Abstract
Although males often display from mixed-species aggregations, the influence of nearby heterospecifics on risks associated with sexual signalling has not been previously examined. We tested whether predation and parasitism risks depend on proximity to heterospecific signallers. Using field playback experiments with calls of two species that often display from the same ponds, túngara frogs and hourglass treefrogs, we tested two hypotheses: (1) calling near heterospecific signallers attractive to eavesdroppers results in increased attention from predatory bats and parasitic midges (collateral damage hypothesis) or (2) calling near heterospecific signallers reduces an individual's predation and parasitism risks, as eavesdroppers are drawn to the heterospecifics (shadow of safety hypothesis). Bat visitation was not affected by calling neighbours. The number of frog-biting midges attracted to hourglass treefrog calls, however, rose threefold when played near túngara calls, supporting the collateral damage hypothesis. We thus show that proximity to heterospecific signallers can drastically alter both the absolute risks of signalling and the relative strengths of pressures from predation and parasitism. Through these mechanisms, interactions between heterospecific guild members are likely to influence the evolution of signalling strategies and the distribution of species at both local and larger scales.
Keywords: mating signals, risk, túngara frogs, hourglass treefrogs, Trachops cirrhosus, Corethrella midges
1. Introduction
The use of conspicuous mating signals that increase attractiveness to females is nearly ubiquitous among animals [1–3]. The evolution of mating signals is, however, shaped by opposing selective pressures that act on different components of fitness—mating and survival. In addition to females, sexual advertisement signals can also attract eavesdropping predators and parasites [4–8]. Signalling behaviours influence the costs imposed by such eavesdroppers. High call rates or signal complexity, for example, increase attractiveness to predators and parasites [4,9–11]. Likewise, proximity to signalling conspecifics can alter selective pressures acting on a calling individual through the dilution of risks posed by signal-oriented predators and parasites, or by increasing the perceptual challenges faced by such eavesdroppers [12–15].
Given that mixed-species aggregations signalling for mates are common across many taxa (e.g. dawn chorus of birds or mixed-species frog choruses: [16,17]), interactions with heterospecifics may also be an important mechanism modulating signalling trade-offs and driving individual signalling behaviour. While prey foraging in multi-species aggregations has been widely documented to enjoy reductions in predation risk [18–20], the ways in which inter-species signalling dynamics influence risks to prey within mating aggregations have not been previously investigated.
Here, we examine how signallers producing signals highly attractive to eavesdroppers alter the predator and parasitism risks suffered by nearby heterospecific individuals signalling within the same aggregation. Specifically, we conducted field playback experiments to determine how proximity to calling heterospecifics alters the responses of eavesdropping predators and parasites to the calls of two species of Neotropical anurans often found calling at the same ponds: túngara frogs (Engystomops pustulosus) and hourglass treefrogs (Dendropsophus ebraccatus).
During the breeding season, túngara frog males, like males of many anuran species, aggregate in puddles and ponds from which they call to attract females [21]. These mating calls are exploited by a broad variety of predators and parasites, including the fringe-lipped bat (Trachops cirrhosus) and parasitic frog-biting midges (Corethrella spp.; reviewed in [22]). Hourglass treefrogs often share their breeding habitat with túngara frogs, and their calls also attract these two species of eavesdroppers. Male hourglass treefrogs, however, attract considerably fewer midges than túngara frogs. For both species, risks posed by eavesdropping predators and parasites are of prime importance. Fringe-lipped bats often impose a lethal cost on signallers. Although parasitism by frog-biting midges has lower immediate costs, it is also important given the high numbers of midges attacking calling males [9], the substantial amounts of blood they can take from each signaller (XE Bernal 2010, unpublished data), and the fact that they can transmit blood parasites such as trypanosomes [23,24].
In this study, we determine whether calling next to a male that produces signals highly attractive to eavesdroppers influences predation and parasitism risks of a neighbouring heterospecific male with less attractive signals. Specifically, we examined how calling túngara frogs alter the eavesdropper attack rates for the hourglass treefrog. We investigate two mutually exclusive hypotheses about the risks of calling near individuals of a species highly attractive to eavesdroppers: (1) an increase in predation or parasitism risk might result due to bats and midges being drawn to the area by túngara frog calls and then attacking nearby heterospecific frogs (collateral damage hypothesis); (2) a decrease in predation or parasitism risk might result due to foraging bats and midges preferentially attacking túngara frogs, overlooking nearby heterospecific frogs (shadow of safety hypothesis). We discuss our results in terms of these alternative hypotheses, the calling behaviour of individual frogs, and the potential implications for the composition of mating aggregations.
