Highlight
Dormancy release by cold stratification depends on jasmonate biosynthesis in wheat grains
Key words: Abscisic acid, acetylsalicylic acid, cold, dormancy, jasmonate, stratification, wheat.
Abstract
Hydration at low temperatures, commonly referred to as cold stratification, is widely used for releasing dormancy and triggering germination in a wide range of species including wheat. However, the molecular mechanism that underlies its effect on germination has largely remained unknown. Our previous studies showed that methyl-jasmonate, a derivative of jasmonic acid (JA), promotes dormancy release in wheat. In this study, we found that cold-stimulated germination of dormant grains correlated with a transient increase in JA content and expression of JA biosynthesis genes in the dormant embryos after transfer to 20 oC. The induction of JA production was dependent on the extent of cold imbibition and precedes germination. Blocking JA biosynthesis with acetylsalicylic acid (ASA) inhibited the cold-stimulated germination in a dose-dependent manner. In addition, we have explored the relationship between JA and abscisic acid (ABA), a well-known dormancy promoter, in cold regulation of dormancy. We found an inverse relationship between JA and ABA content in dormant wheat embryos following stratification. ABA content decreased rapidly in response to stratification, and the decrease was reversed by addition of ASA. Our results indicate that the action of JA on cold-stratified grains is mediated by suppression of two key ABA biosynthesis genes, TaNCED1 and TaNCED2.
Introduction
Timing of germination is one of the most critical adaptation strategies used by plants to ensure reproductive success. In wild plant species, dormancy has a key role in ensuring survival of a population by blocking seed germination until conditions become favourable for germination and seedling establishment (Bewley, 1997). In addition to genetic determinants, environmental signals experienced during seed maturation and following dispersal strongly influence the timing of dormancy loss. Unlike many wild plant species, cultivated crops such as wheat (Triticum aestivum L.) display weak grain dormancy at maturity due to selective breeding against dormancy for uniform and vigorous germination. As a result, modern wheat varieties exhibit increased susceptibility to pre-harvest sprouting (PHS) following cool and moist conditions in the field, which result in serious loss of grain yield and quality (Gubler et al., 2005; Rodríguez et al., 2015). Thus, research aimed at understanding environmental and genetic control of dormancy will assist in developing new strategies for the elimination of PHS worldwide in domesticated crops.
A variety of environmental signals including temperature, light quality, photoperiod, and nitrate have been shown to influence cereal dormancy (Rodríguez et al., 2015), with temperature widely recognized as the major signal (Harrington, 1923; Sawhney and Naylor, 1979; Buraas and Skinnes, 1985; Reddy et al., 1985; Nyachiro et al., 2002; Gu et al., 2006). Interestingly, temperature effects on grain dormancy are very much dependent on the developmental stage. In general, temperature during grain development strongly influences dormancy, with cooler conditions producing more dormant grains (Barrero et al., 2015). Exposure to higher temperatures for brief or extended periods during grain maturation has been shown to reduce dormancy (Gualano and Benech-Arnold, 2009).
There is also a dramatic change in the low temperature responses once grains are imbibed. Imbibition at 4 oC, commonly referred to as cold stratification, is widely used to break dormancy (Bewley and Black, 1994). It is considered that cold stratification mimics the cold and moist conditions experience by cereals in soil during autumn and winter, during which seed dormancy is lost in order to trigger germination prior to spring. In wheat, stratification for 48h at 4 oC in the dark is sufficient to induce germination even in strongly dormant cultivars (Mares, 1984). It is important to note that prematurely harvested grains as early as 18 d post-anthesis are capable of responding to stratification, although the effectiveness of the cold stratification increases with maturity (Gosling et al., 1981). Even though the effect of cold is well known, the hormonal and molecular mechanisms by which cold stratification regulate cereal dormancy remain unknown.
Plant hormones play a key role in dormancy regulation through synergistic and antagonistic interactions. In cereals and other plants, abscisic acid (ABA) is considered to be the primary mediator in dormancy induction and maintenance (Finkelstein et al., 2008; Rodríguez et al., 2015; Shu et al., 2016). Genes encoding ABA metabolism enzymes appear to be major targets for environmental signals that promote dormancy, such as hypoxia, high temperature, and blue light (BL) (Rodríguez et al., 2015). Furthermore, dormancy release by after-ripening is mediated by a decrease in ABA content in imbibed wheat and barley grains as a result of co-ordinated promotion of ABA catabolism and repression of ABA biosynthesis genes (Millar et al., 2006; Jacobsen et al., 2013).
ABA action is regulated in part by crosstalk with other hormones such as gibberellins (GAs) and their associated signalling networks. Applications of GA, an antagonist of ABA, can be effective in breaking dormancy in cereals (Jacobsen et al., 2002; Tuttle et al., 2015). In Arabidopsis, GA is essential for germination, but this has not yet been demonstrated for cereals (Jacobsen et al., 2002; Peng and Harberd, 2002; Ogawa et al., 2003; Gubler et al., 2005; Finkelstein et al., 2008; Hauvermale et al., 2015). A recent study has also demonstrated that jasmonates can act antagonistically to ABA by promoting dormancy release in wheat dormant grains (Jacobsen et al., 2013). Applications of methyl-jasmonate (MeJA) inhibited the expression of the ABA biosynthesis gene, Ta9-cis-EPOXYCAROTENOID DIOXYGENASE1 (TaNCED1), resulting in a decrease in ABA content in imbibed embryos prior to germination. However, in Arabidopsis, jasmonic acid (JA) and its precursor 12-cis-oxophytodienoic acid (OPDA) inhibited seed germination (Dave et al., 2011), indicating that the role of JAs in dormancy varies according to the species. Other hormones such as ethylene, brassinosteroid, and auxin also play roles in germination of Arabidopsis, but as yet there is no information on whether they mediate dormancy processes in cereals (Steber and McCourt, 2001; Xue et al., 2009; X, Liu et al., 2013; Corbineau et al., 2014).
Our current understanding of the role of hormones in cold stratification of seeds is very limited. In non-dormant Arabidopsis seeds, bioactive GA4 content increased in response to low temperature during imbibition, due—at least in part—to increased expression of a key GA biosynthesis gene (Yamauchi et al., 2004). Mutant analysis revealed that the AtGA3ox1 gene is required for cold-stimulated seed germination. However, this work has not been extended to test if this is also the case in imbibed dormant seeds. In wheat, changes in ABA sensitivity have been observed in response to stratification (Mares, 1984; Noda et al., 1994), but it is not clear whether this is due to changes in ABA metabolism or signalling. Recently, a cold-induced increase in JA content was demonstrated in Arabidopsis seedlings exposed to cold. In this study, some genes of the JA biosynthesis pathway such as lipoxygenase (LOX), allene oxide synthase (AOS), and allene oxide cyclase (AOC) were induced by cold (Hu et al., 2013). A cold-induced increase in JA has also been reported in rice roots (Moons et al., 1997). Although we have previously demonstrated that MeJA and JA promote dormancy release in wheat (Jacobsen et al., 2013) and that the JA biosynthesis pathway was up-regulated in the coleorhiza of non-dormant barley embryos compared with dormant grains (Barrero et al., 2009), there was no evidence linking JA with cold responses in grains.
In this study we have undertaken hormonal and molecular analyses to investigate the role of JAs and other hormones in stratification of dormant wheat grains. Applying optimized reversed phase liquid chromatography with electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS), we found that JA and jasmonyl-isoleucine (JA-Ile) contents increased rapidly in embryos of dormant grains following 48h stratification compared with grains that were incubated at 20 oC. Molecular analysis revealed that the increase in JA and JA-Ile correlated with expression of cold-inducible JA biosynthesis genes. Furthermore, using a jasmonate biosynthesis inhibitor, we showed that jasmonate synthesis is required for cold-induced germination in wheat. The results also indicated that the increase in JA triggers a decrease in ABA content in the embryos of cold-stratified grains. The results are in agreement with our earlier study which showed that JA is a repressor of ABA biosynthesis (Jacobsen et al., 2013), and they provide the first evidence that JAs play a central role in the release of seed dormancy by cold stratification.
Materials and methods
Plant materials
Two spring wheats cultivars (Triticum aestivum L. cv. Sunstate and cv. AC Barrie) were grown in a phytotron with the temperature set at 17 °C/9 °C day/night cycle as previously described (Gubler et al., 2008). Sunstate is an Australian white-grained wheat and AC Barrie is a Canadian red-grained wheat. Heads were harvested at maturity, dried at 37 °C for 1 d, and grains were threshed by hand (to avoid grain damage and to remove the husk) and then stored at –20 °C to retain dormancy or after-ripened at 37 °C for 4 weeks.
Chemicals
HPLC-grade acetone, diethyl ether, and methanol; citric acid, acetylsalicylic acid (ASA), ABA, and MeJA were from Sigma-Aldrich Co. JA, cis-OPDA, [2H5]OPDA, N-[(–)-jasmonoyl]-(l)-isoleucine, dihydrojasmonate (DHJA), and [2H6]ABA were from OlChemIm Ltd. N-[(±)–jasmonoyl]-(l)-isoleucine was from Paul E. Staswick.
Germination assays
Germination was performed in Petri dishes each with 20 grains on one Whatman filter paper (No. 598) and 5ml of distilled water. Plates were sealed and incubated at 4 oC or 20 oC either under continuous 20 µmol m−2 s−1 BL-emitting diodes or wrapped in two layers of aluminium foil to block any light (dark treatment). Germinated grains were scored following emergence of coleorhiza (Barrero et al., 2009). The germination index (GI) was calculated over 7 d imbibition as described in Walker-Simmonds (1987).
