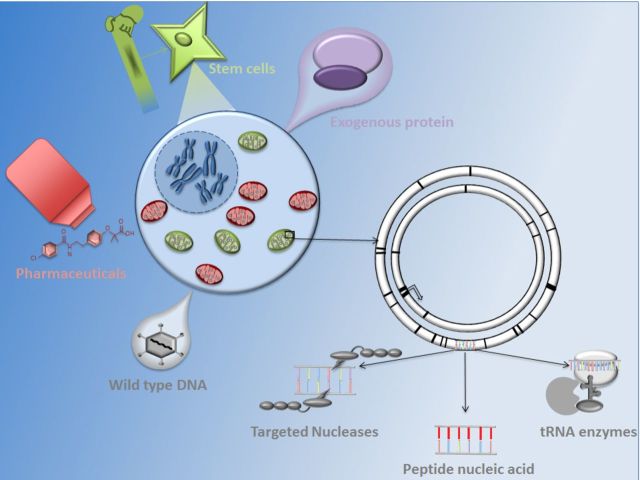
Traditionally, mitochondrial disorders have been treated with vitamins, co-factors and nutritional supplements with no proven benefit. While effective treatments are still lacking, several new molecular and cellular strategies have recently been proposed. Nightingale et al. critically appraise the most promising preclinical developments.
Keywords: mitochondrial disorders, treatment, gene therapies, protein, pharmaceuticals
Traditionally, mitochondrial disorders have been treated with vitamins, co-factors and nutritional supplements with no proven benefit. While effective treatments are still lacking, several new molecular and cellular strategies have recently been proposed. Nightingale et al. critically appraise the most promising preclinical developments.
Abstract
Mitochondrial disorders are a diverse group of debilitating conditions resulting from nuclear and mitochondrial DNA mutations that affect multiple organs, often including the central and peripheral nervous system. Despite major advances in our understanding of the molecular mechanisms, effective treatments have not been forthcoming. For over five decades patients have been treated with different vitamins, co-factors and nutritional supplements, but with no proven benefit. There is therefore a clear need for a new approach. Several new strategies have been proposed acting at the molecular or cellular level. Whilst many show promise in vitro, the clinical potential of some is questionable. Here we critically appraise the most promising preclinical developments, placing the greatest emphasis on diseases caused by mitochondrial DNA mutations. With new animal and cellular models, longitudinal deep phenotyping in large patient cohorts, and growing interest from the pharmaceutical industry, the field is poised to make a breakthrough.
Introduction
Mitochondria are complex intracellular organelles that play a central role in cell homeostasis (Wallace, 1999). They are the principal source of intracellular energy, are intimately involved in both calcium and free radical metabolism, and they can trigger programmed cell death (apoptosis). Tissues and organs that critically dependent on these functions bear the brunt of the pathology in human mitochondrial diseases, which often affect the nervous system, muscle and endocrine organs (Pfeffer et al., 2012). Most mitochondrial disorders are progressive and often result in disability and premature death. Therefore, although they are rare diseases, with a minimum reported prevalence of 1 in 4300 (Schaefer et al., 2004), they have substantial impact on families and healthcare services.
Most mitochondrial disorders are ultimately thought to arise through a bioenergetic defect linked to a deficiency of ATP synthesis. ATP synthesis is the final step of respiration, which is carried out by five oxidative phosphorylation (OXPHOS) complexes situated on the inner mitochondrial membrane. Each complex has multiple protein subunits encoded by two distinct genomes: nuclear chromosomal DNA (nDNA), and the 16.5 kb mitochondrial genome (mtDNA).
Pathogenic mtDNA mutations often cause a subset of classical mitochondrial clinical syndromes (Chinnery and Hudson, 2013). However, an increased number of multisystem mitochondrial disorders are being described in the literature, many of which are yet to be fully characterized clinically and genetically. These include an emerging myriad of nuclear encoded mitochondrial disorders caused by mutations in some of the ∼1500 nuclear genes thought to code for mitochondrial proteins (Chinnery and Hudson, 2013). In the past, the phenotypic and genetic diversity has made clinical diagnosis very challenging. However, with international diagnostic standards (Wolf and Smeitink, 2002), and the widespread availability of molecular genetic techniques, an accurate diagnosis is less challenging than before. Next generation sequencing is revolutionizing the diagnostic approach, with multi-gene panels, whole exome, and whole genome sequencing increasing the pace of diagnosis, and probably reducing the overall costs. As a consequence, more and more patients are being diagnosed with a mitochondrial disorder, placing even greater emphasis on developing treatments.
A recent systematic review identified over 1300 reports using a variety of approaches expected to bypass or enhance components of mitochondrial function. However, the vast majority of these reports are open-labelled case series with less than five subjects. Although ∼30 randomized trials have been carried out to date, no treatment has shown a clear cut benefit on a clinically meaningful end-point (for reviews see Pfeffer et al., 2012; Kerr, 2013). It is therefore likely that components of the traditional ‘mitochondrial cocktail’ do not have a major therapeutic impact on most mitochondrial diseases. There is therefore a clear need for the field to ‘think outside the box’ when developing new treatments, harnessing the massive increase in our understanding of mitochondrial disease pathogenesis. After preclinical evaluation in cellular and animal models, new treatments showing promise should be studied in patients using a rigorous approach (Pfeffer et al., 2013). This review focuses on these new developments, with a particular emphasis on mtDNA diseases, which were previously thought to be intractable. Here we critically appraise each approach, and highlight areas where there is likely to be traction in the future. This is timely, because both small and large pharmaceutical companies are starting to see the potential market in developing treatments for these so-far untreatable disorders.
Capitalizing on the unique properties of mitochondria
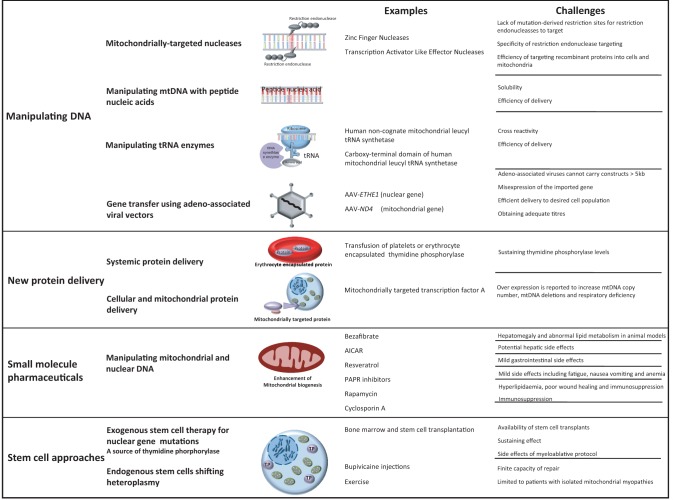
Mitochondria have unusual characteristics that are potentially targetable for treatment at the molecular level. Mitochondria contain numerous copies of their genome, and most patients with mtDNA disease harbour both mutated and wild-type mtDNA (heteroplasmy) (Wallace, 1999). The proportion of mutated mtDNA needs to be in excess to cause a biochemical defect of the respiratory chain. This critical threshold varies from mutation to mutation, and also between tissues and organs. This partly explains the tissue selectivity and clinical heterogeneity of mitochondrial disorders (Macmillan et al., 1993). The overall amount of mtDNA within a cell is also tightly regulated in a tissue-specific manner, and some tissues (such as skeletal muscle) display a close correlation between mtDNA content and mitochondrial activity (Wang et al., 1999). This complexity raises the possibility of enhancing mitochondrial function by manipulating the ratio of mutant to wild-type mtDNA, or by increasing the amount of wild-type mtDNA. Targeting mutated mtDNA is an alternative approach, although this would need to be highly specific to avoid adverse effects on wild-type genomes. The delivery of wild-type nDNA (Di Meo et al., 2012; Bottani et al., 2014; Torres-Torronteras et al., 2014) or mtDNA (Ellouze et al., 2008; Yu et al., 2012a,b) using viral vectors is another possibility, or perhaps the replacement of dysfunctional proteins via the cell nucleus, hitch-hiking on the mitochondrial import mechanism (Perales-Clemente et al., 2011). Moving away from the two genomes, small molecule screens may enhance function of the respiratory chain, stem cell therapies could correct enzyme defects due to nuclear gene defects (Hirano et al., 2006; Hill et al., 2009; Halter et al., 2011; Lenoci et al., 2011; Filosto et al., 2012; Sicurelli et al., 2012; Hussein, 2013), and treatments aimed at non-specifically preventing neurodegeneration may be the way forward. Some of these approaches capitalize on the ‘uniqueness’ of mitochondria, where other approaches build on knowledge acquired from rare and common neurological disorders. Having an open mind is critical at this stage—the first effective treatment may not come from an obvious place. This review considers each, structured into four sections (Fig. 1): manipulating DNA; new protein delivery; small molecule pharmaceuticals; and finally, stem cell approaches. Our discussion focuses on the likelihood of these treatments being used in the clinic for patients with mitochondrial disease, and does not discuss recent work aimed at preventing these disorders.
Figure 1.
Overview of novel therapeutic approaches for the treatment of mitochondrial disorders. AICAR = 5-aminoimidazole-4-carboxamide ribonucleotide; PAPR = poly adenosine diphosphate-ribose polymerase receptor; TP = thymidine phosphorylase.
Nucleic acid-based approaches
Several approaches, described below, have been developed to manipulate or replace mutated nDNA and mtDNA. As for all potential therapies, the multi-organ nature of mitochondrial disorders and difficulties of transferring therapies across cellular and mitochondrial membranes without causing toxic effects (Mingozzi and High, 2011), makes therapeutic targeting difficult. However, as explored further below, methods exist to overcome these difficulties including the use of viral vectors (Mingozzi and High, 2011), harnessing allotopic expression (nuclear expression of mtDNA encoded protein) (Farrar et al., 2013), or fusion of therapeutic molecules to targeting proteins (Iyer et al., 2009).
