Abstract
Background:
The rate of uptake of the Papanicolaou (Pap) smear is generally low. Its causal relationship with human papillomavirus (HPV) DNA allows HPV DNA self-sampling to be used as an alternative screening tool for cervical cancer.
Objectives:
This study explored the acceptability of HPV DNA self-sampling and its impact on the rate of compliance with cervical cancer screening.
Methods:
A crossover randomized clinical trial was conducted in community-based clinics. Participants were allocated to 1 of the following 2 arms: arm 1: self-sampling before a Pap smear; and arm 2: a Pap smear before self-sampling. After completing the 2 screening methods, participants in each arm took part in face-to-face interviews using standardized, structured questionnaire.
Results:
The participants accepted both self-sampling (7.7/10) and a Pap smear (7.8/10) for cervical cancer screening. However, participants without previous experience of Pap smears or who had more than 2 sexual partners preferred self-sampling (P < .05). The participants expressed overall positive feelings toward self-sampling, and there was good agreement in HPV detection between the 2 screening methods (κ = 0.65). We estimate that the introduction of HPV DNA self-sampling could increase the future rate of uptake of cervical cancer screening by 6.5% and would entail lower costs.
Conclusion:
Human papillomavirus DNA self-sampling could be an alternative screening method to increase the coverage of cervical cancer screening.
Implications for Practice:
Human papillomavirus DNA self-sampling could overcome the barriers raised by Pap smears and enhance the coverage of cervical cancer screening. Promotional publicity and education are essential.
KEY WORDS: Cervical cancer screening, HPV self-sampling, Human papillomavirus
Cervical cancer is the sixth leading cause of death worldwide and the second most common cancer in women.1 Cervical screening can detect cancer at an early stage when the initiation of treatment has a high probability of resulting in a cure. Thus, much of the mortality from cervical cancer can be avoided if effective cervical screening programs are implemented. The Papanicolaou (Pap) smear is the most commonly used screening method for cervical cancer, but its uptake rate varies among countries, ranging from 2% to 20% in developing countries and 65% to 90% in developed countries.2–4 In Hong Kong, the government launched the Cervical Screening Program for women on a voluntary basis in 2004 at a cost that ranged from US $12.90 to US $128.50, depending on the service provider.5 Since that time, a decreasing trend in the age-standardized incidence of cervical cancer has been observed from 9.5 to 7.3 per 100 000 of the standard population in 2011.6 However, the uptake rate of the Pap smear for cervical cancer screening is still low in Hong Kong.7 In 2012, 49.1% of women refused to participate in regular cervical screening, of whom 75.9% had never been screened,7compared with some Western countries, where around 70% to 80% eligible women have been screened at least once in the past 5 years.8,9 A number of studies have shown that women who have never been screened or have not had regular screening have a higher risk of developing cervical cancer.10,11 Some factors that may discourage women from attending regular screening sessions that involve Pap smears include a lack of time, discomfort, embarrassment, pain, inconvenience, cultural objections, transportation, and the high cost of screening.12,13 Encouraging these women to participate would save lives and reduce the costs of treatment for invasive cancer.
The causal relation between human papillomavirus (HPV) DNA and cervical cancer enables the use of HPV DNA self-sampling as an alternative method for cervical screening to overcome the perceived barriers raised by the adoption of Pap smears.14 Overseas studies have shown that, by offering the option of self-sampling to nonattendees, the rates of participation in cervical cancer screening programs can be increased.15,16 Furthermore, HPV DNA testing is more sensitive than liquid-based cytology for the detection of cervical intraepithelial neoplasia grade 2 (CIN2+) or higher, and its performance is not influenced by the age of the woman being screened.17,18 European and North American data have confirmed that HPV DNA testing is substantially more sensitive in detecting CIN2+ than cytology.19 To enhance the coverage of cervical cancer screening and the sensitivity of the medical device used, it is important to have further insights into the acceptability and accuracy of self-collected vaginal samples for HPV DNA testing among women. Human papillomavirus DNA self-sampling has been reported to be acceptable and manageable among a group at high risk of cervical cancer––female sexual workers––in our previous study (Wong et al, “Can HPV DNA self-sampling be an alternative strategy for cervical cancer screening in female sex workers? A randomized control trial,”" 2010, unpublished manuscript). To expand the study population, this research aimed to explore the acceptability of HPV DNA self-sampling in the general population, to compare the rates of HPV detection in self-collected samples and Pap smears, to compare the costs of self-sampling and Pap smears, and to predict the future uptake rate of cervical cancer screening. The findings should provide a basis for health policy makers to consider HPV DNA self-sampling as an alternative option to Pap smears in government cervical cancer screening programs.
Methods
This study was a crossover randomized clinical trial to evaluate the acceptability of HPV DNA self-sampling compared with that of Pap smears and its impact on the rate of compliance with cervical cancer screening. The crossover design was used to assess the sequence effect of the 2 screening methods on the outcome. Participants who met the eligibility criteria and provided written consent were randomly allocated into either arm 1 (HPV DNA self-sampling before a Pap smear) or arm 2 (a Pap smear before HPV DNA self-sampling).
Ethics Statement
The trial was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee in Hong Kong. All of the participants were informed of the purpose of the study, the data collection procedures, the rights of participation, and the confidentiality and anonymity of the data, and all provided written informed consent. This trial was registered with the Australian New Zealand Clinical Trials Registry (http://www.anzctr.org.au) as ACTRN12613001003763.
Participants
Participants aged 35 to 65 years were recruited when attending 1 of 3 local community-based clinics for routine Pap smear check-ups between November 2011 and September 2012. Women who were currently pregnant, had had cervical surgery, had had a total hysterectomy, had impaired immunity (eg, systemic lupus erythematosus), had histologically confirmed CIN or cancer, were currently receiving immunosuppressive therapy, or had never had sex were excluded from the study. A minimum of 390 participants in total were recruited based on a 50% acceptance of HPV sampling from our previous pilot study at a power of 95% and a 5% level of statistical significance.
