Abstract
The pruritic skin disease scabies is caused by the burrowing of the itch mite Sarcoptes scabiei (De Geer). It is difficult to diagnose this disease because its symptoms often resemble those of other skin diseases. No reliable blood or molecular diagnostic test is available. The aim of this project was to begin to characterize the scabies proteome to identify scabies mite proteins, including those that may be useful in the development of a diagnostic test or vaccine. Various scabies mite extracts were separated by two-dimensional electrophoresis, and 844 Coomassie Blue-stained protein spots were excised, subjected to trypsin digestion, and analyzed by Matrix Assisted Laser Desorption/Ionization Time-Of-Flight/Time-Of-Flight (MALDI-TOF/TOF) mass spectrometry (MS). Tryptic fragment sequences determined by MS were searched against the recently completed S. scabiei annotated genome, leading to the identification of >150 proteins. Only 10 proteins hit to previously identified scabies proteins including actin, tropomyosin, and several ABC transporters. Thirteen proteins had homology to dust mite allergens (members of groups 8, 10, 13, 17, 20, 25, and 28). Most other sequences showed some homology to proteins in other mites and ticks including homologs of calmodulin, calreticulin, lipocalin, and glutathione-S-transferase. These data will now allow the identification of the proteins to which scabies patients produce antibodies, including those that may be good candidates for inclusion in a diagnostic test and vaccine.
Keywords: Sarcoptes scabiei, itch mite, scabies, genome, proteome
Scabies, caused by the mite Sarcoptes scabiei (De Geer) that burrows in the strateum corneum of the epidermis, has plagued humans and other mammals for millennia. It is a disease that is difficult to diagnose early in its course in humans and other mammals. Early scabies infestations in humans, dogs, and presumably other domestic and wild mammals, involve few mites on the body, but these mites can cause an intense itch (pruritus) and scratching. Scabies left untreated leads to hyperkeratosis and crusting of the skin. In humans this condition is known as Norwegian or crusted scabies. Chronic human scabies may be accompanied by secondary Group A streptococci and Staphylococcus areus bacterial infections that may lead to renal and cardiac disease and death (Hay et al. 2012).
The World Health Organization now lists human scabies as a neglected tropical disease (Anonymous, WHO). Scabies in humans is a skin disease that affects millions of people worldwide, but it is particularly prevalent in developing countries or resource-limited populations where prevalence may be 50% or more in children, some communities, and institutions (Andrews et al. 2009, Chosidow 2012, Hay et al. 2012, Engelman et al. 2013, Thomas et al. 2015). It is not uncommon in the general population and in institutions in developed countries. Close personal contact associated with crowding in communities, living quarters, and institutions, and among health care workers and scabietic patients facilitates the transfer of mites from infected to uninfected individuals. In addition, migration of people from communities with higher scabies prevalence to areas that have lower levels may change the dynamics of the worldwide occurrence or prevalence in specific settings. For example, the Center for Disease Control and Prevention (CDC) reports that many of the unaccompanied children from Central America reaching the US and Mexico border have scabies (Anonymous, CDC).
Although scabies causes an intense itch and scratching, it remains difficult to diagnose (Thomas et al. 2015). A positive diagnosis of scabies is based on recovery of mites, eggs, or fecal material from a skin scrape of the patient’s epidermis. Because early scabies cases typically involve only a few mites on the body, it is difficult to recover the mite material that is necessary for the definitive diagnosis and patients are often treated with an acaricide based on symptoms alone. There is evidence that scabies mites have developed resistance to the acaricide permethrin and this, along with poor treatment compliance, has resulted in treatment failures (Thomas et al. 2015). Other methods of diagnosis, which rely on molecular techniques, have yet to be developed, but their creation will rely on knowledge of proteomic and genomic data.
The parasite has a complex biology and interaction with its host. It must find a host, penetrate and burrow in the stratum corneum of the epidermis to obtain nutrients and shelter, and the sexes must find each other and mate so that females can produce eggs in order to establish a population in the host all the while avoiding elimination by the host’s protective innate and humoral immune responses. Although the mites provoke a defensive response by the host, they are the sources of molecules that down-modulate the initial innate and humoral responses, presumably allowing the mites to survive, reproduce, and establish a population in the host skin (Arlian et al. 1996c, 2003, 2004b, 2006, 2007; Elder et al. 2006, 2009; Lalli et al. 2004; Morgan and Arlian 2009, 2010; Bergstrom et al. 2009; Mullins et al. 2009; Mika et al. 2012; Morgan et al. 2013; Reynolds et al. 2014; Swe and Fischer 2014).
Understanding the biology of the parasite and its interaction with the host and diagnosing, treating, and protecting hosts from this mite-caused disease will require understanding the proteomics and genomics of this parasite. To this end, we have prepared a draft, annotated genome of this parasite (Rider et al. 2015). Here we report a proteomic analysis of extracts of whole mite bodies, eggs, and crust material shed from the infested host that includes mite bodies, exoskeletons, eggs, and fecal material as well as mite saliva and other secretions. Collectively, these materials contain all the various proteins that a mite produces including those involved in obtaining and digesting nutrients from the host, the molting process, modulating the host’s defensive responses, and those that eventually induce a protective response. Elucidation of these proteins and the genes responsible for their production may provide target proteins (secreted, soluble, and hidden—e.g., gut proteins) that could be useful in the development of a test to diagnose this disease or in a vaccine to prevent it.
Materials and Methods
S. scabiei Mite Extracts
Live scabies mites of all active life stages of S. scabiei var. canis were collected after they migrated from crusts of strateum corneum in response to light as previously described (Arlian et al. 1991). Mites were collected by aspiration onto a 38-µm stainless steel mesh (Small Parts Inc., Miami Lakes, FL) and washed to remove host material by drawing sequential 4-ml aliquots of PBST (Dulbecco’s phosphate-buffered saline with 0.05% Tween 20), endotoxin-free water, and 70% ethanol through the mesh. Mites were killed by freezing at −80°C and were stored frozen at this temperature.
An aqueous whole body homogenate was prepared by extracting mites overnight at 4°C in endotoxin-free water (1:20, W:V) followed by grinding 10 strokes on ice in a TenBroeck homogenizer. The homogenate was centrifuged for 10 min at 4°C in 5-ml microtubes in a Flexifuge (Argos Technologies, Inc., Elgin, IL) on the F setting (∼5,700 × g), and the supernatant was collected. The pellet was resuspended in another 20 volumes of water and the extraction–homogenization–centrifugation was repeated. The two supernatants were combined and were sterile filtered (0.22 µm) into a sterile vial for storage at 4°C. The pellet was resuspended as before and stored at −20°C. The protein concentration of the aqueous soluble protein extract was determined using the Bradford (Bradford 1976) method with bovine serum albumin as standard.
