Abstract
Corals are acclimatized to populate dynamic habitats that neighbour coral reefs. Habitats such as seagrass beds exhibit broad diel changes in temperature and pH that routinely expose corals to conditions predicted for reefs over the next 50–100 years. However, whether such acclimatization effectively enhances physiological tolerance to, and hence provides refuge against, future climate scenarios remains unknown. Also, whether corals living in low-variance habitats can tolerate present-day high-variance conditions remains untested. We experimentally examined how pH and temperature predicted for the year 2100 affects the growth and physiology of two dominant Caribbean corals (Acropora palmata and Porites astreoides) native to habitats with intrinsically low (outer-reef terrace, LV) and/or high (neighbouring seagrass, HV) environmental variance. Under present-day temperature and pH, growth and metabolic rates (calcification, respiration and photosynthesis) were unchanged for HV versus LV populations. Superimposing future climate scenarios onto the HV and LV conditions did not result in any enhanced tolerance to colonies native to HV. Calcification rates were always lower for elevated temperature and/or reduced pH. Together, these results suggest that seagrass habitats may not serve as refugia against climate change if the magnitude of future temperature and pH changes is equivalent to neighbouring reef habitats.
Keywords: climate change, coral reefs, ocean acidification, seagrass beds, environmental variance, thermal stress
1. Introduction
Tropical coral reefs are increasingly threatened from both ocean warming and acidification as atmospheric pCO2 concentrations continue to rise [1]. However, how these two factors will interact to drive future productivity and distribution of reef-building corals remains unclear [2,3]. Evidence suggests that a rise in temperature can increase coral metabolic and/or growth rates [4], but eventually push corals past their physiological limits, resulting in mortality [1]. Lower pH appears to enhance the metabolism of select coral endosymbionts [5], but may increase the energetic cost of calcification and growth for the coral host [6,7]. Consequently, research efforts have focused on attempting to de-convolve the impacts of pH and temperature, while also trying to understand the potential synergistic interactions of combined stressors.
Coral reefs can have inherently high or low environmental variance. Deeper outer- and fore-reef habitats typically have relatively stable physio-chemical conditions. In contrast, shallow reefs are highly dynamic; bathymetry, benthic composition, extreme weather events and tidal cycles can create natural oscillations in pH and temperature [8–10]. The scale of these oscillations is habitat-specific (e.g. reef-flat versus seagrass or mangroves [8–12]) with daily temperature fluxes up to 2.5°C and pH variance exceeding 0.5 units [8,9]. Over longer temporal scales, these habitats are subject to greater oscillations [12]. Consequently, corals within shallow habitats routinely experience periods of extreme pH (7.8 [9,13]) and temperature (greater than 33°C [9,12]) considered representative of future reef conditions under Intergovernmental Panel on Climate Change scenarios. It is therefore plausible that corals persisting in high-variance habitats are better conditioned to tolerate periods of less favourable environmental conditions (e.g. enhanced physiological control) through expansion of physiological performance ranges [7].
Increased physiological tolerance to anomalous temperature and/or pH exposure has been shown for corals populating habitats with high environmental variance in some studies [11,14,15], but not all [16,17]. Such contrasting observations could reflect that few studies have included both temperature and pH as experimental variables, despite the simultaneous threat of ocean warming and acidification, with even fewer experiments replicating the natural daily oscillations (frequency and range) of temperature and pH inherent to coral habitats. Experiments have been performed to account for ambient pH variability for coral recruits (Seriatopora caliendrum [18]) and mature corals (Acropora hyacinthus [11]) when grown under dynamic rather than steady-state pCO2 conditions, but do not consider the simultaneous role of temperature variance. Conversely, the interactive role of temperature and acidification has been examined for massive- [2,7,19] and branching-corals [2], but do not replicate the ambient variance in temperature and pH. Only Dove et al. [20] have incorporated daily and seasonal variance for both temperature and pH within their study on patch reef communities, predicting serious implications for coral reef systems under future climate change [20]. However, how their observations hold across systems with inherently different scales of natural variance remains untested.
To date, no study has compared the physiological response of corals from relatively low-variance (LV, outer-reef) versus high-variance (HV, seagrass) habitats, as characterized by natural daily oscillations of both temperature and pH. It is unknown whether corals populating HV pH–temperature habitats have inherently greater tolerance to pH–temperature stress that is predicted for reefs of the future. Whether corals populating LV pH–temperature habitats can acclimatize to HV pH–temperature conditions is also unresolved. Addressing these unknowns is fundamental to understanding how present-day dynamic reef systems will respond to future changes in environmental conditions [18]. To address these unknowns, we conducted a multifactorial manipulative experiment on two dominant Caribbean coral species, one cosmopolitan to HV and LV habitats (Porites astreoides) versus a species only found in the LV habitat (A. palmata). Both species were exposed to current mean temperature and/or pH as well as future predicted mean temperature and/or pH for HV and LV habitats, but superimposing the natural variance of either habitat onto the predicted mean temperature and pH (following [20]). We also assessed the physiological tolerance based on key metabolic traits.
2. Material and methods
(a). Study location and test organisms
The study was conducted on the north coast of Little Cayman (Cayman Islands) at two sites comprising two habitats: outer-reef (LV) on the reef-terrace (19°41.53, 80°03.50) and coral inhabited seagrass (HV) within the adjacent shallow lagoon roughly 0.5 km inshore (19°41.48, 80°03.26). Detailed environmental characterization between March and April 2013 informed the target control conditions within the study (details in §2c).
The manipulation study was conducted between May and July 2014. Two study organisms were selected: A. palmata, which was only found at the LV outer-reef habitat (n = 40) and P. astreoides, which populated both the LV outer-reef and HV seagrass habitats (n = 40 colonies per habitat). On the reef, these two coral species accounted for 45.2 ± 0.3% of relative coral cover. Removed fragments were less than 5 cm, carefully collected from a depth of about 1.5 m.
(b). Experimental design
Eight experimental treatment conditions were created within controlled laboratory conditions (electronic supplementary material, figure S1). For both HV and LV, treatments consisted of: (i) a control, (ii) elevated temperature only, (iii) reduced pH only, and (iv) elevated temperature-reduced pH. Each treatment was superimposed onto the ambient diel variance of temperature and pH for the HV versus LV habitats. The eight experimental treatments were each replicated across five independent aquaria (2 l), with one fragment of (i) A. palmata (LV), (ii) P. astreoides (LV) and (iii) P. astreoides (HV) per aquarium. All aquaria were maintained under an ambient 12 L : 12 D photoperiod [2], with average daylight PAR of 400–500 µmol photons m−2 s−1 (representative of in situ conditions; electronic supplementary material, table S1). Light was measured using three HOBO Pendant temperature/light loggers (Microdaq, USA), with values averaged and converted to PAR using the daylight coefficient [21]. Daily 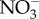 measurements were taken using an
measurements were taken using an 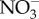 probe (Orion, USA) with all treatments exposed to relatively low levels throughout the experiment (mean ± s.e. of n = 59 per habitat; HV = 0.95 ± 0.03 µM, LV = 1.06 ± 0.04 µM; electronic supplementary material, table S1).
probe (Orion, USA) with all treatments exposed to relatively low levels throughout the experiment (mean ± s.e. of n = 59 per habitat; HV = 0.95 ± 0.03 µM, LV = 1.06 ± 0.04 µM; electronic supplementary material, table S1).
