Abstract
Patch size and isolation are predicted to alter both species diversity and evolution; yet, there are few empirical examples of eco-evolutionary feedback in metacommunities. We tested three hypotheses about eco-evolutionary feedback in a gall-forming fly, Eurosta solidaginis and two of its natural enemies that select for opposite traits: (i) specialization and poor dispersal ability constrain a subset of natural enemies from occupying small and isolated patches, (ii) this constraint alters selection on the gall fly, causing phenotypic shifts towards traits resistant to generalist and dispersive enemies in small and isolated patches, and (iii) reduced dispersal evolves in small, isolated populations. We sampled patches in a natural metacommunity and found support for all hypotheses; Eurosta's specialist wasp parasitoid attacked fewer galls in small and isolated patches, generating a selection gradient that favoured small galls resistant to predation by a dispersive and generalist bird predator. Phenotype distributions matched this selection gradient, and these phenotypic differences were maintained in a common garden experiment. Finally, we found lower dispersal abilities in small and isolated patches, a phenotypic shift that aids in the maintenance of local adaptation. We suggest that the trophic rank and the species traits of consumers are central to evolution in metacommunities.
Keywords: Eurosta solidaginis, metacommunity, Solidago altissima, consumer–resource, trophic dependency, dispersal
1. Introduction
Ecological communities are commonly believed to reflect ongoing feedback between evolutionary dynamics and ecological interactions, yet empirical examples of these types of feedback have only been established for a handful of ecosystems [1,2]. Metacommunities and island-mainland systems describe distinct communities linked by dispersal that may be particularly prone to eco-evolutionary dynamics, owing to the potential for local adaptation, limited dispersal across ecologically distinct patches, and clear effects of species traits on their spatial distributions in these systems [3–5]. The recent focus on eco-evolutionary dynamics in metacommunities raises the challenge of testing relationships among species interactions, selection and phenotypic variation over complex landscapes.
Despite extensive testing of metacommunity theory, we still do not have a thorough understanding of when particular species should be present or absent in a patch, or the evolutionary consequences of these differences. For example, we have only recently begun to understand why trophic groups respond differently to patch size and isolation [4,6]. Predators and parasitoids are usually more sensitive to habitat size and isolation than their prey [3]. While both predators and parasitoids consume prey species, parasitoids reproduce within their host and tend to be specialized [7]. The increased sensitivity of predators and parasitoids to habitat size and isolation is caused by a number of mechanisms including sampling effects, increased detection of large patches by consumers, high metabolic demands necessitating a greater resource base, difficulty finding rare prey [8] and trophic dependencies [4]. Trophic dependency, the reliance of a consumer on its resource, may be particularly important in restricting the distribution of specialist consumers including many parasitoids [3,6,7,9]. Together, these mechanisms reduce colonization and increase extinction rates of consumers, decreasing consumer species richness. Nonetheless, the specific consumers that will be lost from small and isolated patches, and the evolutionary consequences of such losses, have only been superficially explored and only at large spatial scales [10].
Loss of consumers and top predators on islands has been implicated as the cause of several macro-evolutionary patterns. Charles Darwin first noted that finches and marine iguanas on the Galapagos Islands were more docile than their mainland equivalents [11,12], and attributed these differences to the lack of predators on remote islands. Island gigantism, a common trend where island populations of insects and small mammals and lizards evolve increased body size, is similarly thought to result from selection on optimal body size following predator release [13,14]. These evolutionary patterns are extreme examples of the evolutionary consequences of predator loss on island which occur over long timescales when virtually all predators are absent.
A more typical scenario for metacommunities is that consumer species should be found less frequently and be lost at different rates as patch size and connectivity decrease, creating spatial gradients in selection [6,9,15]. Consumer species often select for specific prey phenotypes [16–18]; when different consumer species have distinct responses to patch size and isolation, patches differing in these biogeographic factors should produce alternative selection regimes. One way species may differ in their sensitivity to patch size and isolation is by their degree of specialization. In food webs composed of specialist and generalist consumers, specialists will be more sensitive to patch size owing to stronger trophic dependencies [6], and an inability to occupy the matrix. In the most extreme cases, specialists can attack only one species precluding the possibility of resource switching where generalists can consume alternative prey should one resource become extirpated. For example, island populations of the Glanville fritillary butterfly exhibit a classic metapopulation structure, occurring less frequently in small and isolated patches [19]. Its local abundance or absence also determines the landscape structure of its specialist parasitoids, causing a scaling through trophic levels [20]. However, the Glanville fritillary butterfly also hosts generalist hyperparasitoids that are expected to have broader spatial distributions [21]. These types of patterns are likely common to a wide range of specialist herbivores and parasitoids that are restricted to patchy distributions of their host plants [22]. Differences in the sensitivity of consumers to patch size and the resulting changes in community composition can equally be mediated by differences in consumer behaviour or dispersal ability. For example, more dispersive consumers are usually more common in small and isolated communities [23]. Regardless of the underlying mechanism, spatial shifts in community assemblage are not simply ecological patterns of trophic richness—they commonly alter natural selection and thus the evolution of resource populations [16,17].
Predators may also cause phenotypic differentiation among local prey communities, permitting the development of ecological interactions that differ among patches in a metacommunity. Phenotypic shifts in local populations can be driven by local selection regimes if populations have sufficient reproductive isolation to allow for strong assortative mating [24]. Classic research has shown that islands isolated by only tens of metres, and as small as a few metres across, were colonized by arthropods in ways consistent with predictions made by island biogeography [15], suggesting that the conditions necessary for isolation-based assortative mating may be commonly met for insects. However, dispersal among patches, and thus the importance of assortative mating, may also be subject to evolution in metacommunities. For example, highly dispersive phenotypes of the Glanville butterfly are associated with smaller patches that are prone to extinction [25], a pattern that should decrease local adaptation to small patches. By contrast, many populations evolve reduced dispersal on islands, which is adaptive when populations are locally adapted or dispersal is very costly due to the low success in finding other suitable habitat patches (islands; [26,27]). Although both selective forces may be present in metacommunities, increases in host population sizes in the absence of predators, and local adaptation to predator-free conditions, seem more likely to select for lower dispersal in isolated populations of a resource species.
