Abstract
Dengue virus (DENV) is transmitted by Aedes spp mosquitoes during a bloodmeal uptake. The bloodmeal consists of host cells, immune factors, and possibly blood-borne pathogens, such as arboviruses. Human cells and immune-related factors, like the complement system, can remain active in the bloodmeal and may be able to interact with pathogens in the mosquito. Previous studies have shown that active complement proteins impact Plasmodium parasite viability in the Anopheles midgut. Thus, we investigated the effects of the human complement on DENV infection in the midgut of Aedes aegypti. Our findings indicate that mosquitoes receiving DENV mixed with normal non-inactivated human serum showed significantly lower viremia than those fed with heat-inactivated serum. This implies that human complement may act to limit DENV infection in the mosquito midgut. In addition, we found that human complement C5a protein was able to directly communicate with mosquito cells, affecting the cell antiviral response against DENV. Our results also show that human C5a protein is able to interact with several membrane-bound mosquito proteins. Together these results suggest an important role of human complement protein in DENV transmission.
Keywords: human complement, dengue, mosquito, Aedes
Dengue virus (DENV) is currently the most important arboviral disease of the tropics, causing high morbidity and mortality in pediatric populations (Gubler 2011, Hagenlocher et al. 2013). There are four distinct DENV serotypes, DENV 1–4, all transmitted by Aedes mosquitoes (Diptera: Culicidae), which become infected while taking a bloodmeal from a DENV-infected mammalian host (Watts et al. 1987, Yamashiro et al. 2004). The virus must first infect the mosquito midgut, which is the initial barrier of infection. If successful, the virus spreads via hemolymph throughout the body and finally invades the salivary glands before transmission to a new host. During mosquito feeding, the virus is transferred to the midgut along with many host factors, including antiviral immune molecules (Tempelis 1975, Washino and Tempelis 1983, Stowers and Carter 2001). Previous studies have demonstrated that human complement and other immune factors can remain highly active in the Anopheles midgut several hours after blood feeding and are able to act on and clear certain pathogens (Margos et al. 2001, Simon et al. 2013). Interestingly, previous research has even shown that human serum components are able to induce gene expression in mosquito tissues, modulating transmission of vector-borne pathogens (Kang et al. 2008, Surachetpong et al. 2011, Drexler et al. 2014).
Animals have evolved multiple immune mechanisms to fight pathogenic virus infections (Blandin and Levashina 2007, Waterhouse et al. 2007). The innate immune response plays a crucial role in the early stages of infection and it is initiated by the recognition of pathogen membrane components. In the human host, the complement system is very effective in recognizing such components (Dunkelberger and Song 2010). The human complement system is composed of more than 30 soluble proteins and membrane-bound receptors (Cummings et al. 2007, Stoermer and Morrison 2011). Evidence suggests that vertebrates and invertebrates may share a common ancestor for complement-like proteins as an immune defense mechanism (Levashina et al. 2001, Dimopoulos 2003, Osta et al. 2004, Buresova et al. 2011). Human complement and complement-like proteins are part of the thioester-containing protein (TEP) family (Nonaka 2000). TEPs are mainly divided into three major families: alpha-2-macroglobulins, C3/C4/C5 complement factors, and insect TEPs (iTEPs; Bou Aoun et al. 2011, Sekiguchi et al. 2012). The mosquito complement-like system is mainly composed of iTEPs, which are similar to the mammalian complement C3/C4/C5 proteins (Sekiguchi et al. 2012). A subtype of iTEPs, known as macroglobulin complement-related factors (MCRs), are able to interact with scavenger receptors, leading to the expression of antimicrobial peptide (AMP) genes, including defensin A (DEFA) and cecropins (Sekiguchi et al. 2012, Xiao et al. 2014). Recently, the Ae. aegypti MCR (AaMCR) has been found to participate in the anti-DENV immune response (Xiao et al. 2014).