After use for mite collection as described above, the crusts from scabies-infested hosts were stored at −20°C. Microscopic examination of the crusts revealed that they were composed of heavily burrowed strateum corneum and contained mite bodies and exoskeletons, mite eggs, and fecal material. Presumably, mite saliva and other secretions were also present in this material. Crusts were cut into small pieces (∼ 1 cm3) and were ground in a mortar and pestle to the consistency of a fine powder. The crust powder was suspended in 10 volumes of endotoxin-free water and was processed once as described above. The supernatant was collected, filter sterilized, and stored as above and the protein content was determined. The crust pellet was discarded.
Microscopic examination of the scabies crust powder revealed the presence of intact scabies mite eggs. With the aid of a stereomicroscope, a fine needle probe was used to remove ∼4,200 eggs from the crust powder. Eggs were collected in a Protein LoBind Tube (Eppendorf North America, Inc., Westbury, NY) in endotoxin-free water and frozen. The volume was adjusted to ∼1 µl per 11 eggs, and the sample was placed in an ultrasonic bath (Model FS20; Fisher Scientific, Pittsburgh, PA) on ice for 15 min. The sample was then centrifuged for 10 min at 14,000 × g and the supernatant collected into a fresh LoBind Tube. The pellet was resuspended in an equal volume of water and ground 3 min on ice using a Kontes pellet pestle and motor (Fisher). Both mite egg samples (aqueous supernatant and resuspended pellet homogenate) were stored frozen at −20°C.
Two-Dimensional Gel Electrophoresis (2D-GE)
Prior to first-dimension isoelectric focusing (IEF), all samples were cleaned using the ReadyPrep 2-D Cleanup Kit (Bio-Rad Laboratories, Inc., Hercules, CA) essentially as described in Section 4.2 of the Instruction Manual. All samples were processed in Protein LoBind tubes. Aqueous scabies mite and crust extract samples ranged from 250–400 µg of protein, depending on the experiment. For the scabies whole body homogenate pellet, 100 µl of the resuspended material was treated. The entire mite egg aqueous supernatant and resuspended pellet homogenate samples were subjected to cleaning. The various protein pellets resulting from the cleaning processes were extracted into 200 µl of ReadyPrep Rehydration/Sample Buffer (Bio-Rad Cat. no. 163-2106) containing the chaotropic agent 8 M urea and 2% of the zwitterionic detergent CHAPS (3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate). Undissolved material was removed by centrifugation.
2D-GE was performed using the reagents and protocols supplied with the ReadyPrep 2-D Starter Kit (Bio-Rad). ReadyStrip 11 cm IPG strips (Bio-Rad) were loaded with 185 µl of sample using overnight passive rehydration. Most of the experiments reported here were performed using pH 3–10 linear gradient strips. IEF was carried out in a Bio-Rad PROTEAN IEF cell using the run parameters as prescribed in the Starter Kit instruction manual. At the conclusion of the run, strips were immediately frozen at −80°C to prevent diffusion.
Proteins separated by IEF and immobilized on the IPG strips were reduced and alkylated using Equilibration Buffers I and II from the kit. Strips were rinsed with 1× Tris/Glycine/SDS Buffer (Bio-Rad) and loaded onto Criterion TGX Any kD Precast Gels (Bio-Rad) overlaid with agarose. Precision Plus Protein Standards (Bio-Rad) were run in parallel to provide molecular weight markers. Electrophoresis was performed at a constant 200 V.
Following electrophoresis, gels were fixed for 15 min in 50% methanol, 7% acetic acid, washed with water, and stained with GelCode Blue Stain Reagent (Thermo Scientific, Rockford, IL). Gels were destained with water and imaged with a ChemiDoc MP Imaging System (Bio-Rad).
Spot Selection and Trypsin Digestion
Gel images were overlaid with a 1,000-cell grid with 25 rows of 40 cells each with cells numbered sequentially from left to right (e.g., row 1 = cells 1–40, row 2 = cells 41–80, etc., therefore cell 1,000 was at the extreme lower right corner of the gel, row 25, column 40). Blue-stained proteins were excised using a 1-mm spot picker, expelled into LoBind tubes, and frozen. Spots were assigned identification numbers based on the number of the cell in which they were located on the image with the 1,000-cell grid overlay (e.g., spot number 414 was in the 14th cell from the left on the 11th row of cells).
Samples were subjected to in-gel digestion with trypsin using the reagents and protocol supplied in the Thermo Scientific In-Gel Tryptic Digestion Kit. Briefly, gel pieces were destained with 25 mM ammonium bicarbonate in 50% acetonitrile (ACN). Proteins were reduced with 50 mM TCEP (Tris(2-carboxyethyl)phosphine), alkylated with 100 mM iodoacetamide, and gel pieces were washed then shrunk in ACN. Trypsin was activated by dilution to 10 ng/µl in 25 mM ammonium bicarbonate digestion buffer, and gel pieces were rehydrated with 10 µl. Following the addition of 25 µl of digestion buffer, samples were allowed to digest overnight at 30°C with shaking.
The next day, tryptic digests were collected into clean LoBind tubes, and 20 µl of 1% trifluoroacetic acid (TFA) was added to the gel to extract additional peptides and to neutralize the trypsin. This was collected and added to the original digest.
Matrix Assisted Laser Desorption/Ionization Time-Of-Flight/Time-Of-Flight (MALDI-TOF/TOF) Mass Spectrometry (MS)
Peptides resulting from the trypsin digestion were desalted, concentrated, and prepared for MS analysis using Millipore C18 ZipTips (obtained through Fisher). Peptides were eluted in 0.3% TFA in 95% ACN. Samples were mixed with an equal volume of α-cyano-4-hydroxycinnamic acid matrix (Bruker Daltronics, Inc., Billerica, MA), 10 mg/ml in 60% ACN, 10% acetone, 0.3% TFA, and applied to a brushed-steel target plate. Fresh Peptide Calibration Standards II for MS (Bruker) were loaded with every set of samples and were used to calibrate the MS before every run.
MALDI-TOF/TOF MS analyses were performed using a Bruker Autoflex III spectrometer. Bruker LIFT and CID were used to generate protein and peptide sequences for peaks of interest. Briefly, the software averaged 10,000 randomized laser shots over the target area (200 shots per position). Other parameters (e.g., laser power, mass range, and lag focusing) were adjusted as appropriate. MS/MS-based laser-induced dissociation (Bruker LIFT) was used for fragmentation. Parent ions were acquired using 2,000 shots followed by 10,000 shots for fragmentation.
