Abstract
The intestinal immune system remains unresponsive to beneficial microbes and dietary antigens while activating pro-inflammatory responses against pathogens for host defence. In intestinal mucosa, abnormal activation of innate immunity, which directs adaptive immune responses, causes the onset and/or progression of inflammatory bowel diseases. Thus, innate immunity is finely regulated in the gut. Multiple innate immune cell subsets have been identified in both murine and human intestinal lamina propria. Some innate immune cells play a key role in the maintenance of gut homeostasis by preventing inappropriate adaptive immune responses while others are associated with the pathogenesis of intestinal inflammation through development of Th1 and Th17 cells. In addition, intestinal microbiota and their metabolites contribute to the regulation of innate/adaptive immune responses. Accordingly, perturbation of microbiota composition can trigger intestinal inflammation by driving inappropriate immune responses.
Keywords: adaptive immunity, commensal bacteria, gut homeostasis, inflammatory bowel disease, innate immunity
In the intestine, a refined balance is maintained between inflammatory responses and tolerance against multiple environmental factors, including microflora and food antigens. This is because aberrant inflammatory responses can lead to the development of inflammatory bowel disease (IBD), such as Crohn’s disease (CD) and ulcerative colitis. The number of effector T cells such as T helper (Th)1 cells and Th17 cells is increased in the gut mucosa of patients with IBD compared with healthy individuals, suggesting that Th1/Th17 responses contribute to the development or/and pathogenesis of IBD (1). Thus, the number and activity of effector T cells are tightly regulated by several mechanisms in the gut mucosa. For example, Foxp3+ regulatory (Treg) cells, abundantly present in the intestinal lamina propria, suppress inflammatory responses by producing anti-inflammatory cytokines including interleukin (IL)-10 and transforming growth factor (TGF)-β. IL-10 derived from Treg cells is thought to regulate the activity of intestinal myeloid cells.
Recently, several innate immune subsets possessing the ability to modulate intestinal homeostasis were identified in human and murine intestinal mucosa (2–4). In the gut, the activity of innate immune cells such as dendritic cells (DCs) and macrophages is tightly regulated by several mechanisms, and the excessive and inappropriate initiation of innate immunity causes development of IBD. Some innate myeloid subsets have been reported to promote intestinal inflammation by colitogenic effector T cells through upregulation of gut homing receptor α4β7 (5) and production of colitogenic cytokines such as IL-6 and IL-23 via high expression of TLRs (6). Accordingly, patients with CD have abnormal innate immune cell functions, including cytokine production, pathogen clearance and recruitment of neutrophils. This indicates that intestinal innate immunity is responsible for maintaining gut homeostasis and dysregulation of innate cell activity can lead to the development of IBD by driving colitogenic effector T cells.
Accumulating evidence has revealed that microbiota play a crucial role in maintaining gut homeostasis by controlling nutritional metabolism, epithelial barrier integrity and host immunity. Alterations of the microbial community structure, termed dysbiosis, are implicated in IBD because of increased pathogen invasion and the overgrowth of pathobionts (7–9).
In this review, we focus on a variety of intestinal innate immune subsets, which are implicated in the maintenance of gut homeostasis, and the impact of microbiota on development of host immune systems.
Innate Immunity and Gut Homeostasis
Several studies have identified a variety of mononuclear phagocytes that maintain gut homeostasis by enhancing or suppressing T cell responses (10–13). In particular, CD103+ DCs and CX3CR1+ cells have been well characterized in the murine intestinal mucosa (14–16) (Fig. 1). In addition, the human counterparts to murine intestinal macrophages, CD103+ DCs and CX3CR1+ cells were recently identified (Fig. 2).
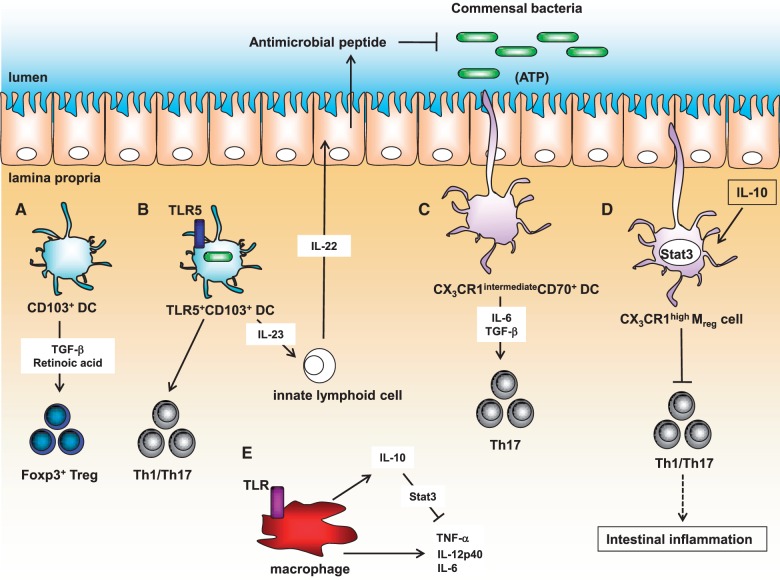
Fig. 1.
