The canon of knowledge on the catalytic properties of the photosynthetic enzyme Rubisco has shackled efforts to understand its diversity. Now the chains are off. While investigating the variability in Rubisco function among diatoms, Young et al. (see pages 3445–3456 in this issue) have demonstrated that it is our thinking, not Rubisco catalysis, that has been constrained.
The photosynthetic CO2-fixing enzyme, ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), is renowned for its slow catalytic rate and difficulty in distinguishing between the substrate of photosynthesis, CO2, and one of the products, O2. The oxygenase activity was discovered 45 years ago (Bowes et al., 1971), nearly 20 years after the discovery of the carboxylase activity (Quayle et al., 1954; Weissbach et al., 1954). The photorespiratory losses associated with Rubisco oxygenation are substantial (Sharkey, 1988), and the persistence of oxygenation throughout 3.5 billion years of evolution – particularly the last 400 million years when the O2 and CO2 pressures in the atmosphere have favored substantial oxygenation and photorespiration – is still a mystery (Hagemann et al. 2016).
It is conceivable that evolutionary constraints related to the origins of Rubisco from enzymes with roles in sulfur metabolism (Ashida et al., 2003) may have imposed limitations on the catalytic mechanism for CO2 fixation, though none have come to light. Oxygenation has also been proposed as an energy dissipative mechanism that would be beneficial under some stressful conditions (Osmond, 1981), but this does not explain why it exists in all Rubiscos, including those from anaerobic organisms (Andrews and Lorimer, 1987).
The inefficiency of Rubisco is due not only to the strong competitive interaction of O2 and CO2 at the active site, but also to a slow catalytic turnover rate per active site (k cat) and a low affinity for CO2 (high K C). Substrate-saturated k cat values (maximum CO2 fixation per catalytic site) occur in the range 1–12s–1 and the k cat/K C ratios (which reflect the ability of Rubisco to function when CO2 is limiting) occur in the range 2–40×104 M–1 s–1 (Badger et al., 1998). This feeble catalytic potency at limiting CO2 is several orders of magnitude slower than the diffusion limit to catalysis that many enzymes approach (108–109 M–1 s–1) and it means that plants need copious amounts of Rubisco (as much as 50% of soluble leaf protein, consuming up to 25% of leaf nitrogen) to support adequate rates of photosynthesis in our current atmosphere (Andrews and Lorimer, 1987). The requirement for such large quantities of Rubisco gives it the dubious honor of being the most abundant protein on Earth (Ellis, 1979). Even with so much Rubisco present, it is still often the rate-limiting enzyme in photosynthesis.
Target for manipulation
Scientists around the world who were interested in increasing plant productivity saw this suite of catalytic problems and recognized that it made Rubisco a good target for manipulation (Andrews and Lorimer, 1987; Parry et al., 2013). However, even after the first 25 crystal structures were available for Rubiscos from eight divergent species (Form I Red and Green types, and Form II) along with a plethora of sequences, there was next to nothing that could explain observed variation in catalysis (Andersson and Taylor, 2003). Furthermore, efforts to genetically modify the enzyme were hampered by its complex structure and the difficulty of assembling functional enzymes in workhorse organisms like Escherichia coli. This has increased the need to study kinetic properties of Rubisco from a more diverse range of species (Parry et al., 2013), while simultaneously convincing many that improvements are highly unlikely. The few surveys of a handful of catalytic properties showed some patterns of improvement from cyanobacteria to plants within the green lineage, along with trade-offs between increased k cat and decreased selectivity of Rubisco in species that express a carbon-concentrating mechanism (CCM). This favoring of speed over affinity and selectivity works because CCMs elevate the level of CO2 near the active sites of Rubisco (Badger et al., 1998). These observations led to the hypothesis that Rubisco was already optimized in photosynthetic organisms (Tcherkez et al., 2006) and that the kinetics were constrained in a narrow, one-dimensional landscape (Savir et al. 2010).
The findings of Young et al. (2016) now challenge the generality of this hypothesis. The work describes the survey of Rubisco catalytic properties as a diagnostic tool for the enzymes’ subcellular environment in diatoms (Box 1), providing a proxy for examining CCM efficiency. They found a remarkable range of diversity in Rubisco kinetics within this Form I Red-type lineage, suggesting a wide range of cellular environments and CCM function. This in itself is an interesting and important finding since it has been challenging to study CCM function in diatoms. However, the astonishing discovery is that the canonical linear relationship between speed and affinity does not exist, for either CO2 or O2, in Rubisco from these species (Young et al., 2016; Fig. 3A, D), and their data greatly weakens or eliminates the broader relationship when examined together with Rubiscos from other species.
