Abstract
Objective
Lymph node (LN) metastasis is widely accepted as a poor prognosis indicator in patients with gastric cancer. An accurate preoperative prediction of LN status is of crucial importance for the planning treatment. The aim of the present study was to assess the predictive value of the preoperative platelet/lymphocyte (PLR) and neutrophil/lymphocyte rates (NLR) on the LN metastasis in gastric cancer patients and to develop a new preoperative score system to predict LN metastasis.
Patients and methods
A total of 492 operable patients with gastric cancer were enrolled in our study. The clinical utility of the PLR and NLR was evaluated by receiver operating characteristic (ROC) curves. The logistic analysis was used to identify the independent parameters associated with LN metastasis. Then, a score system including those independent parameters that can be detected preoperatively was established, which was also tested by an ROC curve.
Results
The ideal cutoff values for predicting LN metastasis were 1.59 for NLR and 155.67 for PLR according to the ROC curve. Multivariate analyses showed that both PLR and NLR are significantly associated with LN metastasis independent of depth of invasion, lymphatic invasion, macroscopic type, and tumor size. The area under the ROC curve of the score system was 0.830 (95% confidence interval 0.782–0.878), showing a reliable ability to evaluate the status of nodal involvement.
Conclusion
Preoperative PLR and NLR are useful biomarkers to predict LN metastasis and the score system in our study may serve as a reliable instrument to predict LN metastasis in gastric cancer patients.
Keywords: combination, gastric cancer, lymph node metastasis, neutrophil/lymphocyte rate, platelet/lymphocyte rate
Introduction
Gastric cancer is a common malignant tumor of the gastrointestinal tract and is considered a major public health threat worldwide, with about 1 million new patients diagnosed each year, ranked the second most common cause of cancer-related mortality because of its poor prognosis 1–3. In most countries, the 5-year survival rates of gastric cancer are about 20% because of the lacking of screening programs 1,2. Among all the prognostic factors related to gastric cancer, it is well known that lymph node (LN) metastasis is confirmed to be an independent prognostic indicator 4,5. Clinically, the prediction of nodal involvement is a key point for identifying a reasonable treatment strategy.
Recently, neoadjuvant therapy has been proposed as a new treatment standard for certain gastric cancer patients, especially for those with LN metastasis 6,7. Even though controversies exist on the optimal timing and regimens of neoadjuvant therapies, the beneficial effects of this strategy, such as preoperative chemotherapy or chemoradiotherapy, have been proved in some randomized-controlled trials (RCTs) 8–10. The current National Comprehensive Cancer Network guidelines have also recommended a previous option of perioperative chemotherapy or an alternate approach of preoperative chemoradiotherapy for LNs-positive patients 11. There is no doubt that the accuracy of preoperative prediction of LN status will affect the selection of this treatment.
Nowadays, although several imaging techniques, such as endoscopic ultrasonography (EUS), computed axial tomography, and MRI, are used to evaluate nodal status in gastric cancer patients, all have limited success because of their widely inconsistent sensitivities and specificities reported in different studies 12–14. Several researches have also succeeded in finding some novel molecular biomarkers to detect nodal involvement in gastric cancer patients 15,16. However, their clinical applications are unrealistic because of their high cost, complicated personal allocation, and technology. Therefore, to date, no convenient, reproducible, and useful preoperative biomarkers have been reported to accurately predict nodal involvement in gastric cancer patients.
Researches have indicated that inflammation activities play critical roles in each stage of cancer development, from the initiation of cancer cells until the spread of distant metastasis 17–19. The Glasgow Prognostic Score, as one of the typical systemic inflammatory response (SIR) markers, in combination with hypoalbuminemia (albumin<35 g/l) with increased C-reactive protein level (CRP) (>10 mg/l), has been shown to be an independent prognostic factor for numerous types of cancer 20. However, the CRP concentration is not a part of the routine measurement of preoperative evaluations in gastric cancer patients 21. Nowadays, platelet/lymphocyte (PLR) and neutrophil/lymphocyte rates (NLR) are receiving increasingly more attention as new SIR indicators in some clinical situations. Several investigators have already recognized that preoperative NLR and PLR can be considered advanced-stage predictive markers, treatment response, or prognostic indicators in some types of carcinoma 22,23, but with respect to the predictive capability of LN metastasis in gastric cancer patients, previous English-language studies of these ratios have not been published.
Therefore, the aim of our retrospective research is to determine whether PLR and NLR are preoperative predictive indicators of LN metastasis in patients with gastric cancer and to construct a more instructive score system to assess the likelihood of nodal involvement by combining them with tumor-related factors, thus providing additional evidence to enable clinicians to offer an individualized multimodality treatment.
Materials and methods
Patients
We retrospectively evaluated 927 consecutive gastric cancer patients who underwent gastrectomy at the First Affiliated Hospital of Wenzhou Medical University between January 2009 and December 2011. Sex, age, macroscopic tumor types, lymphatic invasion, tumor size, tumor location, the number of harvested LNs and positive LNs, pathological type, tumor, node, metastasis (TNM) stage 24, and routine preoperative laboratory measurements including full blood count [albumin, carcinoembryonic antigen (CEA), neutrophils, lymphocytes, platelets] were recorded in our study. Two doctors independently reviewed the medical data of all patients. CEA was divided into two groups, up to 5 and more than 5, with respect to the normal level in our institution. The pathological types of gastric adenocarcinoma were classified as follows: type 1, well differentiated; type 2, moderately differentiated; type 3, poorly differentiated; and type 4, signet-ring cell or mucinous carcinoma. All patients were histologically confirmed in our study and the exclusion criteria were as follows: (a) insufficient number of retrieved LNs (<15), (b) history of gastric resection, (c) liver cirrhosis, (d) synchronous and metachronous malignancies, (e) severe inflammation, (f) severe bleeding or immune-system disease, and (g) preoperative chemotherapy or irradiation. The number of each excluded category of patients was 388, 3, 4, 5, 9, 19, and 7, respectively. Finally, 492 gastric cancer patients were included. The Institutional Review Board of the First Affiliated Hospital of Wenzhou Medical University approved our research.
PLR and NLR evaluation
Complete blood count test was a part of routine preoperative inspection in the surgery of gastric cancer. A blood sample was obtained within 14 days (median time 4.3 days) before the surgery. Venous blood was sampled in EDTA-containing tubes and then analyzed by the hemocounter (XE2100, Sysmex Co., Kobe, Japan) to calculate the count of differential leukocyte and platelet. The definition of NLR or NLR was neutrophil count or platelet count divided by lymphocyte count, respectively. To avoid predetermined a cutoff threshold on the basis of the prognosis of the gastric cancer or treatment response, we constructed a receiver operating characteristic (ROC) curve to establish a new cutoff point of preoperative PLR and NLR. Values with the maximal Youden index were selected 25. Our patients were then divided into two groups on the basis of the cutoff value.