2. Material and methods
(a). Study sites and field methods
We conducted two field playback experiments between October 2012 and February 2013. For both experiments, we set up speakers (Tympany Peerless 2.5 inch driver, Sausalito, USA connected to Kemo 12 W amplifier (M032N), Geestland, Germany, and Sony mp3-player (Sony NWZ-B162F) playing .wav files) at eight different sites within the vicinity of streams, pools or ponds around Gamboa, Soberanía National Park, Panama. Hourglass treefrogs and túngara frogs call from small ponds and puddles throughout Soberanía, overlapping at many of these sites. Hourglass treefrogs usually call from vegetation growing in or near pools of water, whereas túngara frogs can call from vegetated as well as non-vegetated pools. All sites were at least 1 km apart from each other to maximize the probability of testing different frog-eating bats in each location, given their known home ranges [25]. We also assigned a random number to the sites [1–8] and subsequently cycled through that order so that there was a minimum of an 8-day gap in between subsequent trials at any single site (electronic supplementary material, S1). Each site was initially planned to be tested a total of four times (32 nights), but we retested some sites an extra time because the videocamera or IR light batteries shut down before the trial was over. In these cases, we had a minimum of a 4-day gap between subsequent trials. Each night, we played calls of each species from the speakers within the range of their natural call amplitudes, preserving the mean relative amplitudes of the two call types (78 dB SPL re. 20 µP for túngara frog calls and 89 dB re. 20 µP for the hourglass treefrog calls, at a distance of 1 m from each speaker; C-weighted, fast response) [21,26]. To increase the probability that bats had an opportunity to hear each call type before attacking, to more closely approximate conditions on high activity nights, and to increase comparability with previously published studies, call periods were standardized to 1 s.d. below (2 s for túngara, and 3.55 s for hourglass treefrogs) the published mean periods for each species [21,27,28]. Stimuli for each species consisted of call exemplars from one of 10 individuals, each containing between two and five unique calls. One of these stimuli from each species was randomly selected for use in all treatments within a given night.
(b). Experiment I: the influence of nearby signallers on parasitism and predation risks
To investigate the effect of nearby túngara calls on calling hourglass treefrogs, at each site we placed four playback stations separated by 25 m in a transect line bordering the edge of the streams, pool or ponds, each broadcasting a different experimental treatment. We rotated the relative locations of the treatments along the transect each night. Each treatment consisted of two speakers, separated 2 m apart for the bat portion of the experiment, and by 1 m for the midge portion of the experiment. These distances are typical inter-individual spacing at choruses of these species in nature [21,29]. Each speaker set-up consisted of a wooden circle, 33 cm in diameter, with a circular opening in the centre, in which we positioned a speaker, flush with the platform. We attached a plastic frog model (approx. 2.50 cm long and 1.3 cm wide) adjacent to the speaker on each platform. Frog models were identical for all treatments and were of an intermediate size between the two focal frog species, within the size range of each. The treatments in this experiment were as follows: (i) conspecific treatment (HH): an hourglass treefrog calling next to another hourglass treefrog, with calls played alternately from the two speakers; (ii) heterospecific treatment (HT): an hourglass treefrog calling next to a calling túngara, with calls played antiphonally from the two speakers; (iii) hourglass alone treatment (HA): an hourglass treefrog call playing from one speaker, adjacent to a speaker platform with a silent frog model; (iv) Túngara alone treatment (TA): a túngara call playing from one speaker, adjacent to a speaker platform with a silent frog model.
To examine incoming predators to the calling speakers, we played stimuli for 80 min and recorded with Sony Nightshot (DCR-SR45, Tokyo, Japan) cameras with supplemental IR lights positioned 3 m away from the centre of each two-platform treatment (methods modified from [29]). Predator playbacks were set up first every experimental night in order to coincide with times of peak bat foraging activity. After these initial 80 min, we set up modified CDC miniature light traps (without the light bulb), following Bernal et al. [9] to collect incoming insects attracted to the calls. We broadcasted the same stimuli used in the previous portion of the experiment for 40 more minutes. Based on previous studies, 40 min is enough to collect hundreds of flies per insect trap in speakers broadcasting túngara calls, whereas 80 min is necessary to account for enough bat visitations [9,30]. Following playback, we euthanized the captured insects in a freezer for later identification and quantification. Throughout the night, we removed any live frogs attracted to the speakers and relocated them to nearby breeding areas.
(c). Experiment II: baseline attractiveness of túngara frog and hourglass treefrog calls to predators and parasites
To establish the baseline attractiveness of calls of both species to eavesdropping bats in the wild without any potential influence of nearby treatments, we conducted an additional playback experiment, in which calls of only one species were played at site on a given night. We placed three playback speakers in a line transect, spaced by 25 m, in the same eight sites and positions used before. Each night, we chose three out of the four potential positions randomly. The túngara frog call only treatment (TO) included túngara frog calls played from a single speaker at two different locations and a silent speaker at a third location within the site. The hourglass treefrog call only treatment (HO) included hourglass treefrog calls played from a single speaker at two different locations and a silent speaker at a third location within the site. We set up single species trials on two sites each night, with a different species played at each site. We conducted trials at each site on two nights, switching the species played between nights, for a total of eight nights and eight sites per species. The order in which sites were used was random.
