Supplemental Digital Content is available in the text.
Keywords: anti-tumour necrosis factor-α agent, biologic, clinician survey, Crohn’s disease, questionnaires, review, systematic, ulcerative colitis
Abstract
Objectives
Comparative outcomes of patients with ulcerative colitis (UC) and Crohn’s disease (CD) prescribed a biologic therapy are inconclusive. The aim of this research was to characterize the degree of unmet medical need in patients with UC or CD and to identify the potential role for new therapies.
Methods
A systematic literature review was undertaken of studies reporting outcomes associated with the use of existing biologic therapies in patients with UC or CD, focusing on the nature and rate of treatment failure. To complement the systematic review, contemporaneous data were obtained from a survey of practising gastroenterologists in the UK and France. Data were qualitatively combined in a narrative framework to evaluate the degree of unmet medical need among patients with UC or CD.
Results
Studies identified in the systematic review (n=120) were heterogeneous, particularly with respect to the definitions of treatment failure; estimates of treatment failure were high but uncertain. On the basis of standardized definitions, estimates of treatment failure provided by clinicians (n=102) were high, and they were higher for second-line treatment failure (primary: ≤37%; secondary: ≤41%) compared with first-line treatment failure (primary: ≤26%; secondary: ≤28%). The majority of the systematic review and survey data were reflective of outcomes with infliximab and adalimumab.
Conclusion
High treatment failure rates associated with existing biologics, identified by the review and clinician surveys, indicate a need for other biologic treatment options to improve the management and outcomes for people with UC and CD. Outcomes associated with existing and new biologic treatments should be investigated in head-to-head randomized trials in the context of their likely uses in clinical practice.
Introduction
The aim of treatment of ulcerative colitis (UC) and Crohn’s disease (CD), the two most common forms of inflammatory bowel disease (IBD) 1, is achievement of remission and, once that is achieved, maintaining remission. Conventional therapies, such as aminosalicylates, corticosteroids, general immunosuppressants or antibiotics, are used to treat the symptoms of active disease 2. However, patients unable to receive conventional therapies due to intolerance, lack of response or ineligibility, or those with moderate or severe disease, commonly receive biologic therapy. Available biologic therapies include the anti-tumour necrosis factor-α (anti-TNFα) agents, infliximab (IFX), adalimumab (ADA), certolizumab pegol (CTZ) and golimumab (GOL), and the anti-α4 integrin natalizumab (NAT) 3–7.
Blockade of TNFα signalling has several limitations; biologic agents with this mechanism of action (IFX, ADA, CTZ and GOL) are unable to solely block the immune system from attacking the body’s own tissue (such as intestinal tissue in UC and CD) while leaving the rest of the immune response intact. Consequently, anti-TNFα biologics may reduce the body’s ability to fight other infections, leaving UC and CD patients receiving these therapies susceptible to other diseases and infections 3–6. For instance, anti-TNFα biologics have been associated with the recurrence of tuberculosis in patients with latent tuberculosis infection, atypical mycobacterial infections and invasive fungal infections, such as by histoplasmosis, as well as other opportunistic infections 8,9. On rare occasions, blood disorders have been observed with IFX, ADA and CTZ treatment 3–6, and, although reports of lymphoma in patients taking IFX, ADA and CTZ are rare, it does occur more often than in the general population 10,11.
A biologic with a different mechanism of action may be more suitable for patients who have experienced primary or secondary failure with anti-TNFα biologics. Currently, NAT is specifically available for the population of patients with CD, but not for UC, and is the only non-anti-TNFα biologic approved by the US Food and Drug Administration 7. However, similar to anti-TNFα biologics, NAT does not specifically and solely target gut inflammation, instead hindering the ability of immune cells to be recruited to sites of inflammation throughout the body, which may lower the ability of the immune system to fight infections, increasing the risk for infections in NAT-treated patients 7. Vedolizumab, a humanized IgG1 monoclonal antibody, is also a biologic targeting the alpha-4 integrin, but it has a new mechanism of action that leads to the selective inhibition of immune cell recruitment to the gut 12; although not currently routinely available, vedolizumab is under evaluation for use in clinical practice in the UK 13,14.
The efficacies of available biologic treatments have been evaluated against placebo in relevant clinical trials 15–26; however, the comparative effectiveness of these treatments, in particular, outcomes observed in clinical practice outside of the trial setting, is not conclusive. Empirical studies and meta-analyses on the comparative effectiveness of existing biologic treatments have indicated the potential for patient-relevant differences in outcomes, whereas other studies have concluded that there is no evidence of clinical superiority among anti-TNFα agents 27–31. Consequently, the extent of unmet medical need among patients who have, or will have, an inadequate response to, have lost response to, or have intolerance to, either conventional therapy or existing biologics, is not established.
The objective of this research was to characterize the degree of unmet medical need among patients with UC or CD and to identify the potential role for newer therapeutic options.
Methods
A systematic literature review and narrative summary of studies reporting outcomes associated with the use of existing biologic therapies in patients with UC or CD was undertaken. To complement the systematic review, contemporaneous data were obtained from a survey of practising gastroenterologists in the UK and France. Data obtained from the systematic review and surveys were qualitatively combined in a narrative framework to evaluate the degree of unmet medical need (defined by the rate of treatment failure) and the potential role for new biologic therapies.
The principle outcomes assessed by the review and surveys were the rate of treatment failure and the clinician response to failure during the induction phase (primary failure) or maintenance phase (secondary failure). Where possible, failure rates were summarized by type of biologic, therapy line (first or second), timing of treatment failure from biologic initiation (months) and clinicians’ likely therapeutic response to treatment failure. Secondary outcomes assessed in the review were resource use, adverse events (AEs) and quality of life of patients with UC or CD treated with a biologic agent; these outcomes and further details on treatment patterns are reported in full detail in Supplementary digital content 1, http://links.lww.com/EJGH/A23.
Systematic literature review
The systematic literature review was conducted in accordance with the PRISMA statement 32, and in accordance with the methodology described by the Cochrane Collaboration and the National Institute for Health and Care Excellence 33,34. The following databases were searched to identify relevant studies on biologic agents for UC and CD: Embase, Medline (through PubMed), the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, and the NHS Economic Evaluation Database (NHS EED). A search for human studies in these databases from the inception of individual databases to the date of the search (9 October 2013) was performed using controlled vocabulary descriptors and specific keywords to represent the concept of UC, CD, IBD and therapeutic use of biologic agents. The studies of interest were randomized controlled trials, nonrandomized trials and observational studies. Bibliographic searches of the identified systematic reviews were conducted to identify any potentially relevant study not identified in the original search.
