Highlight
Transketolase 7 and 10 of Craterostigma plantagineum participate in the synthesis of octulose phosphate in an alternative Calvin cycle. Octulose is the main transport sugar in fully hydrated plants.
Key words: Octulose, photosynthesis, resurrection plants, sugar transport, transketolase.
Abstract
Phylogenetic analysis revealed that Craterostigma plantagineum has two transketolase genes (transketolase 7 and 10) which are separated from the other transketolase genes including transketolase 3 from C. plantagineum. We obtained recombinant transketolase 3, 7, and 10 of C. plantagineum and showed that transketolase 7 and 10 of C. plantagineum, but not transketolase 3, catalyse the formation of octulose-8-phosphate in vitro. Transketolase 7 and 10 of C. plantagineum performed the exchange reaction that produces octulose-8-phosphate using glucose-6-phosphate and fructose-6-phosphate as substrates. Octulose is localized in the cytosol and phloem exudate analysis showed that octulose was the dominant sugar exported from the leaves to the roots.
Introduction
d-Glycero-d-ido-octulose (octulose) is a rare monosaccharide which accumulates in the desiccation-tolerant plant Craterostigma plantagineum Hochst. The conversion between octulose and sucrose occurs when C. plantagineum undergoes dehydration and subsequent rehydration (Bianchi et al., 1991). Considerable research has revealed that sucrose has a fundamental function in regulating osmotic potential and protecting membranes as well as macro-molecules in resurrection plants (Hoekstra et al., 1997; Vicré et al., 2004; Peters et al., 2007; Dinakar and Bartels, 2013). Some efforts have been made to explain how sucrose is produced in C. plantagineum (Ingram et al., 1997; Kleines et al., 1999). In contrast to sucrose metabolism, the generation and physiological function of free octulose is still a mystery.
Octulose is an eight carbon monosaccharide, its mono-phosphate octulose-8-phosphate is an intermediate in the pentose phosphate pathway and may be synthesized via a novel alternative photosynthesis pathway (Flanigan et al., 2006; Williams and MacLeod, 2006). Transketolase acts as a key enzyme in the pentose phosphate pathway and, in photosynthesis, it catalyses the formation of various sugar phosphates (Krüger and von Schaewen, 2003). It has been suggested to participate in octulose metabolism in C. plantagineum. Three isoforms of transketolase (tkt3, tkt7, and tkt 10) have been characterized at the molecular level in C. plantagineum (Bernacchia et al., 1995). Transketolase extracted from rehydrated leaves of C. plantagineum catalysed the formation of octulose-8-phosphate using glucose-6-phosphate and β-hydroxypyruvate as substrates (Willige et al., 2009). Similarly, Williams and MacLeod (2006) obtained octulose-8-phosphate in extracts from spinach through the exchange reaction with glucose-6-phosphate and fructose-6-phosphate as substrates. They proposed that the exchange reaction catalysed by transketolase is part of an alternative Calvin cycle (also called the alternative scheme). Although there is still a dispute about the exchange reaction, Clasquin et al. (2011) provided new evidence for this alternative scheme. They showed that d-glycero-d-altro-octulose-1,8-bisphosphate might be synthesized in yeast by the aldol addition of dihydroxyacetone phosphate and ribose-5-phosphate which was catalysed by the glycolytic enzyme fructose bisphosphate aldolase. This is consistent with another part of the alternative scheme proposed by Flanigan et al. (2006). Therefore, it is necessary to examine whether the exchange reaction is performed by transketolase isolated from C. plantagineum. Storage and transport are important components of sugar metabolism which are involved in carbon allocation in plants. The appearance of octulose in the roots of C. plantagineum (Norwood et al., 2003) raises the question, could octulose be transported?
Transketolase protein was extracted from C. plantagineum leaves and the recombinant C. plantagineum transketolases 3, 7, and 10 were obtained from the corresponding over-expression construct. Proteins were used to test the exchange reaction. GC/MS analysis demonstrated that transketolases 7 and 10 can perform the exchange reaction and octulose was synthesized. Octulose is accumulated in the cytosol, acting as an excellent antioxidant (Zhang and Bartels, 2016). Octulose is also exported from the leaves to the roots in C. plantagineum.
Materials and methods
Plant material
C. plantagineum plants were grown as previously described by Bartels et al. (1990).
Gene cloning and protein purification
The cDNA fragments encoding the C. plantagineum genes TKT3, TKT7, and TKT10 were amplified by PCR and cloned into the PJET1.2 vector according to the manual of molecular cloning (Sambrook et al., 2001), the guide to the CloneJET PCR cloning kit (Thermo scientific, #k1231), and the Thermo scientific protocol of DNA digestion and ligation. Detailed information on the gene sequences of TKT3, TKT7, and TKT10 of C. plantagineum is given by Bernacchia et al. (1995). Enzyme-digested fragments were ligated to the digested pET28a+ vector and transformed into the Escherichia coli expression strain BL21 (DE3). All of the primers used were designed by software Oligo 7 and their sequences are listed in Supplementary Table S1 at JXB online. Protein expression and purification were conducted according to Kirch and Röhrig (2010). Purified proteins were dissolved in the elution buffer: 50mM HEPES/NaOH (pH 7.4), 300mM NaCl, 250mM imidazole, 10% (v/v) glycerol, 0.1% (v/v) Triton X-100, and 1.5mM β-mercaptoethanol. The empty vector pET 28a+ was used as the control in the transformation and protein purification from E. coli BL21 (DE3) cells. Protein concentrations were determined according to Bradford (1976) using the Bio-Rad kit (Bio-Rad Laboratories, Inc. USA).
Transketolase extraction from plant tissue and protein analysis
Transketolase from plant leaves was purified according to Bernacchia et al. (1995). The pellet that was obtained after 50–70% (w/v) (NH4)2SO4 precipitation was dissolved in 500 µl buffer A (50mM TRIS–HCI, pH 7.5, 10% (v/v) glycerol, 10mM MgCl2) and used for enzymatic assay.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS–PAGE) of the proteins was conducted according to Laemmli (1970). Protein Western blot analysis was performed as described by Bartels et al. (1991) and the transketolase antiserum was prepared by Bernacchia et al. (1995).
