Highlight
Rice exposed to a toxic cadmium concentration recovers from toxicity symptoms after supplementation of the medium with silicon, and transcript levels of marker genes are readjusted to that in unstressed conditions.
Key words: Ascorbate, Cd toxicity, glutathione, oxidative stress, photosynthesis, rice, SAP, silicon, transcript regulation.
Abstract
Silicon (Si) modulates tolerance to abiotic stresses, but little is known about the reversibility of stress effects by supplementing previously stressed plants with Si. This is surprising since recovery experiments might allow mechanisms of Si-mediated amelioration to be addressed. Rice was exposed to 10 µM CdCl2 for 4 d in hydroponics, followed by 0.6mM Si(OH)4 supplementation for 4 d. Si reversed the effects of Cd, as reflected in plant growth, photosynthesis, elemental composition, and some biochemical parameters. Cd-dependent deregulation of nutrient homeostasis was partially reversed by Si supply. Photosynthetic recovery within 48h following Si supply, coupled with strong stimulation of the ascorbate–glutathione system, indicates efficient activation of defense. The response was further verified by transcript analyses with emphasis on genes encoding members of the stress-associated protein (SAP) family. The transcriptional response to Cd was mostly reversed following Si supply. Reprogramming of the Cd response was obvious for Phytochelatin synthase 1, SAP1 , SAP14, and the transcription factor genes AP2/Erf020, Hsf31, and NAC6 whose transcript levels were strongly activated in roots of Cd-stressed rice, but down-regulated in the presence of Si. These findings, together with changes in biochemical parameters, highlight the significance of Si in growth recovery of Cd-stressed rice and indicate a decisive role for readjusting cell redox homeostasis.
Introduction
Cadmium (Cd) is almost ubiquitously present as an environmental pollutant in the rice-growing regions of the world and threatens the quality of harvested rice grains for human food (Zhu et al., 2008; Williams et al., 2009; Zhuang et al., 2009). Excessive use of phosphate fertilizers and sewage sludge in irrigated rice areas are the major sources of Cd contamination. Cd accumulation in rice grains can cause severe human health problems especially in areas where rice is a dietary staple (Kikuchi et al., 2007; Takahashi et al., 2011). Recent risk assessments suggest that there is no safety margin between current dietary Cd exposure and potential development of adverse health effects (Clemens et al., 2013).
Plants exposed to Cd encounter toxicity effects by interference with proteins and lipids and production of reactive oxygen species (ROS). Disturbances in the functional components of thylakoid membranes, integral for photosynthetic activity, are also considered as potential sites for metal-induced toxicity (Shah et al., 2001; Lin, 2005; Maksymiec, 2007).
Silicon (Si) is the second most abundant element in the earth’s crust. Si nutrition alleviates various kinds of biotic and abiotic stresses (Marschner, 1995; Exley, 1998; Epstein, 1999; Ma, 2004; Balakhnina et al., 2012; Farooq and Dietz, 2015). High silica contents in plant tissues, particularly in graminaceous plants such as rice, maize, or barley, activate physical and biochemical defense mechanisms for increased stress tolerance (Ma et al., 2006; Currie and Perry, 2007; Mitani et al., 2009a, b ). Silica depositions underneath leaf cuticles form the subcuticular double layer which contributes to biotic stress resistance and reduces water loss by transpiration, thereby improving water use efficiency particularly under abiotic stresses (Lux et al., 2002; Hattori et al., 2005). Furthermore, decreased lipid peroxidation, reduced membrane permeabilization, and higher activities of stress defense enzymes could be linked to Si nutrition under drought, cold, and salt stress (Liang et al., 2007; Farooq et al., 2015). Savvas et al. (2008) reported increased CO2 assimilation rates and a substantial decrease in the uptake and translocation of sodium (Na+) and chloride (Cl−) ions into leaves of salt-stressed zucchini in the presence of Si in the growth medium. Similarly, Si deposition in the cell wall of roots correlated with immobilization of toxic metals such as aluminum (Al) in barley (Hammond et al., 1995), manganese (Mn) in cucumber (Shi et al., 2005), and Cd in maize (Vaculik et al., 2009, 2012). Hence Si affects entry and detoxification of metal ions in the plant body. Improved antioxidative capacity and increased concentrations of ascorbate and glutathione are also suggested to explain Si-mediated metal stress tolerance (Shi et al., 2005; Ma and Yamaji, 2006). These nutritional benefits support the view that Si functions as a beneficial element for plants. Its inclusion in the list of elements essential for higher plants is debatable. Most studies elucidate the importance of Si in a specific physiological and biochemical context, and for this Si is added either prior to or simultaneously with the stressor. Thus, questions as to the reversibility of stress effects by treating plants afterwards with Si but also in relation to the molecular mechanisms involved remain unanswered.
To understand the beneficial Si syndrome in its entirety we need to target and identify genes involved in signaling or regulatory pathways. Recently, a new family of genes with 18 members termed SAPs (stress-associated proteins) was identified in rice. An important role for SAPs in abiotic stress acclimation is indicated by expression profiling and transgenic approaches. SAP family members are characterized by the presence of an A20/AN1 zinc-finger domain. Proteins with such a domain are present in all eukaryotes and are also well characterized in animals (Mukhopadhyay et al., 2004). Members of the SAP gene family present in rice and other plant species show specific inducibility to one or the other abiotic stresses (Vij and Tyagi, 2008; Solanke et al., 2009; Giri et al., 2011). Little is known about their modulation under heavy metal stress, in particular Cd toxicity. In Arabidopsis thaliana, expression of AtSAP10 containing multiple cysteine and histidine residues in the AN1 and A20 domains could be linked to metal binding and confers tolerance to nickel (Ni), zinc (Zn), and Mn toxicity (Dixit and Dhankher, 2011). Increasing evidence suggests that SAPs play decisive roles in stress acclimation; for example, OsSAP1 overexpression improves drought tolerance of transgenic rice (Dansana et al., 2014).
To address the question of Si-induced reversibility of Cd stress effects and the suitability of SAPs as readout for stress intensity, a hydroponic study was conducted to characterize the expression profiles of 18 SAP gene family members in rice exposed to Cd stress and their respective modulation by Si application. We hypothesized that post-stress application of Si recovers growth impairment caused by Cd toxicity through altering stress-related proteins. The results will provide kinetic insight into the Si effect in plant stress tolerance and address early response mechanisms.
Materials and methods
Plant material and growth conditions
Seeds of rice (Oryza sativa L.) cv. IR64 were obtained from the International Rice Research Institute (IRRI, Los Baños, Phillipines). After surface sterilization with 5% NaOCl solution, and thorough rinsing and soaking in distilled water in darkness for 48h, the seeds were germinated on vermiculite with 0.5× Hoagland solution: 3mM KNO3, 0.5mM (NH4)H2PO4, 1mM MgSO4, 2mM Ca(NO3)2, 35 µM Fe-EDTA, and microelements (0.1 µM Na2MoO4, 0.32 µM CuSO4, 0.77 µM ZnSO4, 5 µM MnCl2, and 20 µM H3BO3) (Golldack et al., 2002). After 10 d, eight uniform seedlings were selected and transferred to 5 liter plastic pots containing 0.5× Hoagland solution. Seedlings were grown for another 28 d in a growth chamber with 14h light (300 µmol m−2 s−1, 25 oC) and 10h dark (21 oC) with 50% relative humidity. Hydroponic solution was renewed every 5 d for the first 20 d, then every 3 d for the remainder of the experiment, and the pH was adjusted to 6.2 by using either 1M HCl or 0.5M KOH on a daily basis. At the age of 38 d, plants were stressed with 10 µM CdCl2 added to the nutrient solution for 8 d, while the control plants were maintained in Hoagland medium lacking Cd. Silicon treatments (0 or 0.6mM Si) were introduced 4 d after Cd stress by using sodium silicate (Na2O3Si) solution. An equivalent amount of NaCl was added to the Si-free plants to compensate for the Na content of the Na2O3Si-treated plants. The steady-state quantum yield of photosystem II (ΦPSII) was measured in atmospheric CO2 at 0, 12, 24, 36, 48, and 96h after Si supplementation (Mini-PAM Fluorometer, Walz, Germany) under light conditions as indicated above. Both young and mature leaves were selected randomly from each treatment and measured several times. The photosynthetic yield was calculated according to the manufacturer’s instructions. For biochemical parameters, both leaves and roots were harvested 4 d after Cd stress [i.e. prior to Si application (42 d)] and also at the end of the experiment (46-day-old plants; 4 d after Si supply), immediately frozen in liquid nitrogen, and stored at –80 oC until further analyses. For assessing plant growth, roots were initially drained out between paper towels and plants were separated into leaves, shoots, and roots. Thereafter, plant tissues were dried at 65 °C to constant weight for dry biomass yield.