Data Collection
Once the participants had been randomly allocated into either arm 1 or 2 based on computer-generated tables, they underwent both screening methods in different sequences on the same day at 30- to 45-minute intervals between methods and were then approached for individual face-to-face interviews conducted using a standardized, structured questionnaire. The participants were given supermarket coupons as a token of our appreciation of their participation.
Instrument
The structured questionnaire to capture the attitudes of the participants on HPV DNA self-sampling and Pap smears comprised 3 sections including (1) acceptability, that is, confidence in their skill, comfort of the procedure, trust in the test result, recommendation of the tests to friends, and a score of their overall perceived feelings about the 2 screening methods; (2) sociodemographics; and (3) their intention to use HPV DNA self-sampling in the future. The questionnaire took approximately 15 minutes to complete. A 5-point Likert scale was used to gauge the response of acceptability and ranged from “completely disagree” (1) to “completely agree” (5) for all items except for the score of overall perceived feelings about the 2 screening methods, for which a scale range of 0 to 10 was used.
The questionnaire was derived from a literature review and our research experience in this topic.20–23 A face validity test was carried out with 10 middle- and older-aged women to ensure the questionnaire was understandable and comprehensive. For the structure of the questionnaire regarding attitudes toward 2 sampling methods, internal consistency was determined using Cronbach’s α coefficient, which checks whether items within the scale measure the same concept or construct. The coefficient ranges from 0 to 1, where lower values of the coefficient indicate a poorer interrelatedness among the items or with the construct. A coefficient of at least 0.7 generally indicates good reliability of the scale.24 The Cronbach’s α coefficient of our tool was .87, indicating that the tool had sufficient internal consistency.
Screening Tests
Self-sampling for the HPV DNA Test
The participants were directed to a private, well-lit room. An HPV DNA self-sampling kit with verbal explanations and written instructions with diagrams was given to each participant by our researcher. The self-sampling kit comprised a long-handled, sterile Dacron swab in a wrapper, a plastic vial, and a specimen bag with the participant’s identification number. The participant was instructed to insert the swab into her vagina to a depth of at least 2 inches and to rotate the swab fully 5 times. The swab was then placed in the plastic vial, which was in turn placed in a sealed specimen bag that was passed to the research staff for HPV DNA testing.
Pap Smear
To ensure the consistency of the procedures and concordance of the findings, Pap smears were performed by the assigned clinicians using a cytobrush. The cytobrush was then placed into a 20-mL vial containing phosphate-buffered saline for cytology and HPV DNA testing.
Laboratory Test for HPV Detection and Typing
The self-collected samples and Pap smear samples for HPV DNA testing were transported to the laboratory. Cells were collected by centrifugation at 750g for 10 minutes, resuspended in 200 μL of phosphate-buffered saline and kept at −70°C before HPV detection and typing.
The presence of HPV DNA in the self-collected samples and Pap smear samples was detected by polymerase chain reaction (PCR) using the consensus primer PGMY09/11 that targets a 450-base-pair region of the L1 gene. PGMY-positive specimens were typed using the Linear Array HPV Genotyping Test (Roche Molecular Systems, Inc, Pleasanton, California), which can differentiate between HPV types 6, 11, 16, 18, 26, 31, 33, 34, 35, 39, 40, 42, 45, 51, 52, 53, 54, 44, 56, 58, 59, 61, 62, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, and 89. The participants were regarded as HPV-positive when the sample showed positive results in the PGMY PCR, and the corresponding genotyping was also shown. For those samples in which HPV DNA was present and the Linear Array HPV Genotyping Test gave negative results, the PGMY PCR product was then subjected to DNA sequencing to determine the nucleotide sequence. The sequence obtained was analyzed using the BLAST (Basic Local Alignment Search Tool) program based on all HPV sequences available in GenBank. A sequence similarity of 95% or greater was regarded as identification of the type. Samples that showed a suspicious band by PGMY PCR but subsequently tested negative in both the Linear Array HPV Genotyping Test and sequencing were regarded as HPV-negative.25
Statistical Analyses
The data were entered into the PASW 18.0 (formerly SPSS) database using dual data entry, and greater than 10% of the database was further audited to ensure accuracy and completeness. Statistical significance was considered to have been achieved when P < .05. The attitudes toward the screening methods and the detection rate of HPV in the 2 arms were compared to determine a sequence effect in the 2 arms. Once the sequence effect was confirmed to be minimal, the data were managed as a whole data set for analysis.
For data analysis, the responses regarding the participants’ attitudes toward self-sampling and the Pap smear were presented descriptively and compared by parametric and nonparametric tests. The agreement of the results in percentages of discordant pairs between self-sampling and Pap smear was calculated. The strength of the agreement was categorized into 6 levels according to the κ statistic, where κ < 0 was poor, 0 to 0.20 was slight, 0.21 to 0.40 was fair, 0.41 to 0.60 was moderate, 0.61 to 0.80 was substantial, and 0.81 to 1.00 was almost perfect.26 A binary variable of paired results was created, where 0 indicated a variation in HPV detection between self-sampling and the Pap smear, and 1 indicated no variation. Logistic regression of the binary variable was performed to assess whether the probabilities of achieving the same results or different results were influenced by the sequence effects of the 2 screening methods. The types of HPV infection for the participants with positive results were further analyzed by reporting the percentage of high-risk types.
The direct screening costs of the Pap smear and HPV DNA self-sampling per person were calculated from the initial screening to the point before referral for colposcopy and biopsy. The costs of the staff (clinical and clerical), sampling kits, laboratory tests, disposal materials, the women’s time off work, and transportation were all considered to estimate the cost, which was presented in US dollars. For the ThinPrep liquid-based Pap smear screening (Hologic, USA), it was assumed that the women would take half a day (4 hours) off work to attend a clinic. For the HPV self-sampling, it was assumed that the Evalyn Brush (Rovers Medical Devices B.V., Oss, The Netherlands) would be delivered from the women’s home to the laboratory at room temperature by post.27
The future uptake rate of cervical cancer screening was predicted from the preferences of the participants for future screening methods. The percentages of nonattendees shifting to Pap smear and self-sampling screening from a British study were adopted as reference, in which 3.8% of nonattendees returned to the clinics for Pap smear screening and 6.4% returned HPV self-collected samples after receiving the self-sampling kits.15 The overall uptake rate of cervical cancer screening was therefore calculated as the sum of the uptake rates of using the Pap smear and HPV self-sampling methods.