In this paper, we test how trophic rank, differences among top predators, and the spatial structure of a natural metacommunity impact evolutionary processes and the ecological distributions of species and anti-predator traits. We use patches of tall goldenrod (Solidago altissima) plants that support a specialist, gall-forming fly parasite (Eurosta solidaginis) and the fly's specialist parasitoid, Eurytoma gigantea [28]. Research in this well-studied system has shown that gall size of Eurosta is under stabilizing selection when Eurytoma and generalist bird consumers are present (woodpeckers and chickadees), with Eurytoma selecting for larger gall sizes and bird predators selecting for smaller galls [16,29]. We quantified attack rates and selection imposed by Eurytoma and bird predators in patches of goldenrod that varied in size (less than 10 to more than 250 stems) and distance (3–88 m) from a large ‘mainland’ field of goldenrod. Our study tested the above ideas by hypothesizing that (i) Eurosta will be less abundant in small and isolated goldenrod patches, (ii) Eurosta attack by Eurytoma, the specialist parasitoid, will decline in small and isolated goldenrod patches but attack by birds will be unchanged, (iii) the decline in Eurytoma attack frequency will shift selection gradients to favour galls that are more susceptible to parasitoid attack but less susceptible to attack by downy woodpeckers, (iv) changes in selection gradients will cause morphological shifts towards Eurosta phenotypes with decreased susceptibility to downy woodpecker attack in small and isolated patches, and (v) Eurosta from isolated goldenrod patches will have reduced dispersal ability.
2. Material and methods
(a). Study system
Solidago altissima (hereafter referred to as goldenrod) is a common old-field plant in much of eastern North America [30] and can be patchily distributed, often in forest clearings [31]. Goldenrod tends to occur in large clonal patches, almost never occurring singly [31]. Goldenrod is attacked by E. solidaginis (hereafter referred to as Eurosta), a univoltine fly (Diptera: Tephitidae) whose larvae cause goldenrod to develop a spherical, tumour-like stem gall [28,32]. Adult females emerge from galls, mate and oviposit in late May in Southern Ontario. The gall appears roughly three weeks later and continues to grow for another month [16]. Galls are attacked by birds (downy woodpeckers and chickadees), and two species of Eurytoma wasp, and Mordellistena beetles, but only downy woodpeckers and E. gigantea preferentially attack galls of a particular size [28]. Eurytoma is a true specialist, attacking only E. solidaginis galls, whereas woodpeckers and chickadees are generalists, both consuming dozens of arthropod species [33].
The size preferences of bird predators and E. gigantea (hereafter referred to as Eurytoma) have been shown to drive selection on gall size. Eurytoma females probe galls of all sizes but can only insert an egg when the width of the gall wall is less than the length of the ovipositor. This parasitoid is therefore limited to smaller galls, causing selection for increased gall size [16,34]. During winter months, downy woodpeckers (Picoides pubescens) and black-capped chickadees (Poecile atricapillus) attack Eurosta galls, preferentially consuming large galls, and imposing selection for small gall size [29,35]. The preferences of bird predators and Eurytoma cause stabilizing selection [16] with the optimum gall size dependent on the attack rate of each [28,34].
(b). Field methods
In Early May 2015, we collected Eurosta galls from 28 goldenrod patches surrounded by a forest matrix at Koffler Scientific Reserve, King Township, Ontario, Canada. We surveyed all goldenrod patches within 150 m of a large ‘mainland’ field which bordered the forest. The goldenrod patches varied in the number of stems (7–266 ramets; hereafter referred to as patch size) and distance from the mainland field (3–88 m, hereafter referred to as isolation). Patch size and isolation were not related to one another (electronic supplementary material figure S1; R2 = 0.002, p = 0.8), and no other large populations of goldenrod were nearby. We collected every gall from each patch, measured the maximum gall diameter then dissected each gall to determine its content. We scored galls containing Eurosta larvae as survivors, and reared the Eurosta larvae for further tests (below). We marked galls with large holes as having been killed by birds, those containing wasp larvae as having been attacked by Eurytoma, and empty galls showing no signs of habitation as early larval death (ELD).
To ensure that any among-patch differences in gall size were driven by underlying differences of Eurosta rather than differences in goldenrod quality or genotype that is related to patch characteristics, we reared populations of Eurosta in a common garden. We reared out all Eurosta from each patch less those used in dispersal trials (below) in centrifuge tubes, then introduced them to cages (diameter = 1.13 m, height = 1.5 m) placed over S. altissima ramets. Each population was housed in a different cage, but all cages were close to each other in a continuous patch of goldenrod. In August, after galls had stopped growing [16], we harvested all galls, measuring diameter then comparing mean gall diameter in the common garden with mean gall diameter in the original population.
To test for differences in dispersal ability among Eurosta from different patches, we used a subset of the collected pupae from each patch as well as from the mainland field, and reared these pupae in 50 ml Falcon tubes until emergence in early June. After emergence, we conducted dispersal assays on up to three individuals per population. We tested individuals within 2 days of emergence after marking them on the wing with a fine point marker. We released focal individuals in equal sex ratios, from the same point in a 9 m2 patch of goldenrod surrounded by forest. This set-up was to assess dispersal among forest patches (i.e. dispersal that would occur in this metacommunity). We started each trial at 9.00 and searched for flies every 2 h for the first 6 h, then once the following day at 9.00. At each time step, we marked the position of each individual using a flag, then measured the distance moved since the previous capture. To standardize the environment between trials, we only released flies on days that were rainless with little wind (i.e. less than 10 km h−1). Dispersal estimates may be biased when long-distance dispersal goes undetected, which may occur when distances are not recorded for individuals that disappear from a trial. To ensure that this was not influencing dispersal estimates from our study, we quantified the number of Eurosta we failed to re-sight.