Statistical analysis
The Kolmogorov–Smirnov test and a box-blot were used to describe the normality of each continuous parameter’s distribution. Data between these groups were presented as medians (interquartile range) and compared using the Mann–Whitney U-test for all variables with non-normal distributions. The ROC curve was used to assess the performance of SIR markers. The features of clinicopathological and categorical variables associated with NLR or PLR were analyzed using the χ2-test, which was also used for univariate analysis of LN metastasis. On the basis of the univariate analysis, those variables with P value less than 0.05 were considered the inclusion criteria in the multivariate logistic regression analysis to confirm independent variables. The forward stepwise method was used to eliminate variables that did not yield significant information. The hazard ratio (HR) and 95% confidence interval (CI) of each independent variable were calculated routinely. A comparison of the score system and other clinicopathologic characteristics was also performed according to the area under the ROC curve (AUC). A P value less than 0.05 was identified to be statistically significant and the statistical analysis was carried out using SPSS software (version 22.0; SPSS Inc., Chicago, Illinois, USA).
Results
Patient characteristics
Of the 492 patients selected, 379 were men and the rest were women. The median age of the patients was 63.0 (interquartile range 56.0–71.5) years. According to the TNM stage, the largest proportion of the patients (284, 57.7%) had stage III disease, whereas 97 (19.7%), 67 (13.6%), and 44 (8.9%) patients had stage I, stage II, and stage IV disease, respectively. A total of 363 (77.4%) patients had a tumor at least 3 cm in size. Lymphatic infiltration was detected in 146 (29.7%) patients. More than half of the disease (322, 65.4%) was located in the gastric antrum. Histology results showed that the majority of patients had poorly differentiated (336, 68.3%) or moderately differentiated adenocarcinoma (85, 17.3%) and 362 (73.6%) patients had advanced disease (T3/T4). Hypoalbuminemia was observed in 80 (16.3%) patients. A total of 136 patients (27.6%) were LN negative and 356 (72.4%) were positive.
The features of preoperative systemic inflammatory response markers in gastric cancer with nodal involvement
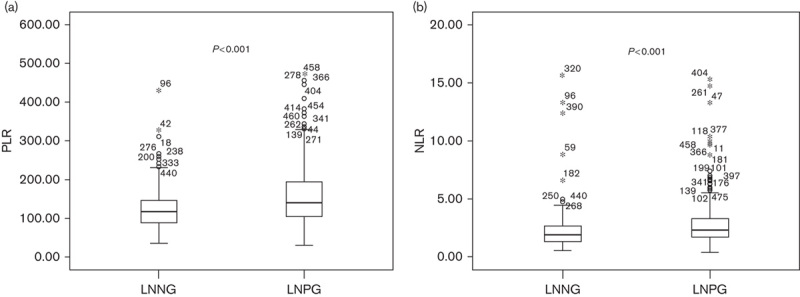
As shown in Table 1, platelet count (P=0.017) and neutrophil count (P=0.044) were significantly higher in those gastric cancer patients with nodal involvement than those without nodal involvement. Although the white blood cell count showed no significant association (P=0.741) with LN metastasis, the lymphocyte count showed a negative relationship (P=0.001). On combining a positive indicator and a negative indicator together, PLR and NLR were also significantly higher in those patients with nodal involvement (P<0.001) (Table 1 and Fig. 1). The ROC curves were further used to evaluate those variables that differed significantly. Figure 2 shows that the AUC of PLR (0.629, 95% CI 0.576–0.682) and NLR (0.619, 95% CI 0.564–0.674) were wider than neutrophils (0.559, 95% CI 0.503–0.615), platelet (0.570, 95% CI 0.515–0.624), and lymphocyte (0.595, 95% CI 0.539–0.650), which indicated that the ability of preoperative PLR and NLR values to differentiate LN metastasis is more powerful than individual indicators of lymphocyte, neutrophils, and platelet.
Table 1.
Systemic inflammatory response markers according to lymph node involvement
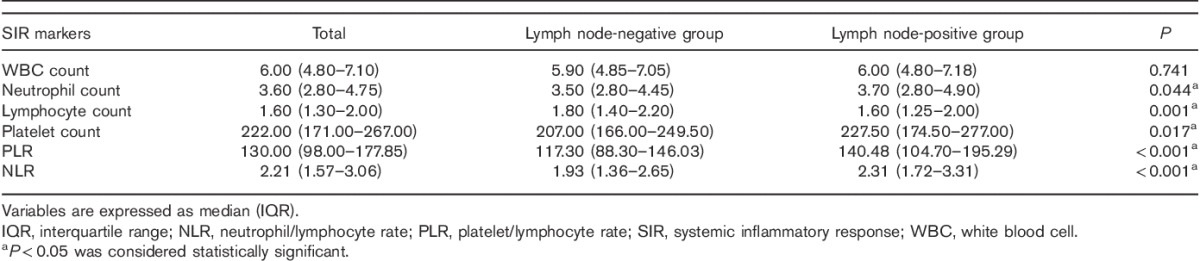
Fig. 1.
Distributions of PLR (a) and NLR (b) between LNNG and LNPG. LNNG, lymph node-negative group; LNPG, lymph node-positive group; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Fig. 2.
ROC curves for systemic inflammatory response makers in patients with gastric cancer according to lymph node metastasis. NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ROC, receiver operating characteristic.