Results
A total of 392 participants were recruited, with a response rate of 78.1%, of whom 197 were assigned to arm 1 and 195 to arm 2. No adverse event was reported during the study. A flow diagram of the recruitment is shown in Figure 1.
Figure 1.
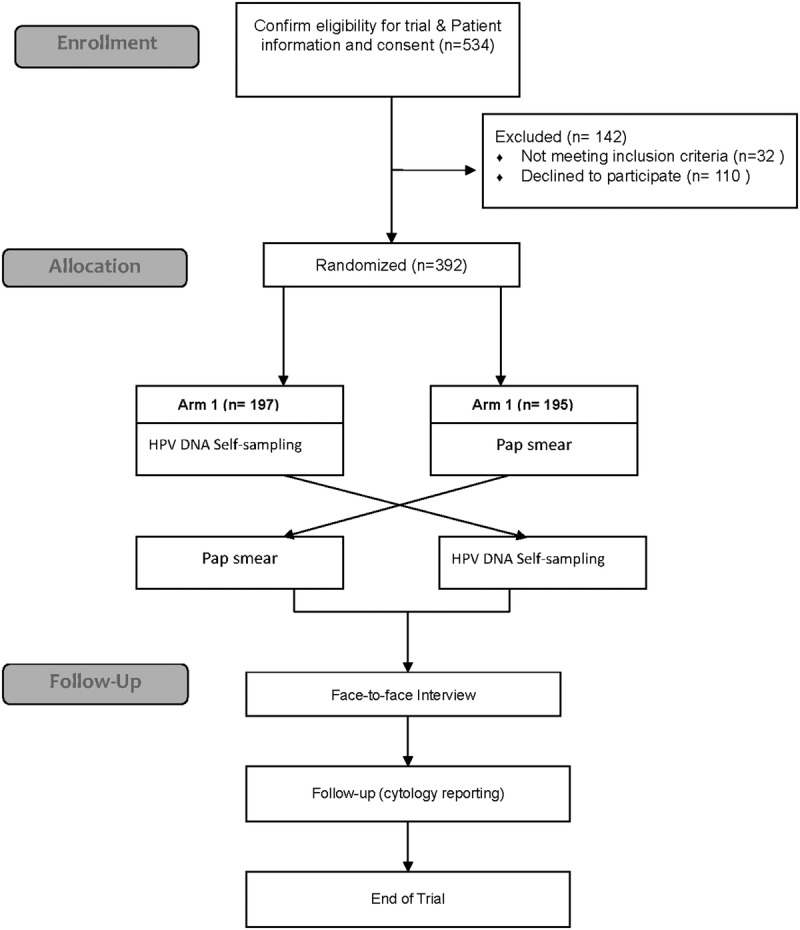
Study Flowchart.
Characteristics of the Subjects
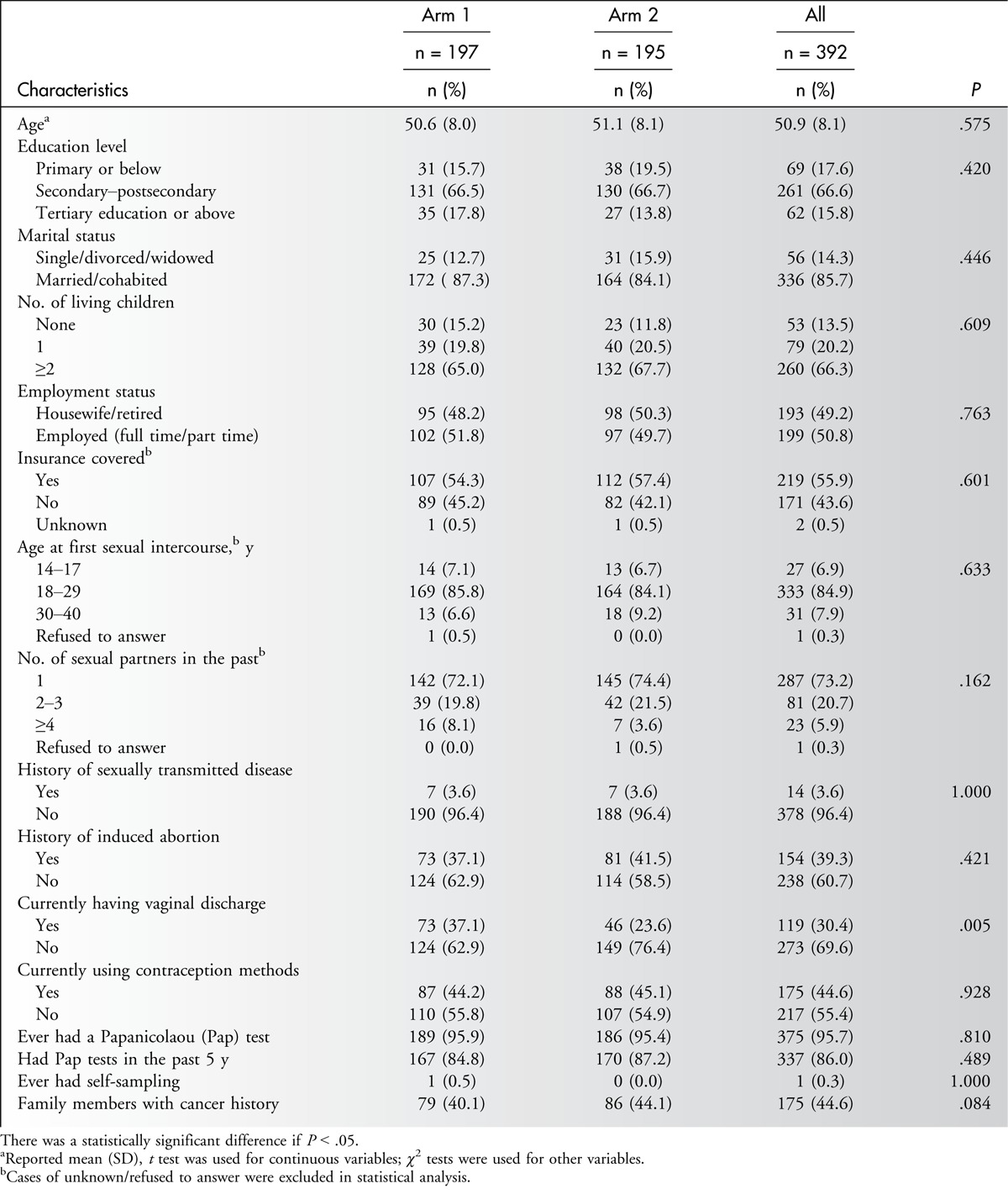
In general, no significant difference in demographic characteristics, acceptability of sampling, or detection rate of HPV was observed between the 2 arms. Therefore, a homogeneous and minimal sequence effect of the 2 screening methods was assumed, and thus the data were analyzed as a whole data set (Table 1). Overall, the average age of the participants was 50.9 years; the majority had completed a secondary education or higher (82.4%), were married or cohabited (85.7%), and had at least 1 child (86.5%). Approximately 44.6% of the participants had family members with a history of cancer, and 95.7% had had a Pap test previously, of whom 86.0% had undergone a test in the previous 5 years. Only 1 woman had experience of HPV DNA self-sampling (Table 1).