To quantify the baseline attractiveness of túngara frog and hourglass treefrog calls to midges, we assumed the distance between treatments in Experiment I (greater than or equal to 25 m) as large enough relative to the size of these small insects to consider the treatments independent form one another. We, therefore, examined midge numbers attracted to the túngara frog call alone (TA) treatment and the hourglass treefrog call alone (HA) treatments in the first experiment.
(d). Video analysis and midge identification
During video analysis, visits were scored only for bat passes that were directly above the speaker, below 50 cm in height; and had a change of flight direction in relation to the platform. Changes in flight path that met these criteria included curving at least 90° around the platform, diving down and hovering on top of platform, circling one, two or three times around the platform; and landing on the platform. Subsequent passes to the same speaker separated by less than 1 min were counted as the same visit. Because it was not possible to identify individual bats, a single bat may have been attracted to a speaker multiple times. Nonetheless, as we were testing whether there was greater predation pressure against calls produced next to conspecific or heterospecific signallers, we focused on the nightly difference between visits to each call treatment. This relative difference should be maintained even if multiple visitations increase the absolute number of visits recorded [30].
Frog-biting midges collected in the insect traps were counted and stored in ethanol 75%. A sub-sample of the midges collected were identified using the key for Neotropical Corethrellidae published by Borkent [31]. Similar to previous studies [32], the most common species of frog-biting midges found in traps broadcasting túngara and hourglass frog calls were the same. For the purpose of this study, and following previous work [9], we pooled all midge species together to examine the effect of these eavesdroppers as a guild on the calling frogs.
(e). Statistical analysis
All statistical analyses were conducted using the program R v. 2.15.1 (R Development Core Team: www.R-project.org). To investigate any changes in the predation or parasitism risks faced by hourglass treefrogs calling next to túngara frogs, we compared nightly bat and midge visits to the hourglass treefrog call in the heterospecific treatment (HHT), hourglass treefrog call in the conspecific treatment (HHH) and the hourglass treefrog call in the single treatment (HA). We used a generalized linear mixed effect model function (GLMM) in the glmmADMB package [33] with a negative binomial error structure and a log link. We included call treatment as a fixed factor, site as a random factor, and date as a random factor nested within site. Because we were interested in the relative risk posed to calls in each treatment, rather than in quantifying absolute risk to frogs in particular contexts, and we did not wish to score predator preferences when no predators were present, nights without at least one bat visit to any of the treatments were excluded from the analysis. To determine which treatments were significantly different from each other, we used the glht function in the multcomp R package [34] and the mcp linear function with a Tukey contrasts test.
We also investigated whether changes in risk were due to a heterospecific transfer of risks from individuals of one species to individuals of the other. To determine this, we compared the number of bat visits and the number of flies attracted to the túngara frog call alone (TA) treatment with visits to the túngara frog call when played next to the hourglass treefrog call (THT) using the GLMM function (negative binomial error structure; call treatment as fixed factor, site as random factor and date nested within site). If risks were transferred from túngara frogs to hourglass treefrogs, or vice versa, we expected to see a difference between these two treatments.
Finally, to assess any differences in baseline bat visitations to túngara frogs and hourglass treefrog calls, we compared the number of nightly bat visits to the túngara frog only (TO) and hourglass treefrog only (HO) treatments in the second experiment (GLMM function; negative binomial error structure; species treatment as fixed effect and site as random effect). Similarly, to assess baseline midge visitations to calls of the two frog species, we compared the number of flies attracted nightly to the túngara frog alone (TA) and hourglass treefrog alone (HA) treatments in the first experiment (GLMM function; negative binomial error structure, call treatment as fixed factor, site as the random factor and date nested within site).
3. Results
(a). Experiment I: the influence of nearby signallers on parasitism and predation risks
Playback experiments successfully attracted predators and parasites to the speakers. The most common predators were bats. These were likely Trachops cirrhosus (Spix, 1823), identified in some videos by their ear and wing morphology. Other less common visitors to the speakers were opossums (three nights) and caiman (one night). Bats approached our speakers on 20 of 32 nights (62.5%).
Analysis of the playback videos showed that hourglass treefrog calls attracted a similar numbers of bat visits in all treatments (χ2 = 0.062, p > 0.969; mean number of bat visits ± s.d. for HHT = 0.632 ± 1.34; HHH = 0.684 ± 1.003; and HA = 0.632 ± 0.895, n = 19; figure 1). Similarly, we found no differences in the number of bat visits to túngara frog calls in the heterospecific (THT) versus single (TA) treatments (χ2 = 0.532, p > 0.53; THT = 0.769 ± 0.599 and TA = 0.538 ± 0.967, n = 13; figure 1).
Figure 1.