Enzymatic assays and product dephosphorylation
The enzymatic reactions were performed as described by Willige et al. (2009): 25 µg purified protein, 58mM glycylglycine (pH 7.7), 0.01% (w/v) Na-azide, 0.002% thiamine pyrophosphate, 15mM MgCl2, 5.3mM acceptor substrate (ribose-5-phosphate or glucose-6-phosphate), and 16mM donor substrate (β-hydroxypyruvate or fructose-6-phosphate).
After the catalysing reaction, sugar phosphates were dephosphorylated according to Willige et al. (2009). The dephosphorylated products were purified by passing through a column containing ion-exchange bed resin AG 501-X8(D) (BIO-RAD). The flow-through fractions were used for GC/MS analysis.
Carbohydrate extraction and sugar analysis by GC/MS
Sugars were extracted from plant tissues as described by Willige et al. (2009). Freeze-dried plant material was ground to a fine powder and extracted twice with 80% (v/v) methanol (3ml g–1) at 4 °C. The homogenates were cleared by centrifugation for 5min at 5 000 g and 4 °C. The methanol was evaporated to dryness under reduced pressure at 25 °C. The sediment was taken up in water and washed three times with chloroform to remove lipophilic substances. The aqueous phase was centrifuged for 30min at 10 000 g and 4 °C to remove any particles. A cation (Dowex 50 WX8) and an anion (Dowex IX8) exchange resin were added to the aqueous phase (5g resin per 100ml) to remove organic acids, amino acids or other charged molecules. After stirring for 1h the aqueous phase was transferred into a reaction vial for GC analysis.
The extracted sugar fractions were further separated and identified by coupled gas chromatography (GC)/flame ionization detection (GC/FID) and coupled gas chromatography/mass spectrometry (GC/MS). Ten µl of extract, prepared as described above, and 10 µg of xylitol (used as the internal standard) were dried at 60 °C under N2 gas. 30 µl pyridine and 30 µl N,O-Bis(trimethylsilyl)-trifluoracetamide (BSTFA) were then added and the sample was diluted with 50 µl chloroform to reach a mass between 1ng and 20ng. The samples were heated for 40min at 70 °C. The trimethylsilyl (TMS) sugar derivatives were separated on a DB1 column (J&W Scientific, Folsom, CA, USA). Qualitative GC/MS analysis was carried out with a gas chromatograph 7890B, detector 5977A MS Detection HP (Agilent Technologies, Böblingen, Germany); quantitative analysis was carried out with GC/FID (5890Series II Plus, HP, Agilent Technologies) (Willige et al., 2009). One µl of each sample was injected and the H2 flow was set to 37 kpa. The initial temperature was 65 °C for 3min, after which the temperature was raised at a rate of 8 °C min–1 to a temperature of 240 °C, after which the rate was increased by 12 °C min–1 to a final temperature of 310 °C for 35min. Data analysis was performed with GC ChemStation [Rev.B.03.02(341), Agilent Technologies, Böblingen, Germany].
Chloroplast isolation
Chloroplasts of C. plantagineum were isolated according to Rowan and Bendich (2011). Plant leaves were rinsed briefly with 70% (v/v) ethanol, followed by distilled water. Four grams of C. plantagineum leaves were mixed into 100ml XPI solution (50mM HEPES pH 7.5, 330mM sorbitol, 5mM ascorbic acid, 1mM MgCl2, 1mM MnCl2, and 2mM EDTA) and homogenated on ice using a blender. The homogenate was filtered through three layers of Miracloth. The filtered liquid was centrifuged at 4 °C at 3 000 g for 5min. The pellet was suspended in 5ml XPI solution and layered on to the prepared 30/70% Percoll gradient (70% Percoll at the bottom, 30% Percoll on top). The mix was centrifuged at 4 °C at 12 000 g for 10min using a swing-out rotor. The purified chloroplasts were collected from the band at the interface between the 30% and 70% Percoll layers using glass Pasteur pipettes. The chloroplasts were washed twice with 10 vols of XPI solution and centrifuged at 12 000 g for 20s. Finally, the isolated chloroplasts were freeze-dried and kept at –70 °C.
Thin layer chromatography (TLC) and octulose purification
Sugars were separated by TLC silica gel 60 (CAS 105553, Merck Millipore) using n-butanol:H2O:n-propanol (9:1:0.5 by vol.) as the developing solution for about 2h. The plate was then dried at 37 °C and the sugars were detected by spraying the plates with a mixture of EtOH:H2SO4:HAc:anisaldehyde (90:5:1:5 by vol.) and heating to 100 °C for 5min (Kutzer, 2004).
The area containing the sugars was excised from the silica gel and saturated in ethanol overnight. After centrifuging at maximum speed for 10min and filtration through a 40 µm filter membrane, the ethanol solution was evaporated to dryness under reduced pressure at 25 °C. Finally, the syrup was weighed and dissolved in water for GC/MS analysis.
Phloem exudate analysis
The analysis was performed according to Wingenter et al. (2010). At the end of the illumination, the petiole ends of mature leaves from 6-week-old plants were cut under water and then transferred quickly into reaction tubes containing 100 µl of 15mM EDTA solution (pH 7.25). The sugar export experiment was done in a water-saturated atmosphere overnight. The solution was then heated to 95 °C for 3min in the reaction tubes before being cooled down for GC analysis (5mM CaCl2 inthe reaction tubes was used as a control). To increase the sugar concentration for the GC analysis, the leaf exudate solutions were condensed to about 20 µl by evaporating under reduced pressure at 25 °C. The sugar content is presented as a percentage of the total sugars.
Phylogenetic analysis of transketolase genes
Transketolase sequences were retrieved by Blast from the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov/) and obtained from the RNA sequencing of Lindernia brevidens and Lindernia subracemosa (unpublished data). The phylogenetic tree was generated from aligned sequences of predicted proteins from 51 plant transketolase genes by Maximum Likelihood Analysis using 2 000 bootstrap predictions and the 50% majority rule in MEGA6 (Hall, 2013).