Murine innate immune subsets in the gut mucosa. (A) CD103+ DCs facilitate the differentiation of Foxp3+ Treg cells through the production of retinoic acid and TGF-β. (B) TLR5+CD103+CD11c+ DCs can induce the development of Th1/Th17 cells and IL-22 production in innate lymphoid cells by secreting IL-23 followed by antimicrobial peptide expression in intestinal epithelial cells. (C) CX3CR1intermediateCD70+CD11b+ DCs promote Th17 cell development by producing IL-6 and TGF-β upon ATP stimulus produced by commensal bacteria. (D) CX3CR1highMreg cells can prevent intestinal inflammation by inhibiting effector T cell proliferation. (E) Macrophages suppress production of pro-inflammatory cytokines by myeloid cells due to IL-10 production.
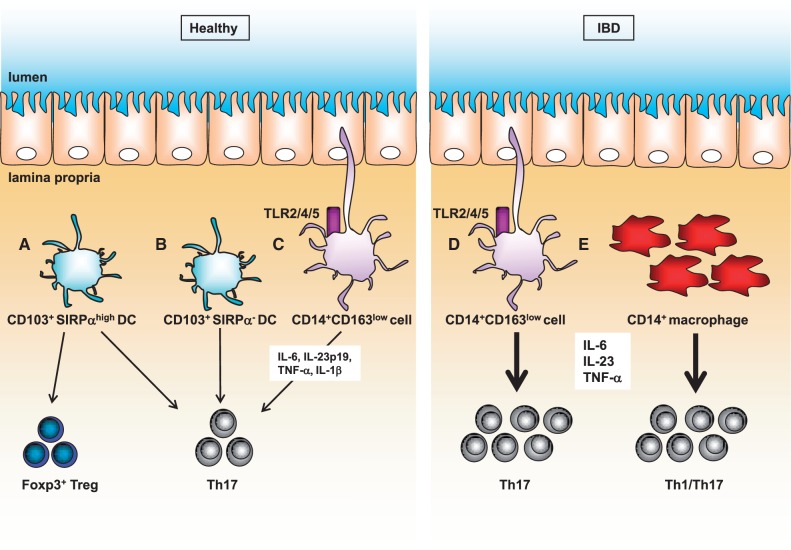
Fig. 2.
Human innate immune subsets in the gut mucosa. (A) CD103+SIRPαhigh DCs can induce Foxp3+ Treg cells. (B) CD103+SIRPαhigh DCs and CD103+SIRPα- DCs initiate Th 17 cell development. (C) CD14+CD163low cells induce Th17 differentiation by expression of IL-6, IL-23p19, TNF-α and IL-1β via TLR2, TLR4, and TLR5. (D and E) Colitogenic IL-17 and IFN-γ-producing T cells are induced by CD14+CD163low cells and macrophages in the gut mucosa of patients with IBD.
(i) CD103+ DCs
In the murine intestine, CD103+ DCs possess a variety of functions, including: inducting cytotoxic T lymphocytes (17); CD4+ and CD8+ T cell proliferation (17, 18) and gut immune tolerance by facilitating differentiation of Foxp3+ Treg cells through the production of retinoic acid and TGF-β (12, 19, 20). In addition, Toll-like receptor (TLR) 5-activated CD103+CD11c+ DCs induce the development of Th1/Th17 cells (21). Adequate Th1 and Th17 responses mediate host defence against invaded pathogens. Th1 cells-produced interferon (IFN)-γ activates macrophages to kill intracellular pathogens (22) whereas Th17-related cytokines such as IL-17 and IL-22 promote the production of anti-microbial peptides (23–25) and regulate integrity of epithelial cells (26, 27), respectively. Moreover, flagellin-dependent IL-23 production by CD103+ DCs induces IL-22 production from innate lymphoid cells followed by antimicrobial peptide expression in intestinal epithelial cells (28). Furthermore, common probiotic, Bifidobacterium breve activates intestinal CD103+ DCs to produce IL-10 and IL-27 via the TLR2/MyD88 pathway thereby inducing IL-10-producing Tr1 cells in the large intestine (29).
Human CD103+ DCs as well as murine CD103+ DCs induce gut homing receptors such as CCR9 and integrin α4β7 on T cells (18). In addition, transcription factor IRF4-expressing CD103+CD141-SIRPαhigh DCs induce Foxp3+ Treg cells by producing retinoic acid in human and murine small intestines (30). Both CD103+CD141−SIRPαhigh DCs and CD103+CD141−SIRPα- DCs initiate the development of Th 17 cells, which are equivalent to murine CD103+CD11b+ DCs that promote effector T cell differentiation.
Together, CD103+ DCs contribute to the maintenance of gut homeostasis by inducing immune tolerance to intestinal antigens, while promoting protective immune responses through the induction of Th1/Th17 cells in human and murine intestine.
(ii) Th17-inducing myeloid cells
Currently, several subsets of CX3CR1+ cells have been characterized in the murine lamina propria, including CD11c-CX3CR1+, CD11c+CX3CR1+CD68+F4/80+, and CD11c+CX3CR1+CD68−F4/80- cells (16). CX3CR1+ cells contribute to the induction of oral tolerance by transferring fed antigens to CD103+ DCs via gap junction molecule connexin 43 (31). In addition, CX3CR1+ cells help Th17 cells development (32, 33). In particular, CX3CR1intermediateCD70+CD11b+ DCs drive Th17 cell development by producing IL-6 and TGF-β upon ATP stimulus, which is derived from commensal bacteria (32). In the human intestinal mucosa, HLA-DRhighCD14+CD163low cells were identified as human counterparts to murine CX3CR1intermediateCD70+CD11b+ DCs. These cells induce Th17 cell differentiation by producing high levels of IL-6, IL-23p19, tumour necrosis factor (TNF)-α, and IL-1β via TLR2, TLR4 and TLR5 signalling pathways. Furthermore, the Th17 cell-inducing activity of HLA-DRhighCD14+CD163low cells is increased in patients with CD, suggesting that this cell subset might play a crucial role in the pathogenesis of CD.