Ambient in situ diel variance of pH and temperature for the LV and HV habitats were recreated for experimentation. In situ diel records of temperature and pH were used to determine seven time points representing the range of conditions and hence scale of variance. Mean temperature and pH at each time point served as the basis of recreating ambient habitat variance ex situ (see electronic supplementary material, figure S2) in one of two ways: (i) for the LV habitat, four 250 l reservoirs of non-filtered outer-reef habitat (LV) natural seawater supplied all treatment aquaria; (ii) for the HV seagrass habitat, water from the reservoir was pumped into a 45 l sump containing seagrass and carbonate sediment (collected from the in situ seagrass habitat; electronic supplementary material, figure S1). The sump contained a pump and aerator, which subsequently supplied water to all HV treatment aquaria. Temperature was controlled for both the LV and HV treatments using a water bath with heaters (Aquael, Poland) to achieve the target conditions for each time period.
Additional elevated temperature and/or reduced pH ‘future scenario’ treatments were created by superimposing the pH and temperature conditions predicted for 2100 onto the natural diurnal trends from the LV and HV reservoirs (as per [20]; figure 1). Elevated temperature was achieved using additional heaters to recreate the diurnal oscillations. Temperature was continuously measured over the duration of the experiment using a HOBO temperature/light logger verified daily with a temperature probe (Ocean Optics, UK).
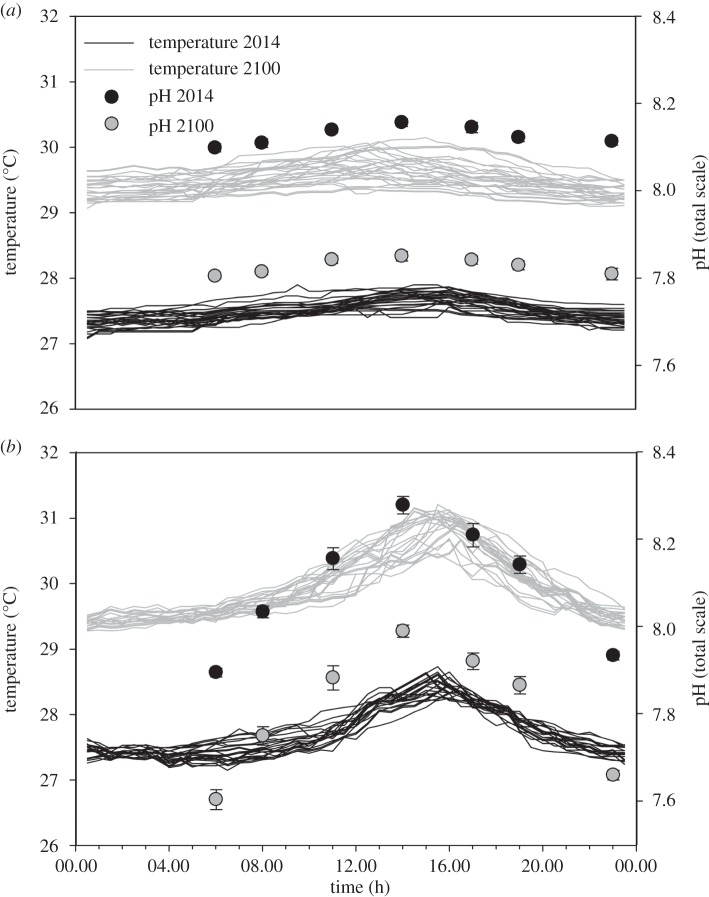
Figure 1.
The average pH and temperature aquarium conditions for: (a) the outer-reef low-variance (LV) and (b) seagrass high-variance (HV) habitats of Little Cayman, Cayman Islands, BWI, for both present day and 2100 (under A1B scenario estimates). The 2100 conditions (grey) represent an approximate temperature increase ± s.e. of 2.2 ± 0.03°C and pH decrease of 0.3 ± 0.02 units. pH was measured from daily discrete water samples over the seven time periods, while temperature was measured continuously with a HOBO pendant temperature/light 64 K data logger set at a 30 min interval.
For the reduced pH treatments, feeds from either the LV or HV source water were tapped into aquaria where pH was altered by subsequently manipulating the seawater by equimolar additions of strong acid (1 mol l−1 HCl), NaOH and Na2CO3 (Thermo-Fisher Scientific, USA) [22]. Volumes of HCl and HCO3−, required to adjust pCO2 and pH to the chosen target values, were first calculated from the measured ambient state of the carbonate system in seawater using CO2SYS [22]. For each of the seven daily time periods (electronic supplementary material, figure S2), water within each aquarium was flushed with new manipulated seawater. To ensure that the target pH was achieved and that total alkalinity (TA) was maintained, pH and TA were tested before every water exchange from discrete water samples. pH (total) was measured using the Orion Ross Ultra Glass Triode Combination Electrode (Fisher Scientific, UK) calibrated with Tris buffers (accuracy approx. ±0.002) using the potentiometric technique [23]. An open-cell potentiometric titration procedure was used to measure TA using a Titrino titrator (Metrohm, UK) with accuracy and precision of less than or equal to 2 µmol kg−1 (verified with Dickson standards).
Based on these manipulations, the future scenario treatments achieved a temperature increase of 2.2 ± 0.03°C and pH decrease of 0.3 ± 0.02 units (mean ± s.e. conditions at the seven diurnal time points over the 59-day experimental period; n = 413; electronic supplementary material, figure S2). Our method of manipulating the pH and temperature was able to recreate the predicted changes of 2100 under IPCC A1B scenarios incorporating the natural diel trends of each habitat (figure 1), including aragonite saturation state (Ωarg) (electronic supplementary material, figure S3 and table S1). Together, these treatments yielded a daily mean (±s.e.) (n = 59) Ωarg for the HV and LV habitats of 3.79 ± 0.04 and 2.94 ± 0.02, respectively, for present-day conditions versus 2.33 ± 0.08 and 2.24 ± 0.03 for 2100 scenarios. Under both the HV and LV treatments, greater daily variance in both temperature and pH was experienced for the tanks exposed to the 2100 level conditions (temperature: t6 = 6.36, p < 0.001; pH: t6 = 4.44, p < 0.005; see the electronic supplementary material).