Mitochondrially-targeted nucleases
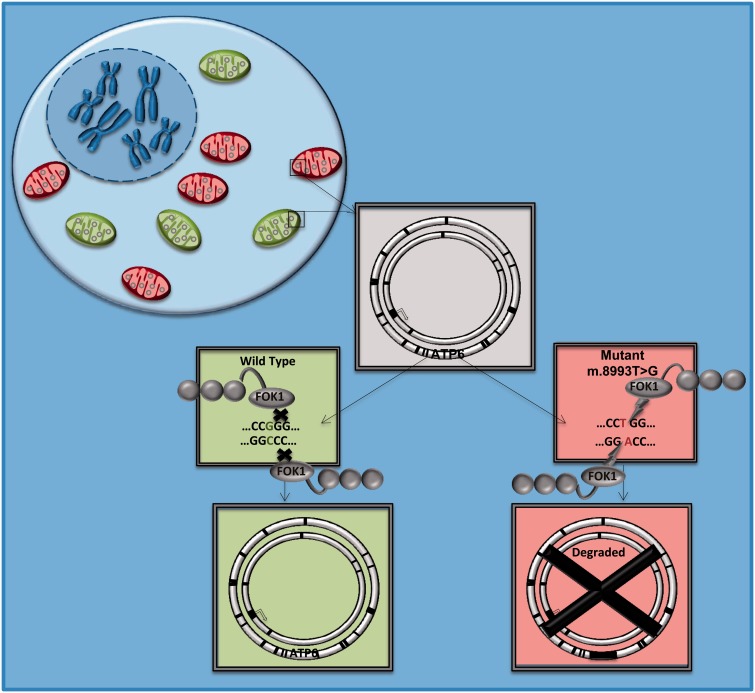
Restriction endonuclease approaches were developed to recognize specific DNA sequences, produce double-stranded DNA breaks, and thereby initiate target molecule degradation. Unlike other gene therapy approaches, restriction endonucleases are not contingent on stable vector integration into the nuclear genome. In theory, a single treatment could induce a stable modulation of mtDNA heteroplasmy, correcting the biochemical defect within diseased cells (Minczuk et al., 2008; Bacman et al., 2013; Gammage et al., 2014). Restriction endonucleases recognize a broad repertoire of mtDNA sequences (‘restriction sites’) and some pathogenic mtDNA mutations add restriction sites that restriction endonucleases specifically target.
The m.8993T > G mtDNA mutation causes Leigh syndrome or neuropathy ataxia retinitis pigmentosa, and mitochondrially-targeted restriction endonuclease SmaI has been shown to selectively eliminate m.8993T > G from patient-derived cybrid cells. Over time, the treatment resulted in repopulation with wild-type mtDNA, and normalization of cellular ATP content (Tanaka et al., 2002).
Unfortunately, a key limitation of restriction endonucleases is that very few human pathogenic mutations create restriction sites amenable to this form of targeted destruction. This can in part be overcome by restriction endonucleases custom designed to bind specific DNA sequences (Klug, 2010). A zinc finger nuclease (ZFN) consists of tandem repeat zinc fingers, each binding approximately three DNA bases, combined with a FokI endonuclease domain functioning as the DNA cleavage module (Klug, 2010). Specificity is achieved through different combinations of zinc fingers. ZFNs have also been shown to selectively eliminate the m.8993T > G mutation in a cybrid model (Fig. 2) (Minczuk et al., 2008). Early ZFNs caused significant cytotoxicity, homodimerization (Ramalingam et al., 2011) and off-target binding. This would be detrimental in vivo, thereby limiting their clinical use (Radecke et al., 2010), but are being addressed by improved design (Gammage et al., 2014). The architecture of dimeric ZFNs ensures that the active cleavage reagent is only assembled at desired restriction sites, overcoming targeting difficulties and concerns about constitutive nuclease activity (Gammage et al., 2014). Customized ZFNs targeted to mitochondria cause shifts in heteroplasmy through the selective degradation of mtDNA containing the m.8993T > G point mutation, and the large scale (4977 bp) mtDNA ‘common deletion’, which is the most common cause of chronic progressive external ophthalmoplegia, Kearns-Sayre syndrome, and Pearson marrow pancreas syndrome (Gammage et al., 2014). This work opens up the opportunity to develop a library of bespoke ZFNs against more common pathogenic mtDNA mutations, but the shifts in heteroplasmy have been limited to date. Longer-term studies, particularly using animal models, will hopefully show that ZFNs can improve biochemical function in vivo—but this will be technically demanding, not least because of the challenges delivering these agents at an appropriate concentration to affected tissues.
Figure 2.
Endonucleases. Endonucleases are used to target specific sequences in mtDNA causing double-strand breaks and degradation of mtDNA. For example the endonuclease ZFN has been shown to reduce mutation load in a cybrid model of Leigh and NARP syndrome, which are caused by the mtDNA mutation m.8933T > G within the ATP6 domain. ZFN binds specifically to the mutant form of the mtDNA and the FOK1 endonuclease domain cleaves the DNA molecule, which is then degraded.
When combined with a non-specific nuclease (e.g. FokI), TALEs (transcription activator-like effectors) become target-specific DNA nucleases (TALENs) (Christian et al., 2010). TALENs are potentially much more potent than ZFNs, but their larger size limits their use with adeno-associated viral vectors (AAVs) (Ellis et al., 2013), TALEs contain repetitive domain modules, each of which bind individual nucleotides (Moscou and Bogdanove, 2009), thus different domain module combinations can be used to ensure specificity.
A recent report described the use of mitochondrially localized TALENs targeted for mtDNA species with the ‘common deletion’ (m.8483_13459del4977), and a separate TALEN that recognizes a point mutation (m.14459G > A in the MT-ND6 gene) in an osteosarcoma cybrid cell model (Bacman et al., 2013). The TALEN markedly reduced the level of deleted mtDNA species allowing compensatory mitochondrial biogenesis to preferentially restore wild-type mtDNA (Bacman et al., 2013). In studies with the point mutation, relative specificity was demonstrated for the mutant over the wild-type sequence (Bacman et al., 2013). Interestingly the heteroplasmy shift persisted after the TALENs were no longer detectable, suggesting long-lasting effects after a single treatment (Bacman et al., 2013). However, the impact of the rapid reduction in mtDNA copy number following TALEN treatment raises serious concerns about their potential toxicity, not least because several fatal mitochondrial diseases are caused by mtDNA depletion. However, the co-induction of mitochondrial biogenesis could circumvent this issue, but will need careful evaluation in animal models before any clinical studies.
The most recent addition to this group of endonucleases is Cas9 nuclease. Unlike the ZFN and TALE proteins that target the FokI endonuclease, Cas9 is targeted using a much smaller short RNA sequence CRISPR (Hsu et al., 2014). This RNA sequence can more easily be altered to change target site than for TALEs and distinct RNA sequences can be used target Cas9 to induce multiple double-stranded DNA breaks (Hsu et al., 2014; Cox et al., 2015). Furthermore this system is not hindered by context-specific binding reported for ZFNs and TALENs (Hsu et al., 2014; Cox et al., 2015). Although there has been controversy surrounding this approach, recently Cas9/CRISPR has been demonstrated to restore the weight loss phenotype of a mouse model of fatal disease hereditary tyrosinaemia type 1 (Yin et al., 2014).
The potential use of restriction endonucleases to prevent germline transmission has also been investigated. MitoTALENs have been demonstrated to reduce levels of human mutated mtDNA responsible for Leber hereditary optic neuropathy (LHON) and NARP (neurogenic muscle weakness, ataxia, and retinitis pigmentosa) in oocytes (Reddy et al., 2015).
Peptide nucleic acids
Sequence-specific peptide nucleic acids selectively bind mutant mtDNA and induce direct mtDNA strand degradation (Mukherjee et al., 2008). Although conjugation with mitochondrial targeting peptides promotes importation and successful targeting into human cells in culture (Chinnery et al., 1999), this initially failed to modulate heteroplasmy in patient-derived cell lines (Kyriakouli et al., 2008). In part this is due to a failure to transmit the peptide nucleic acids across the mitochondrial membrane, so techniques of traversing the cell membrane are being explored, such as ‘cell membrane crossing oligomers’ (Kyriakouli et al., 2008), which possess greater polarity, and RNA vectors (Comte et al., 2013). Although these examples show promise, evidence for therapeutic benefit remains sparse despite nearly two decades of research. Targeted delivery will be a common problem, and potential toxic effects need to be excluded in long-term animal studies. Again, limited availability of animal models of human mtDNA diseases has hindered progress.
Manipulating tRNAs
The large array of pathogenic mtDNA mutations affecting tRNA genes prompted the exploration of tRNA-targeted therapies (Yarham et al., 2011). Of particular interest are tRNA synthetases that catalyse the addition of specific amino acid molecules to cognate tRNA molecules during protein translation. Early work demonstrated that the overexpression of cognate aminoacyl mt-tRNA synthetase stabilized mt-tRNA (mitochondrial tRNA) molecules (Rorbach et al., 2008). More recently overexpression of human non-cognate mitochondrial leucyl tRNA synthetase and its small carboxy-terminal domain were shown to partially rescue the biochemical dysfunction secondary to mt-tRNA defects (Hornig-Do et al., 2014; Perli et al., 2014).
Gene transfer using adeno-associated viral vectors
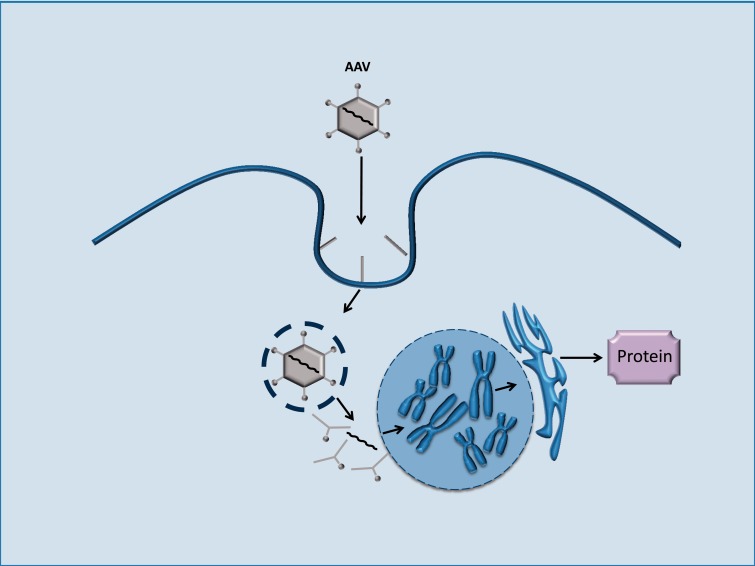
Harnessing viral vectors to transfer wild-type genes into the cell nucleus dates back to the late 1990’s. Although initial excitement was tempered by the death of a patient in an early clinical trial (Raper et al., 2003), much progress has been made in developing new, less immunogenic vectors such as AAV (Wang et al., 2012) (Fig. 3).