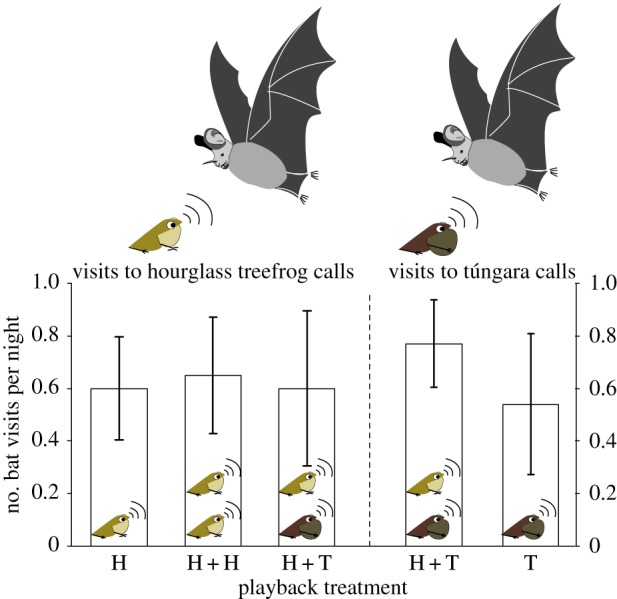
Mean number (±1 s.e.) of approaches per night by bats to speakers playing recorded hourglass treefrog (left side of the figure) and túngara frog (right side) calls. Treatments were as follows: HA, hourglass treefrog calls played alone; HH, hourglass treefrog calls played next to conspecific calls; HT, hourglass treefrog calls played next to túngara frog calls; T, túngara frog calls played alone.
On the other hand, we found that hourglass treefrogs calling next to túngara frogs faced stronger parasitism risk, with a threefold increase in the number of midges attracted to hourglass treefrog calls when they were in heterospecific (HHT) treatments as compared to when they were in conspecific (HHH) or single (HA) treatments (χ2 = 54.38, p < 0.0001; mean number of flies ± s.d. for HHT = 86.937 ± 83.75; HHH = 29.312 ± 37.25; and HA = 27.31 ± 29.53; Tukey contrasts: HHT–HHH, p < 0.0001, HHT–HA, p < 0.0001, HHH–HA, p = 0.998; n = 32; figure 2). Interestingly, we found no difference in the number of midges attracted to túngara frog calls in heterospecific versus single túngara call treatments (χ2 = 0.636, p < 0.425; THT = 443.437 ± 388.868 and TA = 424.906 ± 420.436, n = 32; figure 2).
Figure 2.
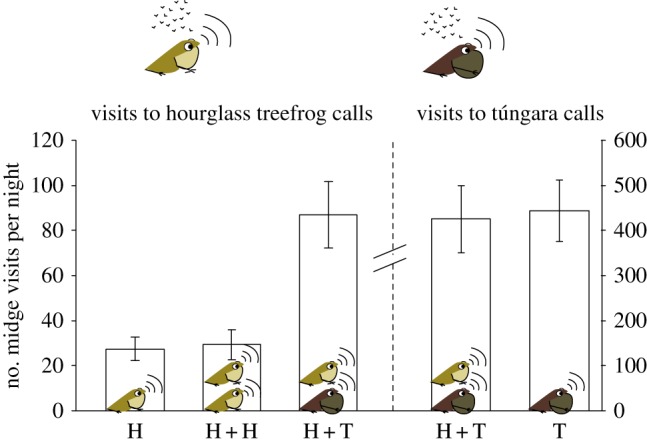
Mean number (±1 s.e.) of approaches per night by midges to speakers playing recorded hourglass treefrog (left side of the figure) and túngara frog (right side) calls. Treatments were as follows: HA, hourglass treefrog calls played alone; HH, hourglass treefrog calls played next to conspecific calls; HT, hourglass treefrog calls played next to túngara frog calls; T, túngara frog calls played alone.
(b). Experiment II: baseline attractiveness of túngara frog and hourglass treefrog calls to predators and parasites
We found no differences in the number of bat visits to speakers where only túngara frog calls were played compared with speakers where only hourglass treefrog calls were played (χ2 = 0.192, p < 0.7411; mean number of bat visits ± s.d. for HO = 1.625 ± 2.065 and TO = 1.875 ± 1.96; n = 8). By contrast, túngara frogs calling alone attracted 15 times more Corethrella spp. midges than hourglass treefrogs calling alone (χ2 = 86.144, p < 0.0001; HA = 27.312 ± 29.531; TA = 424.906 ± 420.435; n = 32).
4. Discussion
Signalling in mixed-species choruses may have important consequences for an individual's risk of predation or parasitism, in particular when these risks are species-specific and asymmetrical. We tested whether calling in the vicinity of heterospecifics that are attractive to eavesdroppers reduces (shadow of safety hypothesis), or increases (collateral damage hypothesis) an individual's own signalling risk. Our data confirm that both túngara frogs and hourglass treefrogs face considerable predation and parasitism risk. More importantly, we found that parasitism risk varied strongly with the presence of nearby calling heterospecifics, but predation risk did not. The observed heterospecific effects were asymmetrical, with parasitism risk to hourglass treefrogs being strongly affected by the presence of neighbouring túngara, but not the converse. Thus, our results support the collateral damage hypothesis for parasitic midges attracted to hourglass treefrogs calling near túngara frogs, but suggest that proximity to heterospecifics has no effect on bat predation.