Hydrogen peroxide (H2O2) quantification
Plant material for H2O2 quantification was immediately frozen in liquid nitrogen and then stored at –80 oC. H2O was quantified as described by Pérez and Rubio (2006). Stored leaves (0.1g) were pulverized with a pestle and mortar in liquid nitrogen, and then H2O2 was extracted with 0.5ml of 5% trichloroacetic acid (TCA). The homogenate was centrifuged at 13 000 g for 10min. After dilution with 0.1M sodium carbonate buffer, 20 µl aliquots were incubated with 50U of catalase (bovine liver, Sigma, USA) or with the same volume of water for 10min at 30 oC as control. H2O2 was determined by chemiluminescence (CL) with luminol. The sample (2 µl) was added to 1ml of reagent solution [stock luminol and stock Co(II) solution diluted in 0.1M sodium carbonate buffer, pH 10.2]. The emitted photons were counted over 7s with a luminometer (Mini Lumat LB 9506, Berthold, D-Bad Wildbad). The difference between catalase-treated and untreated samples (∆CL) was considered as H2O2-specific CL. A standard curve was generated using appropriate dilutions of 30% H2O2 (Carl Roth, Germany).
Ascorbate
Ascorbate and dehydroascorbate (DHA) were determined as described by Horling et al. (2003). Leaves were pulverized in liquid N2 and extracted with 1ml of 1M HClO4. After centrifugation at 13 000rpm (5min at 4 °C), 400 μl of supernatant was transferred to 200 μl of 1M HEPES/KOH buffer (pH 7.0). The pH of the solution was adjusted to pH 5.0–6.0 with 5M K2CO3. After centrifugation, the supernatant was used for measuring the contents of reduced and total ascorbate spectrophotometrically.
Ascorbate was measured after adding 150 μl of supernatant to 850 μl of 0.1M sodium phosphate buffer (pH 5.6) by monitoring the decrease in A 265 in the presence of 5U of ascorbate oxidase (Sigma, Deisenhofen, Germany). For measuring total ascorbate, DHA was reduced with 50mM DTT in four volumes of 0.1M sodium phosphate (pH 7.0) during 30min of incubation on ice and ascorbate was analyzed as described above. DHA was calculated as the difference of ascorbate contents determined in the presence and absence of DTT according to identically treated ascorbate and DHA standards.
Glutathione and non-protein thiols
Glutathione was quantified with an enzyme-cycling assay based on oxidation of GSH by DTNB (2,2'-dinitro-5'5-dithiodibenzoic acid) and reduction of GSSG by NADPH in the presence of glutathione reductase (GR) (Griffith, 1980) with a few modifications. A 200mg aliquot of frozen plant material was extracted in 1ml of 0.1M HCl and 0.1mM EDTA. For total GSH, 200 µl of neutralized supernatant was incubated with 6mM DTNB for 5min followed by 15min incubation with 5 µl of 2-vinylpyridine. After centrifugation, the reaction was started by adding GR, and changes in DTNB absorbance were monitored at 412nm for 8min. For GSSG, the neutralized supernatant was incubated with 2-vinylpyridine for 15min followed by 5min DTNB incubation and subsequently GR and NADPH. The difference between total glutathione and GSSG contents is presented as the GSH content.
Non-protein thiols (NPTs) in leaf and root samples were determined as described by Sharma et al. (2004). A 0.1g aliquot of the plant material was extracted with 1ml of 1M HCl and 1mM EDTA. The extract was added to 0.8ml of assay buffer (0.12M Na-phosphate, pH 7.8) and 100 µl of 6mM DTNB. The absorbance was recorded at 412nm and compared with a calibration curve with GSH.
Elemental analyses
Leaf sheaths, roots, and shoots (including leaf blades) were separated, and apoplastic Cd from roots was desorbed with 5mM PbNO3 at 4 °C for 30min. Samples were dried at 65 °C, homogenized, and microwave digested (START 1500; MLS GmbH, Leutkirch, Germany) in 2ml of 30% (w/v) H2O2 and 4ml of 65% HNO3 with the following temperature protocol: 12min 30s ramping to 80 °C, 5min 30s at 80 °C, 4min ramping to 180 °C, 12min at 180 °C. Plastic labware was used to prevent Si contamination. Element compositions (including Si) were determined with an inductively coupled plasma atomic emission spectrometer (ICP-AES, iCAP 6500, Thermo Scientific, Waltham, MA, USA).
Targeted transcript analyses
Total RNA was extracted with the Trizol reagent (Life Technologies, Karlsruhe, Germany) and reverse transcribed (Wormuth et al., 2006). Semi-quantitative RT-PCR was performed to optimize equal loading of cDNA using actin primers as reference (Finkemeier et al., 2005). For each transcript, root cDNA from control plants was used for annealing temperature and cycle number optimization. Supplementary Table S1 at JXB online contains the list of gene-specific primers designed by Primer3Plus software (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi/). Quantitative real-time PCR (qRT-PCR) was then performed using the iCycler™ Thermal Cycler (Bio-Rad, USA) with the iQTM SYBR Green Supermix (Bio-Rad, USA) in a final volume of 20 µl using actin as an internal control. The standard thermal program consisted of the following steps: 95 °C for 1min; 45× (95 °C for 30s, 58 °C for 40s, 72 °C for 45s), 72 °C for 10min followed by a melting curve program (55–95 °C in increasing steps of 0.5 °C). Samples from each treatment were run in duplicate, and values in Supplementary Fig. S2 represent the average from two independent experiments. Efficiencies of each reaction were calculated using LinRegPCR software (Ruijter et al., 2009). The relative expression level was calculated as values relative to corresponding control samples at the indicated time points, after normalization to actin and α-tubulin using the threshold cycles (average background subtracted) according to the equation of Pfaffl (2001).
Statistical analysis
The data were subjected to statistical analysis by using the t-test, and treatments were compared by calculating means with SD at P≤5%.
Results
Plant growth and photosynthetic response
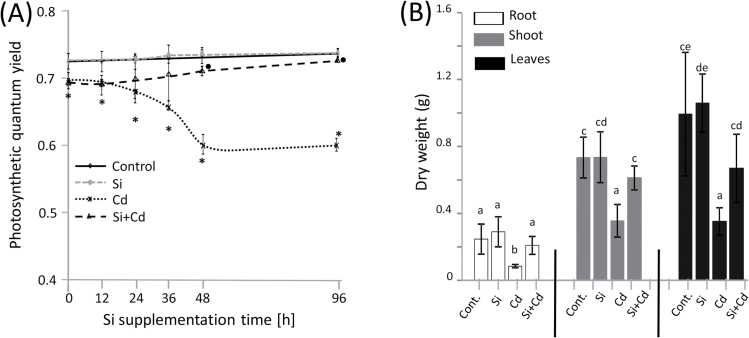
Thirty-eight-day old IR64 rice plants were treated with 10 µM CdCl2 for 4 d prior to addition of 0.6mM Si. In order to test the hypothesis that Si supplementation enables recovery from established Cd stress, ΦPSII was determined at regular time intervals between 0h and 96h. Both young and old leaves from each treatment were measured several times. Differences were not detected between control plants grown with and without Si supply (Fig. 1A). The 4 d period of Cd exposure had significantly decreased the ΦPSII, indicating progressing Cd toxicity. The decline continued until a minimum value was recorded 48h after onset of measurement (6 d after Cd addition) and remained at this level during the next 2 d. The minimum yield under Cd stress was ~21% lower than in control plants. Contrastingly, photosynthetic efficiency of Cd-treated plants receiving Si nutrition increased with time and reached the ΦPSII of untreated controls after 96h and hence was ~18% higher than in stressed plants without an extra Si supply.
Fig. 1.
Plant growth and photosynthetic characteristics of rice genotype IR64 grown in hydroponic nutrient solution with or without Cd and supplementary Si. (A) Kinetic changes of photosynthetic quantum yield (ΦPSII) of rice treated with Cd for 4 d prior to inititation of recovery by addition of Si (t=0h). Also depicted are untreated control, and single treatments with Cd and Si. Significant differences between Cd-treated and control rice are denoted by an asterisk, while a filled circle indicates a significant difference between Cd/Si-treated and Cd-treated plants. (B) Dry weight of rice roots, shoots, and leaves recorded at harvest (46-day-old plants). Data groups of significant difference are labeled with different letters (Student’s t-test, P<0.05). The data are means ±SD of n=108 (A) and n=3 (B) from three independent experiments.
Further, the plants were investigated for growth performance (Fig. 1B). Growing rice for 8 d on nutrient solution supplemented with 10 µM Cd severely inhibited growth of all tissues. Roots most sensitively responded to Cd. Thus, root biomass of Cd-treated plants was 35% of that of the control, while Cd-treated shoots and leaves reached 40–50% that of untreated controls. Si supplementation substantially ameliorated the growth inhibition by Cd. Dry biomass of Cd-treated plants was 1.7- to 2.4-fold higher in the presence of Si compared with Cd-stressed plants lacking Si (Fig. 1B), while there was no significant difference between control plants supplied or not with Si.