Table 1.
Demographic Characteristic of Study Participants
Acceptability of HPV DNA Self-sampling
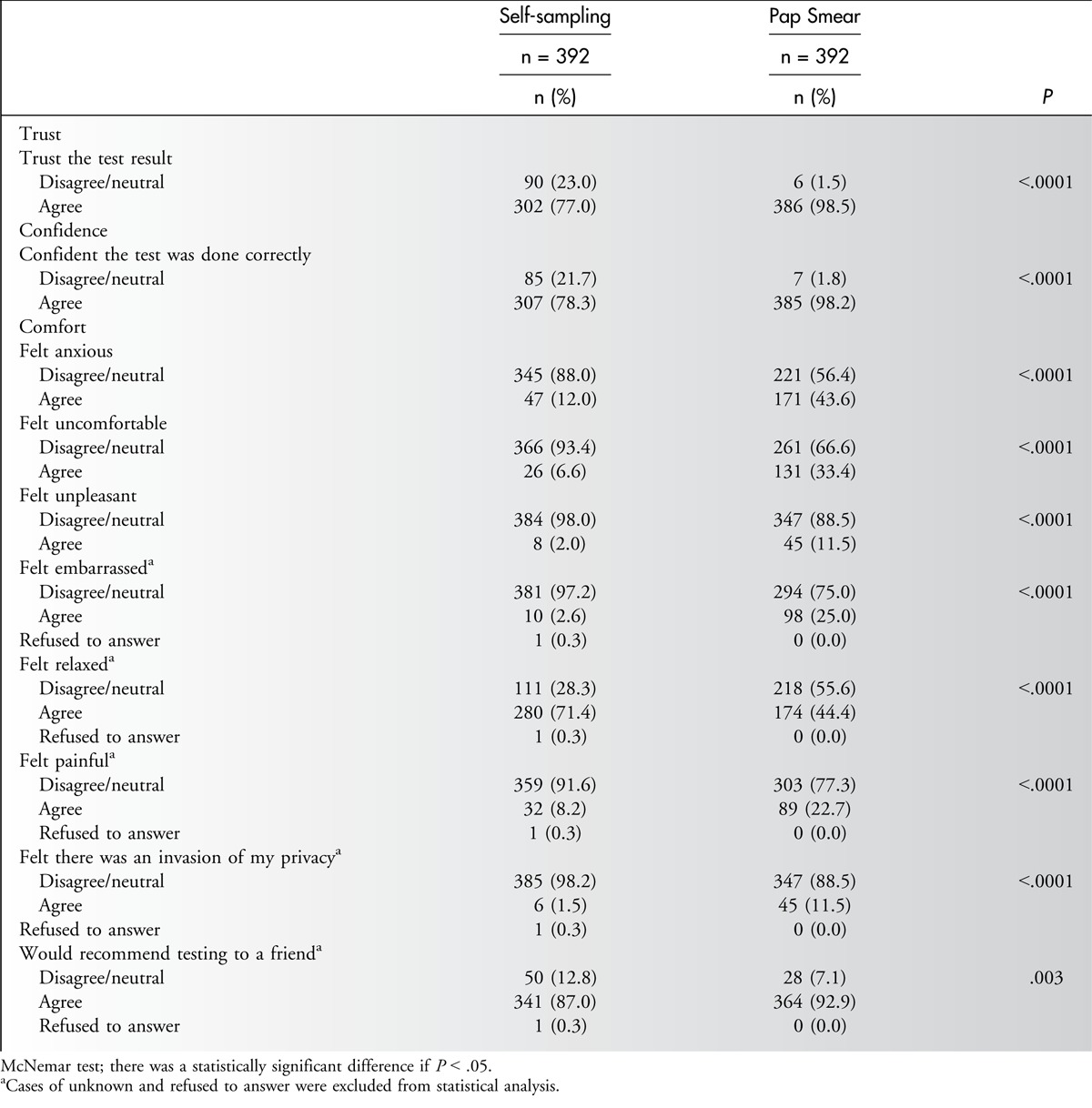
The participants found the self-sampling (7.7/10) and Pap smear (7.8/10) to be equally acceptable for cervical cancer screening. With regard to the comfort of the procedure of self-sampling, the majority reported feeling no anxiety (88.0%), no discomfort (93.4%), no unpleasantness (98.0%), no embarrassment (97.2%), no pain (91.6%), and no invasion of privacy (98.2%) and were relaxed (71.4%). Among all of the participants, 78.3% were confident that the test would be carried out correctly with the self-sampling, and 77.0% trusted its result; however, more participants trusted and had more confidence in the Pap smear test (98.5% and 98.2%, respectively). Furthermore, the proportion of participants who would recommend self-sampling (87.0%) to friends was smaller than the proportion who would recommend the Pap smear (92.9%) (Table 2).
Table 2.