(a). Predation risk and the proximity of heterospecific signallers
We observed no changes in bat visitations to hourglass treefrog calls when played in close proximity to the calls of túngara frogs, with similar numbers of bats visiting hourglass treefrogs in all treatments. We also found no difference in the number of bats attracted to túngara versus hourglass treefrog calls when played alone at separate sites. When predator preferences are symmetrical, heterospecific and conspecific neighbours may have similar effects on the risks of predation faced by signallers. It remains plausible, however, that collateral damage or shadow of safety effects exist for other species calling in mixed-species choruses, especially when one prey species is highly preferred. Furthermore, the preferences of fringe-lipped bats for prey can change seasonally [35]; and these bats have been shown to alter their foraging behaviour in captivity when prey cues from multiple modalities are present, or if trained only to approach túngara frog calls, when hourglass treefrog calls are also played nearby [15]. A temporary or permanent change in preference across the population could give rise to risk transfer due to the collateral damage or shadow of safety effects.
Finally, predation risk does not only depend on attractiveness, but also capture success. Hourglass treefrogs call from vegetation growing in or near pools of water, whereas túngara frogs can call from vegetated as well as non-vegetated pools. Vegetation is known to interfere with the bats' echolocation system and can result in a reduction in successful attacks [36]. Túngara frogs could experience an indirect benefit by using calling hourglass treefrogs to locate safer vegetated sites with reduced predation risk. Further tests of the collateral damage and shadow of safety hypotheses under these contexts are needed.
(b). Parasitism risk and the proximity of heterospecific signallers
In contrast to our predation results, our parasitism results show that frog-biting midges strongly preferred the calls of túngara frogs. Moreover, parasitism risk increased significantly for hourglass treefrog calls presented next to those of túngara frogs, as compared to those presented next to the calls of conspecifics, or to hourglass treefrog calls presented alone (figure 2). We found no difference between the number of midges attracted to hourglass treefrog calls played next to conspecific calls and hourglass treefrog calls played alone. This result indicates that the differences in parasitism risk were due to the identity of the neighbour and not due to changes in local frog density (1 versus 2 calling individuals). Thus, consistent with the collateral damage hypothesis, it is riskier for an hourglass treefrog to call next to a túngara frog than to call next to a conspecific or alone.
It is unlikely that inter-speaker separation accounts for the starkly contrasting results we observed in parasite and predator eavesdroppers. Although speakers in the predator portion of Experiment I were slightly further apart than in the parasite portion of the experiment (2 m versus 1 m), previous experiments have demonstrated that frog-eating bats are capable of assessing speakers separated by up to 7 m [10,37]. Furthermore, typical localization errors quantified for these bats are far below the speaker separation distances used in either portion of Experiment I [6,38].
Interestingly, the number of midges attracted to túngara frog calls in the heterospecific treatment did not decrease as compared to the number of midges observed in túngara frog alone treatment. The risk to túngara frogs, therefore, did not decrease in conjunction with the increased risk to hourglass treefrogs. Thus, the alteration of risk experienced by hourglass treefrogs occurred via an overall increase in the number of flies attracted to the heterospecific treatment and not via a reallocation of parasitic midges from túngara frogs to hourglass treefrogs.
(c). Implications for signalling behaviour and the diversity of mating assemblages
Where and when should an individual frog choose to call? From the predation perspective, our results suggest that when preferences are symmetrical, it does not make much difference whether or not male hourglass treefrogs call near signalling heterospecifics. From the parasitism perspective, site selection based on heterospecific presence or abundance clearly benefits the signaller. In order to avert a large increase in parasitism, hourglass treefrogs should avoid calling in the vicinity of túngara frogs, or should call only when nearby túngara are silent.
When considering larger spatial scales, collateral damage and shadow of safety phenomena have the potential to influence the species composition of individual breeding aggregations or the geographical distribution of species predated upon and parasitized by eavesdroppers. Signallers may be particularly attracted to, or avoid, breeding sites where certain heterospecifics display if the presence of those heterospecific signallers alters the risks posed by eavesdroppers. Similarly, populations experiencing strong collateral damage effects could suffer reduced population sizes or, in extreme cases, a limited geographical range due to heightened predation and parasitism risks associated with spatial variation in the presence of heterospecific signallers.