Hydrogen peroxide and ascorbate contents
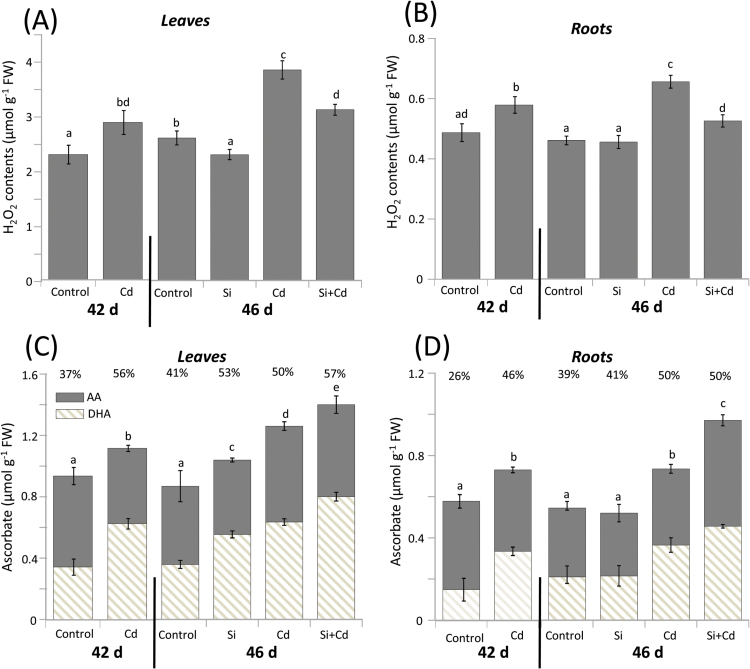
As measure of oxidative stress, H2O2 was quantified in both leaves and roots. Compared with control plants, the 4 d Cd stress before Si application caused a significant increase in H2O2 contents in leaves and roots by 22% and 26%, respectively (Fig. 2A, B). H2O2 accumulation in leaves and roots increased further during the next 4 d of Cd exposure, while Si supplementation to Cd-stressed plants reversed the Cd-induced H2O2 accumulation to a large extent. However, H2O2 contents in non-stressed Si-supplemented plants were marginally lower in leaves and unaltered in root tissues, when compared with control plants (Fig. 2A, B).
Fig. 2.
Hydrogen peroxide and ascorbate levels dependent on Cd and Si treatment of rice. Leaf (A, C) and root (B, D) contents of H2O2 (A, B) and ascorbate (C, D) were determined in 42-day-old plants stressed with Cd for 4 d prior to Si application and subsequently treated with Si for another 4 d (labeled as 46-day-old plants), with appropriate controls. In (C and D), contents of ascorbate in its reduced (AA; solid) and oxidized (DHA; hatched) form are given. The percentage values above the columns represent the oxidation state of the ascorbate pool. The data are means ±SD from three independent experiments and n=18 (A and B) and n=6 (C and D) determinations. Data groups of significant difference were calculated by t-test and are labeled with different letters (P<0.05).
Ascorbate levels were analyzed as a major low molecular mass antioxidant linked to redox homeostasis. The ascorbate and DHA levels significantly increased in leaves and roots during the first 4 d of Cd exposure (Fig. 2C; 42 d) and in the subsequent 4 d until 46 d. Leaf ascorbate levels of control plants were slightly increased after Si application. However, the most pronounced increase in ascorbate levels was recorded when Si was applied to Cd-stressed rice. The oxidation state of the ascorbate pool ranged between 37% and 57%, and, importantly, the upper range of oxidation was noticed under combined application of Cd and Si, suggesting involvement of Si in modulating ascorbate homeostasis. Root total ascorbate response was significant when plants either experienced Cd toxicity alone or were supplemented with Si under Cd stress (Fig. 2D). As compared with controls, ascorbate levels reached 127% and 137% after 4 d and 8 d of Cd stress, respectively (Fig. 2D). In the presence of Si and Cd, the size of the ascorbate pool reached up to 176% as compared with untreated control plants. Again, the oxidation level was higher in the Cd-treated plant material.
Glutathione and non-protein thiol contents
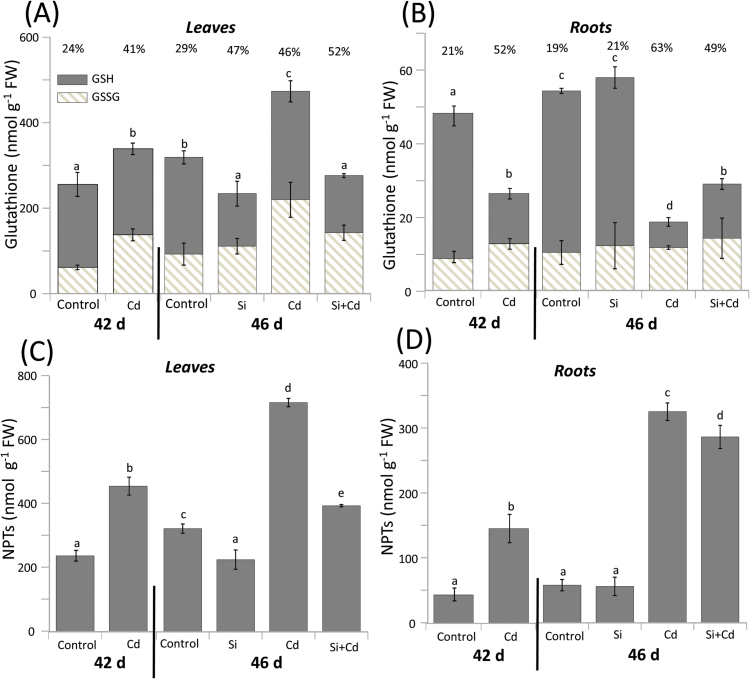
The response pattern of glutathione differed greatly from that of ascorbate. Leaf glutathione levels were 30% increased after 4 d of Cd exposure (42 d) and 50% after 8 d (Fig. 3A). Interestingly, in Cd-stressed roots, total glutathione was 43% below that of control roots after 4 d and 70% after 8 d (Fig. 3B). Si application alone decreased the total glutathione contents by 27% in leaves, while it was ineffective in roots (Fig. 3A, B). Si reversed the contrasting Cd effects in leaves and roots (i.e. decrease in leaves and increase in roots; Fig. 3A, B). The proportion of oxidized glutathione ranged between 19% and 63%. The highly oxidized state was observed in the Cd-stressed roots.
Fig. 3.
Glutathione and non-protein thiol levels dependent on Cd and Si treatment of rice. Leaf (A, C) and root (B, D) contents of glutathione (A, B) and non-protein thiols (NPTs) (C, D) were measured in 42-day-old plants stressed with Cd for 4 d prior to Si application and subsequently treated with Si for another 4 d (labeled as 46-day-old plants), with appropriate controls. In (A and B), contents of glutathione in its reduced (GSH; solid) and oxidized (GSSG; hatched) form are presented, as is the oxidation state as a percentage above the columns. The data are means ±SD from three independent experiments and n=6 (A and B) and n=15 (C and D) determinations. Data groups of significant difference were calculated by t-test and are labeled with different letters (P<0.05).
Phytochelatin synthesis represents the major Cd detoxification mechanism in plants (Clemens and Persoh, 2009; Rea, 2012). The possible impact of Si on Cd chelation by phytochelatins was addressed by quantifying NPTs. Subtracting glutathione from NPT gives a reasonable estimate of phytochelatins. Roots responded to Cd exposure more strongly than leaves, with an ~3.3- and 5.6-fold increment in NPTs after 4 d and 8 d stress, respectively (Fig. 3D). In Cd-treated plants, leaf NPT contents were ~1.9-fold higher after 4 d, and 2.2-fold after 8 d exposure than in control leaves (Fig. 3C). The response strength in roots of Cd-treated plants slightly decreased upon Si supplementation, but decreased by 45% in leaves which compensated for ~80% of the Cd-induced effect (Fig. 3C, D). Si supplementation to control plants slightly decreased leaf NPTs, while no change occurred in roots.