The eight experimental treatments were conducted over three phases: (i) recovery (3 days), where corals were initially removed from their in situ environment and left to recover in the laboratory under their present-day ambient (i.e. native LV versus HV treatment conditions), (ii) acclimatization (21 days, as per [24]) where the full set of present-day or future scenario pH and temperature treatments were applied; and finally, (iii) experimentation (35 days), where corals continued to be exposed to all treatments (total experimental duration, n= 59 days; electronic supplementary material, figure S4). All corals were sampled for zooxanthellae and chlorophyll a concentrations, and incubated to measure rates of photosynthesis, respiration and calcification (see §2d), (i) at the end of the experimental period (te) to evaluate for the treatment effect, but also (ii) at the end of the recovery period (t0) to determine that physiological properties had not drifted in the controls as a result of the experimental set-up. Buoyant mass measurements were taken at the end of the acclimatization period (ti) and te to establish an average daily growth rate (electronic supplementary material, figure S4).
(c). Carbonate chemistry baseline
At each location, discrete water sampling was conducted over 18 days between March and April 2012 to establish the natural diurnal trends in pH and temperature. Samples were collected over 24 h, at 3 h intervals starting at sunrise (n = 144 per site). Seawater carbonate chemistry was measured following the Carbon Dioxide Information Analysis Centre protocols [23], with carbonate parameters (pCO2, TCO2 and Ωarg) calculated from TA and pH, as described in [21]. Discrete water samples were also collected weekly at the seagrass (HV) and outer-reef (LV) sites during the experimental period (n = 8; electronic supplementary material, table S1).
(d). Physiological measurements
Coral replicates were weighed in seawater (buoyant weight) at ti and te with a Ohaus Scout-Pro balance (accuracy 0.01 g). Skeletal dry weight was demined as per [25] and normalized to surface area calculated using the advanced geometric technique (AGT) [26]. Density was determined at the end of the experimental period using the calculated mass and volume determined from three-dimensional scans [27]. Growth rates established from buoyant mass corresponded closely to rates established from the TA method (electronic supplementary material, figure S5), and we therefore present data obtained only from the TA method. Daily-integrated rates were simultaneously obtained for all colonies from 8 × 3 h incubations (and three control chambers that contained only seawater) sequentially conducted throughout a 24 h period at t0 and te (as previously detailed [21]). Each colony was incubated in a closed 500 ml chamber, which was manually stirred every hour using a stir-bar and magnet. Net photosynthesis (PN) and respiration rates (R) were determined (each 4 × 3 h incubations at t0 and te), where O2 was measured at the start and end of each incubation using a Foxy-R O2 probe (Ocean Optics). Dark incubations were conducted immediately after exposure to light by covering the incubation chambers with custom-made blackout bags (see [21]). Changes in TA and O2 for each chamber and 3 h incubation were corrected for any changes in TA or O2 from the seawater controls (n = 3), to yield hourly rates for calcification (G, mmol CaCO3 m−2 h−1) or PN and R (mmol O2 m−2 h−1) [21]. Gross productivity (PG) was calculated by the addition of net photosynthesis and respiration.
Three colonies of each species from each experimental treatment were randomly selected for surface area determination by three-dimensional scans [27]. This method showed that AGT underestimated surface area, and thus a species-specific correction factor was applied to all surface area measurements to account for this difference: A. palmata: r2 = 0.974, n = 24, p = 0.001, AGT = −0.93 + (0.97 · three-dimensional scan); P. astreoides: r2 = 0.965, n = 48, p = 0.010, AGT = −1.08 + (0.97 · three-dimensional scan).
(e). Chlorophyll a and zooxanthellae counts
Tissue was removed from each nubbin with a water pik using GF/F-filtered seawater; the area of tissue removed was quantified through corrected AGT [26]. Tissue slurry was homogenized using a Pasteur pipette and a small aliquot taken for cell quantification using microscopy [28]. A second aliquot was filtered and extracted in methanol for 24 h at 4°C, and chlorophyll a quantified on the pigment extracts using a USB 2000+ Spectrophotometer (Mikropack, HL-2000) and the equations of [29] for dinoflagellates.
(f). Statistics
Linear regression was used to compare the rates of calcification between t0 and te, to relate calcification calculated with the TA anomaly method to the buoyant mass technique, and to relate surface area measured by AGT and the three-dimensonal scanning method. A t-test was conducted to assess whether rates of calcification at t0 were different from rates of calcification at te.
(i). Multi-model comparison
Within the study, P. astreoides was found at both habitats, whereas A. palmata was only found at one, and thus the experiment was not fully factorial. In addition, exploratory analysis of the data identified significant third-order interaction terms (such as pH, temperature and species). The large number of terms in a third-order ANOVA and the unbalanced design raised concerns that an ANOVA may be affected by over-fitting or ill-conditioning [30]. For these reasons, our results were analysed in two ways: (i) by ANOVA with restrictions on the variables (electronic supplementary material, table S2 and S3), and (ii) with a set of nested nonlinear models using the multi-model selection framework (electronic supplementary material). The Akaike information criterion of linear models (ANOVA) and nonlinear models was compared (electronic supplementary material, table S4–S6), with a difference of 0–2 considered negligible [30]. Model simplification, by removing non-significant variables (e.g. tank variance of 2100 versus present-day; see the electronic supplementary material), was undertaken to compare models with progressively simplified fixed effects to select the most appropriate model (see the electronic supplementary material). Confidence intervals (CIs) were calculated by the log-profile method [30], and from these we obtained bounds on the corresponding p-values. pH and temperature variation for the present-day and 2100 treatments were tested by Kolmogorov–Smirnov on the residuals of both the ANOVA and nonlinear model (electronic supplementary material, table S7–S8 and figure S6).
3. Results
(a). Carbonate chemistry manipulation
To ensure that experimental HV (seagrass) and LV (outer-reef) present-day control treatments were comparable to the ambient habitat conditions, we compared calcification rates at t0 with those at te of the experimental period. Calcification rates across all species and treatments were unchanged between te and t0 (slope not significantly different to one, i.e. 1 : 1), thus demonstrating that ambient conditions were well conserved throughout the experimental period (figure 2). Calcification rates for all species based on the TA anomaly method versus buoyant mass were significantly correlated (r2 = 0.791, n = 120, p = 0.001), with their relationship described by the equation: buoyant mass (mmol m−2 d−1) = 4.33 + (0.864 · alkalinity depletion (mmol m−2 d−1)) (electronic supplementary material, figure S5 and table S9).
Figure 2.
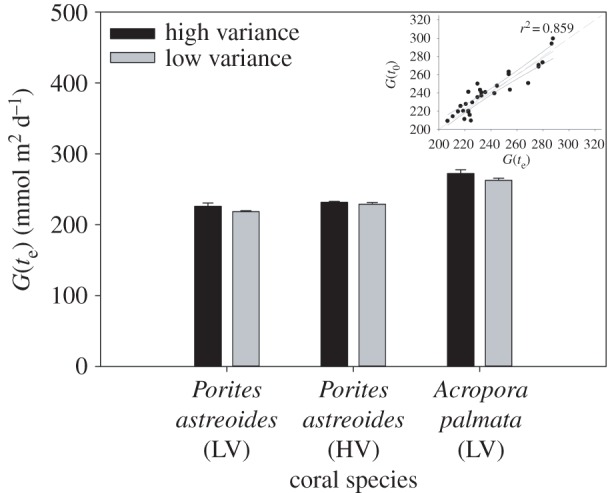
Average (±s.e.) daily calcification rates (G) at the end of the experiment (te) for: A. palmata (outer-reef, low-variance (LV) habitat), P. astreoides (LV), and P. astreoides (seagrass, high-variance (HV) habitat) within the HV and LV control tanks. A regression between rates of G at the start (t0) and te of the experiment show strong colinearity between rates (r2 = 0.859, n = 30, p = 0.001, G(t0) (mmol m−2 d−1) = 18.55 + 0.93G(te) (mmol m−2 d−1).