A previous study with captive bats suggests that heterospecific signallers could affect the evolution of signal structure by producing considerable acoustic noise [15]. This may result in increased reliance on active echolocation of motion cues produced by prey and selection against the associated visual signal components. Collateral damage and shadow of safety effects may likewise have the potential to influence signal structure. The evolution of mating signals and signalling behaviours is shaped both by female choice and by the costly attention of eavesdroppers. Previous studies have demonstrated that geographical variation in the relative strengths of these selective pressures can alter trade-offs and result in locally adapted signals [30,39]. Rather than variation in the overall intensity of local eavesdropper activity itself, our results suggest that spatial or temporal heterogeneity in the presence of signalling heterospecific guild members could modulate eavesdropping risks, and in turn, shift the balance of selective forces acting on male displays. This may lead to variation in signal structure that is correlated not only with local female preferences or eavesdropper abundance [30], but also with the density of particular heterospecifics that share signalling habitat.
(d). Collateral damage and shadows of safety in other contexts
It should be possible to extend the collateral damage and shadow of safety concepts to our understanding of interactions beyond those between predators or parasites and prey—for example, in the context of female choice. Should males vying for female attention call adjacent to particularly attractive conspecifics, and perhaps benefit from the highly stimulating effects of their neighbour's calls on the sensory systems of potential mates? Alternatively, should males call next to low-quality rivals, who make the focal male's signals seem attractive by comparison? Attractive males of the prairie mole cricket (Gryllotalpa major) are known to call from sites with above-average distances to their nearest rivals [40]. On the other hand, large males of the short-winged meadow katydid (Conocephalus brevipennis) approach speakers broadcasting calling aggregations more closely than smaller males [41]. To better understand the importance of signal quality-mediated variation in signaller spacing, independent of territorial interactions between neighbours, further studies are needed, aimed at determining how the relative attractiveness of males affects the location and reproductive success of signallers within mating aggregations.
Similarly, the collateral damage and shadow of safety phenomena may play an important role in sympatric species known to hybridize. As a result of the collateral damage, an individual calling near a heterospecific signaller may bear the costs of an increased encounter rate with heterospecific females, wasting time, gametes or more profitable mating opportunities. On the other hand, the shadow of safety effect may allow this same individual to avoid detrimental matings with heterospecific females that would otherwise be attracted to his own call if he calls close to a more suitable neighbour. Which of these two alternatives is dominant for the species in question should have a strong influence on whether signallers choose to call next to males of a species for which hybridization is a risk.
This study demonstrates that an individual's risk of parasitism can be strongly influenced by the species identity of individuals calling nearby. This effect could help shape the species composition of breeding aggregations and the spatial arrangement of individuals within those aggregations. Species composition will, in turn, alter the spatial and acoustic contexts in which mate choice takes place, and may ultimately influence the evolution of signal structure [41–44]. There has been considerable work on how interactions between conspecifics shape signalling behaviour [3]. We suggest a novel mechanism by which heterospecific interactions may exert significant selective pressures and influence the evolution of signalling strategies and the distribution of species at both local and larger scales.
Supplementary Material
Acknowledgements
We thank the Smithsonian Tropical Research Institute for logistical support, Santiago Meneses, Ernesto Bolívar, Mariana Franco and Natalia Lozano for help with fieldwork, and Justin Touchon for his assistance with the manuscript. The work was facilitated by ongoing collaborations with the Page Lab Research Group.
Data accessibility
All data used in this manuscript have been archived in the Dryad digital repository at: http://dx.doi.org/10.5061/dryad.mq06h.
Authors' contributions
P.A.T. conceived of the study, participated in the experimental design, coordinated and carried out the data collection, data analysis and drafting of the manuscript. M.O.W. participated in the experimental design and carried out the data collection; M.S.C. and W.H.H. conceived of the study, participated in the experimental design, assembled part of the field equipment and sound files and revised the manuscript; X.E.B. and R.A.P. participated in the experimental design, provided equipment and revised the manuscript. X.E.B. identified the midge species for this study. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by a Smithsonian Tropical Institute and Butler University post-doctoral fellowship to P.A.T. X.E.B. was funded by NSF IOS no. 1433990.