Elemental composition
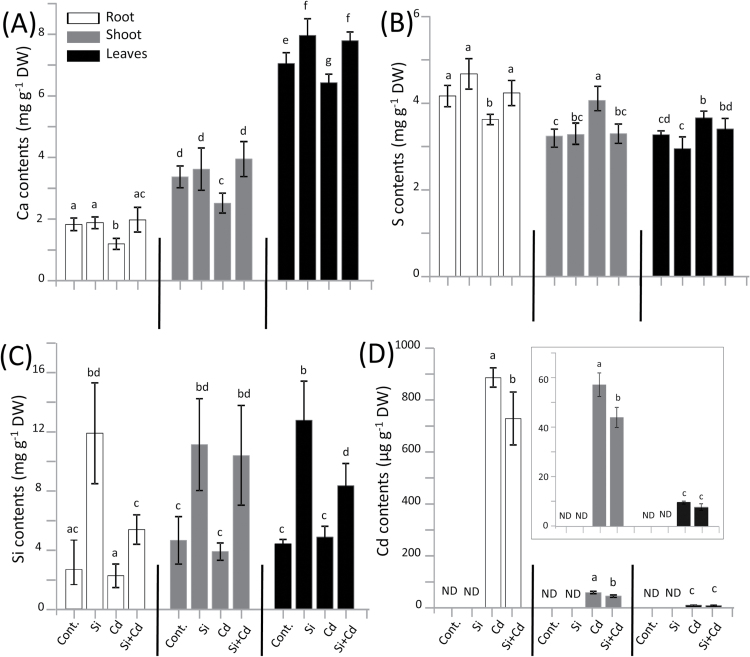
Heavy metal accumulation and compartmentation represent decisive parameters in heavy metal tolerance. The elemental composition in roots, shoots, and leaves of Cd-stressed rice varied significantly between plants grown in the presence or absence of a Si supply (Fig. 4; Supplementary Fig. S1). As expected, tissue Si contents were substantially elevated following their addition to the hydroponic culture (Fig. 4C). Interestingly, a major proportion of Si applied under stress was translocated to above-ground rice tissues. Cd was undetectable in control plants. Cd accumulated less in roots in the presence of Si. Also 24% less Cd was translocated to shoots (Fig. 4D). In contrast, mean Cd contents of leaf blades were insignificantly lower following Si supply. Furthermore, the presence of Cd in the growth medium significantly lowered the accumulation of essential macro- and micronutrients such as calcium (Ca), potassium (K), magnesium (Mg), and Zn (Fig. 4A; Supplementary Fig. S1). This effect was significantly dampened by the inclusion of Si in the nutrient formulation of stressed plants. For instance, root Ca accumulation was improved to control levels, enabling higher root/shoot translocation and similarly 21% more Ca accumulated in leaves of Cd-stressed rice receiving Si supply (Fig. 4A). Similar but smaller effects were seen for K, Mg, and Zn contents (Supplementary Fig. S1). In contrast, Cd caused an increase of leaf and shoot S contents by 12% and 25%, respectively (Fig 4B). The addition of Si lowered the shoot S contents in the presence of Cd. The root S response was opposite, with 13% less S accumulation under Cd stress. This effect was partly reversed upon Si addition as indicated by the 17% increase (Fig. 4B).
Fig. 4.
Calcium (Ca), sulfur (S), silicon (Si), and cadmium (Cd) contents in roots, shoots, and leaves of rice genotype IR64 grown in hydroponic nutrient solution with or without Cd and supplementary Si. Data are means ±SD (n=4) from four independent experiments. ND, not detected. Data groups of significant difference were calculated by t-test and are labeled with different letters (P<0.05).
Transcript analyses
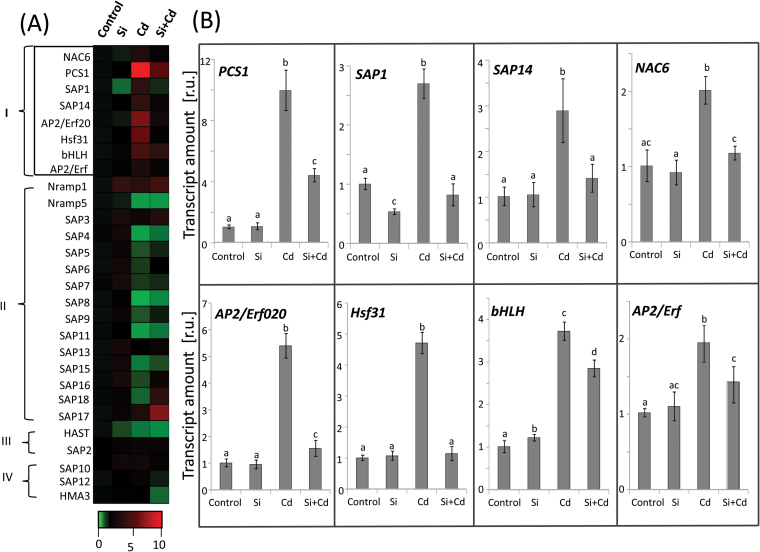
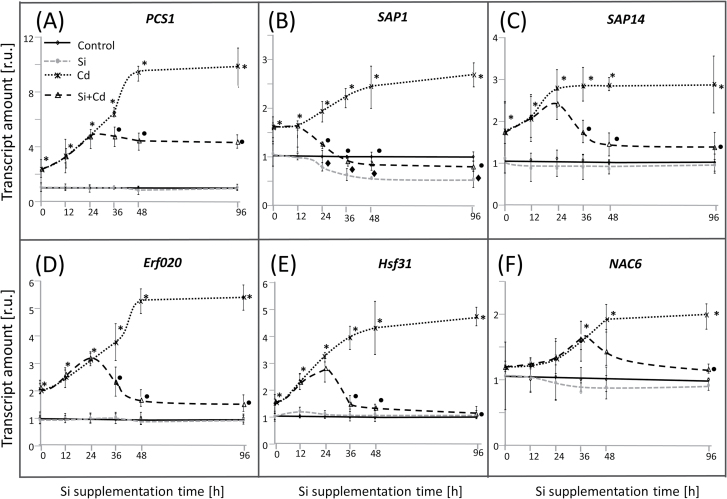
The redox state-related data described so far indicated a high efficiency of Si in reversing Cd-induced disturbances of biochemical homeostasis. Transcript analysis was performed to identify markers of Si-mediated alleviation of Cd stress. We focused on genes encoding the 18 members of stress-inducible SAPs (Vij and Tyagi, 2006), Cd-related metal transporters, and detoxification mechanisms, namely phytochelatin synthase (PCS1, LOC_Os05g34290.1), Nramp1 (LOC_Os07g15460.1), Nramp5 (LOC_Os07g15370.1), HMA3 (LOC_Os07g12900.1), and HAST (LOC_Os03g09970.1) (Ogawa et al., 2009; Miyadate et al., 2010; Takahashi, 2011; Sasaki et al., 2012; Zhou et al., 2014). Additionally, Cd-responsive transcription factor (TF) genes, namely NAC6 (LOC_Os03g60800.1)], AP2/Erf020 (LOC_Os05g34730.1), Hsf31 (LOC_Os02g32590.1), bHLH (LOC_Os01g06640.1), and AP2/Erf (LOC_Os07g22730.1), were analyzed in order to identify signaling components potentially involved in Cd toxicity and Si/Cd antagonism. These TFs were selected from two previous experiments with Cd-stressed rice roots where transcriptomes had been profiled using gene chips and RNA-seq (http://genevestigator.com/gv/; Hruz et al., 2008; Ogawa et al., 2009; He et al., 2015). The transcripts were quantified 4 d after Si supply. Obtained response patterns to Cd and/or Si were categorized into four groups: (I) antagonistic effects of Si on Cd toxicity (Fig. 5B); (II) independent effects of Si and Cd; (III) additive effects (positive or negative) of Si and Cd; and (IV) complex patterns (Fig. 5A; Supplementary Fig. S2). Six promising targets from group I (Fig. 5B) showing the Si-induced reversal of the Cd effect were selected for a time course analysis until 96h after Si addition in order to describe the recovery phase initiated by Si supplementation. PCS1 was selected as a marker for Cd stress and revealed an ~10-fold up-regulation at t=48h which was unchanged until the end at day 4. In the presence of Si, the changes first followed the kinetics observed in the Cd-treated sample until 24h, but then started to decline without reaching the value of the non-stressed control (Fig. 6A). A similar reversal pattern was detected for the expression of both TF genes. AP2/Erf020 was induced 5.4-fold, while Hsf31 revealed a 4.7-fold up-regulation at t=96h as compared with the control (Fig. 6D, E). In contrast, in the presence of Si under Cd stress, the Hsf31 transcript level dropped already after 36h, near to the level of the control (Fig. 6E). The AP2/Erf020 accumulation in Si/Cd plants first followed the kinetics observed in the Cd plants until 24h, then slightly declined at t=36h, and subsequently reached the control level (Fig. 6D). SAP1 and SAP14 transcript levels also increased in parallel in Cd and Cd/Si tissue, however only for 12h. Si supplementation reversed the Cd effect almost completely (Fig. 6B, C). The maximal delay was detected for NAC6 mRNA (Fig 6F). Following the parallel increase in mRNA in the Cd and Cd/Si plants until 36h, NAC6 levels continued to rise in the Cd-stressed sample, while they decreased to levels close to the control in the Cd/Si sample (Fig. 6F). For all transcripts, extra Si supply to control plants caused no change in mRNA levels, except for SAP1 which was down-regulated by almost 2-fold as compared with the respective control (Fig. 6B).
Fig. 5.
Response pattern of transcripts in rice roots grown with or without Cd and supplementary Si. (A) Expression profiles of transcripts 4 d after Si supply represented as a heat map. Based on their differential response to Cd and Si, transcripts were classified into four groups (see text for details, and Supplementary Fig. S2 with Group II–Group IV category members). In (B), the transcriptional response of targets following the recovery pattern (Group I) are shown. Data are means ±SD (n=6) from three independent experiments. Data groups of significant difference were calculated by t-test and are labeled with different letters (P<0.05). (This figure is available in colour at JXB online.)