(b). Native habitat and species response
Daily mean calcification rates for colonies maintained under their native conditions were 9.3% higher (CI: 7.8–10.7%) for A. palmata (table 1 and electronic supplementary material, S9) (268.5 ± 1.2 mmol m−2 d−1) compared with both native HV and LV P. astreoides (231.6 ± 5.6 mmol m−2 d−1, 218.6 ± 7.4 mmol m−2 d−1, respectively; table 1 and figure 2). Respiration and photosynthesis rates were 9.0% (CI: 7.2–10.3%) and 7.0% (CI: 5.2–7.9%) higher, respectively, for P. astreoides compared with A. palmata (table 1). Transplantation of P. astreoides colonies from HV and LV habitats into LV and HV control tanks, respectively, did not induce a change in calcification (figure 2). Thus, colonies native to the LV outer-reef sustained calcification in the HV seagrass control tanks. Colonies originating from HV, however, showed no enhanced calcification rates under the more stable LV control (electronic supplementary material, table S9).
Table 1.
Statistical results for the interval estimate and significance of temperature, pH and temperature–pH combined on the physiology (photosynthesis, respiration and calcification rates) for the corals A. palmata (native to outer-reef), P. asteoides (native to outer-reef) and P. astreoides (native to seagrass), under both high and low experimental treatment variance. For each of the metabolic parameters (photosynthesis, respiration and calcification) model estimates are shown for different variable interactions (species, habitat, treatment variance, temperature and pH). Models were simplified to remove any non-significant parameters (see the electronic supplementary material). Confidence intervals (CIs) are indicated at 95% for the model estimates as are significance (p) values. Experimental parameters represented in the model are indicated by dummy-coding.
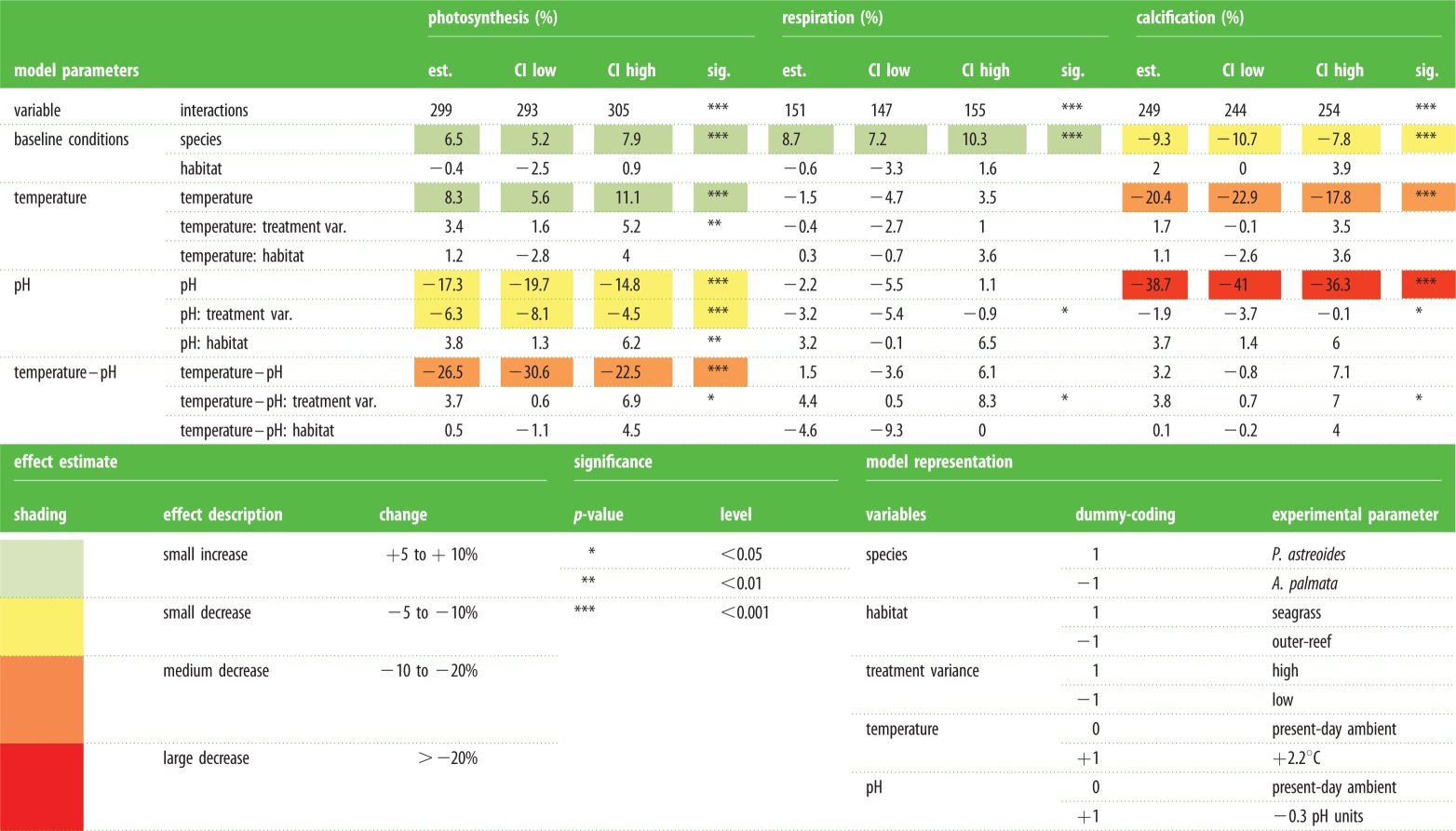 |
Consistently, across all observations (photosynthesis, respiration and calcification), native habitat gave a small positive effect (less than +4% for corals native to HV seagrass) which in only a single case (photosynthesis) rose above the threshold for statistical significance (table 1). Thus, the response to ‘future scenarios’ was independent of LV (outer-reef) or HV (seagrass) growth history. However, colonies from the LV habitat all experienced an increase in skeletal density under the reduced pH treatments (t = −3.79, p = 0.005; electronic supplementary material, figure S7). No significant difference was detected in the skeletal densities of the controls between the HV and LV treatments, independent of species.
