Abstract
Adequate waste management is vital for the success of social life, because waste accumulation increases sanitary risks in dense societies. We explored why different leaf-cutting ants (LCA) species locate their waste in internal nest chambers or external piles, including ecological context and accounting for phylogenetic relations. We propose that waste location depends on whether the environmental conditions enhance or reduce the risk of infection. We obtained the geographical range, habitat and refuse location of LCA from published literature, and experimentally determined whether pathogens on ant waste survived to the high soil temperatures typical of xeric habitats. The habitat of the LCA determined waste location after phylogenetic correction: species with external waste piles mainly occur in xeric environments, whereas those with internal waste chambers mainly inhabit more humid habitats. The ancestral reconstruction suggests that dumping waste externally is less derived than digging waste nest chambers. Empirical results showed that high soil surface temperatures reduce pathogen prevalence from LCA waste. We proposed that LCA living in environments unfavourable for pathogens (i.e. xeric habitats) avoid digging costs by dumping the refuse above ground. Conversely, in environments suitable for pathogens, LCA species prevent the spread of diseases by storing waste underground, presumably, a behaviour that contributed to the colonization of humid habitats. These results highlight the adaptation of organisms to the hygienic challenges of social living, and illustrate how sanitary behaviours can result from a combination of evolutionary history and current environmental conditions.
Keywords: Acromyrmex, ant waste, ant behaviour, Atta, group living, waste management
1. Introduction
Sanitary risks of living in societies have favoured the evolution of physiological and behavioural responses aimed at preventing pathogen spread. Two major sanitary risks of living in densely populated societies are the increased rate of pathogen infection and transmission [1–5] and the generation of huge amounts of waste [6], a common pathogen reservoir. These two risks act in concert because the accumulation of waste increases the risk of infection, while the frequent contact with conspecifics, typical of social groups, facilitates the spread of pathogens among individuals [5]. Social animals prevent the spread of pathogens through prophylactic behaviours [6–12]. To prevent infection and spread of pathogens from refuse disposals, social organisms have strategies of waste management, including the location of refuse piles (external refuse piles or internal nest chambers; [6,13–17]) and waste handling by specialized individuals [13,18–20]. Adequate waste management is essential for the survival of societies and studying waste management therefore enlightens the understanding of the evolution and maintenance of group living [21,22].
Leaf-cutting ants (genera Atta and Acromyrmex, hereafter LCA) harvest plant material for growing a symbiotic fungus and are ideal organisms for studying the adaptive nature of waste management for several reasons. First, colonies have large densities of closely related individuals (up to 8 million in the genus Atta and hundreds of thousands in Acromyrmex), who are constantly interacting with each other, which potentially increases disease transmission [5,23]. Second, the combination of large colony size and fungus agriculture produces huge amounts of pathogenic waste [6,20,24,25]. One of the major sanitary risks for LCA is the specialized fungal pathogen Escovopsis (Ascomycota: anamorphic Hypocreales), a highly virulent parasite that potentially overgrows the fungus gardens leading to the death of the ant colony [26,27]. Finally, LCA perform sophisticated and diverse prophylactic behaviours, such as avoiding pathogens while nesting [28,29], applying antimicrobial compounds while grooming [10,30,31], having specialized workers managing waste [8,9,18,32], and disposing of refuse material [6,20]. In summary, all these characteristics make LCA a good model to better understand how social organisms deal with the sanitary risk of waste accumulation.
One of the most efficient methods of social prophylaxis in LCA is the isolation of accumulated waste from the fungus garden. As explained above, waste from the fungus garden is an important source of infection because it often contains the virulent fungal parasite Escovopsis, as well as other harmful microorganisms [6,8,28,33]. For example, Escovopsis has been isolated from waste samples in several species of Atta and Acromyrmex [6,25,34], and contact with waste materials increases mortality of ant workers [6,35]. Accordingly, LCA workers avoid contact with waste [36,37] and show a clear division of labour between waste workers and foragers [9,18,19]. Because there is an explicit link between waste and infection [20], selection could act on the final destination of waste to reduce the potential spread of diseases.
Despite having similar foraging habits, fungi-culture traits and general biology, LCA show two contrasting strategies to isolate waste from the brood and the fungus garden [38]. Some LCA species dump waste on refuse piles outside the nest on the soil surface, but others species locate waste in specialized refuse chambers inside the nest ([6,39]; electronic supplementary material, figure S1). Both strategies involve costs and benefits. Storing refuse in underground chambers is an effective way to isolate larger quantities of waste, because ants often seal the access tunnels of full refuse chambers [40]. However, digging the waste chambers demands large energy investments [41,42]. As LCA are continuously producing vast quantities of waste [24], they need to remove considerable amounts of soil to build waste-storage chambers [43]. Moreover, digging refuse chambers is more costly than building other nest chambers because waste chambers are typically located at the deepest part of the nest [40,44,45]. Soil particles are transported over large distances against gravity, and thus workers are expected to incur large time and energy costs in carrying soil [43]. Also, digging effort increases ant mortality [46]. Conversely, dumping waste in external refuse dumps avoids the digging costs, but the waste is located closer to the nest entrances, increasing the risk of contamination because of rain and wind, and restricting foraging areas, because foragers often avoid the vicinities of refuse piles [20,37]. It is still unclear why closely related LCA species evolved and maintain contrasting solutions to the problem of where to locate waste.