Results
Molecular phylogeny of plant transketolases
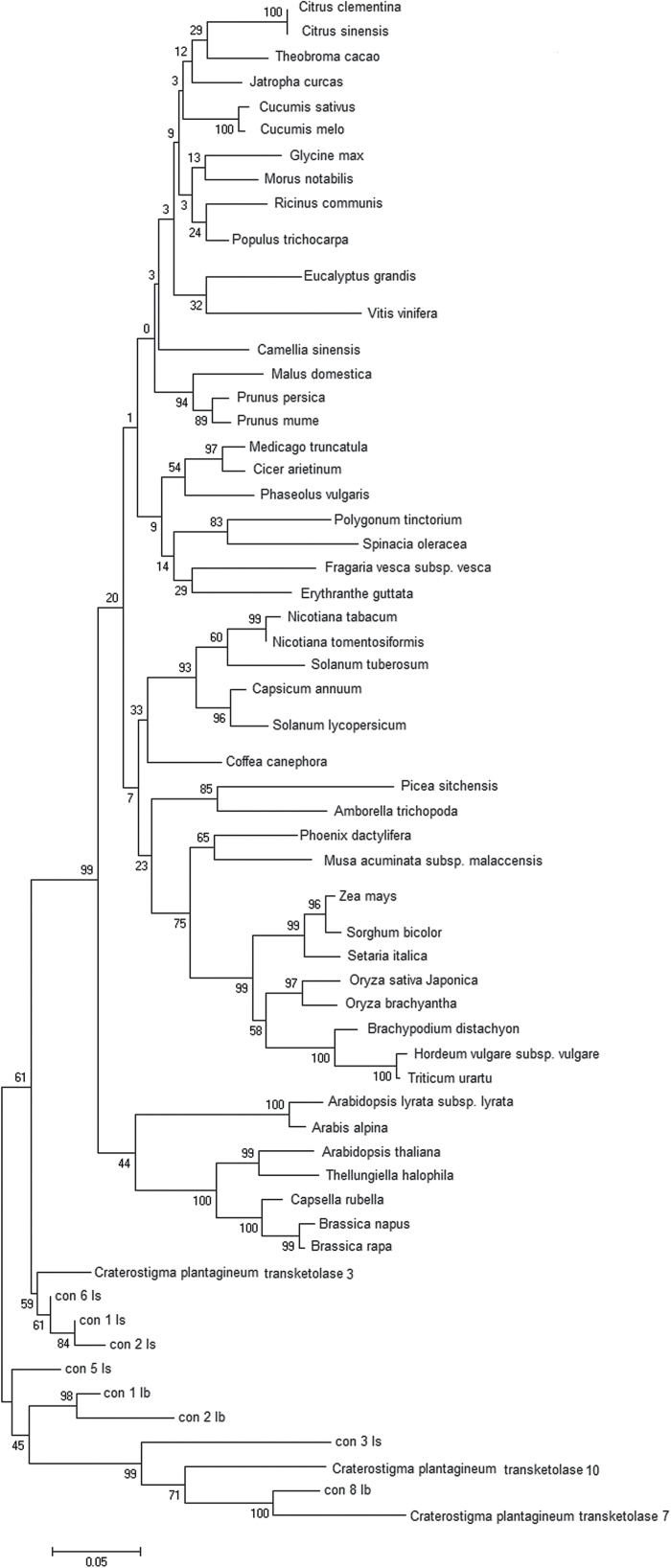
Phylogeny analysis of the transketolase genes demonstrates that TKT7 and TKT10 are closely related in C. plantagineum and have diverged from other transketolase genes of desiccation-sensitive species while TKT3 shares a higher identity with other plant species (Fig. 1). Using RNA sequencing, we have found eight possible homologous genes for TKT3, TKT7, and TKT10 of C. plantagineum in another two octulose-producing plants L. brevidens and L. subracemosa (Phillips et al., 2008). In the phylogenetic tree, TKT7 and TKT10 and their five homologues from L. brevidens and L. subracemosa (con 8 lb, con 3 ls, con 2 lb, con 1 lb, and con 5 ls) form a separate branch. Three homologues from L. brevidens and L. subracemosa (labelled as con 6 LS, con 1 LS, and con 2 LS) show highly similar evolutionary characteristics with TKT3 in another branch. The branch with C. plantagineum TKT3 appears closer to the big group that is composed of transketolases from another 48 angiosperm species. This shows the specificity of the TKT7 and TKT10 genes in octulose-producing plants in evolution.
Fig. 1.
Phylogenetic tree of plant transketolases constructed by Mega 6 software using amino acid sequences obtained from NCBI databases and the transcriptomes of Lindernia brevidens and Lindernia subracemosa. The homologues of transketolase genes from L. brevidens are con 1 lb, con2 lb, and con 8 lb and from L. subracemosa are con 1 ls, con 2 ls, con 3 ls, con 5 ls, and con 6 ls.