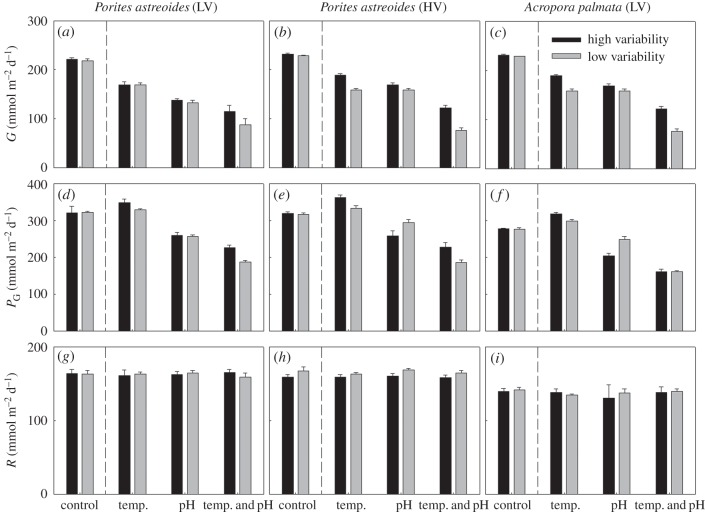
Overall, physiological responses to experimental treatments were similar for both A. palmata and P. astreoides (figure 3; electronic supplementary material, table S9). pH had a larger influence on coral calcification than temperature (table 1). Reduced pH treatments decreased calcification by 38.7% (CI: 36.3–41.0%), whereas elevated temperature treatments decreased calcification by 20.4% (CI: 17.8–22.9%). The greatest decrease in calcification was observed under the combined elevated temperature–reduced pH treatment. However, the nonlinear model indicated that any interactive effect of pH and temperature was negligible (3.2%, CI: −0.8 to 7.1%; table 1), because an additive response of temperature and pH independently explained the majority of trends within the dataset (see the electronic supplementary material). Consequently, the additive response of temperature and pH stress resulted in a decrease in calcification of 59.1%.
Figure 3.
The metabolic response of corals across experimental treatments. Daily rates of respiration (R), gross productivity (PG) and calcification (G) for (a,d,g) Porites astreoides (outer-reef, low-variance (LV) habitat), (b,e,h) P. astreoides (seagrass, high-variance (HV) habitat) and (c,f,i) A. palmata (LV) relative to the controls, for both the HV and LV treatments. Daily rates (±s.e., n = 5) were determined at the end of the experiemnt (te). Corals were from Little Cayman, Cayman Islands, BWI, with control conditions resprentative of present-day in situ conditions for the seagrass and outer-reef, while experimental conditions best represent the temperature increases and pH decreases estimated under the IPCC A1B scenario.
Respiration did not change in response to any treatment (within 5.5% of the control; figure 3 and table 1) and therefore changes in net photosynthesis were mirrored as changes in gross photosynthesis. Net photosynthesis fell under all reduced pH treatments (pH: 17.3%, CI: 14.8–19.7; pH + temp: 26.5% CI: 22.5–30.6%). When temperature was elevated to 2100 scenarios but pH was maintained at present-day levels, net photosynthesis was elevated (8.3%, CI: 5.6–11.1%, table 1).
(c). Treatment variance
Corals native to both HV (seagrass) and LV (outer-reef) environments experienced significant, but very small (less than 5%) reductions in all metabolic parameters under the HV treatment conditions (table 1). The treatment response was the same for LV-grown A. palmata exposed to HV treatment conditions, as well as the reciprocal transplantation of HV- or LV-grown P. astreoides (figure 3 and table 1).
(d). Metabolic coupling
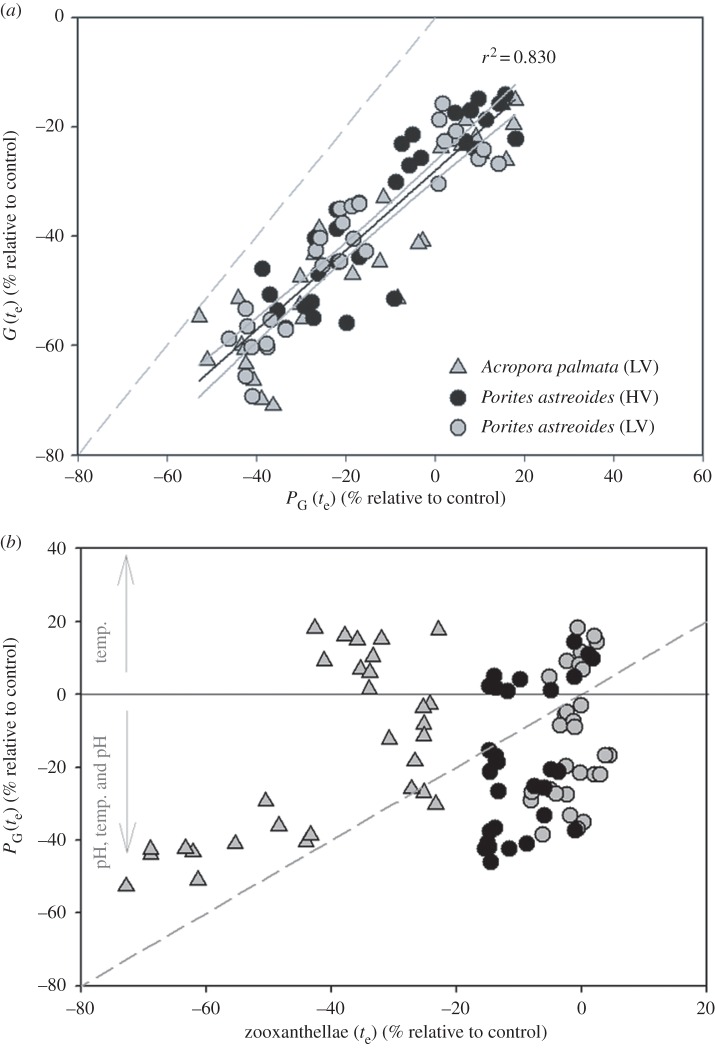
Across treatments, calcification rates were closely coupled to gross photosynthesis rates, independent of species and native habitat, with 83% of calcification explained by the covariance with gross photosynthesis (r2 = 0.831, n = 90, p = 0.01; figure 4). For P. astreoides, changes in gross photosynthesis were generally independent of the zooxanthellae density (zooxanthellae never declined by less than 10% of the control; figure 4). Similarly, A. palmata colonies exposed to the elevated temperature treatment exhibited a decrease in gross photosynthesis that was independent of zooxanthellae density. However, A. palmata exposed to reduced pH or combined elevated temperature–reduced pH treatments exhibited a decrease in gross photosynthesis that corresponded with a loss in zooxanthellae density (figure 4), as well as decrease in chlorophyll a, 10–23% (electronic supplementary material, table S9). Visual observations support these trends, with paling observed among the A. palmata fragments beginning on day 14 of the experiment (E. Camp 2014, personal observation). Thus, while the treatment induced declines in calcification that were independent of host taxa (figure 4), they correspond with very different modes of symbiont response: a loss of productivity per zooxanthellae cell for P. asteoides (and A. palmata under elevated temperature alone) versus a loss of zooxanthellae cells (but general maintenance of productivity per zooxanthellae cell) under reduced pH for A. palmata (figure 4).