Here, we assessed whether the current ecological context of the LCA accounted for the variation among species in waste location (internal waste chambers or external refuse piles), considering phylogenetic effects. We propose that the location of waste depends on whether the environmental conditions enhance or reduce the risk of infection from waste. As pathogen proliferation and contamination risk is higher in warm and humid conditions compared with dry conditions [32,47,48], we predict that LCA species living in xeric environments (i.e. desert and dry habitats) more often dump their refuse on external piles. Dry habitats with high daily ground temperatures may reduce the contamination potential of waste on external refuse dumps, enabling ants to save the cost of digging and without exposing the colony to high-infection risks. Conversely, the humid and warm conditions of tropical and subtropical forests or savannahs represent a suitable environment for the proliferation and spread of waste pathogens (i.e. Escovopsis). Therefore, we expect LCA living in warm and humid habitats to locate their waste inside underground chambers, because the reduced risk of contamination of waste stored inside internal nest chambers may outweigh the costs of digging those chambers. To test this idea, we: (i) reviewed the literature to determine which LCA species locate their waste inside or outside the nest, (ii) determined the geographical range and habitat occupied by these species, (iii) evaluated the effect of phylogeny on the location of waste, and (iv) performed an experiment to determine whether LCA waste experimentally exposed to a warming regime typical of desert habitats would still have the fungal pathogen Escovopsis.
2. Material and methods
(a). Data collection
To obtain the geographical range, taxonomic status, phylogeny and waste location of LCA, we consulted experts and examined published literature on these topics. The database included 70 studies describing LCA geographical range and refuse location (electronic supplementary material, table S1 and dataset), and seven studies describing LCA phylogeny (see below). From these sources, we recorded: (i) waste location (inside or outside the nest); (ii) geographical range (i.e. latitudinal distribution limits); (iii) main ecological habitats, categorized as (1) forest, (2) savannah, shrubland and grassland, and (3) desert and dry grasslands; and (iv) phylogenetic relation among the species. Finally, we obtained estimations of population size of different LCA species where the data were available.
(b). Phylogenetic signal and phylogenetic correction
To test whether the phylogeny had an effect on the behaviour to locate refuse inside or outside the nest, we estimated the value of phylogenetic signal D [49], using the function phylo.d of the ‘caper’ package [50] for R software [51]. We constructed a tree with the Atta and Acromyrmex species of known phylogenetic relations and refuse location. We used phylogenetic logistic regressions (PLR [52,53]) to test whether having internal refuse dumps was associated with the lower or the higher latitude of the species distribution, and with the latitudinal range, or with living on xeric habitats (see details in the electronic supplementary material, Materials and Methods). We also reconstructed the ancestral state of refuse dumps using parsimony (unordered model, equal probabilities of gains and losses) in Mesquite [54] (details in the electronic supplementary material, Materials and Methods).
(c). Heat tolerance laboratory experiment
To determine whether the typical temperature regimes of xeric habitats may reduce the pathogenicity level of ant waste located in external piles, we heated samples of external refuse dumps from eight mature nests of Atta colombica and monitored the presence of the LCA fungal parasite Escovopsis. Refuse samples in the heat treatment were subjected to 4 h at 50°C and 20 h at room temperature (approx. 28°C) over 2 days. This warming regime simulated the temperature regime typical of soil and refuse dump surfaces at the geographical limits of LCA distribution ([55]; see the electronic supplementary material, table S2). We estimated the prevalence of Escovopsis on refuse per nest as the number of subsamples with Escovopsis divided by all subsamples of each nest (n = 30). We compared this proportion between heat and control treatments with a t-test, using nests as replicates (i.e. n = 8). More details are provided in the electronic supplementary material.
3. Results
We obtained data of refuse location and geographical range of 32 LCA species (electronic supplementary material, table S1 and dataset): 12 Atta and 20 Acromyrmex, which represents approximately 85% of all described LCA species in [45]. Atta and Acromyrmex species differed in the location of waste: 80% of the Acromyrmex species have external refuse dumps (11 exclusively external, five both internal and external), whereas only 17% of Atta species have external refuse dumps. In summary, 13 LCA species only locate waste on external refuse dumps (13 Acromyrmex and two Atta spp.) and 15 species only locate waste inside refuse chambers (five Acromyrmex and 10 Atta spp.). Only four LCA species (all in Acromyrmex) showed both strategies; in two cases different subspecies were associated with different waste location (Acromyrmex lundii and Acromyrmex subterraneus; electronic supplementary material, table S1), and in two other cases different authors described contrasting refuse location for the same species without referring to the existence of subspecies (Acromyrmex coronatus and Acromyrmex crassispinus).
For the species with an available phylogeny, we found that the behaviour of storing refuse in underground chambers had a strong phylogenetic signal (D = 0.13), and therefore it had a high probability of resulting from Brownian phylogenetic structure (p = 0.44; i.e. where more distant species are more likely to diverge in waste location), and a low probability of resulting from randomness (p = 0.04). That is, few Acromyrmex have unequivocal internal refuse chambers, whereas most Atta have internal refuse chambers. Besides the phylogenetic effect, the location of waste was also associated with the habitat of the species: LCA species with external refuse dumps mainly occur in xeric habitats, whereas species with internal refuse chambers mainly occur in humid habitats (χ2 = 18.7, p < 0.001; figure 1), and this effect holds when corrected for phylogeny (PLR, Z = 1.99, p = 0.04). Locating the waste inside the nest was not clearly associated with the extent of the geographical range (t = 1.4, p = 0.10) or with the latitudinal limit (t = 0.15, p = 0.88), and the results are consistent after phylogenetic correction (PLR, Z = 1.4, p = 0.16 and Z = 1.2, p = 0.23, respectively).
Figure 1.
