Supplemental Digital Content is available in the text.
Key Words: cytotoxic T-lymphocyte–associated antigen 4, CTLA-4, programmed death 1, PD-1, immune checkpoint
Abstract
The cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) immune checkpoints are negative regulators of T-cell immune function. Inhibition of these targets, resulting in increased activation of the immune system, has led to new immunotherapies for melanoma, non–small cell lung cancer, and other cancers. Ipilimumab, an inhibitor of CTLA-4, is approved for the treatment of advanced or unresectable melanoma. Nivolumab and pembrolizumab, both PD-1 inhibitors, are approved to treat patients with advanced or metastatic melanoma and patients with metastatic, refractory non-small cell lung cancer. In addition the combination of ipilimumab and nivolumab has been approved in patients with BRAF WT metastatic or unresectable melanoma. The roles of CTLA-4 and PD-1 in inhibiting immune responses, including antitumor responses, are largely distinct. CTLA-4 is thought to regulate T-cell proliferation early in an immune response, primarily in lymph nodes, whereas PD-1 suppresses T cells later in an immune response, primarily in peripheral tissues. The clinical profiles of immuno-oncology agents inhibiting these 2 checkpoints may vary based on their mechanistic differences. This article provides an overview of the CTLA-4 and PD-1 pathways and implications of their inhibition in cancer therapy.
A key requirement of the immune system is to distinguish self from nonself. While the concept is simple, the implementation is a complex system that has taken decades to understand. At the center of this process is recognition and binding of a T-cell receptor (TCR) to an antigen displayed in the major histocompatibility complex (MHC) on the surface of an antigen-presenting cell (APC). Multiple other factors then influence whether this binding results in T-cell activation or anergy.
The life of a T cell begins in the thymus, where immature cells proliferate and create a wide repertoire of TCRs through recombination of the TCR gene segments. A selection process then begins, and T cells with strong reactivity to self-peptides are deleted in the thymus to prevent autoreactivity in a process called central tolerance.1 T cells with insufficient MHC binding undergo apoptosis, but those that can weakly respond to MHC molecules and self-peptides are not deleted and are released as naive cells to circulate through the blood, spleen, and lymphatic organs. There they are exposed to professional APCs displaying foreign antigens (in the case of infection) or mutated self-proteins (in the case of malignancy). Some TCRs may have specificity that is cross-reactive with self-antigens. To prevent autoimmunity, numerous immune checkpoint pathways regulate activation of T cells at multiple steps during an immune response, a process called peripheral tolerance.1,2 Central to this process are the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) immune checkpoint pathways.3 The CTLA-4 and PD-1 pathways are thought to operate at different stages of an immune response. CTLA-4 is considered the “leader” of the immune checkpoint inhibitors, as it stops potentially autoreactive T cells at the initial stage of naive T-cell activation, typically in lymph nodes.2,4 The PD-1 pathway regulates previously activated T cells at the later stages of an immune response, primarily in peripheral tissues.2 A core concept in cancer immunotherapy is that tumor cells, which would normally be recognized by T cells, have developed ways to evade the host immune system by taking advantage of peripheral tolerance.5,6 Inhibition of the immune checkpoint pathways has led to the approval of several new drugs: ipilimumab (anti-CTLA-4), pembrolizumab (anti-PD-1), and nivolumab (anti-PD-1). There are key similarities and differences in these pathways, with implications for cancer therapy.
CTLA-4 PATHWAY
T-cell activation is a complex process that requires >1 stimulatory signal. TCR binding to MHC provides specificity to T-cell activation, but further costimulatory signals are required. Binding of B7-1 (CD80) or B7-2 (CD86) molecules on the APC with CD28 molecules on the T cell leads to signaling within the T cell. Sufficient levels of CD28:B7-1/2 binding lead to proliferation of T cells, increased T-cell survival, and differentiation through the production of growth cytokines such as interleukin-2 (IL-2), increased energy metabolism, and upregulation of cell survival genes.
CTLA-4 is a CD28 homolog with much higher binding affinity for B77,8; however, unlike CD28, binding of CTLA-4 to B7 does not produce a stimulatory signal. As such, this competitive binding can prevent the costimulatory signal normally provided by CD28:B7 binding7,9,10 (Fig. 1). The relative amount of CD28:B7 binding versus CTLA-4:B7 binding determines whether a T cell will undergo activation or anergy.4 Furthermore, some evidence suggests that CTLA-4 binding to B7 may actually produce inhibitory signals that counteract the stimulatory signals from CD28:B7 and TCR:MHC binding.11,12 Proposed mechanisms for such inhibitory signals include direct inhibition at the TCR immune synapse, inhibition of CD28 or its signaling pathway, or increased mobility of T cells leading to decreased ability to interact with APCs.9,12,13
FIGURE 1.