References
- 1.Andersson M, Simmons LW. 2006. Sexual selection and mate choice. Trends Ecol. Evol. 21, 296–302. ( 10.1016/j.tree.2006.03.015) [DOI] [PubMed] [Google Scholar]
- 2.Andersson S, Pryke SR, Ornborg J, Lawes MJ, Andersson M. 2002. Multiple receivers, multiple ornaments, and a trade-off between agonistic and epigamic signaling in a widowbird. Am. Nat. 160, 683–691. ( 10.1086/342817) [DOI] [PubMed] [Google Scholar]
- 3.Bradbury JW, Vehrencamp SL. 2011. Principles of animal communication, 2nd edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- 4.Hedrick AV. 2000. Crickets with extravagant mating songs compensate for predation risk with extra caution. Proc. R. Soc. B 267, 671–675. ( 10.1098/rspb.2000.1054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endler JA. 1980. Natural selection on color patterns in Poecilia reticulata. Evolution 34, 76–91. ( 10.2307/2408316) [DOI] [PubMed] [Google Scholar]
- 6.Ryan MJ, Tuttle MD, Rand AS. 1982. Bat predation and sexual advertisement in a neotropical anuran. Am. Nat. 119, 136–139. ( 10.1086/283899) [DOI] [Google Scholar]
- 7.Zuk M, Kolluru GR. 1998. Exploitation of sexual signals by predators and parasitoids. Q. R. Biol. 73, 415–438. ( 10.1086/420412) [DOI] [Google Scholar]
- 8.Cade W. 1975. Acoustically orienting parasitoids: fly phonotaxis to cricket song. Science 190, 1312–1313. ( 10.1126/science.190.4221.1312) [DOI] [Google Scholar]
- 9.Bernal XE, Rand AS, Ryan MJ. 2006. Acoustic preferences and localization performance of blood-sucking flies (Corethrella Coquillett) to tungara frog calls. Behav. Ecol. 17, 709–715. ( 10.1093/beheco/arl003) [DOI] [Google Scholar]
- 10.Akre KL, Farris HE, Lea AM, Page RA, Ryan MJ. 2011. Signal perception in frogs and bats and the evolution of mating signals. Science 333, 751–752. ( 10.1126/science.1205623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuttle MD, Ryan MJ. 1981. Bat predation and the evolution of frog vocalizations in the neotropics. Science 214, 677–678. ( 10.1126/science.214.4521.677) [DOI] [PubMed] [Google Scholar]
- 12.Tuttle MD, Ryan MJ. 1982. The role of synchronized calling, ambient light, and ambient noise in anti-bat-predator behavior of a treefrog. Behav. Ecol. Sociobiol. 11, 125–131. ( 10.1007/BF00300101) [DOI] [Google Scholar]
- 13.Hamilton WD. 1971. Geometry for the selfish herd. J. Theoret. Biol. 31, 295–311. ( 10.1016/0022-5193(71)90189-5) [DOI] [PubMed] [Google Scholar]
- 14.Alem S, Koselj K, Siemers BM, Greenfield MD. 2011. Bat predation and the evolution of leks in acoustic moths. Behav. Ecol. Sociobiol. 65, 2105–2116. ( 10.1007/s00265-011-1219-x) [DOI] [Google Scholar]
- 15.Rhebergen F, Taylor RC, Ryan MJ, Page RA, Halfwerk W. 2015. Multimodal cues improve prey localization under complex environmental conditions. Proc. R. Soc. B 282, 20151403 ( 10.1098/rspb.2015.1403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells KD. 2010. The ecology and behavior of amphibians. Chicago, IL: University of Chicago Press. [Google Scholar]
- 17.Marler P, Slabbekoorn H. 2004. Nature's music: the science of birdsong. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 18.Sridhar H, Beauchamp G, Shanker K. 2009. Why do birds participate in mixed-species foraging flocks? A large-scale synthesis. Anim. Behav. 78, 337–347. ( 10.1016/j.anbehav.2009.05.008) [DOI] [Google Scholar]
- 19.Stensland EVA, Angerbjörn A, Berggren PER. 2003. Mixed species groups in mammals. Mamm. Rev. 33, 205–223. ( 10.1046/j.1365-2907.2003.00022.x) [DOI] [Google Scholar]
- 20.Grand TC, Dill LM. 1999. Predation risk, unequal competitors and the ideal free distribution. Evol. Ecol. Res. 1, 389–409. [Google Scholar]
- 21.Ryan MJ. 1985. The túngara frog: a study in sexual selection and communication. Chicago, IL: University of Chicago Press. [Google Scholar]
- 22.Page RA, Ryan MJ, Bernal XE. 2013. Be loved, be prey, be eaten. In Animal behavior case studies: integration and application of animal behavior, vol. 3 (ed Yasakawa K.), pp. 123–154. Santa Barbara: ABC-CLIO Editorial. [Google Scholar]
- 23.Bernal XE, Pinto CM. 2016. Sexual differences in prevalence of a new species of trypanosome infecting túngara frogs. Int. J. Parasitol.: Parasites Wildl. 5, 40–47. ( 10.1016/j.ijppaw.2016.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calsbeek R, Sinervo B. 2004. Within-clutch variation in offspring sex determined by differences in sire body size: cryptic mate choice in the wild. J. Evol. Biol. 17, 464–470. ( 10.1046/j.1420-9101.2003.00665.x) [DOI] [PubMed] [Google Scholar]