Figure 3.
Adeno-associated viral vectors expressing wild-type gene constructs. Gene constructs can be introduced into host cells by AAV and transcribed within the nucleus of the host. The end product is a functional protein, which can replace or bypass dysfunctional proteins resulting from mutations in the host’s nDNA or mtDNA.
Gene therapy for nuclear-mitochondrial disorders
Ethylmalonic encephalopathy classically has heterogeneous phenotypes with neurological, gastrointestinal, metabolic and psychiatric sequelae (Tiranti et al., 2004) and results from mutations in the ETHE1 gene (Tiranti et al., 2004). ETHE1 encodes a ubiquitous mitochondrial sulphur dioxygenase that detoxifies hydrogen sulphide (Tiranti et al., 2004). AAV-mediated hepatic ETHE1 gene expression restores sulphur dioxygenase activity in a murine model of ethylmalonic encephalopathy, correcting biochemical abnormalities within liver, muscle, and brain; and increasing survival from a few weeks to more than 6 months (Di Meo et al., 2012). However, targeting human skeletal muscle and other large tissues is extremely challenging, given currently achievable viral titres. Targeting the relatively impenetrable blood–brain barrier presents another hurdle in the treatment of nuclear-encoded mitochondrial diseases. However, there are exceptions where the approach has real potential for therapeutic benefit in the near future.
Mitochondrial neurogastrointestinal encephalopathy (MNGIE) is a rare defect of the thymidine phosphorylase encoding gene TYMP (Nishigaki et al., 2003). Mutations in TYMP cause high circulating levels of thymidine, which in turn lead to mtDNA depletion and the formation of secondary mtDNA deletions and point mutations. Treating a single organ, such as the liver, has been proposed to generate enough enzyme to ‘detoxify’ the systemic circulation and thereby prevent disease progression (Torres-Torronteras et al., 2014). In keeping with this idea, the permanent and dose-dependent reduction of systemic toxic metabolite levels in a murine model of MNGIE has been demonstrated with the construct, AAV2/8-hcTYMP, which possesses a liver-specific promoter (Torres-Torronteras et al., 2014). Interestingly, recent evidence that the liver can synthesize potentially therapeutic thymidine phosphorylase levels has prompted the suggestion that liver transplantation would be an effective therapy for MNGIE (Boschetti et al., 2014).
The Harlequin mouse model of mitochondrial complex 1 deficiency results from a pro-viral X-linked gene insertion causing severely reduced apoptosis-inducing factor 1 (AIF1) expression. These mice exhibit retinal and optic nerve respiratory chain complex 1 deficiency causing glial and microglial cell activation, retinal ganglion loss and optic atrophy. The intra-vitreal administration of an AAV2/2_AIF1 vector has been shown to counteract these changes (Bouaita et al., 2012).
Finally, mutations in the MPV17 gene result in a hepatocerebral mtDNA depletion syndrome that is also potentially amenable to gene therapy (Bottani et al., 2014). MPV17 knockout (KO) mice exhibit hepatic mtDNA depletion and liver failure when subjected to a ketogenic diet (Bottani et al., 2014). This phenotype is rescued by AAV viral vector expression of human MPV17 cDNA with a hepatic specific promoter (Bottani et al., 2014). However, it is unclear whether the tissue-specific expression of the transgene will correct the cerebral disorder. This is critical because the CNS features of the disorder are fatal.
Gene therapy for mitochondrial DNA disorders
Delivering gene therapy into mitochondria presents an even greater challenge. Many cells vulnerable to mitochondrial disease contain thousands of mitochondria, and the mitochondrial membrane is relatively impermeable. There is little evidence that AAVs penetrate into the mitochondrial matrix, so achieving the high titres required to intercalate with multiple copies of mtDNA seems almost insurmountable at present. An alternative approach is to harness well-established techniques for nuclear gene therapy to ‘allotopically express’ mtDNA encoded proteins. If carefully engineered, and with a targeting peptide presequence, this has the potential to deliver wild-type proteins to the mitochondrial membrane.
After early in vitro work expressing the subunits of the mitochondrial ATPase (Bokori-Brown and Holt, 2006), several laboratories have focused on the allotropic expression of complex I subunits with a view to treating the most common mtDNA disorder: LHON. The mtDNA m.11778G > A mutation affects nicotinamide adenine dinucleotide (NADH) dehydrogenase subunit 4 (ND4 of complex 1, and accounts for ∼50% of LHON patients). Not surprisingly, this mutation has been the focus for most of the studies to date. In vitro and preclinical work (Guy et al., 2002; Qi et al., 2007; Ellouze et al., 2008; Yu et al., 2012a,b) provide some evidence base for this approach, and preparations for early phase clinical (Lam et al., 2010; Bin, 2015; Vignal, 2015) studies are well under way in the USA, China and Europe.
The allotopic expression of a synthetic ND4 gene is reported to increase ATP synthesis in a human cell harbouring the m.11778G > A mutation (Guy et al., 2002). More recently, in murine LHON models (Qi et al., 2007; Ellouze et al., 2008; Yu et al., 2012a,b), intraocular injection of human nuclear ND4 gene constructs expressed by AAV has been shown to be safe (Ellouze et al., 2008; Yu et al., 2012a); non-mutagenic to host mtDNA (Yu et al., 2013); prevent retinal ganglion cells loss; and improve optic atrophy and vision (Ellouze et al., 2008; Yu et al., 2012a). However, as explored further below, this contradicts the longstanding evidence of interspecies protein incompatibility (McKenzie et al., 2003; Perales-Clemente et al., 2011). Furthermore, although the allotopically-expressed proteins appear to be imported, it is not clear whether the new peptides integrate within the respiratory chain (Figueroa-Martinez et al., 2011). Indeed, one study demonstrated that mitochondrially-encoded NADH (reduced nicotinamide adenine dinucleotide) dehydrogenase (with an additional mitochondrial transduction domain) was expressed in the nucleus and partially rescued the phenotype of cells with a homoplasmic knockout mutation for mt-Nd6 (Perales-Clemente et al., 2011). However, the expressed protein seemed to lie largely outside the outer mitochondrial membrane (Perales-Clemente et al., 2011). Despite these concerns, groups in Miami (Lam et al., 2010) and China (Bin, 2015) are currently recruiting for a trial to examine intraocular AAV-ND4 for LHON. A further safety trial is currently recruiting in France (Vignal, 2015).
Expression of non-mammalian genes
There is interest in using AAV expressing mature yeast complexes (not troubled by issues of integration) to bypass mutations in multi-subunit mammalian equivalents. This ‘transkingdom approach’ has been shown to preserve retinal ganglion cells, improve optic nerve function and improve vision in a rotenone-induced LHON rodent model (Marella et al., 2010; Chadderton et al., 2013). Lentiviral vectors expressing a yeast alternative oxidase have also been demonstrated to rescue COX (cytochrome c oxidase) deficiency in a mouse model (El-Khoury et al., 2013).
Despite the emerging evidence supporting transkingdom protein expression, it remains a contentious issue. The co-evolution of nDNA and mtDNA has led to species-specific genome compatibility (Kenyon and Moraes, 1997). Incompatibilities between nDNA and mtDNA explain why Human-Gorilla xenomitochondrial cybrids have a respiratory chain deficiency (Barrientos et al., 1998), and incompatibles of interspecies mtDNA gene products (McKenzie et al., 2003; Perales-Clemente et al., 2011), suggest that caution is needed with this approach, which is very much at the early preclinical phase.
Improving mitochondrial DNA copy number in mitochondrial DNA depletion syndromes
In vitro and preclinical evidence support the therapeutic potential of increasing deoxyribonucleotides to treat rate autosomal recessive mtDNA depletion syndromes. One approach involves deoxyribonucleoside supplementation, which increased mtDNA levels (copy number) in primary cell cultures from patients with deoxyguanosine kinase deficiency due to DGUOK mutations (Bulst et al., 2009; Camara et al., 2014). A similar effect has also been seen in an in vitro model of MNGIE (Camara et al., 2014). Another strategy involves inhibition of deoxyribonucleoside catabolism, which has also been shown to improve mtDNA copy number in vitro (Camara et al., 2014). More recently, deoxyribonucleoside supplementation in a knock-in mouse model of thymidine kinase 2 (TK2) deficiency increased mtDNA copy number, improved mitochondrial respiratory chain function, and prolonged life span (Garone et al., 2014).
New protein delivery
Systemic protein delivery
The identification of a specific protein dysfunction or deficiency resulting in mitochondrial disease has led to the possibility of protein replacement or the removal of accumulated toxic metabolites.
In MNGIE, haemodialysis has been used to remove toxins (Spinazzola et al., 2002) and the transfusion of platelets (Lara et al., 2006) or erythrocyte-encapsulated thymidine phosphorylase (EE-TP) has been explored as a means of delivering exogenous thymidine phosphorylase (Table 2) (Moran et al., 2008; Bax et al., 2013; Hussein, 2013). At present there is no convincing evidence of sustained clinical benefit from either approach. However, drawing on experience from other inborn errors of metabolism such as adenosine deaminase deficiency (Bax et al., 2007), direct enzyme replacement could proceed into early phase studies in the short to medium term. Indeed, a recent preclinical trial has demonstrated adequate safety of recombinant EE-TP in mice and beagle dogs (Levene et al., 2013). However, the reported antibody generation (Levene et al., 2013) may preclude long-term use, and based on experience with other rare inherited enzyme defects, this approach is likely to be extremely expensive.
Table 2.