In the intestine, commensal bacteria including Enterococcus mundtii, Enterococcus gallinarum, Escherichia coli and Salmonella secrete ATP, thereby mediating several immune responses (34–36). Thus, extracellular ATP is tightly regulated by ATP-hydrolyzing ecto-enzymes on epithelial cells and immune cells, such as ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases) and ecto-nucleotide pyrophosphatase/phosphodiesterases (E-NPPs) in the intestine. For example, E-NTPD7 on epithelial cells contributes to inhibiting Th17 development through ATP hydrolysis in the small intestine (37). In addition, E-NPP3 on mast cells is responsible for preventing allergen-induced diarrhoea by hydrolyzing ATP. ATP is secreted by mast cells upon FcεRI stimulation; thereby IL-6 production is elevated through purinergic receptor P2X7. In this context, E-NPP3 is also induced in mast cells and contributes to inhibiting ATP-dependent inflammatory responses (38).
(iii) CX3CR1high regulatory myeloid cells
In the murine colonic lamina propria, CX3CR1highCD11b+CD11c+ cells termed regulatory myeloid (Mreg) cells have a negative regulatory function (39). Mreg cells suppress CD4+ T cell proliferation by a cell-cell contact-dependent mechanism, and inhibit intestinal inflammation. Mreg cells preferentially associate with CD4+ T cells through highly expressed adhesion molecules such as ICAM-1 and VCAM-1, but do not activate CD4+ T cells because expression of CD80/CD86 was severely suppressed via IL-10/Stat3 signalling. LysM-cre; Stat3flox/flox mice, which harbour a Stat3 mutation specifically in myeloid cells, spontaneously develop colitis and show defective Mreg cell function. Transfer of wild-type Mreg cells to Stat3 mutant mice ameliorated intestinal inflammation, suggesting that the dysfunction of Mreg cells is involved in the pathogenesis of intestinal inflammation. However, human counterparts to murine Mreg cells remain elusive.
(iv) Macrophages
Intestinal CD11b+CD11c– macrophages produce large amounts of IL-10 in response to microbiota (40–42). Intestinal macrophage-derived IL-10 inhibits the production of pro-inflammatory cytokines including IL-12 and TNF-α produced by activated intestinal myeloid cells against microbiota by an IL-10/Stat3 signal-dependent mechanism. In addition, IL-10 produced by intestinal macrophages prevents intestinal inflammation by maintaining the persistence of Foxp3 expression in Treg cells (43). Accordingly, IL-10-deficient mice and LysM-cre; Stat3flox/flox mice spontaneously develop enteric inflammation accompanied by enhanced effector T cell activity (40, 44).
In patients with CD, numbers of CD14+ macrophages are increased and produce greater amounts of colitogenic cytokines such as IL-6, IL-23 and TNF-α against microbiota compared with healthy individuals (45). In addition, CD14+ macrophage-derived IL-23 affects the accumulation of colitogenic IL-17 and IFN-γ-producing T cells, suggesting that the abnormal activity of macrophages causes the development and/or pathogenesis of IBD (45–48).
(v) Innate lymphoid cell
In addition to innate myeloid cells, innate lymphoid cells (ILCs) comprising type 1 ILCs (ILC1s), ILC2s, and ILC3s are responsible for immune homeostasis. In particular, RORγt+ ILC3s predominantly produce IL-22 and/or IL-17 in the intestine. In both human and mice, IL-23 produced by DCs activates ILC3s responses (49). ILC3s-derived IL-22 promotes production of anti-microbial peptide and mucus production in epithelial cells (50–52), leading to host defence against pathogen such as Citrobacter rodentium (49, 50, 53, 54). In contrast, accumulation of IL-17- and IFN-γ- producing ILC3s is associated with development of colitis during Helicobacter hepaticus infection (55). These results indicate that ILC3s responses induce either host defence or inflammation in accordance with context.
Commensal Bacteria and Gut Homeostasis
The mammalian gastrointestinal tract harbours a huge number of microbial species. Recent studies report that intestinal microbiota mediate the maintenance of gut homeostasis by modulating both nutrient metabolism and host immune responses (56–58) (Fig. 3). Thus, perturbation of the microbiota composition is linked to the pathogenesis of IBD (59–62). Recently, several studies reported that host genetic alterations are implicated in the perturbation of intestinal microbiota composition leading to the development of IBD. Accordingly, IBD patients with NOD2 or ATG16L1 mutations showed altered intestinal microbial composition characterized by decreased amounts of Bacteroidetes and Firmicutes (63).