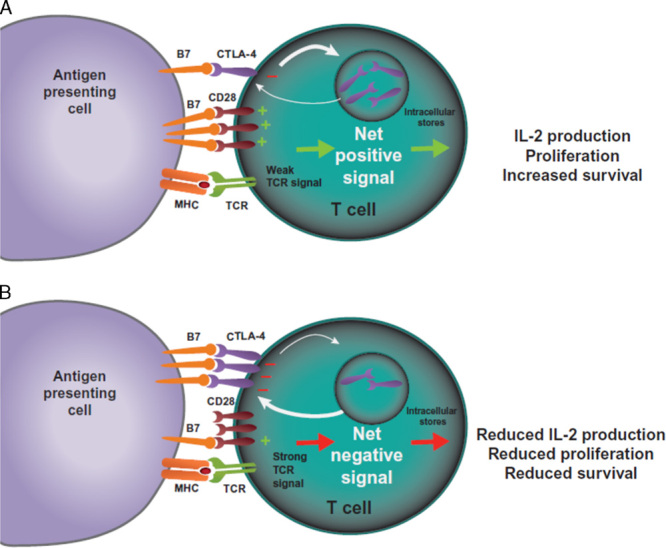
CTLA-4-mediated inhibition of T cells. T cells are activated when TCRs bind antigen displayed in the MHC on antigen-presenting cells in concert with CD28:B7-mediated costimulation. A, In the case of a weak TCR stimulus, CD28:B7 binding predominates, resulting in a net positive activating signal and IL-2 production, proliferation, and increased survival. B, In the case of a strong TCR stimulus, CTLA-4 expression is upregulated by increased transport to the cell surface from intracellular stores and decreased internalization. CTLA-4 competes with CD28 for binding of B7 molecules. Increased CTLA-4:B7 binding can result in a net negative signal, which limits IL-2 production and proliferation, and limits survival of the T cell. CTLA-4 indicates cytotoxic T-lymphocyte–associated antigen 4; IL-2, interleukin-2; MHC, major histocompatibility complex; TCR, T-cell receptor.
CTLA-4 itself is subject to regulation, particularly by localization within the cell. In resting naive T cells CTLA-4 is located primarily in the intracellular compartment.14 Stimulatory signals resulting from both TCR and CD28:B7 binding induce upregulation of CTLA-4 on the cell surface by exocytosis of CTLA-4-containing vesicles.14 This process operates in a graded feedback loop whereby stronger TCR signaling elicits more CTLA-4 translocation to the cell surface. In case of a net negative signal through CTLA-4:B7 binding, full activation of T cells is prevented by inhibition of IL-2 production and cell cycle progression.15
CTLA-4 is also involved in other aspects of immune control. Regulatory T cells (Tregs) control functions of the effector T cells, and thus are key players in maintaining peripheral tolerance.16,17 Unlike effector T cells, Tregs constitutively express CTLA-4, and this is thought to be important for their suppressive functions.17 In animal models, genetic CTLA-4 deficiency in Tregs impaired their suppressive functions.17,18 One mechanism whereby Tregs are thought to control effector T cells is downregulation of B7 ligands on APCs, leading to reduced CD28 costimulation (Fig. 2).18,19
FIGURE 2.
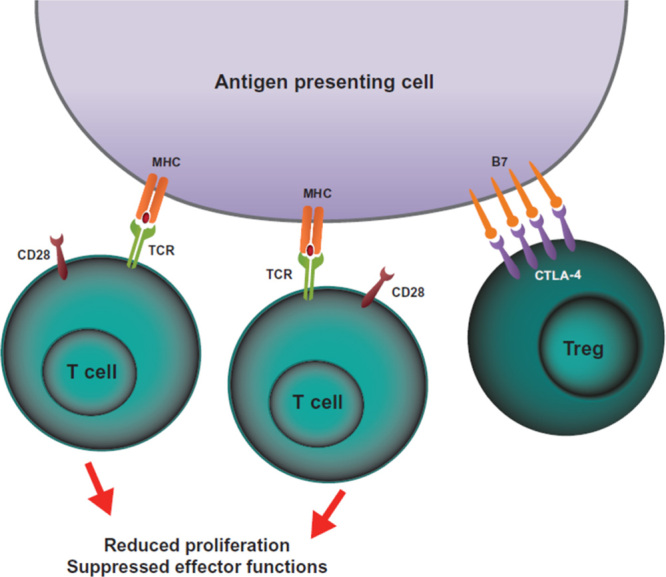
CTLA-4-mediated inhibition of Tregs. One hypothesis of how CTLA-4 expression on Tregs can inhibit T-cell activation is depicted. Constitutive expression of CTLA-4 on Tregs can sequester or cause internalization (not depicted) of B7 molecules on antigen-presenting cells. The lack of CD28:B7-mediated costimulation leads to reduced T-cell proliferation and reduced effector functions. CTLA-4 indicates cytotoxic T-lymphocyte–associated antigen 4; MHC, major histocompatibility complex; TCR, T-cell receptor; Tregs, regulatory T cells.
PD-1 PATHWAY
PD-1 is a member of the B7/CD28 family of costimulatory receptors. It regulates T-cell activation through binding to its ligands, programmed death ligand 1 (PD-L1) and programmed death ligand 2 (PD-L2).20 Similar to CTLA-4 signaling, PD-1 binding inhibits T-cell proliferation, and interferon-γ (IFN-γ), tumor necrosis factor-α, and IL-2 production, and reduces T-cell survival20 (Fig. 3). If a T cell experiences coincident TCR and PD-1 binding, PD-1-generated signals prevent phosphorylation of key TCR signaling intermediates, which terminates early TCR signaling and reduces activation of T cells.10,21 PD-1 expression is a hallmark of “exhausted” T cells that have experienced high levels of stimulation or reduced CD4+ T-cell help.22 This state of exhaustion, which occurs during chronic infections and cancer, is characterized by T-cell dysfunction, resulting in suboptimal control of infections and tumors.
FIGURE 3.