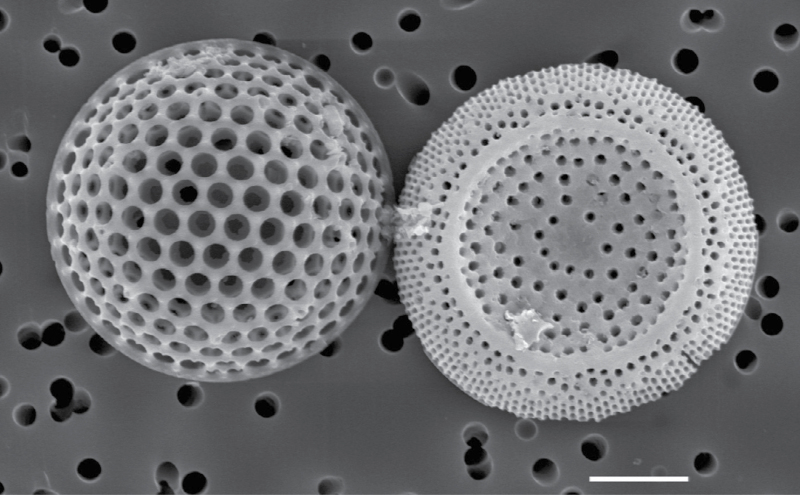
Box 1. Ancient phytoplankton
Centric diatom frustules from ancient marine sediments at site 1090 in the Atlantic sector of the Southern Ocean (samples obtained from the Integrated Ocean Drilling Program at the Bremen Core Repository in Bremen, Germany). These diatoms are from the Eocene–Oligocene boundary (about 35 million years ago). SEM; scale bar = 10 μm. Courtesy of Dr Ana Heureux.
Some might dismiss the significance of their findings since the affinities are low for CO2, but they are also lower for O2 and to a different extent. However, it is not the direction of the variation that is of greatest significance but rather the magnitude. Within the community of ‘Rubiscologists’, the enormous pile of proposals that have died on the reviewer’s sword – because of the idea that the trade-off between speed and affinity is narrow and immutable – is often lamented. Many have argued that the tight relationship was caused more by a sampling bias than a fundamental constraint, and the data of Young et al. (2016) now show they are correct. Their data also show that diversity in subcellular environment will be as or more important than diversity in Rubisco sequence when selecting organisms whose Rubiscos can shed light on the catalytic mechanism.
‘Absence of evidence is not evidence of absence’: the old cliché highlights the largest problem facing those trying to improve plant productivity through the manipulation of Rubisco catalysis. The desire for a simple solution, i.e. the single magic catalytic parameter that everyone can measure, and an irrational fear of exploratory research efforts (oft derided as fishing trips) has severely impeded advancement of our understanding of Rubisco catalysis for over 30 years. This has had the odd effect of stifling the generation of new data despite an ever-increasing interest in the small amount of data available (Box 2). Early surveys of Rubisco catalytic properties from photosynthetic organisms (see references in Badger et al., 1998) received a great deal of interest, but were conducted prior to our current knowledge about the diversity of Rubisco sequences and modern methods for accurately measuring catalysis. By focusing on the evolutionary line leading to land plants, they over-looked the Red Form I enzymes found in the highly diverse non-green algae. This led to the assumption that the most useful Rubiscos would be found in land plants, and this idea has crept into scientific dogma. Even when later studies were published showing that Red Form I Rubisco from non-green algae were different and potentially better, little attention was paid.
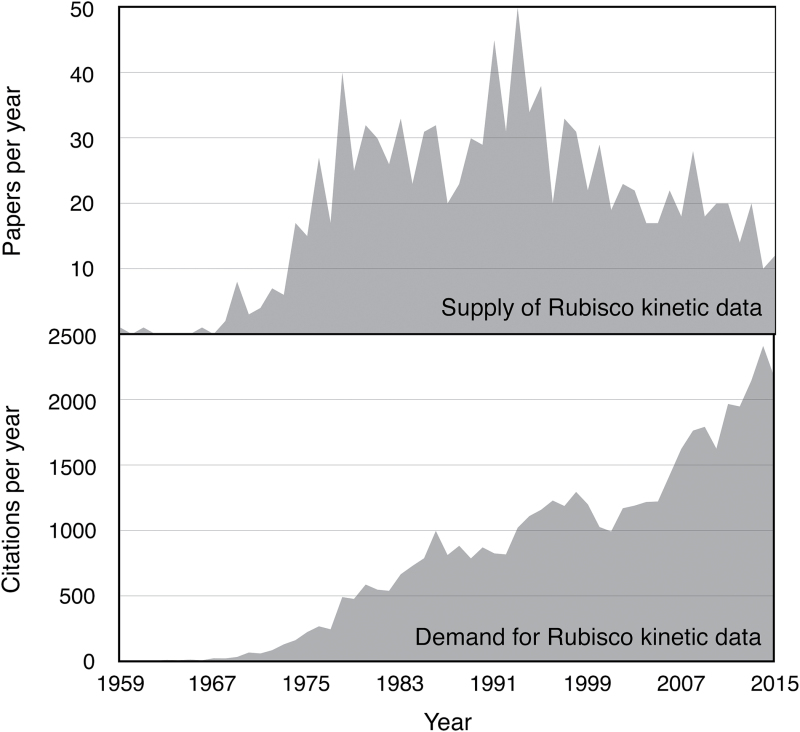
Box 2. Demand for kinetic data is outstripping supply
Interest in Rubisco kinetics is ever increasing yet the number of papers forming the canon (containing the words ribulose, carboxylase and kinetic* in Web of ScienceTM) peaked two to three decades ago. A new peak in data is overdue.