In vitro synthesis of octulose
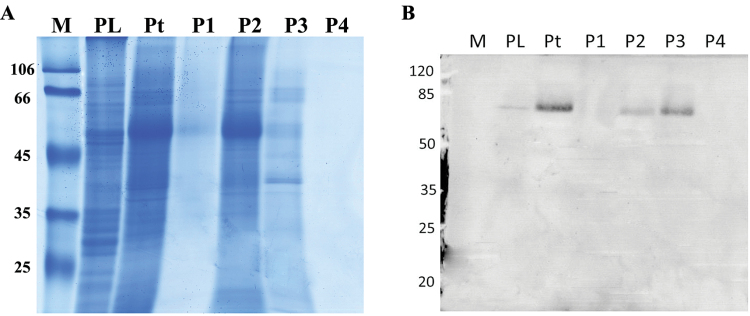
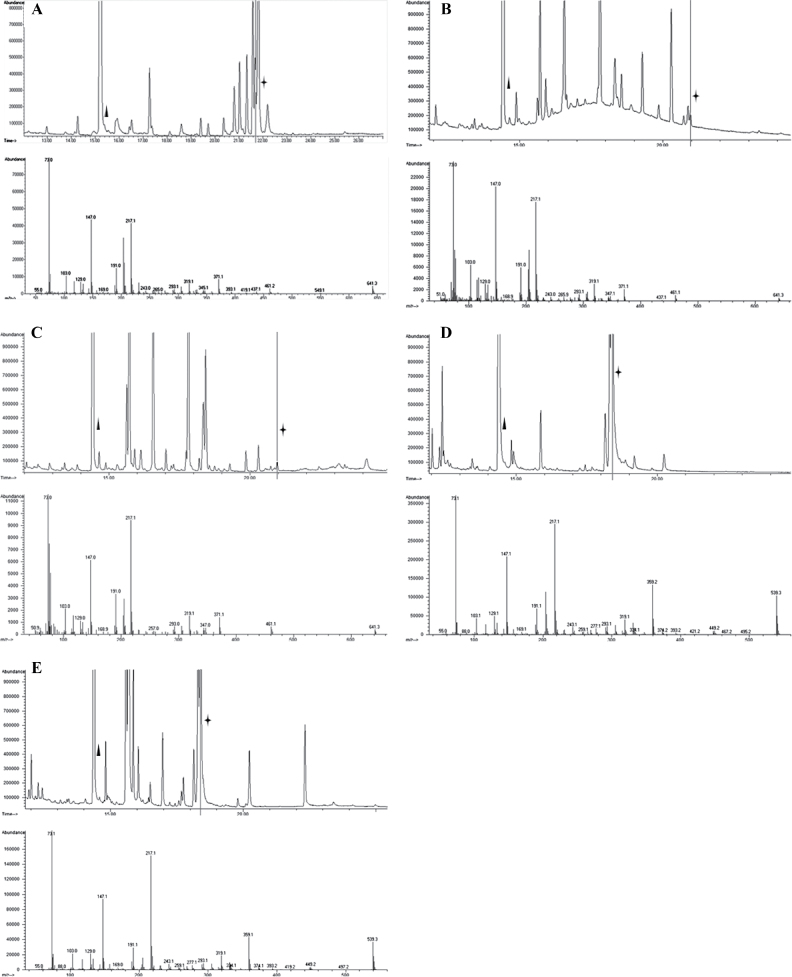
Protein extracts enriched in transketolase were obtained from leaves of C. plantagineum by fractionated ammonium sulphate precipitation. Immunoblotting showed that transketolase was concentrated in the fractions precipitated by 50–70% (w/v) ammonium sulphate (Fig. 2). This transketolase containing protein fraction was used in reactions with glucose-6-phosphate and β-hydroxypyruvate as substrates. Octulose was detected in the reaction by verifying that its trimethylsilyl derivative has the same mass spectrum as that of the octulose standard purified from C. plantagineum leaves (Fig. 3A, B). Octulose-8-phosphate was synthesized in the reaction which confirmed a previous result that octulose-8-phosphate was synthesized (Willige et al., 2009). Octulose-8-phosphate was also synthesized when β-hydroxypyruvate was substituted by fructose-6-phosphate (Fig. 3C). However, when ribose-5-phosphate was the acceptor substrate in the reaction, sedoheptulose-7-phosphate was formed, despite β-hydroxypyruvate or fructose-6-phosphate being the donor (Fig. 3 D, E).
Fig. 2.
SDS-PAGE (a) and immuno blotting (b) of proteins extracted from leaves of C. plantagineum. Note: M, protein size markers (given in kDa); PL, proteins not soluble in extraction buffer; Pt, total proteins extracted by the extraction buffer; P1, proteins precipitated by 0–25% (w/v) (NH4)2SO4; P2, proteins precipitated by 25–50% (w/v) (NH4)2SO4; P3, proteins precipitated by 50–70% (w/v) (NH4)2SO4; P4, proteins remaining in extraction buffer after precipitation by 50–70% (w/v) (NH4)2SO4; all pellets were dissolved in buffer A of the same volume as used in the extraction. Samples were loaded with SDS buffer on the gel.
Fig. 3.
(A) The chromatogram of the trimethylsilyl (TMS) derivatives of octulose purified from C. plantagineum leaves by TLC (upper part) and the mass spectra of the TMS derivative of octulose (lower part). The peak corresponding to the internal standard xylitol is labelled by a triangle and that of octulose is labelled by an asterisk. (B) The chromatogram of the TMS derivatives of the dephosphorylated reaction products with glucose-6-phosphate as acceptor substrate and β-hydroxypyruvate as donor (upper part) and the mass spectra of the TMS derivative of octulose (lower part). The peak corresponding to the internal standard xylitol is labelled by a triangle and that of octulose is labelled by an asterisk. The reaction contains 25 µg purified protein, 58mM glycylglycine (pH 7.7), 0.01% (w/v) Na-azide, 0.002% thiamine pyrophosphate, 15mM MgCl2, 5.3mM donor, and 16mM acceptor. After 24h of the catalysing reaction, sugar phosphates in the product were dephosphorylated using acid phosphatase. The dephosphorylated products were purified on a column containing ion-exchanging bed resin AG 501-X8(D) (Bio-Rad). The flow-through fractions were used for GC/MS analysis. (C) The chromatogram of the TMS derivatives of the dephosphorylated reaction products with glucose-6-phosphate as acceptor substrate and fructose-6-phosphate as donor (upper part) and mass spectra of the TMS derivative of octulose (lower part). The peak corresponding to the internal standard xylitol is labelled by a triangle and that of octulose is labelled by an asterisk. The reaction conditions are the same as described in (B). (D) The chromatogram of the TMS derivatives of the dephosphorylated reaction products with ribose-5-phosphate as acceptor substrate and β-hydroxypyruvate as donor (upper part) and the mass spectra of the TMS derivative of sedoheptulose (lower part). The peak corresponding to the internal standard xylitol is labelled by a triangle and that of sedoheptulose is labelled by an asterisk. The reaction conditions are the same as described in (B). (E) The chromatogram of the TMS derivatives of the dephosphorylated reaction products with ribose-5-phosphate as acceptor substrate and fructose-6-phosphate as donor (upper part) and the mass spectra of the TMS derivative of sedoheptulose (lower part). The peak corresponding to the internal standard xylitol is labelled by a triangle and that of sedoheptulose is labelled by an asterisk. The reaction conditions are the same as described in (B).
The genes encoding transketolases 3, 7, and 10 were cloned in the expression vector pET28a+ and the corresponding recombinant proteins were expressed and purified from isopropyl β-d-1-thiogalactopyranoside-induced E. coli (BL21 DE3) cells. The quality and identity of the proteins was validated by SDS-PAGE (see Supplementary Fig. S1 at JXB online). Various reactions were carried out with the purified recombinant proteins and the dephosphorylated products of the reactions were analysed by GC/MS (Table 1). Results showed that transketolase 7 and transketolase 10 of C. plantagineum had the ability to catalyse the formation of octulose-8-phosphate using glucose-6-phosphate as acceptor and β-hydroxypyruvate or fructose-6-phosphate as donor, whereas transketolase 3 of C. plantagineum could not perform this function. However, all three recombinant proteins catalysed the formation of sedoheptulose-7-phosphate with ribose-5-phosphate as acceptor substrate and β-hydroxypyruvate or fructose-6-phosphate as donor. Neither octulose nor sedoheptulose were detected in the dephosphorylated products of reactions catalysed by the proteins purified from E. coli (BL21 DE3) cells transformed with the vector pET28a+.