As in some experiments, grains were imbibed with ASA, MeJA, ABA, and OPDA. ASA was diluted with water to a 30mM stock solution; MeJA was prepared as a 2000 µM stock solution as previously described (Jacobsen et al., 2013); and ABA was stored as a 30 µM stock and diluted prior to use.
JA, JA-Ile, and ABA analysis
The extraction method (Dave et al., 2011) was adapted to quantify the contents of JA, JA-Ile, and ABA in wheat embryos as follow.
With the aid of a microscope, biological replicates (n=4, each replicate weighing ~80mg) of 40 embryos were dissected from dry or imbibed wheat grains, weighed, and immediately frozen on dry ice. Samples were stored at –80 oC until extraction. Subsequently, a 5mm stainless steel bead was placed in each tube to lyse the frozen embryos mechanically using a tissue lyser (Qiagen) for 15s at a speed of 30 cycles s–1. Then samples were spiked with 20 µl of the stock internal standard mixture (1 µg ml−1 dihydrojasmonic acid, [2H5]OPDA, and [2H6]ABA) and extracted with 1.8ml of acetone/50mM citric acid (70:30, v/v) for 3h at 4 oC on an orbital shaker. Tubes were moved to a fumehood, uncapped, and left in darkness overnight for the acetone to evaporate. The remaining aqueous phase was extracted with diethyl ether (3×500 µl) and the extracts were combined in a 2ml glass vial and dried prior to reconstitution with 50 µl of methanol. Samples were filtered through a 0.45 µm GHP membrane (hydrophilic polypropylene) NanoSep MF centrifuge tube before LC/MS analysis.
Samples and standards (7 μl) were injected onto an Agilent Zorbax Eclipse 1.8 μm XDB-C18 2.1×50mm column. Solvent A consisted of 0.1% aqueous formic acid and solvent B, methanol with 0.1% formic acid. The plant hormones were eluted with a linear gradient from 10% to 50% solvent B over 8min, 50% to 70% solvent B from 8min to 12min (then held at 70% from 12min to 20min) at a flow rate of 200 μl min–1. The column effluent was analysed by an Agilent 6 530 Accurate Mass LC/MS QTOF with an ESI Jetstream ion source interface. Optimized ESI negative ion polarity parameters were as follow: gas temperature 250 ºC, drying gas flow 9 litres min–1, nebulizer 25 psig [pounds per square inch (gauge)], sheath gas temperature 250 ºC and sheath gas flow 11 litres min–1, capillary voltage 2500V, nozzle voltage 500V, and fragmentor voltage 145V. The positive ion polarity parameters were: gas temperature 250 ºC, drying gas flow 7 litres min–1, nebulizer 35 psig, sheath gas temperature 325 ºC and sheath gas flow 11 litres min–1, capillary voltage 3500V, nozzle voltage 500V, and fragmentor voltage 125V. The QTOF was operated in the extended dynamic range mode and data were acquired using targeted MS/MS (see Supplementary Table S1 at JXB online) with an m/z 1.3 isolation window prior to collision-induced dissociation (CID; N2 collision gas supplied at 18 psi). Mass spectra were acquired at three spectra s–1 and MS/MS at three spectra s–1 over a range of m/z 50–1000). The m/z values were corrected against two reference ions {purine, [MH]+ m/z 121.050873, [M-H]− m/z 119.036320 and hexakis(1H, 1H, 3H-tetra fluoropropoxy)phosphazine, [MH]+ m/z 922.009798, [M+HCO2]− m/z 966.000725}. Data were acquired and analysed using the Agilent Technologies MassHunter software (ver. B.5.0).
The extraction protocol of Dave et al. (2011) was validated by assessing absolute extraction recovery and matrix effects (i.e. ion suppression or ionization enhancement) using embryo samples that were unfortified (n=3) and fortified (n=3) with a known amount (50ng) of each analyte, and recovery (%) was calculated. Calibration standards (0.01–5.0ng µl–1), quality control (QC) standards (0.1ng µl–1 and 1.0ng µl–1), and samples were analysed, in both optimized positive and negative ion modes, for cis-OPDA, JA, JA-Ile, and ABA. Limits of detection (LOD) were determined by a signal to noise ratio of 3:1 based on the 7 µl injection volume, and the lower limits of quantification (LLOQ) were determined by a signal to noise ratio of 5:1 (see Supplementary Table S1). Calibration and QC standards had all internal standards fixed at a concentration of 0.4ng µl–1. Matrix effects were negligible. Absolute quantification was based on analyte peak areas normalized against peak areas of the corresponding isotope-labelled internal standard as shown in Supplementary Table S1. Quantification of JA and JA-Ile [(+)-7-iso-JA-l-Ile and (–)-JA-l-Ile] was performed against the analogue (±)-9,10-dihydrojasmonic acid internal standard.
GA analysis
Samples were frozen in liquid nitrogen and lyophilized. GA analysis was carried out at the Plant Biotechnology Institute of the National Research Council of Canada (http://www.nrc-cnrc.gc.ca/eng/solutions/advisory/plant_hormone.html) using LC-MS/MS (Chiwocha et al., 2003; Zaharia et al., 2005). Four biological replicates were analysed.
Quantitative PCR analysis
For RNA extraction, 10 embryos for each sample were ground with a tissue lyser and the powder added to 5ml of hot RNA lysis buffer as described by Chang et al. (1993). Following purification, the RNA was treated with RNase-free DNase. A 2 µg aliquot of total RNA was reverse transcribed in a 20 µl reaction. cDNA samples were diluted and used in 10 µl PCRs as described in Jacobsen et al. (2013). The reactions were run on a 7900HT fast real-time PCR system (Applied Biosystems). The expression of TaACTIN (CJ961169) (Ji et al., 2011) was used as an internal control to normalize gene expression. For the analysis, a linear standard curve was generated using a series of dilutions for each PCR product; the levels of the transcript in all unknown samples were determined according to the standard curve. The sequence of primers and gene accession number are listed in Supplementary Table S2. Two biological repeats were performed with similar results. Data from one of the replicates are shown.
Accession numbers
Full-length or EST sequence data in this work can be found in the EMBL/GenBank database under the following accession numbers: wheat TaAOS1 (AY196004), TaAOS2 (BT009396), TaAOC1 (KF573524), TaAOC2 (BJ241555), TaAOC3 (CK163974), TaNCED1 (CD884104), and TaNCED2 (CA731387).
Results
Endogenous JA and JA-Ile increase in response to cold stratification
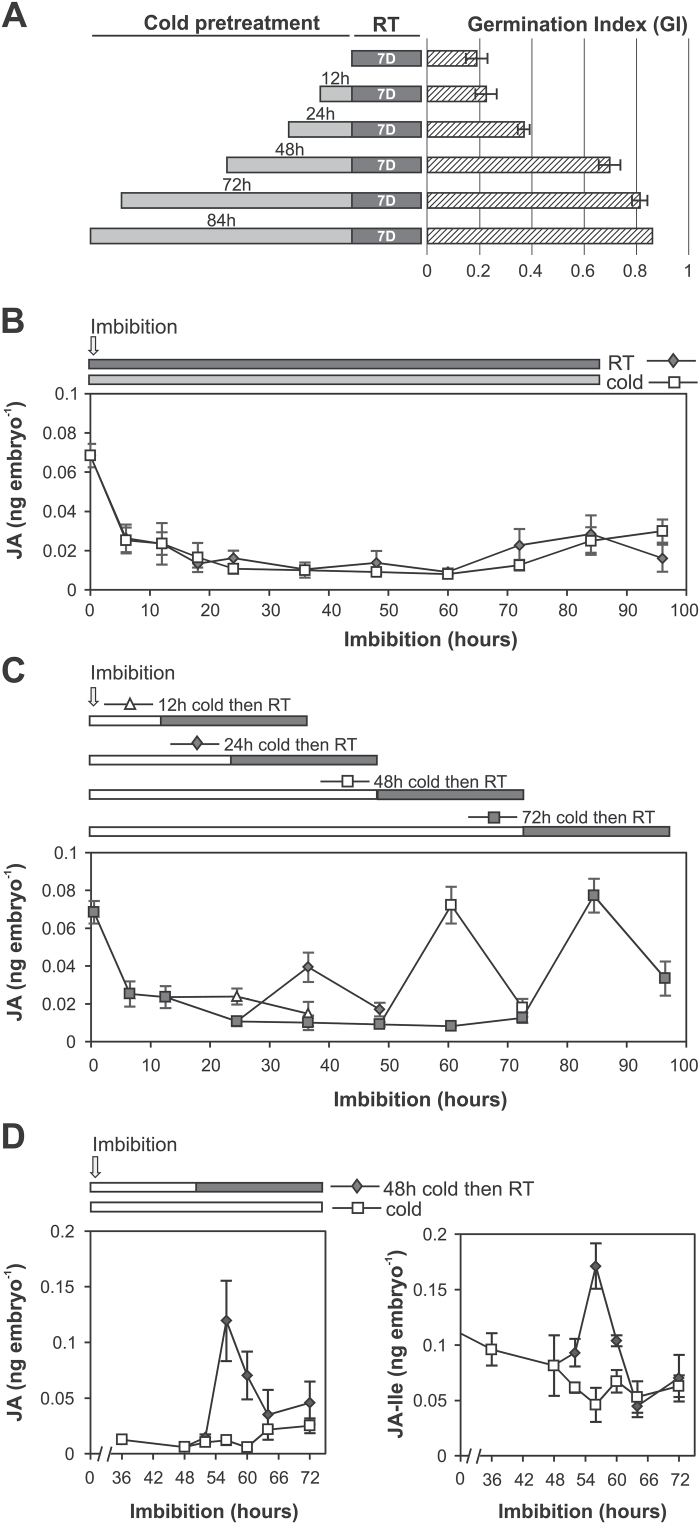
To characterize the responsiveness to stratification, dormant wheat grains (cv. Sunstate) were imbibed under BL (conditions in which dormancy is manifested to a greater extent) in the cold (4 oC) for periods of up to 84h prior to transfer to room temperature (20 oC) for 7 d (Jacobsen et al., 2013). Germination was assessed over the 7 d period and the effect of stratification time on the GI was measured (Fig. 1A; Supplementary Fig. S1). GI increased with duration of cold stratification until 48h when dormancy was almost completely lost. Similarly, longer stratification for 72h and 84h resulted in only further small increases in GI. The observed response to stratification is similar to that previously published for other wheat cultivars (Mares, 1984; Noda et al., 1994).