All peptide mass fingerprinting, CID, and LIFT data were submitted using BioTools and analyzed by searching the MASCOT server at the Wright State University Proteomics Analysis Laboratory. Database searching was done using the MASCOT Search Engine (Matrix Science, London, United Kingdom). The data were searched against the National Center for Biotechnology Information Non-redundant (NCBInr) database with a mass accuracy of 0.7 Da for the parent ion (MS) and 0.3 for the fragment ions (MS/MS). The peptides were constrained to be tryptic with a maximum of two missed cleavages. Fixed modifications were carbamidomethylation of Cys, whereas oxidation of Met and phosphorylation of Ser were set as variable modifications. Taxonomy was restricted to “Other Metazoa” to increase the likelihood of identifying matches to arthropod proteins and to “Other Mammals” to identify proteins of host origin. Most data were also searched against the SwissProt database.
Database Searching Based in the S. scabiei Genome
To assist in identifying scabies mite proteins, we undertook a parallel project to sequence the genome of S. scabiei (Rider et al. 2015). Briefly, a draft genome of Sarcoptes scabiei var. canis was generated from paired end sequences using DeBruijn graph-based assembly methods. Assembled contigs covered 56.2 megabases with a contig N50 of 11.5 kb. These data were deposited into the NCBI database as Bioproject PRJNA268368. The assembly was then used to predict the S. scabiei proteome. Maker was used for structural annotations of 10,644 protein-coding genes. Roughly 70% of the predicted proteins could be assigned to an orthologous group, and were given natural language identifiers based on their homology to other proteins. The assembled genome and predicted proteome were then used to distinguish mite proteins from those of host origin and to identify the mite proteins whose predicted presence was confirmed by MS sequencing.
Tryptic fragment amino acid sequences determined by MS/MS were searched against the S. scabiei whole-genome shotgun contigs using the NCBI tBLASTn search algorithm (Altschul et al. 1997). This allowed us to find the contig on which the gene for a given protein sequence was located and to eliminate host proteins since only peptide sequences of mite origin should result in significant alignment scores. With a few exceptions, only fragment amino acid sequences that aligned to deduced protein sequences with E values ≤ 1.0E-03 were defined as significant matches. These deduced protein sequences were then searched by DELTA-BLASTp vs. the NBCInr database (with taxonomy restricted to Acari) to determine homology to other proteins and to identify conserved protein domains (Altschul et al. 1997, Schaffer et al. 2001, Boratyn et al. 2012). Gene Ontology (GO) annotations (Ashburner et al. 2000) for the cellular processes in which a predicted protein is expected to be involved were obtained by searching the UniProt database for homologous proteins.
Results
Over the course of this study, a total of 844 Coomassie Blue-stained protein spots were excised from various 2D electrophoresis gels, subjected to trypsin digestion, and analyzed by MALDI-TOF/TOF mass spectrometry. Spots were excised from gels used to separate aqueous extract of scabies mite homogenate (609 samples), scabies homogenate pellet solubilized in IEF sample buffer (165 spots), scabies mite egg homogenate (45 spots), and aqueous extract of crusts collected from scabies-infested hosts (25 spots). Of these, 420 (49.8%) yielded sequence data for one or more tryptic fragments. Initial efforts at protein identification utilized the MASCOT search engine to conduct an MS/MS ion search of sequence data submitted directly using BioTools. Data were searched against the NCBInr database which contained >4.6 million sequences. In order to increase the likelihood of identifying mite proteins, the taxonomy of the searches was restricted to “other metazoa” which limited the database to ∼206,000 proteins. Parallel searches were conducted using the “other mammals” restriction to identify proteins that may have originated from the rabbit host.
MASCOT search of the databases existing prior to October 2014 resulted in the unambiguous identification of very few scabies mite proteins. This was largely due to the paucity of data deposited into NCBI at the time of the original searches. In November of 2014, there were only 166 S. scabiei protein sequences available, and of these, multiple sequences for six different proteins, submitted for a variety of mite strains, accounted for 120 of the sequences (72%). Additionally, it was difficult to determine if protein sequences were of mite or host origin since often a single sequence yielded similar MASCOT scores for arthropod and rabbit proteins.
To assist in identifying scabies mite proteins, we undertook a parallel project to sequence the genome of S. scabiei (Rider et al. 2015). The assembled genome and predicted proteome were then used to help deduce the origins of peptides identified by mass spectrometry. This resulted in the identification of over 150 predicted S. scabiei proteins with amino acid sequences that aligned to tryptic fragment amino acid sequences determined by MS/MS (Supp. Table 1 [online only]). Only 10 of the proteins identified aligned to previously reported S. scabiei proteins, six of which were ATP-binding cassette (ABC) transporters. An additional six proteins had homology to proteins from the astigmatid house dust mite Dermatophagoides farinae Hughes. Seventy-two proteins had tryptic fragment and deduced amino acid sequences that best matched proteins predicted from the genome of Metaseiulus occidentalis Nesbitt, the western predatory mite, while 29 others aligned to predicted proteins from other Acari—most from the tick Ixodes scapularis Say. Twenty-seven other proteins had tryptic fragment amino acid sequences aligning to deduced sequences that contained conserved protein domains but that did not align with other Acari proteins. The identified proteins are involved in a broad spectrum of cellular processes (Fig. 1). The largest portion (35%) of the proteins are involved in metabolic processes while those involved in phosphorylation, oxidation–reduction, and translation are also well-represented.
Fig. 1.
Representation of the cellular processes in which the proteins identified by mass spectrometry are involved.
Proteins in the Aqueous Extract of Scabies Mite Homogenate
Most of the proteins identified in this study (109/152 = 72%) were isolated from spots excised from 2D gels used to separate aqueous extracts of whole scabies mite body homogenates (Supp. Table 1 [online only]). This set included the proteins most prominent on the Coomassie blue-stained 2-D gels—tropomyosin, heat-shock proteins, and arginine kinase (Fig. 2; Table 1). Since these are the most abundant proteins on the gels, several of the proteins in this set had two or more fragments that aligned to the deduced sequences, resulting in a higher percentage of sequence coverage. In our genome sequence (Rider et al. 2015), the gene for tropomyosin was split between two different contigs (contig 10523 containing sequence for residues 1–80 and contig 10317 coding for residues 81–284) that align to the previous NCBI entry AFH08744. Twelve different tryptic fragments aligned to this sequence yielding 47% sequence coverage.
