOSH-defined safety levels, a substantial proportion of the positive samples would expose consumers to levels higher than the safety limits.
Flavorings in ECs
The issue of flavoring use in EC products is a matter of strong debate, mostly in terms of being appealing to youth. A survey of more than 4,000 dedicated users determined that the reason for the availability of a large variety of flavors is the market demand by existing consumers (vapers), and showed that sweet flavors were the most popular category used by this population.4 Less attention has been given to the issue of safety when inhaling food-approved substances. While many food flavorings have never been tested for inhalation safety, the focus here was on known inhalation toxins that are flavor compounds.
Toxicity of DA and AP
DA is a water soluble volatile α-diketone that is both a natural constituent of numerous foods and an added ingredient used by the flavoring industry. In 1995, an estimated 96,000kg of diacetyl were used in the food industry.22 It has been identified as a prominent volatile organic compound in air samples from microwave popcorn plants and flavoring manufacturing plants.23,24 DA exposure through inhalation has been associated with a decline in respiratory function (characterized by a declined in FEV1) and the development of bronchiolitis obliterans, a rare irreversible obstructive disease involving the respiratory bronchioles. Kreiss et al.13 evaluated 117 workers in a microwave popcorn production plant in Missouri and found that these workers had 2.6 times the expected rate of respiratory symptoms such as chronic cough and shortness of breath and 3.3 times the expected rate of airway obstruction. Kanwal et al.11 examined workers in six popcorn plants and found that exposure to flavorings mixing for more than 12 months was associated with higher prevalence of decline in respiratory function, while three cases of bronchiolitis obliterans were documented by lung biopsy. Similar findings were observed by Lockey et al.25 Three cases of clinical bronchiolitis obliterans were also diagnosed in a diacetyl facility in the Netherlands.26 Finally, a cross-sectional analysis of medical surveillance data from 16 companies confirmed the risk of lung disease among workers at companies using diacetyl.27
AP is chemically and structurally almost identical to DA, has a similar buttery, creamy flavor, and has been used as a DA substitute in many flavoring manufacturing facilities.14 Toxicological studies in animals have shown that it has adverse effects on respiratory epithelium similar to DA and at similar levels.15,28
Study Implications
A wide range of DA and AP concentrations were found in the samples, indicating that in some cases the chemicals were used deliberately as ingredients while in others they were probably contaminants. Overall the estimated daily exposure from EC use was approximately 100 times lower for DA and 10 times lower for AP compared to tobacco cigarettes; therefore, it is still plausible to classify ECs as tobacco harm reduction products.29 However, the major source of DA and AP in tobacco cigarette smoke is the combustion process;21 thus, it is an unavoidable risk. In EC liquids, these chemicals are introduced during the production process, since there is no combustion. Production of DA and AP from thermal decomposition is unlikely, and was not detected in this study. Since 25.8% of the samples of similar flavors were DA and AP free, the findings indicate that vapers are exposed to an avoidable risk. It is imperative that appropriate removal measures should be undertaken. The major source of flavorings for EC liquid manufacturers is the food-flavoring industry, with DA and AP being approved as ingredients. Establishment of an inhalation-specific flavoring industry is recommended, with dedication to evaluate and choose appropriate flavoring compounds for EC liquids, based on inhalation safety profiles. In any case, it is of high priority for every manufacturer to properly examine the flavorings used in the production process. The results of the aerosol analysis, showing that DA and AP are readily delivered from the liquid to the aerosol, indicate that analysis of the liquid is sufficient.
Limitations
Our selection was targeted to sweet-only flavors because it was expected that these are more likely to contain DA and AP. Other classes of flavorings available in the market, such as tobacco, mint/menthol, fruits, beverages, and nuts, probably have lower prevalence of DA and AP. However, we cannot exclude the possibility that there may be liquids from other flavor types (besides sweets) which contain these compounds.
Fewer samples contained AP compared to DA. This was unexpected, since it has been common practice for the flavoring industry to substitute DA with alternative chemicals due to the criticism for the adverse effects of DA exposure to workers. It is unknown whether this is a generalized finding in the EC liquid market or it is attributed to chance related to the selection of the samples.