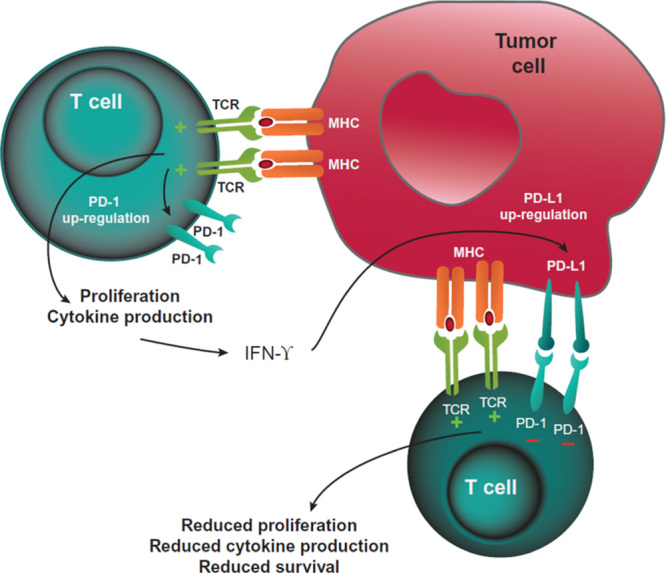
PD-1-mediated inhibition of T cells. T cells recognizing tumor antigens can be activated to proliferate, secrete inflammatory cytokines, and resist cell death. Prolonged TCR stimulation during an ongoing immune response can cause upregulated PD-1 expression. Tumor cells can express PD-L1 (and PD-L2, not shown) as a consequence of inflammatory cytokines and/or oncogenic signaling pathways. PD-1:PD-L1 binding inhibits TCR-mediated positive signaling, leading to reduced proliferation, reduced cytokine secretion, and reduced survival. IFN-γ indicates interferon-γ; MHC, major histocompatibility complex; PD-1, programmed death protein 1; PD-L1, programmed death ligand 1; PD-L2, programmed death ligand 2; TCR, T-cell receptor.
Both CTLA-4 and PD-1 binding have similar negative effects on T-cell activity; however, the timing of downregulation, the responsible signaling mechanisms, and the anatomic locations of immune inhibition by these 2 immune checkpoints differ. Unlike CTLA-4, which is confined to T cells, PD-1 is more broadly expressed on activated T cells, B cells, and myeloid cells.2,20 While CTLA-4 functions during the priming phase of T-cell activation, PD-1 functions during the effector phase, predominantly within peripheral tissues.20
The distribution of PD-1 ligands also differs from those for CTLA-4. The B7 ligands for CTLA-4 are expressed by professional APCs, which typically reside in lymph nodes or spleen2; however, PD-L1 and PD-L2 are more widely expressed.2,10,23,24 PD-L1 is expressed on leukocytes, on nonhematopoietic cells, and in nonlymphoid tissues, and can be induced on parenchymal cells by inflammatory cytokines (IFN-γ) or tumorigenic signaling pathways.25 PD-L1 expression is also found on many different tumor types, and is associated with an increased amount of tumor-infiltrating lymphocytes (TILs) and poorer prognosis.26–28 PD-L2 is primarily expressed on dendritic cells and monocytes, but can be induced on a wide variety of other immune cells and nonimmune cells, depending on the local microenvironment.29 PD-1 has a higher binding affinity for PD-L2 than for PD-L1, and this difference may be responsible for differential contributions of these ligands to immune responses.30 Because PD-1 ligands are expressed in peripheral tissues, PD-1–PD-L1/PD-L2 interactions are thought to maintain tolerance within locally infiltrated tissues.2
As might be expected, the plurality of ligands for PD-1 leads to variation in biological effects, depending upon which ligand is bound. One model showed opposing roles of PD-L1 and PD-L2 signaling in activation of natural killer T cells.31 Inhibition of PD-L2 binding leads to enhanced TH2 activity,32 whereas PD-L1 binding to CD80 has been shown to inhibit T-cell responses.33 These different biological effects are likely to contribute to differences in activity and toxicity between antibodies directed at PD-1 (preventing binding to both ligands) as opposed to those directed at PD-L1, and therefore have potential therapeutic implications.
Although Tregs express PD-1 as well as CTLA-4, the function of PD-1 expression on these cells remains unclear. PD-L1 has been shown to contribute to the conversion of naive CD4+ T cells to Treg cells34 and to inhibit T-cell responses by promoting the induction and maintenance of Tregs.35 Consistent with these findings, PD-1 blockade can reverse Treg-mediated suppression of effector T cells in vitro.36
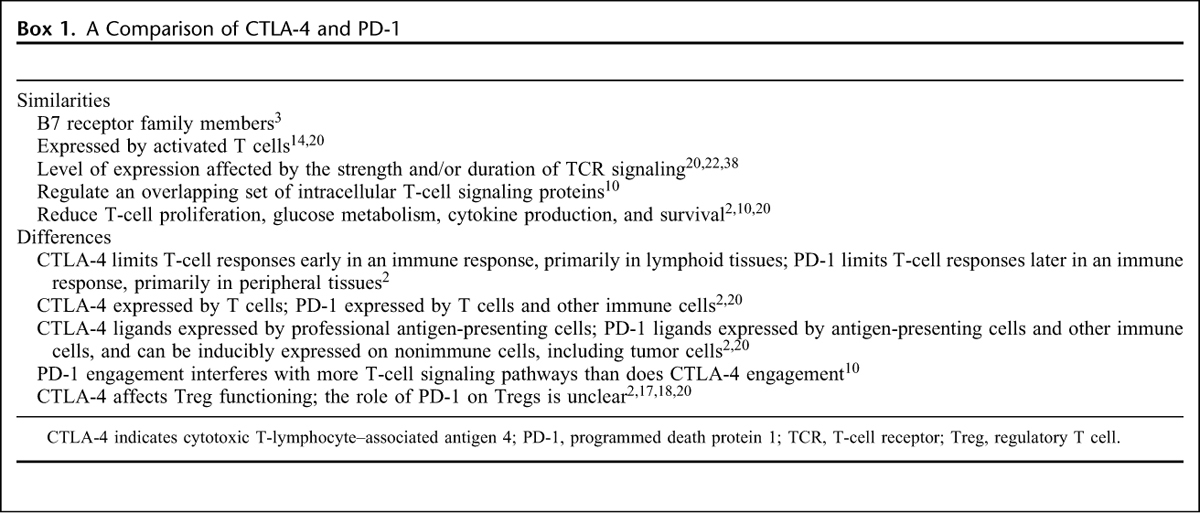
PD-1 binding with its ligands decreases the magnitude of the immune response in T cells that are already engaged in an effector T-cell response.22 This results in a more restricted spectrum of T-cell activation compared with CTLA-4 blockade, which may explain the apparently lower incidence of immune-mediated adverse events (AEs) associated with PD-1 compared with a CTLA-4 blockade (see below).37 Similarities and differences between the CTLA-4 and PD-1 receptors, and the consequences of their engagement, are detailed in Box 1.