Table 1.
Summary of the dephosphorylated products obtained in transketolase-catalysed reactions in combination with different acceptor and donor substrates
| Enzymea | Donor+acceptor | Donor+acceptor | Donor+acceptor | Donor+acceptor |
|---|---|---|---|---|
| HP+Glu-6-Pb | HP+Rib-5-Pb | Fru-6-P+Glu-6-Pb | Fru-6-P+Rib-5-Pb | |
| CK | – | – | – | – |
| CKe | – | – | – | – |
| Tkt3 | – | Sedc | – | Sed |
| Tkt7 | Octc | Sed | Oct | Sed |
| Tkt10 | Oct | Sed | Oct | Sed |
| Tktp | Oct | Sed | Oct | Sed |
a CK: reaction without protein; Cke: proteins purified from E. coli (BL21) transformed with vector pET28a+; Tktp: the protein enriched from leaf extracts of C. plantagineum.
b HP refers to β-hydroxypyruvate, Glu-6-P to glucose-6-phosphate, Rib-5-P to ribose-5-phosphate, and Fru-6-P to fructose-6-phosphate.
c Sed: sedoheptulose; Oct: octulose; –: no sedoheptulose or octulose found.
These results indicate that the exchange reaction is feasible with a transketolase-enriched protein fraction from C. plantagineum. The recombinant C. plantagineum transketolases 7 and 10 also demonstrate the feasibility of the exchange reaction (Table 1). The differentiation of the catalysing functions of the three transketolase isoforms demonstrates that they have different catalytic properties which are connected with different roles in sugar metabolism in C. plantagineum.
Localization and transport of octulose
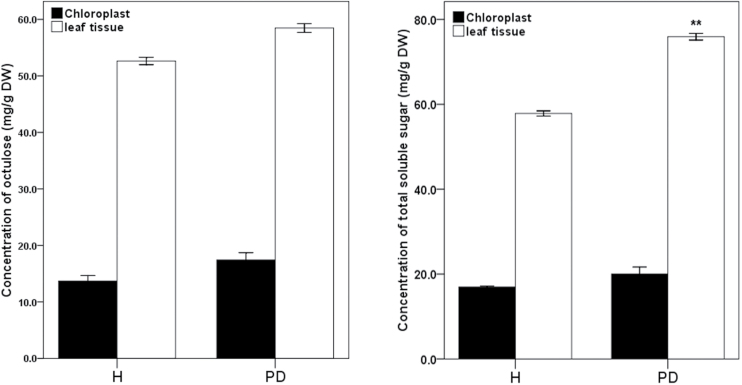
Although octulose accumulates to a high level, its cellular localization is as yet unknown. Therefore we attempted to identify the cellular compartment where octulose accumulates and how it is transported. Experimental results showed that the amount of octulose in isolated chloroplasts was lower than in whole leaf tissues in hydrated conditions or partially dehydrated conditions (Fig. 4). Partial dehydration led to a significant increase in total soluble sugars in leaf tissue tissues but it did not affect the levels of octulose and total soluble sugars in the chloroplast. This implies that octulose mainly accumulates in the cytosol.
Fig. 4.
The amounts of sugars in chloroplasts and leaf tissues in hydrated (H) and partially dehydrated (PD) conditions. In PD, plants were slowly dehydrated for 2 d when an RWC of 75% was reached in the leaves. All data represent means ±SD (n=3). Asterisks indicate significant differences determined with Student’s t test (*P <0.05, **P <0.01).
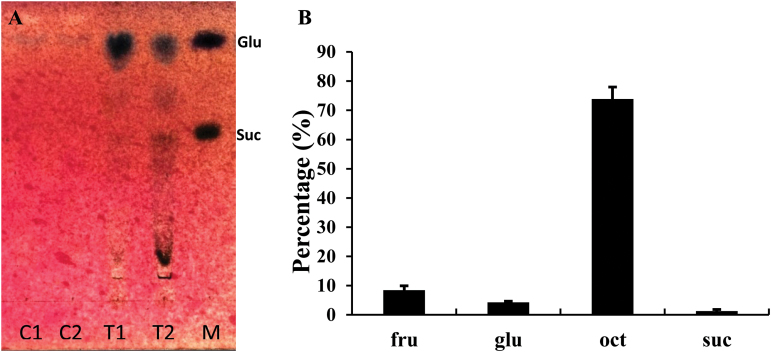
Octulose was found to be the most abundant sugar in fully hydrated plants. However, a dedicated analysis showed that octulose levels fluctuate and are subject to a circadian rhythm (Supplementary Fig. S2). To explore the circadian change in octulose levels, phloem exudate analysis was conducted which demonstrated that 75–80% of the exported sugars is octulose (Fig. 5).
Fig. 5.
Thin layer chromatogram (a) and GC analysis of leaf exudates (b) of C. plantagineum. C1 and C2 are two replicates of the sugars exported into 5mM CaCl2 from leaves (control); T1 and T2 are two replicates of the sugars exported into 15mM EDTA (leaf exudates); Lane M contains glucose (Glu) and sucrose (Suc) standards. The GC analysis shows the percentage of sugars relative to total sugars. All data represent means ±SD (n=3).
Discussion
As an important component of the photosynthesis reaction and the pentose phosphate pathway, the evolution of transketolase in plants might reflect the strategy of plants in adapting sugar metabolism to environmental requirements. Phylogeny analysis of transketolase genes demonstrates that TKT7 and TKT10 in C. plantagineum have diverged from the transketolase genes of many desiccation-sensitive species. The sequence analysis showed that C. plantagineum TKT7 and TKT10 and their homologous genes (e.g. con 8 lb from L. brevidens and con 3 ls from L. subracemosa) lack recognizable transit peptides that transport the transketolase to the chloroplast. The transit peptide targeting transketolase to plastids is characteristic for most transketolase genes and is also present in TKT3 of C. plantagineum (Lange et al. 1998; Willige et al., 2009). This may explain the diversification of C. plantagineum TKT7 and TKT10 and their homologous genes from L. brevidens and L. subracemosa and may be related to the fact that they encode enzymes involved in octulose metabolism in these plants.