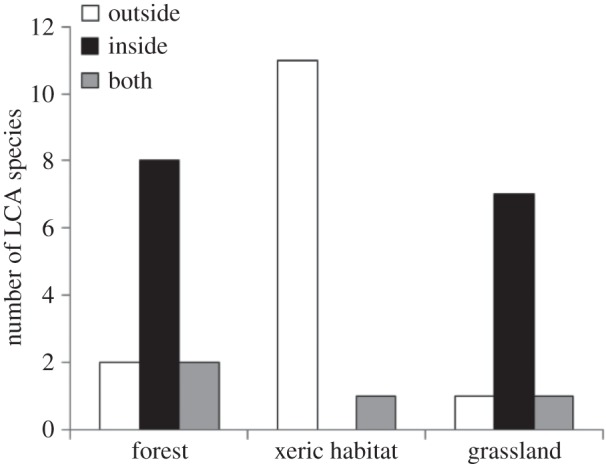
Number of species of leaf-cutting ants that locate waste inside the nest (black), in external refuse dumps (white) or both (grey), according to the habitat where they occur.
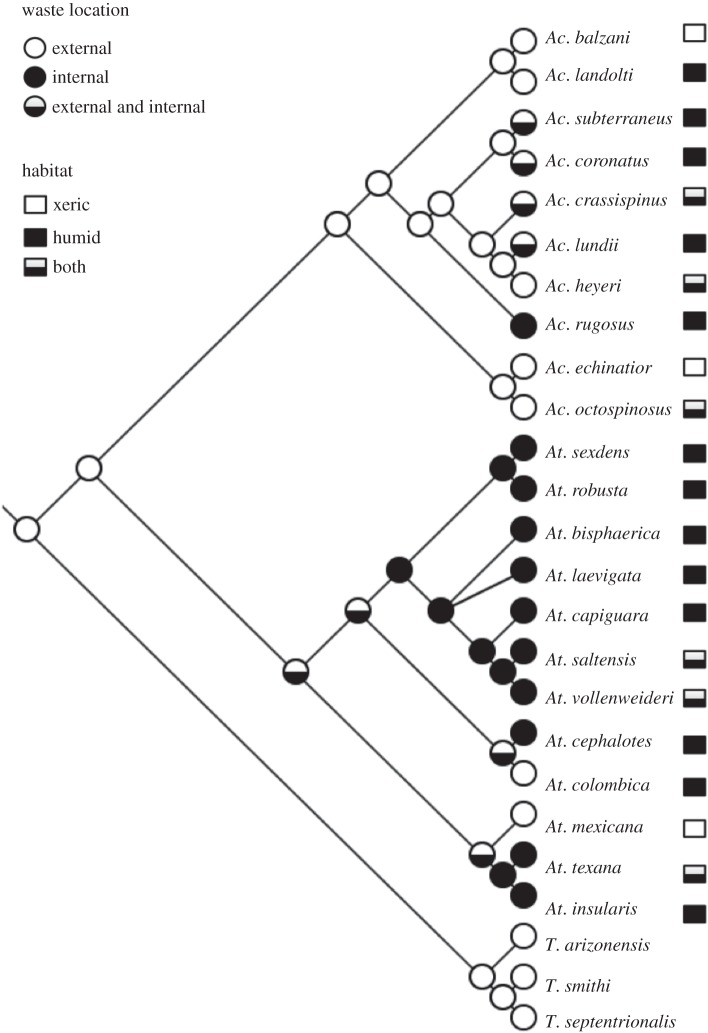
The ancestral state reconstruction suggests that external refuse dumps preceded the evolution of Atta and Acromyrmex (figure 2). If the proposed phylogeny is assumed to be true, in Acromyrmex the evolution of internal refuse chambers correlates with present occurrence of the species on humid environments, and Acromyrmex landolti and Acromyrmex octospinosus are the only two species living in forest or grasslands with external refuse dumps. In Atta, conclusions should be drawn more carefully, because the state of the refuse is ambiguous at several nodes. The analysis cannot solve whether the two Atta species with external refuse dumps (At. colombica and Atta mexicana) evolved it independently or inherited this trait from the ancestor. Nonetheless, it is clear that eight species living in forest with internal refuse chambers also share a single common ancestor (subgenus Epiatta and Neoatta, following the nomenclature of [56]). The inclusion of the other species of Acromyrmex in the cladogram (especially those with internal waste chambers) would allow us to make stronger conclusions, but the present evidence supports this hypothesis for the evolution of waste location.
Figure 2.
Cladogram of Atta and Acromyrmex species (based on [56,60,71,72]) showing the location of waste and the species habitat (xeric environments: deserts and dry forests; humid environments: forest and grasslands). Nodes on the cladogram depict the most parsimonious ancestral state. Three species of Trachymyrmex were used as an out-group. Some species of Acromyrmex could not be included in the cladogram because the phylogenetic relation with the other species is still unknown (see Material and methods).
(a). Laboratory experiment
Exposing At. colombica waste to high temperatures for short time periods over 2 days negatively affected the survival of the fungal parasite Escovopsis, as it was detected in 0–13% of the refuse subsamples from the heat treatment, whereas it was in 27–53% (min–max) of the control refuse subsamples (0.05 ± 0.01 versus 0.32 ± 0.02, respectively; mean ± s.e., t = 6.25, n = 8, p < 0.001). Moreover, all control nests showed refuse with some level of contamination with Escovopsis, whereas only 50% of the nests under the heat treatment showed refuse with this fungal parasite (electronic supplementary material, dataset).