Comparison of Perception and Attitudes Toward Their Experiences on Self-sampling Method and Pap Smear
After undergoing the 2 tests, all of the participants were asked about their preferences for future screening methods and indicated a distinct preference for self-sampling (56.9% [223/392]) compared with the Pap smear (37.8% [148/392]), with 5.4% (21/392) expressing no preferences. Participants with no history of Pap smears and more than 2 sexual partners stated a preference for self-sampling (P < .05). No statistically significant differences in the preference for self-sampling were observed for other demographic characteristics or settings, either at home or at a clinic.
Comparison of the HPV Detection of Self-sampling and the Pap Smear
Among the 392 participants, 12 (3.1%) were diagnosed with abnormal results from the Pap smear, of whom 10 had atypical squamous cells of undetermined significance (ASCUS), and 2 had a low-grade squamous intraepithelial lesion, one of which was diagnosed as CIN1 by colposcopy.
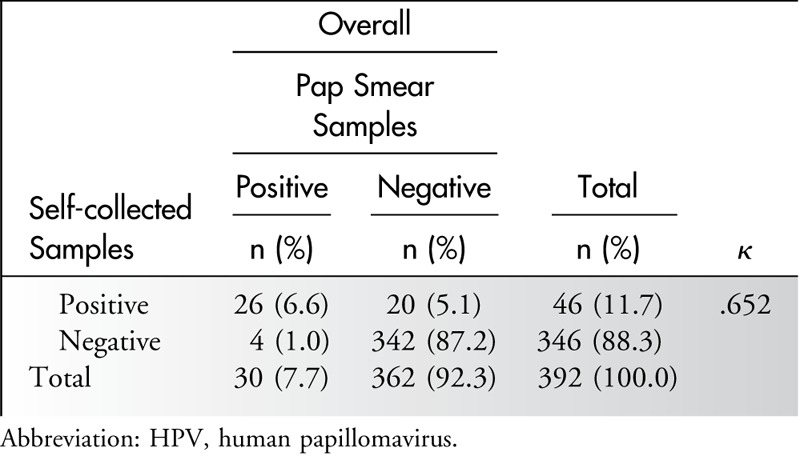
The detection rate for HPV positivity was 7.7% (30/392) with the Pap smear and 11.7 % (46/392) with self-sampling. There were a total of 24 discordant pairs (6.1%) of HPV-positive results between the Pap smear and self-sampling: 20 samples that were negative in the Pap smear showed a positive result by self-sampling, and four samples that were negative by self-sampling showed a positive result in the Pap smear. The κ measurement for the agreement of HPV detection between the 2 sampling methods was 0.65, which was considered to represent a reasonable agreement (Table 3).26 With adjustments for alcohol consumption and vaginal discharge, logistic regression of the paired test results showed no significant difference in the proportion of HPV detection between the 2 arms (P = .981).
Table 3.
Agreement of HPV DNA Results Between Self-collected and Pap Smear Samples
Multiple types of HPV infection occurred more frequently in the self-sampling (45.7% [21/46 positive cases]) than with the Pap smear (30.0% [9/30 positive cases]). Among the self-sampling tests, 5.6% (22/392) had at least 1 probable or high-risk HPV type, the most prevalent of which were HPV types 52 (1.8%), 58 (1.5%), and 18 (1%), whereas in the Pap smear tests, 3.6% (14/392) had at least 1 probable or high-risk HPV type, the most prevalent of which were HPV types 52 (1.3%), 58 (0.8%), and 39 (0.8%). Details are given in Figure 2. In terms of the characteristics of the participants with an HPV infection, those who had a history of sexually transmitted disease were more liable to have an infection than those who did not (P = .027). Other demographic and lifestyle characteristics did not show significant results.
Figure 2.

Proportions of HPV genotypes in HPV-positive cases. Abbreviation: HPV, human papillomavirus.
Costing of Self-sampling and Pap Smears
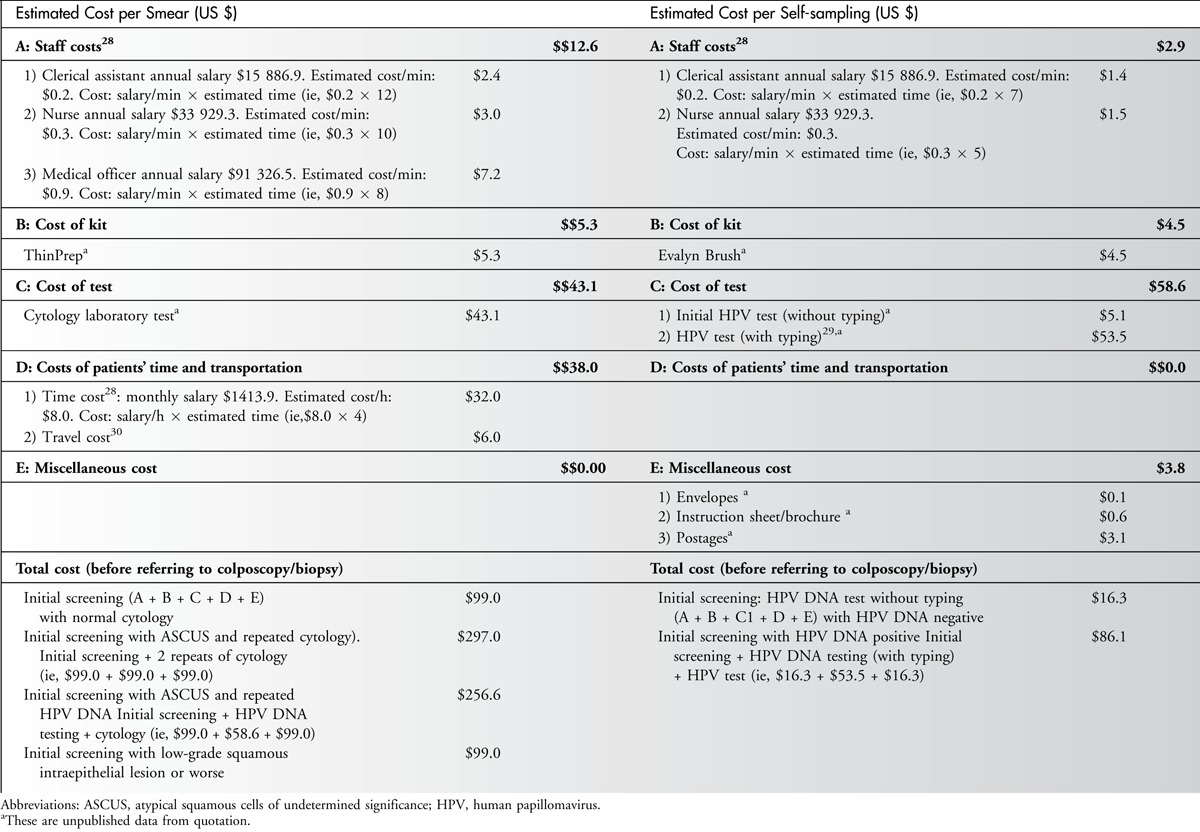
The total cost of each Pap smear was US $99.00; however, for cases with abnormal cytology results with triage for repeated cytology or HPV DNA, the cost ranged from US $99.00 to US $297.00 before referral for colposcopy. For HPV DNA self-sampling, the total cost of each HPV DNA self-sampling case was US $16.30; however, for cases that were HPV DNA-positive, the costs for typing were US $86.10. The cost of the Pap smear involved more professional time, staff manpower, and time for the women’s attendance than self-sampling. On the assumption that self-sampling would be performed at home, the costs of time away from work and transportation were not included in the cost estimates of self-sampling. This analysis shows that time and money (US $82.70) could be saved per case by implementing HPV self-sampling at home compared with Pap smears for initial cervical cancer screening. Comparing the triage repeated follow-up for self-sampling HPV-positive cases with cases of abnormal of Pap smears, US $210.90, US $170.50, and US $12.90 would be saved for ASCUS with repeated cytology, ASCUS with repeated HPV DNA typing, and low-grade squamous intraepithelial lesion or more serious findings, respectively. The details are shown in Table 4.
Table 4.