Clinicopathologic characteristics of gastric cancer associated with preoperative NLR and PLR
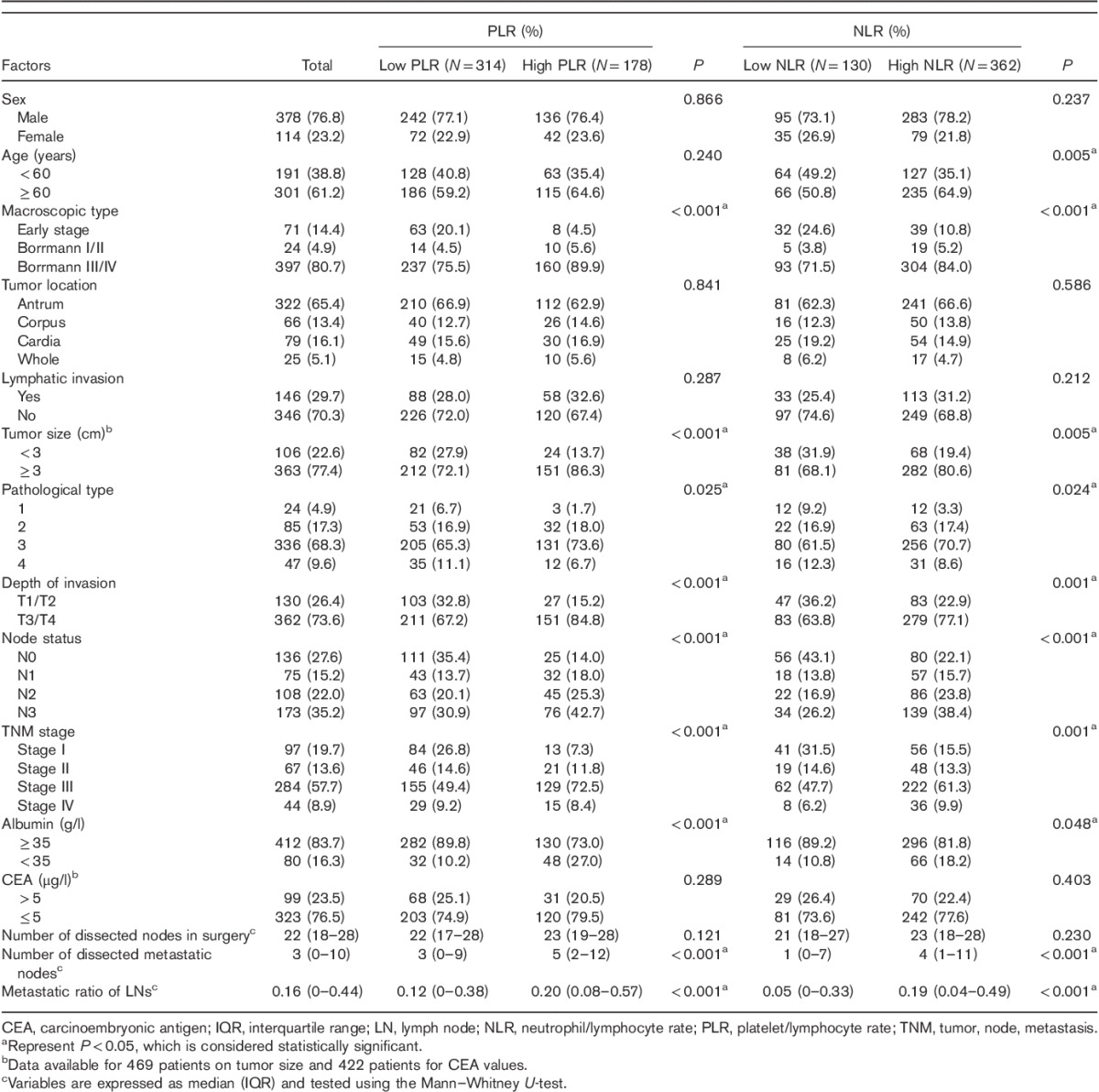
According to the ROC curve plotted above (Fig. 2), the cutoff values of the PLR and NLR for LN metastasis were set to 155.67 and 1.59, respectively. On the basis of the cutoff value, the diagnostic sensitivity and specificity were 42.9 and 81.6%, respectively, for PLR and 80.4 and 41.2%, respectively, for NLR. Thus, we dichotomized the patients into groups of ‘high PLR (>155.67)’ and ‘low PLR (≤155.67)’ or ‘high NLR (>1.59)’ and ‘low NLR (≤1.59)’. Of 492 patients, the number of patients with high PLR and high NLR was 178 (36.2%) and 362 (73.6%), respectively.
Clinicopathologic features of gastric cancer associated with preoperative NLR and PLR were further analyzed. As shown in Table 2, the number of dissected metastatic nodes and metastatic ratio of LNs 4 were statistically higher in high PLR (both P<0.001) and NLR (both P<0.001) groups, whereas the total number of dissected nodes showed no significant difference in the group of PLR (P=0.121) or NLR (P=0.230). With respect to the other clinicopathologic characteristics examined, high PLR was associated significantly with poorly pathological type (P=0.025), advanced TNM stage, larger tumor size, hypoalbuminemia, higher node status, and depth of invasion (all P<0.001). NLR was significantly increased with old age (P=0.005), poorly pathological type (P=0.024), hypoalbuminemia (P=0.048), depth of invasion (P=0.001), advanced TNM stage (P<0.001), high node status (P<0.001), and large tumor size (P=0.002). No significant difference was observed in our study in terms of the rest of the clinicopathological characteristics.
Table 2.
Clinicopathologic features of patients according to the platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios
Univariate and multivariate analysis of clinicopathological characteristics and predictive value of the score system
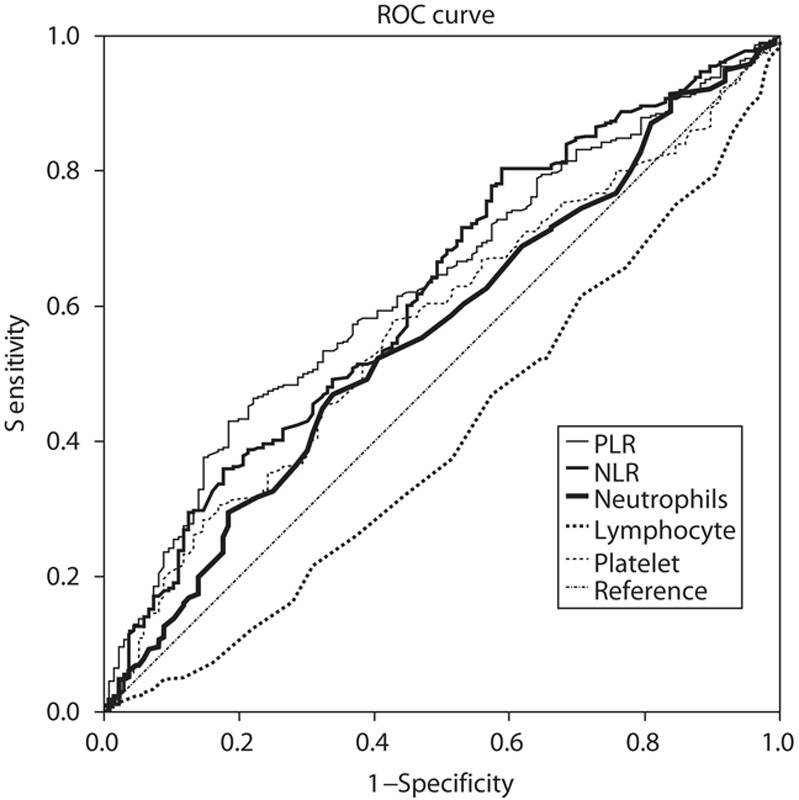
Univariate analysis of clinicopathological characteristics indicated that tumor location (χ2=10.799, P=0.013), tumor size (χ2=87.181, P<0.001), lymphatic invasion (χ2=57.481, P<0.001), albumin level (χ2=9.218, P=0.002), macroscopic type (χ2=134.242, P<0.001), depth of invasion (χ2=147.188, P<0.001), pathological type (χ2=50.174, P<0.001), NLR (χ2=21.044, P<0.001), and PLR (χ2=25.781, P<0.001) showed significant differences according to the nodal involvement (Table 3). There was no significant relationship between the LN-negative and LN-positive groups with respect to sex (χ2=0.126, P=0.722) and CEA values (χ2=0.093, P=0.760). Therefore, nine variables examined in univariate analysis (P<0.05) were selected as potential independent risk factors in multivariate analysis and the results showed (Table 4) that six of these differed significantly (P<0.05). Finally, we identified that NLR (HR 1.920; 95% CI 1.040–3.543; P=0.037), PLR (HR 1.918; 95% CI 1.028–3.578; P=0.041), depth of invasion (HR 3.980; 95% CI 2.017–7.852; P<0.001), macroscopic type [(Bormann I/II vs. early stage; HR 2.103, 95% CI 0.499–8.857; P=0.311); (Bormann III/IV vs. early stage; HR 3.888; 95% CI 1.473–10.266; P=0.006)], lymphatic invasion (HR 7.104; 95% CI 2.885–17.492; P<0.001), and tumor size (HR 2.443; 95% CI 1.296–4.607; P=0.006) were independent predictive indicators of LN metastasis. The score system that aimed to predict LN metastasis preoperatively only consists of those variables that could be detected precisely before surgical intervention. Considering that the lymphatic invasion can only be evaluated in specimens after surgery and preoperative endoscopic ultrasound showed a high accuracy to distinguish advanced T stages (T3–T4) between early T stages (T1–T2) 14,26, the variable of lymphatic invasion was excluded from this system and depth of invasion was retained. According to the HR of logistic regression analysis, the predictive score system was determined by logarithmically transforming it for each selected variable and multiplying by 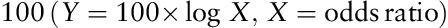 . Different points were calculated for each parameter (Table 5). ROC curves were constructed to compare the score system with other preoperative variables (Fig. 3). It is obvious that the AUC of the score system (0.830, 95% CI 0.782–0.878) was larger than tumor size (0.705, 95% CI 0.646–0.764), depth of invasion (0.762, 95% CI 0.707–0.817), macroscopic type (0.696, 95% CI 0.635–0.757), NLR (0.619, 95% CI 0.564–0.674), and PLR (0.629, 95% CI 0.576–0.682). The cutoff value of this score system was 156.0 determined by the Youden Index. The positive predictive value, negative predictive value, specificity, and sensitivity of the score system were 88.7, 61.5, 72.4, and 82.7%, respectively, showing a reliable discrimination ability to act as a predictor of LN metastasis in gastric cancer.