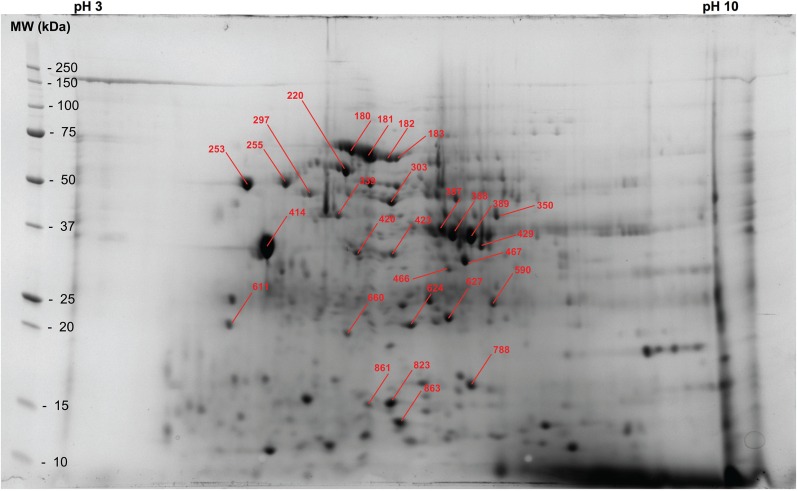
Fig. 2.
S. scabiei homogenate supernatant proteins separated by two-dimensional gel electrophoresis. Spot numbers correspond to those in Table 1. A catalog of all proteins identified in the supernatant sample is provided in Supp. Table 1 (online only).
Table 1.
Identification of proteins excised from a two-dimensional gel used to separate proteins in the scabies homogenate supernatant
| Fig. 2 spot no. | pI | MW | S. scabiei protein identification | NCBI access. no. |
|---|---|---|---|---|
| 180 | 5.9 | 62.4 | Sar s 28 (heat-shock protein 70-like protein 3) | KPM07069 |
| 180–181 | 6.0 | 59.4 | Sar s 28 (heat-shock protein 70-like protein 4), partial | KPM07870 |
| 180–181 | 6.0 | 59.4 | Sar s 28 (heat-shock protein 70-like protein 6) | KPM10172 |
| 180–182 | 6.3 | 57.9 | Sar s 28 (heat-shock protein 70-like protein 2) | KPM03927 |
| 180–182 | 6.3 | 57.9 | Sar s 28 (heat-shock protein 70-like protein 8) | KPM11560 |
| 183 | 6.3 | 57.9 | Glutamate receptor, ionotropic kainate-like protein 2 | KPM05237 |
| 220 | 5.8 | 51.3 | 60 kDa heat-shock protein, mitochondrial-like protein | KPM06690 |
| 253 | 4.7 | 45.8 | Calreticulin-like protein | KPM04170 |
| 255 | 5.1 | 46.2 | ABC transporter sub-family C-like protein 7 | KPM08018 |
| 297 | 5.4 | 42.6 | ATP synthase subunit beta, mitochondrial-like protein, partial | KPM07577 |
| 303 | 6.3 | 39.9 | Enolase-like protein | KPM02829 |
| 339 | 5.7 | 36.3 | Actin-like protein 6, partial | KPM11937 |
| 350 | 7.4 | 36.2 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 2, mitochondrial-like protein | KPM10735 |
| 387–389 | 6.8–7.2 | 32.9–31.8 | Sar s 20 allergen (arginine kinase-like protein 2) | KPM07362 |
| 414 | 4.9 | 29.8 | Sar s 10 allergen (tropomyosin-like protein 1) residues 1–80 | KPM05881 |
| 414 | 4.9 | 29.8 | Sar s 10 allergen (tropomyosin-like protein 2) residues 81–284 | KPM09025 |
| 420 | 5.9 | 28.6 | Actin-like protein 5 | KPM08943 |
| 423, 429 | 6.3, 7.3 | 28.3, 29.8 | Sar s 20 allergen (arginine kinase-like protein 1) | KPM11902 |
| 466 | 6.9 | 26.1 | Cytochrome c oxidase subunit 2 (mitochondrion) | KPL93356 |
| 467 | 7.1 | 27.4 | Elongation factor 2-like protein | KPM11996 |
| 590 | 7.4 | 21.8 | Enoyl-CoA hydratase, mitochondrial-like protein | KPM02479 |
| 611 | 4.5 | 19.8 | ABC transporter sub-family G-like protein 20 | KPM09942 |
| 624 | 6.5 | 19.7 | Glutathione S-transferase delta class 3 | KPM09608 |
| 627 | 6.9 | 20.4 | Sar s 25 allergen (triosephosphate isomerase-like protein) | KPM10468 |
| 660 | 5.8 | 19.0 | Leucine-rich repeat-containing protein 17 | KPM04329 |
| 788 | 7.1 | 15.4 | Nucleoside diphosphate kinase B-like protein | KPM06968 |
| 823 | 6.3 | 14.5 | Alanyl-tRNA synthetase-like protein 2 | KPM10943 |
| 861 | 6.0 | 14.4 | Elongation factor 1-alpha 1-like protein | KPM11350 |
| 863 | 6.4 | 13.5 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 7, mitochondrial-like protein | KPM08178 |
Spot numbers correspond to those in Fig. 2. A catalog of all proteins identified in the supernatant sample is provided in Supp. Table 1 (online only).
Additionally, genomic analysis revealed that there were two different homologs of arginine kinase and eight different heat-shock protein 70 (HSP70) homologs, with the predicted sequences of the various homologs differing. Both proteins were present on 2D gels as a group of similarly migrating spots and tryptic fragment sequences aligning to both arginine kinase homologs and to six of the eight HSP70 homologs were identified, confirming that most of the various homologs of these two proteins were translated into protein.
The scabies homogenate supernatant also contained six proteins that were labeled “hypothetical proteins” since they showed no significant homology to other previously identified or predicted proteins nor did they contain any identifiable conserved domains. This suggests that these proteins are unique to S. scabiei.
Proteins in the Scabies Homogenate Pellet
Treatment of the aqueous scabies homogenate pellet with the IEF sample buffer containing a chaotropic agent and detergent, allowed for the extraction of a very different set of proteins (Fig. 3; Table 2; Supp. Table 1 [online only]). Most of these proteins (21/24) were not present in the original aqueous extract. This set of proteins contained an assortment of membrane proteins, especially those involved in transport.
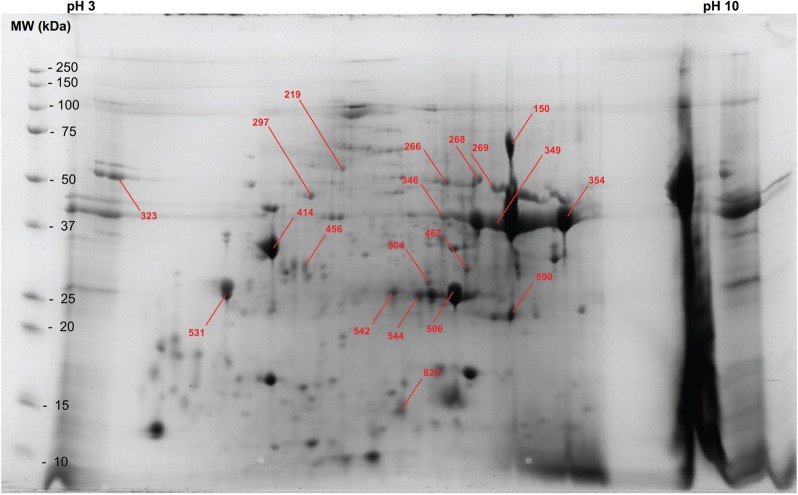
Fig. 3.