4. Discussion
Waste management is one of the major challenges of social life. High population densities generate large quantities of pathogenic waste that increase the risk of spreading diseases [57]. Accordingly, social insects have several strategies for managing waste that include dumping refuse outside the nest or storing it inside specific underground chambers. Here, we analysed these strategies in LCA, an insect group with one of the most advanced grades of eusociality [45]. Our results suggest that the contrasting strategies of waste location showed by different LCA species can be interpreted as a prophylactic behaviour that depends on both the evolutionary history of the species and the environmental conditions of its current habitat.
We found that LCA phylogeny has a strong effect on waste location. Several lines of evidence suggest that dumping waste outside the nest preceded the behaviour of storing it inside nest chambers. First, lower attines (e.g. Trachymyrmex and Serycomyrmex) deposit waste externally ([45,58–60]; H. Fernández-Marín 2012, unpublished data; electronic supplementary material, table S1). Second, the ancestral state reconstruction suggests that external refuse dumps precede the evolution of Atta and Acromyrmex. Five of the eight Acromyrmex species that we could not include in the ancestral state reconstruction have also external refuse dumps. The ancestral state for the common ancestor of all Atta species was ambiguous in the parsimony analysis, but almost all species from the most derived genus Atta have underground refuse chambers. Also, Atta species with internal waste chambers have external refuse dumps as incipient colonies (A. G. Farji-Brener and H. Fernández-Marín 2004, personal observations). Finally, building underground chambers to isolate waste is considered a more derived form of nest hygiene than dumping refuse outside [34]. This suggests that the external location of waste is the ancestral condition and to store waste in internal nest chambers is a more derived behaviour.
Present environmental conditions also regulate the final destination of waste, despite an important influence of phylogeny. In xeric habitats, almost all LCA species show external refuse dumps, whereas grasslands and subtropical and tropical forests are dominated by LCA with internal refuse chambers. Controlling for phylogeny, the habitat explained the variation in the location of waste, supporting our hypothesis that the dry and hot conditions of xeric habitats allow ants to locate their waste externally with minimum infection risk. Several lines of evidence suggest that dry environments prevent the prevalence of waste pathogens that are harmful to the fungus garden of LCA [34,48]. First, ants prefer drier chambers for waste disposal when offered a choice between dry and humid chambers in laboratory conditions [61]. Second, LCA workers actively reduce the humidity content of waste. Experiments in Atta sexdens showed that increased debris humidity triggers strong recruitment of workers to dig tunnels through waste piles. These tunnels reduce humidity by increasing the rate of water loss, and digging ceases as the waste becomes drier [62]. Third, drier waste is less repellent for workers than humid waste. Refuse placed around seedlings in an arid steppe significantly delayed the leaf-cutting activity of Acromyrmex lobicornis, and the repellence gradually decreased after a few days [37]. Conversely, refuse samples are repellent to workers for longer time in more humid habitats [36]. Fourth, in dry environments LCA use their own waste to repair nest-mound damages [63], a behaviour never seen in LCA species occurring in more humid habitats. Finally, we demonstrated that one of the main specialized parasites of LCA fungus gardens is greatly reduced when the waste suffers the temperature regime of desert habitats; in laboratory conditions, Escovopsis germinated six times less in waste exposed to a few hours of hot temperature compared with unheated control samples. It is logical to speculate that in field conditions, this fungal pathogen is negatively affected by heating during the 90 day period of an entire summer. Accordingly, Escovopsis appears to be absent in refuse samples from LCA species that inhabit temperate deserts ([64], A. G. Farji-Brener 2014, unpublished data). These facts strongly suggest that xeric environments decrease or eliminate the hazardous properties of ant waste. Because the risk of pathogen infection is increased in humid environments, it is logical to hypothesize that the selective pressure for isolating waste in underground chambers is stronger in humid than in xeric habitats.
(a). The evolution of waste location: a hypothesis
We propose that the location of waste in LCA was shaped by natural selection to reduce sanitary risks, a threat that in turn depended on the quantity of waste (i.e. colony size) and the environmental conditions. Small amounts of waste probably do not represent a sanitary threat and can be placed outside the nest with little infection risks, as this waste can be rapidly degraded or dispersed by wind and rain. For small and short-lived ant colonies, it is therefore more adaptive to locate waste outside the nest, regardless of the habitat, as they avoid the costs of digging a chamber to store refuse. Accordingly, fungus gardening species (non-LCA) with colonies of fewer than 100 to 1000 individuals, produce small amounts of waste that are often externally deposited ([45,58–60], H. Fernández-Marín 2012, unpublished data). This relationship between population size and location of waste is also reflected in the colony ontogeny; as far as it is known, all incipient LCA colonies dump waste outside the nest, and only mature nests of some Atta and Acromyrmex species build refuse chambers (H. Fernández-Marín and A. G. Farji-Brener 2004–2015, unpublished data). The origin of colonies with large population size in Atta and Acromyrmex approximately 12 Ma represented a major sanitary challenge [65]. We proposed that the behaviour of isolating waste in underground chambers was key in the success of large colony-sized LCA species in humid habitats, where the environmental conditions favour the spread of disease from refuse dumps. This assumes that in these habitats the cost of infection from external refuse dumps exceeds the cost of digging a waste chamber, a plausible assumption.