Although we tried to define the “acceptable” levels of DA and AP in EC liquids, there is no clinical evidence indicating that the limit set by NIOSH is applicable to EC use. This limit is set for occupational exposure, and no exposure limit has been set for continuous or recreational exposure to EC aerosols. Therefore, this assessment should be approached with caution. The cut-off level of risk calculated by NIOSH for the TWA limit is for 1 in 1,000 chance of suffering reduced lung function associated with lifelong diacetyl exposure. This is a very conservative estimation; however, a significant proportion of the samples had >5 times higher levels of DA and AP than NIOSH limits. Moreover, the finding that more than 25% of the samples tested did not contain any of the two chemicals shows that it is feasible to prepare sweet flavorings with alternative chemicals; thus, there is no need to exclude them from the market, since they have been found to be quite popular among dedicated users.
A recent study raised doubts about the association between DA and AP exposure and development of bronchiolitis obliterans;21 high levels of these chemicals were found in tobacco smoke while smoking is not a risk factor for development of the disease. However, cigarette smoke contains many respiratory irritants, which probably act synergistically and cause a different pattern of lung disease. The prevalence of chronic obstructive lung disease in active smokers is estimated to be 15.4%,30 by far higher than the prevalence of bronchiolitis obliterans in patients exposed to diacetyl. Moreover, it is quite common that the condition is often misdiagnosed.13 Finally, post-mortem examinations have shown that many smokers have histopathological features of respiratory bronchiolitis.31
Conclusion
In conclusion, DA and AP were present in a large proportion of sweet-flavored EC liquid samples from both European and US manufacturers and retailers, and are readily delivered to the aerosol inhaled by the users. The median level of exposure is lower compared to tobacco cigarettes by 1–2 orders of magnitude, confirming their role as tobacco harm reduction products. However, any risk from exposure to DA and AP by EC use is totally avoidable, by using alternative compounds, and this was evident from the samples of similar flavor in which no DA or AP was detected. Manufacturers and flavoring suppliers should take the necessary steps to make sure that these chemicals are not present in EC liquid products, by regularly testing their products and changing formulations, without the need to limit the availability of sweet flavors in the market.
Funding
This study was funded through an open internet crowd-funding campaign which was conducted in the website www.indiegogo.com.
Declaration of Interests
Some of the studies by KF and VV were performed using funds provided to the institution by e-cigarette companies.
Acknowledgments
We would like to thank Dimitris Agrafiotis (a volunteer vaping advocate) for his assistance in organizing the crowd-funding campaign and in the selection of EC liquid samples.
References
- 1. Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the ‘e-cigarette’ in the USA. Tob Control. 2013;22:19–23. [DOI] [PubMed] [Google Scholar]
- 2. Farsalinos KE, Stimson GV. Is there any legal and scientific basis for classifying electronic cigarettes as medications? [published online ahead of print April 07, 2014]. Int J Drug Policy. 10.1016/j.drugpo.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 3. Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V. Impact of flavour variability on electronic cigarette use experience: an internet survey. Int J Environ Res Public Health. 2013;10:7272–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flavors and Extracts Manufacturers’ Association (FEMA). Safety assessment and regulatory authority to use flavors: focus on e-cigarettes 2014. http://www.femaflavor.org/safety-assessment-and-regulatory-authority-use-flavors-focus-e-cigarettes. Accessed May 31, 2014.
- 6. Farsalinos KE, Romagna G, Allifranchini E, et al. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health. 2013;10:5146–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Romagna G, Allifranchini E, Bocchietto E, Todeschi S, Esposito M, Farsalinos KE. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhal Toxicol. 2013;25:354–361. [DOI] [PubMed] [Google Scholar]
- 8. Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34:529–537. [DOI] [PubMed] [Google Scholar]
- 9. Mathews JM, Watson SL, Snyder RW, Burgess JP, Morgan DL. Reaction of the butter flavorant diacetyl (2,3-butanedione) with N-α-acetylarginine: a model for epitope formation with pulmonary proteins in the etiology of obliterative bronchiolitis. J Agric Food Chem. 2010;58:12761–12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Institute for Occupational Safety and Health (NIOSH). Criteria for a recommended standard: occupational exposure to diacetyl and 2,3-Pentanedione 2011. http://www.cdc.gov/niosh/docket/archive/pdfs/NIOSH-245/DraftDiacetylCriteriaDocument081211.pdf. Accessed May 27, 2014.