Figure 4.
Gross productivity (PG) versus calcification (G) and zooxanthellae percentage change for A. palmata (outer-reef, low-variance (LV) habitat), P. astreoides (seagrass, high-variance (HV) habitat) and P. astreoides (LV) from Little Cayman, Cayman Islands, BWI. (a) Percentage change in PG versus G and (b) percentage change in PG plotted against the per cent change in zooxanthellae. Relative percentage change (±s.e.) was determined by averaging the experimental replicates per treatment (n = 5) and standardizing by the control at the end of the experiment (te).
4. Discussion
Recent studies have suggested that populations grown under more variable pH [11] or temperature [15] environments enhance resistance of corals to anomalous pH and temperature stress. Shallow reef habitats are often characterized by variable temperature and pH as a result of coupling between environmental factors and benthic metabolism [20]. However, we observed the same impact of elevated mean temperature and/or reduced pH on metabolic rates, regardless of acclimatization to HV (seagrass) or LV (outer-reef) habitats. This was true for P. astreoides found in both habitats, but also for A. palmata found only in the LV outer-reef, which under our experimental conditions acclimatized to present-day HV conditions. Therefore, the absence of A. palmata from the HV seagrass habitat would suggest that some factor other than variance itself restricts its ability to survive in this environment (e.g. low sexual reproductive success of A. palmata [31]).
Within our study, reduced pH had a larger negative impact on photosynthesis and calcification rates relative to the elevated temperature treatment (figure 3). This outcome is consistent with recent observations that calcification rates decline under lower seawater pH scenarios [1], with little to no upregulation in calcification from elevated temperature [2]. Both A. palmata and P. astreoides in our experiment exhibited a large decrease in calcification across all treatments (independently of LV and HV). These observations are consistent with previous reports of a significant decrease in calcification (approx. 40%) of P. astreoides along an increasingly acidic gradient [16] and under lowered pH conditions within laboratory studies [32]. Similarly, Acropora spp. have exhibited reduced calcification in experimental acidification scenarios [33]. Such responses are not always observed, with feeding on particulate organics [19] and/or elevated inorganic nutrient availability [34] in particular found to ameliorate the impacts of low pH on Porites spp. calcification. However, our experimental set-up was designed to replicate the in situ conditions with low inorganic nutrient availability (NO3− concentrations less than 1.1 µM) and supplied with organic particulate by the regular exchange of native ambient seawater.
The reduced pH treatments resulted in denser coral calcification for the HV populations, although it is not currently clear why HV corals were more densely calcified when they experienced similar reductions in calcification to the LV populations. HV conditions did result in periods of extended low pH (at night), and consequently low Ωarg (close to/below the saturation threshold). This response appears to mirror abiotic aragonite precipitation, where low Ωarg (pH) conditions have been shown to induce shorter and wider crystal formation in tightly packed bundles, compared with longer, thinner crystals under ‘normal’ Ωarg [6].
For the pH and combined pH–temperature treatments, calcification and photosynthesis were coupled across all treatments. Interestingly, however, the elevated temperature treatment stimulated photosynthesis (as per [35]) for both species examined, but was not accompanied by enhanced calcification rates, resulting in an uncoupling of the photosynthesis–calcification relationship. While the cause of this latter response is not entirely clear, Anthony et al. [2] observed increased productivity for Acropora sp. without an overall decrease in calcification rates for an intermediate warming scenario, roughly equivalent to future treatment levels employed within our study [2]. This is potentially indicative of exceeding thermal windows that govern metabolic processes (e.g. inorganic carbon acquisition [2]). Thermal tolerance windows are species-specific [36], and perhaps explain why some studies [35] but not others [37] have observed an increase in calcification with increased temperature. Coral species can potentially sustain calcification through upregulation of pH of the calcifying fluid [7], although this probably comes with an energetic cost [6]. Consequently, corals that are unable to maintain photosynthesis owing to reduced thermal tolerance are likely to experience a decrease in calcification rates, when respiration also remains unchanged as our results demonstrate.
For P. astreoides, changes of gross photosynthesis rates over the experimental treatments were independent of zooxanthellae density as cells become less productive with the various stressors. In contrast, A. palmata under reduced pH exhibited a loss of productivity that was associated with a loss of symbiont cell density (and coloration). Such acidification-induced bleaching has been observed by Anthony et al. [2] for both massive and branching corals, but the cause has not been fully resolved [2]. For example, depigmentation could stem from direct impacts of acidosis [38], disruption to the carbon-concentration mechanisms [39] or disruption to the photoprotective mechanism of corals through reduction of PGPase [40]. Even so, a key difference between the two species we tested appears to be an inherent association with different symbiont genotypes. A search of the ‘Coral Trait’ database (https://coraltraits.org; search 11 November 2015) demonstrates that A. palmata has a highly conserved association with Symbiodinium ITS2 type A3 throughout the Caribbean [41], whereas P. astreoides has a wider symbiont pool, including types A3, A4, A4a, B1, C3 and Cla-j [41,42]. Symbiodinium genotypes, in particular those in Clade A have very different responses to pH [5] and/or temperature [43], and may partly explain why A. palmata and P. astreoides are affected by temperature and pH stress in very different ways (despite the same net outcome of a reduction in photosynthesis and calcification); this notion warrants more targeted investigation.
Central to how corals will survive pH/temperature stress predicted for reefs under future climate scenarios is whether the rate of adaptation exceeds the rate of environmental change [44]. Within our experiment, we exposed corals to altered environmental conditions of 2.2 ± 0.03°C and a pH decrease of 0.3 ± 0.02 units over 59 days; consequently, any adaptation process exceeding this period will not have been identified. The overall lack in effect of environmental growth history (LV versus HV) on the metabolic rate measurements is consistent with Comeau et al. [11], who observed that coral calcification was independent of native Ωarg variance [11]. Similarly, coral populations from the Florida Reef tract exposed to large highly dynamic diel and seasonal fluctuations in pH experienced no reduced effect to elevated pCO2 conditions expected under acidification [17]. It is conceivable that the differences in environmental growth history may be too small to affect coral physiology (i.e. we observed the same physiological response for corals native to HV and LV in terms of metabolic rate measurements).
Variance in pH and/or temperature did not influence calcification rates, despite the very different Ωarg profiles of each habitat. Under present-day conditions, the seagrass habitat could thus be described as providing a ‘buffering’ service [9,12], because the daily net Ωargwas elevated by 1.52 units compared with the reef system, thereby maintaining favourable conditions that are being lost elsewhere (a refuge, sensu [45]). However, when 2100 pH and temperature conditions were superimposed, the HV seagrass and LV outer-reef achieved similar daily net Ωarg, because the daytime elevation in pH and Ωarg of the seagrass habitat did not compensate for the low night-time conditions. Similarity in coral metabolic response when exposed to 2100 conditions, irrespective of their environmental history, questions whether seagrass habitats can sustain a buffering role if they ultimately experience the same changes in pH as predicted for the open ocean.