. Different points were calculated for each parameter (Table 5). ROC curves were constructed to compare the score system with other preoperative variables (Fig. 3). It is obvious that the AUC of the score system (0.830, 95% CI 0.782–0.878) was larger than tumor size (0.705, 95% CI 0.646–0.764), depth of invasion (0.762, 95% CI 0.707–0.817), macroscopic type (0.696, 95% CI 0.635–0.757), NLR (0.619, 95% CI 0.564–0.674), and PLR (0.629, 95% CI 0.576–0.682). The cutoff value of this score system was 156.0 determined by the Youden Index. The positive predictive value, negative predictive value, specificity, and sensitivity of the score system were 88.7, 61.5, 72.4, and 82.7%, respectively, showing a reliable discrimination ability to act as a predictor of LN metastasis in gastric cancer.
Table 3.
Univariate analysis of clinical characteristics according to nodal involvement
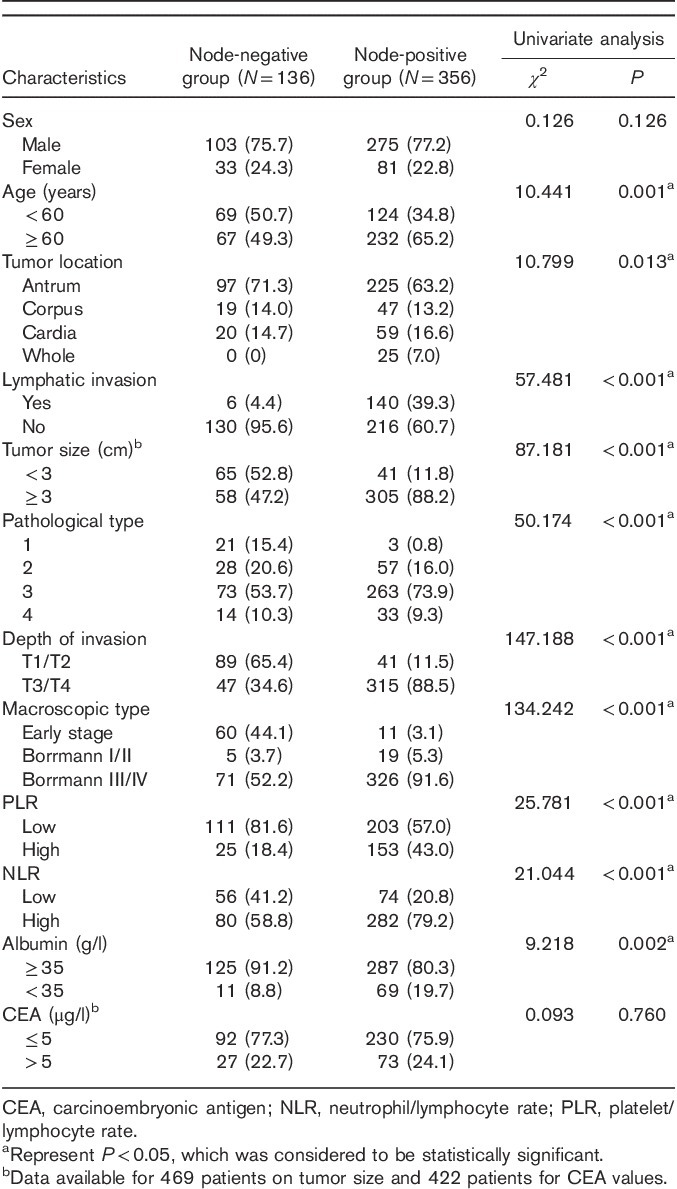
Table 4.
Results of the clinicopathological parameters for gastric cancer with nodal involvement by multivariate logistic analyses
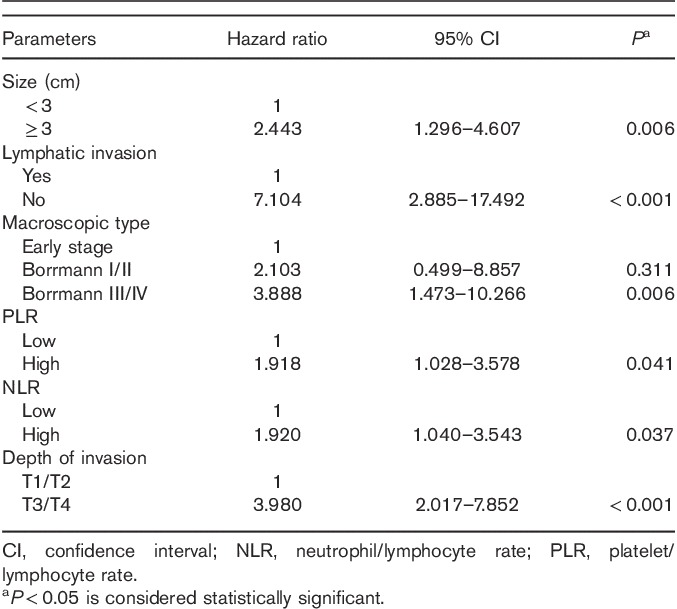
Table 5.