- 11. Kanwal R, Kullman G, Piacitelli C, et al. Evaluation of flavorings-related lung disease risk at six microwave popcorn plants. J Occup Environ Med. 2006;48:149–157. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention. Fixed obstructive lung disease in workers at a microwave popcorn factory--Missouri, 2000–2002. JAMA. 2002;287:2939–2940. [PubMed] [Google Scholar]
- 13. Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347:330–338. [DOI] [PubMed] [Google Scholar]
- 14. Day G, LeBouf R, Grote A, et al. Identification and measurement of diacetyl substitutes in dry bakery mix production. J Occup Environ Hyg. 2011;8:93–103. [DOI] [PubMed] [Google Scholar]
- 15. Hubbs AF, Cumpston AM, Goldsmith WT, et al. Respiratory and olfactory cytotoxicity of inhaled 2,3-pentanedione in Sprague-Dawley rats. Am J Pathol. 2012;181:829–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. European Commission. Recommendation from the Scientific Committee on Occupational Exposure Limits for diacetyl 2013. http://www.ser.nl/documents/82310.pdf. Accessed May 27, 2014.
- 17. Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA). CORESTA Recommended Method No. 75: determination of selected carbonyls in mainstream cigarette smoke by HPLC 2013. http://www.coresta.org/Recommended_Methods/CRM_75.pdf. Accessed March 19, 2014.
- 18. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10:2500–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barrett KE, Ganong WF. Ganong’s review of medical physiology. London, UK: McGraw-Hill Medical; 2012. [Google Scholar]
- 20. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Characteristics, perceived side effects and benefits of electronic cigarette use: a worldwide survey of more than 19,000 consumers. Int J Environ Res Public Health. 2014;11:4356–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pierce JS, Abelmann A, Spicer LJ, Adams RE, Finley BL. Diacetyl and 2,3-pentanedione exposures associated with cigarette smoking: implications for risk assessment of food and flavoring workers. Crit Rev Toxicol. 2014;44:420–435. [DOI] [PubMed] [Google Scholar]
- 22. Harber P, Saechao K, Boomus C. Diacetyl-induced lung disease. Toxicol Rev. 2006;25:261–272. [DOI] [PubMed] [Google Scholar]
- 23. Akpinar-Elci M, Travis WD, Lynch DA, Kreiss K. Bronchiolitis obliterans syndrome in popcorn production plant workers. Eur Respir J. 2004;24:298–302. [DOI] [PubMed] [Google Scholar]
- 24. Parmet AJ, Von Essen S. Rapidly progressive, fixed airway obstructive disease in popcorn workers: a new occupational pulmonary illness? J Occup Environ Med. 2002;44:216–218. http://journals.lww.com/joem/Citation/2002/03000/Rapidly_Progressive,_Fixed_Airway_Obstructive.2.aspx. Accessed June 4, 2014. [DOI] [PubMed] [Google Scholar]
- 25. Lockey JE, Hilbert TJ, Levin LP, et al. Airway obstruction related to diacetyl exposure at microwave popcorn production facilities. Eur Respir J. 2009;34:63–71. [DOI] [PubMed] [Google Scholar]
- 26. van Rooy FG, Rooyackers JM, Prokop M, Houba R, Smit LA, Heederik DJ. Bronchiolitis obliterans syndrome in chemical workers producing diacetyl for food flavorings. Am J Respir Crit Care Med. 2007;176:498–504. [DOI] [PubMed] [Google Scholar]
- 27. Kim TJ, Materna BL, Prudhomme JC, et al. Industry-wide medical surveillance of California flavor manufacturing workers: cross-sectional results. Am J Ind Med. 2010;53:857–865. [DOI] [PubMed] [Google Scholar]
- 28. Morgan DL, Jokinen MP, Price HC, Gwinn WM, Palmer SM, Flake GP. Bronchial and bronchiolar fibrosis in rats exposed to 2,3-pentanedione vapors: implications for bronchiolitis obliterans in humans. Toxicol Pathol. 2012;40:448–465. [DOI] [PubMed] [Google Scholar]
- 29. Polosa R, Rodu B, Caponnetto P, Maglia M, Raciti C. A fresh look at tobacco harm reduction: the case for the electronic cigarette. Harm Reduct J. 2013;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev. 2009;18:213–221. [DOI] [PubMed] [Google Scholar]
- 31. Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974;291:755–758. [DOI] [PubMed] [Google Scholar]