Box 1: A Comparison of CTLA-4 and PD-1.
IMPLICATIONS OF CTLA-4 AND PD-1 PATHWAY BLOCKADE IN CANCER
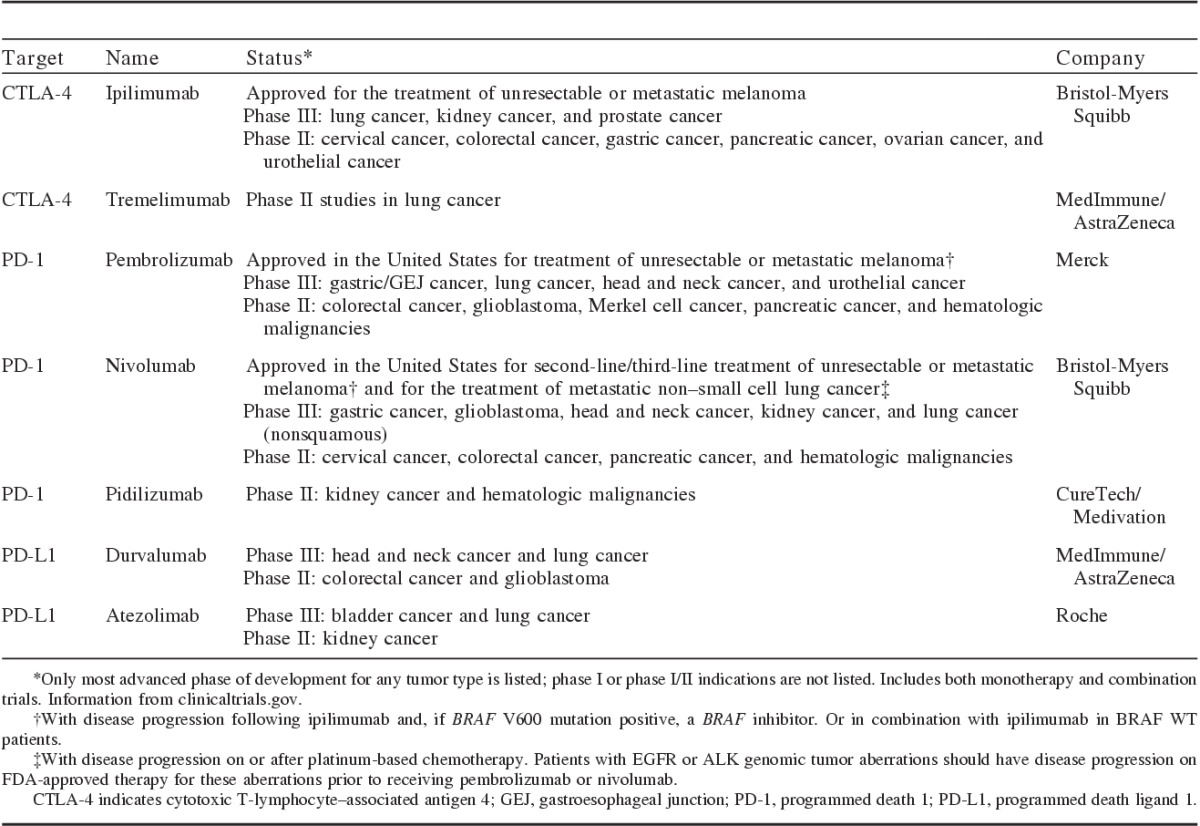
Preclinical studies showing decreased tumor growth and improved survival with CTLA-4 or PD-1 pathway blockade provide the rationale for immune checkpoint inhibition for cancer treatment.39,40 Monoclonal antibodies that block CTLA-4 or PD-1 are now approved for melanoma and lung cancer, and are in development for other tumor types, including kidney cancer, prostate cancer, and head and neck cancer (Table 1).41–44 Other agents targeting PD-L1 specifically are also in development (Table 1).41–44
TABLE 1.
CTLA-4 and PD-1 Pathway Inhibitors Approved or in Phase II and/or III Clinical Trial Stage of Development41–44
The exact mechanism by which anti-CTLA-4 antibodies induce an antitumor response is unclear, although research to date suggests that CTLA-4 blockade affects the immune priming phase by supporting the activation and proliferation of a higher number of effector T cells, regardless of TCR specificity, and by reducing Treg-mediated suppression of T-cell responses (Fig. 4).2 An increase in the diversity of the peripheral T-cell pool following CTLA-4 blockade in patients with melanoma has recently been reported.45 An ipilimumab study in patients with melanoma or prostate cancer provided evidence that baseline T-cell profile may also be important. An immediate turnover of the T-cell repertoire on initial treatment was shown, and it continued to evolve with further treatment; both expansion and loss of individual T-cell clonotypes were identified, but there was a net increase in TCR diversity.46 Overall survival, however, was associated with the maintenance of clones present in high frequency at baseline. In patients with shorter overall survival, numbers of these highest frequency clones decreased with treatment. These findings suggest that effective CTLA-4 blockade may depend on the ability to retain preexisting high-avidity T cells with relevance to the antitumor response.