(c). Statistical analyses
We used a series of linear models (LMs) to test for the effects of patch size and isolation on the attack rate of natural enemies, selection gradients caused by differences in attack rate and phenotypic shifts in anti-predator traits. We first standardized all independent variables by subtracting the mean and dividing by the standard deviation. We then used a generalized linear mixed model (GLMM) with a binomial distribution and logit link function to test for differences in the rate of goldenrod parasitism by Eurosta among patches differing in size and isolation. To avoid trends caused by correlations among individuals in a patch, we included patch as a random effect. We included patch size, isolation and their interaction as independent variables, and removed the interaction term and reanalysed the model if the interaction term was not significant. We used the same model to test for differences in attack rate of bird predators and the parasitoid Eurytoma. We used log-likelihood ratios to test for significance of all models, with likelihoods determined from the maximum-likelihood solution.
We calculated selection coefficients both across all patches (globally) and for each patch individually (locally). Before analysing selection coefficients, we calculated relative fitness by dividing an individual's survival by the mean survival measured in the population [36]. Fitness was relativized across all patches for the global selection analysis, but within each patch when calculating local selection gradients. Relativizing fitness globally assumes that populations are panmictic, whereas local fitness assumes that individuals interact only within their patch.
We used separate models to estimate significance values and regression coefficients because coefficients from GLMMs cannot be directly related to selection, and LMs of transformed binary data cannot be assessed for statistical significance, because distribution and variance assumptions are violated. We tested for significant selection coefficients by regressing survival against standardized gall size using a GLMM with a logit link function for all data together (global selection), and for each patch separately (local selection). We included patch as a random effect in the global selection analysis. We then used an LM with relative fitness as the response variable to estimate the selection coefficient and standard error of each coefficient. To estimate nonlinear selection coefficients, we included the squared standardized gall size in the models [36], and then doubled the coefficients from LMs to calculate the actual magnitude of nonlinear selection [37]. We then re-ran LMs without the quadratic term to estimate the coefficient of directional selection.
We tested for systematic differences in selection among patches by regressing isolation and patch size against local selection coefficients using an LM, again removing the isolation by patch size interaction if it was non-significant. To prevent significant patterns being driven by non-significant selection coefficients, we weighted each coefficient by the inverse of its standard error. We independently tested for differences in nonlinear and linear selection by separately modelling quadratic and linear selection coefficients.
After testing for differences in selection coefficients, we investigated phenotypic differences across patch sizes and isolation. We tested for an effect of patch size and isolation on standardized gall size while including patch as a random effect in an LME. To ensure that any difference in gall size resulted from underlying differences among Eurosta populations (rather than goldenrod populations), we used a LM to regress the mean gall size of each Eurosta population measured in the spring against the mean gall size of the corresponding population in the common garden. We also tested for differences in distance dispersed by individual flies using an LME with patch size and isolation as main effects, and time nested in individual after release as a random effect.
To explore the likely contribution of gene flow from nearby patches (versus from the mainland), we used a metapopulation model that calculates the proportion of immigrants that come from nearby patches versus the ‘mainland’ patch. We calculated connectivity as the negative exponent of distance between two patches divided by α, the mean dispersal distance of an individual. We then weighted those connectivity values by multiplying by the number of stems in a given patch (following [38]). We generated connectivity calculations for several values of α (2, 10 and 100 m), with the lowest value reflecting mean observed dispersal distances over a 1-day period. Although these estimates do not give an absolute estimate of immigration or gene flow, they quantify the relative importance of different sources (patches and the mainland) to the immigrant pool. Statistical analyses were conducted in R (v. 3.1.1, 2014) using the base package and the ‘lme4’ package [39].
3. Results
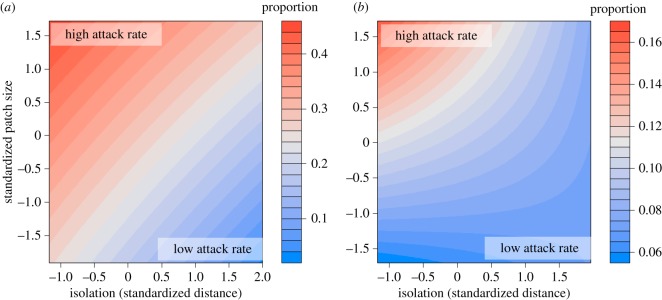
A large proportion of Eurosta galls were attacked by Eurytoma and downy woodpeckers, with attack rates depending on gall size and patch isolation and size (figures 1 and 2). Eurosta was more common in large patches that were near to the large ‘mainland’ patch (figure 2; both p < 0.001). Eurytoma preferentially attacked small galls (figure 1; p < 0.001) and were rarer in small and isolated patches (figure 2; electronic supplementary material, figure S2; both p < 0.003). Downy woodpeckers attacked large galls (figure 1; p < 0.001) but attack rates did not differ with patch size or isolation (electronic supplementary material, figure S2, both p > 0.6). All bird predation recorded was from downy woodpeckers.
Figure 1.
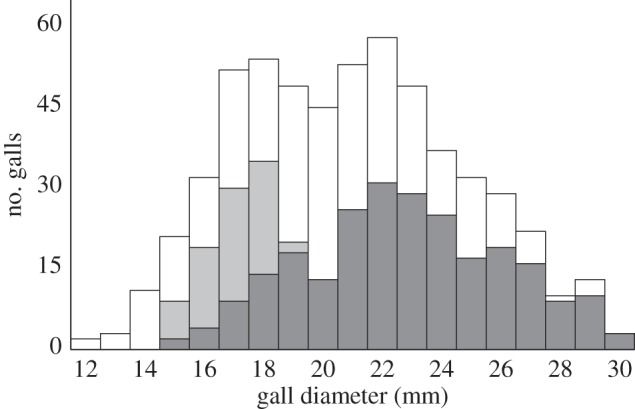
The number of galls attacked by Eurytoma (light grey), downy woodpeckers (dark grey) and surviving (white). Eurytoma preferentially parasitized small galls, downy woodpeckers attacked large galls, and most survivors had an intermediate gall size. The smallest galls were not attacked, but had high rates of ELD (not shown here).