Fig. 6.
Time course analysis of transcript regulation for selected targets in roots of control and Si-supplied rice plants with or without Cd exposure. mRNA levels were quantified by qPCR from three independent experiments with duplicate determinations. Data are means ±SD, asterisks denote significant differences between Cd-treated and control rice, filled circles indicate a significant difference between Cd/Si-treated and Cd-treated plants, and filled diamonds mark significant differences between Si-treated and control plants. Student’s t-test, P<0.05.
Discussion
Avoidance and repair as a combined strategy for Si-induced reversibility of Cd toxicity
Si modulates tolerance levels to biotic and abiotic stresses and also ameliorates heavy metal toxicity (Nwugo and Huerta, 2008; Kaya et al., 2009). Si-dependent recovery from pre-established stress conditions has not been analyzed in detail. This experimental design promises access to a kinetic and mechanistic understanding. The first two questions addressed in this work concerned the appropriate experimental design and the correctness of the hypothesis that Si administered post-stress ameliorates the negative effects of pre-established Cd toxicity.
Cd added to the hydroponic medium significantly reduced ΦPSII after 4 d of treatment and therefore clearly established Cd toxicity effects which were further aggravated until 8 d (Fig. 1A). Photosynthesis is also a known sensitive target of Cd toxicity in rice (Moya et al., 1993). In contrast, Si supply prevented further development of damage and improved ΦPSII within 48h, reaching maximal values close to those of non-stressed plants. Apparently Si application after onset of Cd stress allowed for recovery of the rice plants. Previously, Si was shown to reduce the inhibitory effects of Cd on the photosynthetic machinery of cucumber by increasing the contents of photosynthetic pigments and reducing the damage to thylakoid membranes (Feng et al., 2010). In a recent screening for dominant changes in the rice leaf proteome, Nwugo and Huerta (2011) identified 60 proteins that were differentially regulated in response to Cd treatment in plants lacking or pre-treated with Si. With a 30% share, polypeptides with functions in photosynthetic processes represented the largest functional category among the identified proteins. This study goes beyond our present knowledge since the photosynthetic performance of Cd-stressed rice recovered by post-stress application of Si.
H2O2 as a typical Cd toxicity symptom accumulated in leaves and roots after 4 d. The accumulation was significantly reduced in Si-treated plants (Fig. 2A, B). Ascorbate and glutathione play decisive roles in cell redox homeostasis, antioxidant defense, and plant development in normal metabolism and under stress (Mittler et al., 2004; Dowdle et al., 2007; Schlaeppi et al., 2008; Mhamdi et al., 2010). The results indicate a lowered oxidative load of Cd-stressed rice in the presence of Si (Figs 2C, D, 3A, B). The major difference between ascorbate and glutathione response was that ascorbate levels further increased in both leaves and roots under Si/Cd treatment beyond the already elevated levels found in Cd-treated rice. The enlarged ascorbate pool size indicates stimulated defense capacity by providing additional substrate for the water–water cycle, and for quenching of the tocopherol radical and ROS, particularly H2O2 (Song et al., 2009; Gest et al., 2013). This type of positive Si effect on the water–water cycle was described in the context of salinity stress and mycorrhization (Garg and Bhandari, 2016). NTPs under non-stress conditions tentatively match the glutathione pool (Metwally et al., 2005) as also seen in this study (Fig. 3). As previously reported for barley (Finkemeier et al., 2003), Cd stress reduced root contents of glutathione which was converted to phytochelatins (Fig. 3B). This is apparent from the greater difference between NPTs and glutathione which reached >244 nmol g–1 FW in leaves, and 306 nmol g–1 FW in roots after 8 d exposure to Cd stress. The value dropped to 118 nmol g–1 FW in leaves and 257 nmol g–1 FW in roots from Cd/Si plants. The Si-induced drop in phytochelatin-bound thiols indicates decreasing concentrations of free Cd in the cytosol. Phytochelatins mediate sequestration into the vacuole. For this study, it is important that Si supply to Cd-stressed plants significantly increased the glutathione proportion within the NPT pool of roots. It is concluded that lower Cd levels in the cytoplasm reduced the PCS1 activity and the drainage of GSH into phytochelatin synthesis (Fig. 3B). The recovery experiment advances the study of Song et al. (2009) who reported similar increases in the glutathione pool of Cd-stressed Brassica plants when Si and Cd were supplied simultaneously. Apparently Cd-induced phytochelatin synthesis drained more glutathione than could be synthesized in roots, leading to a >5-fold increase in NPTs. The opposite pattern in leaves, namely up-regulation of glutathione in Cd-treated tissue and down-regulation upon Cd/Si treatment, indicates that stimulation of glutathione synthesis was able to overcompensate for the drainage into phytochelatin synthesis. Song et al. (2009) reported synergistic effects of simultaneous Cd and Si supply on glutathione contents of Brassica chinensis. The discrepancies might be caused by the entirely different experimental design and distinct nutrient solution, as well as species differences.
The elemental analysis revealed reduced Cd accumulation in roots in the presence of Si (Fig. 4). Cd is first absorbed apoplastically and then transported across the plasma membrane with the help of secondary cation transporters such as IRT1 (Clemens et al., 2002; Clemens, 2006). Except for Cd-hyperaccumulating species, Cd accumulates more in roots than in shoots and leaves, which is in line with our observations (Kirham, 2006). Si uptake is an active process particularly in silica-accumulating species such as rice (Ma et al., 2006). Si strongly binds to cell wall components and contributes to cross-linking of cell wall structures. Si-induced structural alterations and blockage of the apoplasmic transport route are suggested to reduce Cd uptake by roots and translocation to shoots (Liang et al., 2001, 2007; Lukacova et al., 2013). However, Cd concentrations in leaves did not differ significantly between plants with or without Si. Apparently substantial Cd amounts had already accumulated during the first 4 d of Cd exposure before the start of the Si treatment. Thus, Si-dependent blockage of uptake and transfer of Cd from roots to shoots cannot explain the recovery of photosynthesis in the recovery experiment. About 80% of the Si absorbed under Cd stress was transferred to shoots and leaves. As a consequence, Si levels were highly similar in different tissues of Si-treated control plants (Fig. 4). This suggests that the positive effects of Si administration on above-ground tissue are caused by local effects of Si accumulating in the shoot and not by effects on long-distance Cd transport. Enhanced defense as described above for ascorbate and compatible compartmentation, for example in the vacuole, are probably involved in the process.
Recently, Pavlovic et al. (2013) reported that Si alleviates Fe deficiency in cucumber at two mechanistic levels, namely indirectly by increasing root apoplastic iron (Fe) uptake and directly by modulation of strategy-I-responsive genes involved in synthesis of Fe-mobilizing compounds. Controlled uptake, long-distance transport, and utilization of the functionally important micro- and macroelements plays crucial roles in maintaining optimal metabolism, plant growth, and high productivity. Element analyses suggest that differential Ca translocation in the presence or absence of Cd and Si contributes to the protection of the photosynthetic apparatus. However, it should be noted that Ca contents do not equate to Ca concentrations. Ca is known to alleviate Cd toxicity (Suzuki, 2005). In addition, the repressed delivery of other elements such as Mg, K, Zn, and S was also partially improved by Si supply under Cd stress. Improved utilization of micronutrients (Fe and Zn) and macronutrients (Ca, Mg, and K) upon Si supply to chromium (Cr)- and Cd-stressed plants was previously reported by Tripathi et al. (2012a, b). However, in their study, Cd and Si were supplied simultaneously, thus recovery was not a point of interest. Here it is shown that already established negative effects of Cd are reversed upon Si supplementation. This is important since it indicates the action of mechanisms beyond uptake in roots and long-distance transport. Apparently, Si also facilitated detoxification of Cd that had already been incorporated during the first 4 d. The results also exclude a major function for Si in developmental processes underlying the Cd acclimation response, since the effects were observed in adult leaves.
Si-mediated regulation of Cd-induced gene expression
The biochemical data provided compelling evidence for the beneficial effects of Si on the physiology of Cd-stressed rice. The response was further characterized by transcript analyses in order to identify genes involved in signaling or regulatory pathways. Transcript regulation provides fast, sensitive, and specific readouts of altered signaling pathways (e.g. as fast as 20s after light shift experiments; Moore et al., 2014). The quantitative response of transcripts belonging to the SAP family and other Cd stress markers including TFs/signaling molecules could be grouped into four response patterns, among which the antagonistic effect seen for PCS1 SAP1, SAP14, NAC6, AP2/Erf020, Hsf31, bHLH, and AP2/Erf most tightly followed and thus confirmed the hypothesis of reversibility of stress effects by post-stress addition of Si. The kinetics of biochemical and molecular changes as monitored for PCS1, SAP1, SAP14, NAC6, AP2/Erf020, and Hsf31 transcripts gave additional insight into the Si/Cd interference. Levels of each of the six transcripts increased in response to Cd, but the distinct bifurcation kinetics of the response curves of Cd-treated plants with and without Si hints at different sensitivity thresholds, signaling pathways, and involved mechanisms (Fig. 6). Levels of none of the transcripts differed within the first 12h after Si addition.