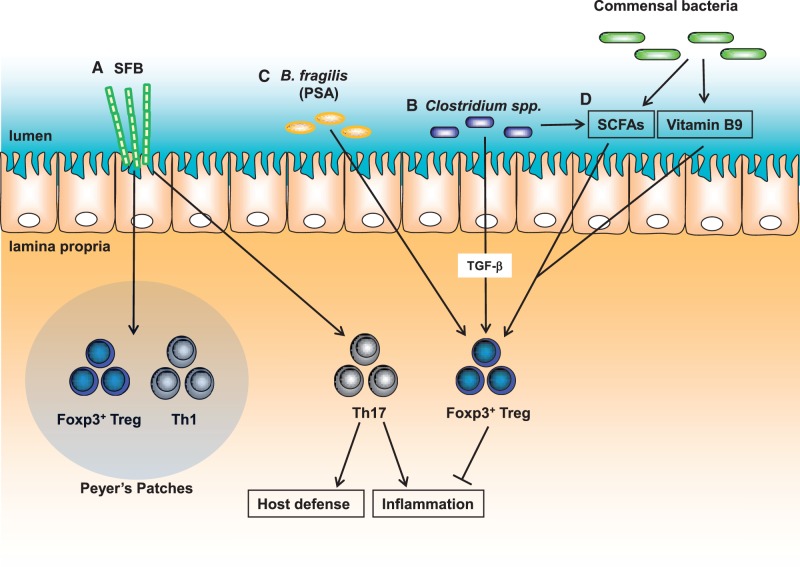
Fig. 3.
Functions of commensal bacteria on the host immunity. (A) SFB mediate the induction of Th1/Foxp3+ Treg cells and development of Th17 cells in Peyer’s Patches and the small intestine, respectively. (B) Clostridium species initiate the development of Foxp3+ Treg cells in the colon. (C) Bacteroides fragilis-derived polysaccharide A (PSA) contribute to induction of Foxp3+ Treg cells. (D) Micronutrients produced by commensal bacteria including SCFAs and Vitamin 9 participate in maintenance of Foxp3+ Treg cells in the gut.
Th17 Cell Generation by Commensal Bacteria
Segmented filamentous bacteria (SFB) mediate Th17 cell development in the small intestine (64) and induce Th1 cells and Foxp3+ Treg cells in Peyer’s patches (65), indicating that SFB may extensively regulate the intestinal adaptive immune system. Adhesion of SFB induces production of serum amyloid A protein and reactive oxygen species (ROS) in intestinal epithelial cells, leading to induction of antigen-specific Th17 cell development (66). SFB colonization has been reported to protect hosts from C.rodentium infection via the generation of Th17 cells. In contrast, SFB monocolonization of K/BxN mice triggered autoimmune arthritis (67) and enhanced experimental autoimmune encephalomyelitis (68, 69), indicating that the accumulation of Th17 cells by SFB colonization participates in developing autoimmune diseases while contributing to mucosal protection against pathogens.
Foxp3+ Treg cell Generation by Commensal Bacteria
Clostridium species belonging to cluster XIVa and IV initiate the development of Foxp3+ Treg cells in the colon (70). These Clostridium species activates production of matrix metalloproteinase (MMPs) and indoleamine 2, 3-dioxygenase. MMPs generate biologically active TGF-β, leading to the development and maintenance of Foxp3+ Treg cells. In addition, Bacteroides fragilis protect animals from experimental colitis by initiating Foxp3+ Treg cell development (71). The beneficial effect of B.fragilis depends on the expression of polysaccharide A, which is a unique surface polysaccharide that binds to TLR2 on CD4+ T cells (72).
Metabolites Derived from Microbiota and Gut Immunity
Micronutrients produced by commensal bacteria, such as short chain fatty acids (SCFAs), lipids and vitamins, can modulate the host immune system and further participate in shaping the microbiota architecture (60, 73). For instance, vitamin B9 also called folic acid derived from diet and commensal bacteria is involved in maintaining immunological homeostasis by promoting the survival of gut Foxp3+ Treg cells with high levels of folate receptor 4 (74, 75). In addition, bile acids synthesized by commensal bacteria suppress the production of IL-6, TNF-α, IL-1β and IFN-γ by inhibiting NF-κB activity via the G protein-coupled bile acid receptor (GPBAR1) and nuclear receptors subfamily 1, group H, member4 (NR1H4, also known as FXR) (76–78) in macrophages. IBD patients show intestinal dysbiosis, which is associated with lower concentrations of bile acid in the faeces and periphery compared with healthy individuals (79), indicating that altered bile acid production by microbiota might contribute to the development of IBD.
SCFAs, such as butyrate, acetate and propionate, are the major metabolites produced by commensal bacteria during fermentation of dietary fibre in the colon. They contribute to the maintenance of gut homeostasis through G protein-coupled receptors (GPRs) including GPR43 and GPR109A (80–83). Acetate produced by Bacteroides thetaiotaomicron facilitates goblet cell development and mucus secretion (84). Bifidobacterium longum can suppress the translocation of E. coli O157:H7 Shiga toxin by promoting intestinal epithelial integrity through the production of acetate (85). In addition, acetate promotes the development of Th1 and Th17 cells by inhibiting histone deacetylases (HDACs) activity that regulates the mTOR pathway (86). In contrast, butyrate and propionate promote the accumulation of Foxp3+ Treg cells and their suppressive activity by inhibiting HDAC activity through GPR43 (87, 88). Butyrate also negatively regulates the production of pro-inflammatory cytokines including IL-12, IL-6, TNF-α, and nitric oxide from macrophages through HDAC inhibition (89, 90). Accordingly, HDAC inhibitors have potential therapeutic benefit for IBD.