Whether dissolution of carbonate sediment [10] or enhanced daytime productivity of photoautrotrophs [12] can offset the influence of future acidification on inshore habitats remains to be seen. Seagrass beds, as for our HV habitat, typically appear to respond favourably to high CO2 levels, with increased reproduction, rhizome biomass and growth of new shoots [46]. As a result, a positive feedback scenario might be expected as ocean acidification progresses, whereby the amplification of pH by seagrass may increase to moderate the decline in pH. Future research and long-term monitoring is necessary to determine if the status of seagrass as ‘winners’ with ocean acidification can counteract the decline in pH and provide refuge to corals within or near seagrass beds. A true ‘ecological scale’ experiment will clearly be required to resolve whether acidification and warmer waters combined will drive greater metabolic variance in shallow habitats; however, we have shown for the first time that variance does not enhance tolerance of A. palmata or P. astreoides when maintained under present-day variance regimes of pH and temperature. Species appear to have very different photosynthesis responses coupled to a common calcification response across pH and temperature treatments. Shallow water systems with inherently variable pH and/or temperature have been proposed as possible future climate change refugia [12], but the results from our species and system examined would not support this view.
Supplementary Material
Acknowledgements
Sincere thanks also go to the Cayman Islands Department of the Environment, in particular Keith Neale, Croy McCoy, John Bothwell, Tim Austin and Phillipe Bush for their continued support. In addition, the logistical support of William England, Richard McElhannon and Frank Roulstone is acknowledged. Finally, thanks to Graham Kolodziej at NOAA for assistance with sample processing.
Competing interests
We declare we have no competing interests.
Funding
D.J.S. was supported by an Australian Research Council Future Fellowship (FT130100202).
References
- 1.Hoegh-Guldberg O, Bruno JF. 2010. The impact of climate change on the world's marine ecosystems. Science 328, 1523–1528. ( 10.1126/science.1189930) [DOI] [PubMed] [Google Scholar]
- 2.Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. 2008. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl Acad. Sci. USA 105, 17 442–17 446. ( 10.1073/pnas.0804478105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Hooidonk RJ, Maynard JA, Manzello D, Planes S. 2014. Opposite latitudinal gradients in projected ocean acidification and bleaching impacts on coral reefs. Glob. Change Biol. 20, 103–112. ( 10.1111/gcb.12394) [DOI] [PubMed] [Google Scholar]
- 4.McNeil BI, Matear RJ, Barnes DJ. 2004. Coral reef calcification and climate change: the effect of ocean warming. Geophy. Res. Lett. 31, 1733–1743. ( 10.1029/2004GL021541) [DOI] [Google Scholar]
- 5.Brading P, Warner ME, Davey P, Smith DJ, Achterberg EP, Suggett DJ. 2011. Differential effects of ocean acidification on growth and photosynthesis among phototypes of Symbiodinum (Dinophyceae). Limnol. Oceanogr. 56, 927–938. ( 10.4319/lo.2011.56.3.0927) [DOI] [Google Scholar]
- 6.Cohen AL, Holcomb MH. 2009. Why corals care about ocean acidification, uncovering the mechanism. Oceanography 22, 118–127. ( 10.5670/oceanog.2009.102) [DOI] [Google Scholar]
- 7.Georgiou L, Falter J, Trotter J, Kline DI, Holcomb M, Dove SG, Hoegh-Guldberg O, McCulloch M. 2015. pH homeostasis during coral calcification in a free ocean CO2 enrichment (FOCE) experiment, Heron Island reef flat, Great Barrier Reef. Proc. Natl Acad. Sci. USA 112, 13 219– 13 224. ( 10.1073/pnas.1505586112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw EC, McNeil BI, Tilbrook B, Matear R, Bates ML. 2013. Anthropogenic changes to seawater buffer capacity combined with natural reef metabolism induce extreme future coral reef CO2 conditions. Glob. Change Biol. 19, 1632–1641. ( 10.1111/gcb.12154) [DOI] [PubMed] [Google Scholar]
- 9.Camp E, Suggett DJ, Gendron G, Jompa A, Manfrino C, Smith DJ. 2016. Mangrove and seagrass beds provide different biogeochemical services for corals threatened by climate change. Front. Mar. Sci. 3, 52 ( 10.3389/fmars.2016.00052) [DOI] [Google Scholar]
- 10.Anthony KRN, Diaz-Pulido G, Verlinden N, Andersson AJ. 2013. Benthic buffers and boosters of ocean acidification on coral reefs. Biogeoscience 10, 4897–4909. ( 10.5194/bg-10-4897-2013) [DOI] [Google Scholar]
- 11.Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. 2014. Diel pCO2oscillations modulate the response of the coral Acropora hyacinthus to ocean acidification. Mar. Ecol. Prog. Ser. 501, 99–111. ( 10.3354/meps10690) [DOI] [Google Scholar]
- 12.Manzello DP, Enochs IC, Melo N, Gledhill DK, Johns EM. 2012. Ocean acidification refugia of the Florida Reef Tract. PLoS ONE 7, e41715 ( 10.1371/journal.pone.0041715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price NN, Martz TR, Brainard RE, Smith JE. 2012. Diel variability in seawater pH relates to calcification and benthic structure on coral reefs. PLoS ONE 7, e43843 ( 10.1371/journal.pone.0043843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoepf V, Stat M, Falter JL, McCulloch MT. 2015. Limits to the thermal tolerance of corals adapted to a highly fluctuating, naturally extreme temperature environment. Sci. Rep. 5, 17639 ( 10.1038/srep17639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker AC, Starger CJ, McClanahan TR, Glynn PW. 2004. Corals’ adaptive response to climate change. Nature 430, 741 ( 10.1038/430741a) [DOI] [PubMed] [Google Scholar]
- 16.Crook ED, Cohen AL, Rebolledo-Vieyra M, Hernandez L, Paytan A. 2013. Reduced calcification and lack of acclimatisation by coral colonies growing in areas of persistent natural acidification. Proc. Natl Acad. Sci. USA 110, 1144–1149. ( 10.1073/pnas.1301589110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okazaki RR, Swart PK, Langdon C. 2013. Stress-tolerant corals of Florida Bay are vulnerable to ocean acidification. Coral Reefs 32, 671–683. ( 10.1007/s00338-013-015-3) [DOI] [Google Scholar]
- 18.Dufault AM, Cumbo VR, Fan TY, Edmunds PJ. 2012. Effects of diurnally oscillating pCO2 on the calcification and survival of coral recruits. Proc. R. Soc. B. 279, 2951–2958. ( 10.1098/rspb.2011.2545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edmunds PJ. 2011. Zooplanktivory ameliorates the effects of ocean acidification on the reef coral Porites spp. Limnol. Oceanogr. 56, 2402–2410. ( 10.4319/lo.2011.56.6.2402) [DOI] [Google Scholar]