(b). Striking exceptions: Atta colombica and Atta texana
These two species are remarkable exceptions for the hypothesis that refuse location in large-population societies mainly depend on whether habitat features increase sanitary risks. At. colombica lives in tropical rainforest, a habitat favourable for the growth of pathogens on waste and its potential spread to the colony, yet this species dumps the refuse in external piles. Also, Atta texana lives in xeric habitats that hinder pathogenicity of external waste, yet workers dig underground chambers to deposit waste. We hypothesize that the behaviour of dumping the refuse outside the nest implies costs for At. colombica. Waste of this species is colonized by Escovopsis more often than waste of other LCA species. Infection rates of Escovopsis in refuse samples from Atta and Acromyrmex in Central America vary between 43% and 51% [26], and 27% in Brazil [66]. By contrast, Escovopsis has been isolated from 70% [6] or 100% (this study) of refuse samples from At. colombica. Possibly as a consequence of fungus garden contamination, colonies of At. colombica relocate their nests approximately every 4 years, a migration rate much higher than that for other Atta species occurring in the same region [34,67–69]. The migration of a colony to a new nest is a vast, energy-demanding and dangerous task for LCA. It involves the excavation of large quantities of soil, the movement of millions of workers and brood and exposes the queen to predation. These facts may explain the restricted geographical range of At. colombica. Whereas this species occupies the narrowest geographical range of LCA (3° of latitude), its sister species Atta cephalotes—with very similar natural history but with internal refuse chambers—occupies one of the largest geographical ranges in the genus (33° of latitude). It is less clear why At. texana locates its waste in underground chambers despite living in an environment that hinders pathogen proliferation on waste. Possibly At. texana evolved to locate waste inside the nest in subtropical refuge areas in Mexico during the last Pleistocene glaciation, maintaining this behaviour when it later colonized drier habitats from Central and East Texas [65]. If this is the case, the cost of digging in more sterile environments should be lower than the cost of disease spread from locating waste externally in warm, wet environments. These two species deserve special attention to better understand the evolution and maintenance of the different strategies of waste management in LCA.
(c). Concluding remarks: be shallow or be deep?
It has been proposed that behavioural responses are one of the most important strategies to prevent disease spread in eusocial insects [70]. We combined the analysis of a large published dataset with empirical data to better understand why different LCA species locate their waste inside or outside the nest. Our results suggest that the environment of the current habitat determines waste location, although this behaviour is also influenced by phylogeny. In environmental conditions detrimental for pathogens (i.e. desert habitats), LCA often avoid digging costs and reduce sanitary risk by dumping their waste outside the nest. Conversely, humid habitats provide suitable conditions for the proliferation of pathogens living in waste, which selects for the isolation of waste in specific underground chambers, despite digging costs. In addition, waste location also depends on the amount of waste the colony generates; small quantities are dumped externally with little sanitary risks, but the large amounts of waste produced by larger colonies require different handling. In summary, we propose that the final destination of waste depends on the natural history of the species, colony size (i.e. waste production) and habitat features. Our results highlight a behavioural adaptation of group living organisms to the hygienic challenges of sociality, and illustrate how different sanitary behaviours can be better understood as the interaction between past traits and present environmental conditions.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgement
We thank D. Barrios, J. Queiroz, M. Nickele, R. Clark, T. Della Lucia and U. Mueller for providing information about natural history of some LCA species.
Data accessibility
Datasets supporting this article can be found in the electronic supplementary material.
Authors' contributions
A.G.F.-B. conceived the idea, designed and coordinated the study, participated in data analysis and drafted the manuscript. S.A.-V. carried out the phylogenetic analyses and collaborated in writing the manuscript. L.E. searched the information of LCA. H.F.-M. carried out the experimental laboratory work. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
A.G.F.-B. was partially funded by a fellowship of the Smithsonian Tropical Research Institute and a grant of FONCYT, Argentina (PICT1406). H.F.-M. was supported by SNI funds.
References
- 1.Côté IM, Poulinb R. 1995. Parasitism and group size in social animals: a meta-analysis. Behav. Ecol. 6, 159–165. ( 10.1093/beheco/6.2.159) [DOI] [Google Scholar]
- 2.Brown CR, Brown MB. 1986. Ectoparasitism as a cost of coloniality in cliff swallows (Hirundo pyrrhonota). Ecology 67, 1206–1218. ( 10.2307/1938676) [DOI] [Google Scholar]