Conclusion
Recent advances have revealed the crucial roles of innate immune cells for immunological tolerance and protection against invading pathogens. Abnormal activity of innate immunity in the intestinal lamina propria can lead to the development of IBD by inducing inappropriate Th1/Th17 responses whereas normal Th1 and Th17 responses protect against pathogens for host defence. Thus, Foxp3+ Treg cells, which abundantly exist in the intestinal mucosa, inhibit innate and adaptive immune responses to prevent intestinal inflammation. In addition, recent studies showed that microbiota and nutritional metabolites derived from commensal bacteria are implicated in the maintenance of gut homeostasis and an imbalance in the composition of gut microbiota influences the pathogenesis of IBD. Thus, further studies to characterize the mechanisms by which commensal bacteria regulate the innate/adaptive immune systems may promote advances in therapeutic approaches for IBD.
Glossary
Abbreviation
- CCR9
CC chemokine receptor 9
- CX3CR1
CX3C chemokine receptor 1, Foxp3, Forkhead box P3
- HDAC
histone deacetylase
Funding
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology; the Japan Science and Technology Agency, and by the Ministry of Health, Labour, and Welfare.
Conflict of Interest
None declared.
References
- 1.Maloy K.J., Kullberg M.C. (2008) IL-23 and Th17 cytokines in intestinal homeostasis. Mucosal Immunol. 1, 339–349 [DOI] [PubMed] [Google Scholar]
- 2.Garrett W.S., Gordon J.I., Glimcher L.H. (2010) Homeostasis and inflammation in the intestine. Cell 140, 859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renz H., Brandtzaeg P., Hornef M. (2012) The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat. Rev. Immunol. 12, 9–23 [DOI] [PubMed] [Google Scholar]
- 4.Maloy K.J., Powrie F. (2011) Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474, 298–306 [DOI] [PubMed] [Google Scholar]
- 5.Do J.S., Visperas A., Freeman M.L., Iwakura Y., Oukka M., Min B. (2014) Colitogenic effector T cells: roles of gut-homing integrin, gut antigen specificity and gammadelta T cells. Immunol. Cell Biol. 92, 90–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddiqui K.R., Laffont S., Powrie F. (2010) E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity 32, 557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamada N., Chen G.Y., Inohara N., Nunez G. (2013) Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 14, 685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willing B., Halfvarson J., Dicksved J., Rosenquist M., Jarnerot G., Engstrand L., Tysk C., Jansson J.K. (2009) Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn's disease. Inflamm. Bowel Dis. 15, 653–660 [DOI] [PubMed] [Google Scholar]
- 9.Li E., Hamm C.M., Gulati A.S., Sartor R.B., Chen H., Wu X., Zhang T., Rohlf F.J., Zhu W., Gu C., Robertson C.E., Pace N.R., Boedeker E.C., Harpaz N., Yuan J., Weinstock G.M., Sodergren E., Frank D.N. (2012) Inflammatory bowel diseases phenotype, C. difficile and NOD2 genotype are associated with shifts in human ileum associated microbial composition. PLoS One 7, e26284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strober W. (2009) The multifaceted influence of the mucosal microflora on mucosal dendritic cell responses. Immunity 31, 377–388 [DOI] [PubMed] [Google Scholar]
- 11.Laffont S., Powrie F. (2009) Immunology: dendritic-cell genealogy. Nature 462, 732–733 [DOI] [PubMed] [Google Scholar]
- 12.Coombes J.L., Powrie F. (2008) Dendritic cells in intestinal immune regulation. Nat. Rev. Immunol. 8, 435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varol C., Zigmond E., Jung S. (2010) Securing the immune tightrope: mononuclear phagocytes in the intestinal lamina propria. Nat. Rev. Immunol. 10, 415–426 [DOI] [PubMed] [Google Scholar]
- 14.Varol C., Vallon-Eberhard A., Elinav E., Aychek T., Shapira Y., Luche H., Fehling H.J., Hardt W.D., Shakhar G., Jung S. (2009) Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 31, 502–512 [DOI] [PubMed] [Google Scholar]
- 15.Bogunovic M., Ginhoux F., Helft J., Shang L., Hashimoto D., Greter M., Liu K., Jakubzick C., Ingersoll M.A., Leboeuf M., Stanley E.R., Nussenzweig M., Lira S.A., Randolph G.J., Merad M. (2009) Origin of the lamina propria dendritic cell network. Immunity 31, 513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niess J.H., Adler G. (2010) Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J. Immunol. 184, 2026–2037 [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto K., Karuppuchamy T., Takemura N., Shimohigoshi M., Machida T., Haseda Y., Aoshi T., Ishii K.J., Akira S., Uematsu S. (2011) A new subset of CD103+CD8alpha+ dendritic cells in the small intestine expresses TLR3, TLR7, and TLR9 and induces Th1 response and CTL activity. J. Immunol. 186, 6287–6295 [DOI] [PubMed] [Google Scholar]
- 18.Jaensson E., Uronen-Hansson H., Pabst O., Eksteen B., Tian J., Coombes J.L., Berg P.L., Davidsson T., Powrie F., Johansson-Lindbom B., Agace W.W. (2008) Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 205, 2139–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson-Lindbom B., Svensson M., Pabst O., Palmqvist C., Marquez G., Forster R., Agace W.W. (2005) Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202, 1063–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun C.M., Hall J.A., Blank R.B., Bouladoux N., Oukka M., Mora J.R., Belkaid Y. (2007) Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uematsu S., Fujimoto K., Jang M.H., Yang B.G., Jung Y.J., Nishiyama M., Sato S., Tsujimura T., Yamamoto M., Yokota Y., Kiyono H., Miyasaka M., Ishii K.J., Akira S. (2008) Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 9, 769–776 [DOI] [PubMed] [Google Scholar]