FIGURE 4.
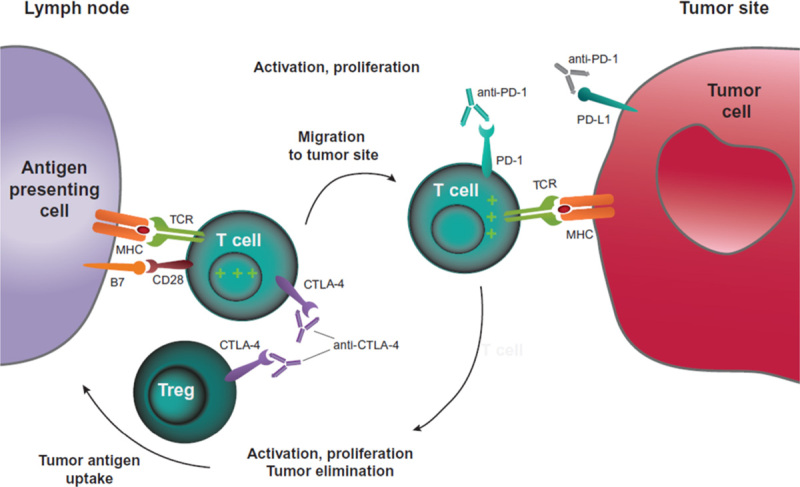
CTLA-4 and PD-1 pathway blockade. CTLA-4 blockade allows for activation and proliferation of more T-cell clones, and reduces Treg-mediated immunosuppression. PD-1 pathway blockade restores the activity of antitumor T cells that have become quiescent. A dual pathway blockade could have a synergistic effect, resulting in a larger and longer lasting antitumor immune response. CTLA-4 indicates cytotoxic T-lymphocyte–associated antigen 4; MHC, major histocompatibility complex; PD-1, programmed death 1; PD-L1, programmed death ligand 1; TCR, T-cell receptor; Treg, regulatory T cell.
PD-1 blockade works during the effector phase to restore the immune function of T cells in the periphery that have been turned off following extended or high levels of antigen exposure, as in advanced cancer.22,23 As mentioned above, the ligands for PD-1 can be expressed by tumor cells as well as tumor-infiltrating immune cells. PD-L1 expression on tumor cells varies by tumor type and also within a given tumor type, but appears to be particularly abundant in melanoma, non–small cell lung cancer (NSCLC), and ovarian cancer.27,28,47 In a recent study, PD-L1 expression on tumor cells was shown to be significantly associated with PD-1 expression on TILs, and was locally associated with PD-L2 expression when this ligand was also expressed.27 In the same study, tumor PD-L1 expression was the single factor most closely correlated with response to anti-PD-1 blockade, whereas PD-L1 expression on TILs was not associated with response.27 Another study, however, found that patient response to anti-PD-L1 blockade was strongest when PD-L1 was expressed by tumor-infiltrating immune cells.48
Inhibiting PD-L1 specifically, as opposed to PD-1 inhibition, will block PD-1:PD-L1 interactions while preserving PD-1:PD-L2 interactions. This has the potential to provide a more targeted signal with less unwanted toxicity, as self-tolerance mediated through PD-1:PD-L2 interactions should be preserved.37,49 Furthermore, as PD-L1 is known to bind CD80 as well as PD-1 to deliver inhibitory signals to T cells,33 PD-L1 inhibition with an appropriate antibody could in theory also prevent PD-L1 reverse signaling and its resulting T-cell downregulation through CD80; a PD-L1-directed antibody could also interrupt the PD-L1:CD80 axis on other cells where they are coexpressed, such as dendritic cells.20,23
The differences in timing, location, and nonredundant effects of their actions suggest that anti-CTLA-4–targeted therapies and anti-PD-1 therapies have the potential for additive or possibly synergistic effects in the treatment of advanced malignancy. Further evidence that supports this theory and highlights the different role of each immune checkpoint comes from a study that investigated the biological effect of CTLA-4 and PD-1 blockade in patients undergoing single-agent or combination treatment.50 While CTLA-4 inhibition induced a proliferative signal found predominantly in a subset of transitional memory T cells, PD-1 inhibition was associated with changes in genes thought to be involved in cytolysis and natural killer cell function; dual blockade led to nonoverlapping changes in gene expression. The 2 treatment types also produced different effects on levels of circulating cytokines. This study confirms that CTLA-4 and PD-1 blockade lead to distinct patterns of immune activation, supporting the rationale for the investigation of immune checkpoint combinations in the clinic.