Search terms and systematic review methodology, including inclusion and exclusion criteria, are provided as Supplemental digital content 2, http://links.lww.com/EJGH/A24. Searches were limited to articles published in English and including a human adult population (≥18 years) with IBD (UC/CD/IBD unknown), treated with an anti-TNFα agent and reporting data pertinent to clinical outcomes, treatment patterns, AEs and economic outcomes. Searches were limited by country of origin, to characterize clinical practice and degree of unmet need in countries in which new biologic therapies with different mechanisms of action will likely become available as treatment alternatives to conventional treatment options for UC and CD 12,35. Search terms identified studies from 15 European Union countries [Austria, Belgium, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Luxembourg, the Netherlands, Portugal, Spain, Sweden, UK (England, Wales, Scotland)] and from seven other countries (Australia, Brazil, Canada, Mexico, New Zealand, Russia and USA).
After performing the searches, two investigators independently reviewed the titles and abstracts of all identified citations to generate a list of potentially relevant articles for further review. Full texts were obtained for the relevant articles identified, which were also reviewed independently by two reviewers using a prespecified data extraction grid with a positive inclusion strategy. Discrepancies in the data extracted were resolved through consensus. Where more than one publication was identified describing a single study, data were compiled into a single entry to avoid double counting. Only data from full-text publications were included. Studies included in the evidence synthesis were those prioritized as relevant to the study objectives, on the basis of publication year, publication type, country, disease type, interventions and outcomes assessed.
Reporting of the systematic literature review was qualitative (a narrative summary of published studies) and quantitative (a numerical summary of reported ranges). Published rates of primary failure, secondary failure, dose escalation and switching of biologics were also reported where available.
Clinician surveys
A cross-sectional survey was conducted to elicit expert clinical opinion among gastroenterologists practising in a secondary-care setting in the UK (n=50) and France (n=52) in relation to the failure rates seen among patients treated with existing biologic products and to understand how these patients were subsequently treated. The aim of this survey was to inform gaps in the published literature and clarify uncertainties identified following the systematic literature review.
UK and France were chosen as the study countries to capture current clinical practice with IFX and ADA (the mainstays of biologic therapy in the UK), and GOL and CTZ, which are used in clinical practice in France but not in the UK. Senior-level gastroenterologists, who had agreed to participate in market research run by Cegedim Strategic Data (CSD database), were invited to participate; all gastroenterologists agreeing to participate in this research completed the questionnaire. To be eligible for participation, the clinicians had to be a practising gastroenterologist treating patients with either CD (n>10) or UC (n>5) with a biologic product at the time of the survey. The fieldwork periods for the UK and France were 27 January–12 February 2014, and 14 February–24 February 2014, respectively.
The gastroenterologists were asked to answer a series of multiple choice, closed-ended and open-ended questions related to the study objectives. The questionnaire was administered as an online survey (see Supplemental digital content 3, http://links.lww.com/EJGH/A25). Pretesting of the questionnaire by seven gastroenterologists was undertaken to validate the survey questions, ensuring that the survey was reliable and met its objectives; no modifications to the survey were suggested. The survey questions predominantly required a numerical response. A minority of the questions were in free-text format. Numerical data were summarized as means and SDs, and number of clinicians who completed the questionnaire (N); plots are provided for illustrative purposes, reporting the median and interquartile range of the data. Qualitative responses were summarized by combining like responses into a common numerical format (i.e. categorical variable) and producing associated summary statistics. Analyses were carried out in Microsoft Excel and Stata version 11 36,37.
The majority of survey questions elicited data on primary failure and secondary failure. Survey respondents were provided with the following working definitions of treatment failure, on the basis of the definitions provided by Yanai and Hanauer 38. Primary failure: lack of improvement in clinical signs and symptoms during induction therapy, leading to discontinuation of a given biologic therapy. Reduced response: loss of response during maintenance therapy, defined as the occurrence of disease activity after achieving an appropriate induction response, without immediate discontinuation of a given biologic therapy. Secondary failure: loss of response during maintenance therapy, defined as recurrence of disease activity after achieving an appropriate induction response, leading to discontinuation of a given biologic therapy.
Results
Systematic literature review
Of the 120 full-text publications included for evidence synthesis (PRISMA diagram presented as Fig. 1; identified publications listed within Supplemental digital content 1, http://links.lww.com/EJGH/A23), 24 were identified for UC, 76 for CD, 15 for IBD combined (both UC and CD) and five for IBD unspecified (UC, CD or indeterminate).
Fig. 1.
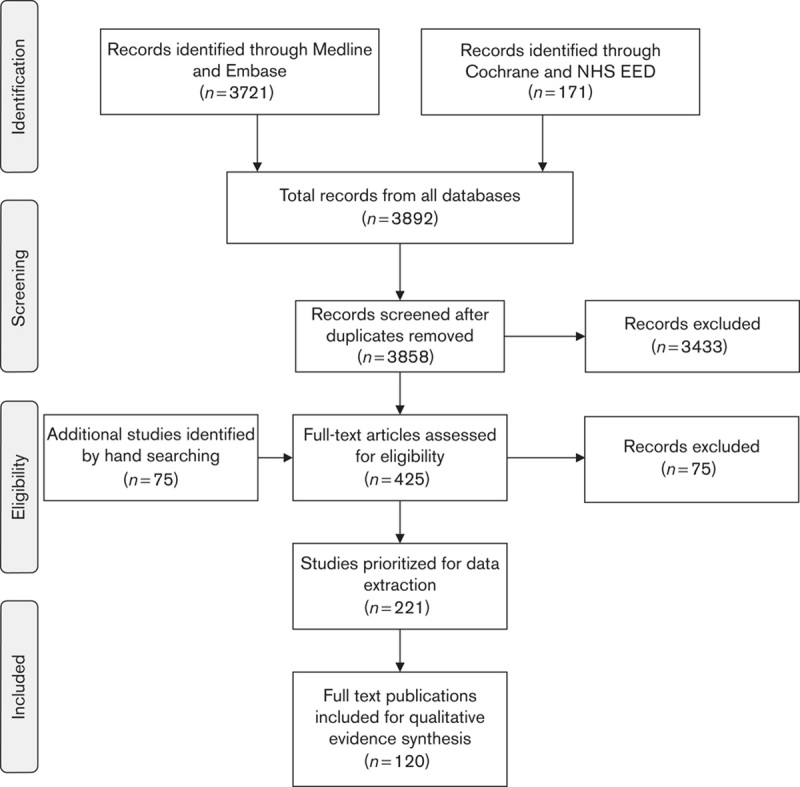
The trial flow of the review process (per PRISMA statement 32). NHS EED, NHS Economic Evaluation Database.