SAP1, SAP14, and Hsf31 in Cd and Cd/Si treatments diverged already within 24h; in fact, SAP1 significantly more than SAP14 and Hsf31 (Fig. 6B, C). SAP1 and 14 belong to the recently identified SAP gene family with 18 members in rice. Overexpression of SAPs in rice and other plant species confers tolerance to various abiotic stresses. For example, Mukhopadhyay et al. (2004) reported the up-regulation of OsSAP1 upon mechanical injury, submergence, abscisic acid treatment, drought, salt, and cold stress. Similarly, OsSAP9 was found to be involved in stress response against cold, heat, and oxidative stress (Huang et al., 2008). Their involvement in response to multiple stresses has been reported also for, for example, maize, Arabidopsis, tomato, and banana (Jin et al., 2007; Vij and Tyagi, 2008; Solanke et al., 2009; Giri et al., 2011). The stimulatory action of Cd on SAP1 and SAP14 probably indicates a role in Cd-related stress response, and the reversal by Si in parallel with improved growth tentatively underscores this hypothesis (Fig. 6B, C). SAP1 and SAP14 have been reported to be up-regulated under cold, salt, and dehydration stress (Vij and Tyagi, 2006). Their early response indicates that the involved signaling pathway is linked to a rather fast and possibly linearly Cd-dependent stress-sensing mechanism such as deviation from redox and ROS homeostasis. Si would then support re-establishment of redox homeostasis.
Phytochelatins synthesized from GSH have long been known for their role in heavy metal binding and detoxification (Finkemeier et al., 2003; Yadav, 2010). Therefore, up-regulation of PCS1 is a rather specific response to Cd toxicity. The Cd-induced up-regulation of PCS1 was reversed after 36h of Si supplementation (Fig. 6A). In Arabidopsis, Khandekar and Leisner (2011) found that the relative expression of PCS1 in leaves is enhanced by Si under Cu stress as compared with stressed plants lacking a Si supply, while expression of the metallothionein gene MT1a is down-regulated in the simultaneous presence of Si and Cu. Thus there exist species-specific differences. It may be speculated that Si activated a more efficient compartmentalization and detoxification of Cd. The element analyses support this scenario (Fig. 4). On the other hand, Si supplementation stopped the further accumulation of PCS1 transcript but failed to lower it to the control level. This may either indicate a long half-life of the PCS1 transcript or sustained Cd stress in the cytosol.
In order to address potential signaling elements and regulators, we selected five Cd-responsive TFs, namely AP2/Erf020, AP2/Erf, bHLH, NAC6, and Hsf31, from rice transcriptome analyses, and subsequently focused on three of them which revealed an almost complete reversal upon Si application to Cd-stressed rice. He et al. (2015) reported up-regulation of AP2/Erf020-TF under Cd stress. Likewise a genome-wide transcriptome analysis in Arabidopsis revealed strong (>30-fold) up-regulation upon Cd stress of AP2/Erf019-TF (At1g22810), a close homolog of AP2-Erf020 in rice (Weber et al., 2006). Further, Hsf31 was chosen because its Arabidopsis homolog HsfA3 (At5g03720) was implicated in controlling the expressional up-regulation of ascorbate peroxidase 2. This mechanism was suggested to promote tolerance to oxidative stress in Arabidopsis (Hwang et al., 2012). Hsf31 significantly responded to the Si supplementation within 36h and returned to the level of the Cd-free control plants within 2 d. Hsf31 is a member of the antioxidant regulatory network. The rapid and complete reversal of Hsf31 transcript to the control level with Si probably indicates efficient readjustment of redox homeostasis.
The most delayed bifurcation was seen for OsNAC6 (Fig. 6F). OsNAC6 is drought induced and targets downstream stress-responsive genes such as apetala 2-TFs, Zn-finger proteins, and MYB TFs. Its overexpression enhances drought and salt stress tolerance in transgenic rice (Rachmat et al., 2014). The late response of NAC6 may tentatively be explained by ionic or osmotic imbalances under Cd stress (Hassan et al., 2009) which may be reversed by Si only slowly. Si is known to affect drought tolerance, for example in sorghum by increased silicification or altering internal barriers (Hassan et al., 2009).
Si effects on regulation of metal transporters in higher plants have only been addressed in a few studies such as that of Li et al. (2008) who found that Si application under Cu stress reduced the activity of HMA5 in roots of Arabidopsis. In our study, the response of metal transporters to Cd and Cd/Si treatments was barely distinguishable, except for OsHMA3 which is a member of heavy metal ATPases (Fig. 5A; Supplementary Fig. S2). Here, expression of HMA3 in roots of Cd-stressed rice was not different from that in controls which tentatively fits a report by Kim et al. (2014) who found a significant up-regulation of HMA3 in roots of Cd-stressed rice only during the early stress period at day 1, but the difference largely disappeared during extended exposure. Here, we observed that the expression of HMA3 was significantly suppressed due to the presence of Si under Cd stress, which indicates that Si might be involved in Cd sequestration; therefore, less HMA3 would be needed. This finding is in line with our results from element analyses where we found reduced Cd levels in roots associated with Si application.
In conclusion, the synergistic and antagonistic responses of H2O2, metabolites, and element levels, as well as PCS, SAP genes, AP2/Erf020, Hsf31, NAC6, and transporter transcripts to Cd and Cd/Si supplementation, and the distinct kinetics of the antagonistic response indicate that Si interferes with Cd stress via several mechanisms. Decreased Cd uptake and translocation, as well as improved compartmentation, readjustment of redox homeostasis, and strengthened antioxidant capacity as indicated by elevated ascorbate levels contribute to the Si effect. Among the members of the SAP family, SAP1 and SAP14 are promising candidates for involvement in the Cd toxicity response, while SAP3, 4, 5, 6, 7, 9, 13, 15, and 16 responded to Si alone and thus might be of interest for further consideration in general stress responses and Si-dependent stress amelioration. Recently, overexpression of OsSAP1 in rice was shown to improve water stress tolerance (Dansana et al., 2014). The overexpressing rice lines revealed alterations in their transcriptome, with many transcripts assigned to the gene ontology group of stress-responsive genes. These findings support the conclusion that the up-regulation of SAP1 in Cd-stressed rice and the reversal of this effect by Si indicate efficient stress relief by Si supplementation. The Si-induced recovery of Cd-stressed rice will allow for identifying early signaling responses by, for example, transcriptome profiling in the future.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. K, Mg, and Zn contents in rice genotype IR64 grown in hydroponic nutrient solution with or without Cd and supplementary Si.
Figure S2. Transcript abundance in roots exposed to Cd toxicity and changes in response 96h after Si supply.
Table S1. Sequence of primers used for real-time PCR analysis.
Acknowledgements
This work was supported by the Higher Education Commission (HEC) of Pakistan in collaboration with the German Academic Exchange Service (DAAD). MAF designed the study, conducted all experiments and measurements except of element analysis, discussed the data, and wrote the paper, AD and SC performed element analysis, discussed data, and commented on the paper, KJD designed the study, discussed the data, and wrote the paper.
Glossary
Abbreviations:
- bHLH
basic helix–loop–helix
- Cd
cadmium
- DHA
dehydroascorbate
- DTNB
2,2'-dinitro-5'5-dithiodibenzoic acid
- ERF
ethylene response factor
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- Hsf
heat shock transcription factor
- NPT
non-protein thiol
- ΦPSII
quantum yield of photosystem II
- ROS
reactive oxygen species
- SAP
stress-associated protein
- Si
silicon
- TF
transcription factor.