CLINICAL EFFICACY AND CHARACTERISTICS OF RESPONSES WITH IMMUNE CHECKPOINT INHIBITORS
Anti-CTLA-4 blockade with ipilimumab was the first treatment to prolong overall survival in patients with advanced melanoma in a randomized setting.51,52 Analysis of long-term survival data pooled across several phase II and phase III trials showed that the survival curve begins to plateau at about 3 years, with 3-year survival rates of 22%, 26%, and 20% in all patients with sufficient follow-up, in treatment-naive patients, and in previously treated patients, respectively.53 Consistent with its survival benefit, CTLA-4 blockade is associated with durable responses in a proportion of patients treated, with some responses reported to last >3 years.51,54
More recently, PD-1 blockade has been shown to improve survival and progression-free survival in patients with metastatic melanoma and in patients with previously treated metastatic squamous and nonsquamous NSCLC.55–62 The longest follow-up data available indicate that highly durable responses can also occur with PD-1 blockade in patients with melanoma, NSCLC, or renal cell carcinoma (RCC).48,63–66 The response rates with PD-1 pathway blockade were higher than with CTLA-4 blockade in advanced melanoma: 33% to 34% versus 12% of patients in a phase III head-to-head trial of pembrolizumab versus ipilimumab. This trial also reported higher 1-year survival rates with pembrolizumab versus ipilimumab: 68% to 74% versus 58%.54
Because immune checkpoint inhibitors work by restarting an effective antitumor immune response, response patterns can differ from those seen with chemotherapy or targeted agents. Delayed or unconventional responses may be related to variations in the kinetics and efficacy of each patient’s individual immune system, as well as its interplay with tumors and metastases. An initial increase in target lesion tumor volume could be because of true tumor growth before the generation of effective antitumor response. Conversely, faster activation of an antitumor immune response could lead to inflammation and an influx of immune cells into the tumor site, which could masquerade as tumor progression. In clinical trials of ipilimumab, approximately 10% of patients were initially characterized as having progressive disease by World Health Organization criteria, but subsequently had favorable survival.67 Approximately 4% to 8% of patients with advanced melanoma receiving nivolumab or pembrolizumab in clinical trials had unconventional responses that did not meet Response Evaluation Criteria in Solid Tumors (RECIST) criteria, but were nevertheless associated with patient benefit.55,66,68,69 Unconventional response patterns have also been observed in patients with lung cancer or RCC receiving PD-1 pathway inhibitors.56,64,65,70 These atypical responses have led to the development of modified response criteria called immune-related response criteria (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/AJCO/A11067,68,71).
BIOMARKERS
A frustration with ipilimumab has been the inability to predict prospectively which patients are most likely to benefit from treatment. The low level of inducible CTLA-4 expression and the widespread expression of its B7 ligands are not useful as predictive biomarkers. Retrospective studies have identified several markers associated with response, including absolute lymphocyte count, upregulation of the T-cell activation maker-inducible costimulator (ICOS), and the development of a polyfunctional T-cell response to the tumor antigen NY-ESO-1.72 To date, none of these potential markers have been validated prospectively. An association between melanoma mutational load and clinical benefit with CTLA-4 blockade has been shown, but was insufficient alone to predict patients who are likely to respond to treatment73; however, work examining tumor neoantigens has shown promise, with the identification of a neoantigen signature present in tumors that correlated with overall survival of individuals treated with CTLA-4 blockade.73
In contrast, the upregulation of PD-1 on exhausted cells and of PD-L1 ligands on tumor cells or tumor-infiltrating immune cells may offer the potential for identifying patients responsive to PD-1 or PD-L1 blockade.27,48 Preliminary data across tumor types suggest that patients with PD-L1-expressing tumors or infiltrating immune cells typically have a higher response rate to anti-PD-1 or anti-PD-L1 therapy and may also have improved survival outcomes compared with patients with low or negative PD-L1 expression.27,48,55,63,64,70,74,75 However, in most studies, responses have also been seen in patients with PD-L1-low or PD-LI-negative tumors, and thus these patients should not be excluded from treatment. In a trial comparing the combination of ipilimumab and nivolumab against each agent alone, responses in PD-L1-positive patients were similar with the combination versus nivolumab alone, whereas PD-L1-negative patients did better receiving the combination.58 While all of these results are provocative, more research is needed to establish the validity and utility of PD-L1 expression as a predictive biomarker.
Other markers of response to anti-PD-1 or PD-L1 therapy have also been explored and include features associated with PD-L1-mediated suppression of preexisting immunity.48,76 As with CTLA-4, mutational burden and higher neoantigen burden have recently been shown to be associated with efficacy in patients with NSCLC treated with PD-1 blockade.77
IMMUNOLOGIC TOXICITIES
Immune checkpoint blockade is associated with AEs with potential immunologic etiologies, so-called immune-mediated AEs. Commonly reported immune-mediated AEs include rash or pruritus, gastrointestinal disorders, and endocrinopathies.54,55,57,66,69,78,79
The overall rate of grade ≥3 AEs was higher with ipilimumab (20%) compared with pembrolizumab (10% to 13%) in a phase III trial.54 Theoretically, this could be a consequence of a greater magnitude of T-cell proliferation or reduced Treg-mediated immunosuppression with CTLA-4 blockade, or activation of a smaller number of T-cell clones with PD-1 blockade.
Hypophysitis is reported in about 2% to 4% of patients receiving ipilimumab but in <1% of patients receiving PD-1 inhibitors51,54,55,69,80; however, this variation in incidence may not be related to differences in immune mechanism of action, but may be explained by ectopic expression of CTLA-4 in the pituitary gland, leading to ipilimumab binding to endocrine cells, followed by complement fixation and inflammation.80
Inhibiting PD-L1 rather than PD-1 may result in a slightly different toxicity profile, although clinical data are currently limited. Treatment-related grade 3-4 AEs were reported in 4% to 13% of patients receiving PD-L1 inhibitors in phase I/II trials across 2 different agents and multiple tumor types.48,74,75,81 While data from comparative trials are not yet available, the incidence of grade 3-4 treatment-related AEs may trend lower with PD-L1 inhibitors than with PD-1 inhibitors; however, the immune-mediated AEs reported to date have been similar between the 2 types of agents.