Risk score assigned on the basis of preoperative variables to predict lymph node metastasis
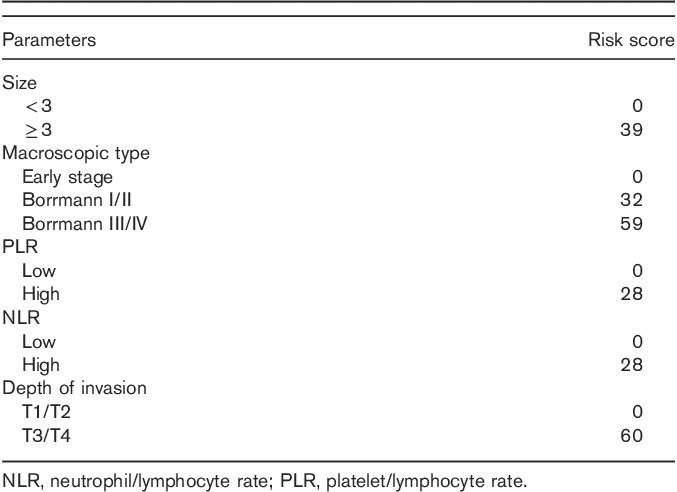
Fig. 3.
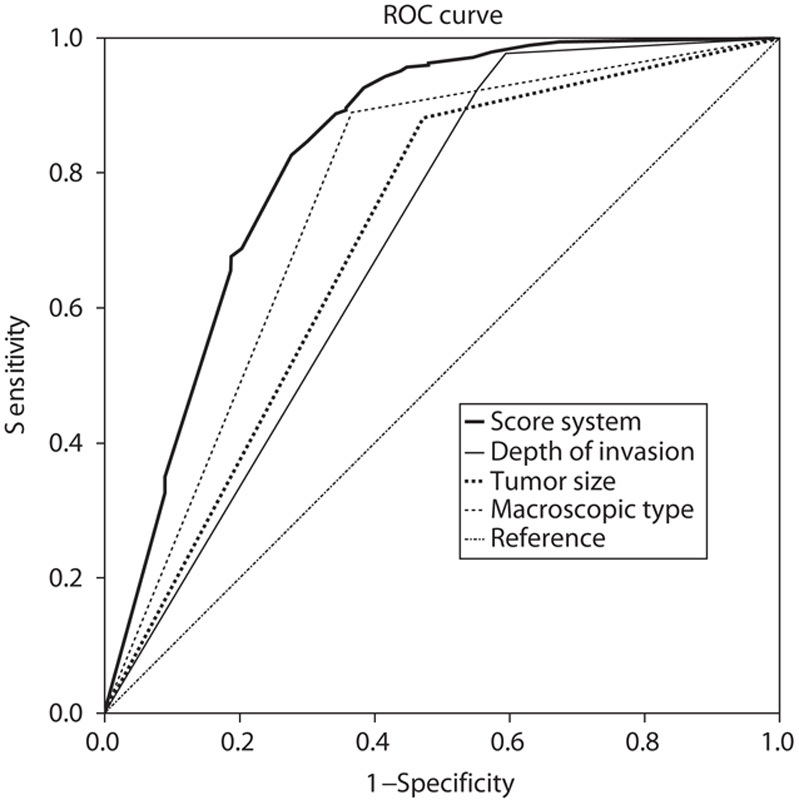
Comparison of ROC curves for depth of invasion, tumor size, macroscopic type, and the score system to evaluate the probability of lymph node metastasis. ROC, receiver operating characteristic.
Discussion
Worldwide, neoadjuvant therapy is increasingly being advocated to treat patients with gastric cancer, showing a survival advantage compared with surgery only, without increasing postoperative morbidity and mortality 8,27–29. The theoretical benefits of neoadjuvant therapy have been analyzed in previous studies 30,31. The potential of LN metastasis is a crucial preoperative consideration in administering this type of treatment. Increasingly more clinical experts have recommended preoperative therapies for gastric cancer patients with nodal involvement 6,7,32. Findings have indicated that preoperative therapies are effective in controlling LN metastasis in gastric cancer, thereby reducing tumor N stage and increasing complete resection 32–34. Therefore, the estimation of LN status before surgery seems to be critical to select a reasonable therapeutic schedule for patients. In addition, in terms of the lymphadectomy, although D2 LN dissection is considered the standard care, it is not performed uniformly under all conditions. Some previous RCTs have shown that extended (D2) lymphadectomy was related to a higher possibility of reoperation, morbidity, and mortality and no prolonged survival was observed compared with D1 lymphadectomy 35,36. Another recent Italian RCT suggested that D2 resection may be more suitable than D1 resection for advanced gastric cancer patients with LN metastasis 37. In USA, a D1 or modified D2 gastrectomy (≥15 LNs harvested) was recommended by the National Comprehensive Cancer Network guidelines 11. Therefore, we believe that D1 gastrectomy may be appropriate for localized gastric cancer without LN metastasis, especially for early-stage gastric cancer; suitable cases can also be cured by endoscopic resection. However, for other curable disease, D2 gastrectomy may be necessary.
Given the above, it is clear that identification of a preoperative model to discriminate the metastasis of LN in gastric cancer is valuable to establish a personalized treatment plan in clinical work. Nowadays, EUS is a very commonly studied instrument for local staging of gastric cancer, showing a powerful ability to describe T stages and a less reliable performance in predicting LN status 26. Similar to EUS, all of other current imaging modalities achieved limited success in staging preoperative LN status 12,14. As a result, numerous methods including gene microarray, comparative proteomic, and histological analysis have been used to discover new biomarkers recognizing the LN metastasis and several predictive markers have been found successfully, such as miR-1207-5p expression, LYVE-1 antibody, 14-3-3β and profilin-1 protein, suppressor of cytokine signaling-3, and so on 15,16,38,39. However, the usefulness of these assessments depends on expensive equipment, difficult technology, and some biomarkers that cannot be detected before surgery. The search for a reliable and affordable preoperative biomarker to predict this clinical behavior of gastric cancer is still ongoing.
Our current research showed the significance of preoperative PLR and NLR in the peripheral blood on LN metastasis in gastric cancer. The ROC curve indicated that the utility of the PLR and NLR as predictive markers for LN metastasis in gastric cancer is the best among all preoperative SIR makers. Given the fact that platelet and neutrophil showed a positive link with LN metastasis and lymphocyte showed a negative link with LN metastasis, it is not difficult to understand that combining a positive indicator with a negative indicator together is better than just using one of them. Although preoperative NLR and PLR were significant in terms of the number of dissected positive LNs and metastatic ratio of LNs, the total number of LNs dissected in surgeries showed no obvious difference, thereby indicating that NLR and PLR have a close relationship with the metastatic LNs. In our research, a significant correlation was found between NLR and age, whereas PLR showed no statistically significant difference, which is in agreement with a previous study that found that older age was an independent risk factor for high NLR 40. This change may be because of an age-related dysfunction of immunosurveillance for cancers 41,42. Moreover, comparison between NLR and PLR with other clinical characteristic has shown that both elevated NLR and PLR was statistically significant in terms of larger tumor size, advanced TNM stage, poorly differentiated, higher node status, and depth of invasion, reflecting aggressive behaviors of gastric cancer. Consistent with previous studies 43,44, lymphatic invasion, macroscopic type, depth of invasion, and tumor size retained significance in the logistic regression model in our study. Importantly, the model first identified that both PLR and NLR are independent predictors of LN metastasis in gastric cancer.