Treatment patterns and pathways
Variations in treatment patterns were reported for both UC and CD. Studies typically reported the use of conventional treatment pathways for IBD (both UC and CD), including initiation of therapy with corticosteroids, followed by the use of biologics in patients who failed corticosteroid therapy. Among the anti-TNFα agents, the published literature provided evidence of a preference for IFX over ADA as first-line biologic therapy for both UC and CD. Failure with an anti-TNFα agent was managed by dose escalation of the same biologic or switching to another anti-TNFα agent or corticosteroids. Surgery was used as an option at any stage to manage therapy failure and disease progression.
Response rates and treatment failures
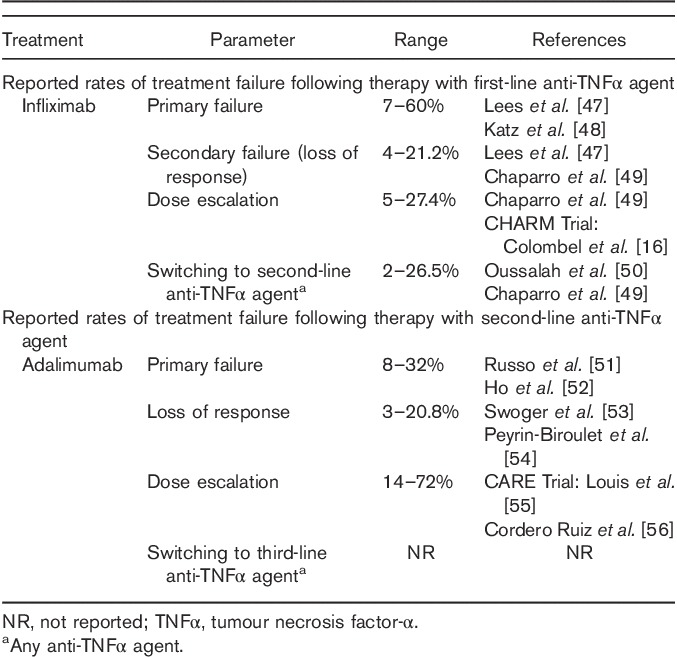
The systematic literature review found that primary failure and secondary failure with first-line IFX (the most common first-line anti-TNFα agent) was common in both UC and CD patients, and that rates of failure with second-line anti-TNFα agents were also high. However, definitive conclusions on differences in the rates of failure between first-line and second-line biologic therapies were difficult to make, given the wide ranges of reported values and heterogeneity across studies (Tables 1 and 2). There was some evidence to suggest that treatment failure (as measured by the proportion of patients undergoing dose escalation) was higher at second-line biologic treatment compared with first-line biologic treatment (see Tables S1 and S2, Supplemental digital content 1, http://links.lww.com/EJGH/A23). This is in line with evidence from several clinical studies, supporting the conclusion that response to second-line anti-TNFα agents tends to decrease in patients exposed to prior anti-TNFα agents 31,57,58.
Table 1.
Range of reported treatment failure rates in ulcerative colitis identified in the systematic literature review
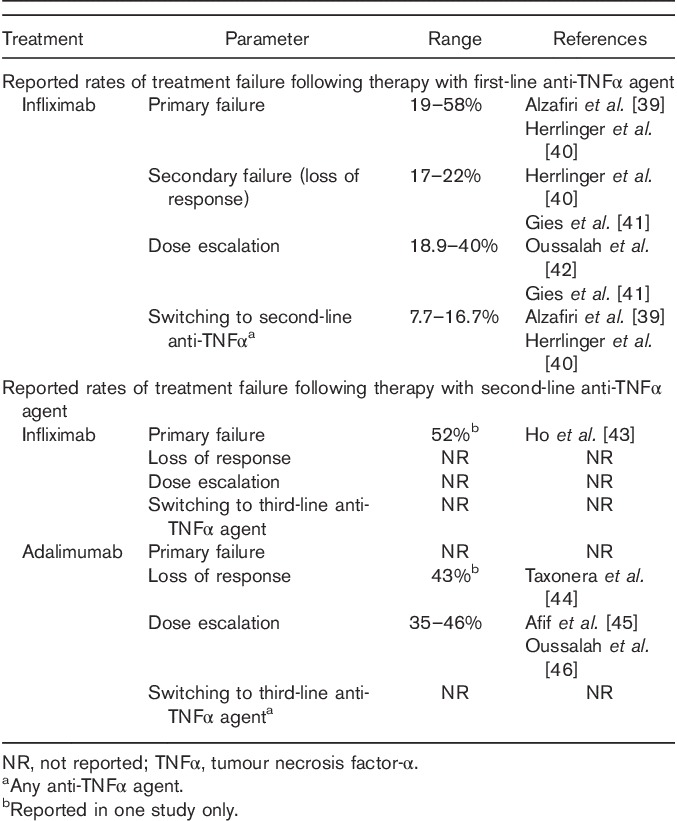
Table 2.
Range of reported treatment failure rates in Crohn’s disease identified in the systematic literature review
Adverse events, resource use and quality of life
The literature review also sought to characterize published data on AEs, resource use and quality of life in patients with UC/CD who received biologic treatments. These data are summarized in Supplemental digital content 1, http://links.lww.com/EJGH/A23 (Tables S5–S10). The most commonly reported AEs were infections and infusion reactions. In terms of resource utilization, most of the studies reported data at an aggregated level for direct healthcare costs. Secondary failure to an anti-TNFα agent was associated with an increase in total cost; the cost of anti-TNFα agents was the highest contributing factor to overall costs (see Tables S3–S5, Supplemental digital content 1, http://links.lww.com/EJGH/A23).