- 25.Kalko EK, Friemel D, Handley CO, Schnitzler H. 1999. Roosting and foraging behavior of two neotropical gleaning bats, Tonatia silvicola and Trachops cirrhosus (Phyllostomidae). Biotropica 31, 344–353. ( 10.1111/j.1744-7429.1999.tb00146.x) [DOI] [Google Scholar]
- 26.Schwartz JJ, Wells KD. 1984. Interspecific acoustic interactions of the neotropical treefrog Hyla ebraccata. Behav. Ecol. Sociobiol. 14, 211–224. ( 10.1007/BF00299621) [DOI] [Google Scholar]
- 27.Reichert MS. 2011. Aggressive calling in treefrogs. Columbia, MO: University of Missouri. [Google Scholar]
- 28.Bosch J, Rand AS, Ryan MJ. 2000. Signal variation and call preferences for whine frequency in the túngara frog, Physalaemus pustulosus. Behav. Ecol. Sociobiol. 49, 62–66. ( 10.1007/s002650000280) [DOI] [Google Scholar]
- 29.Wells KD, Schwartz JJ. 1984. Vocal communication in a neotropical treefrog, Hyla ebraccata: aggressive calls. Behaviour 91, 128–145. ( 10.1163/156853984X00254) [DOI] [Google Scholar]
- 30.Trillo PA, Athanas KA, Goldhill DH, Hoke KL, Funk WC. 2013. The influence of geographic heterogeneity in predation pressure on sexual signal divergence in an Amazonian frog species complex. J. Evol. Biol. 26, 216–222. ( 10.1111/jeb.12041) [DOI] [PubMed] [Google Scholar]
- 31.Borkent A. 2008. The frog-biting midges of the world (Corethrellidae: Diptera). Purrujas de las ranas del Mundo (Corethrellidae: Diptera). Zootaxa 1804, 1–456. [Google Scholar]
- 32.de Silva P, Jaramillo C, Bernal XE. 2014. Feeding site selection by frog-biting midges (Diptera: Corethrellidae) on anuran hosts. J. Insect Behav. 27, 302–316. ( 10.1007/s10905-013-9428-y) [DOI] [Google Scholar]
- 33.Skaug H, Fournier D, Nielsen A, Magnusson A, Bolker B. 2012. Generalized linear mixed models using AD model builder. R package Version 0.7.4. See http://glmmadmb.r-forge.r-project.org.
- 34.Hothorn T, Bretz F, Westfal P. 2012. Simultaneous inference in general parametric models. R package. Version 1.2-14. See http://multcomp.r-forge.r-project.org/.
- 35.Birkhead TR, Pizzari T. 2002. Postcopulatory sexual selection. Nat. Rev. Genet. 3, 262–273. ( 10.1038/nrg774) [DOI] [PubMed] [Google Scholar]
- 36.Halfwerk W, Jones P, Taylor R, Ryan M, Page R. 2014. Risky ripples allow bats and frogs to eavesdrop on a multisensory sexual display. Science 343, 413–416. ( 10.1126/science.1244812) [DOI] [PubMed] [Google Scholar]
- 37.Jones PL, Ryan MJ, Flores V, Page RA. 2013. When to approach novel prey cues? Social learning strategies in frog-eating bats. Proc. R. Soc. B 280, 20132330 ( 10.1098/rspb.2013.2330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page RA, Ryan MJ. 2008. The effect of signal complexity on localization performance in bats that localize frog calls. Anim. Behav. 76, 761–769. ( 10.1016/j.anbehav.2008.05.006) [DOI] [Google Scholar]
- 39.Endler JA. 1995. Multiple-trait coevolution and environmental gradients in guppies. Trends Ecol. Evol. 10, 22–29. ( 10.1016/S0169-5347(00)88956-9) [DOI] [PubMed] [Google Scholar]
- 40.Howard DR, Lee N, Hall CL, Mason AC. 2011. Are centrally displaying males always the centre of female attention? Acoustic display position and female choice in a lek mating subterranean insect. Ethology 117, 199–207. ( 10.1111/j.1439-0310.2010.01858.x) [DOI] [Google Scholar]
- 41.Guerra PA, Mason AC. 2005. Male competition and aggregative behaviour are mediated by acoustic cues within a temporally unstructured aggregation. Behaviour 142, 71–90. ( 10.1163/1568539053627730) [DOI] [Google Scholar]
- 42.Schmidt AKD, Römer H, Riede K. 2013. Spectral niche segregation and community organization in a tropical cricket assemblage. Behav. Ecol. 24, 470–480. ( 10.1093/beheco/ars187) [DOI] [Google Scholar]
- 43.Jain M, Diwakar S, Bahuleyan J, Deb R, Balakrishnan R. 2013. A rain forest dusk chorus: cacophony or sounds of silence? Evol. Ecol. 28, 1–22. ( 10.1007/s10682-013-9658-7) [DOI] [Google Scholar]
- 44.Sinsch U, Lümkemann K, Rosar K, Schwarz C, Dehling M. 2012. Acoustic niche partitioning in an anuran community inhabiting an Afromontane wetland (Butare, Rwanda). Afr. Zool. 47, 60–73. ( 10.3377/004.047.0122) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this manuscript have been archived in the Dryad digital repository at: http://dx.doi.org/10.5061/dryad.mq06h.