S. scabiei homogenate pellet proteins separated by two-dimensional gel electrophoresis. Spot numbers correspond to those in Table 2. A catalog of all proteins identified in the pellet sample is provided in Supp. Table 1 (online only).
Table 2.
Identification of proteins excised from a two-dimensional gel used to separate proteins in the scabies homogenate pellet
| Fig. 3 spot no. | pI | MW | S. scabiei protein identification | NCBI access. no. |
|---|---|---|---|---|
| 150 | 7.6 | 67.5 | ABC transporter ABCF1 | KPM04972 |
| 219 | 5.8 | 53.6 | Succinate dehydrogenase [ubiquinone] flavoprotein subunit, mitochondrial-like protein | KPM08759 |
| 266 | 6.9 | 47.4 | DFP2-like protein 20 | KPM06360 |
| 268 | 7.2 | 48.5 | Sodium-independent sulfate anion transporter-like protein | KPM06808 |
| 269 | 7.4 | 45.1 | Partitioning defective 6-like protein gamma-like protein | KPM05730 |
| 297 | 5.4 | 42.2 | ATP synthase subunit beta, mitochondrial-like protein, partial | KPM07577 |
| 323 | 3.3 | 36.6 | Histidine decarboxylase | KPM10342 |
| 346 | 6.9 | 36.5 | Cytochrome c oxidase subunit 1 | KPL93357 |
| 349 | 7.4 | 34.3 | Malate dehydrogenase, mitochondrial-like protein, partial | KPM12024 |
| 354 | 8.2 | 34.7 | Polysaccharide Biosynthesis Domain-Containing Protein | KPM05230 |
| 414 | 5.0 | 29.2 | Sar s 10 allergen (tropomyosin-like protein 1) residues 1–80 | KPM05881 |
| 414 | 5.0 | 29.2 | Sar s 10 allergen (tropomyosin-like protein 2) residues 81–284 | KPM09025 |
| 456 | 5.4 | 26.6 | Acetylcholinesterase-like protein 6 | KPM11082 |
| 467 | 7.1 | 26.0 | Glutathione S-transferase delta class 3 | KPM09608 |
| 504 | 6.7 | 24.1 | Bestrophin-2 (Vitelliform macular dystrophy 2-like protein 1)-like protein | KPL98654 |
| 506 | 7.0 | 23.1 | Matrix metalloproteinase-like protein 2 | KPM05304 |
| 531 | 4.5 | 22.7 | Cysteine-tRNA ligase, cytoplasmic-like protein | KPM06744 |
| 542 | 6.3 | 22.9 | SPFH domain-containing protein 2 | KPM06464 |
| 544 | 6.6 | 22.6 | ABC transporter sub-family F-like protein 2 | KPM10024 |
| 590 | 7.6 | 20.4 | Fatty acid synthase-like protein 3 | KPM05489 |
| 823 | 6.4 | 13.8 | ABC transporter ABCC1 | KPM07300 |
Spot numbers correspond to those in Fig. 3. A catalog of all proteins identified in the pellet sample is provided in Supp. Table 1 (online only).
Proteins in Scabies Mite Eggs
An additional 14 proteins were identified only in the homogenate prepared from scabies mite eggs. The supernatant contained too little material to identify any proteins; all 14 of the identified proteins were present in the resuspended egg homogenate pellet (Supp. Table 1 [online only]). Most notable among this set of proteins were several proteins involved in transcription and translation (activities to be expected in a developing organism) including a histone-binding protein, an RNA polymerase, three helicases, and a 28S ribosomal protein involved in protein synthesis. Two actin moieties were also present among these proteins as were cullin (a protein involved in embryogenesis), lipocalin (a fatty acid-binding protein), and chitin deacetylase (an enzyme that may play a role in cuticle and peritrophic membrane formation).
Scabies Proteins in Crust Extract
Although the majority of crust proteins were of host origin, we were able to identify 11 mite proteins by excluding all protein sequences that did not yield significant alignments to the scabies contigs (Supp. Table 1 [online only]). Eight of these proteins were unique to the crust extract including a glycerol kinase, HSP90, cytoplasmic malate dehydrogenase, and a fragment of myosin heavy chain.
Other Proteins of Interest
Nine potential ABC transporters, representing subfamilies A, B, C, E, F, and H have previously been reported (Mounsey et al. 2006). Analysis of our scabies genome (Rider et al. 2015) predicted the presence of 77 proteins belonging to this family, including potential members of all subfamilies from A–G. Our proteomic analysis identified seven different family members (Supp. Table 1 [online only]). A and C subfamily members were present in soluble mite extract as was a G subfamily protein not previously predicted. Representatives of the C and F subfamilies were identified in crust extract along with another protein containing a distinctive transmembrane domain motif.
Scabies mites are closely related phylogenetically to other astigmatid mites including the Dermatophagoides spp. house dust mites that are known to be the sources of at least 33 allergen molecules (Thomas 2015). A search of our scabies genome revealed the presence of genes coding for homologs of 27 of these 33 proteins (Rider et al. 2015). Proteomic analysis confirmed the presence of at least seven of these proteins (members of groups 8, 10, 13, 17, 20, 25, and 28) in the mite preparations separated by 2D-GE (Table 3).
Table 3.
S. scabiei homologs of known dust mite allergens identified by mass spectrometry
| Allergen name | Protein identification | NCBI access. no. |
|---|---|---|
| Sar s 8 | Glutathione S-transferase delta class 3 | KPM09608 |
| Sar s 10 | Tropomyosin, residues 1–80 | KPM05881 |
| Tropomyosin, residues 81–284 | KPM09025 | |
| Sar s 13 | Lipocalin-like protein 1 | KPM06293 |
| Sar s 17 | Calcium-binding EGF-like domain-containing protein | KPM06260 |
| Sar s 20 | Arginine kinase-like protein 1 | KPM11902 |
| Sar s 20 | Arginine kinase-like protein 2 | KPM07362 |
| Sar s 25 | Triosephosphate isomerase-like protein | KPM10468 |
| Sar s 28 | Heat-shock protein 70-like protein 2 | KPM03927 |
| Sar s 28 | Heat-shock protein 70-like protein 3 | KPM07069 |
| Sar s 28 | Heat-shock protein 70-like protein 4, partial | KPM07870 |
| Sar s 28 | Heat-shock protein 70-like protein 5 | KPM08853 |
| Sar s 28 | Heat-shock protein 70-like protein 6 | KPM10172 |
| Sar s 28 | Heat-shock protein 70-like protein 8 | KPM11560 |
We also identified a number of proteins with homology to putative tick proteins that are suspected to be involved in host–parasite molecular interactions (Radulovic et al. 2014). Among these were homologs of calmodulin, calreticulin, lipocalin, and glutathione-S-transferase (Supp. Table 1 [online only]).