Our results showed that TKT3 catalysed the transfer of a two-carbon ketol group from β-hydroxypyruvate or fructose-6-phosphate to ribose-5-phosphate to form sedoheptulose-7-phosphate. Besides catalysing the same transfer reaction, TKT7 and TKT10 also catalyse the formation of octulose-8-phosphate using glucose-6-phosphate as acceptor and β-hydroxypyruvate/fructose-6-phosphate as donor. This means that the exchange reaction is possible in C. plantagineum as it is in spinach (Williams and MacLeod, 2006). Willige et al. (2009) reported that TKT3 is localized in the chloroplasts while TKT7 and TKT10 are localized in the cytoplasm. Combined with the alternative Calvin cycle (Flanigan et al., 2006), it is reasonable to suggest that TKT3 is only involved in carbon reactions in photosynthesis and the pentose phosphate pathway, while TKT7 and TKT10 are responsible for the accumulation of octulose in C. plantagineum. TKT7 and TKT10 could also participate in the pentose phosphate pathway, as transketolases can accept various substrates (Williams et al., 1987; Krüger and von Schaewen, 2003) which is also shown for both TKT7 and TKT10 in our study. Expression analysis of transketolase isoforms showed that TKT3 is constitutively expressed in leaves and roots while TKT7 and TKT10 are up-regulated in leaves during rehydration of the desiccated plant (Bernacchia et al., 1995). The up-regulation of TKT7 and TKT10 positively correlates with octulose accumulation during rehydration. This supports the notion that TKT7 and TKT10 catalyse the exchange reaction to produce octulose. However, we are still left with the question: Is the conversion from octulose to sucrose during rehydration achieved through the reverse exchange reaction or is there another pathway for the conversion? Although the transcript levels of TKT7 and TKT10 decrease during dehydration (Rodriguez et al., 2010), dehydration treatments generally take more than 2 weeks compared with rehydration that is completed within 48h. It is possible to achieve the conversion during the longer time period. It is also possible that octulose is converted to sucrose by another pathway that might comprise phosphotransferase, aldolase, triosephosphate isomerase, and fructose 1,6-bisphosphatase as proposed by Williams and MacLeod (2006). Further experimentation is necessary to verify this hypothesis.
Except in C. plantagineum, octulose was also found to accumulate in several closely related Linderniaceae, such as Lindernia brevidens, Lindernia subracemosa, Lindernia philcoxii, Lindernia numilarifolia, Lindernia exilis, and Lindernia acicularis (Kutzer, 2004; Phillips et al., 2008). Thus it can be speculated that the exchange reaction catalysed by transketolase is also present in these plant species. Similarly, the other isomers of octulose, such as d-glycero-dmanno-octulose isolated from avocado and sedum species (Charlson and Richtmyer, 1960) and d-glycero-l-galacto-octulose isolated from Persea gratissima, Sedum spectabile, and Primula oficinalis (Sephton and Richtmyer, 1963; Begbie and Richtmyer, 1966), may also be synthesized by the exchange reactions catalysed by transketolase with substrates of specific stereo configurations.
Many monosaccharides do not occur naturally in the free state but are commonly found as phosphate-ester derivatives that are important intermediates in the breakdown and synthesis of carbohydrates in living organisms (Robyt, 1998). For some free monosaccharides, specific phosphatases exist, catalysing the dephosphorylation of their phosphate-ester derivatives, e. g. glucose and sedoheptulose (Van Schaftingen and Gerin, 2002; Ceusters et al., 2013). It is likely that a phosphatase exists which dephosphorylates octulose-8-phosphate or its isomers to produce octulose in C. plantagineum or d-glycero-d-manno-octulose/d-glycero-l-galacto-octulose in Persea gratissima/Sedum spectabile.
Octulose was shown to be the dominant sugar in leaf exudates by phloem exudate analysis. This indicates that C. plantagineum leaves transport octulose to roots as an energy supply. Although, in general, sucrose is the main transport form in plants, some plants also transport raffinose and stachyose and/or sugar alcohols (Turgeon and Wolf, 2009). Some studies have also proposed sedoheptulose as the sugar transport form (Liu et al., 2002; Ceusters et al., 2013) and, therefore, octulose is a good candidate for sugar transport in C. plantagineum and related plants. Our study showed that octulose accumulation follows a circadian rhythm (Supplementary Fig. S2) as already indicated by Norwood et al. (2003). The circadian fluctuation in octulose levels might be explained by octulose transport or by the circadian regulation of biosynthetic enzyme activities.
Sugars are often seen as an energy resource and as signalling molecules in plant cells (Eveland and Jackson, 2012). In the present study, we compared octulose levels in isolated chloroplasts and intact leaf tissues which suggests that octulose is localized in the cytosol. It is also possible that octulose is stored in the vacuole which occupies >95% of the cell volume in fully turgid plants and could serve as sites for storing soluble carbohydrates. This hypothesis could not be proved, as a protocol for vacuole isolation for C. plantagineum could not yet be established. Additional experiments showed that partial dehydration did not affect octulose levels in the leaf tissues. This can be explained by the study of Kutzer (2004) which showed that, during dehydration, sucrose starts to be rapidly synthesized in C. plantagineum only when the RWC is below 75%. Similarly, Cooper and Farrant (2002) found that sucrose substantially accumulated in Craterostigma wilmsii only during the late stages of dehydration (below 25% RWC). Increasing evidence also suggests that sugar molecules counteract oxidative stress by acting as genuine ROS scavengers (Van den Ende and Valluru, 2009; Peshev et al., 2013). Our analysis with the octulose isolated from TLC plates suggested that octulose has a stronger hydroxyl radical (OH−) scavenging ability than sucrose (Zhang and Bartels, 2016). Previous studies showed that sucrose is a more efficient scavenger than glucose and fructose (Nishizawa et al., 2008; Peshev et al., 2013). The superior antioxidant properties of octulose might also be a reason for the high accumulation in C. plantagineum.