Control of viral infection via complement activation in the human host includes direct lysis of virions and infected cells (Stoermer and Morrison 2011). This occurs due to the interaction of activated complement proteins with targeted membranes or with specific complement receptors on effector cells (Fujita 2002). The human complement C5 protein plays an important role in inflammatory and cell death processes (Guo and Ward 2005), and previous studies have shown its involvement in DENV infection and pathogenesis (Malasit 1987, John et al. 2015). C5 is cleaved into C5a and C5b by the C5 converses (Pangburn and Rawal 2002). C5a not only works as a chemoattractant of neutrophils and macrophages but also enhances phagocytosis (Guo and Ward 2005, Niederbichler et al. 2006). C5b forms the first part of the membrane attack complex on targeted pathogens and infected cells, leading to cell lysis (Xiong et al. 2003). Although the role of active complement proteins in human DENV infection and pathogenesis is well-known, little information is available on the role of these factors in DENV transmission to its main vector, Ae. aegypti. Here, we investigate the effects of ingested human complement proteins on DENV infection of mosquitoes and their role on gene expression of mosquito immune factors. In particular, we examined the role played by C5a on DENV infection and expression of both mosquito complement-like proteins and antimicrobial peptides.
Materials and Methods
Cell Lines
The Ae. aegypti cell line, Aag2, was used for in vitro studies. The cells were grown at 30°C and 5% CO2 in DMEM supplemented with 10% heat-inactivated fetal bovine serum (Gemini, CA), 1% penicillin-streptomycin, and 1% tryptose phosphate broth (Sigma, MO). DENV stock was grown in C6/36 Aedes albopictus cell line using the same media. The dengue strain used was DENV-2 New Guinea C. Cells were infected at a multiplicity of infection (MOI) of 1.0, and the virus was allowed to propagate for 6-8 days. Supernatant was removed, spun down, and virus stock was stored at −80°C until use.
Human Samples
Human red blood cells and serum were obtained from expired blood purchased at the Blood Center (New Orleans, LA). Red blood cells (RBCs) were washed three times in 1 × DPBS and kept at 4°C until use. Heat inactivation of serum was accomplished by heating the serum for 1 h at 56°C. Inactivation of all complement pathways was verified using the Complement System Screen kit (Euro Diagnostica, Sweden) according to the manufacturer’s instructions.
Mosquito Infection With DENV
Aedes aegypti mosquitoes (Rockefeller strain) were reared at 25–28°C, 70–80% RH, and a photoperiod of 16:8 (L:D) h, and maintained on a 10% sucrose solution once they turned into adults. Female mosquitoes from 5- to 10-d-old were infected by blood feeding, using 500 μL of DENV-infected C6/36 cell supernatant added to 250 μL of heat-inactivated or non-inactivated human serum, and 250 μL of washed human RBCs (Das et al. 2007). Mosquitoes were fed for 20 min at room temperature using 1 mL of virus–blood mixture in a Hemotek feeder maintained at 37°C. Midguts were dissected 3 h post blood feeding and individually transferred to 1.5-mL tubes. Midguts were stored in 1 × PBS for protein assays and homogenized in RLT buffer (Qiagen, CA) with BME. RNA from mosquito midguts was purified using an RNeasy kit (Qiagen, CA), according to the manufacturer’s instructions, and kept at −20°C until testing for gene expression assays. The quantitative real-time polymerase chain reaction (qRT-PCR) analysis was done using the QuantiFast kit, according to the manufacturer’s instructions (Qiagen, CA). Oligos for the qRT-PCR reactions were: DENV-2 envelope (E) protein: F: 5′-CATTCCAAGTGAGAATCTCTTTGTCA-3′ R: 5′-CAGATCTCTGATGAATAACCAACG-3′; Ae. aegypti Actin: F: 5′-GAACACCCAGTCCTGCTGACA-3′, R: 5′-TGCGTCATCTTCTCACGGTTAG-3′. AaMCR and DEFA were amplified as described by Xiao et al. (2014). Each sample was tested in duplicates.
In Vitro Test of Complement Effects on Mosquito Cells
Aag2 cells were incubated for 48 h at 37°C in 96-well plates with 1 μg/mL of either recombinant human C5a protein (R&D Systems, MN) with anti-human C5a antibodies (R&D Systems, MN) or heat-treated recombinant C5a (C5a protein incubated for 1 h at 56°C). Both treatments were in the presence or absence of DENV-2 (MOI of 1.0) and were performed in sextuplets. Cells were washed three times and RNA was isolated using the FastLane Cell cDNA Kit (Qiagen, CA). Purified RNA was kept at −20°C until testing.