New protein delivery to treat MNGIE
| Reference | Age/gender | Intervention | Outcome measures | Outcome | Reported adverse events |
|---|---|---|---|---|---|
| Bax et al., 2013 | M/28 | Encapsulated TP | Plasma dThd and dUrd levels between cycles | Thd and dUrd levels reduced to 8.1 and 12.6 mmol/l from 20.5 and 30.6 mmol/l, respectively after 27 cycles. | Mild transient reaction to infusion with coughing and head and neck erythema. |
| Urinary dThd and dUrd levels between cycles | Urinary dThd and dUrd levels reduced to 192–282 and 0.0–184 mmol/24 from 421 and 324 mmol/24 h, respectively from cycle 21. | ||||
| Plasma creatine kinase | Creatine kinase level reduced from 1200 U/l pre-therapy to 254 U/l (normal range 40–320 U/l) at 23 months. | ||||
| Clinical condition | Increase in MRC power sum score 56 (baseline) to 74 (23 months post-infusion). Improvements in gait, balance, sensory ataxia and finger dexterity. Weight increased from 57.4 kg (baseline) to 61.2 kg (post-infusion). No change in EMG or nerve conduction studies. Patient self reported an increased walking distance from 1 to 10 km. | ||||
| Moran et al., 2008 | F/21 | Encapsulated TP | Plasma dThd and dUrd levels | 3 days post-infusion plasma dThd and dUrd levels reduced (but not to within normal range) but then began to rise. | |
| Urinary dThd and dUrd levels | 3 days post-infusion urinary dThd and dUrd levels fell to 6% and 13% of the pre-therapy values respectively (these values were still not within normal range). | None reported. | |||
| Clinical condition | Clinical condition remained poor and the patient died 21 days post-infusion. |
Systemic protein delivery: thymidine phosphorylase (TP) encapsulated in erythrocytes. Thymidine phosphorylase can be encapsulated in autologous blood erythrocytes by reversible hypo-osmotic dialysis, these erythrocytes can then used for infusion to increase the functional thymidine phosphorylase levels of the recipient. Results from two case reports of infusion of thymidine phosphorylase encapsulated in erythrocytes for patients with MNGIE demonstrated initial reductions in toxic product levels post transplantation and some reported clinical improvement. MRC = Medical Research Council; dTHd = thymidine; dURD = deoxythymidine.
Table 1.
Exogenous stem cell therapy for nuclear gene mutations in MNGIE
| Reference | Age/Gender | Intervention | Outcome measures | Outcome | Reported adverse events |
|---|---|---|---|---|---|
| Hussein 2013 | Patient 1: M/24 | HLA fully matched BMT | TP activity | TP activity initially rose from 1 to 180 nmol/h/mg/protein but then dropped to 10 nmol/h/mg/protein 10 months post-transplantation. | GVHD |
| dThd and dUrd levels | Normalized. | ||||
| Clinical condition | Subjective improvement in walking, hearing, abdominal pain, dysphagia, vomiting and diarrhoea and weight gain 3 months post-transplantation. No improvement in peripheral neuritis or foot drop. | ||||
| Filosto et al., 2012 | Patient 1: F/34 | HLA matched HSCT | TP activity | Patient 1 and 2: TP activity rose from 0 nmol/h/mg/protein to within normal range. | Patient 1: Pyrexia of unknown origin, hyperglycaemia, pancreatitis, CMV reactivation, GVHD and complications of immunodeficiency. |
| Patient 2: F/22 | Clinical condition | Patient 1: no improvement in neurological assessment, nerve conduction studies or gastrointestinal symptoms. Patient died 15 months post-transplantation. | Patient 2: mild GVHD. Posterior reversible encephalopathy syndrome and C. difficile diarrhoea, followed by septic shock and ARDS. | ||
| Patient 2: reduced gastrointestinal discomfort but no improvement in neurological symptoms. Died 8 months post-transplantation. | |||||
| Sicurelli et al., 2012 | Patient 1: F/23 | HLA matched HSCT | TP activity | TP activity rose from 0 nmol/h/mg/protein to within normal range. | Worsening of sensory neuropathy. |
| dThd and dUrd levels | dThd and dUrd levels normalized. | ||||
| Blood lactate | Blood lactate decreased from 2.3 to 1.5 mmol/l. | ||||
| Clinical condition | Resolution of diarrhoea, vomiting and pain and fatigability 1 month post-transplantation. MRC muscle strength score increased by 16 points and proximal motor conduction velocities improved 12 months post-transplantation. Sensory neuropathy worsened and MRI defined leukoencephaolopathy was unchanged. | ||||
| Hirano et al., 2006 | Patient 1: F/21 | HLA matched placental cord blood transplant | TP activity | Patient 1 and 2: TP activity increased to peaks of 104 and 165 nmol/h/mg/protein after 1-2 months in patient 1 and 2 respectively but then decreased. | Patient 1: non-engraftment. |
| Patient 2: F/30 | HLA matched related SCT | dThd and dUrd levels | Patient 1 and 2: dThd and dUrd levels were reduced in both patients and to within normal range for patient 2 after 2 months. | ||
| Clinical condition | Patient 1: No symptomatic improvement was documented. Died 86 days Post-transplantation from disease progression. | ||||
| Patient 2: subjective improvement in abdominal pain, swallowing and distal limb numbness 6.5 months post-transplantation. Biceps and ankle reflexes returned | |||||
| Lenoci et al., 2011 (abstract only) | Patient 1: F/21 | HLA matched related HSCT | TP activity | TP activity normalized 55 days post-transplantation. | None reported. |
| dThd and dUrd levels | dThd and dUrd levels decreased. | ||||
| Clinical condition | Subjective improvement in vomiting, diarrhoea and abdominal pain with increase in body weight. No improvement in neurological symptoms was documented. | ||||
| Hill et al., 2009 | Patient 1: F/41 | HLA matched related peripheral blood SCT | TP activity | TP activity normalized by day 25 post-transplantation and was still normal 14 months post-transplantation. | Idiopathic thrombocytopenic purpura and gastrointestinal haemorrhage. |
| Clinical condition | Improvement in oral intake and increase in weight. Neurological performance status, repeat EMG and cerebral MRI scan were unchanged. |
A series of case reports of stem cell therapies in eight patients with MNGIE showed variable results. Overall thymidine phosphorylase activity tended to increase post-transplantation and some improvements in gastrointestinal and neurological symptoms were reported.
ARDS = acute respiratory distress syndrome; BMT = bone marrow transplant; CMV = cytomegalovirus; EMG = electromyogram; F = female; GVHD = graft versus host disease; HLA = human leukocyte antigen; HSCT = haematopoietic stem cell transplantation; M = male; MRC = Medical Research Council; SCT = stem cell transplant; TP = thymidine phosphorylase; dTHd = thymidine; dURD = deoxythymidine.
Cellular and mitochondrial protein delivery
Delivering proteins across the mitochondrial membrane is challenging because of their hydrophobicity, but harnessing endogenous mitochondrial import machinery provides a solution.
The mitochondrial protein TFAM (transcription factor A, mitochondrial) is important for mtDNA expression and replication (Iyer et al., 2009). Treating LHON cybrids with mitochondrially-targeted TFAM increased respiratory chain protein levels and enhanced cellular respiration (Iyer et al., 2009). Furthermore, the systemic injection of this construct into mice increased aerobic respiration in muscle and brain, and improved overall motor endurance (Iyer et al., 2009). However, caution is needed when considering translation into human trials because transgenic mice overexpressing TFAM show increased mtDNA copy number, mtDNA deletions and respiratory chain deficiency—all of which could lead to long-term toxicity (Ylikallio et al., 2010). This work highlights the difficulty in interpreting the results from different cellular and animal models that can be misleading. Studying a new treatment in a range of cellular and animal systems will reduce the chance of inappropriately rejecting a treatment based on adverse effects in one model. On the other hand, studying more than one model will reduce the chance of pursuing a drug that will never make it into clinical use.
Small molecule pharmaceuticals
Future pharmaceutical development focusing on disease-specific or patient-specific molecular targets aimed at boosting residual mitochondrial function is likely to be more successful than previous approaches, which were generally based on a non-specific bypass or amelioration of defective components of the respiratory chain.
Systematic screening of small molecules has recently increased in popularity, in part driven by the widespread availability of patient cell lines, and technological advances in medium-to-high throughput screening (Golubitzky et al., 2011; Pfeffer et al., 2013; Soiferman et al., 2014). Although the complexity of the diverse genotype–phenotype relationship still complicates drug discovery (Macmillan et al., 1993; Wallace, 1999; Wang et al., 1999; Koopman et al., 2012), this could be circumvented by screening a panel of drugs on specific patient cell lines, leading to a personalized or precision medicine approach (Koopman et al., 2012), which can be optimized for testing in animals models then clinical studies (Koopman et al., 2012). So called ‘N-of-one’ clinical trials provide one way of moving forward in patients, proving the drugs have a measurable effect in a short time period.
Induction of mitochondrial biogenesis
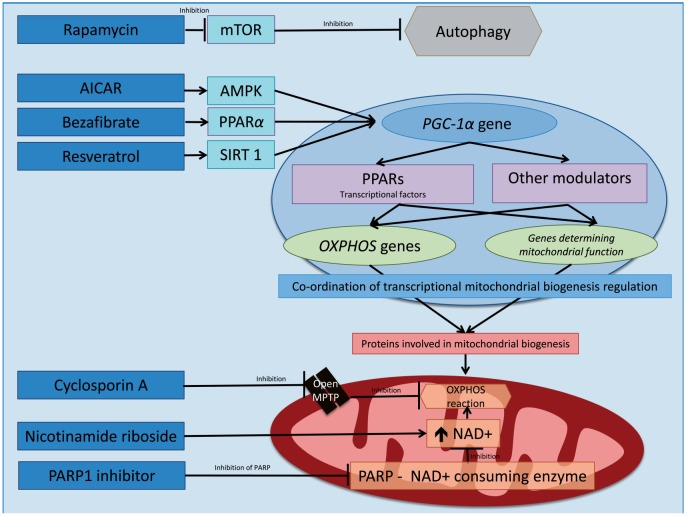
Bezafibrate
Several studies have focussed on peroxisome proliferator-activated receptor (PPAR) gamma co-activator 1 alpha (PGC-1α, encoded by PPARGC1A) as a potential treatment for mitochondrial disease. PGC-1α co-ordinates the vast majority transcriptional mitochondrial biogenesis regulation in accordance with metabolic demand (Scarpulla et al., 2012). The array of novel pharmaceuticals discussed below either modulate PCG-1α mtDNA or protein expression or target its downstream pathways (Fig. 4). Bezafibrate is pan-agonist for the PPAR family of transcriptional factors. PPAR-α activation promotes mitochondrial biogenesis by upregulating PPARGC1A gene expression (Kanabus et al., 2014). Although bezafibrate was reported to increase COX activity and ameliorate the disease phenotype in a muscle-specific Cox10 knockout mouse (Wenz et al., 2008), this observation has been retracted. Bezafibrate was not effective in two other murine models of COX deficiency Surf1 knockout (Viscomi et al., 2011) or Deletor mice (Yatsuga and Suomalainen, 2012). Bezafibrate induced hepatomegaly and abnormal lipid metabolism is reported in Surf1 knockout (Viscomi et al., 2011) and Deletor mice (Yatsuga and Suomalainen, 2012). Importantly this does not appear to occur at comparable doses in humans.