Fig. 1.
JA and JA-Ile increase in embryos of imbibed wheat grains in response to cold. (A) The effect of stratification on the germination of dormant grain. The GI was calculated over 7 d imbibition at 20 oC after different periods of cold treatment (0–84h). (B) JA content in embryos isolated from Sunstate wheat grains imbibed for 96h continuously in the cold or at 20 oC. (C) Changes in JA content in embryos isolated from wheat grains that have been stratified for different times (12, 24, 48, and 72h) prior to imbibition at 20 oC. (D) Changes in JA and JA-Ile content in embryos of wheat grains in response to 48h stratification. Grains were imbibed at 4 oC for 48h and then transferred to room temperature (RT) or kept at 4 oC. Values are means ±SE (n=4), and each replicate was obtained from 20 grains (A) and 40 embryos (B–D).
To assess JA changes in response to continuous cold treatment, we compared JA content in embryos of dormant grains imbibed continuously in the cold and at room temperature over a 96h period (Fig. 1B). The JA content was highest in dry embryos and it decreased during the first 6h of imbibition both in the cold and at room temperature, and thereafter remained low in both treatments up to 96h imbibition.
We then measured JA production in wheat embryos following stratification. JA was quantified in dormant wheat grains that were imbibed in the cold for different times (12, 24, 48, and 72h) and transferred to room temperature for 24h (Fig. 1C). JA content increased in wheat embryos that had been stratified for longer than 12h. The increase in JA content in the stratified grains correlated with the loss of dormancy, with close to maximal effect observed in grains that were stratified for 48h or longer prior to transfer to 20 oC (Fig. 1A, C).
To understand further the induction of JA production, we repeated the analysis of 48h stratified grains with more time points to better define changes in the content of JA and its associated metabolite, JA-Ile, following the cold treatment. As shown in Fig. 1D, JA content in embryos increased (~10-fold) between 4h and 8h after transfer to 20 oC, and thereafter the content decreased rapidly back to levels similar to those found in wheat grains imbibed continuously in the cold. JA-Ile, one of the JA bioactive forms, also increased (~2-fold) in a pattern similar to JA, reaching its maximum between 4h and 8h after transfer to room temperature (Fig. 1D).
We also examined the effect of dark on germination and JA content in cold-treated grains. In contrast to BL, imbibition of cv. Sunstate grains under dark conditions strongly promoted germination (Supplementary Fig. S2A). Analysis of JA content in grains imbibed in the dark revealed that following 48h stratification, JA content increased rapidly 12h after transfer to room temperature (Supplementary Fig. S2B). We were also interested in determining if the cold-induced increase in JA could be observed in a more highly dormant wheat variety. Wheat cv. AC Barrie was found to be dormant even when imbibed under dark conditions (Supplementary Fig. S2A); however, germination of cv. AC Barrie grains was strongly promoted by 48h imbibition at 4 oC in the dark prior to transfer to room temperature (Supplementary Fig. S2A). As shown in Supplementary Fig. S2B, changes in JA content were similar to those found in cv. Sunstate grains, indicating that the cold-induced increase in JA content is not cultivar specific.
Effect of stratification on the jasmonate biosynthesis pathway
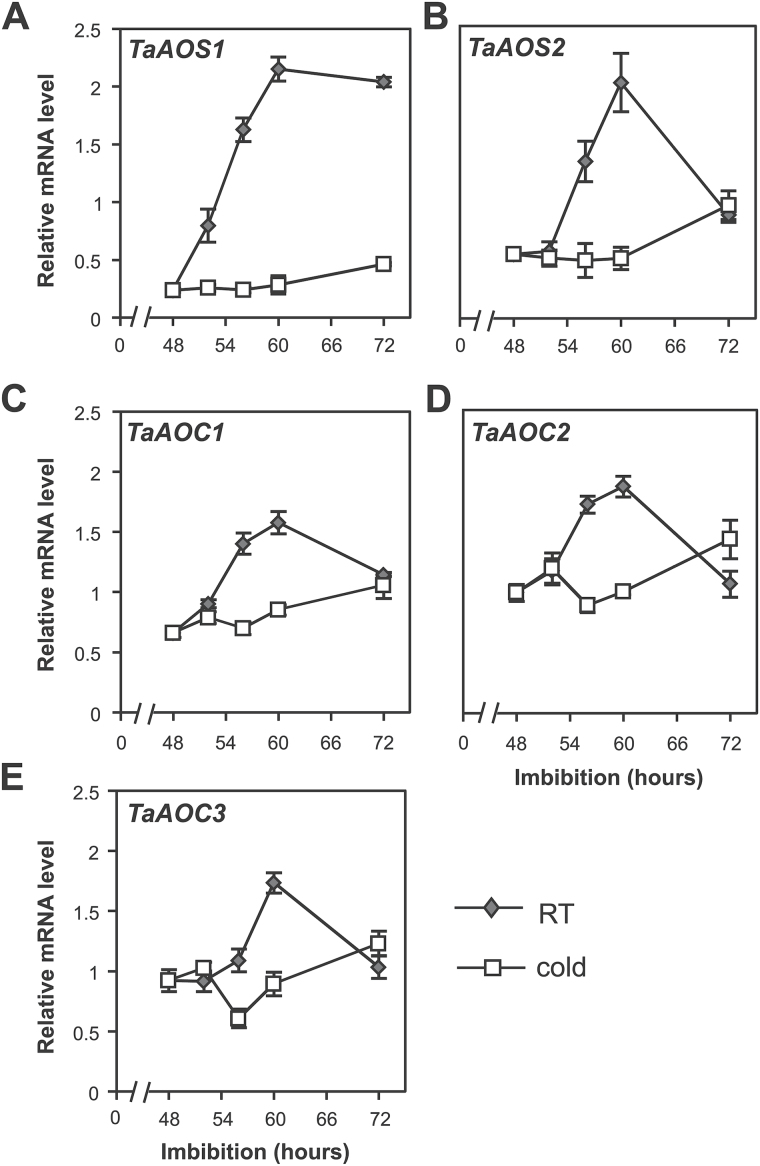
To understand further the effect of stratification on JA production, we examined the responses of several genes in the jasmonate biosynthesis pathway to cold. In Arabidopsis, AOS and AOC genes are considered to encode key enzymes in jasmonate biosynthesis and to be regulated by cold (Hu et al., 2013). On the basis of published sequences of AOS and AOC genes in wheat (Wu et al., 2004; Liu et al., 2011; A. Liu et al., 2013; Zhao et al., 2014), we selected TaAOS1, TaAOS2, TaAOC1, TaAOC2, and TaAOC3 genes and monitored their expression after stratification. In response to stratification, the expression of TaAOS (Fig. 2A, B) and TaAOC (Fig. 2C, E) genes was up-regulated within 8h after transfer to room temperature. These data suggest that the increase in JA content is due, at least in part, to up-regulation of the expression of TaAOS and TaAOC genes in wheat embryos (Fig. 2).
Fig. 2.
Expression of JA biosynthesis genes is induced by 48h stratification in wheat grains. Relative expression of TaAOS1 (A), TaAOS2 (B), TaAOC1 (C), TaAOC2 (D), and TaAOC3 (E) in embryos of wheat grains following 48h stratification. Grains were imbibed at 4 oC for 48h and then either transferred to room temperature (RT) or kept at 4 oC up to 24h (52, 56, 60, and 72h). Values are means ±SE (n=4), and each replicate was obtained from 10 embryos.