Figure 2.
Predicted values of Eurosta and Eurytoma attack rate from GLMMs of patches differing in size and isolation. (a) Proportion of goldenrod stems galled by Eurosta and (b) proportion of Eurosta galls and proportion parasitized by Eurytoma. GLMMs used a logit link function with isolation and patch size as main effects, and patch as a random effect. High and low attack rates are depicted in red and blue, respectively, although the scales differ between plots. In both cases, attack was most frequent in large and near patches, but the attack rate of Eurosta was consistently higher.
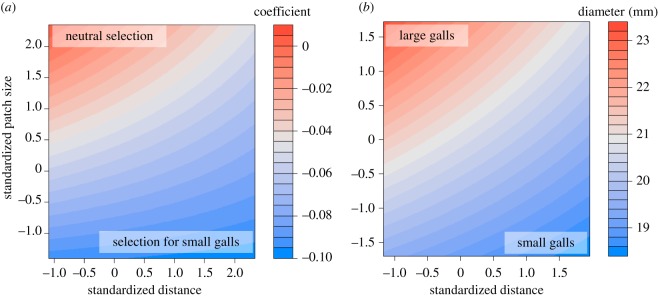
Selection analysis revealed significant patterns of directional selection with patch size and isolation. Globally, there was selection for small galls (selection coefficient = −0.08683, p < 0.001), but nonlinear selection was non-significant (selection coefficient = −0.0659, p = 0.577). Locally, nonlinear selection did not differ across an isolation gradient (β = 0.0062, p = 0.3223), but there was a marginal trend for stabilizing selection to be stronger in larger patches (β = −0.0876, p = 0.0967). Selection for small galls was the strongest in small and isolated patches (figure 3; both p < 0.015), with mean selection coefficients ranging from 0 (neutral) in large, connected patches to –0.1 in small, isolated patches.
Figure 3.
Predicted values of directional selection coefficients and gall sizes from an LM and LME, respectively. (a) Selection on gall diameter and (b) gall diameter (mm). Patch size and isolation were included in both models, and patch was included as a random effect when analysing gall sizes. Low selection coefficients indicate directional selection for smaller gall sizes, while the highest selection coefficients represent neutral selection. High selection coefficients and large galls are represented by red, while low selection coefficients and small galls are shown in blue. Patterns of selection and gall size were congruent, with low selection coefficients and small galls being present in small and isolated patches.
Observed phenotypic shifts were consistent with both theoretical expectations and the results of the selection analysis; Eurosta galls were smaller in both small and isolated patches (figure 3; both p < 0.04). Moreover, there was a significant correlation between gall sizes in the field and the common garden (figure 5; electronic supplementary material, figure S3; p < 0.001, R2 = 0.716) indicating that among population differences in gall size have an underlying heritable component.
Figure 5.
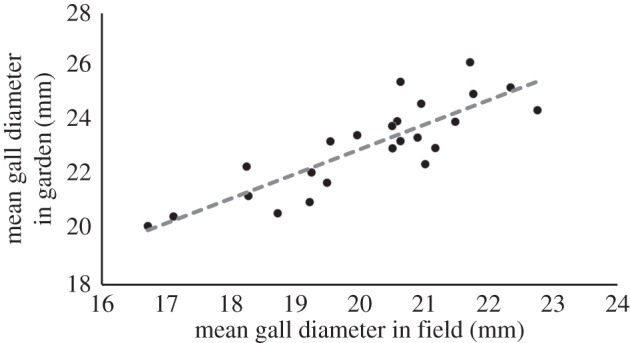
Mean gall diameter in the common garden was positively correlated with mean gall diameter in the field, indicating a genetic component to the observed variation in gall size. The line of best fit was estimated using an LM.
Dispersal distances of Eurosta from different natal populations were consistent with expected selection on dispersal ability. Eurosta from isolated patches dispersed shorter distances (figure 4; p < 0.001), but the size of the natal patch had no effect on dispersal (p = 0.2125). There were no patch level differences in the number of Eurosta we failed to re-sight (electronic supplementary material, table S1). Our metapopulation model of patch connectivity showed that the vast majority of immigrants to distant patches would be from surrounding patches rather than the mainland (figure 4b). This result was clear at the observed mean dispersal distance (2 m) and larger (10 m), but disappeared at very high mean dispersal distances of 100 m (figure 4b). In other words, the low dispersal from patches in the forest that we documented appears to be sufficient to cause spatially assortative mating.
Figure 4.
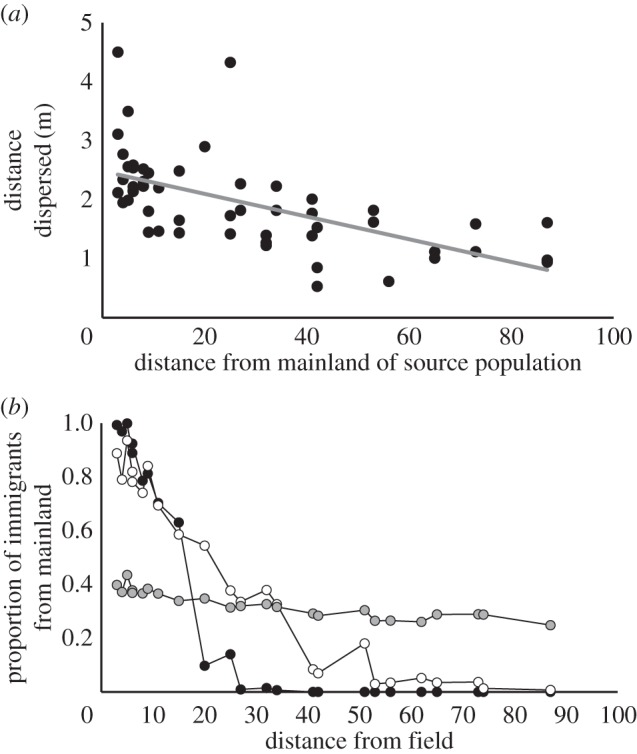
(a) The distance dispersed in 24 h by individual Eurosta from natal patches differing in distance from a mainland. The line of best fit was estimated using a linear mixed effects model with patch size and isolation as main effects, and patch as a random effect. Eurosta from distant patches were poorer dispersers than individuals from near patches. (b) Estimates of the proportion of immigrants originating in the mainland for each patch. Estimates were calculated with mean dispersal distances (α) of 2 (black), 10 (white) and 100 (grey) metres. The genetic contribution from the mainland is initially high but declines quickly when dispersal distances are short but is relatively constant when individuals are dispersive.