Estimated Screening Costs of per Smear and HPV Self-sampling
Predicted Future Uptake of Cervical Cancer Screening
The proportion of participants who underwent Pap smears and chose this method for future screening was 37.8% (148/392), whereas 62.2% (244/392) of the participants chose HPV DNA self-sampling or had no preference. By projecting our findings to the general population, the acceptance rate for Pap smears and HPV DNA self-sampling by those attending clinics would be 23.3% and 38.4%, respectively, which was achieved by the multiplication of 37.8% and 62.2% by the uptake rate for cervical cancer screening of 61.7% (women with experience of Pap smears) in Hong Kong in 2012.7 Among 38.3% of those not attending clinics in Hong Kong, the estimations of the uptake rate for Pap smears and HPV DNA self-sampling were 1.5% and 2.5%, respectively, calculated by adopting the values of 3.8% (nonattendees adopting the Pap smear) and 6.4% (nonattendees adopting HPV DNA self-sampling) from a British study.15 Thus, the overall uptake rate of cervical cancer screening was estimated to be 65.7% by the summation of the uptake rates for Pap smears (24.8%, including 23.3% attendees and 1.5% nonattendees) and HPV DNA self-sampling (40.9%, including 38.4% attendees and 2.5% nonattendees). An increase of 6.5%, from 61.7% to 65.7%, was estimated. The details are shown in Figure 3.
Figure 3.
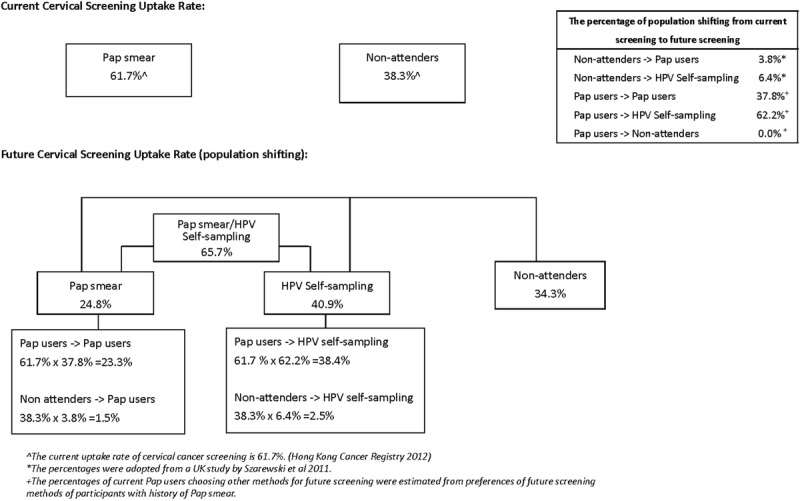
Predicted future cervical screening uptake rate.
Discussion
This was the first randomized controlled trial to measure the acceptability of HPV DNA self-sampling and its impact on the rate of compliance with cervical cancer screening in the general female population of Hong Kong. In Hong Kong, where screening coverage was achieved for only half of the female population (61.7%) in 2012, alternative strategies should be adopted to overcome the possible barriers raised by Pap smears to increase the coverage rate of cervical cancer screening programs further. Our findings show that HPV DNA self-sampling was well accepted and a feasible alternative tool for cervical cancer screening. Furthermore, the study shows that HPV DNA self-sampling would markedly improve the rate of participation of women in cervical screening.
The participants generally accepted self-sampling in preference to the Pap smear. Their experiences of pain, discomfort, unpleasantness, embarrassment, and lack of privacy were much better with self-sampling than with Pap smears. Self-sampling enables women to attend regular screening either in a clinic or at home without encountering embarrassment and other cultural barriers. In addition, it reduces the cost of screening and time of travelling to clinics. These findings echo those from overseas studies.20,21,23,31,32 Consistent with our previous study on female sex workers, women who had never had a Pap smear were more liable to choose self-sampling (Wong et al, “Can HPV DNA self-sampling be an alternative strategy for cervical cancer screening in female sex workers? A randomized control trial”2010, unpublished manuscript), which adds value to the existing evidence that HPV DNA self-sampling could be an important alternative strategy to increase cervical screening rates. Although acceptance of and attitudes toward self-sampling were more positive, the confidence in the proper handling of procedures and trust in the results were more positive for the Pap smear. Interestingly, the proportion of participants who would recommend self-sampling (87.0%) to friends was about 6% less than those who would recommend the Pap smear, which may imply inadequate confidence in self-sampling skills. Similar findings in a British study showed that women from various ethnic groups also reported uncertainty about handling the test properly.33 This could be explained by some women lacking confidence in their self-sampling techniques or having more confidence in clinician sampling for the Pap smears. The promotion of self-sampling to the public by the government or family physicians is needed, and educational campaigns to introduce and explain the significance of self-sampling as an alternative tool for cervical screening are crucial.