Inhibition of jasmonate biosynthesis blocks the stratification response
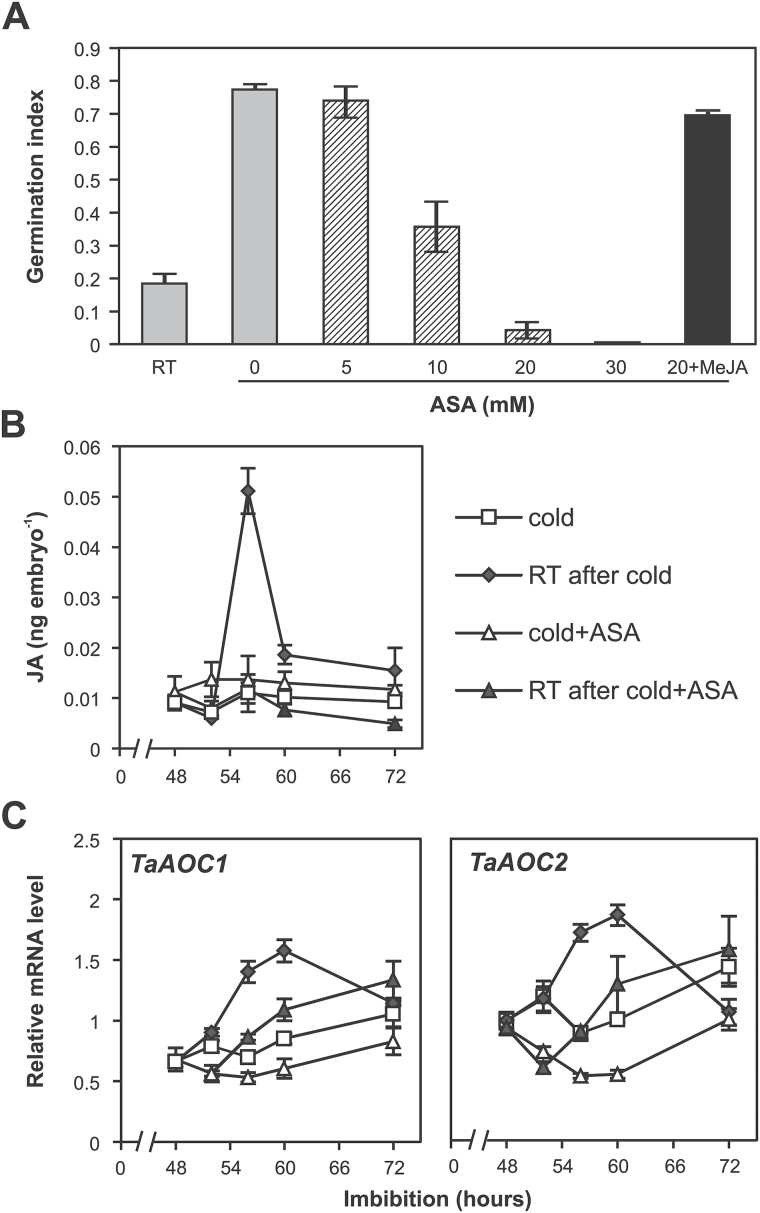
ASA has been shown to block JA synthesis in tomato and flax leaves (Pena-Cortés et al., 1993; Harms et al., 1998). We have used ASA to test whether the increase in JA content is required for the stratification response in wheat. Figure 3A shows that different concentrations of ASA (5, 10, 20, and 30mM) suppressed the cold effect on germination in a dose-dependent manner. In addition, we tried to rescue the effect of ASA by adding 100 µM MeJA. The addition of MeJA reversed the inhibitory effect of ASA, which suggests that JA biosynthesis is essential for the stratification response and the effect is not due to non-specific effects of ASA. We also confirmed that ASA blocked the cold-induced increase in JA content (Fig. 3B). Addition of 20mM ASA blocked the cold-induced peak of JA production, which provided a direct link between the increase in JA and the cold effect on germination. ASA temporally blocked the cold-induced expression of TaAOC1 and TaAOC2, indicating that they may play an essential role in mediating the effect of cold on JA content (Fig. 3C). We did not detect significant down-regulation of TaAOS1, TaAOS2, and TaAOC3 genes (Supplementary Fig. S3) in response to ASA.
Fig. 3.
JA biosynthesis is required for cold-induced germination in dormant wheat. (A) Effect of ASA and MeJA on the GI of wheat following a 48h cold treatment. Different concentrations of ASA (5, 10, 20, and 30mM) and 100 µM MeJA were added from the beginning of cold imbibition. Values are means ±SE (n=4), and each replicate was obtained from 20 grains. (B) Effect of ASA on JA content in embryos of imbibed wheat grains following a 48h cold treatment. Grains were imbibed for 2 d with or without ASA in the cold before being transferred to room temperature (RT). For comparison, grains were kept in the cold after the 48h cold treatment. Embryos were isolated for JA quantification after 48 (end of cold treatment), 52, 56, 60, and 72h imbibition. Values are means ±SE (n=4), and each replicate was obtained from 40 embryos. (C) Effect of ASA on expression of TaAOC1 and TaAOC2 in embryos of imbibed wheat grains in response to a 48h cold treatment. Grains were imbibed for 2 d with or without ASA in the cold before being transferred to RT. For comparison, grains were kept in the cold after the 48h cold treatment. Embryos were isolated for gene expression test after 48 (end of cold treatment), 52, 56, 60, and 72h imbibition. Values are means ±SE (n=4), and each replicate was obtained from 10 embryos.
Stratification induces a decrease in ABA content
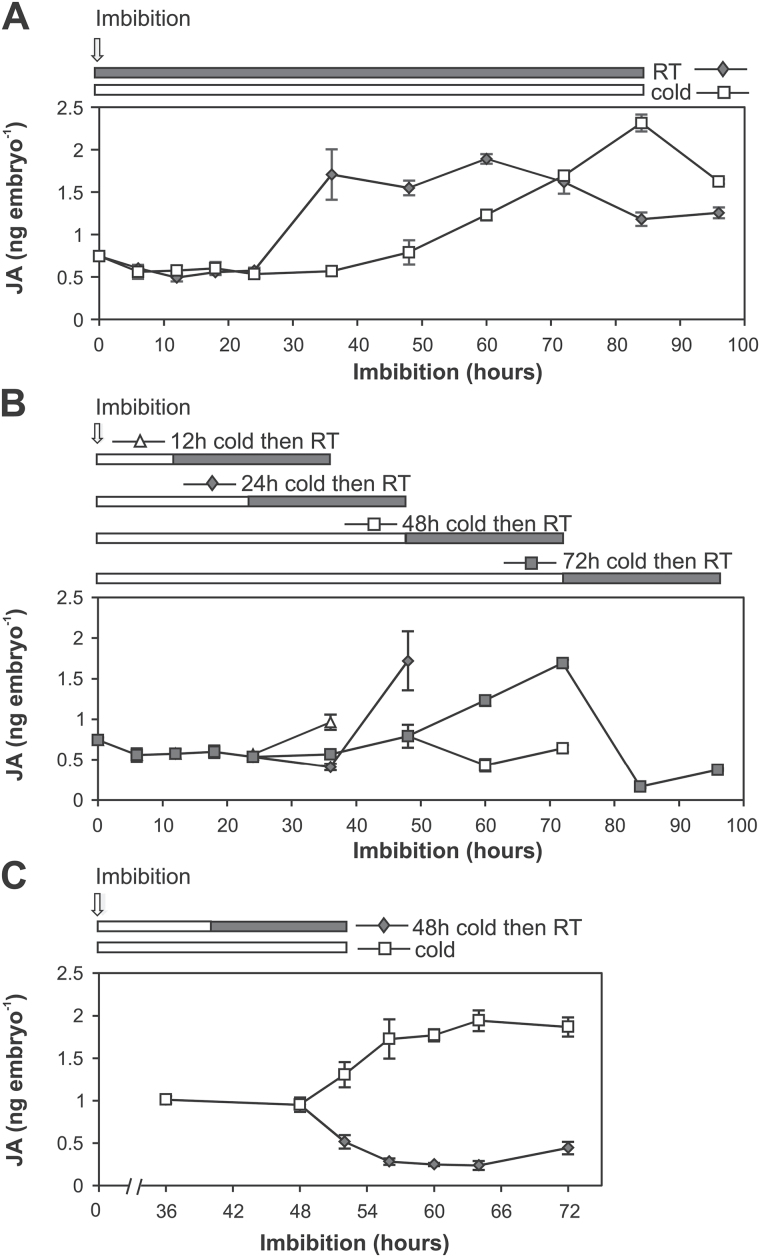
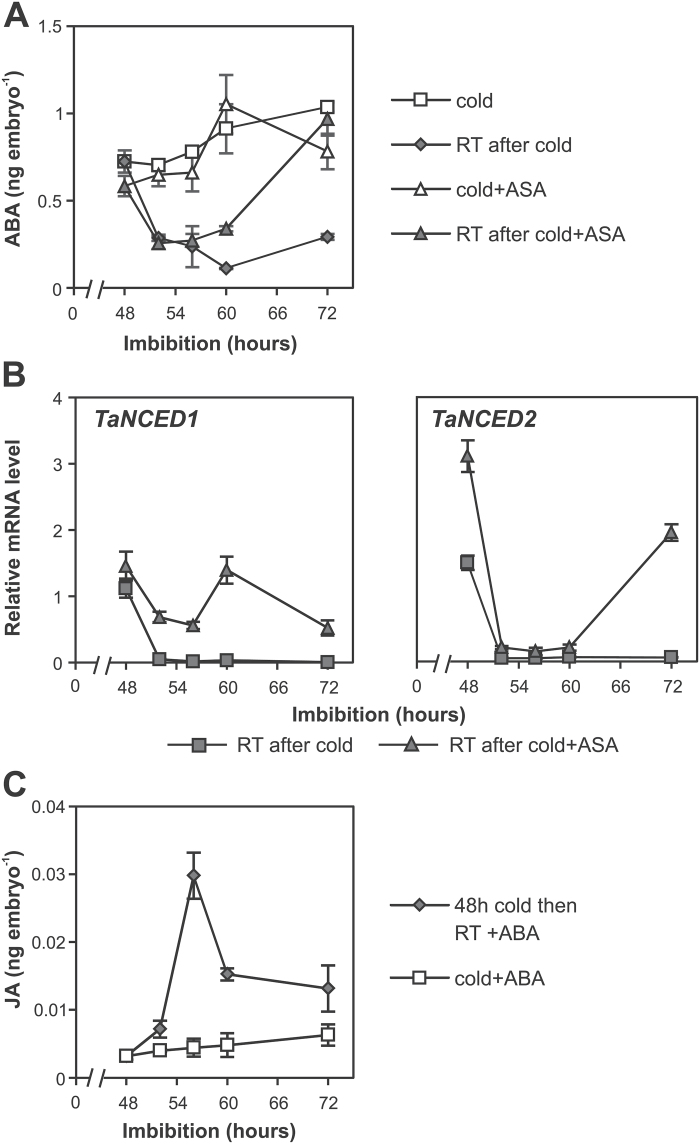
In order to understand the effect of cold on other hormones, we also investigated the role of ABA and GA in the stratification response. In contrast to changes observed with JA, ABA content increased in embryos of wheat grains that were imbibed continuously at either 4 oC or 20 oC but the increase was delayed in grains imbibed at the lower temperature (Fig. 4A). As demonstrated previously, ABA content of embryos of dormant grains increased after 24h imbibition at room temperature and peaked by 48h (Jacobsen et al., 2013). ABA content in cold-imbibed grains increased after 48h imbibition and then continued to rise until 84h. When we examined the effect of stratification time on ABA content, it was observed that stratification times longer than 24h resulted in a decrease in ABA content in the 24h period after transfer to room temperature. Shorter stratification times (12h and 24h) resulted in increased ABA content in the embryo (Fig. 4B) similar to what happened in the room temperature control.