- 3.Rifkin JL, Nunn CL, Garamszegi LZ. 2012. Do animals living in larger groups experience greater parasitism? A meta-analysis. Am. Nat. 180, 70–82. ( 10.1086/666081) [DOI] [PubMed] [Google Scholar]
- 4.Hoi H, Darolova A, König C, Kristofík J. 1998. The relation between colony size, breeding density and ectoparasite loads of adult European bee-eaters (Merops apiaster). Écoscience 5, 156–163. [Google Scholar]
- 5.Schmid-Hempel P. 1998. Parasites in social insects. Princeton, NJ: Princeton University Press. [Google Scholar]
- 6.Bot A, Currie C, Hart A, Boomsma JJ. 2001. Waste management in leaf-cutting ants. Ethol. Ecol. Evol. 13, 225–237. ( 10.1080/08927014.2001.9522772) [DOI] [Google Scholar]
- 7.Dunbar RIM. 1991. Functional significance of social grooming in primates. Folia Primatol. 57, 121–131. ( 10.1159/000156574) [DOI] [Google Scholar]
- 8.Brown MJF, Bot ANM, Hart AG. 2006. Mortality rates and division of labor in the leaf-cutting ant, Atta colombica. J. Insect Sci. 6, 1–8. ( 10.1673/2006_06_18.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballari S, Farji-Brener AG, Tadey M. 2007. Waste management in the leaf-cutting ant Acromyrmex lobicornis : division of labour, aggressive behaviour, and location of external refuse dumps. J. Insect Behav. 20, 87–98. ( 10.1007/s10905-006-9065-9) [DOI] [Google Scholar]
- 10.Cremer S, Armitage SAO, Schmid-Hempel P. 2007. Social immunity. Curr. Biol. 17, R693–R702. ( 10.1016/j.cub.2007.06.008) [DOI] [PubMed] [Google Scholar]
- 11.Diez L, Deneubourg J-L, Detrain C. 2012. Social prophylaxis through distant corpse removal in ants. Naturwissenschaften 99, 833–842. ( 10.1007/s00114-012-0965-6) [DOI] [PubMed] [Google Scholar]
- 12.Diez L, Lejeune P, Detrain C. 2014. Keep the nest clean: survival advantages of corpse removal in ants. Biol. Lett. 10, 20140306 ( 10.1098/rsbl.2014.0306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medina-Medina LA, Hart AG, Ratnieks FLW. 2014. Waste management in the stingless bee Melipona beecheii Bennett (Hymenoptera: Apidae). Sociobiology 61, 435–440. ( 10.13102/sociobiology.v61i4.435-440) [DOI] [Google Scholar]
- 14.Kerr AS, Kerr WE. 1999. Melipona garbage bees release their cargo according to a Gaussian distribution. Rev. Bras. Biol. 59, 119–123. ( 10.1590/S0034-71081999000100015) [DOI] [Google Scholar]
- 15.Sommeijer MJ. 1984. Distribution of labour among workers of Melipona favosa F.: age-polyethism and worker oviposition. Insect. Soc. 31, 171–184. ( 10.1007/BF02232713) [DOI] [Google Scholar]
- 16.Bruijn de L, Sommeijer M, Dijkstra E. 1989. Behaviour of workers on waste dumps in the nest of Melipona favosa (Apidae, Meliponini). Insect. Soc. 5, 31–37. [Google Scholar]
- 17.Czaczkes TJ, Heinze J, Ruther J. 2015. Nest etiquette: where ants go when nature calls. PLoS ONE 10, e0118376 ( 10.1371/journal.pone.0118376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart AG, Ratnieks FLW. 2001. Task partitioning, division of labour and nest compartmentalisation collectively isolate hazardous waste in the leafcutting ant Atta cephalotes. Behav. Ecol. Sociobiol. 49, 387–392. ( 10.1007/s002650000312) [DOI] [Google Scholar]
- 19.Waddington SJ, Hughes WOH. 2010. Waste management in the leaf-cutting ant Acromyrmex echinatior: the role of worker size, age and plasticity. Behav. Ecol. Sociobiol. 64, 1219–1228. ( 10.1007/s00265-010-0936-x) [DOI] [Google Scholar]
- 20.Hart AG, Ratnieks FLW. 2002. Waste management in the leaf-cutting ant Atta colombica. Behav. Ecol. 13, 224–231. ( 10.1093/beheco/13.2.224) [DOI] [Google Scholar]
- 21.Burnstein DE. 1990. Progressivism and urban crisis: ‘The New York City garbage workers’ strike of 1907’. J. Urban Hist. 16, 386–423. ( 10.1177/009614429001600404) [DOI] [Google Scholar]
- 22.Nicolopoulou-Stamati P, Hens L, Howard CV. 2000. Health impacts of waste management policies. Dordrecht, The Netherlands: Springer Netherlands. [Google Scholar]
- 23.Boomsma JJ, Schmid-Hempel P, Hughes WOH. 2005. Life histories and parasite pressure across the major groups of social insects. In Insect evolutionary ecology (eds MDE Fellowes, GJ Holloway, J Rolff), pp. 139–175. Wallingford, UK: CABI Publishing. [Google Scholar]
- 24.Herz H, Beyschlag W, Hölldobler B. 2007. Assessing herbivory rates of leaf-cutting ant (Atta colombica) colonies through short-term refuse deposition counts. Biotropica 39, 476–481. ( 10.1111/j.1744-7429.2007.00283.x) [DOI] [Google Scholar]
- 25.Augustin JO, Groenewald JZ, Nascimento RJ, Mizubuti ESG, Barreto RW, Elliot SL, Evans HC. 2013. Yet more ‘weeds’ in the garden: fungal novelties from nests of leaf-cutting ants. PLoS ONE 8, e82265 ( 10.1371/journal.pone.0082265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Currie CR, Mueller UG, Malloch D. 1999. The agricultural pathology of ant fungus gardens. Proc. Natl Acad. Sci. USA 96, 7998–8002. ( 10.1073/pnas.96.14.7998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Currie CR. 2001. Prevalence and impact of a virulent parasite on a tripartite mutualism. Oecologia 128, 99–106. ( 10.1007/s004420100630) [DOI] [PubMed] [Google Scholar]
- 28.Currie CR, Stuart AE. 2001. Weeding and grooming of pathogens in agriculture by ants. Proc. R. Soc. Lond. B 268, 1033–1039. ( 10.1098/rspb.2001.1605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernández-Marín H, Zimmerman JK, Wcislo WT. 2003. Nest-founding in Acromyrmex octospinosus (Hymenoptera, Formicidae, Attini): demography and putative prophylactic behaviors. Insect. Soc. 50, 304–308. ( 10.1007/s00040-003-0687-z) [DOI] [Google Scholar]