Concurrent with GPS, NLR and PLR are gaining interest as SIR markers in various clinical circumstances 45. Numerous causes of cancer development show an essential relationship with the condition of inflammation. Tumor cells have been shown to overproduce proinflammatory mediators, such as tumor necrosis factor-α, interleukin-3 (IL-3), and IL-6 46, which stimulate the hepatic production of CRP 47,48, increase peripheral blood neutrophil and platelet counts, and decrease lymphocyte counts, thus leading to a relative neutrophilia, thrombocytosis, and lymphocytopenia 49,50. The lymphocyte response plays a pivotal role in the suppression of cancer progression 51. Cancer with lymphocytopenia has been linked to the status of innate cellular immune defects 52. Because of the antitumor activities of lymphocytes, a better prognosis was found in patients with peritumoral lymphocytes infiltration 53. In contrast, the presence of neutrophilia may antagonize the antitumor immune response by impairing the antitumor effect of lymphocyte or provide a conductive tumor microenvironment, thereby promoting tumor growth and metastasis 54–56. It has been noted that circulating vascular endothelial growth factors are mostly secreted by neutrophil, which is of crucial importance in relation to tumor angiogenesis 57. In addition, neutrophilia is considered an index of poor prognosis in some types of cancer 58,59. Platelet count is also a member of SIR markers associated with the tumor 60. Studies have already reported that platelets have the ability to protect tumor cells from immune responses and promote tumor cell growth, extravasation, and migration 61,62. Furthermore, it was observed that platelet count shows a significant relationship with nodal involvement in patients with gastric cancer, colon cancer, and non-small-cell lung cancer 49,63,64.
The role of PLR or NLR in nodal staging of cancer has been investigated in a few studies. Recently, a study of 353 non-small-cell lung cancer patients recommended a novel preoperative COCT-NLR model (a combination of NLR and contrast-enhanced computed tomography) to detect LN metastasis with a high sensitivity (70.59%) and specificity (74.89%) 65. Ertas et al. 66 carried out a study to determine whether preoperative NLR and PLR could provide useful information on LN metastasis in 64 vulvular squamous cell sarcoma patients, concluding that NRL higher than 2.81 and PRL more than 139.5 had a significant independent effect on the LN metastasis (odds ratio 14.18, 95% CI 2.54–79.03, P=0.002; odds ratio 10.4, 95% CI 1.82–59.39, P=0.008, respectively). Another study comprising data on 319 endometrial adenocarcinoma patients concluded that the predictive accuracy of the nodal involvement of NLR and PLR is not better than serum CA125, although PLR and NLR values were significantly elevated in the LN-positive group (P=0.003, 0.012, respectively) 67. Several studies have attempted to explore the relationship between gastric cancer and PLR, NLR. Most of them were designed to determine their roles in the prognosis or chemotherapeutic response rather than LN metastasis 21,50,56,68,69. This is the first research that has attempted to evaluate whether PLR and NLR can be used as predictive indicators for LN metastasis in gastric cancer and to test the clinical utility of a new method with combined tumor-related and host-related factors to identify the risk of LN metastasis.
Several other score models have been developed by researchers to detect LN metastasis in gastric cancer. For example, a nodal status predictive score system including those differentially expressed proteins in pT3 stage gastric cancer with LN metastasis was established by Li et al. 70. The limitation of specific materials and a complex procedure influenced the clinical use of this model and the requirement of more tumor tissues and detecting lymphatic/vascular invasion made its use before surgery difficult. Recently, Shida et al. 71 recommended a preoperative score system to predict LN metastasis in early gastric cancer. Only tumor-related factors were analyzed and the accuracy, specificity, and sensitivity rates of the model were only 70, 61.6, and 63.2%, respectively. In comparison with the above studies, the score system devised in the present study, which included more convenient and available preoperative variables of tumor size, macroscopic type, depth of invasion, PLR, and NLR, showed a reliable and stable power (AUC 0.830, 95% CI 0.782–0.878) to predict LN metastasis with a relatively moderate specificity (72.4%) and a high sensitivity (82.7%), which was also definitely superior to the unstable performance of EUS and other imaging tools, with inconsistent sensitivities, specificities, and accuracies reported in various studies 12,72. However, our score system was only a complementary tool for the prediction of nodal involvement. Given that the positive and negative predictive values of the model were 88.7 and 61.5%, respectively, its clinical application should be combined with traditional imaging protocols to decrease false estimations. Moreover, for those patients who were understaged before the surgery, salvage treatment of adjuvant therapy was urgently needed 7.
In terms of the cutoff value of PLR and NLR, the optimal cutoff levels identified in the previous studies are inconsistent 22,23. Various methods have been used to calculate the cutoff value in different tumors. For gastric cancer, some studies just used their best cutoff values according to the median value 69 or previous studies 68,73. However, several studies applied the ROC curve to assess the ideal cutoff value. For instance, a retrospective study of 1986 patients found that both PLR and NLR can predict the overall survival and recurrence of gastric cancer with a cutoff level for PLR of 126 and 200, respectively, and NLR of 2, 3, respectively, by constructing the ROC curve 50. In contrast, Lee et al. 60 suggested a PLR of 160 and an NLR of three as the optimal threshold as a prognostic indicator by selecting values with the most remarkable difference in the univariate analysis of overall survival curves. However, in the same way, Shimada et al. 40 reported that the ideal cutoff value of NLR was 4.0. Considering the heterogeneity of thresholds proposed in previous studies and the fact that this is the first research to evaluate optimal thresholds according to the nodal involvement of gastric cancer, we established a PLR of 155.67 and an NLR of 1.59 as the ideal cutoff value using the ROC curve. On the basis of our cutoff values, although a slight trend of increased number of patients was noted in line with the extent of nodal metastasis, it did not reach significance among N1, N2, and N3 (data not shown). This result may be attributed to the small number of samples with LN metastasis in our study classified as three different groups. It is obvious that more verification is warranted to confirm these cutoff values, but our study suggested that a PLR of 155.67 and an NLR of 1.59 may be reliable thresholds to detect nodal involvement in gastric cancer patients.
The current study also had some limitations that should not be ignored. First, this was a retrospective study carried out in a single hospital to search the date from a collected computerized database. Second, after excluding lymphatic invasion, the variables of depth of invasion and macroscopic type in the score system were also observed from postoperative specimen examinations. Although we could obtain these tumor variables before surgery, it may have influenced the accuracy of our results. Finally, the relationship between intratumor inflammatory reaction and peripheral hematological component alteration of the SIR was not assessed in our study. Therefore, our results need to be confirmed in further large-scale prospective studies.