Fig. 4.
Changes in ABA content in embryos of imbibed wheat grain in response to stratification. (A) Time course of endogenous ABA content in embryos imbibed at 4 oC (cold) and 20 oC [room temperature (RT)). (B) Changes in ABA content in embryos of grains imbibed for different periods of time in the cold (12, 24, 48, and 72h) prior to transfer to RT. (C) More detailed time course of changes in ABA content in embryos of wheat grains in response to 48h stratification. Dormant grains were imbibed at 4 oC for 48h before transfer to RT. For comparison, grains were also kept in the cold after 48h. Values are means ±SE (n=4), and each replicate was obtained from 40 embryos.
To examine this more closely, changes in ABA content were monitored every 4h following a 48h stratification treatment. As shown in Fig. 4C, ABA content started to decrease within 4h of transfer from 4 oC to 20 oC, and, by 8h, it reached the minimum. The decrease in ABA content correlated with the decrease in expression of TaNCED1 and TaNCED2, both key ABA biosynthesis genes expressed in wheat embryos (Jacobsen et al., 2013) (Fig. 5). The decrease in ABA levels was also observed in grains that were imbibed in the dark (Supplementary Fig. S2C).
Fig. 5.
Expression of ABA biosynthesis genes, TaNCED1 and TaNCED2, is repressed in embryos of wheat grains following stratification. Dormant grains were imbibed at 4 oC for 48h before transfer to room temperature (RT).The grains were imbibed in the cold for 48h prior to transfer to RT. For comparison, grains were also kept in the cold after the 48h cold treatment. Values are means ±SE (n=4), and each replicate was obtained from 10 embryos.
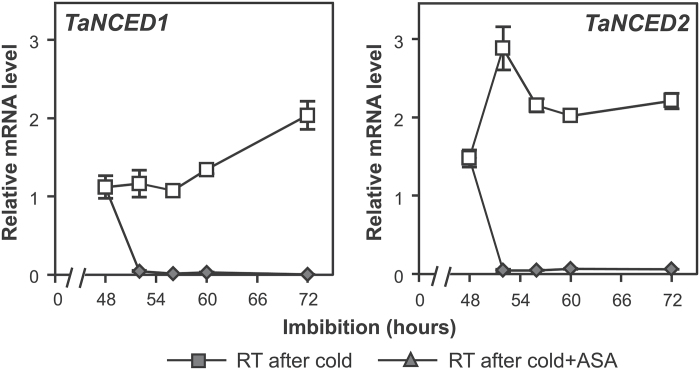
Since it had been previously shown that MeJA represses TaNCED1 expression and ABA content in wheat embryos (Jacobsen et al., 2013), we tested whether the decrease in ABA content following the 48h stratification is directly linked to the increase in JA content. Changes in ABA content were monitored in 48h stratified wheat grains imbibed in the presence of ASA. As shown in Fig. 6A, ABA content in embryos was higher in the ASA-treated grains imbibed at room temperature for 24h following the stratification compared with no ASA treatment. The higher ABA content correlated with the increase in expression of TaNCED1 and TaNCED2 in ASA-treated grains (Fig. 6B). To assess further the relationship between the JA and ABA during the cold-stimulated germination process, JA content was examined after addition of a high concentration of ABA (100 µM). The induction peak of JA still occurred in the presence of ABA (Fig. 6C). These data led to the conclusion that JA acts upstream of ABA during wheat germination of stratified grains.
Fig. 6.
JA regulates ABA content in embryos of wheat grains in response to stratification. (A) ASA promotes an increase in ABA content in embryos of imbibed wheat grains at room temperature (RT) following a 48h cold treatment. Grains were imbibed in the cold for 48h and then either transferred to RT or kept in the cold. (B) ASA-induced changes in relative TaNCED1 and TaNCED2 transcript abundance in embryos following a 48h stratification. Grains were imbibed in the cold for 48h and then either transferred to RT or kept in the cold. Two biological repeats were performed with similar results. Data from one of the replicates are shown. (C) Addition of 100 µM ABA to the imbibition media does not block cold-induced increases in JA content in embryos of stratified wheat grains. Grains were imbibed in the cold for 48h and then either transferred to RT or kept in the cold. Values are means ±SE (n=4), and each replicate was obtained from 40 embryos (A and C) and 10 embryos (B).
Examination of changes in GA and GA metabolites identified significant increases in the content of some GA precursors (GA53 and GA44) and of a GA catabolite (GA8) in embryos of grains imbibed at room temperature following 48h stratification (Supplementary Fig. S4). However, no bioactive GAs (GA1, GA3, or GA4) were detected in this experiment (Supplementary Table S3). To test whether GA biosynthesis is required for cold-induced promotion of germination in dormant grains, grains were imbibed in paclobutrazol, a well known inhibitor of GA biosynthesis, including in wheat grains (Lenton et al., 1994). Addition of 10 μM paclobutrazol to the imbibition medium failed to block the effect of cold stratification on germination yet it reduced the maximum elongation rate of leaf 1 compared with control treatments (Supplementary Fig. S5A–C). It is also of interest to note that addition of GA alone failed to promote germination of stratified and non-stratified dormant grains.
JAs have no detectable role in dormancy release by after-ripening and darkness
Following the demonstration that JA plays a role in cold-induced dormancy release, we looked to see whether the role could be extended to other dormancy release mechanisms. To determine whether JA had a role in dormancy release by dry after-ripening, JA content was monitored over a 60 d after-ripening period at 37 oC during which grains progressively lost dormancy (Supplementary Fig. S6A). As shown in Supplementary Fig. S6B, little or no change in JA, JA-Ile, and ABA content in embryos of dry grains was observed over the 60 d period. We also determined whether there were any differences in JA and JA-Ile content during imbibition of dormant and after-ripened grain (Supplementary Fig. S7B, C). Apart from a difference in JA content at 36h imbibition between dormant and after-ripened grains, there was no evidence that JAs had a role in the after-ripening response. Imbibition in the dark also promoted germination of dormant grains (Supplementary Fig. S7A), but again no difference in JA content was observed between dormant grains imbibed in the dark or in the light (Supplementary Fig. S7C, C).
Discussion
JA and its metabolites are ubiquitous signalling compounds known to regulate multiple processes including defence responses (Browse, 2009), root elongation (Staswick et al., 1992; Pauwels et al., 2010), freezing responses (Hu et al., 2013), male fertility (McConn and Browse, 1996; Cheng et al., 2009), senescence (Ueda and Kato, 1980; Shan et al., 2011), anthocyanin accumulation (Franceschi and Grimes, 1991; Shan et al., 2009), and flowering time (Diallo et al., 2014). In the present work, we have identified several lines of evidence that point to a new role for JA in dormancy release by cold stratification. First, we have found that JA biosynthesis in wheat embryos is induced following cold stratification of dormant grains and that the increase in JA content correlates with the effect on germination. The effect of cold stratification on JA accumulation was observed in wheat cultivars of different dormancy and was independent of light conditions used during imbibition. Importantly, the increase in JA content in cold-stratified grains, which happened at 4–8h (Fig. 1D; Supplementary Fig. S2B) after transfer to room temperature, precedes the emergence of the coleorhiza (the earliest visual indication of grain germination after 8h; Supplementary Fig. S8), thus suggesting a causal link between the two events. We explored the possibility of JA being produced as a consequence of germination; however, that scenario was excluded as our results showed that there is no consistent change in JA and JA-Ile content in embryos of non-dormant grains that are undergoing germination (Supplementary Fig. S7A, B). Secondly, experiments with a JA biosynthesis inhibitor showed that JA production is sufficient and necessary for dormancy release by stratification. This is consistent with our previous results which showed that application of MeJA and JA reduces dormancy of wheat grains (Jacobsen et al., 2013). Thirdly, our study provided evidence that the action of JA on dormancy release is at least in part mediated by reduced ABA content in embryos caused by reduced expression of ABA biosynthesis genes.
Cold-induced increases in JA content have been previously reported in plants. A small increase in JA-Ile (<25%) and no change in JA content have been reported in embryos of wheat grains imbibed for 18h at 30 oC following a 24h stratification treatment compared with embryos from non-stratified grains (Tuttle et al., 2015). It is important to note that the 24h stratification used in the study only had a small effect on the GI of wheat grains imbibed in water. However, the authors reported that the short stratification time impacted on the sensitivity of the grains to GA and ABA. In general, our results support this result but also extend it to demonstrate that the transient increases in JA and JA-Ile occur much earlier, with the maxima achieved 8h after the stratification. Our data show a 10-fold increase in JA content and a 2-fold increase in JA-Ile content in embryos within the first 8h following transfer to 20 oC after stratification. Similar differences are found when the JA content in embryos 8h after stratification are compared with grains that are incubated continuously at 20 oC (non-stratified grains) or 4 oC (compare Fig. 1B and D).