- 22.Szabo S.J., Sullivan B.M., Stemmann C., Satoskar A.R., Sleckman B.P., Glimcher L.H. (2002) Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science 295, 338–342 [DOI] [PubMed] [Google Scholar]
- 23.Chan Y.R., Liu J.S., Pociask D.A., Zheng M., Mietzner T.A., Berger T., Mak T.W., Clifton M.C., Strong R.K., Ray P., Kolls J.K. (2009) Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J. Immunol. 182, 4947–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kao C.Y., Chen Y., Thai P., Wachi S., Huang F., Kim C., Harper R.W., Wu R. (2004) IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J. Immunol. 173, 3482–3491 [DOI] [PubMed] [Google Scholar]
- 25.Liang S.C., Tan X.Y., Luxenberg D.P., Karim R., Dunussi-Joannopoulos K., Collins M., Fouser L.A. (2006) Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dann S.M., Spehlmann M.E., Hammond D.C., Iimura M., Hase K., Choi L.J., Hanson E., Eckmann L. (2008) IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J. Immunol. 180, 6816–6826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebeis S.L., Powell K.R., Merlin D., Sherman M.A., Kalman D. (2009) Interleukin-1 receptor signaling protects mice from lethal intestinal damage caused by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 77, 604–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinnebrew M.A., Buffie C.G., Diehl G.E., Zenewicz L.A., Leiner I., Hohl T.M., Flavell R.A., Littman D.R., Pamer E.G. (2012) Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 36, 276–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeon S.G., Kayama H., Ueda Y., Takahashi T., Asahara T., Tsuji H., Tsuji N.M., Kiyono H., Ma J.S., Kusu T., Okumura R., Hara H., Yoshida H., Yamamoto M., Nomoto K., Takeda K. (2012) Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 8, e1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlitzer A., McGovern N., Teo P., Zelante T., Atarashi K., Low D., Ho A.W., See P., Shin A., Wasan P.S., Hoeffel G., Malleret B., Heiseke A., Chew S., Jardine L., Purvis H.A., Hilkens C.M., Tam J., Poidinger M., Stanley E.R., Krug A.B., Renia L., Sivasankar B., Ng L.G., Collin M., Ricciardi-Castagnoli P., Honda K., Haniffa M., Ginhoux F. (2013) IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38, 970–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazzini E., Massimiliano L., Penna G., Rescigno M. (2014) Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity 40, 248–261 [DOI] [PubMed] [Google Scholar]
- 32.Atarashi K., Nishimura J., Shima T., Umesaki Y., Yamamoto M., Onoue M., Yagita H., Ishii N., Evans R., Honda K., Takeda K. (2008) ATP drives lamina propria T(H)17 cell differentiation. Nature 455, 808–812 [DOI] [PubMed] [Google Scholar]
- 33.Denning T.L., Wang Y.C., Patel S.R., Williams I.R., Pulendran B. (2007) Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 8, 1086–1094 [DOI] [PubMed] [Google Scholar]
- 34.Mempin R., Tran H., Chen C., Gong H., Kim Ho K., Lu S. (2013) Release of extracellular ATP by bacteria during growth. BMC Microbiol. 13, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwase T., Shinji H., Tajima A., Sato F., Tamura T., Iwamoto T., Yoneda M., Mizunoe Y. (2010) Isolation and identification of ATP-secreting bacteria from mice and humans. J. Clin. Microbiol. 48, 1949–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hironaka I., Iwase T., Sugimoto S., Okuda K., Tajima A., Yanaga K., Mizunoe Y. (2013) Glucose triggers ATP secretion from bacteria in a growth-phase-dependent manner. Appl. Environ. Microbiol. 79, 2328–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kusu T., Kayama H., Kinoshita M., Jeon S.G., Ueda Y., Goto Y., Okumura R., Saiga H., Kurakawa T., Ikeda K., Maeda Y., Nishimura J., Arima Y., Atarashi K., Honda K., Murakami M., Kunisawa J., Kiyono H., Okumura M., Yamamoto M., Takeda K. (2013) Ecto-nucleoside triphosphate diphosphohydrolase 7 controls Th17 cell responses through regulation of luminal ATP in the small intestine. J. Immunol. 190, 774–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai S.H., Kinoshita M., Kusu T., Kayama H., Okumura R., Ikeda K., Shimada Y., Takeda A., Yoshikawa S., Obata-Ninomiya K., Kurashima Y., Sato S., Umemoto E., Kiyono H., Karasuyama H., Takeda K. (2015) The ectoenzyme E-NPP3 negatively regulates ATP-dependent chronic allergic responses by basophils and mast cells. Immunity 42, 279–293 [DOI] [PubMed] [Google Scholar]
- 39.Kayama H., Ueda Y., Sawa Y., Jeon S.G., Ma J.S., Okumura R., Kubo A., Ishii M., Okazaki T., Murakami M., Yamamoto M., Yagita H., Takeda K. (2012) Intestinal CX3C chemokine receptor 1(high) (CX3CR1(high)) myeloid cells prevent T-cell-dependent colitis. Proc. Natl. Acad. Sci. U. S. A. 109, 5010–5015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeda K., Clausen B.E., Kaisho T., Tsujimura T., Terada N., Forster I., Akira S. (1999) Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10, 39–49 [DOI] [PubMed] [Google Scholar]