4. Discussion
Our study demonstrates how the spatial distribution of patches in a metacommunity structures species occurrence patterns, shaping species interactions, selection and phenotypic variation. Although it is well understood that trophic dependencies, sampling effects, dispersal limitation and foraging behaviour can limit the occurrence of natural enemies in small and isolated patches, the evolutionary consequences of these differences in natural enemy presence have, until now, been unexplored. Our study shows that natural enemies are less likely to occupy small and isolated habitats, and that reduced enemy abundance can alter selection. However only the distribution of Eurytoma, a Eurosta specialist, was affected by habitat size and isolation, while attack by dispersive and generalist downy woodpeckers was unchanged. The predictable loss of a subset of consumers created gradients of selection that depended on habitat size and isolation. The adaptive phenotypic shifts that we observed were consistent with these selection gradients, and can feedback to affect ecological dynamics by influencing an individual's propensity for dispersal and susceptibility to attack.
Our study suggests that metacommunity theory may be a powerful tool for understanding feedback between ecology and evolution. As predicted by trophic dependency [3,6], we found that the distribution of species was nested by trophic level, with Eurosta only surviving in goldenrod patches, and the specialist parasitoid Eurytoma only surviving in patches with Eurosta (figure 2). These spatial patterns of trophic dependency are likely common in a wide range of ecosystems. For example, isolated grassland fragments contained fewer predators than large or well-connected patches, and these differences were attributed to trophic dependencies [40]. Even in ecosystems where trophic dependencies do not seem to drive predator distributions, the relatively low regional abundances of consumers and biased detection of large habitats can still cause them to be absent from smaller patches [4], suggesting that multiple causal mechanisms such as sampling effects or behavioural differences may lead to the pervasive pattern of fewer predators occurring in small habitats.
In our study system, two traits appear to determine the sensitivity of consumers to patch size and isolation: dispersal ability and diet specialization. In particular, the highly dispersive downy woodpecker, a generalist insect predator, attacked Eurosta at a consistent rate across patches, whereas the specialist parasitoid Eurytoma was more common in large and well-connected patches (figure 2b). These differences suggest that Eurytoma was either unable to immediately disperse to patches from which it was absent, that it was constrained by the low abundance of Eurosta in small and isolated patches (figure 2a), or both. In contrast to Eurytoma, downy woodpeckers could easily disperse over the small spatial extent of our study, and even if dispersal limited, downy woodpeckers could feed on alternate resources in the absence of Eurosta. Differences in both dispersal ability [23] and diet breadth [41–43] are typical in many food webs, suggesting that different predator species will commonly have distinct responses to patch size and isolation. For example, Gravel et al. [6] found that across 50 Adirondack lakes, consumers with generalist diets were more likely to be found in a given lake, and that this effect was magnified in small lakes. Similarly, invertebrate diversity declined in isolated rock pools, but this pattern was caused by the absence of poorly dispersing species [23]. Differences in dispersal ability may vary by species but also be dependent upon the habitat separating patches or other conditions affecting an individual's likelihood of dispersing [7]. Together, these and other studies show that species traits can create disparate consumer communities in patches differing in size and isolation. These changes in consumer community composition may create novel patterns of species interactions and ultimately selection.
The loss of a subset of natural enemies in small and isolated patches may systematically alter selection on prey species in metacommunities. In many cases, resisting consumption has a cost, creating selection against resistance if consumers are absent. For example, Van Buskirk & Schmidt [44] found that newts with anti-consumer defences had improved survival in the presence of dragonfly consumers but lower growth rates in their absence. In our study, woodpeckers and Eurytoma selected for opposite defensive traits, a pattern that is fairly general when several consumers are present—traits that confer an advantage against one consumer may be costly when defending against another [45–47]. When consumers select for alternate anti-predator traits, those traits should be under stabilizing selection when all consumers are present, but the adaptive peak will shift when one consumer is lost. Overall, the widespread observation that consumers induce selection for specific defensive traits in their prey suggests that the effects of consumer specialization and dispersal ability observed in our study may be general to evolving metacommunities.
Differences in selection across patches can either increase standing genetic variation across all patches or mediate local adaptation. Spatial variation in selection should confer increased standing genetic variation when populations are panmictic, but increasingly promote local adaptation as populations have higher levels of assortative mating [24]. We found phenotypic shifts in Eurosta gall size, consistent with selection imposed by constant levels of downy woodpecker consumption but reduced Eurytoma attack in small and isolated patches (figure 3). These shifts in gall size suggest that local adaptation is occurring, causing the mean phenotype present in each patch to reflect the optimal gall size in that patch. In our system, local adaptation is made more likely both by the poor dispersal ability of Eurosta and the barrier imposed by forests to many old-field insects [48,49]. While phenotypic shifts are largely due to heritable differences in gall size (figure 5), differences in both plant genotype and plant nutritive quality can equally alter patterns of gall size among patches [17]. Among-patch differences in the environment, including differences in plant genotype or quality may themselves create differences in selection and local adaptation among patches [50]. While spatial variation in selection often causes local adaptation, the small spatial scale at which we detected phenotypic shifts consistent with local adaptation is exceptional and likely driven by poor dispersal ability through the forest matrix [17,51,52].