ELISAs
Two types of enzyme-linked immunosorbent assay (ELISA)-based assays were performed to evaluate the binding of C5a protein to mosquito proteins. Aag2 cells were cultured in 96-well plates until confluent, and an in-cell ELISA was performed using fixed Aag2 cells as antigen. A standard ELISA was performed using Aag2 cell lysate prepared with 0.1% Triton X 100. Cell lysate was stored at −80°C until use. Lysate (1 and 10 μg/mL) was diluted in coating buffer (KPL, MD), placed on 96-well plates, and incubated overnight at 4°C. Confluent cells were washed twice with 1 × PBS and fixed with 4% paraformaldehyde, then treated with 0.1% Triton X 100. Both, intact cells and cell lysate were incubated with dilutions of recombinant human C5a in DMEM (at 10, 1, 0.1, and 0.01 μg/mL) in quadruplets at 4°C overnight. Control wells were incubated with blocking buffer in the absence of C5a. After three washes with 1 × PBS, cells and cell lysate were incubated with goat anti-human C5a antibodies (1:1000) for 1 h at room temperature. Plates were washed three times and incubated for 1 h at room temperature with a dilution of 1:2000 mouse anti-goat horseradish peroxidase (HRP; Santa Cruz Biotechnology, TX). Plates were then incubated with the substrate 3,3,5,5-tetramethylbenzidine (TMB), 1-Step™ Turbo TMB-ELISA Substrate Solution (Life Technologies Corporation, NY), for 3 min at room temperature. The reaction was stopped with 2 N sulfuric acid and plates were read at 450 nm in a Synergy HT plate reader (Biotek Instruments Inc., VT).
Immunofluorescence
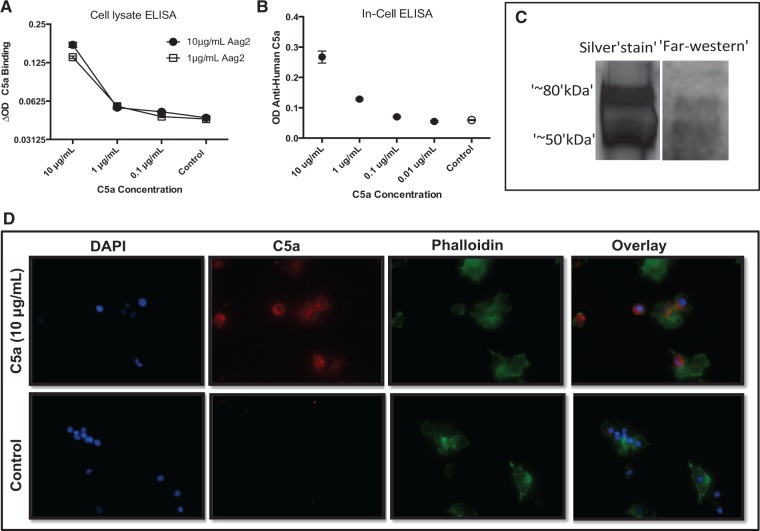
Aag2 cells were grown in 96-well plates until confluent, washed three times with 1 × PBS, and fixed with 4% paraformaldehyde. Cells were then permeabilized with 0.1% Triton X 100 and incubated with human C5a in DMEM (10 μg/mL) at 4°C overnight. Plates were again washed three times with 1 × PBS and incubated with goat anti-human C5a antibodies (1:1000) for 1 h at room temperature. After three additional washes, plates were incubated with 1:5000 Alexa Fluor® 594 chicken anti-goat antibodies (immunofluorescence antibody assay [IFA]; Invitrogen™, MA). An additional incubation was made to the immunofluorescence plates with a 1:5000 dilution of Alexa Fluor® 488 Phalloidin for 1 h at room temperature to selectively label cellular F-actin. Imagines in Fig. 4D were captured using the EVOS® Cell Imaging System (Thermo-Fisher, NY).
Fig. 4.
Human C5a binds proteins in Aag2 mosquito cells. (A) An ELISA was performed to examine the binding of human C5a (10, 1, and 0.1 μg/mL) with two concentrations of Aag2 cell lysate: 1 and 10 μg/mL. (B) An in-cell ELISA (ELISA performed on fixed and plated Aag2 cells) was performed to evaluate human C5a binding with Aag2 cells. Error bars represent values tested in quadruplets. (C) The Far-Western blot identifies two bands with bound human C5a. Aag2 lysate was run on SDS-PAGE gel and proteins were transferred to PVDF membrane. The membrane was incubated with C5a, washed, and then incubated with goat anti-human C5a antibodies. (D) Immunofluorescence of Aag2 cells incubated with or without C5a (10 μg/mL). After 48 h, cells were fixed and stained with DAPI, anti-human C5a (Alexa Fluor® 594 dye), and Alexa Fluor® 488 Phalloidin.