Cold-induced increases in MeJA have also been reported in wheat seedlings (Diallo et al., 2014). MeJA accumulates in winter wheat during vernalization and then decreases rapidly once the plants are transferred to inductive flowering conditions. The decrease in MeJA precedes the rise in expression of TaVRN1 and TaFT1, indicating that MeJA may suppress expression of flowering time genes during vernalization and thus delaying flowering until the plants are exposed to warmer temperatures (Diallo et al., 2014). It is now clear that vernalization and stratification in wheat are both mediated at least in part by the accumulation of JAs. It is intriguing to note that in both instances accumulation in JAs precedes activation of growth processes both in the embryonic axis and in the vegetative apex.
The role of JAs may not only vary depending on the developmental stage of various tissue types and between seeds from different species. The JA response to stratification in wheat grains appears to be fundamentally different from that in Arabidopsis, since it only occurs after the cold treatment, once the grains are transferred to room temperature. In Arabidopsis seedlings, JA accumulated rapidly within 1.5h of transfer from 22 oC to 4 oC, triggering the expression of the INDUCER OF CBF EXPRESSION-C-REPEAT BINDING FACTOR transcriptional pathway (Hu et al., 2013). This response appeared to be related to cold stress responses, and the increase in JA enhanced the seedling freezing tolerance.
Our data indicate that in wheat dormancy, the role of JAs is restricted to cold stratification and not other dormancy release mechanisms such as after-ripening or darkness. We found that JA and JA-Ile contents were very similar in embryos from dormant and after-ripened grain imbibed over 24h at 20 oC, with content declining 3- to 4-fold over this period. This is in agreement with an earlier study of wheat grain dormancy which found that JA and JA-Ile content declined in whole dormant and after-ripened grains over 24h hydration at room temperature (A. Liu et al., 2013). Similarly, we found little difference between dormant grains that were imbibed in the dark or BL even though the grains had different germination outcomes (Supplementary Fig. S7A, B).
We have previously shown that wheat grains have different responses to MeJA depending on the dormancy status. Application of MeJA to dormant grain strongly promoted dormancy release, but in after-ripened grain the application of MeJA inhibited the growth of the embryo (Jacobsen et al., 2013). The dependence on the dormancy status in determining the role of JA may also explain why there are reports of JAs promoting or repressing germination in a number of other plant species (Daletskaya and Sembdner, 1989; Berestetsky et al., 1991; Ranjan and Lewak, 1992; Jarvis et al., 1997; Krock et al., 2002; Yildiz et al., 2007, 2008; Preston et al., 2009). This dual role of JA could explain why the cold-induced JA production is transitory; otherwise it would affect embryo growth once dormancy is released.
The role of JAs in seeds hase been previously studied in Arabidopsis, but their functions seem different from those in wheat grains. For example, large differences in JA and JA-Ile content have been reported in dry seeds from Arabidopsis ecotypes which differ in their level of dormancy, the content higher being in non-dormant than in dormant ecotypes (Preston et al., 2009). However, so far, there is no functional evidence supporting a role for that correlation. Moreover, the role of JA and OPDA has also been examined in non-dormant Arabidopsis seeds. In that work, using single and double mutants, it was established that OPDA has an inhibitory effect on germination by increasing ABA sensitivity, and that JA and JA-Ile had no effect (Dave et al., 2011). In wheat, the application of OPDA has small effects promoting germination (Supplementary Fig. S9), and the content of this molecule was below the level of detection in our stratification assays. Taken together, these results suggest that the role of JA, JA-Ile, and OPDA in dormancy and germination could be different between dicot and monocot species, although more genetic work is needed to confirm this conclusion.
In Arabidopsis seedlings, cold rapidly up-regulates expression of a number of key genes in the JA biosynthesis pathway including LOX1–LOX3, AOS, AOC1–AOC4, and JAR1 (Hu et al., 2013). We found in wheat that the expression of TaAOS1-2 and TaAOC1-3 increased rapidly only after the cold stratification treatment and that this correlated with the rapid rise in JA content. ASA blocked the cold-induced increase in JA content and germination, possibly in part by temporally suppressing the cold-induced expression of TaAOC1 and TaAOC2 genes. Furthermore this evidence confirms the contribution of both TaAOC genes to the cold-induced transient increase in JA. In flax leaves, ASA has been shown to block JA production via inhibition of AOS expression (Harms et al., 1998), indicating that ASA is able to alter the expression of different JA biosynthesis genes in different plants. It is important to note that recent work has shown that salicylic acid inhibition of JA biosynthesis is a consequence of feedback inhibition caused by repression of JA signalling (Leon-Reyes et al., 2010; van der Does et al., 2013).
The role of ABA as a principal dormancy promoter and germination inhibitor has been extensively reported (Finkelstein et al., 2008). However, little is known about crosstalk between JA and ABA signalling pathways in cereals. ABA and JA show a synergistic relationship in response to several abiotic and biotic stresses such as wounding, salinity, or pathogen attack (Wang et al., 2001; Forcat et al., 2008; Wang et al., 2012). In contrast, an antagonistic interaction between the signalling pathways for those hormones has been recorded for other stresses (Moons et al., 1997; Anderson et al., 2004; Nakata et al., 2013), thus indicating that the nature of ABA and JA crosstalk is complex and stress dependent. In relation to wheat grains, we have identified an inverse relationship between JA and ABA content in response to 48h stratification, and we have asked if the two responses are inter-related. Our results show that the decrease in ABA content precedes the increase in JA content and that ABA content remains low after the transient spike in JA content. Our results are consistent with a recent report which found that a 24h cold stratification treatment of wheat grains was associated with a small decrease in ABA and a small increase in JA-Ile content (Tuttle et al., 2015). We explored the possibility that ABA might suppress JA synthesis in wheat embryos by determining whether ABA could suppress JA production. Addition of 100 μM ABA to wheat grains undergoing cold stratification failed to block the production of a transient spike in JA, indicating that the increase in JA and the initial rapid decrease in ABA content in response to cold stratification are independent events. However, we were able to show that JA may have a key role in maintenance of low ABA content following the rapid initial decrease in response to cold. By blocking JA production in cold-stratified grains with ASA, we were able to show that ABA content rapidly increased to levels similar to those found before stratification. We were also able to show that the increase in ABA content in ASA-treated grains can be attributed to a promotion of expression of the key ABA biosynthesis genes, TaNCED1 and TaNCED2. This was consistent with our previous finding that MeJA promotion of dormancy release in wheat acts via inhibition of ABA biosynthesis by suppressing TaNCED1 expression in embryos of dormant grains (Jacobsen et al., 2013). Therefore, the decline and maintenance of low ABA content in stratified grains is, at least in part, directly attributed to JA suppression of ABA synthesis. It has been shown that dormant wheat grains lose ABA sensitivity in response to cold (Noda et al., 1994; Tuttle et al., 2015), but it has yet to be determined if the change in ABA sensitivity is due to cold-induced changes in ABA signalling mechanisms. However, it is important to note that manipulation of expression of ABA metabolism genes can also lead to alterations in ABA sensitivity (Okamoto et al., 2006). Thus, JA-induced changes in ABA content may be responsible, at least in part, for changes in ABA sensitivity.
Previous studies of seed germination have established a link between cold stratification and GA. In particular, increases in GA content during cold stratification have been observed in seeds of several plant species (West, 1970; Rudnioki et al., 1972; Yamauchi et al., 2004; Chen et al., 2008). This evidence, along with the demonstration that GA application is often effective in breaking dormancy, has led to the proposal that cold-induced increases in GA content are responsible for increased germination (Yamauchi et al., 2004). However, in agreement with a previous study (Tuttle et al., 2015), we were not able to detect any bioactive GAs in our cold-stratified grains, indicating that the content of these GAs is very low and thus likely not to have a role. The demonstration that the GA biosynthesis inhibitor paclobutrazol did not inhibit cold stratification promotion of germination provides evidence that GA biosynthesis is not critical for the response in wheat. In contrast, we were able to detect changes in some GA precursors and catabolites, indicating that GA metabolism is active in cold-treated grains but its role remains unknown.
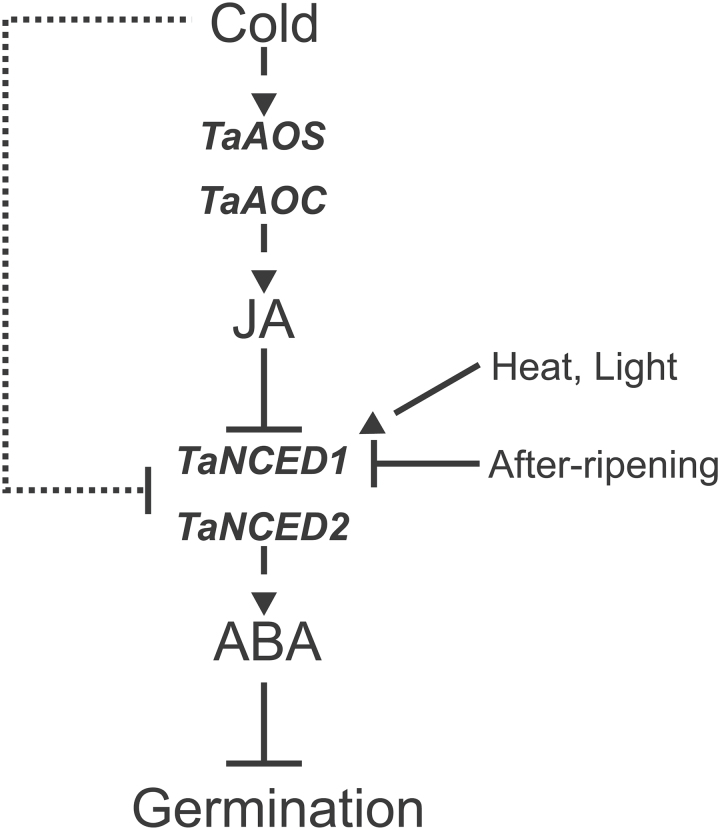
In conclusion, our study in wheat leads us to propose a model showing that JA and ABA have opposing roles in the regulation of dormancy release by stratification (Fig. 7). ABA repression of germination acts through two signalling pathways, one independent of JA (dotted line) and one dependent on JA signalling mechanisms. We showed that the cold-induced increase in JA content is necessary for dormancy release and that its action is mediated, at least in part, by repression of ABA biosynthesis genes, TaNCED1 and TaNCED2. NCED1 in wheat and barley has been previously shown to be regulated by a number of other environmental conditions such as high temperature stress (Leymarie et al., 2008), BL (Jacobsen et al., 2013), and after-ripening (Gubler et al., 2008). In addition, we have now shown that both TaNCED1 and TaNCED2 are down-regulated in response to cold in a JA-independent pathway (dotted line). Further investigations are now needed to determine how cold stratification promotes JA biosynthesis and at the same time inhibits ABA biosynthesis.