Conclusion
The present study showed that NLR and PLR could be simple, repeatable, and inexpensive preoperative indicators of LN metastasis in gastric cancer patients. As a potential therapeutic target, knowledge of their mechanisms may be useful for cancer prevention and therapy. In addition, the score system that combined PLR and NLR and tumor-related factors is a reliable and economical predictive tool to distinguish gastric cancer patients with nodal involvement between those without nodal involvement in the study, which is useful for further planning of selective neoadjuvant therapy or nodal dissection before surgery. In future, more studies on clinically available preoperative NLR and PLR, together with our score system, such as in combination with imaging systems and other known sera markers, should be explored to find a more accurate preoperative strategy to predict LN metastasis in patients with gastric cancer.
Acknowledgements
Authors contribution: Wenyang Pang: acquired data, analyzed, and interpreted the data, and drafted the manuscript; Neng Lou: acquired data; Cancan Jin, Changyuan Hu: carried out the statistical analysis; Chandoo Arvine: analyzed and interpreted the data, and revised the manuscript; Guangbao Zhu: designed the study, analyzed, and interpreted the data, supervised the study, revised the manuscript, and finally approved the version of the manuscript for publication; Xian Shen: contributed toward the conception of the study, designed the study, analyzed, and interpreted the data, revised the manuscript, and finally approved the version of the manuscript for publication.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006; 24:2137–2150. [DOI] [PubMed] [Google Scholar]
- 2.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006; 24:2903–2909. [DOI] [PubMed] [Google Scholar]
- 3.Shang J, Pena AS. Multidisciplinary approach to understand the pathogenesis of gastric cancer. World J Gastroenterol 2005; 11:4131–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol 2002; 9:775–784. [DOI] [PubMed] [Google Scholar]
- 5.Deng J, Zhang R, Pan Y, Wang B, Wu L, Hao X, Liang H. N stages of the seventh edition of TNM classification are the most intensive variables for predictions of the overall survival of gastric cancer patients who underwent limited lymphadenectomy. Tumour Biol 2014; 35:3269–3281. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz RE. Current status of management of malignant disease: current management of gastric cancer. J Gastrointest Surg 2015; 19:782–788. [DOI] [PubMed] [Google Scholar]
- 7.Newton AD, Datta J, Loaiza-Bonilla A, Karakousis GC, Roses RE. Neoadjuvant therapy for gastric cancer: current evidence and future directions. J Gastrointest Oncol 2015; 6:534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu T, Bu ZD, Li ZY, Zhang LH, Wu XJ, Wu AW, et al. Neoadjuvant chemoradiation therapy for resectable esophago-gastric adenocarcinoma: a meta-analysis of randomized clinical trials. BMC Cancer 2015; 15:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009; 27:851–856. [DOI] [PubMed] [Google Scholar]
- 10.Kim YW, Kim MJ, Ryu KW, Lim HS, Lee JH, Kong SY, et al. A phase II study of perioperative S-1 combined with weekly docetaxel in patients with locally advanced gastric carcinoma: clinical outcomes and clinicopathological and pharmacogenetic predictors for survival. Gastric Cancer 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11.National comprehensive cancer network guidelines version 3; 2015, gastric cancer. Available at: http://www.nccn.org. [Accessed 23 March 2015].
- 12.Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer 2009; 12:6–22. [DOI] [PubMed] [Google Scholar]
- 13.Seevaratnam R, Cardoso R, McGregor C, Lourenco L, Mahar A, Sutradhar R, et al. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastric Cancer 2012; 15 (Suppl 1):S3–S18. [DOI] [PubMed] [Google Scholar]
- 14.Shim CN, Lee SK. Endoscopic submucosal dissection for undifferentiated-type early gastric cancer: do we have enough data to support this? World J Gastroenterol 2014; 20:3938–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang KH, Lan YT, Fang WL, Chen JH, Lo SS, Li AF, et al. The correlation between miRNA and lymph node metastasis in gastric cancer. Biomed Res Int 2015; 2015:543163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morita H, Ishikawa Y, Akishima-Fukasawa Y, Ito K, Akasaka Y, Nishimura C, et al. Histopathological predictor for regional lymph node metastasis in gastric cancer. Virchows Arch 2009; 454:143–151. [DOI] [PubMed] [Google Scholar]
- 17.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357:539–545. [DOI] [PubMed] [Google Scholar]
- 20.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013; 39:534–540. [DOI] [PubMed] [Google Scholar]
- 21.Jiang N, Deng JY, Liu Y, Ke B, Liu HG, Liang H. The role of preoperative neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after radical resection for gastric cancer. Biomarkers 2014; 19:444–451. [DOI] [PubMed] [Google Scholar]
- 22.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013; 88:218–230. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One 2014; 9:e101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112. [DOI] [PubMed] [Google Scholar]
- 25.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J 2005; 47:458–472. [DOI] [PubMed] [Google Scholar]
- 26.Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: a meta-analysis. Gastrointest Endosc 2011; 73:1122–1134. [DOI] [PubMed] [Google Scholar]
- 27.Badgwell B, Ajani J, Blum M, Ho L, Fournier K, Chiang YJ, et al. Postoperative morbidity and mortality rates are not increased for patients with gastric and gastroesophageal cancer who undergo preoperative chemoradiation therapy. Ann Surg Oncol 2016; 23:156–162. [DOI] [PubMed] [Google Scholar]
- 28.Shum H, Rajdev L. Multimodality management of resectable gastric cancer: a review. World J Gastrointest Oncol 2014; 6:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Z, Nussbaum DP, Speicher PJ, Czito BG, Tyler DS, Blazer DG., 3rd Neoadjuvant radiation therapy does not increase perioperative morbidity among patients undergoing gastrectomy for gastric cancer. J Surg Oncol 2015; 112:46–50. [DOI] [PubMed] [Google Scholar]
- 30.Leong T, Smithers BM, Michael M, Gebski V, Boussioutas A, Miller D, et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer 2015; 15:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schirren R, Reim D, Novotny AR. Adjuvant and/or neoadjuvant therapy for gastric cancer? A perspective review. Ther Adv Med Oncol 2015; 7:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong S, Yu JR, Zhang Q, Liu XS. Neoadjuvant chemotherapy in controlling lymph node metastasis for locally advanced gastric cancer in a Chinese population. J Chemother 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33.Wu ZM, Teng RY, Shen JG, Xie SD, Xu CY, Wang LB. Reduced lymph node harvest after neoadjuvant chemotherapy in gastric cancer. J Int Med Res 2011; 39:2086–2095. [DOI] [PubMed] [Google Scholar]
- 34.Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010; 28:5210–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999; 79:1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004; 22:2069–2077. [DOI] [PubMed] [Google Scholar]