Looking forward
Roughly half a century after discovering the importance of Rubisco in photosynthesis and photorespiration, kinetic properties have only been comprehensively studied for three Form I Rubiscos – tobacco, spinach and the cyanobacterium Synechococcus – and even those could benefit from more work. Young et al. (2016) demonstrate the need for studying Rubisco from a greater diversity of species and the value of measuring Rubisco kinetic parameters for both CO2 and O2 catalysis. However, to make a truly quantum leap in our understanding, a wider range of parameters should be measured. In addition to k cat and K m for CO2 and O2, and specificity for CO2 over O2, it is essential to include assays of the K m for the other substrate, RuBP, along with binding kinetics of other known inhibitors and activators, frequency and forms of catalytic misfires, and ideally fractionation between isotopologues of CO2 and O2. These assays should be conducted using different combinations of large and small subunits and paired with measures of activation by multiple forms of Rubisco activase. Finally, given the paramount role of environment it will be critical to conduct assays across biologically relevant temperatures along with other subcellular properties like changes in molecular crowding. Now the chains are off and the kinetics research can get moving again.
References
- Andersson I, Taylor TC. 2003. Structural framework for catalysis and regulation in ribulose-1,5-bisphosphate carboxylase/oxygenase. Archives of Biochemistry and Biophysics 414, 130–140. [DOI] [PubMed] [Google Scholar]
- Andrews TJ, Lorimer GH. 1987. Rubisco: structure, mechanisms, and prospects for improvement. In: Stumpf PK, Conn EE, eds. The biochemistry of plants, Vol 10 San Diego: Academic Press, 131–218. [Google Scholar]
- Ashida H, Saito Y, Kojima C, Kobayashi K, Ogasawara N, Yokota A. 2003. A functional link between RuBisCO-like protein of Bacillus and photosynthetic RuBisCO. Science 302, 286–290. [DOI] [PubMed] [Google Scholar]
- Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD. 1998. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Canadian Journal of Botany 76, 1052–1071. [Google Scholar]
- Bowes G, Ogren WL, Hageman RH. 1971. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochemical and Biophysical Research Communications 45, 716–722. [DOI] [PubMed] [Google Scholar]
- Ellis JR. 1979. The most abundant protein in the world. Trends in Biochemical Science 4, 241–244. [Google Scholar]
- Hagemann M, Kern R, Maurino VG, Hanson DT, Weber APM, Sage RF, Bauwe H. 2016. Evolution of photorespiration from cyanobacteria to land plants, considering protein phylogenies and acquisition of carbon concentrating mechanisms. Journal of Experimental Botany doi: 10.1093/jxb/erw063. [DOI] [PubMed] [Google Scholar]
- Osmond C. 1981. Photo-respiration and photoinhibition: some implications for the energetics of photosynthesis. Biochimica et Biophysica Acta 639, 77–98. [Google Scholar]
- Parry MAJ, Andralojc PJ, Scales JC, Salvucci ME, Carmo-Silva AE, Alonso H, Whitney SM. 2013. Rubisco activity and regulation as targets for crop improvement. Journal of Experimental Botany 64, 717–730. [DOI] [PubMed] [Google Scholar]
- Quayle JR, Fuller RC, Benson AA, Calvin M. 1954. Enzymatic carboxylation of ribulose diphosphate. Journal of the American Chemical Society 76, 3610–3611. [Google Scholar]
- Savir Y, Noor E, Milo R, Tlusty T. 2010. Cross-species analysis traces adaptation of Rubisco toward optimality in a low-dimensional landscape. Proceedings of the National Academy of Sciences, USA 107, 3475–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD. 1988. Estimating the rate of photorespiration in leaves. Physiologia Plantarum 73, 147–152. [Google Scholar]
- Tcherkez GGB, Farquhar GD, Andrews TJ. 2006. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proceedings of the National Academy of Sciences, USA 103, 7246–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach A, Smyrniotis PZ, Horecker BL. 1954. Pentose phosphate and CO2 fixation with spinach extracts. Journal of the American Chemical Society 76, 3611–3612. [Google Scholar]
- Young JN, Heureux AMC, Sharwood RE, Rickaby REM, Morel FMM, Whitney SM. 2016. Large variation in the Rubisco kinetics of diatoms reveals diversity among their carbon concentrating mechanisms. Journal of Experimental Botany 67, 3445–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]