There was moderate to good agreement in HPV detection between the self-sampling and Pap smear samples (κ= 0.65). Another study showed that the agreement between self-sampling and Pap smear samples ranged from 0.24 to 0.96 using various self-sampling devices, including vaginal swabs, vaginal brushes, vulvar swabs, tampons, pads, cervicovaginal lavage, and urine specimens. The majority used the vaginal swabs, which were adopted for the self-sampling in our study, and showed a higher agreement (κ > 0.6),34 which may imply that swabs would be easier for women to handle during the self-sampling procedure.
Human papillomavirus infections are the primary cause of precancerous cervical lesions. The detection of HPV infection was greater after self-sampling than after the Pap smears, although this difference was not statistically significant. This result is consistent with those of other overseas studies31,32 and could be due to a potentially larger proportion of HPV-infected cells being collected from the vagina in self-sampling. Echoing these findings, a moderately lower prevalence of HPV has been observed in women with normal cytological findings in sub-Saharan African regions, Latin America and the Caribbean, Eastern Europe, and Southeast Asia.35 However, the detection of high-risk HPV types in this study was similar for the 2 screening methods.
The preference of the majority of participants shifted from Pap smears to HPV DNA self-sampling, which may reflect that HPV DNA self-sampling would be well accepted among Pap smear users and could enhance the adherence of women to cervical cancer screening. With regard to coverage, a 6.5% increase in the rate of acceptance of cervical cancer screening was predicted if HPV DNA self-sampling were to be adopted in future screening; this prediction was made using a model constructed by applying the British reference value because no related data were found for Hong Kong. The difference in the costs of Pap smears and HPV DNA self-sampling was estimated at US $82.7 per case for an initial screening. The direct cost of adopting HPV DNA self-sampling was lower than that of the Pap smear. Taking into consideration the increased overall coverage and lower direct costs, HPV DNA self-sampling could add value to cancer prevention programs, particularly in developing or low-income countries. If HPV DNA self-sampling were to be implemented at home, certain costs would be reduced by decreasing the amount of time spent dealing with patients. In addition, the distance travelled from home to clinic is 1 of the barriers that deter women from undergoing a Pap smear. Self-sampling would not only further reduce the cost of screening, but would also save the time and cost of travelling to clinics.
This study has 2 limitations. The study population was recruited from women attending community-based clinics for gynecological check-ups, and all 3 territories of Hong Kong and various age groups were well covered, but the attitudes of the participants toward HPV self-sampling may differ from those who do not attend gynecological clinics. A prospective cohort study is recommended to evaluate the feasibility of HPV self-sampling in nonattendees of clinics. Another possible limitation is the follow-up period. The study reported that HPV self-sampling could result in the detection of more cases of CIN2+ than the use of Pap smears.36 However, because of the small number of cytological abnormalities and the short follow-up period, the sensitivity and specificity of the self-collected samples could not be estimated, which may limit the comparison of cancer detection by self-sampling and colposcopy.
Conclusion and Implications
Coverage is an important factor in determining the benefit of cervical cancer screening programs. The high rate of detection and acceptance and the lower cost of HPV DNA self-sampling suggest that it could be used as an alternative to conventional screening. Our study shows that HPV DNA self-sampling was well accepted and could therefore increase cervical screening coverage and further decrease mortality from cervical cancer, because most cases of cervical cancer are associated with an absence of screening. Our study findings should facilitate the implementation of policies and guidelines for new cervical screening programs. However, promotional publicity and education are crucial steps to the success of such programs.
AcknowledgmentS
The authors thank the staff and patients at the United Christian Nethersole Community Health Service for providing them with the sites for data collection. They also thank the Food and Health Bureau for funding and supporting this project.
Footnotes
The Food and Health Bureau funded and supported this project.
The authors have no conflicts of interest to disclose.
References
- 1.IARC. Estimated age-standardized incidence and mortality rates: women. 2012. http://globocan.iarc.fr/factsheet.asp. Accessed September 1, 2013.
- 2. Louie KS, Sanjose S, Mayaud P. Epidemiology and prevention of human papillomavirus and cervical cancer in sub-Sahara Africa: a comprehensive review. Trop Med Int Health. 2009; 14: 1287– 1302. [DOI] [PubMed] [Google Scholar]
- 3. Nelson W, Moser RP, Gaffey A, et al. Adherence to cervical cancer screening guidelines for U.S. women aged 25–64: data from the 2005 Health Information National Trends Survey (HINTS). J Womens Health (Larchmt). 2009; 18(11): 1759– 1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paskett ED, McLaughlin JM, Reiter PL, et al. Psychosocial predictors of adherence to risk-appropriate cervical cancer screening guidelines: a cross sectional study of women in Ohio Appalachia participating in the Community Awareness Resources and Education (CARE) project. Prev Med. 2010; 50(1–2): 74– 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Department of Health. Cervical screening program report. The Government of the Hong Kong Special Administrative Region. 2014. http://www.cervicalscreening.gov.hk/english/sr/sr_statistics_cc.html. Accessed September 1, 2013.
- 6.Hospital Authority. Hong Kong Cancer Registry. 2011. http://www3.ha.org.hk/cancereg/. Accessed September 1, 2013.
- 7.Department of health. Statistics on behavioural risk factors. 2012. http://www.chp.gov.hk/en/data/4/10/280/387.html. Accessed November 1, 2013.
- 8.NHS. Statistics for the NHS Cervical Screening Programme. 2013. http://www.cancerscreening.nhs.uk/cervical/statistics.html. Accessed September 1, 2013.