Fig. 7.
Model summarizing the role of JA and ABA in dormancy release by cold stratification in wheat. Stratification stimulates rapid changes in two hormones known to regulate dormancy. First, there is a decrease in ABA content, independent of JA (dotted line), which is driven by the repression of TaNCED1 and TaNCED2. Secondly, there is a cold-induced increase in JA content that also decreases ABA by repressing the NCED genes. The increase in JA is driven by the cold-induced expression of TaAOS and TaAOC. Blockage of JA biosynthesis using ASA results in high ABA content; thus, the maintenance of low ABA after stratification is dependent on JA production. In cereals, NCED1 is a common key target of environmental factors (cold, heat, light, and after-ripening) that affect germination.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. The effect of stratification on the germination of dormant grain.
Figure S2. Changes in JA and ABA content in embryos of imbibed Sunstate and AC Barrie grain in response to stratification in darkness.
Figure S3. Effect of ASA on expression of TaAOC1, TaAOC2, and TaAOS3 in embryos of imbibed wheat grains in response to 48h stratification.
Figure S5. Effect of paclobutrazol and gibberellic acid on GI and leaf 1 elongation rate.
Figure S4. Changes in GA content in embryos of Sunstate wheat grains in response to 48h stratification.
Figure S6. JA and ABA contents in dry embryos during after-ripening.
Figure S7. Time course of JA content in dormant and after-ripened embryos in BL and darkness.
Figure S8. Time course of germination of Sunstate wheat grains in response to 48h stratification.
Figure S9. Effect of ODPA on germination on dormant and after-ripened wheat grains.
Table S1. Validation results for extraction and LC-ESI-MS/MS analytical methodologies for the detection and absolute quantification of plant hormones in wheat embryos.
Table S2. Primer sequences used for qRT–PCR.
Table S3. Changes in GA content in embryos of Sunstate wheat grains in response to 48h stratification in BL.
Acknowledgements
This project was funded by a CSIRO Office of the Chief Executive PDF scheme. We thank Will Heaslip, Trijntje Hughes, Jasmine Rajamony, and Carol Harding for their excellent technical assistance. We also gratefully acknowledge helpful comments from Dr J. Finnegan and Dr B. Ford in preparation of the manuscript, and the gift of jasmonoyl-l-isoleucine and jasmonoyl-[13C6]isoleucine from Paul E. Staswick.
References
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K. 2004. Antagonistic interaction between abscisic acid and jasmonate–ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. The Plant Cell 16, 3460–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrero JM, Talbot MJ, White RG, Jacobsen JV, Gubler F. 2009. Anatomical and transcriptomic studies of the coleorhiza reveal the importance of this tissue in regulating dormancy in barley. Plant Physiology 150, 1006–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrero JM, Cavanagh C, Verbyla KL, et al. 2015. Transcriptomic analysis of wheat near-isogenic lines identifies PM19-A1 and A2 as candidates for a major dormancy QTL. Genome Biology 16, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berestetsky V, Dathe W, Daletskaya T, Musatenko L, Sembdner G. 1991. Jasmonic acid in seed dormancy of Acer tatarium . Biochemie und Physiologie der Pflanzen 187, 13–19. [Google Scholar]
- Bewley JD. 1997. Seed germination and dormancy. The Plant Cell 9, 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Black M. 1994. Seeds: physiology of development and germination. New York: Plenum Press. [Google Scholar]
- Browse J. 2009. Jasmonate passes muster: a receptor and targets for the defense hormone. Annual Review of Plant Biology 60, 183–205. [DOI] [PubMed] [Google Scholar]
- Buraas T, Skinnes H. 1985. Development of seed dormancy in barley, wheat and triticale under controlled conditions. Acta Agricultarea Scandinavica 35, 233–244. [Google Scholar]
- Chang S, Puryear J, Cairney J. 1993. A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reports 11, 113–116. [Google Scholar]
- Chen SY, Kuo SR, Chien CT. 2008. Roles of gibberellins and abscisic acid in dormancy and germination of red bayberry (Myrica rubra) seeds. Tree Physiology 28, 1431–1439. [DOI] [PubMed] [Google Scholar]
- Cheng H, Song S, Xiao L, Soo HM, Cheng Z, Xie D, Peng J. 2009. Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis . PLoS Genetics 5, e1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiwocha SD, Abrams SR, Ambrose SJ, Cutler AJ, Loewen M, Ross AR, Kermode AR. 2003. A method for profiling classes of plant hormones and their metabolites using liquid chromatography-electrospray ionization tandem mass spectrometry: an analysis of hormone regulation of thermodormancy of lettuce (Lactuca sativa L) seeds. The Plant Journal 35, 405–417. [DOI] [PubMed] [Google Scholar]
- Corbineau F, Xia Q, Bailly C, El-Maarouf-Bouteau H. 2014. Ethylene, a key factor in the regulation of seed dormancy. Frontiers in Plant Science 5, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daletskaya T, Sembdner G. 1989. Effect of jasmonic acid on germination of nondormant and dormant seeds. (In Russian.) Fiziologia Rastenii 36, 1118–1123. [Google Scholar]
- Dave A, Hernandez ML, He Z, Andriotis VM, Vaistij FE, Larson TR, Graham IA. 2011. 12-Oxo-phytodienoic acid accumulation during seed development represses seed germination in Arabidopsis. The Plant Cell 23, 583–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo AO, Agharbaoui Z, Badawi MA, Ali-Benali MA, Moheb A, Houde M, Sarhan F. 2014. Transcriptome analysis of an mvp mutant reveals important changes in global gene expression and a role for methyl jasmonate in vernalization and flowering in wheat. Journal of Experimental Botany 65, 2271–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R, Reeves W, Ariizumi T, Steber C. 2008. Molecular aspects of seed dormancy. Annual Review of Plant Biology 59, 387–415. [DOI] [PubMed] [Google Scholar]
- Forcat S, Bennett MH, Mansfield JW, Grant MR. 2008. A rapid and robust method for simultaneously measuring changes in the phytohormones ABA, JA and SA in plants following biotic and abiotic stress. Plant Methods 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi VR, Grimes HD. 1991. Induction of soybean vegetative storage proteins and anthocyanins by low-level atmospheric methyl jasmonate. Proceedings of the National Academy of Sciences, USA 88, 6745–6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling PG, Butler RA, Black M, Chapman JM. 1981. The onset of germination ability in developing wheat. Journal of Experimental Botany 32, 621–627. [Google Scholar]
- Gu XY, Kianian SF, Foley ME. 2006. Dormancy genes from weedy rice respond divergently to seed development environments. Genetics 172, 1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualano NA, Benech-Arnold RL. 2009. Predicting pre-harvest sprouting susceptibility in barley: looking for ‘sensitivity windows’ to temperature throughout grain filling in various commercial cultivars. Field Crops Research 114, 35–44. [Google Scholar]
- Gubler F, Hughes T, Waterhouse P, Jacobsen J. 2008. Regulation of dormancy in barley by blue light and after-ripening: effects on abscisic acid and gibberellin metabolism. Plant Physiology 147, 886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler F, Millar AA, Jacobsen JV. 2005. Dormancy release, ABA and pre-harvest sprouting. Current Opinion in Plant Biology 8, 183–187. [DOI] [PubMed] [Google Scholar]
- Harms K, Ramirez II, Pena-Cortes H. 1998. Inhibition of wound-induced accumulation of allene oxide synthase transcripts in flax leaves by aspirin and salicylic acid. Plant Physiology 118, 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington GT. 1923. Forcing the germination of freshly harvested wheat and other cereals. Journal of Agricultural Research 23, 79–100. [Google Scholar]
- Hauvermale AL, Tuttle KM, Takebayashi Y, Seo M, Steber CM. 2015. Loss of Arabidopsis thaliana seed dormancy is associated with increased accumulation of GID1 gibberellin hormone receptors. Plant and Cell Physiology 56, 1773–1785. [DOI] [PubMed] [Google Scholar]
- Hu Y, Jiang L, Wang F, Yu D. 2013. Jasmonate regulates the INDUCER OF CBF EXPRESSION-C-REPEAT BINDING FACTOR/DRE BINDING FACTOR1 cascade and freezing tolerance in Arabidopsis . The Plant Cell 25, 2907–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JV, Barrero JM, Hughes T, Julkowska M, Taylor JM, Xu Q, Gubler F. 2013. Roles for blue light, jasmonate and nitric oxide in the regulation of dormancy and germination in wheat grain (Triticum aestivum L). Planta 238, 121–138. [DOI] [PubMed] [Google Scholar]
- Jacobsen JV, Pearce DW, Poole AT, Pharis RP, Mander LN. 2002. Abscisic acid, phaseic acid and gibberellin contents associated with dormancy and germination in barley. Physiologia Plantarum 115, 428–441. [DOI] [PubMed] [Google Scholar]
- Jarvis SB, Taylor MA, Bianco J, Corbineau F, Davies HV. 1997. Dormancy-breakage in seeds of Douglas fir (Pseudotsuga menziesii (Mirb) Franco). Support for the hypothesis that LEA gene expression is essential for this process. Journal of Plant Physiology 151, 457–464. [Google Scholar]