Data gaps and uncertainty in the evidence base
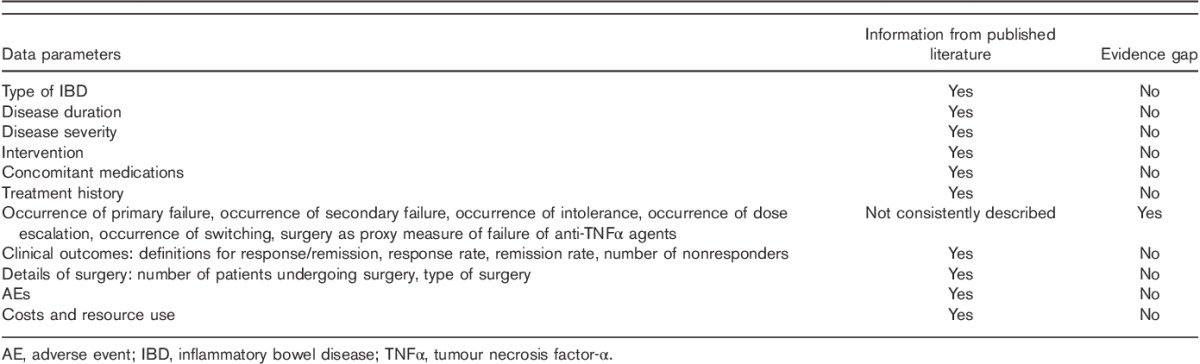
Although a significant amount of data was uncovered from the published literature, it was apparent that there was significant between-study heterogeneity and a number of evidence gaps (Table 3). Definitions for primary failure and secondary failure varied and were not consistently reported. There was relatively more evidence characterizing the use and outcomes of IFX therapy, and lesser evidence for ADA, with few studies providing evidence on the use of other available biologics such as NAT, GOL and CTZ. Furthermore, there remain gaps in the literature in the reporting of therapeutic rates of primary failure and secondary failure, or the response to treatment failure (such as dose escalation or therapy switching) across treatments, which need to be characterized to fully ascertain the extent of unmet need in patients with UC and CD.
Table 3.
Evidence gaps
Clinician survey
The expert opinion of gastroenterologists (respondents) in the UK (n=50) and France (n=52) was elicited to clarify and extend the evidence base identified in the systematic literature review.
Respondents from both countries had more experience in the treatment of CD than in the treatment of UC. The mean number of patients with CD that a clinician in the UK reported to have treated with biologic therapy was 70, versus 34 patients with UC; by comparison, French clinicians treated a mean of 44 patients with CD using biologic therapies, compared with 27 patients with UC. However, whereas the experience of UK clinicians was restricted to IFX and ADA, French clinicians had experience in the use of IFX, ADA, GOL and CTZ.
Clinical practice and unmet need
In the UK context, fewer CD compared with UC patients were classified as having severe disease, rather than moderate disease, at onset (57 vs. 69% were classified as having severe CD and UC, respectively). However, on average, it was estimated that CD patients were treated with a biologic 8 months sooner than UC patients (15 vs. 23 months from disease onset to first biologic therapy). The experience of UK clinicians was restricted to IFX and ADA, and in this context, clinicians were asked a series of questions related to treatment failure and response with first-line and second-line biologic therapy with these two anti-TNFα agents.
In France, the proportion of patients classified as having severe disease rather than moderate disease were similar among UC and CD patients, with more patients categorized as severe for both (UC: 58% severe vs. 40% moderate; CD: 55% severe vs. 44% moderate). However, consistent with UK clinician experience, it was estimated that CD patients were treated with a biologic 6 months sooner than UC patients (12 vs. 18 months on average from disease onset to administration of first-line anti-TNFα agent). French clinician experience was reported for IFX, ADA, GOL and CTZ, and in this context, clinicians were asked a series of questions related to treatment failure and response with first-line and second-line anti-TNFα agents; clinician experience was predominantly in treatment with IFX and ADA, with clinicians having limited experience with GOL and CTZ.
Rate and timing of therapy failure
When asked to estimate treatment failure on the basis of their own experience, UK clinicians estimated that 18–26% of patients fail and discontinue therapy with a first-line anti-TNFα agent during the induction phase (primary failure), and that 22–26% lose response and discontinue that anti-TNFα agent over time – that is, secondary failure of the first-line anti-TNFα agent. Estimates of treatment failure were even higher for second-line treatment with an anti-TNFα agent: 28–37% of patients fail and discontinue treatment during the induction phase, and 31–41% lose response and discontinue their second-line anti-TNFα agent treatment over time (Fig. 2). Among patients who lost response, it was estimated that 68–77% of patients discontinued treatment by the end of the first year, and 82–90% of patients discontinued treatment by the end of the second year of treatment due to loss of response.
Fig. 2.
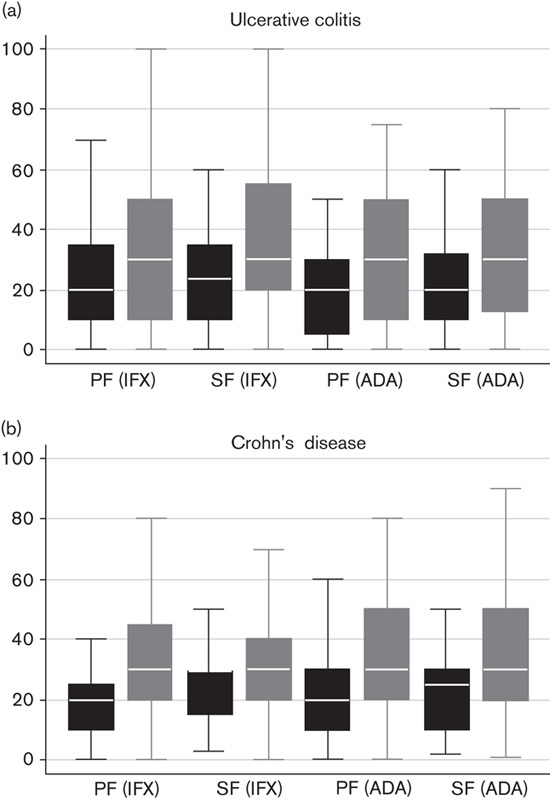
Treatment failure during anti-TNFα therapy as estimated by UK clinicians. Estimates of treatment failure are consistently lower for first-line biologic therapy (black boxes) compared with second-line biologic therapy (grey boxes) in (a) UC and (b) CD, on the basis of the experience of UK gastroenterologists. Median line with box of 25th/75th percentiles and upper/lower adjacent values; while reading the figure, please observe that each category on the x-axis is assigned two boxes. ADA, adalimumab; anti-TNFα, anti-tumour necrosis factor-α; CD, Crohn’s disease; IFX, infliximab; PF, primary failure; SF, secondary failure; UC, ulcerative colitis.