- 9.Centers for Disease Control and Prevention (CDC). Cancer screening—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012; 61(3): 41– 45. [PubMed] [Google Scholar]
- 10. Palmisano ME, Gaffga AM, Daigle J, et al. Detection of human papillomavirus DNA in self-administered vaginal swabs as compared to cervical swabs. Int J STD AIDS. 2003; 14(8): 560– 567. [DOI] [PubMed] [Google Scholar]
- 11. Budge M, Halford J, Haran M, et al. Comparison of a self-administered tampon ThinPrep test with conventional pap smears for cervical cytology. Aust N Z J Obstet Gynaecol. 2005; 45(3): 215– 219. [DOI] [PubMed] [Google Scholar]
- 12. Petignat P, Vassilakos P. Is it time to introduce HPV self-sampling for primary cervical cancer screening? J Natl Cancer Inst. 2012; 104(3): 166– 167. [DOI] [PubMed] [Google Scholar]
- 13. Fu SN, Ip WS, Chan YS, et al. Comparison of characteristics of women undertaking cervical cancer screening in Hong Kong and Beijing. H K Pract. 2008; 30: 12– 23. [Google Scholar]
- 14.World Health Organization. Comprehensive Cervical Cancer Control—A Guide to Essential Practice. Geneva, Switzerland: World Health Organization; 2006. [PubMed] [Google Scholar]
- 15. Szarewski A, Cadman L, Mesher D, et al. HPV self-sampling as an alternative strategy in non-attenders for cervical screening - a randomised controlled trial. Br J Cancer. 2011; 104(6): 915– 920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Virtanen A, Nieminen P, Luostarinen T, et al. Self-sample HPV tests as an intervention for nonattendees of cervical cancer screening in Finland: a randomized trial. Cancer Epidemiol Biomarkers Prev. 2011; 20(9): 1960– 1969. [DOI] [PubMed] [Google Scholar]
- 17. Zhao FH, Lewkowitz AK, Chen F, et al. Pooled analysis of a self-sampling HPV DNA test as a cervical cancer primary screening method. J Natl Cancer Inst. 2012; 104(3): 178– 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lazcano-Ponce E, Lorincz AT, Cruz-Valdez A, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011; 378(9806): 1868– 1873. [DOI] [PubMed] [Google Scholar]
- 19. Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006; 119(5): 1095– 1101. [DOI] [PubMed] [Google Scholar]
- 20. Dzuba IG, Diaz EY, Allen B, et al. The acceptability of self-collected samples for HPV testing vs. the pap test as alternatives in cervical cancer screening. J Womens Health Gend Based Med. 2002; 11(3): 265– 275. [DOI] [PubMed] [Google Scholar]
- 21. Anhang R, Nelson JA, Telerant R, et al. Acceptability of self-collection of specimens for HPV DNA testing in an urban population. J Womens Health (Larchmt). 2005; 14(8): 721– 728. [DOI] [PubMed] [Google Scholar]
- 22. Kahn JA, Bernstein DI, Rosenthal SL, et al. Acceptability of human papillomavirus self testing in female adolescents. Sex Transm Infect. 2005; 81(5): 408– 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Waller J, McCaffery K, Forrest S, et al. Acceptability of unsupervised HPV self-sampling using written instructions. J Med Screen. 2006; 13(4): 208– 213. [DOI] [PubMed] [Google Scholar]
- 24. Nunnally J, Bernstein L. Psychometric Theory. New York: McGraw-Hill Higher, Inc; 1994. [Google Scholar]
- 25. Chan PK, Cheung TH, Li WH, et al. Attribution of human papillomavirus types to cervical intraepithelial neoplasia and invasive cancers in Southern China. Int J Cancer. 2012; 131(3): 692– 705. [DOI] [PubMed] [Google Scholar]
- 26. Landis LR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33: 159– 174. [PubMed] [Google Scholar]
- 27. Van Baars R, Bosgraaf RP, ter Harmsel BW, et al. Dry storage and transport of a cervicovaginal self-sample by use of the Evalyn Brush, providing reliable human papillomavirus detection combined with comfort for women. J Clin Microbiol. 2012; 50(12): 3937– 3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang AR, Hazlett CB. A regional cervical screening programme evaluation. Health Services Research Committee. 2002. Report no. 421017. [Google Scholar]
- 29. Wu J. Cervical cancer prevention through cytologic and human papillomavirus DNA screening in Hong Kong Chinese women. Hong Kong Med J. 2011; 17(suppl 3): S20– S24. [PubMed] [Google Scholar]
- 30. Kim JJ, Leung GM, Woo PP, et al. Cost-effectiveness of organized versus opportunistic cervical cytology screening in Hong Kong. J Public Health (Oxf). 2004; 26: 130– 137. [DOI] [PubMed] [Google Scholar]
- 31. Safaeian M, Kiddugavu M, Gravitt PE, et al. Comparability of self-collected vaginal swabs and physician-collected cervical swabs for detection of human papillomavirus infections in Rakai. Uganda. Sex Transm Dis. 2007; 34(7): 429– 436. [DOI] [PubMed] [Google Scholar]
- 32. Karwalajtys T, Howard M, Sellors JW, et al. Vaginal self sampling versus physician cervical sampling for HPV among younger and older women. Sex Transm Infect. 2006; 82(4): 337– 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Forrest S, McCaffery K, Waller J, et al. Attitudes to self-sampling for HPV among Indian, Pakistani, African-Caribbean and white British women in Manchester. UK. J Med Screen. 2004; 11(2): 85– 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stewart DE, Gagliardi A, Johnston M, et al. Self-collected samples for testing of oncogenic human papillomavirus: a systematic review. J Obstet Gynaecol Can. 2007; 29(10): 817– 828. [DOI] [PubMed] [Google Scholar]
- 35. Bruni L, Diaz M, Castellsague X, et al. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010; 202(12): 1789– 1799. [DOI] [PubMed] [Google Scholar]
- 36. Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003–2006. J Infect Dis. 2011; 204(4): 566– 573. [DOI] [PubMed] [Google Scholar]