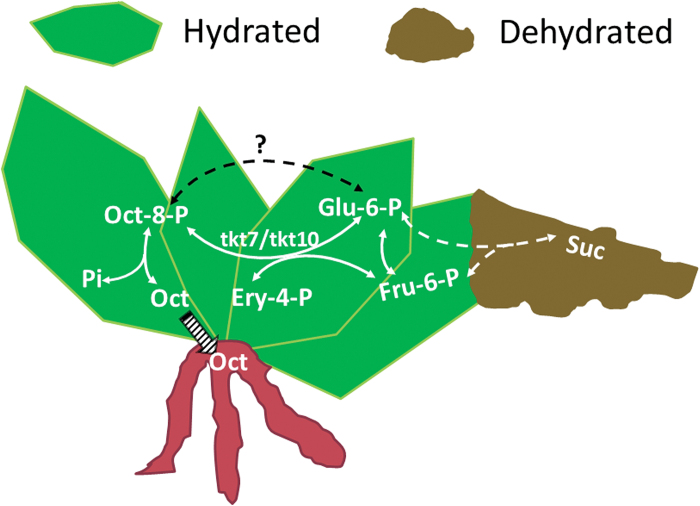
In conclusion, our study suggests that the TKT7 and TKT10 isoforms of transketolase show distinct specificity in function and evolution. They catalyse the synthesis of octulose-8-phosphate using glucose-6-phosphate and fructose-6-phosphate as substrate (Fig. 6). Octulose acts as a transport sugar in C. plantagineum and could play a role in antioxidant defence in the vacuole because of its stronger OH− scavenging ability than other abundant sugars. To date, a scheme of the metabolism of octulose in C. plantagineum can be formed to explain the conversion between octulose and sucrose based on the cellular water status.
Fig. 6.
A scheme of octulose metabolism in C. plantagineum. In hydrated conditions, octulose-8-phosphate (Oct-8-P) is synthesized through the exchange reaction that is catalysed by transketolase 7 (tkt7) and transketolase 10 (tkt10) using glucose-6-phosphate (Glu-6-P) and fructose-6-phosphate (Fru-6-P) as substrates. Oct-8-P is dephosphorylated to produce octulose (Oct). Oct can be transported from leaves to roots. In dehydrated conditions, Oct-8-P is transformed into Glu-6-P or Fru-6-P that is used to synthesize sucrose (Suc). Pi refers to phosphate. The striped arrow indicates the transport of octulose from leaves to roots. The dashed arrow only indicates the overall reaction direction (the details of the reaction are not shown).
Supplementary data
Supplementary data can be found at JXB online.
Figure S1. SDS-PAGE of purified proteins: transketolase 3 (a), transketolase 7 (b), and transketolase 10 (c).
Figure S2. Diurnal variation of octulose levels in leaves of C. plantagineum.
Table S1. List of primers used in this study.
Acknowledgements
Qingwei Zhang was sponsored by the China Scholarship Council. The authors would like to thank Christiane Buchholz for growing the plants for this study.
References
- Bartels D, Engelhardt K, Roncarati R, Schneider K, Rotter M, Salamini F. 1991. An ABA and GA modulated gene expressed in the barley embryo encodes an aldose reductase related protein. The EMBO Journal 10, 1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels D, Schneider K, Terstappen G, Piatkowski D, Salamini F. 1990. Molecular cloning of abscisic acid-modulated genes which are induced during desiccation of the resurrection plant Craterostigma plantagineum . Planta 181, 27–34. [DOI] [PubMed] [Google Scholar]
- Begbie R, Richtmyer NK. 1966. The isolation of some heptoses, heptuloses, octuloses, and nonuloses from Primula officinalis Jacq. Carbohydrate Research 2, 272–288. [Google Scholar]
- Bernacchia G, Schwall G, Lottspeich F, Salamini F, Bartels D. 1995. The transketolase gene family of the resurrection plant Craterostigma plantagineum: differential expression during the rehydration phase. The EMBO Journal 14, 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi G, Gamba A, Murelli C, Salamini F, Bartels D. 1991. Novel carbohydrate metabolism in the resurrection plant Craterostigma plantagineum . The Plant Journal 1, 355–359. [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Ceusters J, Godts C, Peshev D, Vergauwen R, Dyubankova N, Lescrinier E, De Proft MP, Van den Ende W. 2013. Sedoheptulose accumulation under CO2 enrichment in leaves of Kalanchoë pinnata: a novel mechanism to enhance C and P homeostasis? Journal of Experimental Botany 64, 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson AJ, Richtmyer NK. 1960. The isolation of an octulose and an octitol from natural sources:d-glycero-d-manno-octulose anddD-erythro-d-galacto-octitol from the avocado andd-glycero-d-manno-octulose from Sedum species. Journal of the American Chemical Society 82, 3428–3434. [Google Scholar]
- Clasquin MF, Melamud E, Singer A, et al 2011. Riboneogenesis in yeast. Cell 145, 969–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K, Farrant JM. 2002. Recovery of the resurrection plant Craterostigma wilmsii from desiccation: protection versus repair. Journal of Experimental Botany 53, 1805–1813. [DOI] [PubMed] [Google Scholar]
- Dinakar C, Bartels D. 2013. Desiccation tolerance in resurrection plants: new insights from transcriptome, proteome, and metabolome analysis. Frontiers in Plant Science 4, 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveland AL, Jackson DP. 2012. Sugars, signalling, and plant development. Journal of Experimental Botany 63, 3367–3377. [DOI] [PubMed] [Google Scholar]
- Flanigan IL, MacLeod JK, Williams JF. 2006. A re-investigation of the path of carbon in photosynthesis utilizing GC/MS methodology. Unequivocal verification of the participation of octulose phosphates in the pathway. Photosynthesis Research 90, 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BG. 2013. Building phylogenetic trees from molecular data with MEGA. Molecular Biology and Evolution 30, 1229–1235. [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, Wolkers WF, Buitink J, Golovina EA, Crowe JH, Crowe LM. 1997. Membrane stabilization in the dry state. Comparative Biochemistry and Physiology Part A: Physiology 117, 335–341. [Google Scholar]
- Ingram J, Chandler JW, Gallagher L, Salamini F, Bartels D. 1997. Analysis of cDNA clones encoding sucrose-phosphate synthase in relation to sugar interconversions associated with dehydration in the resurrection plant Craterostigma plantagineum Hochst. Plant Physiology 115, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirch HH, Röhrig H. 2010. Affinity purification and determination of enzymatic activity of recombinantly expressed aldehyde dehydrogenases. Methods in Molecular Biology 639, 282–291. [DOI] [PubMed] [Google Scholar]