Figure 4.
Schematic representation of pharmaceutical modulators of mitochondrial biogenesis. There are multiple signalling pathways involved in mitochondrial biogenesis. PGC-1α (encoded by PPARGC1A), which is a co-activator for a family of transcriptional factors known as PPARs, co-ordinates via a cascade of nuclear encoded proteins the vast majority transcriptional mitochondrial biogenesis. Novel pharmacological therapies aim to modulate PCG-1α mtDNA expression (e.g. PPARα) and protein expression or target downstream pathways. Bezafibrate is pharmacological ligand for the transcriptional co-factor PGC-1α. AICAR activates AMP-activated protein kinase (AMPK) and is thought to modulate increased mitochondrial biogenesis through PGC-1α. The natural polyphenol resveratrol activates sirtuin 1 (SIRT1). Sirtuins are part of a group of oxidizing NAD-dependent protein deacetylases. Upon activation, for example, by PGC-1α or transcription factor A, mitochondrial (TFAM) they promote mitochondrial respiratory chain activities and the transcription of genes modulating mitochondrial biogenesis and function. Nicotinamide riboside can be used to supplement NAD+ levels. PARP1 functions as a NAD+ consuming enzyme. Thus in turn inhibition of PARP1 has been demonstrated to increase NAD+ bioavailability and SIRT1 activity (not shown above) promoting oxidative phosphorylation. Rapamycin inhibits mTOR, which in turn releases mTOR inhibition of autophagy. Cyclosporin A inhibits the mitochondrial permeability transition pore (MPTP). Opening of the mitochondrial permeability transition pore is thought to deplete pyridine nucleotides thus impairing mitochondrial oxidative respiration.
AICAR
5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) is an adenosine monophosphate (AMP) analogue (Merrill et al., 1997) causing AMPK to initiate catabolic mechanisms to produce ATP and inhibit anabolic processes (Canto and Auwerx, 2009), promote PPARGC1A gene transcription (Terada et al., 2002) and PGC-1α activity by direct phosphorylation (Jager et al., 2007). AICAR has been shown to increase mitochondrial biogenesis and ATP production alongside decreasing reactive oxygen species human complex I deficient fibroblasts (Golubitzky et al., 2011). AICAR also causes partial correction of COX defects in mice (Viscomi et al., 2011). The low apparent toxicity in humans supports its further investigation (Dixon et al., 1991), although some caution is needed as increased liver weight is suggested with prolonged use in mice (Winder et al., 2000).
Resveratrol
Resveratrol is a natural polyphenol, known to increase NAD+ (oxidized nicotinamide adenine dinucleotide) levels, which activate the protein deacetylase SIRT1 (Kanabus et al., 2014). SIRT1 activates PGC-1α by deacetylation sparking a chain of signalling pathways promoting mitochondrial biogenesis and function (Canto and Auwerx, 2009; Kanabus et al., 2014).
Resveratrol activation of SIRT1 has been demonstrated to restore normal function in human fibroblasts with inborn errors of mitochondrial fatty acid β-oxidation (Bastin et al., 2011). Such preclinical data have prompted investigation of resveratrol for diseases with mitochondrial pathogenesis such as Friedreich’s ataxia (Delatycki, 2012; Yiu et al., 2013).
A recently completed open-label clinical pilot study investigating resveratrol in Friedrich’s ataxia has presented preliminary data (full publication is awaited) showing improvement in disease severity and oxidative stress markers (Delatycki, 2012; Yiu et al., 2013). Resveratrol is reported to have only mild gastrointestinal side effects (Patel et al., 2011).
Modulating NAD+ bioavailability
The balance between NAD+ and NADH is crucial to the process of oxidative phosphorylation. With increased NAD+ bioavailability enhancing oxidative phosphorylation. Indeed NAD+ supplementation with nicotinamide riboside has produced promising biochemical and clinical improvements in two mitochondrial myopathy murine models; a nuclear gene (Sco2) knockout/knockin mouse (Cerutti et al., 2014), and the Deletor mouse possessing a nuclear gene mutation resulting in mtDNA deletions (Khan et al., 2014).
PARP inhibitors
Inhibition of the NAD+-consuming enzyme poly ADP (adenosine diphosphate-ribose) polymerase 1 (PARP1) increases NAD+ bioavailability, SIRT1 activity and subsequently oxidative metabolism (Bai et al., 2011). There is convincing evidence from murine models of myopathy (Cerutti et al., 2014) and mitochondrial encephalopathy (Felici et al., 2014), of the potential benefit of PARP inhibition in human mitochondrial disease. Toxicity is also reported to be low (Tutt et al., 2010).
Inhibiting cytosolic translation and autophagy
During starvation states damaged cellular components are degraded to maintain cellular activity and viability (Kim et al., 2011). This process termed autophagy is inhibited by mammalian target of rapamycin (mTOR) (Kim et al., 2011).
Administration of the mTOR inhibitor rapamycin in a murine model of Leigh syndrome delayed the onset of symptoms, reduced neuroinflammation and prevented brain lesions, leading to an increased life span (Johnson et al., 2013). Although the safety profile and clinical use of rapamycin is well documented, the associated immunosuppressive effects, reduced wound healing and hyperlipidaemia may limit its use for mitochondrial disease (Johnson et al., 2013). In keeping with this, recent evidence in patient cell lines also showed benefit from the partial inhibition of intra-mitochondrial translation using cycloheximide, and the inhibition of autophagy by lithium chloride (Peng et al., 2015).
Inhibiting opening of the mitochondrial permeability transition pore
Opening of the mitochondrial permeability transition pore (PTP) is thought to deplete pyridine nucleotides thus impairing mitochondrial oxidative respiration. Cyclosporin A inhibits the mitochondrial PTP and has been shown to induce muscle regeneration, improve mitochondrial function, and suppress muscle apoptosis (Merlini et al., 2008) in both a murine model of collagen VI-deficiency myopathy and five patients with the same disorder. These findings lend support to the use of cyclosporin A in other mitochondrial disorders such as LHON, where a clinical trial is currently recruiting patients (Milea, 2014). However, like rapamycin, this widely used immunosuppressant may have a non-acceptable side effect profile in chronic use.
Stem cell approaches
Substantial preclinical, and increasing clinical evidence exists to support the use of stem cell therapies in several neurological disorders where mitochondrial dysfunction plays a role (e.g. Parkinson’s disease) (Lunn et al., 2011). The proposed therapeutic mechanisms of stem cells are summarized in Fig. 5 (Parr et al., 2007; Kemp et al., 2010, 2014). Although not specific to mitochondrial dysfunction, harnessing these approaches could be of therapeutic benefit. Unfortunately the relative lack of animal models for primary mitochondrial disorders (Farrar et al., 2013) has limited investigation of stem cell therapies for mtDNA disease.
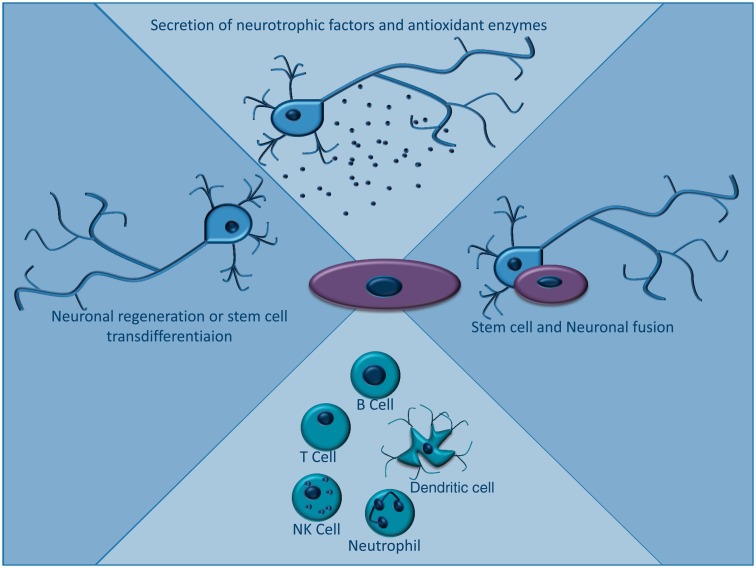
Figure 5.
Mechanism of action of stem cell therapies. Various mechanisms have been described for the therapeutic action of stem cells for neurodegenerative conditions. These include secretion of neurotrophic factors and antioxidant enzymes such as superoxide dismutase, modulation of the immune system, regeneration of neurons and more controversially, stem cell transdifferentiation into neurons. Recently mesenchymal stem cells have been demonstrated to fully fuse with native cells to form heterokaryons or partially fuse via junction formation and transfer cellular organelles and factors.
Exogenous stem cell therapy for nuclear DNA mutations
A number of case reports and small case series, discussed below, have described the effects of allogenic stem cell treatment in patients with MNGIE (Table 2) (Hirano et al., 2006; Hill et al., 2009; Lenoci et al., 2011; Filosto et al., 2012; Sicurelli et al., 2012; Hussein, 2013). The rationale behind the treatment is based on work where haemo/peritoneal dialysis or platelet transfusions reduced circulating levels of toxic thymidine, presumably by introducing cells with normal thymidine phosphorylase activity (Moran et al., 2008; Bax et al., 2013; Hussein, 2013). Restoring thymidine phosphorylase activity in the bone marrow has been shown to reduce toxic thymidine levels post-transplantation, although the results were highly variable (Hirano et al., 2006; Hill et al., 2009; Halter et al., 2011; Lenoci et al., 2011; Filosto et al., 2012; Sicurelli et al., 2012; Hussein, 2013), and there is no convincing evidence that objective clinical benefit was actually achieved.
Interestingly, a recent retrospective analysis of all the 24 patients with MNGIE known to undergo a haematopoietic stem cell transplantation between 2005 and 2011 reported that in the nine survivors, thymidine phosphorylase activity rose from undetectable to normal levels (Halter et al., 2015).
Two trials at Columbia University, USA are currently recruiting; one to map the natural history study of MNGIE (Hirano, 2015a) and the other to asses the safety of stem cell transplant for MNGIE in a proposed 12 patients (Hirano, 2015b). These should help inform future clinical trial design and begin to address safety concerns highlighted by a consensus report published by Halter et al. (2011).