Just as dispersal influences patterns of genetic diversity, the spatial structure of selection gradients may alter dispersal. Selection often favours reduced dispersal ability in isolated habitats, first because the cost of dispersal is high, and later because an individual is more likely to be maladapted in other patches once local adaptation has occurred [53,54]. Increased assortative mating has been shown to result from maladaptation driving reduced immigrant fitness in a wide range of systems [55] suggesting that this mechanism may be common. Our findings are consistent with both high costs of dispersal and immigrant maladaptation—Eurosta from isolated natal populations were poorer dispersers than individuals from less isolated patches (figure 4), and tended to produce galls that were maladapted to patches with greater numbers of Eurytoma (figures 3b and 5). Widespread loss of dispersal structures in isolated populations [26,56] suggests that feedback between local adaptation and selection for reduced dispersal ability may be common in natural populations.
One question that arises from our results, is why the phenotypic distribution of gall sizes does not perfectly match the optimal gall size predicted by selection gradients. Put otherwise, why has selection not fixed the optimal phenotype in each habitat? One possibility is that ELD is more prevalent in small galls, and by excluding ELD from our analyses we biased our calculations of selection coefficients [16]. Indeed, ignoring some component of fitness is an issue for virtually all studies of selection [57–59], and in our study could not be avoided because we cannot know when small galls cause ELD and vice versa. Other reasons for imperfect phenotype–environment matching include temporally variable selection [60–62], immigration from populations differing in optimal gall size [24,63], inadequate time for populations to fully adapt to local conditions [64], or failing to account for other agents of selection. How these mechanisms interact to produce observed patterns of phenotypic variation remains a focus of evolutionary ecology. Nonetheless, the clear trends in selection and phenotypic distributions that we observed suggest that patch size and isolation play a strong role in structuring gall sizes in this system.
By incorporating well-understood spatial and trophic processes with evolutionary dynamics, our study has begun to elucidate the intricacies of eco-evolutionary dynamics in metacommunities. We have demonstrated that accounting for trophic structure, and species-specific differences in specialization and dispersal ability, predictably alters spatial patterns of species distributions. These differences alter species interactions, selection and local phenotypes, potentially affecting ecological dynamics and generating feedback between dispersal and local adaptation. The small spatial scales over which these patterns manifest suggests that these types of dynamics are likely to be widespread in nature.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank Arthur Weis and Colin Bonner for helping to classify galls, and for their advice on working with Eurosta. We thank Stephen de Lisle for help with selection analyses. We also thank the staff of Koffler Scientific Reserve for their hospitality and technical support.
Data accessibility
Data are available from Dryad: http://dx.doi.org/10.5061/dryad.vr588.
Authors' contributions
D.S. conceived the study and collected the data. D.S. and B.G. analysed the data, wrote and approved the manuscript.
Competing interests
We have no competing interests.
Funding
Research funding was provided by NSERC (B.G.) and personal funding was awarded by NSERC-CGS (D.S.).
References
- 1.Fussmann GF, Loreau M, Abrams PA. 2007. Eco-evolutionary dynamics of communities and ecosystems. Funct. Ecol. 21, 465–477. ( 10.1111/j.1365-2435.2007.01275.x) [DOI] [Google Scholar]
- 2.Schoener TW. 2011. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331, 426–429. ( 10.1126/science.1193954) [DOI] [PubMed] [Google Scholar]
- 3.Holt RD, Lawton JH, Polis GA, Martinez ND. 1999. Trophic rank and the species–area relationship. Ecology 80, 1495–1504. [Google Scholar]
- 4.Srivastava DS, Trzcinski MK, Richardson BA, Gilbert B. 2008. Why are predators more sensitive to habitat size than their prey? Insights from bromeliad insect food webs. Am. Nat. 172, 761–771. ( 10.1086/592868) [DOI] [PubMed] [Google Scholar]
- 5.Bell G, Gonzalez G. 2011. Adaptation and evolutionary rescue in metapopulations experiencing environmental deterioration. Science 332, 1327–1330. ( 10.1126/science.1203105) [DOI] [PubMed] [Google Scholar]
- 6.Gravel D, Massol F, Canard E, Mouillot D, Mouquet N. 2011. Trophic theory of island biogeography. Ecol. Lett. 14, 1010–1016. ( 10.1111/j.1461-0248.2011.01667.x) [DOI] [PubMed] [Google Scholar]
- 7.Cronin JT, Haynes KJ. 2004. An invasive plant promotes unstable host–parasitoid patch dynamics. Ecology 85, 2772–2782. ( 10.1890/04-0303) [DOI] [Google Scholar]
- 8.Pimm SL, Lawton JH. 1977. Number of trophic levels in ecological communities. Nature 268, 329–331. ( 10.1038/268329a0) [DOI] [Google Scholar]
- 9.Pillai P, Loreau M, Gonzalez A. 2009. A patch-dynamic framework for food web metacommunities. Theor. Ecol. 3, 223–237. ( 10.1007/s12080-009-0065-1) [DOI] [Google Scholar]