French clinicians estimated that up to 26% of patients failed and discontinued first-line biologic therapy during the induction phase, and up to 28% lose response and discontinue therapy with an anti-TNFα agent over time. Estimates of treatment failure were even higher with second-line anti-TNFα agents, where it was estimated that up to 29% of patients failed and discontinued treatment during the induction phase, and up to 40% lose response and discontinue an anti-TNFα agent over time (Fig. 3). Among patients who lose response and discontinue biologic treatment, it was estimated that 45–60% discontinue treatment by the end of their first year of treatment. By the end of the third year of treatment, 73–87% of patients discontinue treatment due to loss of response.
Fig. 3.
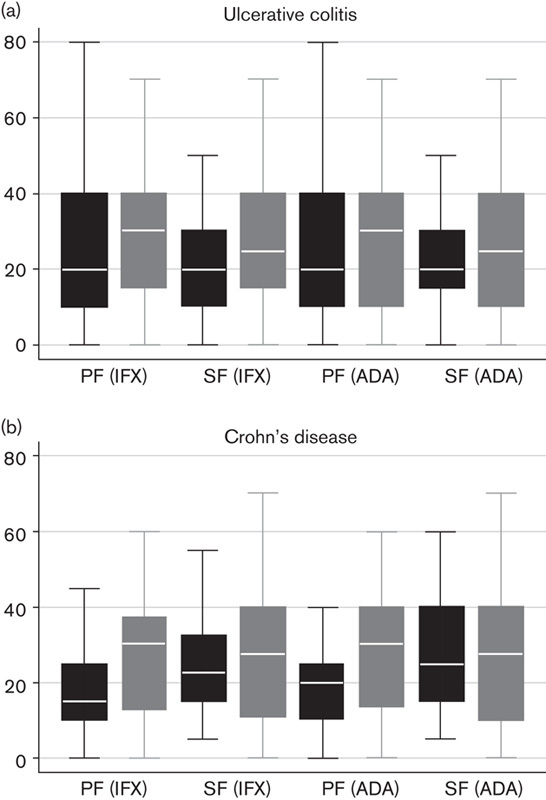
Treatment failure during anti-TNFα therapy as estimated by French clinicians. Estimates of treatment failure are consistently lower for first-line biologic therapy (black boxes) compared with second-line biologic therapy (grey boxes) in (a) UC and (b) CD, on the basis of the experience of French gastroenterologists. Median line with box of 25th/75th percentiles and upper/lower adjacent values; while reading the figure please observe that each category on the x-axis is assigned two boxes. ADA, adalimumab; anti-TNFα, anti-tumour necrosis factor-α; CD, Crohn’s disease; IFX, infliximab; PF, primary failure; SF, secondary failure; UC, ulcerative colitis.
Clinician response to treatment failure
UK clinicians estimated that in patients with first-line treatment failure who showed signs of a reduced response, their response would be to add another (nonbiologic) treatment, such as corticosteroids, in around 25% of cases, increase the dose of the existing biologic therapy in 21–24% of cases, increase the administration frequency in 17–25% of cases, and offer surgery as a treatment option in 20–22% of cases. When treatment alternatives in patients failing first-line biologic therapy were restricted to surgery or a switch to a second-line biologic, UK clinicians estimated that among UC patients failing IFX as a first-line anti-TNFα agent, around 50% would switch to ADA as a second-line anti-TNFα agent and the other 50% would pursue surgery as a treatment option. Similarly among ADA failures, around 50% would switch to IFX as a second-line anti-TNFα agent and 50% would pursue surgery. Among CD patients failing IFX, clinicians estimated that around 70% would switch to ADA as a second-line anti-TNFα agent and the other 30% would pursue surgery as a treatment option. For ADA failures, around 50% would switch to IFX as a second-line anti-TNFα agent and 50% would pursue surgery. French clinicians estimated that among patients with first-line treatment failure or a reduced response, around 18–39% would receive an additional nonbiologic treatment, such as corticosteroids, around 12–33% would be administered an increased dose of the existing anti-TNFα agent, administration frequency would be increased in around 9–27% of patients and surgery would be offered to around 7–23% of patients. When treatment alternatives in patients failing first-line biologic therapy were restricted to surgery or a switch to a second-line anti-TNFα agent, it was estimated that among UC patients, around 20% would switch to surgery from biologic therapy (IFX/ADA/CTZ) and 77%would switch between IFX and ADA (i.e. IFX to ADA or ADA to IFX). Among CD patients failing a first-line anti-TNFα agent, 15% would switch to surgery from IFX/ADA, 26% would switch from CTZ to surgery and 80% would switch between IFX and ADA; clinicians estimated that there was limited switching to GOL or CTZ.
Discussion
The impact of UC and CD on the quality of life of patients and on medical expenditure is well understood 59, as is the need for effective treatment options 60. The efficacy of conventional treatments and biologic therapies used to manage cases of UC and CD has been explored in a number of clinical trials 15–26,61–63. Meta-analyses and retrospective analyses of patients receiving the available biologic therapies suggest that their comparative effectiveness is similar, whereas other studies point toward differences between the treatments in terms of patient-relevant outcomes 27–31. The current literature, therefore, does not allow definitive conclusions to be drawn with regard to the comparative outcomes of patients treated with currently available biologic therapies in clinical practice. This research aimed to characterize treatment patterns and outcomes of patients with UC and CD and therein characterize the degree of unmet medical need among patients with these conditions.