- 30.Fernández-Marín H, Zimmerman JK, Rehner SA, Wcislo WT. 2006. Active use of the metapleural glands by ants in controlling fungal infection. Proc. R. Soc. B 273, 1689–1695. ( 10.1098/rspb.2006.3492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-Marín H, Zimmerman JK, Nash DR, Boomsma JJ, Wcislo WT. 2009. Reduced biological control and enhanced chemical pest management in the evolution of fungus farming in ants. Proc. R. Soc. B 276, 2263–2269. ( 10.1098/rspb.2009.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hart A, Anderson C, Ratnieks F. 2002. Task partitioning in leafcutting ants. Acta Ethol. 5, 1–11. ( 10.1007/s10211-002-0062-5) [DOI] [Google Scholar]
- 33.Hughes WOH, Thomsen L, Eilenberg J, Boomsma JJ. 2004. Diversity of entomopathogenic fungi near leaf-cutting ant nests in a neotropical forest, with particular reference to Metarhizium anisopliae var. anisopliae. J. Invertebr. Pathol. 85, 46–53. ( 10.1016/j.jip.2003.12.005) [DOI] [PubMed] [Google Scholar]
- 34.Hart A. 2002. Does disease threat cause colony emigrations in the leaf-cutting ant Atta colombica (Guerin)? Entomol. Mon. Mag. 138, 41–42. [Google Scholar]
- 35.Lacerda FG, Lucia TMCD, DeSouza O, de Souza LM, de Souza DJ. 2013. Task performance of midden workers of Atta sexdens rubropilosa Forel (Hymenoptera: Formicidae). J. Insect Behav. 26, 873–880. ( 10.1007/s10905-013-9403-7) [DOI] [Google Scholar]
- 36.Zeh JA, Zen AD, Zeh DW. 1999. Dump material as an effective small-scale deterrent to herbivory by Atta cephalotes. Biotropica 31, 368–371. ( 10.1111/j.1744-7429.1999.tb00149.x) [DOI] [Google Scholar]
- 37.Ballari SA, Farji-Brener AG. 2006. Refuse dumps of leaf-cutting ants as a deterrent for ant herbivory: does refuse age matter? Entomol. Exp. Appl. 121, 215–219. ( 10.1111/j.1570-8703.2006.00475.x) [DOI] [Google Scholar]
- 38.Weber NA. 1972. Gardening ants, the Attines. Philadelphia, PA: American Philosophical Society. [Google Scholar]
- 39.Farji-Brener AG, Medina CA. 2000. The importance of where to dump the refuse: seed banks and fine roots in nests of the leaf-cutting ants Atta cephalotes and A. colombica. Biotropica 32, 120–126. ( 10.1111/j.1744-7429.2000.tb00454.x) [DOI] [Google Scholar]
- 40.Verza SS, Forti LC, Lopes JFS, Hughes WOH. 2007. Nest architecture of the leaf-cutting ant Acromyrmex rugosus rugosus. Insect. Soc. 54, 303–309. ( 10.1007/s00040-007-0943-8) [DOI] [Google Scholar]
- 41.Fröhle K, Roces F. 2012. The determination of nest depth in founding queens of leaf-cutting ants (Atta vollenweideri): idiothetic and temporal control. J. Exp. Biol. 215, 1642–1650. ( 10.1242/jeb.066217) [DOI] [PubMed] [Google Scholar]
- 42.Römer D, Roces F. 2014. Nest enlargement in leaf-cutting ants: relocated brood and fungus trigger the excavation of new chambers. PLoS ONE 9, e97872 ( 10.1371/journal.pone.0097872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pielström S, Roces F. 2013. Sequential soil transport and its influence on the spatial organisation of collective digging in leaf-cutting ants. PLoS ONE 8, e57040 ( 10.1371/journal.pone.0057040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moser JC. 2006. Complete excavation and mapping of a Texas leafcutting ant nest. Annu. Entomol. Soc. Am. 99, 891–897. ( 10.1603/0013-8746(2006)99%5B891:CEAMOA%5D2.0.CO;2) [DOI] [Google Scholar]
- 45.Hölldobler B, Wilson EO. 2010. The leafcutter ants: civilization by instinct. New York, NY: W. W. Norton. [Google Scholar]
- 46.Camargo RS, Forti LC, Fujihara RT, Roces F. 2010. Digging effort in leaf-cutting ant queens (Atta sexdens rubropilosa) and its effects on survival and colony growth during the claustral phase. Insect. Soc. 58, 17–22. ( 10.1007/s00040-010-0110-5) [DOI] [Google Scholar]
- 47.Ribeiro PL, Navas CA. 2007. The leaf-cutting ant Atta sexdens rubropilosa, FOREL, 1908 prefers drier chambers for garbage disposal. J. Insect Behav. 20, 19–24. ( 10.1007/s10905-006-9052-1) [DOI] [Google Scholar]
- 48.Waller DA, Moser JC. 1990. Invertebrate enemies and nest associates of the leaf-cutting ant Atta texana (Buckley) (Formicidae, Attini). In Applied myrmecology: a world perspective (eds RK Van der Meer, K Jaffe, A Cedeño), pp. 256–273. Boulder, CO: Westview Press. [Google Scholar]
- 49.Fritz SA, Purvis A. 2010. Selectivity in mammalian extinction risk and threat types: a new measure of phylogenetic signal strength in binary traits. Conserv. Biol. 24, 1042–1051. ( 10.1111/j.1523-1739.2010.01455.x) [DOI] [PubMed] [Google Scholar]
- 50.Orme D. 2013. caper: comparative analyses of phylogenetics and evolution in R.
- 51.R Core Team. 2014. R: a language and environment for statistical computing. Austria, Vienna: R Core Team.
- 52.Ives AR, Garland T. 2010. Phylogenetic logistic regression for binary dependent variables. Syst. Biol. 59, 9–26. ( 10.1093/sysbio/syp074) [DOI] [PubMed] [Google Scholar]
- 53.Ho LST, Ané C. 2014. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst. Biol. 63, 397–408. ( 10.1093/sysbio/syu005) [DOI] [PubMed] [Google Scholar]
- 54.Madison W, Madison DR.2008. Mesquite: a modular system for evolutionary analysis v. 3.04. See http://mesquiteproject.org .