- Kleines M, Elster R-C, Rodrigo M-J, Blervacq A-S, Salamini F, Bartels D. 1999. Isolation and expression analysis of two stress-responsive sucrose-synthase genes from the resurrection plant Craterostigma plantagineum (Hochst.). Planta 209, 13–24. [DOI] [PubMed] [Google Scholar]
- Krüger NJ, von Schaewen A. 2003. The oxidative pentose phosphate pathway: structure and organisation. Current Opinion in Plant Biology 6, 236–246. [DOI] [PubMed] [Google Scholar]
- Kutzer M. 2004. Untersuchung zum Zuckerstoffwechsel der Wiederauferstehungspflanze Craterostigma plantagineum und einiger Lindernia-Arten. PhD thesis, University of Bonn, Germany. [Google Scholar]
- Laemmli U. 1970. Most commonly used discontinuous buffer system for SDS electrophoresis. Nature 227, 680–685.5432063 [Google Scholar]
- Lange MB, Wildung MR, McCaskill D, Croteau R. 1998. A family of transketolases that directs isoprenoid biosynthesis via a mevalonate-independent pathway. Proceedings of the National Academy of Sciences, USA 95, 2100–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Sievert J, Arpaia ML, Madore MA. 2002. Postulated physiological roles of the seven-carbon sugars, mannoheptulose, and perseitol in avocado. Journal of the American Society for Horticultural Science 127, 108–114. [Google Scholar]
- Nishizawa A, Yabuta Y, Shigeoka S. 2008. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiology 147, 1251–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwood M, Toldi O, Richter A, Scott P. 2003. Investigation into the ability of roots of the poikilohydric plant Craterostigma plantagineum to survive dehydration stress. Journal of Experimental Botany 54, 2313–2321. [DOI] [PubMed] [Google Scholar]
- Peshev D, Vergauwen R, Moglia A, Hideg E, Van den Ende W. 2013. Towards understanding vacuolar antioxidant mechanisms: a role for fructans? Journal of Experimental Botany 64, 1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Mundree SG, Thomson JA, Farrant JM, Keller F. 2007. Protection mechanisms in the resurrection plant Xerophyta viscosa (Baker): both sucrose and raffinose family oligosaccharides (RFOs) accumulate in leaves in response to water deficit. Journal of Experimental Botany 58, 1947–1956. [DOI] [PubMed] [Google Scholar]
- Phillips JR, Fischer E, Baron M, Van Den Dries N, Facchinelli F, Kutzer M, Rahmanzadeh R, Remus D, Bartels D. 2008. Lindernia brevidens: a novel desiccation-tolerant vascular plant, endemic to ancient tropical rainforests. The Plant Journal 54, 938–948. [DOI] [PubMed] [Google Scholar]
- Robyt JF. 1998. Essentials of carbohydrate chemistry. Springer Science & Business Media. [Google Scholar]
- Rodriguez MCS, Edsgärd D, Hussain SS, Alquezar D, Rasmussen M, Gilbert T, Nielsen BH, Bartels D, Mundy J. 2010. Transcriptomes of the desiccation-tolerant resurrection plant Craterostigma plantagineum . The Plant Journal 63, 212–228. [DOI] [PubMed] [Google Scholar]
- Rowan B, Bendich A. 2011. Isolation, quantification, and analysis of chloroplast DNA. In: Jarvis RP, ed. Chloroplast research in Arabidopsis: methods and protocols, Vol. 1 Humana Press: New York, 151–170. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW, Irwin N. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY. [Google Scholar]
- Sephton HH, Richtmyer NK. 1963. The isolation of a second octulose and of a heptose from the Avocado:d-glycero-l-galacto-octulose andd-glycero-d-galacto-heptose. The Journal of Organic Chemistry 28, 1691–1694. [Google Scholar]
- Turgeon R, Wolf S. 2009. Phloem transport: cellular pathways and molecular trafficking. Annual Revivew of Plant Biology 60, 207–221. [DOI] [PubMed] [Google Scholar]
- Van den Ende W, Valluru R. 2009. Sucrose, sucrosyl oligosaccharides, and oxidative stress: scavenging and salvaging? Journal of Experimental Botany 60, 9–18. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E, Gerin I. 2002. The glucose-6-phosphatase system. Biochemical Journal 362, 513–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicré M, Farrant J, Driouich A. 2004. Insights into the cellular mechanisms of desiccation tolerance among angiosperm resurrection plant species. Plant, Cell and Environment 27, 1329–1340. [Google Scholar]
- Williams JF, Arora KK, Longenecker JP. 1987. The pentose pathway: a random harvest: impediments which oppose acceptance of the classical (F-type) pentose cycle for liver, some neoplasms and photosynthetic tissue. The case for the L-type pentose pathway. International Journal of Biochemistry 19, 749–817. [DOI] [PubMed] [Google Scholar]
- Williams JF, MacLeod JK. 2006. The metabolic significance of octulose phosphates in the photosynthetic carbon reduction cycle in spinach. Photosynthesis Research 90, 125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willige BC, Kutzer M, Tebartz F, Bartels D. 2009. Subcellular localization and enzymatic properties of differentially expressed transketolase genes isolated from the desiccation tolerant resurrection plant Craterostigma plantagineum . Planta 229, 659–666. [DOI] [PubMed] [Google Scholar]
- Wingenter K, Schulz A, Wormit A, Wic S, Trentmann O, Hoermiller II, Heyer AG, Marten I, Hedrich R, Neuhaus HE. 2010. Increased activity of the vacuolar monosaccharide transporter TMT1 alters cellular sugar partitioning, sugar signaling, and seed yield in Arabidopsis. Plant Physiology 154, 665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Bartels D. 2016. Physiological factors determine the accumulation ofd-glycero-d-ido-octulose (d-g-d-i-oct) in the desiccation tolerant resurrection plant Craterostigma plantagineum . Functional Plant Biology (in press). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.