Studies identified in the systematic review reported high rates treatment failure – around 7–60% primary failure and 4–43% secondary failure – which were highly variable. Heterogeneity between studies with regard to definitions of treatment failure, study sample sizes, study designs and follow-up periods thwarted a robust quantitative synthesis of estimates of treatment failure across studies. The narrative summary of the literature that we report is useful in confirming a general sense of high unmet need among patients with UC and CD; the wide range of reported values for treatment failure in the literature confirms the inconclusive nature of the outcomes of treating patients with existing biologics.
The output of the systematic review also confirmed that there is a paucity of data describing patterns and outcomes of treatment for UC and CD in several important respects, particularly in estimates of treatment failure by type of biologic therapy and disease. Outcomes for patients receiving ADA, GOL or CTZ, as well as data characterizing the time to secondary failure, were not well reported in the published literature.
Similarly to the findings from the systematic literature review, surveyed clinicians estimated that a significant number of patients show failure with existing biologic therapies during the induction phase (up to 37%) and the maintenance phase (up to 41%) of treatment. The survey data provided additional granularity around estimates of treatment failure. The estimated rates of primary and secondary failure were high for first-line and second-line therapies, with higher rates of treatment failure observed among patients switching between available biologic therapies.
The reviewed literature and estimates of treatment failure obtained from clinicians treating patients with biologics in current clinical practice demonstrate that there is significant unmet need among patients with UC and CD. In the significant number of patients who cannot tolerate or who fail their first-prescribed biologic treatment, subsequent pharmacological treatment choices are restricted to the biologic therapies currently available. Data from this study suggest that the outcome for patients who cycle between existing biologic treatment options with similar mechanisms of action (i.e. IFX, ADA, GOL, CTZ) is likely to be suboptimal in around 21–41% of cases.
The findings of the literature review and surveys were generally consistent. For first-line treatment failures, survey estimates were generally within the ranges reported in the published literature, with the exception of secondary failure in IFX-treated patients, where the surveys estimated slightly higher proportions of treatment failure for both UC and CD. Survey estimates were also broadly consistent with observations from a real-world IBD audit, an ongoing nationwide audit of individual patient care and provision concerning the organization of IBD services in the UK 64. The audit has previously reported a primary failure rate of 14.5% among UC patients treated with anti-TNFα agents (on the basis of a reported primary response of 85.5%). This is comparable to the primary failure rates identified by the survey of UK clinicians, which were 26 and 19% for patients treated with IFX and ADA, respectively. Similarly, for CD, a primary failure rate of 12.4% was reported by the IBD audit (on the basis of an overall response of 87.6%), which compares to the primary failure rates of 18 and 20% estimated for IFX and ADA, respectively, by the UK clinician survey. In both cases, the IBD audit reported significantly lower primary failure rates compared with clinician survey results. However, subsequent iterations of the audit reported that 62% of adult patients treated with anti-TNFα agents enter remission: an implied failure rate of 38% 64. These audit data underscore the degree of unmet need identified by the systematic review and surveys, and the potential role of new therapeutic options with an alternative mechanism of action.
A secondary objective of the systematic literature review was to extract and qualitatively assess the evidence for additional treatment outcomes, including costs, AEs and quality of life. The details of and a brief narrative review of the literature for these outcomes are provided in Supplemental digital content 1, http://links.lww.com/EJGH/A23.
Conclusion
This study underscores the apparent need for newer biologic treatments with novel mechanisms of action that may offer greater therapeutic value to patients with UC or CD failing existing biologic therapies. Further evidence is warranted from both placebo-controlled trials and head-to-head comparisons of current anti-TNFα agents versus new therapies to understand the consequences of switching to a biologic with a novel therapeutic target and mechanism of action.
Supplementary Material
Acknowledgements
This study was supported by Cegedim Strategic Data (CSD UK Ltd) and Capita India Pvt. Ltd in the design and implementation of the clinician surveys and systematic literature reviews, respectively. We acknowledge writing support provided by Beverley Jones (HEOR Ltd).
This study was funded by an unrestricted research grant from Takeda Development Centre, Europe. Initial data analyses and writing was undertaken by J.P.G. and P.C.M., who are employees of HEOR Ltd and received funding from Takeda Development Centre, Europe. All named authors contributed to the study design, writing support and interpretation of findings.
Conflicts of interest
J.P.G., P.C.M., A.M. and D.M.S. have served as consultants to and received research funding from Takeda Development Centre, Europe, in relation to this study. J.P.G., P.C.M. and D.M.S. are employees of HEOR Ltd. A.M. is an employee of Oxon Epidemiology. J.P. is an employee of Takeda Development Centre, Europe.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.eurojgh.com).
References
- 1.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet 2007; 369:1627–1640. [DOI] [PubMed] [Google Scholar]
- 2.Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD). Pharmacol Rep 2011; 63:629–642. [DOI] [PubMed] [Google Scholar]
- 3.Janssen Biologics BV. Summary of product characteristics. Remicade. 2013. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000240/WC500050888.pdf. [Accessed 9 January 2015].
- 4.AbbVie Ltd. Summary of product characteristics. Humira. 2014. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000481/WC500050870.pdf. [Accessed 9 January 2015].
- 5.UCB Pharma SA. Summary of product characteristics. Cimzia 2014. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001037/WC500069763.pdf. [Accessed 9 January 2015].
- 6.Janssen Biologics BV. Summary of product characteristics. Simponi. 2014. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000992/WC500052368.pdf. [Accessed 9 January 2015].
- 7.Biogen Idec Ltd. Prescribing information: TYSABRI (natalizumab) injection, for intravenous use. 2013. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125104s840s847s889lbl.pdf. [Accessed 24 October 2014].
- 8.Caviglia R, Boskoski I, Cicala M. Long-term treatment with infliximab in inflammatory bowel disease: safety and tolerability issues. Expert Opin Drug Saf 2008; 7:617–632. [DOI] [PubMed] [Google Scholar]
- 9.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001; 345:1098–1104. [DOI] [PubMed] [Google Scholar]
- 10.Siegel CA, Hur C, Korzenik JR, Gazelle GS, Sands BE. Risks and benefits of infliximab for the treatment of Crohn’s disease. Clin Gastroenterol Hepatol 2006; 4:1017–1024, quiz 976. [DOI] [PubMed] [Google Scholar]
- 11.Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol 2009; 7:874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeda Pharma A/S. Summary of product characteristics. Entyvio 300 mg powder for concentrate for solution for infusion. 2014. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002782/WC500168528.pdf. [Accessed 9 January 2015].