- 55.Farji-Brener AG. 2000. Leaf-cutting ant nests in temperate environments: mounds, mound damages and nest mortality rate in Acromyrmex lobicornis. Stud. Neotropical Fauna Environ. 35, 131–138. ( 10.1076/0165-0521(200008)35:2;1-9;FT131) [DOI] [Google Scholar]
- 56.Bacci M, Solomon SE, Mueller UG, Martins VG, Carvalho AOR, Vieira LGE, Silva-Pinhati ACO. 2009. Phylogeny of leafcutter ants in the genus Atta fabricius (Formicidae: Attini) based on mitochondrial and nuclear DNA sequences. Mol. Phylogenet. Evol. 51, 427–437. ( 10.1016/j.ympev.2008.11.005) [DOI] [PubMed] [Google Scholar]
- 57.Hughes WOH, Eilenberg J, Boomsma JJ. 2002. Trade-offs in group living: transmission and disease resistance in leaf-cutting ants. Proc. R. Soc. Lond. B 269, 1811–1819. ( 10.1098/rspb.2002.2113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waller DA. 1989. Foraging behavior of Trachymyrmex turrifex Wheeler (Formicidae: Attini). Southwest. Nat. 34, 271–275. ( 10.2307/3671737) [DOI] [Google Scholar]
- 59.Seal JN, Tschinkel WR. 2006. Colony productivity of the fungus-gardening ant Trachymyrmex septentrionalis (Hymenoptera: Formicidae) in a Florida pine forest. Ann. Entomol. Soc. Am. 99, 673–682. ( 10.1603/0013-8746(2006)99%5B673:CPOTFA%5D2.0.CO;2) [DOI] [Google Scholar]
- 60.Rabeling C, Cover SP, Johnson RA, Mueller UG. 2007. A review of the North American species of the fungus-gardening ant genus Trachymyrmex (Hymenoptera: Formicidae). Zootaxa 1664, 1–53. [Google Scholar]
- 61.Ribeiro PL, Camacho A, Navas CA. 2012. Considerations for assessing maximum critical temperatures in small ectothermic animals: Insights from leaf-cutting ants. PLoS ONE 7, e32083 ( 10.1371/journal.pone.0032083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leite P, Cunha W. 1985. Construçao com depositos de detritos pela sauva (Atta sexdens rubropilosa Forel, 1908): fatores desencadeantes e provavel funcao. Cienc. Cult. 37, 910. [Google Scholar]
- 63.Farji-Brener AG, Tadey M. 2012. Trash to treasure: leaf-cutting ants repair nest-mound damage by recycling refuse dump materials. Behav. Ecol. 23, 1195–1202. ( 10.1093/beheco/ars101) [DOI] [Google Scholar]
- 64.Meirelles LA, Solomon SE, Bacci M, Wright AM, Mueller UG, Rodrigues A. 2015. Shared Escovopsis parasites between leaf-cutting and non-leaf-cutting ants in the higher attine fungus-growing ant symbiosis. R. Soc. open sci. 2, 150257 ( 10.1098/rsos.150257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mueller UG, Schultz TR, Currie CR, Adams RMM, Malloch D. 2001. The origin of the attine ant-fungus mutualism. Q. Rev. Biol. 76, 169–197. ( 10.1086/393867) [DOI] [PubMed] [Google Scholar]
- 66.Rodrigues A, Bacci M, Mueller UG, Ortiz A, Pagnocca FC. 2008. Microfungal ‘weeds’ in the leafcutter ant symbiosis. Microb. Ecol. 56, 604–614. ( 10.1007/s00248-008-9380-0) [DOI] [PubMed] [Google Scholar]
- 67.Porter SD, Bowers MA. 1980. Emigration of an Atta colony. Biotropica 12, 232–233. ( 10.2307/2387983) [DOI] [Google Scholar]
- 68.Wirth R, Herz H, Ryel R, Beyschlag W, Hölldobler B. 2003. Herbivory of leaf-cutting ants: a case study on Atta colombica in the tropical rainforest of Panama, 230 pp. New York, NY: Springer. [Google Scholar]
- 69.Nickele MA, Pie MR, Reis Filho W. 2012. Emigration of a colony of the leaf-cutting ant Acromyrmex heyeri Forel (Hymenoptera, Formicidae). Rev. Bras. Entomol. 56, 385–386. ( 10.1590/S0085-56262012005000045) [DOI] [Google Scholar]
- 70.Elliot SL, Hart AG. 2010. Density-dependent prophylactic immunity reconsidered in the light of host group living and social behavior. Ecology 91, 65–72. ( 10.1890/09-0424.1) [DOI] [PubMed] [Google Scholar]
- 71.Sumner S, Aanen DK, Delabie J, Boomsma JJ. 2004. The evolution of social parasitism in Acromyrmex leaf-cutting ants: a test of Emery's rule. Insect. Soc. 51, 37–42. ( 10.1007/s00040-003-0723-z) [DOI] [Google Scholar]
- 72.Schultz TR, Sosa-Calvo J, Brady SG, Lopes CT, Mueller UG, Bacci M, Vasconcelos HL. 2015. The most relictual fungus-farming ant species cultivates the most recently evolved and highly domesticated fungal symbiont species. Am. Nat. 185, 693–703. ( 10.1086/680501) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets supporting this article can be found in the electronic supplementary material.