References
- Balakhnina TI, Matichenkov VV, Wlodarczyk T, Borkowska A, Nosalewicz M, Fomina IR. 2012. Effects of silicon on growth processes and adaptive potential of barley plants under optimal soil watering and flooding. Plant Growth Regulation 67, 35–43. [Google Scholar]
- Clemens S. 2006. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochemie 88, 1707–1719. [DOI] [PubMed] [Google Scholar]
- Clemens S, Aarts MGM, Thomine S, Verbruggen N. 2013. Plant science: the key to preventing slow cadmium poisoning. Trends in Plant Sciences 18, 92–99. [DOI] [PubMed] [Google Scholar]
- Clemens S, Palmgren MG, Kraemer U. 2002. A long way ahead: understanding and engineering plant metal accumulation. Trends in Plant Sciences 7, 1360–1385. [DOI] [PubMed] [Google Scholar]
- Clemens S, Persoh D. 2009. Multi-tasking phytochelatin synthases. Plant Science 177, 266–271. [Google Scholar]
- Currie HA, Perry CC. 2007. Silica in plants: biological, biochemical and chemical studies. Annals of Botany 100, 1383–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansana PK, Kothari KS, Vij S, Tyagi AK. 2014. OsiSAP1 overexpression improves water-deficit stress tolerance in transgenic rice by affecting expression of endogenous stress-related genes. Plant Cell Reports 33, 1425–1440. [DOI] [PubMed] [Google Scholar]
- Dixit AR, Dhankher OP. 2011. A novel stress-associated protein ‘AtSAP10’ from Arabidopsis thaliana confers tolerance to nickel, manganese, zinc, and high temperature stress. PLoS One 6, e20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N. 2007. Two genes in Arabidopsis thaliana encoding GDP-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. The Plant Journal 52, 673–689. [DOI] [PubMed] [Google Scholar]
- Epstein E. 1999. Silicon. Annual Review of Plant Physiology and Plant Molecular Biology 50, 641–664. [DOI] [PubMed] [Google Scholar]
- Exley C. 1998. Silicon in life: a bioinorganic solution to bioorganic essentiality. Journal of Inorganic Biochemistry 69, 139–144. [Google Scholar]
- Farooq MA, Dietz KJ. 2015. Silicon as versatile player in plant and human biology: overlooked and poorly understood. Frontiers in Plant Science 6, 994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq MA, Saqib ZA, Akhtar J. 2015. Silicon-mediated oxidative stress tolerance and genetic variability in rice (Oryza sativa L.) grown under combined stress of salinity and boron toxicity. Turkish Journal of Agriculture and Forestry 39, 718–729. [Google Scholar]
- Feng JP, Shi QH, Wang XF, Wei M, Yang FJ, Xu HN. 2010. Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Scientia Horticulturae 123, 521–530. [Google Scholar]
- Finkemeier I, Kluge C, Metwally A, Georgi M, Grothjohann N, Dietz KJ. 2003. Alterations in Cd-induced gene expression under nitrogen deficiency in Hordeum vulgare . Plant, Cell and Environment 26, 821–833. [DOI] [PubMed] [Google Scholar]
- Finkemeier I, Goodman M, Lamkemeyer P, Kandlbinder A, Sweetlove LJ, Dietz KJ. 2005. The mitochondrial type II peroxiredoxin F is essential for redox homeostasis and root growth of Arabidopsis thaliana under stress. Journal of Biological Chemistry 280, 12168–12180. [DOI] [PubMed] [Google Scholar]
- Garg N, Bhandari P. 2016. Interactive effects of silicon and arbuscular mycorrhiza in modulating ascorbate–glutathione cycle and antioxidant scavenging capacity in differentially salt-tolerant Cicer arietinum L. genotypes subjected to long-term salinity. Protoplasma (in press). [DOI] [PubMed] [Google Scholar]
- Gest N, Gautier H, Stevens R. 2013. Ascorbate as seen through plant evolution: the rise of a successful molecule? Journal of Experimental Botany 64, 33–53. [DOI] [PubMed] [Google Scholar]
- Giri J, Vij S, Dansana PK, Tyagi AK. 2011. Rice A20/AN1 zinc-finger containing stress-associated proteins (SAP1/11) and a receptor-like cytoplasmic kinase (OsRLCK253) interact via A20 zinc-finger and confer abiotic stress tolerance in transgenic Arabidopsis plants. New Phytologist 191, 721–732. [DOI] [PubMed] [Google Scholar]
- Golldack D, Su H, Quigley F, Kamasani UR, Munoz-Garay C, Balderas E, Popova OV, Bennett J, Bohnert HJ, Pantoja O. 2002. Characterization of a HKT-type transporter in rice as a general alkali cation transporter. The Plant Journal 31, 529–542. [DOI] [PubMed] [Google Scholar]
- Griffith OW. 1980. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Analytical Biochemistry 106, 207–212. [DOI] [PubMed] [Google Scholar]
- Hammond KE, Evans DE, Hodson MJ. 1995. Aluminium/silicon interactions in barley (Hordeum vulgare L.) seedlings. Plant and Soil 173, 89–95. [Google Scholar]
- Hassan SA, Fariduddin Q, Ali B, Hayat S, Ahmad A. 2009. Cadmium: toxicity and tolerance in plants. Journal of Environmental Biology 30, 165–174. [PubMed] [Google Scholar]
- Hattori T, Inanaga S, Araki H, An P, Morita S, Luxová M, Lux A. 2005. Application of silicon enhanced drought tolerance in Sorghum bicolor . Physiologia Plantarum 123, 459–466. [Google Scholar]
- He F, Liu Q, Zheng L, Cui Y, Shen Z, Zheng L. 2015. RNA-Seq analysis of rice roots reveals the involvement of post-transcriptional regulation in response to cadmium stress. Frontiers in Plant Science 6, 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horling F, Lamkemeyer P, König J, Finkemeier I, Baier M, Kandlbinder A, Dietz KJ. 2003. Divergent light-, ascorbate-, and oxidative stress-dependent regulation of expression of the peroxiredoxin gene family in Arabidopsis thaliana . Plant Physiology 131, 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oerltle L, Widmayer P, Gruissem W, Zimmermann P. 2008. Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes. Advances in Bioinformatics 2008, 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wang MM, Jiang Y, Bao YM. 2008. Expression analysis of rice A20/AN1-type zinc finger genes and characterization of ZFP177 that contributes to temperature stress tolerance. Gene 420, 135–144. [DOI] [PubMed] [Google Scholar]
- Hwang JE, Lim CJ, Chen H, Je JH, Song C, Lim CO. 2012. Overexpression of Arabidopsis dehydration-responsive element-binding protein 2C confers tolerance to oxidative stress. Molecules and Cells 33, 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Wang M, Fu J, Xuan N. 2007. Phylogenetic and expression analysis of ZnF-AN1 genes in plants. Genomics 90, 265–275. [DOI] [PubMed] [Google Scholar]
- Kaya C, Tuna AL, Sonmez O, Ince F, Higgs D. 2009. Mitigation effects of silicon on maize plants grown at high zinc. Journal of Plant Nutrition 32, 1788–1798. [Google Scholar]
- Khandekar S, Leisner S. 2011. Soluble silicon modulates expression of Arabidopsis thaliana genes involved in copper stress. Journal of Plant Physiology 168, 699–705. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Okazaki M, Toyota K, Motobayashi T, Kato M. 2007. The input–output balance of cadmium in a paddy field of Tokyo. Chemosphere 67, 920–927. [DOI] [PubMed] [Google Scholar]
- Kim Y, Khan AL, Kim D, Lee S, Kim K, Waqas M, Jung HY, Shin JH, Kim JG, Lee I. 2014. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biology 14, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham MB. 2006. Cadmium in plants on polluted soil: effects of soil factors, hyperaccumulation, and amendments. Geoderma 137, 19–32. [Google Scholar]
- Li J, Leisner SM, Frantz J. 2008. Alleviation of copper toxicity in Arabidopsis thaliana by silicon addition to hydroponic solutions. Journal of the American Society for Horticultural Science 133, 670–677. [Google Scholar]
- Liang Y, Sun W, Zhu Y, Christie P. 2007. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environmental Pollution 147, 422–428. [DOI] [PubMed] [Google Scholar]
- Liang Y, Yang C, Shi H. 2001. Effects of silicon on growth and mineral composition of barley grown under toxic levels of aluminum. Journal of Plant Nutrition 24, 229–243. [Google Scholar]
- Lin CC, Chen LM, Liu ZH. 2005. Rapid effect of copper on lignin biosynthesis in soybean roots. Plant Science 168, 855–861. [Google Scholar]
- Lukacova Z, Svubova R, Kohanova J, Lux A. 2013. Silicon mitigates the cadmium toxicity in maize in relation to cadmium translocation, cell distribution, antioxidant enzymes stimulation and enhanced endodermal apoplasmic barrier development. Plant Growth Regulation 70, 89–103. [Google Scholar]
- Lux A, Luxova M, Hattori T, Inanaga S, Sugimoto Y. 2002. Silicification in sorghum (Sorghum bicolor) cultivars with different drought tolerance. Physiologia Plantarum 115, 87–92. [DOI] [PubMed] [Google Scholar]
- Ma JF. 2004. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Science and Plant Nutrition 50, 11–18. [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. 2006. A silicon transporter in rice. Nature 440, 688–691. [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N. 2006. Silicon uptake and accumulation in higher plants. Trends in Plant Sciences 11, 392–397. [DOI] [PubMed] [Google Scholar]
- Maksymiec W. 2007. Signalling responses in plants to heavy metal stress. Acta Physiologia Plantarum 29, 177–187. [Google Scholar]