Discussion
Research aimed at developing a serological test for scabies has long been hampered by the lack of information about the proteins produced by this mite. Previous studies have used aqueous extracts of scabies mite whole body homogenates to demonstrate that these mites are the sources of many antigenic and allergenic proteins (Arlian et al. 1988a, 1991, 1994, 1996b, 2004a; Morgan and Arlian 1994; Morgan et al. 1997; Arlian and Morgan 2000; Schumann et al. 2001) as well as to demonstrate that these mites produce molecules that are able to modulate the immune response of an infested host (Arlian et al. 1996c, 2003, 2004a, 2006, 2007; Elder et al. 2006, 2009; Lalli et al. 2004; Bergstrom et al. 2009; Morgan and Arlian 2009, 2010; Mullins et al. 2009; Mika et al. 2012; Morgan et al. 2013; Reynolds et al. 2014; Swe and Fischer 2014). The molecules responsible for the observed antigenic and immunomodulatory activities of scabies mite extracts have not been identified. In this study, we have identified scabies mite proteins in several mite components. This is a first step toward identifying those molecules responsible for the observed antigenic and immunomodulatory activities.
The extracts used in this study were made from whole mite bodies of all active life stages (composed of both aqueous soluble [homogenate supernatant] and insoluble [homogenate pellet] components), mite eggs, and host skin crust. Proteins in the whole body homogenate supernatant presumably contained soluble proteins from the salivary glands, gut lumen (enzymes and fecal material), and hemolymph. Some of the proteins in this material induce innate and humoral host responses and can modulate these systems. Proteins in the mite body homogenate pellet (remaining after extraction of the soluble proteins) include structural and membrane-bound proteins that were solubilized by detergent and chaotropic agent treatment of the mite body homogenate pellet. The proteins in this sample that are antigenic would be considered to be “hidden antigens” if the terminology for similar antigens in ticks is followed. These proteins may include bound digestive enzymes of the gut mucosal cell membranes, those that are involved in transport of nutrients and water, and proteins and chitin associated with the midgut peritrophic membrane and the fore- and hind-gut linings.
Hosts can build antibodies to these various secreted and “hidden” antigens during feeding (salivary secretions), defecation (fecal pellets containing enzymes, peritrophic membrane, and sloughed mucosal cells), and the molting cycle (enzymes in molting fluid). In addition, these and other structural proteins are released when mites die and their bodies and exoskeletons breakdown in the epidermis of the host. Evidence for the later is the surge in host serum antibody titer after treatment that kills the mites that then remain in the epidermis for weeks (Arlian and Morgan 2000). A blood test could be designed to detect host antibody built to proteins from any of these sources using the appropriate antibody isotype (Arlian et al. 2015). Likewise, a vaccine that contained immunogenic proteins from any of these sources could induce host antibody that would bind with any of these proteins and disrupt the processes in which the target protein was involved (digestion and uptake of nutrients and water, metamorphosis and molting, excretion, or reproduction) when ingested. Previous studies show that scabies mites ingest serum that seeps into the burrow and host antibody, some of which remains intact and presumably active (Arlian et al. 1988b, Rapp et al. 2006).
A significant impediment to the development of a diagnostic test for scabies is the extensive immunological cross-reactivity between scabies and house dust mite proteins (Arlian et al. 1988a, 1991, 2015). Our previous analysis of the S. scabiei genome found that it possesses homologs of 27 of the previously characterized 33 house dust mite allergen groups (Rider et al. 2015) and is consistent with the results of immunologic studies demonstrating the cross-reactivity. This study confirms the synthesis of 13 allergen homologs belonging to seven different allergen groups (Table 3). This high level of cross-reactivity complicates efforts to identify scabies-specific antigenic proteins to which hosts (humans) produce antibody that could be used to develop a blood test to scabies or used in a vaccine (Arlian et al. 2015). Our current proteomic analysis clearly shows that the pool of candidate proteins is large. The proteome we have characterized presents a huge catalog of the more abundant proteins that can now be mined to identify immunomodulatory proteins as well as candidates for use in a blood test or vaccine.
A variety of immunoblotting studies have reported the molecular weights of various antigens recognized by the antibodies in the serum from scabies-infested hosts (Morgan and Arlian 1994; Arlian et al. 1996a, 2004a; Morgan et al. 1997; Arlian and Morgan 2000; Schumann et al. 2001; Tarigan and Huntley 2005; Yadav et al. 2006; Zalunardo et al. 2006; Casais et al. 2007; Rambozzi et al. 2007; Rodriguez-Cadenas et al. 2010; Hejduk et al. 2011; Jakubek et al. 2012). Yet little data identifying the proteins involved have been reported. In one study, all of the proteins identified in scabies mites and skin scrapings collected from a scabies-infested pig were determined to be host serum proteins (Zalunardo et al. 2006). And an earlier report characterizing S. scabiei antigens using 2D-GE (Hejduk et al. 2011) showed gels with far fewer spots than those seen here and only three protein spots were identified by MS, all having homology to the dust mite protein DFP1.
Over 1,000 expressed sequence tag sequences for S. scabiei are available through NCBI, but few of these have useful protein identifications associated with them (Ljunggren et al. 2001, Fischer et al. 2003). The present study offers the first comprehensive proteomic analysis of S. scabiei and confirms by MS sequencing the existence of >150 scabies proteins from among the 10,644 proteins that we predicted from genome sequencing (Rider et al. 2015). Together the data generated by these studies and deposited into public database repositories provide powerful tools that will allow the identification of the proteins to which scabies patients produce antibodies, including those that may be good candidates for inclusion in a diagnostic test and vaccine.
Supplementary Data
Supplementary data are available at Journal of Medical Entomology online.