- 20.Dove SG, Kine DI, Pantos O, Angly FE, Tyson GW, Hoegh-Guldberg O. 2013. Future reef decalcification under business-as-usual CO2 emission scenario. Proc. Natl Acad. Sci. USA 110, 15 342–15 347. ( 10.1073/pnas.1302701110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camp EF, Krause S-L, Santos LMF, Naumann MS, Kikuchi RKP, Smith DJ, Wild C, Sugget DJ. 2015. The ‘flexi-chamber’: a novel cost-effective in situ respirometry chamber for coral physiological measurements. PLoS ONE 10, e0138800 ( 10.1371/journal.pone.0138800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richier S, Achterberg EP, Dumousseaud C, Poulton AJ, Suggett DJ, Tyrrell T, Zubkov MV, Moore CM. 2014. Carbon cycling and phytoplankton responses within highly replicated shipboard carbonate chemistry manipulation experiments conducted around Northwest European Shelf Seas. Biogeosci. Discuss. 11, 3489–3534. ( 10.5194/bgd-11-3489-2014) [DOI] [Google Scholar]
- 23.Dickson AG, Sabine CL, Christian JR. 2007. Guide to best practices for ocean CO2 measurements, vol. 3 Sidney, Canada: PICES. [Google Scholar]
- 24.Suggett DJ, Dong LF, Lawson T, Lawrenz E, Torres L, Smith DJ. 2013. Light availability determines susceptibility of reef building corals to ocean acidification. Coral Reefs 32, 327–337. ( 10.1007/s00338-012-0996-7) [DOI] [Google Scholar]
- 25.Davies PS. 1980. Respiration in some Atlantic reef corals in relation to vertical distribution and growth form. Biol. Bull. 158, 187–194. ( 10.2307/1540930) [DOI] [Google Scholar]
- 26.Naumann MS, Jantzen C, Haas AF, Iglesias-Prieto R, Wild C. 2013. Benthic primary production budget of a Caribbean reef lagoon (Puerto Morelos, Mexico). PLoS ONE 8, e82923 ( 10.1371/journal.pone.0082923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enochs IC, Manzello DP, Carlton R, Schopmeyer S, van Hooidonk R, Lirman D.. 2014. Effects of light and elevated pCO2 on the growth and photochemical efficiency of Acropora cervicornis. Coral Reefs 33, 477–485. ( 10.1007/s00338-014-1132-7) [DOI] [Google Scholar]
- 28.Berkelmans R, van Oppen MJH. 2006. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc. R. Soc. B 273, 2305–2312. ( 10.1098/rspb.2006.3567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritchie RJ. 2006. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 89, 27–41. ( 10.1007/s11120-006-9065-9) [DOI] [PubMed] [Google Scholar]
- 30.Burnham KP, Anderson DR. 2003. Model selection and multimodel inference: a practical information-theoretic approach. London, UK: Springer Science and Business Media. [Google Scholar]
- 31.Williams D, Miller M, Kramer K. 2008. Recruitment failure in Florida keys Acropora palmata, a threatened Caribbean coral. Coral Reefs 27, 697–705. ( 10.1007/s00338-008-0386-3) [DOI] [Google Scholar]
- 32.Albright R, Langdon C.. 2011. Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides. Glob. Change Biol. 17, 2478–2487. ( 10.1111/j.1365-2486.2011.02404.x) [DOI] [Google Scholar]
- 33.Albright R, Mason B, Miller M, Langdon C. 2010. Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata. Proc. Natl Acad. Sci. USA 107, 20 400–20 404. ( 10.1073/pnas.1007273107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manzello DP, Enochs IC, Bruckner A, Renaud P, Kolodziej G, Budd D, Carlton R, Glynn PW. 2014. Galápagos Coral Reef persistence after ENSO warming across an acidification gradient. Geophys. Res. Lett. 41, 9001–9008. ( 10.1002/2014GL062501) [DOI] [Google Scholar]
- 35.Reynaud SN, Leclercq N, Romaine-Lioud S, Rier-Page C, Jaubert J, Gattuso JP. 2003. Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification. Glob. Change Biol. 9, 1660–1668. ( 10.1046/j.1365-2486.2003.00678.x) [DOI] [Google Scholar]
- 36.Coles SL, Jokiel PL. 1978. Synergistic effects of temperature, salinity and light on the hermatypic coral Montipora verrucosa. Mar. Biol. 49, 187–195. ( 10.1007/BF00391130) [DOI] [Google Scholar]
- 37.Langdon C, Atkinson MJ. 2005. Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance nutrient and enrichment. J. Geophys. Res. Oceans 110, 1–16. ( 10.1029/2004JC002576) [DOI] [Google Scholar]
- 38.Leggat W, Badger MR, Yellowlees D. 1999. Evidence for an inorganic carbon concentrating mechanism in the symbiotic dinoflagellate Symbiodinium sp. Plant Physiol. 121, 1247–1255. ( 10.1104/pp.121.4.1247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YC, et al. 2004. Structure- and function-based characterization of a new phosphoglycolatephosphatase from Thermoplasma acidophilum. J. Biol. Chem. 279, 517–526. ( 10.1074/jbc.M306054200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crawley A, Kline DI, Dunn S, Anthony K, Dove S.. 2010. The effect of ocean acidification on symbiont photorespiration and productivity in Acropora formosa. Glob. Change Biol. 16, 851–863. ( 10.1111/j.1365-2486.2009.01943.x) [DOI] [Google Scholar]
- 41.La Jeunesse T. 2002. Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar. Biol. 141, 387–400. ( 10.1007/s00227-002-0829-2) [DOI] [Google Scholar]
- 42.Finney JC, Pettay DT, Sampayo EM, Warner ME, Oxenford HA, La Jeunesse TC. 2010. The relative significance of host–habitat, depth, and geography on the ecology, endemism, and speciation of coral endosymbionts in the genus Symbiodinium. Microb. Ecol. 60, 250–263. ( 10.1007/s00248-010-9681-y) [DOI] [PubMed] [Google Scholar]
- 43.Robison JD, Warner ME. 2006. Differential impacts of photoacclimation and thermal stress on the photobiology of four different phylotypes of Symbiodinium (Pyrrhophyta). J. Phycol. 42, 568–579. ( 10.1111/j.1529-8817.2006.00232.x) [DOI] [Google Scholar]
- 44.van Oppen MJ, Oliver JK, Putnam HM, Gates RD. 2015. Building coral reef resilience through assisted evolution. Proc. Natl Acad. Sci. USA 112, 2307–2313. ( 10.1073/pnas.1422301112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keppel G, Wardell-Johnson G.. 2012. Refugia: keys to climate change management. Glob. Change Biol. 18, 2389–2391. ( 10.1111/j.1365-2486.2012.02729.x) [DOI] [Google Scholar]
- 46.Jiang ZJ, Huang X-P, Zhang J-P. 2010. Effects of CO2 enrichment on photosynthesis, growth, and biochemical composition of seagrass Thalassia hemprichii (Ehrenb.) Aschers. Int. J. Plant. Biol. 52, 904–913. ( 10.1111/j.1744-7909.2010.00991.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.