Endogenous stem cells for mitochondrial DNA mutations
There has been ongoing interest in the phenomenon of ‘gene shifting’ as a treatment for mitochondrial myopathy (Clark et al., 1997; Spendiff et al., 2013). In patients with heteroplasmic mtDNA mutations, the muscle satellite cells (myogenic stem cells) typically contain less mutated mtDNA than mature post-mitotic skeletal muscle fibres.
Pharmacological methods and resistance exercise to induce satellite cell proliferation and fusion with mature muscle to deliver wild-type mtDNA into mature muscle (Spendiff et al., 2013) have been demonstrated to reduce mtDNA load in mature muscle (Clark et al., 1997) and improve biochemistry. However, there is currently no evidence of clinical benefit (Andrews et al., 1999). Recent work using somatic cell nuclear transfer adds weight to this approach, showing the correction of the metabolic disturbance with minimal disruption of nuclear-mitochondrial communication using induced pluripotent stem cells, albeit in vitro (Ma et al., 2015).
A series of collaborative trials for patients with mtDNA mutations (comprehensively reviewed) (Kerr, 2013) demonstrated that endurance training increased work capacity and mitochondrial enzyme activity, but did not reduce heteroplasmy. Thus the benefits in this context were unlikely to be attributable to gene shifting and may simply reflect increased oxygen delivery through the development of capillary networks. Moreover, there may be a finite capacity for repair following induction of satellite cell proliferation by these methods; raising concerns about long-term sequelae. Finally, any potential benefits are most likely help the minority of patients with an isolated mitochondrial myopathy. Despite these concerns, there is currently an ongoing crossover trial investigating exercise versus inactivity in a cohort of patients with mitochondrial myopathy (Haller, 2012). This will hopefully provide a definitive answer to the role of exercise training in this context.
Conclusion
The past 5 years have seen several new approaches developing through our understanding of the molecular pathogenesis of mitochondrial diseases. For mtDNA disorders, the early clinical studies attempting to harness gene-shifting some 15 years ago have not really progressed beyond early open labelled studies—probably because the likely clinical impact is limited using current approaches. Although intriguing, other molecular approaches directed against mtDNA disease are very much at the preclinical stage, and will require substantial development to improve efficacy and ensure there is no substantial risk of toxicity before human trials. From a clinical perspective, nuclear-genetic enzyme defects show the greatest promise. Stem cell therapy is already being used in specific contexts, and its efficacy and safety being evaluated, and gene therapy trials in mouse models show clear benefits. Unfortunately, each one of these rare genetic diseases may require their own proprietary approach, and the impact needs to be evaluated long-term. Small molecules are attractive because they have the potential to provide a more generic solution applicable across the mitochondrial disease spectrum, and a greater understanding of cell signalling pathways opens up several unexpected disease targets. For some of these drugs, clinical evaluation is imminent, particularly for those being repurposed or repositioned drugs such as bezafibrate. It is critical at this stage that laboratory and clinical scientists work closely with patient organizations to ensure that the ultimate aims of therapy will actually tackle issues that are important to patients. Given limited resources, this will ensure that new treatments improve quality of life—a prerequisite if these treatments are going to be adopted by healthcare systems worldwide.
Funding
G.P. is the recipient of a Bisby Fellowship from the Canadian Institutes of Health Research D.B. is the recipient of a Kennedy Scholarship. P.F.C is a Wellcome Trust Senior Fellow in Clinical Science (101876/Z/13/Z), and a UK NIHR Senior Investigator, who receives support from the Medical Research Council Mitochondrial Biology Unit (MC_UP_1501/2), the Wellcome Trust Centre for Mitochondrial Research (096919Z/11/Z), the Medical Research Council (UK) Centre for Translational Muscle Disease (G0601943), EU FP7 TIRCON, and the National Institute for Health Research (NIHR) Biomedical Research Centre based at Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Literature search strategy
References for this Review were identified by searches of PubMed for English language articles published between 1970 and September 2015 and references of relevant articles and our personal libraries. The search terms ‘mitochondrial disease’, ‘mitochondrial disorder’, ‘mitochondrial encephalopathy’, ‘Leber’s hereditary optic neuropathy’, ‘mitochondrial neurogastrointestinal encephalopathy’, ‘mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes’, ‘neurogastrointestinal encephalopathy’, ‘myoclonic epilepsy with ragged red fibers’, ‘Kearns Sayre syndrome’, ‘neuropathy, ataxia, and retinitis pigmentosa’, and ‘inborn error metabolism’ were individually combined with ‘treatment’, ‘therapy’, ‘stem cell’, ‘stem cell transplant’, ‘bone marrow transplant’, ‘genome’, ‘genetics’ and ‘gene therapy’ were used. The final reference list was generated based on relevance to the topics covered in this Review.
Glossary
Abbreviations
- AAV =
adeno-associated viral vector
- Cas9 =
clustered regularly interspaced short palindromic repeats associated protein 9
- COX =
cytochrome c oxidase
- LHON =
Leber hereditary optic neuropathy
- MNGIE =
mitochondrial neurogastrointestinal encephalopathy
- PARP =
poly ADP (adenosine diphosphate-ribose) polymerase
- PGC-1α =
peroxisome proliferator-activated receptor gamma coactivator-1-alpha
- PPARs =
peroxisome proliferator-activated receptors
- TALENs =
transcription activator-like effectors nucleases
- ZFN =
zinc finger nuclease
References
- Andrews RM, Griffiths PG, Chinnery PF, Turnbull DM. Evaluation of bupivacaine-induced muscle regeneration in the treatment of ptosis in patients with chronic progressive external ophthalmoplegia and Kearns-Sayre syndrome. Eye 1999; 13: 769–72. [DOI] [PubMed] [Google Scholar]
- Bacman SR, Williams SL, Pinto M, Peralta S, Moraes CT. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat Med 2013; 19: 1111–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 2011; 13: 461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos A, Kenyon L, Moraes CT. Human xenomitochondrial cybrids. Cellular models of mitochondrial complex I deficiency. J Biol Chem 1998; 273: 14210–17. [DOI] [PubMed] [Google Scholar]
- Bastin J, Lopes-Costa A, Djouadi F. Exposure to resveratrol triggers pharmacological correction of fatty acid utilization in human fatty acid oxidation-deficient fibroblasts. Hum Mol Genet 2011; 20: 2048–57. [DOI] [PubMed] [Google Scholar]
- Bax BE, Bain MD, Fairbanks LD, Webster AD, Ind PW, Hershfield MS, et al. A 9-yr evaluation of carrier erythrocyte encapsulated adenosine deaminase (ADA) therapy in a patient with adult-type ADA deficiency. Eur J Haematol 2007; 79: 338–48. [DOI] [PubMed] [Google Scholar]
- Bax BE, Bain MD, Scarpelli M, Filosto M, Tonin P, Moran N. Clinical and biochemical improvements in a patient with MNGIE following enzyme replacement. Neurology 2013; 81: 1269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin L. Safety and efficacy study of rAAV2-ND4 treatment of Leber hereditary optic neuropathy (LHON). China: Huazhong University of Science and Technology; 2015. ClinicalTrials.gov Identifier: NCT01267422. [Google Scholar]
- Bokori-Brown M, Holt IJ. Expression of algal nuclear ATP synthase subunit 6 in human cells results in protein targeting to mitochondria but no assembly into ATP synthase. Rejuvenation Res 2006; 9: 455–69. [DOI] [PubMed] [Google Scholar]
- Boschetti E, D'Alessandro R, Bianco F, Carelli V, Cenacchi G, Pinna AD, et al. Liver as a source for thymidine phosphorylase replacement in mitochondrial neurogastrointestinal encephalomyopathy. PloS One 2014; 9: e96692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottani E, Giordano C, Civiletto G, Di Meo I, Auricchio A, Ciusani E, et al. AAV-mediated liver-specific MPV17 expression restores mtDNA levels and prevents diet-induced liver failure. Mol Ther 2014; 22: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaita A, Augustin S, Lechauve C, Cwerman-Thibault H, Benit P, Simonutti M, et al. Downregulation of apoptosis-inducing factor in Harlequin mice induces progressive and severe optic atrophy which is durably prevented by AAV2-AIF1 gene therapy. Brain 2012; 135: 35–52. [DOI] [PubMed] [Google Scholar]
- Bulst S, Abicht A, Holinski-Feder E, Muller-Ziermann S, Koehler U, Thirion C, et al. In vitro supplementation with dAMP/dGMP leads to partial restoration of mtDNA levels in mitochondrial depletion syndromes. Hum Mol Genet 2009; 18: 1590–9. [DOI] [PubMed] [Google Scholar]
- Camara Y, Gonzalez-Vioque E, Scarpelli M, Torres-Torronteras J, Caballero A, Hirano M, et al. Administration of deoxyribonucleosides or inhibition of their catabolism as a pharmacological approach for mitochondrial DNA depletion syndrome. Hum Mol Genet 2014; 23: 2459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 2009; 20: 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti R, Pirinen E, Lamperti C, Marchet S, Sauve AA, Li W, et al. NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab 2014; 19: 1042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton N, Palfi A, Millington-Ward S, Gobbo O, Overlack N, Carrigan M, et al. Intravitreal delivery of AAV-NDI1 provides functional benefit in a murine model of Leber hereditary optic neuropathy. Eur J Hum Genet 2013; 21: 62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery PF, Hudson G. Mitochondrial genetics. Br Med Bull 2013; 106: 135–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery PF, Taylor RW, Diekert K, Lill R, Turnbull DM, Lightowlers RN. Peptide nucleic acid delivery to human mitochondria. Gene Ther 1999; 6: 1919–28. [DOI] [PubMed] [Google Scholar]
- Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, et al. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 2010; 186: 757–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KM, Bindoff LA, Lightowlers RN, Andrews RM, Griffiths PG, Johnson MA, et al. Reversal of a mitochondrial DNA defect in human skeletal muscle. Nat Genet 1997; 16: 222–4. [DOI] [PubMed] [Google Scholar]
- Comte C, Tonin Y, Heckel-Mager AM, Boucheham A, Smirnov A, Aure K, et al. Mitochondrial targeting of recombinant RNAs modulates the level of a heteroplasmic mutation in human mitochondrial DNA associated with Kearns Sayre Syndrome. Nucleic Acids Res 2013; 41: 418–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DB, Platt RJ, Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med 2015; 21: 121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatycki MA. Study of resveratrol as treatment for Friedreich ataxia. Australia: Murdoch Childrens Research Institute; 2012. ClinicalTrials.gov identifier: NCT01339884. [Google Scholar]
- Di Meo I, Auricchio A, Lamperti C, Burlina A, Viscomi C, Zeviani M. Effective AAV-mediated gene therapy in a mouse model of ethylmalonic encephalopathy. EMBO Mol Med 2012; 4: 1008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R, Gourzis J, McDermott D, Fujitaki J, Dewland P, Gruber H. AICA-riboside: safety, tolerance, and pharmacokinetics of a novel adenosine-regulating agent. J Clin Pharmacol 1991; 31: 342–7. [DOI] [PubMed] [Google Scholar]
- El-Khoury R, Dufour E, Rak M, Ramanantsoa N, Grandchamp N, Csaba Z, et al. Alternative oxidase expression in the mouse enables bypassing cytochrome c oxidase blockade and limits mitochondrial ROS overproduction. PLoS Genet 2013; 9: e1003182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BL, Hirsch ML, Porter SN, Samulski RJ, Porteus MH. Zinc-finger nuclease-mediated gene correction using single AAV vector transduction and enhancement by Food and Drug Administration-approved drugs. Gene Ther 2013; 20: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellouze S, Augustin S, Bouaita A, Bonnet C, Simonutti M, Forster V, et al. Optimized allotopic expression of the human mitochondrial ND4 prevents blindness in a rat model of mitochondrial dysfunction. Am J Hum Genet 2008; 83: 373–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar GJ, Chadderton N, Kenna PF, Millington-Ward S. Mitochondrial disorders: aetiologies, models systems, and candidate therapies. Trends Genet 2013; 29: 488–97. [DOI] [PubMed] [Google Scholar]
- Felici R, Cavone L, Lapucci A, Guasti D, Bani D, Chiarugi A. PARP inhibition delays progression of mitochondrial encephalopathy in mice. Neurotherapeutics 2014; 11: 651–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Martinez F, Vazquez-Acevedo M, Cortes-Hernandez P, Garcia-Trejo JJ, Davidson E, King MP, et al. What limits the allotopic expression of nucleus-encoded mitochondrial genes? The case of the chimeric Cox3 and Atp6 genes. Mitochondrion 2011; 11: 147–54. [DOI] [PubMed] [Google Scholar]
- Filosto M, Scarpelli M, Tonin P, Lucchini G, Pavan F, Santus F, et al. Course and management of allogeneic stem cell transplantation in patients with mitochondrial neurogastrointestinal encephalomyopathy. J Neurol 2012; 259: 2699–706. [DOI] [PubMed] [Google Scholar]
- Gammage PA, Rorbach J, Vincent AI, Rebar EJ, Minczuk M. Mitochondrially targeted ZFNs for selective degradation of pathogenic mitochondrial genomes bearing large-scale deletions or point mutations. EMBO Mol Med 2014; 6: 458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garone C, Garcia-Diaz B, Emmanuele V, Lopez LC, Tadesse S, Akman HO, et al. Deoxypyrimidine monophosphate bypass therapy for thymidine kinase 2 deficiency. EMBO Mol Med 2014; 6: 1016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubitzky A, Dan P, Weissman S, Link G, Wikstrom JD, Saada A. Screening for active small molecules in mitochondrial complex I deficient patient's fibroblasts, reveals AICAR as the most beneficial compound. PloS One 2011; 6: e26883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Qi X, Pallotti F, Schon EA, Manfredi G, Carelli V, et al. Rescue of a mitochondrial deficiency causing Leber Hereditary Optic Neuropathy. Ann Neurol 2002; 52: 534–42. [DOI] [PubMed] [Google Scholar]
- Haller R. The effects of exercise vs. inactivity on people with mitochondrial muscle diseases. University of Texas Southwestern Medical Center Dallas, United States of America. ClinicalTrials.gov Identifier NCT00457314. 2012. https://clinicaltrials.gov/ct2/show/NCT00457314.
- Halter J, Schupbach WMM, Casali C, Elhasid R, Fay K, Hammans S, et al. Allogeneic hematopoietic SCT as treatment option for patients with mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): a consensus conference proposal for a standardized approach. Bone Marrow Transplant 2011; 46: 330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halter JP, Schüpbach WMM, Mandel H, Casali C, Orchard K, Collin M, et al. Allogeneic hematopoietic stem cell transplantation for mitochondrial neurogastrointestinal encephalomyopathy. Brain 2015; 138: 2847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KS, Richardson D, Hammans S, Stroud M, Fine D, Walker V, et al. Successful allogeneic peripheral blood stem cell transplantation from an HLA-identical sibling in a patient with mitochondrial neurogastrointestinal encephalomyelopathy. Bone Marrow Transplant 2009; 43: S184–5. [Google Scholar]
- Hirano M. The Natural History Study of Mitochondrial NeuroGastroIntestinal Encephalopathy (MNGIE) (NAHIM). USA: Columbia University and National Institute of Neurological Disorders and Stroke (NINDS; ); 2015a. ClinicalTrials.gov Identifier: NCT01694953. [Google Scholar]
- Hirano M, Marti R, Casali C, Tadesse S, Uldrick T, Fine B, et al. Allogeneic stem cell transplantation corrects biochemical derangements in MNGIE. Neurology 2006; 67: 1458–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano MNGIE, Allogeneic Hematopoietic. Stem Cell Transplant Safety Study (MASS), Columbia University, New York City, United States of America. ClinicalTrials.gov Identifier: NCT02427178. 2015b. https://clinicaltrials.gov/ct2/show/NCT02427178
- Hornig-Do HT, Montanari A, Rozanska A, Tuppen HA, Almalki AA, Abg-Kamaludin DP, et al. Human mitochondrial leucyl tRNA synthetase can suppress non cognate pathogenic mt-tRNA mutations. EMBO Mol Med 2014; 6: 183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014; 157: 1262–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein E. Non-myeloablative bone marrow transplant and platelet infusion can transiently improve the clinical outcome of mitochondrial neurogastrointestinal encephalopathy: a case report. Transfus Apher Sci 2013; 49: 208–11. [DOI] [PubMed] [Google Scholar]
- Iyer S, Thomas RR, Portell FR, Dunham LD, Quigley CK, Bennett JP, Jr, Recombinant mitochondrial transcription factor A with N-terminal mitochondrial transduction domain increases respiration and mitochondrial gene expression. Mitochondrion 2009; 9: 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 2007; 104: 12017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, et al. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science 2013; 342: 1524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanabus M, Heales SJ, Rahman S. Development of pharmacological strategies for mitochondrial disorders. Br J Pharmacol 2014; 171: 1798–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp K, Hares K, Mallam E, Heesom KJ, Scolding N, Wilkins A. Mesenchymal stem cell-secreted superoxide dismutase promotes cerebellar neuronal survival. J Neurochem 2010; 114: 1569–80. [DOI] [PubMed] [Google Scholar]
- Kemp K, Wilkins A, Scolding N. Cell fusion in the brain: two cells forward, one cell back. Acta Neuropathol 2014; 128: 629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon L, Moraes CT. Expanding the functional human mitochondrial DNA database by the establishment of primate xenomitochondrial cybrids. Proc Natl Acad Sci USA 1997; 94: 9131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DS. Review of clinical trials for mitochondrial disorders: 1997-2012. Neurotherapeutics 2013; 10: 307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsstrom S, et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med 2014; 6: 721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 2011; 13: 132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A. The discovery of zinc fingers and their applications in gene regulation and genome manipulation. Annu Rev Biochem 2010; 79: 213–31. [DOI] [PubMed] [Google Scholar]
- Koopman WJ, Willems PH, Smeitink JA. Monogenic mitochondrial disorders. N Engl J Med 2012; 366: 1132–41. [DOI] [PubMed] [Google Scholar]
- Kyriakouli DS, Boesch P, Taylor RW, Lightowlers RN. Progress and prospects: gene therapy for mitochondrial DNA disease. Gene Ther 2008; 15: 1017–23. [DOI] [PubMed] [Google Scholar]
- Lam BL, Feuer WJ, Abukhalil F, Porciatti V, Hauswirth WW, Guy J. Leber hereditary optic neuropathy gene therapy clinical trial recruitment: year 1. Arch Ophthalmol 2010; 128: 1129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara MC, Weiss B, Illa I, Madoz P, Massuet L, Andreu AL, et al. Infusion of platelets transiently reduces nucleoside overload in MNGIE. Neurology 2006; 67: 1461–3. [DOI] [PubMed] [Google Scholar]
- Lenoci MSF, Bucalossi A, Toraldo F, Tassi M, Tozzi M, Carluccio A, et al. Allogeneic haematopoietic stem cell transplantation in a patient with mitochondrial neurogastrointestinal encephalomyopathy: results of a six-month follow-up. Abstract. 2011. http://registration.akm.ch/einsicht.php?XNABSTRACT_ID= 123780&XNSPRACHE_ID=2&XNKONGRESS_ID= 138&XNMASKEN_ID=900.