BLOCKADE OF BOTH CTLA-4 AND PD-1/PD-L1
Blockade of both CTLA-4 and PD-1 or PD-L1 could, in theory, induce proliferation of a higher number of T cells early in an immune response, restore immune responses of previously activated T cells that have become exhausted, and reduce Treg-mediated immunosuppression (Fig. 4). Preclinical studies showed enhanced antitumor responses using dual blockade compared with single-agent blockade, which was also observed in initial clinical trials.82–85 This synergistic effect validates the different roles these agents play in immune regulation.
An increased response rate and improved progression-free survival were reported with the ipilimumab-nivolumab combination when compared with ipilimumab alone in a randomized phase III trial in treatment-naive patients with metastatic melanoma.58 The objective response rate was 58% versus 19%, and the median progression-free survival was 11.5 versus 2.9 months for the combination and monotherapy, respectively.58 Combinations of CTLA-4 and PD-1 inhibitors are also being investigated in patients with several other tumor types, including advanced NSCLC and RCC. In metastatic RCC, preliminary data suggest that the objective response rate is higher with a combination blockade (38% to 43%) than was seen with PD-1 inhibition alone in a different trial (20% to 22%).70,86 Early data from lung cancer trials do not suggest increased antitumor activity with a combination blockade in NSCLC87,88; however, increased antitumor activity was seen with a combination blockade in small cell lung cancer (SCLC) versus nivolumab.89
Combining CTLA-4 and PD-1 blockade with the aim of increasing efficacy is highly desirable, but combination treatment could prove more toxic. In patients with previously untreated melanoma or recurrent SCLC, the incidence of drug-related grade 3-4 AEs was 54% to 55% with concurrent blockade compared with 24% to 27% with ipilimumab alone and 15% to 16% with nivolumab alone.58,83,89 Prior CTLA-4 inhibition does not appear to predispose patients to development of immune-mediated AEs with PD-1 inhibition,57,90,91 which may therefore support sequential rather than combination treatment.
CONCLUSIONS
The CTLA-4 and PD-1 immune checkpoint pathways downregulate T-cell activation to maintain peripheral tolerance, and can be exploited by tumors to induce an immunosuppressive state that allows the tumors to grow and develop instead of being eliminated by the immune system. The differential patterns of the CTLA-4 and PD-1 ligand expression—found primarily in lymphoid tissue and in peripheral tissues, respectively—are central to the hypothesis that CTLA-4 acts early in tolerance induction and PD-1 acts late to maintain long-term tolerance. Inhibitors of CTLA-4 and PD-1 or its ligand, PD-L1, can restore antitumor immune responses, leading to long-term benefit in a substantial proportion of treated patients. As a likely result of their mechanism of action, immune checkpoint inhibitors are associated with immune-mediated toxicities, most of which can be managed successfully with corticosteroids. Preliminary data suggest that simultaneous blockade of both CTLA-4 and PD-1 pathways leads to increased efficacy over CTLA-4 or PD-1 inhibition alone or in sequence, providing additional evidence of the separate roles of these checkpoints in regulating antitumor immune responses. Further trials are needed to confirm these data and validate a combination strategy.
To date, 3 immune checkpoint inhibitors have been approved for use in melanoma; 2 of the 3 are also approved for lung cancer. These and other investigational CTLA-4, PD-1, and PD-L1 inhibitors are in active clinical development for multiple indications and have the potential to revolutionize future treatment options for many patients with advanced cancer.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.amjclinicaloncology.com.
Professional medical writing assistance was provided by Britt Anderson, PhD, and professional editing assistance was provided by Lisa Sullivan at StemScientific, an Ashfield company, and was funded by Bristol-Myers Squibb. Bristol-Myers Squibb generated the concept for this review article; however, the authors developed the content. Bristol-Myers Squibb reviewed a draft for medical accuracy only. Neither Bristol-Myers Squibb nor StemScientific influenced the content of the manuscript, nor did the authors receive financial compensation for authoring the manuscript.
E.I.B. received research funding from Bristol-Myers Squibb, Genentech, and Merck; spouse previously employed by Merck. A.D. declares no conflicts of interest.