- 41.Ueda Y., Kayama H., Jeon S.G., Kusu T., Isaka Y., Rakugi H., Yamamoto M., Takeda K. (2010) Commensal microbiota induce LPS hyporesponsiveness in colonic macrophages via the production of IL-10. Int. Immunol. 22, 953–962 [DOI] [PubMed] [Google Scholar]
- 42.Kuhn R., Lohler J., Rennick D., Rajewsky K., Muller W. (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 [DOI] [PubMed] [Google Scholar]
- 43.Murai M., Turovskaya O., Kim G., Madan R., Karp C.L., Cheroutre H., Kronenberg M. (2009) Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 10, 1178–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi M., Kweon M.N., Kuwata H., Schreiber R.D., Kiyono H., Takeda K., Akira S. (2003) Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J. Clin. Invest. 111, 1297–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamada N., Hisamatsu T., Okamoto S., Chinen H., Kobayashi T., Sato T., Sakuraba A., Kitazume M.T., Sugita A., Koganei K., Akagawa K.S., Hibi T. (2008) Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J. Clin. Invest. 118, 2269–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahern P.P., Schiering C., Buonocore S., McGeachy M.J., Cua D.J., Maloy K.J., Powrie F. (2010) Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 33, 279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamada N., Hisamatsu T., Honda H., Kobayashi T., Chinen H., Kitazume M.T., Takayama T., Okamoto S., Koganei K., Sugita A., Kanai T., Hibi T. (2009) Human CD14+ macrophages in intestinal lamina propria exhibit potent antigen-presenting ability. J. Immunol. 183, 1724–1731 [DOI] [PubMed] [Google Scholar]
- 48.Lee Y.K., Turner H., Maynard C.L., Oliver J.R., Chen D., Elson C.O., Weaver C.T. (2009) Late developmental plasticity in the T helper 17 lineage. Immunity 30, 92–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cella M., Fuchs A., Vermi W., Facchetti F., Otero K., Lennerz J.K., Doherty J.M., Mills J.C., Colonna M. (2009) A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J., Ouyang W. (2008) Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 [DOI] [PubMed] [Google Scholar]
- 51.Sonnenberg G.F., Fouser L.A., Artis D. (2011) Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 12, 383–390 [DOI] [PubMed] [Google Scholar]
- 52.Ouyang W., Kolls J.K., Zheng Y. (2008) The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28, 454–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sonnenberg G.F., Monticelli L.A., Elloso M.M., Fouser L.A., Artis D. (2011) CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34, 122–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satoh-Takayama N., Vosshenrich C.A., Lesjean-Pottier S., Sawa S., Lochner M., Rattis F., Mention J.J., Thiam K., Cerf-Bensussan N., Mandelboim O., Eberl G., Di Santo J.P. (2008) Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970 [DOI] [PubMed] [Google Scholar]
- 55.Buonocore S., Ahern P.P., Uhlig H.H., Ivanov, Littman D.R., Maloy K.J., Powrie F. (2010) Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee Y.K., Mazmanian S.K. (2010) Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 330, 1768–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Macpherson A.J., Harris N.L. (2004) Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 4, 478–485 [DOI] [PubMed] [Google Scholar]
- 58.Falk P.G., Hooper L.V., Midtvedt T., Gordon J.I. (1998) Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol. Mol. Biol. Rev. 62, 1157–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brestoff J.R., Artis D. (2013) Commensal bacteria at the interface of host metabolism and the immune system. Nat. Immunol. 14, 676–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kau A.L., Ahern P.P., Griffin N.W., Goodman A.L., Gordon J.I. (2011) Human nutrition, the gut microbiome and the immune system. Nature 474, 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nicholson J.K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W., Pettersson S. (2012) Host-gut microbiota metabolic interactions. Science 336, 1262–1267 [DOI] [PubMed] [Google Scholar]
- 62.Tremaroli V., Backhed F. (2012) Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 [DOI] [PubMed] [Google Scholar]
- 63.Frank D.N., Robertson C.E., Hamm C.M., Kpadeh Z., Zhang T., Chen H., Zhu W., Sartor R.B., Boedeker E.C., Harpaz N., Pace N.R., Li E. (2011) Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm. Bowel Dis. 17, 179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ivanov, Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., Tanoue T., Imaoka A., Itoh K., Takeda K., Umesaki Y., Honda K., Littman D.R. (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gaboriau-Routhiau V., Rakotobe S., Lecuyer E., Mulder I., Lan A., Bridonneau C., Rochet V., Pisi A., De Paepe M., Brandi G., Eberl G., Snel J., Kelly D., Cerf-Bensussan N. (2009) The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 [DOI] [PubMed] [Google Scholar]
- 66.Atarashi K., Tanoue T., Ando M., Kamada N., Nagano Y., Narushima S., Suda W., Imaoka A., Setoyama H., Nagamori T., Ishikawa E., Shima T., Hara T., Kado S., Jinnohara T., Ohno H., Kondo T., Toyooka K., Watanabe E., Yokoyama S., Tokoro S., Mori H., Noguchi Y., Morita H., Ivanov, Sugiyama T., Nunez G., Camp J.G., Hattori M., Umesaki Y., Honda K. (2015) Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell 163, 367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu H.J., Ivanov, Darce J., Hattori K., Shima T., Umesaki Y., Littman D.R., Benoist C., Mathis D. (2010) Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berer K., Mues M., Koutrolos M., Rasbi Z.A., Boziki M., Johner C., Wekerle H., Krishnamoorthy G. (2011) Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 [DOI] [PubMed] [Google Scholar]