- 10.Johnson MTJ, Stinchcombe JR. 2007. An emerging synthesis between community ecology and evolutionary biology. Trends Ecol. Evol. 22, 250–257. ( 10.1016/j.tree.2007.01.014) [DOI] [PubMed] [Google Scholar]
- 11.Darwin C. 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life, pp. 134–136. London, UK: John Murray Press. [PMC free article] [PubMed] [Google Scholar]
- 12.Blumstein DT, Daniel JC. 2005. The loss of anti-predator behaviour following isolation on islands. Proc. R. Soc. B 272, 1663–1668. ( 10.1098/rspb.2005.3147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster JB. 1964. Evolution of mammals on islands. Nature 202, 234–235. ( 10.1038/202234a0) [DOI] [Google Scholar]
- 14.Case TJ. 1978. On the evolution and adaptive significance of postnatal growth rates in the terrestrial vertebrates. Q. Rev. Biol. 53, 243–282. ( 10.1086/410622) [DOI] [PubMed] [Google Scholar]
- 15.Simberloff DS, Wilson EO. 1969. Experimental zoogeography of islands: the colonization of empty islands. Ecology 50, 278–296. ( 10.2307/1934856) [DOI] [Google Scholar]
- 16.Weis AE, Abrahamson WG. 1985. Potential selective pressures by parasitoids on a plant–herbivore interaction. Ecology 66, 1261–1269. ( 10.2307/1939179) [DOI] [Google Scholar]
- 17.Craig TP, Itami JK, Horner JD. 2007. Geographic variation in the evolution and coevolution of a tritrophic interaction. Evolution 61, 1137–1152. ( 10.1111/j.1558-5646.2007.00099.x) [DOI] [PubMed] [Google Scholar]
- 18.Losos JB, Schoener TW, Spiller DA. 2004. Predator-induced behaviour shifts and natural selection in field-experimental lizard populations. Nature 432, 505–508. ( 10.1038/nature03039) [DOI] [PubMed] [Google Scholar]
- 19.Hanski I, Thomas CD. 1994. Metapopulation dynamics and conservation: a spatially explicit model applied to butterflies. Biol. Conserv. 68, 167–180. ( 10.1016/0006-3207(94)90348-4) [DOI] [Google Scholar]
- 20.Lei GC, Hanski I. 1997. Metapopulation structure of Cotesia melitaearum, a specialist parasitoid of the butterfly Melitaea cinxia. Oikos 78, 91–100. ( 10.2307/3545804) [DOI] [Google Scholar]
- 21.Lei GC, Vikberg V, Nieminen M, Kuussaari M. 1997. The parasitoid complex attacking Finnish populations of the Glanville fritillary Melitaea cinxia (Lep: Nymphalidae), and endangered butterfly. J. Nat. Hist. 31, 635–648. ( 10.1080/00222939700770301) [DOI] [Google Scholar]
- 22.Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE. 1980. Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Ann. Rev. Ecol. Syst. 11, 41–65. ( 10.1146/annurev.es.11.110180.000353) [DOI] [Google Scholar]
- 23.Vanschoenwinkel B, De Vries C, Seaman M, Brendonck L. 2007. The role of metacommunity processes in shaping invertebrate rock pool communities along a dispersal gradient. Oikos 116, 1255–1266. ( 10.1111/j.0030-1299.2007.15860.x) [DOI] [Google Scholar]
- 24.Slatkin M. 1987. Gene flow and the geographic structure of natural populations. Science 236, 787–792. ( 10.1126/science.3576198) [DOI] [PubMed] [Google Scholar]
- 25.Hanski I, Mononen T. 2011. Eco-evolutionary dynamics of dispersal in spatially heterogeneous environments. Ecol. Lett. 14, 1025–1034. ( 10.1111/j.1461-0248.2011.01671.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adler GH, Levins R. 1994. The island syndrome in rodent populations. Q. Rev. Biol. 69, 473–490. ( 10.1086/418744) [DOI] [PubMed] [Google Scholar]
- 27.Silwood Park UK, Dytham C, Perrin N. 1999. The evolutionary ecology of dispersal. Trends Ecol. Evol. 14, 88–90. ( 10.1016/S0169-5347(98)01571-7) [DOI] [Google Scholar]
- 28.Abrahamson WG, Sattler JF, McCrea KD, Weis AE. 1989. Variation in selection pressures on the goldenrod gall fly and the competitive interactions of its natural enemies. Oecologia 79, 15–22. ( 10.1007/BF00378234) [DOI] [PubMed] [Google Scholar]
- 29.Cane JH, Kurczewski FE. 1976. Mortality factors affecting Eurosta solidaginis (Diptera: Tephritidae). J. N Y Entomol. Soc. 84, 275–282. [Google Scholar]
- 30.Root RB. 1996. Herbivore pressure on goldenrods (Solidago altissima): its variation and cumulative effects. Ecology 77, 1074–1087. ( 10.2307/2265577) [DOI] [Google Scholar]
- 31.Maddox GD, Cook RE, Wimberger PH, Gardescu S. 1989. Clone structure in four Solidago altissima (Asteraceae) populations: rhizome connections within genotypes. Am. J. Bot. 76, 318–326. ( 10.2307/2444674) [DOI] [Google Scholar]
- 32.Uhler LD. 1951. Biology and ecology of the goldenrod gall fly, Eurosta solidaginis (Fitch). Mem. Cornell University Agric. Exp. Station 300, 3–51. [Google Scholar]
- 33.Peters D, Grubb TC Jr. 1983. An experimental analysis of sex-specific foraging in the downy woodpecker, Picoides pubescens. Ecology 64, 1437–1443. ( 10.2307/1937498) [DOI] [Google Scholar]
- 34.Weis AE, Abrahamson WG, McCrea KD. 1985. Host gall size and oviposition success by the parasitoid Eurytoma gigantea. Ecol. Entomol. 10, 341–348. ( 10.1111/j.1365-2311.1985.tb00730.x) [DOI] [Google Scholar]
- 35.Schlichter L. 1978. Winter predation by the black capped chickadees and downy woodpeckers on inhabitants of the goldenrod ball gall (Eurosta solidaginis). Can. Field Nat. 92, 71–74. [Google Scholar]