- 37.Degiuli M, Sasako M, Ponti A, Vendrame A, Tomatis M, Mazza C, et al. Randomized clinical trial comparing survival after d1 or d2 gastrectomy for gastric cancer. Br J Surg 2014; 101:23–31. [DOI] [PubMed] [Google Scholar]
- 38.Ma Y, Li YF, Wang T, Pang R, Xue YW, Zhao SP. Identification of proteins associated with lymph node metastasis of gastric cancer. J Cancer Res Clin Oncol 2014; 140:1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng J, Jiao X, Liu H, Wu L, Zhang R, Wang B, et al. Lymph node metastasis is mediated by suppressor of cytokine signaling-3 in gastric cancer. Tumour Biol 2013; 34:3627–3636. [DOI] [PubMed] [Google Scholar]
- 40.Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer 2010; 13:170–176. [DOI] [PubMed] [Google Scholar]
- 41.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol 2004; 39:687–699. [DOI] [PubMed] [Google Scholar]
- 42.Rashid F, Waraich N, Bhatti I, Saha S, Khan RN, Ahmed J, et al. A pre-operative elevated neutrophil: Lymphocyte ratio does not predict survival from oesophageal cancer resection. World J Surg Oncol 2010; 8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Zhu Z, Sun Z, Wang Z, Zheng X, Xu H. Preoperative predicting score of lymph node metastasis for gastric cancer. Tumour Biol 2014; 35:10437–10442. [DOI] [PubMed] [Google Scholar]
- 44.Jin EH, Lee DH, Jung SA, Shim KN, Seo JY, Kim N, et al. Clinicopathologic factors and molecular markers related to lymph node metastasis in early gastric cancer. World J Gastroenterol 2015; 21:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009; 12:223–226. [DOI] [PubMed] [Google Scholar]
- 46.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer 2007; 121:2373–2380. [DOI] [PubMed] [Google Scholar]
- 47.Ramadori G, Van Damme J, Rieder H, Meyer zum Büschenfelde KH. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur J Immunol 1988; 18:1259–1264. [DOI] [PubMed] [Google Scholar]
- 48.Kim DK, Oh SY, Kwon HC, Lee S, Kwon KA, Kim BG, et al. Clinical significances of preoperative serum interleukin-6 and C-reactive protein level in operable gastric cancer. BMC Cancer 2009; 9:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, et al. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol 2002; 9:287–291. [DOI] [PubMed] [Google Scholar]
- 50.Kim EY, Lee JW, Yoo HM, Park CH, Song KY. The platelet-to-lymphocyte ratio versus neutrophil-to-lymphocyte ratio: which is better as a prognostic factor in gastric cancer? Ann Surg Oncol 2015; 22:4363–4370. [DOI] [PubMed] [Google Scholar]
- 51.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004; 21:137–148. [DOI] [PubMed] [Google Scholar]
- 52.Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S, Tabuchi T. Evaluations of interferon-γ/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol 2010; 102:742–747. [DOI] [PubMed] [Google Scholar]
- 53.Liu H, Tabuchi T, Takemura A, Kasuga T, Motohashi G, Hiraishi K, et al. The granulocyte/lymphocyte ratio as an independent predictor of tumour growth, metastasis and progression: its clinical applications. Mol Med Rep 2008; 1:699–704. [DOI] [PubMed] [Google Scholar]
- 54.Liu H, Ubukata H, Tabuchi T, Takemura A, Motohashi G, Nishimura M, et al. It is possible that tumour-infiltrating granulocytes promote tumour progression. Oncol Rep 2009; 22:29–33. [DOI] [PubMed] [Google Scholar]
- 55.Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol 1985; 134:230–234. [PubMed] [Google Scholar]
- 56.Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology 2007; 73:215–220. [DOI] [PubMed] [Google Scholar]
- 57.Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis 2003; 6:283–287. [DOI] [PubMed] [Google Scholar]
- 58.Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer 2013; 16:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unal D, Eroglu C, Kurtul N, Oguz A, Tasdemir A. Are neutrophil/lymphocyte and platelet/lymphocyte rates in patients with non-small cell lung cancer associated with treatment response and prognosis? Asian Pac J Cancer Prev 2013; 14:5237–5242. [DOI] [PubMed] [Google Scholar]
- 60.Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim KH, Kim HJ. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer 2013; 13:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol 2010; 30:2362–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost 2011; 9:237–249. [DOI] [PubMed] [Google Scholar]
- 63.Liu HB, Gu XL, Ma XQ, Lv TF, Wu Y, Xiao YY, et al. Preoperative platelet count in predicting lymph node metastasis and prognosis in patients with non-small cell lung cancer. Neoplasma 2013; 60:203–208. [DOI] [PubMed] [Google Scholar]
- 64.Lin MS, Huang JX, Zhu J, Shen HZ. Elevation of platelet count in patients with colorectal cancer predicts tendency to metastases and poor prognosis. Hepatogastroenterology 2012; 59:1687–1690. [DOI] [PubMed] [Google Scholar]
- 65.Huang C, Yue J, Li Z, Li N, Zhao J, Qi D. Usefulness of the neutrophil-to-lymphocyte ratio in predicting lymph node metastasis in patients with non-small cell lung cancer. Tumour Biol 2015; 36:7581–7589. [DOI] [PubMed] [Google Scholar]
- 66.Ertas IE, Gungorduk K, Akman L, Ozdemir A, Terek MC, Ozsaran A, et al. Can preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios be used as predictive markers for lymph node metastasis in squamous cell carcinoma of the vulva? Eur J Obstet Gynecol Reprod Biol 2013; 171:138–142. [DOI] [PubMed] [Google Scholar]
- 67.Suh DH, Kim HS, Chung HH, Kim JW, Park NH, Song YS, Kang SB. Pre-operative systemic inflammatory response markers in predicting lymph node metastasis in endometrioid endometrial adenocarcinoma. Eur J Obstet Gynecol Reprod Biol 2012; 162:206–210. [DOI] [PubMed] [Google Scholar]
- 68.Aliustaoglu M, Bilici A, Ustaalioglu BB, Konya V, Gucun M, Seker M, Gumus M. The effect of peripheral blood values on prognosis of patients with locally advanced gastric cancer before treatment. Med Oncol 2010; 27:1060–1065. [DOI] [PubMed] [Google Scholar]
- 69.Cho IR, Park JC, Park CH, Jo JH, Lee HJ, Kim S, et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer 2014; 17:703–710. [DOI] [PubMed] [Google Scholar]
- 70.Li W, Ye F, Wang D, Sun X, Tong W, Lian G, et al. Protein predictive signatures for lymph node metastasis of gastric cancer. Int J Cancer 2013; 132:1851–1859. [DOI] [PubMed] [Google Scholar]
- 71.Shida A, Fujioka S, Kawamura M, Takahashi N, Ishibashi Y, Nakada K, et al. Prediction of lymph node metastasis in patients with submucosa-invading early gastric cancer. Anticancer Res 2014; 34:4471–4474. [PubMed] [Google Scholar]
- 72.Feng XY, Wang W, Luo GY, Wu J, Zhou ZW, Li W, et al. Comparison of endoscopic ultrasonography and multislice spiral computed tomography for the preoperative staging of gastric cancer – results of a single institution study of 610 Chinese patients. PLoS One 2013; 8:e78846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Q, Yang Y, Zhang YP, Zou Z, Qian X, Liu B, et al. Prognostic value of carbohydrate tumor markers and inflammation-based markers in metastatic or recurrent gastric cancer. Med Oncol 2014; 31:289. [DOI] [PubMed] [Google Scholar]