- 69.Lee Y.K., Menezes J.S., Umesaki Y., Mazmanian S.K. (2011) Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 108 (Suppl. 1), 4615–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., Cheng G., Yamasaki S., Saito T., Ohba Y., Taniguchi T., Takeda K., Hori S., Ivanov I.I., Umesaki Y., Itoh K., Honda K. (2011) Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mazmanian S.K., Round J.L., Kasper D.L. (2008) A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453, 620–625 [DOI] [PubMed] [Google Scholar]
- 72.Round J.L., Lee S.M., Li J., Tran G., Jabri B., Chatila T.A., Mazmanian S.K. (2011) The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332, 974–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wood L.G., Garg M.L., Smart J.M., Scott H.A., Barker D., Gibson P.G. (2012) Manipulating antioxidant intake in asthma: a randomized controlled trial. Am. J. Clin. Nutr. 96, 534–543 [DOI] [PubMed] [Google Scholar]
- 74.Kunisawa J., Hashimoto E., Ishikawa I., Kiyono H. (2012) A pivotal role of vitamin B9 in the maintenance of regulatory T cells in vitro and in vivo. PLoS One 7, e32094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kinoshita M., Kayama H., Kusu T., Yamaguchi T., Kunisawa J., Kiyono H., Sakaguchi S., Takeda K. (2012) Dietary folic acid promotes survival of Foxp3+ regulatory T cells in the colon. J. Immunol. 189, 2869–2878 [DOI] [PubMed] [Google Scholar]
- 76.Vavassori P., Mencarelli A., Renga B., Distrutti E., Fiorucci S. (2009) The bile acid receptor FXR is a modulator of intestinal innate immunity. J. Immunol. 183, 6251–6261 [DOI] [PubMed] [Google Scholar]
- 77.Pols T.W., Nomura M., Harach T., Lo Sasso G., Oosterveer M.H., Thomas C., Rizzo G., Gioiello A., Adorini L., Pellicciari R., Auwerx J., Schoonjans K. (2011) TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 14, 747–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y.D., Chen W.D., Yu D., Forman B.M., Huang W. (2011) The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) in mice. Hepatology 54, 1421–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duboc H., Rajca S., Rainteau D., Benarous D., Maubert M.A., Quervain E., Thomas G., Barbu V., Humbert L., Despras G., Bridonneau C., Dumetz F., Grill J.P., Masliah J., Beaugerie L., Cosnes J., Chazouilleres O., Poupon R., Wolf C., Mallet J.M., Langella P., Trugnan G., Sokol H., Seksik P. (2013) Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 62, 531–539 [DOI] [PubMed] [Google Scholar]
- 80.Maslowski K.M., Vieira A.T., Ng A., Kranich J., Sierro F., Yu D., Schilter H.C., Rolph M.S., Mackay F., Artis D., Xavier R.J., Teixeira M.M., Mackay C.R. (2009) Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh N., Gurav A., Sivaprakasam S., Brady E., Padia R., Shi H., Thangaraju M., Prasad P.D., Manicassamy S., Munn D.H., Lee J.R., Offermanns S., Ganapathy V. (2014) Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40, 128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zandi-Nejad K., Takakura A., Jurewicz M., Chandraker A.K., Offermanns S., Mount D., Abdi R. (2013) The role of HCA2 (GPR109A) in regulating macrophage function. FASEB J. 27, 4366–4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gambhir D., Ananth S., Veeranan-Karmegam R., Elangovan S., Hester S., Jennings E., Offermanns S., Nussbaum J.J., Smith S.B., Thangaraju M., Ganapathy V., Martin P.M. (2012) GPR109A as an anti-inflammatory receptor in retinal pigment epithelial cells and its relevance to diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 53, 2208–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wrzosek L., Miquel S., Noordine M.L., Bouet S., Joncquel Chevalier-Curt M., Robert V., Philippe C., Bridonneau C., Cherbuy C., Robbe-Masselot C., Langella P., Thomas M. (2013) Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 11, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fukuda S., Toh H., Hase K., Oshima K., Nakanishi Y., Yoshimura K., Tobe T., Clarke J.M., Topping D.L., Suzuki T., Taylor T.D., Itoh K., Kikuchi J., Morita H., Hattori M., Ohno H. (2011) Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547 [DOI] [PubMed] [Google Scholar]
- 86.Park J., Kim M., Kang S.G., Jannasch A.H., Cooper B., Patterson J., Kim C.H. (2015) Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 8, 80–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., deRoos P., Liu H., Cross J.R., Pfeffer K., Coffer P.J., Rudensky A.Y. (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly Y.M., Glickman J.N., Garrett W.S. (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang P.V., Hao L., Offermanns S., Medzhitov R. (2014) The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. U. S. A. 111, 2247–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vinolo M.A., Rodrigues H.G., Nachbar R.T., Curi R. (2011) Regulation of inflammation by short chain fatty acids. Nutrients 3, 858–876 [DOI] [PMC free article] [PubMed] [Google Scholar]