- 13.National Institute for Health and Care Excellence. Final scope. Vedolizumab for treating moderately to severely active ulcerative colitis. 2014. Available at: http://www.nice.org.uk/guidance/gid-tag450/documents/ulcerative-colitis-moderate-to-severely-active-vedolizumab-final-scope-postreferral2. [Accessed 8 January 2015].
- 14.National Institute for Health and Care Excellence. Final scope. Vedolizumab for treating moderately to severely active Crohn’s disease after prior therapy. 2014. Available at: http://www.nice.org.uk/guidance/gid-tag461/documents/crohns-disease-moderate-to-severe-vedolizumab-final-scope2. [Accessed 8 January 2015].
- 15.Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007; 132:52–65. [DOI] [PubMed] [Google Scholar]
- 16.Colombel JF, Sandborn WJ, Rutgeerts P, Kamm MA, Yu AP, Wu EQ, et al. Comparison of two adalimumab treatment schedule strategies for moderate-to-severe Crohn’s disease: results from the CHARM trial. Am J Gastroenterol 2009; 104:1170–1179. [DOI] [PubMed] [Google Scholar]
- 17.Feagan BG, Panaccione R, Sandborn WJ, D’Haens GR, Schreiber S, Rutgeerts PJ, et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: results from the CHARM study. Gastroenterology 2008; 135:1493–1499. [DOI] [PubMed] [Google Scholar]
- 18.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002; 359:1541–1549. [DOI] [PubMed] [Google Scholar]
- 19.Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 2006; 130:323–333. [DOI] [PubMed] [Google Scholar]
- 20.Lémann M, Mary JY, Duclos B, Veyrac M, Dupas JL, Delchier JC, et al. Groupe d'Etude Therapeutique des Affections Inflammatoires du Tube Digestif (GETAID). Infliximab plus azathioprine for steroid-dependent Crohn's disease patients: a randomized placebo-controlled trial. Gastroenterology 2006; 130:1054–1061. [DOI] [PubMed] [Google Scholar]
- 21.Probert CS, Hearing SD, Schreiber S, Kühbacher T, Ghosh S, Arnott ID, Forbes A. Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut 2003; 52:998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer S, Schreiber S, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 2011; 60:780–787. [DOI] [PubMed] [Google Scholar]
- 23.Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014; 146:85–95, quiz e14-5. [DOI] [PubMed] [Google Scholar]
- 24.Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012; 142:257.e3–265.e3. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber S, Rutgeerts P, Fedorak RN, Khaliq-Kareemi M, Kamm MA, Boivin M, et al. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology 2005; 129:807–818. [DOI] [PubMed] [Google Scholar]
- 26.Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, et al. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology 2007; 132:1672–1683. [DOI] [PubMed] [Google Scholar]
- 27.Dretzke J, Edlin R, Round J, Connock M, Hulme C, Czeczot J, et al. A systematic review and economic evaluation of the use of tumour necrosis factor-alpha (TNF-alpha) inhibitors, adalimumab and infliximab, for Crohn’s disease. Health Technol Assess 2011; 15:1–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behm BW, Bickston SJ. Tumor necrosis factor-alpha antibody for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2008; 1:CD006893. [DOI] [PubMed] [Google Scholar]
- 29.Stidham RW, Lee TC, Higgins PD, Deshpande AR, Sussman DA, Singal AG, et al. Systematic review with network meta-analysis: the efficacy of anti-TNF agents for the treatment of Crohn’s disease. Aliment Pharmacol Ther 2014; 39:1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stidham RW, Lee TC, Higgins PD, Deshpande AR, Sussman DA, Singal AG, et al. Systematic review with network meta-analysis: the efficacy of anti-tumour necrosis factor-alpha agents for the treatment of ulcerative colitis. Aliment Pharmacol Ther 2014; 39:660–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patil SA, Rustgi A, Langenberg P, Cross RK. Comparative effectiveness of anti-TNF agents for Crohn’s disease in a tertiary referral IBD practice. Dig Dis Sci 2013; 58:209–215. [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151:264–269. [DOI] [PubMed] [Google Scholar]
- 33.National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. 2008. Available at: http://www.nice.org.uk/article/pmg9. [Accessed 20 March 2014].
- 34.The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. 2011. Available at: http://handbook.cochrane.org. [Accessed 12 August 2014].
- 35.European Medicines Agency. EMA recommends approval of a locally targeted treatment for ulcerative colitis and Crohn’s disease. 2014. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2014/03/WC500163496.pdf. [Accessed 19 March 2015].