REFERENCES
- 1.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. [DOI] [PubMed] [Google Scholar]
- 2.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. [DOI] [PubMed] [Google Scholar]
- 3.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. [DOI] [PubMed] [Google Scholar]
- 4.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. [DOI] [PubMed] [Google Scholar]
- 6.Poschke I, Mougiakakos D, Kiessling R. Camouflage and sabotage: tumor escape from the immune system. Cancer Immunol Immunother. 2011;60:1161–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers CA, Kuhns MS, Egen JG, et al. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. [DOI] [PubMed] [Google Scholar]
- 8.Collins AV, Brodie DW, Gilbert RJ, et al. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. [DOI] [PubMed] [Google Scholar]
- 9.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. [DOI] [PubMed] [Google Scholar]
- 10.Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fallarino F, Fields PE, Gajewski TF. B7–1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J Exp Med. 1998;188:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masteller EL, Chuang E, Mullen AC, et al. Structural analysis of CTLA-4 function in vivo. J Immunol. 2000;164:5319–5327. [DOI] [PubMed] [Google Scholar]
- 13.Schneider H, Downey J, Smith A, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. [DOI] [PubMed] [Google Scholar]
- 14.Linsley PS, Bradshaw J, Greene J, et al. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. [DOI] [PubMed] [Google Scholar]
- 15.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81–88. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. [DOI] [PubMed] [Google Scholar]
- 19.Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett F, Luxenberg D, Ling V, et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol. 2003;170:711–718. [DOI] [PubMed] [Google Scholar]
- 22.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. [DOI] [PubMed] [Google Scholar]
- 23.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy—inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18:6580–6587. [DOI] [PubMed] [Google Scholar]
- 24.Latchman YE, Liang SC, Wu Y, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci. 2004;101:10691–10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. [DOI] [PubMed] [Google Scholar]
- 26.Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–1766. [DOI] [PubMed] [Google Scholar]
- 27.Taube JM, Klein AP, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. [DOI] [PubMed] [Google Scholar]
- 29.Rozali EN, Hato SV, Robinson BW, et al. Programmed death ligand 2 in cancer-induced immune suppression. Clin Dev Immunol. 2012;2012:656340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youngnak P, Kozono Y, Kozono H, et al. Differential binding properties of B7-H1 and B7-DC to death-1. Biochem Biophys Res Commun. 2003;307:672–677. [DOI] [PubMed] [Google Scholar]
- 31.Akbari O, Stock P, Singh AK, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010;3:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huber S, Hoffmann R, Muskens F, et al. Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood. 2010;116:3311–3320. [DOI] [PubMed] [Google Scholar]
- 33.Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Pino-Lagos K, de Vries VC, et al. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci. 2008;105:9331–9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–856. [DOI] [PubMed] [Google Scholar]
- 37.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300–5309. [DOI] [PubMed] [Google Scholar]
- 38.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23–35. [DOI] [PubMed] [Google Scholar]
- 39.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. [DOI] [PubMed] [Google Scholar]
- 40.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 41.Bristol-Myers Squibb Company. Yervoy (Ipilimumab) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2013. [Google Scholar]
- 42.US National Institutes of Health. Available at: http://www.clinicaltrials.gov. Accessed April 20, 2015.
- 43.Merck & Co Inc. Keytruda (Pembrolizumab) [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2015. [Google Scholar]
- 44.Bristol-Myers Squibb Company. Opdivo (Nivolumab) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2015. [Google Scholar]
- 45.Robert L, Tsoi J, Wang X, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res. 2014;20:2424–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cha E, Klinger M, Hou Y, et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci Transl Med. 2014;6:238–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–856. [DOI] [PubMed] [Google Scholar]
- 48.Herbst RS, Soria JC, Kowanetz M. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Das R, Verma R, Sznol M, et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194:950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. [DOI] [PubMed] [Google Scholar]
- 53.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farolfi A, Ridolfi L, Guidoboni M, et al. Ipilimumab in advanced melanoma: reports of long-lasting responses. Melanoma Res. 2012;22:263–270. [DOI] [PubMed] [Google Scholar]
- 55.Robert C, Schachter J, Long GV, et al. KEYNOTE-006 Investigators. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 56.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 57.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. [DOI] [PubMed] [Google Scholar]
- 59.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. September 27, 2015. [Epub ahead of print]. http://www.nejm.org/doi/full/10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 64.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death-1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDermott DF, Drake CG, Sznol M, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol. 2015;33:2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. [DOI] [PubMed] [Google Scholar]
- 68.Hodi FS, Ribas A, Daud A, et al. Evaluation of immune-related response criteria (irRC) in patients (pts) with advanced melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. J Clin Oncol. 2014;32(suppl):3006. [Google Scholar]
- 69.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. [DOI] [PubMed] [Google Scholar]
- 70.Motzer R, Rini B, McDermott D, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33:1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 72.Callahan MK, Postow MA, Wolchok JD. Immunomodulatory therapy for melanoma: ipilimumab and beyond. Clin Dermatol. 2013;31:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. [DOI] [PubMed] [Google Scholar]
- 75.Segal NH, Ou S-H, Balmanoukian AS, et al. Safety and efficacy of MEDI4736, an anti-PD-L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. J Clin Oncol. 2015;33(suppl):3011. [Google Scholar]
- 76.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. [DOI] [PubMed] [Google Scholar]
- 80.Iwama S, De RA, Callahan MK, et al. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6:230–245. [DOI] [PubMed] [Google Scholar]
- 81.Rizvi N, Brahmer J, Ou S-H, et al. Safety and clinical activity of MEDI4736, an anti-programmed cell death-ligand 1 (PD-L1) antibody, in patients with non-small cell lung cancer (NSCLC). J Clin Oncol. 2015;33(suppl):8032. [Google Scholar]
- 82.Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci. 2010;107:4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Selby M, Engelhardt J, Lu LS, et al. Antitumor activity of concurrent blockade of immune checkpoint molecules CTLA-4 and PD-1 in preclinical models. J Clin Oncol. 2013;31(suppl):3061.23569323 [Google Scholar]
- 85.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hammers H, Plimack ER, Infante JR, et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC). J Clin Oncol. 2015;33(suppl):4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Antonia SJ, Gettinger S, Chow LQ, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: interim phase I results. J Clin Oncol. 2014;32(suppl):8023. [Google Scholar]
- 88.Gettinger S, Hellmann MD, Shepherd FA, et al. First-line monotherapy with nivolumab (NIVO; anti-programmed death-1 [PD-1]) in advanced non-small cell lung cancer (NSCLC): safety, efficacy and correlation of outcomes with PD-1 expression. J Clin Oncol. 2015;33(suppl):8025. [Google Scholar]
- 89.Antonia SJ, Bendell JC, Taylor MH, et al. Phase I/II study of nivolumab with or without ipilimumab for treatment of recurrent small cell lung cancer (SCLC): CA209-032. J Clin Oncol. 2015;33:7503. [Google Scholar]
- 90.Ribas A, Hodi FS, Kefford R, et al. Efficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients (pts) with melanoma (MEL). J Clin Oncol. 2014;32(suppl):LBA9000. [Google Scholar]
- 91.Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