- Levene M, Coleman DG, Kilpatrick HC, Fairbanks LD, Gangadharan B, Gasson C, et al. Preclinical toxicity evaluation of erythrocyte-encapsulated thymidine phosphorylase in BALB/c mice and beagle dogs: an enzyme-replacement therapy for mitochondrial neurogastrointestinal encephalomyopathy. Toxicol Sci 2013; 131: 311–24. [DOI] [PubMed] [Google Scholar]
- Lunn JS, Sakowski SA, Hur J, Feldman EL. Stem cell technology for neurodegenerative diseases. Ann Neurol 2011; 70: 353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Folmes CD, Wu J, Morey R, Mora-Castilla S, Ocampo A, et al. Metabolic rescue in pluripotent cells from patients with mtDNA disease. Nature 2015; 524: 234–238. [DOI] [PubMed] [Google Scholar]
- Macmillan C, Lach B, Shoubridge EA. Variable distribution of mutant mitochondrial DNAs (tRNA(Leu[3243])) in tissues of symptomatic relatives with MELAS: the role of mitotic segregation. Neurology 1993; 43: 1586–90. [DOI] [PubMed] [Google Scholar]
- Marella M, Seo BB, Thomas BB, Matsuno-Yagi A, Yagi T. Successful amelioration of mitochondrial optic neuropathy using the yeast NDI1 gene in a rat animal model. PloS One 2010; 5: e11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie M, Chiotis M, Pinkert CA, Trounce IA. Functional respiratory chain analyses in murid xenomitochondrial cybrids expose coevolutionary constraints of cytochrome b and nuclear subunits of complex III. Mol Biol Evol 2003; 20: 1117–24. [DOI] [PubMed] [Google Scholar]
- Merlini L, Angelin A, Tiepolo T, Braghetta P, Sabatelli P, Zamparelli A, et al. Cyclosporin A corrects mitochondrial dysfunction and muscle apoptosis in patients with collagen VI myopathies. Proc Natl Acad Sci USA 2008; 105: 5225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol 1997; 273: E1107–12. [DOI] [PubMed] [Google Scholar]
- Milea D. Trial of Cyclosporine in the Acute Phase of Leber Hereditary Optic Neuropathy (CICLO-NOHL). France: Centre Hospitalier Universitaire; 2014. ClinicalTrials.gov Identifier: NCT02176733. [Google Scholar]
- Minczuk M, Papworth MA, Miller JC, Murphy MP, Klug A. Development of a single-chain, quasi-dimeric zinc-finger nuclease for the selective degradation of mutated human mitochondrial DNA. Nucleic Acids Res 2008; 36: 3926–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet 2011; 12: 341–55. [DOI] [PubMed] [Google Scholar]
- Moran NF, Bain MD, Muqit MM, Bax BE. Carrier erythrocyte entrapped thymidine phosphorylase therapy for MNGIE. Neurology 2008; 71: 686–8. [DOI] [PubMed] [Google Scholar]
- Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors. Science 2009; 326: 1501. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Mahata B, Mahato B, Adhya S. Targeted mRNA degradation by complex-mediated delivery of antisense RNAs to intracellular human mitochondria. Hum Mol Genet 2008; 17: 1292–8. [DOI] [PubMed] [Google Scholar]
- Nishigaki Y, Marti R, Copeland WC, Hirano M. Site-specific somatic mitochondrial DNA point mutations in patients with thymidine phosphorylase deficiency. J Clin Invest 2003; 111: 1913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant 2007; 40: 609–19. [DOI] [PubMed] [Google Scholar]
- Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K. Clinical trials of resveratrol. Ann NY Acad Sci 2011; 1215: 161–9. [DOI] [PubMed] [Google Scholar]
- Peng M, Ostrovsky J, Kwon YJ, Polyak E, Licata J, Tsukikawa M, et al. Inhibiting cytosolic translation and autophagy improves health in mitochondrial disease. Hum Mol Genet 2015; 24: 4829–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales-Clemente E, Fernandez-Silva P, Acin-Perez R, Perez-Martos A, Enriquez JA. Allotopic expression of mitochondrial-encoded genes in mammals: achieved goal, undemonstrated mechanism or impossible task? Nucl Acids Res 2011; 39: 225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perli E, Giordano C, Pisano A, Montanari A, Campese AF, Reyes A, et al. The isolated carboxy-terminal domain of human mitochondrial leucyl-tRNA synthetase rescues the pathological phenotype of mitochondrial tRNA mutations in human cells. EMBO Mol Med 2014; 6: 169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer G, Horvath R, Klopstock T, Mootha VK, Suomalainen A, Koene S, et al. New treatments for mitochondrial disease-no time to drop our standards. Nat Rev Neurol 2013; 9: 474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer G, Majamaa K, Turnbull DM, Thorburn D, Chinnery PF. Treatment for mitochondrial disorders. Cochrane Database Syst Rev 2012; 4: CD004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Sun L, Lewin AS, Hauswirth WW, Guy J. The mutant human ND4 subunit of complex I induces optic neuropathy in the mouse. Invest Ophthalmol Vis Sci 2007; 48: 1–10. [DOI] [PubMed] [Google Scholar]
- Radecke S, Radecke F, Cathomen T, Schwarz K. Zinc-finger nuclease-induced gene repair with oligodeoxynucleotides: wanted and unwanted target locus modifications. Mol Ther 2010; 18: 743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam S, Kandavelou K, Rajenderan R, Chandrasegaran S. Creating designed zinc-finger nucleases with minimal cytotoxicity. J Mol Biol 2011; 405: 630–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 2003; 80: 148–58. [DOI] [PubMed] [Google Scholar]
- Reddy P, Ocampo A, Suzuki K, Luo J, Bacman SR, Williams SL, et al. Selective elimination of mitochondrial mutations in the germline by genome editing. Cell 2015; 161: 459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorbach J, Yusoff AA, Tuppen H, Abg-Kamaludin DP, Chrzanowska-Lightowlers ZM, Taylor RW, et al. Overexpression of human mitochondrial valyl tRNA synthetase can partially restore levels of cognate mt-tRNAVal carrying the pathogenic C25U mutation. Nucleic Acids Res 2008; 36: 3065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab 2012; 23: 459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AM, Taylor RW, Turnbull DM, Chinnery PF. The epidemiology of mitochondrial disorders–past, present and future. Biochim Biophys Acta 2004; 1659: 115–20. [DOI] [PubMed] [Google Scholar]
- Sicurelli F, Carluccio MA, Toraldo F, Tozzi M, Bucalossi A, Lenoci M, et al. Clinical and biochemical improvement following HSCT in a patient with MNGIE: 1-year follow-up. J Neurol 2012; 259: 1985–7. [DOI] [PubMed] [Google Scholar]
- Soiferman D, Ayalon O, Weissman S, Saada A. The effect of small molecules on nuclear-encoded translation diseases. Biochimie 2014; 100: 184–91. [DOI] [PubMed] [Google Scholar]
- Spendiff S, Reza M, Murphy JL, Gorman G, Blakely EL, Taylor RW, et al. Mitochondrial DNA deletions in muscle satellite cells: implications for therapies. Hum Mol Genet 2013; 22: 4739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinazzola A, Marti R, Nishino I, Andreu AL, Naini A, Tadesse S, et al. Altered thymidine metabolism due to defects of thymidine phosphorylase. J Biol Chem 2002; 277: 4128–33. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Borgeld HJ, Zhang J, Muramatsu S, Gong JS, Yoneda M, et al. Gene therapy for mitochondrial disease by delivering restriction endonuclease SmaI into mitochondria. J Biomed Sci 2002; 9: 534–41. [DOI] [PubMed] [Google Scholar]
- Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T, Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun 2002; 296: 350–4. [DOI] [PubMed] [Google Scholar]
- Tiranti V, D'Adamo P, Briem E, Ferrari G, Mineri R, Lamantea E, et al. Ethylmalonic encephalopathy is caused by mutations in ETHE1, a gene encoding a mitochondrial matrix protein. Am J Hum Genet 2004; 74: 239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Torronteras J, Viscomi C, Cabrera-Perez R, Camara Y, Di Meo I, Barquinero J, et al. Gene therapy using a liver-targeted AAV vector restores nucleoside and nucleotide homeostasis in a murine model of MNGIE. Mol Ther 2014; 22: 901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 2010; 376: 235–44. [DOI] [PubMed] [Google Scholar]
- Vignal C. Safety Evaluation of Gene Therapy in Leber Hereditary Optic Neuropathy (LHON) Patients. France: Agence Nationale de Sécurité du Médicament et des produits de santé; 2015. ClinicalTrials.gov Identifier: NCT02064569. [Google Scholar]
- Viscomi C, Bottani E, Civiletto G, Cerutti R, Moggio M, Fagiolari G, et al. In vivo correction of COX deficiency by activation of the AMPK/PGC-1alpha axis. Cell Metab 2011; 14: 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial diseases in man and mouse. Science 1999; 283: 1482–8. [DOI] [PubMed] [Google Scholar]
- Wang H, Hiatt WR, Barstow TJ, Brass EP. Relationships between muscle mitochondrial DNA content, mitochondrial enzyme activity and oxidative capacity in man: alterations with disease. Eur J Appl Physiol Occup Physiol 1999; 80: 22–7. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang H, Morizono H, Bell P, Jones D, Lin J, et al. Sustained correction of OTC deficiency in spf (ash) mice using optimized self-complementary AAV2/8 vectors. Gene Ther 2012; 19: 404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz T, Diaz F, Spiegelman BM, Moraes CT. Activation of the PPAR/PGC-1alpha pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype. Cell Metab 2008; 8: 249–56. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol (1985) 2000; 88: 2219–26. [DOI] [PubMed] [Google Scholar]
- Wolf NI, Smeitink JA. Mitochondrial disorders: a proposal for consensus diagnostic criteria in infants and children. Neurology 2002; 59: 1402–5. [DOI] [PubMed] [Google Scholar]
- Yarham JW, Al-Dosary M, Blakely EL, Alston CL, Taylor RW, Elson JL, et al. A comparative analysis approach to determining the pathogenicity of mitochondrial tRNA mutations. Hum Mutat 2011; 32: 1319–25. [DOI] [PubMed] [Google Scholar]
- Yatsuga S, Suomalainen A. Effect of bezafibrate treatment on late-onset mitochondrial myopathy in mice. Hum Mol Genet 2012; 21: 526–35. [DOI] [PubMed] [Google Scholar]
- Yin H, Xue W, Chen S, Bogorad RL, Benedetti E, Grompe M, et al. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol 2014; 32: 551–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu GTE, Peverill R, Tai G, Stockley C, Sarsero J, Evans-Galea M, et al. T.P.15 An open label clinical pilot study of resveratrol as a treatment for Friedreich ataxia. Neuromuscular Disorders, 2012; 22: 851.
- Ylikallio E, Tyynismaa H, Tsutsui H, Ide T, Suomalainen A. High mitochondrial DNA copy number has detrimental effects in mice. Hum Mol Genet 2010; 19: 2695–705. [DOI] [PubMed] [Google Scholar]
- Yu H, Koilkonda RD, Chou TH, Porciatti V, Ozdemir SS, Chiodo V, et al. Gene delivery to mitochondria by targeting modified adenoassociated virus suppresses Leber's hereditary optic neuropathy in a mouse model. Proc Natl Acad Sci USA 2012a; 109: E1238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Mehta A, Wang G, Hauswirth WW, Chiodo V, Boye SL, et al. Next-generation sequencing of mitochondrial targeted AAV transfer of human ND4 in mice. Mol Vis 2013; 19: 1482–91. [PMC free article] [PubMed] [Google Scholar]
- Yu H, Ozdemir SS, Koilkonda RD, Chou TH, Porciatti V, Chiodo V, et al. Mutant NADH dehydrogenase subunit 4 gene delivery to mitochondria by targeting sequence-modified adeno-associated virus induces visual loss and optic atrophy in mice. Mol Vis 2012b; 18: 1668–83. [PMC free article] [PubMed] [Google Scholar]