- 36.Microsoft Corporation. Microsoft Excel 2013: Microsoft Corporation, Redmond, Washington, USA. [Google Scholar]
- 37.StataCorp. Stata statistical software: release 11. StataCorp LP, College Station, Texas, USA; 2009. [Google Scholar]
- 38.Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol 2011; 106:685–698. [DOI] [PubMed] [Google Scholar]
- 39.Alzafiri R, Holcroft CA, Malolepszy P, Cohen A, Szilagyi A. Infliximab therapy for moderately severe Crohn’s disease and ulcerative colitis: a retrospective comparison over 6 years. Clin Exp Gastroenterol 2011; 4:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herrlinger KR, Barthel DN, Schmidt KJ, Büning J, Barthel CS, Wehkamp J, et al. Infliximab as rescue medication for patients with severe ulcerative/indeterminate colitis refractory to tacrolimus. Aliment Pharmacol Ther 2010; 31:1036–1041. [DOI] [PubMed] [Google Scholar]
- 41.Gies N, Kroeker KI, Wong K, Fedorak RN. Treatment of ulcerative colitis with adalimumab or infliximab: long term follow-up of a single centre cohort. Aliment Pharmacol Ther 2010; 32:522–528. [DOI] [PubMed] [Google Scholar]
- 42.Oussalah A, Evesque L, Laharie D, Roblin X, Boschetti G, Nancey S, et al. A multicenter experience with infliximab for ulcerative colitis: outcomes and predictors of response, optimization, colectomy, and hospitalization. Am J Gastroenterol 2010; 105:2617–2625. [DOI] [PubMed] [Google Scholar]
- 43.Ho GT, Mowat A, Potts L, Cahill A, Mowat C, Lees CW, et al. Efficacy and complications of adalimumab treatment for medically-refractory Crohn’s disease: analysis of nationwide experience in Scotland (2004–2008). Aliment Pharmacol Ther 2009; 29:527–534. [DOI] [PubMed] [Google Scholar]
- 44.Taxonera C, Estellés J, Fernández-Blanco I, Merino O, Marín-Jiménez I, Barreiro-de Acosta M, et al. Adalimumab induction and maintenance therapy for patients with ulcerative colitis previously treated with infliximab. Aliment Pharmacol Ther 2011; 33:340–348. [DOI] [PubMed] [Google Scholar]
- 45.Afif W, Leighton JA, Hanauer SB, Loftus EV, Jr, Faubion WA, Pardi DS, et al. Open-label study of adalimumab in patients with ulcerative colitis including those with prior loss of response or intolerance to infliximab. Inflamm Bowel Dis 2009; 15:1302–1307. [DOI] [PubMed] [Google Scholar]
- 46.Oussalah A, Laclotte C, Chevaux JB, Bensenane M, Babouri A, Serre AA, et al. Long-term outcome of adalimumab therapy for ulcerative colitis with intolerance or lost response to infliximab: a single-centre experience. Aliment Pharmacol Ther 2008; 28:966–972. [DOI] [PubMed] [Google Scholar]
- 47.Lees CW, Ali AI, Thompson AI, Ho GT, Forsythe RO, Marquez L, et al. The safety profile of anti-tumour necrosis factor therapy in inflammatory bowel disease in clinical practice: analysis of 620 patient-years follow-up. Aliment Pharmacol Ther 2009; 29:286–297. [DOI] [PubMed] [Google Scholar]
- 48.Katz L, Gisbert JP, Manoogian B, Lin K, Steenholdt C, Mantzaris GJ, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis 2012; 18:2026–2033. [DOI] [PubMed] [Google Scholar]
- 49.Chaparro M, Panes J, García V, Mañosa M, Esteve M, Merino O, et al. Long-term durability of infliximab treatment in Crohn's disease and efficacy of dose "escalation" in patients losing response. J Clin Gastroenterol 2011; 45:113–118. [DOI] [PubMed] [Google Scholar]
- 50.Oussalah A, Chevaux JB, Fay R, Sandborn WJ, Bigard MA, Peyrin-Biroulet L. Predictors of infliximab failure after azathioprine withdrawal in Crohn’s disease treated with combination therapy. Am J Gastroenterol 2010; 105:1142–1149. [DOI] [PubMed] [Google Scholar]
- 51.Russo EA, Iacucci M, Lindsay JO, Campbell S, Hart A, Hamlin J, et al. Survey on the use of adalimumab as maintenance therapy in Crohn’s disease in England and Ireland. Eur J Gastroenterol Hepatol 2010; 22:334–339. [DOI] [PubMed] [Google Scholar]
- 52.Ho GT, Smith L, Aitken S, Lee HM, Ting T, Fennell J, et al. The use of adalimumab in the management of refractory Crohn’s disease. Aliment Pharmacol Ther 2008; 27:308–315. [DOI] [PubMed] [Google Scholar]
- 53.Swoger JM, Loftus EV, Jr, Tremaine WJ, Faubion WA, Pardi DS, Kane SV, et al. Adalimumab for Crohn’s disease in clinical practice at Mayo clinic: the first 118 patients. Inflamm Bowel Dis 2010; 16:1912–1921. [DOI] [PubMed] [Google Scholar]
- 54.Peyrin-Biroulet L, Laclotte C, Bigard MA. Adalimumab maintenance therapy for Crohn’s disease with intolerance or lost response to infliximab: an open-label study. Aliment Pharmacol Ther 2007; 25:675–680. [DOI] [PubMed] [Google Scholar]
- 55.Louis E, Löfberg R, Reinisch W, Camez A, Yang M, Pollack PF, et al. Adalimumab improves patient-reported outcomes and reduces indirect costs in patients with moderate to severe Crohn’s disease: results from the CARE trial. J Crohns Colitis 2013; 7:34–43. [DOI] [PubMed] [Google Scholar]
- 56.Cordero Ruiz P, Castro Márquez C, Méndez Rufián V, Castro Laria L, Caunedo Álvarez A, Romero Vázquez J, Herrerías Gutiérrez JM. Efficacy of adalimumab in patients with Crohn’s disease and failure to infliximab therapy: a clinical series. Rev Esp Enferm Dig 2011; 103:294–298. [DOI] [PubMed] [Google Scholar]
- 57.Hoentjen F, Haarhuis BJ, Drenth JP, de Jong DJ. Elective switching from infliximab to adalimumab in stable Crohn’s disease. Inflamm Bowel Dis 2013; 19:761–766. [DOI] [PubMed] [Google Scholar]
- 58.Van Assche G, Vermeire S, Ballet V, Gabriels F, Noman M, D’Haens G, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 2012; 61:229–234. [DOI] [PubMed] [Google Scholar]
- 59.Rocchi A, Benchimol EI, Bernstein CN, Bitton A, Feagan B, Panaccione R, et al. Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol 2012; 26:811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lawrance IC. What is left when anti-tumour necrosis factor therapy in inflammatory bowel diseases fails? World J Gastroenterol 2014; 20:1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rutgeerts P, Diamond RH, Bala M, Olson A, Lichtenstein GR, Bao W, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn’s disease. Gastrointest Endosc 2006; 63:433–442. [DOI] [PubMed] [Google Scholar]
- 62.Rutgeerts P, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology 2004; 126:402–413. [DOI] [PubMed] [Google Scholar]
- 63.D’Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, et al. Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008; 371:660–667. [DOI] [PubMed] [Google Scholar]
- 64.Clinical Effectiveness and Evaluation unit at the Royal College of Physicians. National clinical audit of biological therapies: UK Inflammatory Bowel Disease (IBD) audit. Adult national report. 2013. Available at: https://www.rcplondon.ac.uk/projects/biologics. [Accessed 13 August 2014].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