- Marschner H. 1995. Mineral nutrition of higher plants, 2nd edn San Diego: Academic Press. [Google Scholar]
- Metwally A, Safronova VI, Belimov AA, Dietz KJ. 2005. Genotypic variation of the response to cadmium toxicity in Pisum sativum L. Journal of Experimental Botany 56, 167–178. [DOI] [PubMed] [Google Scholar]
- Mhamdi A, Hager J, Chaouch S, et al. 2010. Arabidopsis GLUTATHIONE REDUCTASE 1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiology 153, 1144–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani N, Chiba Y, Yamaji N, Ma JF. 2009. a Identification and characterization of maize and barley Lsi2-like silicon efflux transporters reveals a distinct silicon uptake system from that in rice. The Plant Cell 21, 2133–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani N, Yamaji N, Ma JF. 2009. b Identification of maize silicon influx transporters. Plant and Cell Physiology 50, 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van-Breusegem F. 2004. Reactive oxygen gene network of plants. Trends in Plant Sciences 9, 490–498. [DOI] [PubMed] [Google Scholar]
- Miyadate H, Adachi S, Hiraizumi A, et al. 2010. OsHMA3, a P1B-type of ATPase affects root to shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytologist 189, 190–199. [DOI] [PubMed] [Google Scholar]
- Moore M, Vogel M, Dietz KJ. 2014. The acclimation response to high light is initiated within seconds as indicated by upregulation of AP2/ERF transcription factor network in Arabidopsis thaliana. Plant Signaling and Behavior 9, 976479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya JL, Ros R, Picazo I. 1993. Influence of cadmium and nickel on growth, net photosynthesis and carbohydrate distribution in rice plants. Photosynthesis Research 36, 75–80. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A, Vij S, Tyagi AK. 2004. Overexpression of a zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgenic tobacco. Proceedings of National Academy of Sciences, USA 101, 6309–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwugo CC, Huerta AJ. 2008. Effects of silicon nutrition on cadmium uptake, growth and photosynthesis of rice plants exposed to low-level cadmium. Plant and Soil 311, 73–86. [Google Scholar]
- Nwugo CC, Huerta AJ. 2011. The effect of silicon on the leaf proteome of rice (Oryza sativa L.) plants under cadmium-stress. Journal of Proteome Research 10, 518–528. [DOI] [PubMed] [Google Scholar]
- Ogawa I, Nakanishi H, Mori S, Nishizawa NK. 2009. Time course analysis of gene regulation under cadmium stress in rice. Plant and Soil 325, 97–108. [Google Scholar]
- Pavlovic J, Samardzic J, Maksimovic V, et al. 2013. Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytologist 198, 1096–1107. [DOI] [PubMed] [Google Scholar]
- Perez FJ, Rubio S. 2006. An improved chemiluminescence method for hydrogen peroxide determination in plant tissues. Plant Growth Regulation 48, 89–95. [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmat A, Nugroho S, Sukma D, Aswidinnoor H, Sudarsono 2014. Overexpression of OsNAC6 transcription factor from Indonesia rice cultivar enhances drought and salt tolerance. Emirate Journal of Food and Agriculture 26, 519–527. [Google Scholar]
- Rea PA. 2012. Phytochelatin synthase: of a protease a peptide polymerase made. Physiologia Plantarum 145, 154–164. [DOI] [PubMed] [Google Scholar]
- Ruijter JM, Ramakers C, Hoogars W, Bakker O, van-den Hoff MJB, Karlen Y, Moorman AFM. 2009. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Research 37, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Yamaji N, Yokosho K, Ma JF. 2012. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. The Plant Cell 24, 2155–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvas E, Giotis D, Chatzieustratiou E, Bakea M, Patakioutas G. 2008. Silicon supply in soilless cultivations of zucchini alleviates stress induced by salinity and powdery mildew infections. Environmental and Experimental Botany 65, 11–17. [Google Scholar]
- Schlaeppi K, Bodenhausen N, Buchala A, Mauch F, Reymond P. 2008. The glutathione-deficient mutant pad2-1 accumulates lower amounts of glucosinolates and is more susceptible to the insect herbivore Spodoptera littoralis . The Plant Journal 55, 774–786. [DOI] [PubMed] [Google Scholar]
- Shah K, Kumar RG, Verma S, Dubey RS. 2001. Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Science 161, 1135–1144. [Google Scholar]
- Sharma SS, Kaul S, Metwally A, Goyal KC, Finkemeier I, Dietz KJ. 2004. Cadmium toxicity to barley (Hordeum vulgare) as affected by varying Fe nutritional status. Plant Science 166, 1287–1295. [Google Scholar]
- Shi Q, Bao Z, Zhu Z, He Y, Qian Q, Yu J. 2005. Silicon-mediated alleviation of Mn toxicity in Cucumis sativus in relation to activities of superoxide dismutase and ascorbate peroxidase. Phytochemistry 66, 1551–1559. [DOI] [PubMed] [Google Scholar]
- Solanke AU, Sharma MK, Tyagi AK, Sharma AK. 2009. Characterization and phylogenetic analysis of environmental stress-responsive SAP gene family encoding A20/AN1 zinc finger proteins in tomato. Molecular Genetics and Genomics 282, 153–164. [DOI] [PubMed] [Google Scholar]
- Song A, Li Z, Zhang J, Xue G, Fan F, Liang Y. 2009. Silicon-enhanced resistance to cadmium toxicity in Brassica chinensis L. is attributed to Si-suppressed Cd uptake and transport and Si-enhanced antioxidant defense capacity. Journal of Hazardous Materials 172, 74–83. [DOI] [PubMed] [Google Scholar]
- Suzuki N. 2005. Alleviation by calcium of cadmium-induced root growth inhibition in Arabidopsis seedlings. Plant Biotechnology 22, 19–25. [Google Scholar]
- Takahashi R, Ishimaru Y, Senoura T, Shimo H, Ishikawa S, Arao T, Nakanishi H, Nishizawa NK. 2011. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. Journal of Experimental Botany 62, 4843–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi DK, Singh VP, Kumar D, Chauhan DK. 2012. a Rice seedlings under cadmium stress: effect of silicon on growth, cadmium uptake, oxidative stress, antioxidant capacity and root and leaf structures. Chemical Ecology 28, 281–291. [Google Scholar]
- Tripathi DK, Singh VP, Kumar D, Chauhan DK. 2012. b Impact of exogenous silicon addition on chromium uptake, growth, mineral elements, oxidative stress, antioxidant capacity, and leaf and root structures in rice seedlings exposed to hexavalent chromium. Acta Physiologia Plantarum 34, 279–289. [Google Scholar]
- Vaculik M, Landberg T, Greger M, Luxova′ M, Stoláriková M, Lux A. 2012. Silicon modifies root anatomy, and uptake and subcellular distribution of cadmium in young maize plants. Annals of Botany 110, 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaculik M, Lux A, Luxová M, Tanimoto E, Lichtscheidl I. 2009. Silicon mitigates cadmium inhibitory effects in young maize plants. Environmental and Experimental Botany 67, 52–58. [Google Scholar]
- Vij S, Tyagi AK. 2006. Genome-wide analysis of the stress associated protein (SAP) gene family containing A20/AN1 zinc finger(s) in rice and their phylogenetic relationship with Arabidopsis. Molecular Genetics and Genomics 276, 565–575. [DOI] [PubMed] [Google Scholar]
- Vij S, Tyagi AK. 2008. A20/AN1 zinc-finger domain-containing proteins in plants and animals represent common elements in stress response. Functional and Integrative Genomics 8, 301–307. [DOI] [PubMed] [Google Scholar]
- Weber M, Trampczynska A, Clemens S. 2006. Comparative transcriptome analysis of toxic metal responses in Arabidopsis thaliana and the Cd(2+)-hypertolerant facultative metallophyte Arabidopsis halleri . Plant, Cell and Environment 29, 950–63. [DOI] [PubMed] [Google Scholar]
- Williams PN, Lei M, Sun GX, Huang Q, Lu Y, Deacon C, Meharg AA, Zhu YG. 2009. Occurrence and partitioning of cadmium, arsenic and lead in mine impacted paddy rice: Hunan, China. Science of the Total Environment 43, 637–642. [DOI] [PubMed] [Google Scholar]
- Wormuth D, Baier M, Kandlbinder A, Scheibe R, Hartung W, Dietz KJ. 2006. Regulation of gene expression by photosynthetic signals triggered through modified CO2 availability. BMC Plant Biology 6, 15–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav SK. 2010. Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African Journal of Botany 76, 167–179. [Google Scholar]
- Zhu YG, Sun GX, Lei M, et al. 2008. High percentage inorganic arsenic content of mining impacted and non-impacted Chinese rice. Environmental Science and Technology 42, 5008–5013. [DOI] [PubMed] [Google Scholar]
- Zhuang P, Zou B, Li NY, Li ZA. 2009. Heavy metal contamination in soils and food crops around Dabaoshan Mine in Guangdong, China: implication for human health. Environmental Geochemistry and Health 31, 707–715. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang Z, Huang Z, Lu C, Han Z, Zhang J, Jiang H, Ge C, Yang J. 2014. Expression of sulfur uptake assimilation-related genes in response to cadmium, bensulfuron-methyl and their co-contamination in rice roots. Journal of Environmental Sciences 26, 650–661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.