- 36.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 37.Stinchcombe JR, Agrawal AF, Hohenlohe PA, Arnold SJ, Blows MW. 2008. Estimating nonlinear selection gradients using quadratic regression coefficients: double or nothing? Evolution 62, 2435–2440. ( 10.1111/j.1558-5646.2008.00449.x) [DOI] [PubMed] [Google Scholar]
- 38.Hanski I. 1994. A practical model of metapopulation dynamics. J. Anim. Ecol. 63, 151–162. ( 10.2307/5591) [DOI] [Google Scholar]
- 39.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 40.Harvey E, MacDougall AS. 2014. Trophic island biogeography drives spatial divergence of community establishment. Ecology 95, 2870–2878. ( 10.1890/13-1683.1) [DOI] [Google Scholar]
- 41.Root R. 1973. Organization of a plant–arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea). Ecol. Monogr. 43, 95–124. ( 10.2307/1942161) [DOI] [Google Scholar]
- 42.Jiang L, Morin PJ. 2005. Predator diet breadth influences the relative importance of bottom-up and top-down control of prey biomass and diversity. Am. Nat. 165, 350–363. ( 10.1086/428300) [DOI] [PubMed] [Google Scholar]
- 43.Wilson SK, Burgess SC, Cheal AJ, Emslie M, Fisher R, Miller I, Polunin NVC, Sweatman J. 2008. Habitat utilization by coral reef fish: implications for specialists vs. generalists in a changing environment. J. Anim. Ecol. 77, 220–228. ( 10.1111/j.1365-2656.2007.01341.x) [DOI] [PubMed] [Google Scholar]
- 44.Van Buskirk J, Schmidt BR. 2000. Predator-induced phenotypic plasticity in larval newts: trade-offs, selection, and variation in nature. Ecology 81, 3009–3028. ( 10.1890/0012-9658(2000)081%5B3009:PIPPIL%5D2.0.CO;2) [DOI] [Google Scholar]
- 45.Peckarsky BL, McIntosh AR. 1998. Fitness and community consequences of avoiding multiple predators. Oecologia 113, 565–576. ( 10.1007/s004420050410) [DOI] [PubMed] [Google Scholar]
- 46.Sih A, Englund G, Wooster D. 1998. Emergent impacts of multiple predators on prey. Trends Ecol. Evol. 13, 350–355. ( 10.1016/S0169-5347(98)01437-2) [DOI] [PubMed] [Google Scholar]
- 47.Teplitsky C, Plenet S, Joly P. 2004. Hierarchical responses of tadpoles to multiple predators. Ecology 85, 2888–2894. ( 10.1890/03-3043) [DOI] [Google Scholar]
- 48.Debinski DM, Holt RD. 2000. A survey and overview of habitat fragmentation experiments. Conserv. Biol. 14, 342–355. ( 10.1046/j.1523-1739.2000.98081.x) [DOI] [Google Scholar]
- 49.Tscharntke T, Brandl R. 2004. Plant–insect interactions in fragmented landscapes. Annu. Rev. Entomol. 49, 405–430. ( 10.1146/annurev.ento.49.061802.123339) [DOI] [PubMed] [Google Scholar]
- 50.Weis AE, Gorman W. 1990. Measuring selection on reaction norms: an exploration of the Eurosta–Solidago system. Evolution 44, 820–831. ( 10.2307/2409548) [DOI] [PubMed] [Google Scholar]
- 51.Egan SP, Ott JR. 2007. Host plant quality and local adaptation determine the distribution of a gall-forming herbivore. Ecology 88, 2868–2879. ( 10.1890/06-1303.1) [DOI] [PubMed] [Google Scholar]
- 52.Richardson JL, Urban MC, Bolnick DI, Skelly DK. 2014. Microgeographic adaptation and the spatial scale of evolution. Trends Ecol. Evol. 29, 165–176. ( 10.1016/j.tree.2014.01.002) [DOI] [PubMed] [Google Scholar]
- 53.Kisdi É. 2002. Dispersal: risk in spreading versus local adaptation. Am. Nat. 159, 579–596. ( 10.1086/339989) [DOI] [PubMed] [Google Scholar]
- 54.Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241. ( 10.1111/j.1461-0248.2004.00684.x) [DOI] [Google Scholar]
- 55.Nosil P, Vines TH, Funk DJ. 2005. Reproductive isolation caused by natural selection against immigrants from divergent habitats. Evolution 59, 705–719. ( 10.1111/j.0014-3820.2005.tb01747.x) [DOI] [PubMed] [Google Scholar]
- 56.Bohonak AJ. 1999. Dispersal, gene flow, and population structure. Q. Rev. Biol. 74, 21–45. ( 10.1086/392950) [DOI] [PubMed] [Google Scholar]
- 57.Reznick DN, Butler MJ, Rodd FH, Ross P. 1996. Life-history evolution in guppies (Poecilia reticulata): differential mortality as a mechanism for natural selection. Evolution 50, 1651–1660. ( 10.2307/2410901) [DOI] [PubMed] [Google Scholar]
- 58.Nagel L, Schluter D. 1998. Body size, natural selection, and speciation in sticklebacks. Evolution 52, 209–218. ( 10.2307/2410936) [DOI] [PubMed] [Google Scholar]
- 59.Gómez JM. 2000. Effectiveness of ants as pollinators of Lobularia maritima: effects on main sequential fitness components of the host plant. Oecologia 122, 90–97. ( 10.1007/PL00008840) [DOI] [PubMed] [Google Scholar]
- 60.Venable DL, Brown JS. 1988. The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. Am. Nat. 131, 360–384. ( 10.1086/284795) [DOI] [Google Scholar]
- 61.Nuismer SL, Gomulkiewicz R, Morgan MT. 2003. Coevolution in temporally variable environments. Am. Nat. 162, 195–204. ( 10.1086/376582) [DOI] [PubMed] [Google Scholar]
- 62.Punzalan D, Rodd FH, Rowe L. 2010. Temporally variable multivariate sexual selection on sexually dimorphic traits in a wild insect population. Am. Nat. 175, 401–414. ( 10.1086/650719) [DOI] [PubMed] [Google Scholar]
- 63.Weis AE. 1996. Variable selection on Eurosta’s gall size, III: can evolutionary response to selection be detected? J. Evol. Biol. 9, 623–640. ( 10.1046/j.1420-9101.1996.9050623.x) [DOI] [Google Scholar]
- 64.Kimura M, Ohta T. 1969. The average number of generations until extinction of an individual mutant gene in a finite population. Genetics 63, 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from Dryad: http://dx.doi.org/10.5061/dryad.vr588.