- Ji X, Dong B, Shiran B, Talbot MJ, Edlington JE, Hughes T, White RG, Gubler F, Dolferus R. 2011. Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiology 156, 647–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krock B, Schmidt S, Hertweck C, Baldwin IT. 2002. Vegetation-derived abscisic acid and four terpenes enforce dormancy in seeds of the post-fire annual Nicotiana attenuata . Seed Science Research 12, 239–252. [Google Scholar]
- Leon-Reyes A, Van der Does D, De Lange ES, Delker C, Wasternak C, Van Wees SCM, Ritsema T, Pieterse CMJ. 2010. Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Planta 232, 1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenton JR, Appleford NEJ, Croker SJ. 1994. Gibberellins and α-amylase gene expression in germinating wheat grains. Plant Growth Regulation 15, 261–270. [Google Scholar]
- Leymarie J, Robayo-Romero ME, Gendreau E, Benech-Arnold RL, Corbineau F. 2008. Involvement of ABA in induction of secondary dormancy in barley (Hordeum vulgare L) seeds. Plant and Cell Physiology 49, 1830–1838. [DOI] [PubMed] [Google Scholar]
- Liu A, Gao F, Kanno Y, Jordan MC, Kamiya Y, Seo M, Ayele BT. 2013. Regulation of wheat seed dormancy by after-ripening is mediated by specific transcriptional switches that induce changes in seed hormone metabolism and signaling. PLoS One 8, e56570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Meng J, Starkey S, Smith CM. 2011. Wheat gene expression is differentially affected by a virulent Russian wheat aphid biotype. Journal of Chemical Ecology 37, 472–482. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang H, Zhao Y, Feng Z, Li Q, Yang HQ, Luan S, Li J, He ZH. 2013. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proceedings of the National Academy of Sciences, USA 110, 15485–15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares DJ. 1984. Temperature dependence of germinability of wheat (Triticum aestivum L) grain in relation to pre-harvest sprouting. Australian Journal of Agricultural Research 35, 115–128. [Google Scholar]
- McConn M, Browse J. 1996. The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. The Plant Cell 8, 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AA, Jacobsen JV, Ross JJ, Helliwell CA, Poole AT, Scofield G, Reid JB, Gubler F. 2006. Seed dormancy and ABA metabolism in Arabidopsis and barley: the role of ABA 8'-hydroxylase. The Plant Journal 45, 942–954. [DOI] [PubMed] [Google Scholar]
- Moons A, Prinsen E, Bauw G, Van Montagu M. 1997. Antagonistic effects of abscisic acid and jasmonates on salt stress-inducible transcripts in rice roots. The Plant Cell 9, 2243–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata M, Mitsuda N, Herde M, Koo AJ, Moreno JE, Suzuki K, Howe GA, Ohme-Takagi M. 2013. A bHLH-type transcription factor, ABA-INDUCIBLE BHLH-TYPE TRANSCRIPTION FACTOR/JA-ASSOCIATED MYC2-LIKE1, acts as a repressor to negatively regulate jasmonate signaling in arabidopsis. The Plant Cell 25, 1641–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K, Kawabata C, Kawakami N. 1994. Response of wheat grain to ABA and imbibition at low temperature. Plant Breeding 113, 53–57. [Google Scholar]
- Nyachiro JM, Clarke FR, DePauw RM, Knox RE, Armstrong KC. 2002. Temperature effects on seed germination and expression of seed dormancy in wheat. Euphytica 126, 123–127. [Google Scholar]
- Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S. 2003. Gibberellin biosynthesis and response during Arabidopsis seed germination. The Plant Cell 15, 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E. 2006. CYP707A1 and CYP707A2, which encode abscisic acid 8'-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiology 141, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L, Barbero GF, Geerinck J, et al. 2010. NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464, 788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Cortés H, Albrecht T, Pra S, Weiler EW, Willmitzer L. 1993. Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta 191, 123–128. [Google Scholar]
- Peng J, Harberd NP. 2002. The role of GA-mediated signalling in the control of seed germination. Current Opinion in Plant Biology 5, 376–381. [DOI] [PubMed] [Google Scholar]
- Preston J, Tatematsu K, Kanno Y, Hobo T, Kimura M, Jikumaru Y, Yano R, Kamiya Y, Nambara E. 2009. Temporal expression patterns of hormone metabolism genes during imbibition of Arabidopsis thaliana seeds: a comparative study on dormant and non-dormant accessions. Plant and Cell Physiology 50, 1786–1800. [DOI] [PubMed] [Google Scholar]
- Ranjan R, Lewak S. 1992. Jasmonic acid promotes germination and lipase activity in non-stratified apple embryos. Physiologia Plantarum 86, 335–339. [Google Scholar]
- Reddy LV, Metzger RJ, Ching TM. 1985. Effect of temperature on seed dormancy of wheat. Crop Science 25, 455–458. [Google Scholar]
- Rodríguez MV, Barrero JM, Corbineau F, Gubler F, Benech-Arnold RL. 2015. Dormancy in cereals (not too much, not so little): about the mechanisms behind this trait. Seed Science Research 25, 99–119. [Google Scholar]
- Rudnioki R, Sinska I, Lewak S. 1972. The influence of abscisic acid on the gibberellin content in apple seeds during stratification. Biologia Plantarum 14, 325–329. [Google Scholar]
- Sawhney R, Naylor JM. 1979. Dormancy studies in seed of Avena fatua. 9. Demonstration of genetic variability affecting the response to temperature during seed development. Canadian Journal of Botany 57, 59–63. [Google Scholar]
- Shan X, Wang J, Chua L, Jiang D, Peng W, Xie D. 2011. The role of Arabidopsis rubisco activase in jasmonate-induced leaf senescence. Plant Physiology 155, 751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Zhang Y, Peng W, Wang Z, Xie D. 2009. Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. Journal of Experimental Botany 60, 3849–3860. [DOI] [PubMed] [Google Scholar]
- Shu K, Liu X, Xie Q, He Z. 2016. Two faces of one seed: hormonal regulation of dormancy and germination. Molecular Plant 9, 34–45. [DOI] [PubMed] [Google Scholar]
- Staswick PE, Su W, Howell SH. 1992. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proceedings of the National Academy of Sciences, USA 89, 6837–6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steber CM, McCourt P. 2001. A role for brassinosteroids in germination in Arabidopsis . Plant Physiology 125, 763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle KM, Martinez SA, Schramm EC, Takebayashi Y, Seo M, Steber CM. 2015. Grain dormancy loss is associated with changes in ABA and GA sensitivity and hormone accumulation in bread wheat, Triticum aestivum (L). Seed Science Research 25, 179–193. [Google Scholar]
- Ueda J, Kato J. 1980. Isolation and identification of a senescence-promoting substance from wormwood (Artemisia absinthium L). Plant Physiology 66, 246–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Does D, Leon-Reyes A, Koornneef A, et al. 2013. Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59. The Plant Cell 25, 744–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M. 1987. ABA levels and sensitivity in developing wheat embryos of sprouting resistant and susceptible cultivars. Plant Physiology 84, 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Liu HY, Xu HM, Li M, Kang ZS. 2012. Analysis of differential transcriptional profiling in wheat infected by Blumeria graminis f. sp. tritici using GeneChip. Molecular Biology Reports 39, 381–387. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mopper S, Hasenstein KH. 2001. Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona . Journal of Chemical Ecology 27, 327–342. [DOI] [PubMed] [Google Scholar]
- West WCF, Fratterelli FJ, Russin KJ. 1970. Effect of stratification and gibberellin on seed germination in Ginkgo biloba . Bulletin of the Torrey Botanical Club 97, 380–384. [Google Scholar]
- Wu JS, Chong K, Xu YY, Tan KH. 2004. Cloning and characteristics of an allene oxide synthase gene (TaAOS) of winter wheat. Journal of Plant Physiology and Molecular Biology 30, 413–420. [PubMed] [Google Scholar]
- Xue LW, Du JB, Yang H, Xu F, Yuan S, Lin HH. 2009. Brassinosteroids counteract abscisic acid in germination and growth of Arabidopsis . Zeitschrift fur Naturforschung C 64, 225–230. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, Yamaguchi S. 2004. Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. The Plant Cell 16, 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz K, Muradoglu F, Yilmaz H. 2008. The effect of jasmonic acid on germination of dormant and nondormant pear (Pyrus communis L) seeds. Seed Science and Technology 36, 569–574. [Google Scholar]
- Yildiz K, Yazici C, Muradoglu F. 2007. Effect of jasmonic acid on germination of dormant and non-dormant apple seeds. Asian Journal of Chemistry 19, 1098–1102. [Google Scholar]
- Zaharia LI, Galka MM, Ambrose SJ, Abrams SR. 2005. Preparation of deuterated abscisic acid metabolites for use in mass spectrometry and feeding studies. Journal of Labelled Compounds and Radiopharmaceuticals 48, 435–445. [Google Scholar]
- Zhao Y, Dong W, Zhang N, Ai X, Wang M, Huang Z, Xiao L, Xia G. 2014. A wheat allene oxide cyclase gene enhances salinity tolerance via jasmonate signaling. Plant Physiology 164, 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.