Acknowledgments
We thank DiAnn Vyszenski-Moher for technical assistance in culturing the mites used in this study and in collecting the scabies mite eggs. We are also grateful for the use of the equipment in the Wright State University Proteomics Analysis Laboratory. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI017252. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References Cited
- Altschul S. F., Madden T. L., Schaffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews R. M., Kearns T., Connors C., Parker C., Carville K., Currie B. J., Carapetis J. R. 2009. A regional initiative to reduce skin infections amongst Aboriginal children living in remote communities of the Northern Territory, Australia. PLoS Negl. Trop. Dis. 3: e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlian L. G., Morgan M. S. 2000. Serum antibody to Sarcoptes scabiei and house dust mite prior to and during infestation with S . scabiei. Vet. Parasitol. 90: 315–326. [DOI] [PubMed] [Google Scholar]
- Arlian L. G., Runyan R. A., Vyszenski-Moher D. L. 1988a. Water balance and nutrient procurement of Sarcoptes scabiei var. canis (Acari: Sarcoptidae). J. Med. Entomol. 25: 64–68. [DOI] [PubMed] [Google Scholar]
- Arlian L. G., Vyszenski-Moher D. L., Gilmore A. M. 1988b. Cross-antigenicity between Sarcoptes scabiei and the house dust mite, Dermatophagoides farinae (Acari: Sarcoptidae and Pyroglyphidae). J. Med. Entomol. 25: 240–247. [DOI] [PubMed] [Google Scholar]
- Arlian L. G., Vyszenski-Moher D. L., Ahmed S. G., Estes S. A. 1991. Cross-antigenicity between the scabies mite, Sarcoptes scabiei, and the house dust mite, Dermatophagoides pteronyssinus. J. Invest. Dermatol. 96: 349–354. [DOI] [PubMed] [Google Scholar]
- Arlian L. G., Morgan M. S., Vyszenski-Moher D. L., Stemmer B. L. 1994. Sarcoptes scabiei: the circulating antibody response and induced immunity to scabies. Exp. Parasitol. 78: 37–50. [DOI] [PubMed] [Google Scholar]
- Arlian L. G., Morgan M. S., Arends J. J. 1996a. Immunologic cross-reactivity among various strains of Sarcoptes scabiei. J. Parasitol. 82: 66–72. [PubMed] [Google Scholar]
- Arlian L. G., Morgan M. S., Rapp C. M., Vyszenski-Moher D. L. 1996b. The development of protective immunity in canine scabies. Vet. Parasitol. 62: 133–142. [DOI] [PubMed] [Google Scholar]
- Arlian L. G., Vyszenski-Moher D. L., Rapp C. M., Hull B. E. 1996c. Production of IL-1 alpha and IL-1 beta by human skin equivalents parasitized by Sarcoptes scabiei. J. Parasitol. 82: 719–723. [PubMed] [Google Scholar]
- Arlian L. G., Morgan M. S., Neal J. S. 2003. Modulation of cytokine expression in human keratinocytes and fibroblasts by extracts of scabies mites. Am. J. Trop. Med. Hyg. 69: 652–656. [PubMed] [Google Scholar]
- Arlian L. G., Morgan M. S., Estes S. A., Walton S. F., Kemp D. J., Currie B. J. 2004a. Circulating IgE in patients with ordinary and crusted scabies. J. Med. Entomol. 41: 74–77. [DOI] [PubMed] [Google Scholar]
- Arlian L. G., Morgan M. S., Neal J. S. 2004b. Extracts of scabies mites (Sarcoptidae: Sarcoptes scabiei) modulate cytokine expression by human peripheral blood mononuclear cells and dendritic cells. J. Med. Entomol. 41: 69–73. [DOI] [PubMed] [Google Scholar]
- Arlian L. G., Morgan M. S., Paul C. C. 2006. Evidence that scabies mites (Acari: Sarcoptidae) influence production of interleukin-10 and the function of T-regulatory cells (Tr1) in humans. J. Med. Entomol. 43: 283–287. [DOI] [PubMed] [Google Scholar]
- Arlian L. G., Fall N., Morgan M. S. 2007. In vivo evidence that Sarcoptes scabiei (Acari: Sarcoptidae) is the source of molecules that modulate splenic gene expression. J. Med. Entomol. 44: 1054–1063. [DOI] [PubMed] [Google Scholar]
- Arlian L. G., Feldmeier H., Morgan M. S. 2015. The potential for a blood test for scabies. PLoS Negl. Trop. Dis. 9: e0004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Ball C. A., Blake J. A., Botstein D., Butler H., Cherry J. M., Davis A. P., Dolinski K., Dwight S. S., Eppig J. T., et al. 2000. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom F. C., Reynolds S., Johnstone M., Pike R. N., Buckle A. M., Kemp D. J., Fischer K., Blom A. M. 2009. Scabies mite inactivated serine protease paralogs inhibit the human complement system. J. Immunol. 182: 7809–7817. [DOI] [PubMed] [Google Scholar]
- Boratyn G. M., Schaffer A. A., Agarwala R., Altschul S. F., Lipman D. J., Madden T. L. 2012. Domain enhanced lookup time accelerated BLAST. Biol. Direct 7: 12–6150-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Casais R., Prieto M., Balseiro A., Solano P., Parra F., Martin Alonso J. M. 2007. Identification and heterologous expression of a Sarcoptes scabiei cDNA encoding a structural antigen with immunodiagnostic potential. Vet. Res. 38: 435–450. [DOI] [PubMed] [Google Scholar]
- (CDC) Center for Disease Control and Prevention. 2015. Factsheet on Unaccompanied Children: Health Information for Public Health Partners. http://www.cdc.gov/usmexicohealth/pdf/unaccompanied-children-factsheet.pdf. Last accessed 4 January 2016. [Google Scholar]
- Chosidow O. 2012. Scabies and pediculosis: Neglected diseases to highlight. Clin. Microbiol. Infect. 18: 311–312. [DOI] [PubMed] [Google Scholar]
- Elder B. L., Arlian L. G., Morgan M. S. 2006. Sarcoptes scabiei (Acari: Sarcoptidae) mite extract modulates expression of cytokines and adhesion molecules by human dermal microvascular endothelial cells. J. Med. Entomol. 43: 910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder B. L., Arlian L. G., Morgan M. S. 2009. Modulation of human dermal microvascular endothelial cells by Sarcoptes scabiei in combination with proinflammatory cytokines, histamine, and lipid-derived biologic mediators. Cytokine 47: 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D., Kiang K., Chosidow O., McCarthy J., Fuller C., Lammie P., Hay R., Steer A., and Members Of The International Alliance For The Control Of Scabies. 2013. Toward the global control of human scabies: Introducing the International Alliance for the Control of Scabies. PLoS Negl. Trop. Dis. 7: e2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K., Holt D. C., Harumal P., Currie B. J., Walton S. F., Kemp D. J. 2003. Generation and characterization of cDNA clones from Sarcoptes scabiei var. hominis for an expressed sequence tag library: Identification of homologues of house dust mite allergens. Am. J. Trop. Med. Hyg. 68: 61–64. [PubMed] [Google Scholar]