Western Blotting
Aag2 lysate (10 μg/mL) was mixed 1:1 with 2 × Laemmli buffer and run on a 4-12% SDS-PAGE gel (Bio-Rad, CA) along with molecular weight marker (Precision Plus Protein™ 10–250 kDa Kaleidoscope™, Bio-Rad, CA). Proteins were transferred using Trans-Blot® Turbo™ Transfer System to PVDF membranes. Membranes were blocked with 5% milk and incubated with 10 μg/mL C5a solution overnight at 4°C. Membranes were washed and incubated with anti-human C5a antibodies diluted 1:1000 in blocking buffer for 1 h at 37°C. HRP-conjugated secondary antibodies diluted 1:1000 in blocking buffer were incubated with the membrane for 1 h at 37°C. Color development was obtained with True Blue TMB (KPL, MD; Invitrogen, MA).
Immunoprecipitation and Mass Spectrometry
Aag2 cell lysate at a concentration of 10 μg/mL and Ae. aegypti midgut tissues were incubated with 1 μg/mL of human C5a protein; bound proteins were then harvested with anti-human C5a coated beads (Life Technologies Corporation, NY), and sent for tandem mass spectrometry (MS/MS) analysis to the ICBR Proteomics Core Facility (University of Florida). All MS/MS samples were analyzed using Mascot (Matrix Science, London, UK; version 2.4.1). The Scaffold software version 4.4.3 (Proteome Software Inc., Portland, OR) was used to validate MS/MS-based protein identifications. Accepted protein identifications were required to be of >80.0% identification probability and contain at least one identified peptide. Protein probabilities were assigned by the ProteinProphet algorithm (Nesvizhskii et al. 2003).
Data Analysis
Statistical differences between two independent groups (i.e., mosquitoes fed with inactivated vs non-inactivated serum) or between gene expression from infected and uninfected groups were tested using Mann–Whitney U test. Analysis of variance (ANOVA) was performed to test the difference of the various C5a concentrations binding among Aag2 cell proteins concentrations and control groups. All differences were considered significant with a P < 0.05. All statistical tests were computed using Prism version 6.0 (Graph Pad Software Inc., CA).
Results
Inactivating Human Complement Increases DENV Infection in Mosquitoes
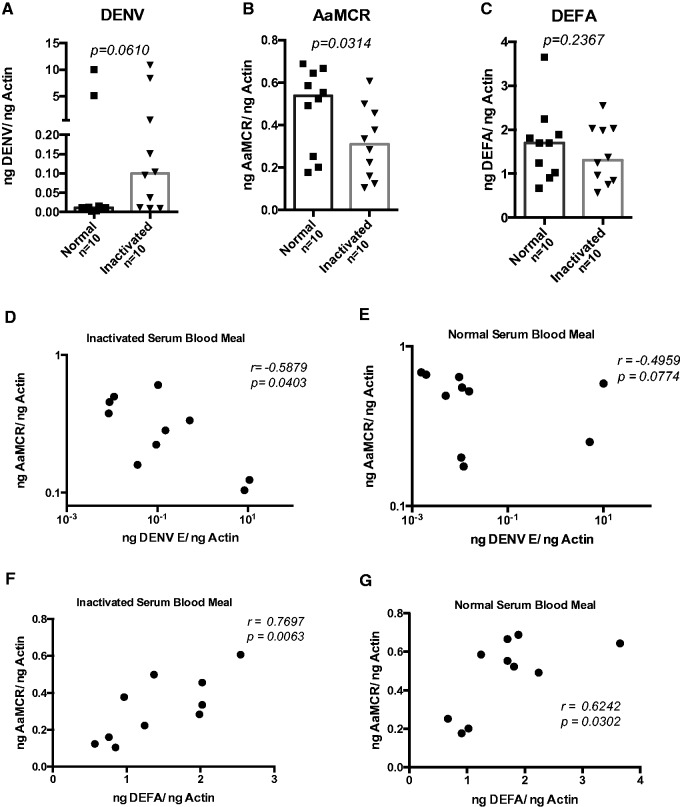
To investigate the effect of active human complement factors in Ae. aegypti mosquito midgut infections with DENV, we fed female mosquitoes with either heat-inactivated or normal non-inactivated human serum. Using qRT-PCR, we measured DENV infection of midguts at 3 h post feeding. Our results showed that mosquitos fed with DENV plus heat-inactivated serum had 89% higher relative viral loads than those fed non-inactivated serum (0.0990 [inactivated serum-fed median] − 0.0108 [normal serum-fed median] = 0.0883 ng DENV/ng Actin; Fig. 1A), but this difference was not statistically significant (P = 0.0610).