- Hay R. J., Steer A. C., Engelman D., Walton S. 2012. Scabies in the developing world–its prevalence, complications, and management. Clin. Microbiol. Infect. 18: 313–323. [DOI] [PubMed] [Google Scholar]
- Hejduk G., Hofstatter K., Lowenstein M., Peschke R., Miller I., Joachim A. 2011. Characterisation of Sarcoptes scabiei antigens. Parasitol. Res. 108: 309–315. [DOI] [PubMed] [Google Scholar]
- Jakubek E. B., Mattsson R., Morner T., Mattsson J. G., Gavier-Widen D. 2012. Potential application of serological tests on fluids from carcasses: detection of antibodies against Toxoplasma gondii and Sarcoptes scabiei in red foxes (Vulpes vulpes). Acta Vet. Scand. 54: 13–0147-54-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli P. N., Morgan M. S., Arlian L. G. 2004. Skewed Th1/Th2 immune response to Sarcoptes scabiei. J. Parasitol. 90: 711–714. [DOI] [PubMed] [Google Scholar]
- Ljunggren E. L., Nilsson D., Naslund K., Mattsson J. G. 2001. Expressed sequence tag analysis of the parasitic mite Sarcoptes scabiei. Parasitology 127: 139–145. [DOI] [PubMed] [Google Scholar]
- Mika A., Reynolds S. L., Mohlin F. C., Willis C., Swe P. M., Pickering D. A., Halilovic V., Wijeyewickrema L. C., Pike R. N., Blom A. M., et al. 2012. Novel scabies mite serpins inhibit the three pathways of the human complement system. PLoS ONE 7: e40489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. S., Arlian L. G. 1994. Serum antibody profiles of Sarcoptes scabiei infested or immunized rabbits. Folia. Parasitol. (Praha) 41: 223–227. [PubMed] [Google Scholar]
- Morgan M. S., Arlian L. G. 2009. Response of human skin equivalents to Sarcoptes scabiei mites and extract. Am. J. Trop. Med. Hyg. 81: S218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. S., Arlian L. G. 2010. Response of human skin equivalents to Sarcoptes scabiei. J. Med. Entomol. 47: 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. S., Arlian L. G., Estes S. A. 1997. Skin test and radioallergosorbent test characteristics of scabietic patients. Am. J. Trop. Med. Hyg. 57: 190–196. [DOI] [PubMed] [Google Scholar]
- Morgan M. S., Arlian L. G., Markey M. P. 2013. Sarcoptes scabiei mites modulate gene expression in human skin equivalents. PLoS ONE 8: e71143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounsey K. E., Holt D. C., McCarthy J., Walton S. F. 2006. Identification of ABC transporters in Sarcoptes scabiei. Parasitology 132: 883–892. [DOI] [PubMed] [Google Scholar]
- Mullins J. S., Arlian L. G., Morgan M. S. 2009. Extracts of Sarcoptes scabiei De Geer downmodulate secretion of IL-8 by skin keratinocytes and fibroblasts and of GM-CSF by fibroblasts in the presence of proinflammatory cytokines. J. Med. Entomol. 46: 845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic Z. M., Kim T. K., Porter L. M., Sze S. H., Lewis L., Mulenga A. 2014. A 24-48 h fed Amblyomma americanum tick saliva immuno-proteome. BMC Genomics 15: 518–2164-15-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambozzi L., Menzano A., Molinar Min A. R., Rossi L. 2007. Immunoblot analysis of IgG antibody response to Sarcoptes scabiei in swine. Vet. Immunol. Immunopathol. 115: 179–183. [DOI] [PubMed] [Google Scholar]
- Rapp C. M., Morgan M. S., Arlian L. G. 2006. Presence of host immunoglobulin in the gut of Sarcoptes scabiei (Acari: Sarcoptidae). J. Med. Entomol. 43: 539–542. [DOI] [PubMed] [Google Scholar]
- Reynolds S. L., Pike R. N., Mika A., Blom A. M., Hofmann A., Wijeyewickrema L. C., Kemp D., Fischer K. 2014. Scabies mite inactive serine proteases are potent inhibitors of the human complement lectin pathway. PLoS Negl. Trop. Dis. 8: e2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider S. D., Morgan M. S., Arlian L. G. 2015. Draft genome of the scabies mite. Parasites Vectors 8: 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Cadenas F., Carbajal-Gonzalez M. T., Fregeneda-Grandes J. M., Aller-Gancedo J. M., Rojo-Vazquez F. A. 2010. Clinical evaluation and antibody responses in sheep after primary and secondary experimental challenges with the mange mite Sarcoptes scabiei var . ovis. Vet. Immunol. Immunopathol. 133: 109–116. [DOI] [PubMed] [Google Scholar]
- Schaffer A. A., Aravind L., Madden T. L., Shavirin S., Spouge J. L., Wolf Y. I., Koonin E. V., Altschul S. F. 2001. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 29: 2994–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann R. J., Morgan M. S., Glass R., Arlian L. G. 2001. Characterization of house dust mite and scabies mite allergens by use of canine serum antibodies. Am. J. Vet. Res. 62: 1344–1348. [DOI] [PubMed] [Google Scholar]
- Swe P. M., Fischer K. 2014. A scabies mite serpin interferes with complement-mediated neutrophil functions and promotes staphylococcal growth. PLoS Negl. Trop. Dis. 8: e2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarigan S., Huntley J. F. 2005. Failure to protect goats following vaccination with soluble proteins of Sarcoptes scabiei: evidence for a role for IgE antibody in protection. Vet. Parasitol. 133: 101–109. [DOI] [PubMed] [Google Scholar]
- Thomas W. R. 2015. Hierarchy and molecular properties of house dust mite allergens. Allergology Intl. 64: 304–311. [DOI] [PubMed] [Google Scholar]
- Thomas J., Peterson G. M., Walton S. F., Carson C. F., Naunton M., Baby K. E. 2015. Scabies: An ancient global disease with a need for new therapies. BMC Infect. Dis. 15: 250–015-0983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (WHO) World Health Organization. List of neglected tropical diseases (NTDs) of the Western Pacific Region. http://www.wpro.who.int/topics/neglected_tropical_diseases/en/ Last accessed 4 January 2016. [Google Scholar]
- Yadav A., Elder B. L., Morgan M. S., Vyszenski-Moher D. L., Arlian L. G. 2006. Prevalence of serum IgE to storage mites in a southwestern Ohio population. Ann. Allergy Asthma Immunol. 96: 356–362. [DOI] [PubMed] [Google Scholar]
- Zalunardo M., Cargill C. F., Sandeman R. M. 2006. Identification of auto-antigens in skin scrapings from scabies-infected pigs. Int. J. Parasitol. 36: 1133–1141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.