Fig. 1.
Serum heat inactivation affects DENV infection in Ae. aegypti female mosquitoes. Mosquitoes were fed human blood containing either normal (non-inactivated) or inactivated serum plus DENV-2. DENV levels were measured by qRT-PCR at 3 h post infection in duplicates and normalized to mosquito Actin gene. (A) DENV-2 E protein expression, (B) AaMCR, and (C) DEFA gene expression levels. Correlation analysis between DENV-2 E protein gene and AaMCR expressions in mosquito midgut fed with either inactivated (D) or normal (E) human serum. (F) and (G) represent the correlation analysis between AaMCR and DEFA gene expression in both study groups. P-values are indicated on each panel.
We next chose to evaluate if active human complement could alter the expression of immune-related mosquito genes in the dissected midguts. Previous studies have demonstrated that the insect complement like-protein gene AaMCR and the AMP gene DEFA participate in the immune response against DENV infection. Thus, we tested the expression of these two genes in the infected mosquito midguts 3 h post infection by qRT-PCR. Our results showed a higher expression of both AaMCR and DEFA in mosquitoes fed with normal non-inactivated serum (Fig. 1B and C). However, only AaMCR expression showed a significant difference between groups. In addition, a negative correlation was observed between DENV envelope (E) protein and AaMCR gene expression (Fig. 1D and E), and there was a positive correlation between AaMCR and DEFA expression in DENV-2-infected midguts (Fig 1F and G). Interestingly, AaMCR and DENV-2 E protein gene expression was significantly linked with the inactivated serum-fed mosquitoes, whereas the AaMCR and DEFA expression correlation was significant in both normal and inactivated serum-fed mosquitoes.
Human Complement Protein C5a Reduces DENV Infection in Mosquito Cells
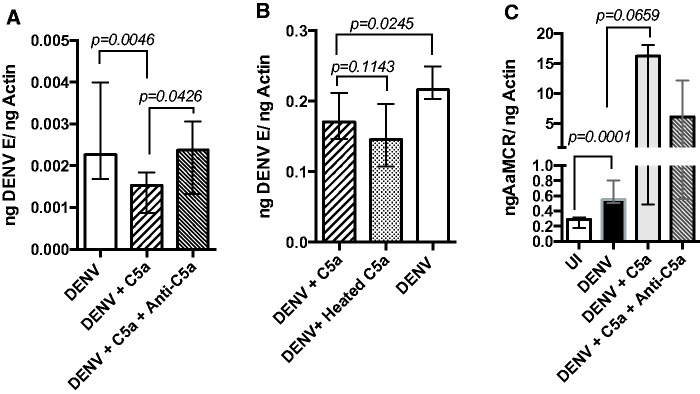
As normal, active human serum lowered DENV infection in mosquitoes, we investigated whether specific complement proteins alone could reduce infection. The Ae. aegypti cell line, Aag2, has been validated as a reliable model to study Aedes immune responses against pathogens (Barletta et al. 2012). Using this cell line, we found that DENV-infected cells incubated with C5a protein had significantly lower viral levels than cells without C5a treatment (P = 0.0046; Fig. 2A). The addition of anti-C5a antibodies, but not the addition of heat-treated C5a, restored DENV infection levels in C5a-treated cells to that of untreated cells (P = 0.0426; Fig. 2B). Furthermore, expression of AaMCR was considerably increased in DENV-infected cells when compared with uninfected cells (P = 0.0001; Fig. 2C). This indicates that human C5a protein can impact DENV infection in mosquitoes.
Fig. 2.
Human C5a protein reduces DENV infection by modulating mosquito gene expression. (A) DENV-2 levels 48 h post infection in Aag2 cells treated with either C5a or specific human anti-C5a antibodies, (B) DENV-2 infection levels in cells exposed to heat-treated recombinant C5a and (C) AaMCR expression in DENV-2 infected cells (DENV) in comparison with uninfected cells (UI) and treated with either C5a or anti-C5a antibodies. P-values are indicated on each panel. Samples were tested in sextuplets. Bars show median and interquartile range.
C5a Protein Induces Expression of AaMCR in the Absence of DENV Infection
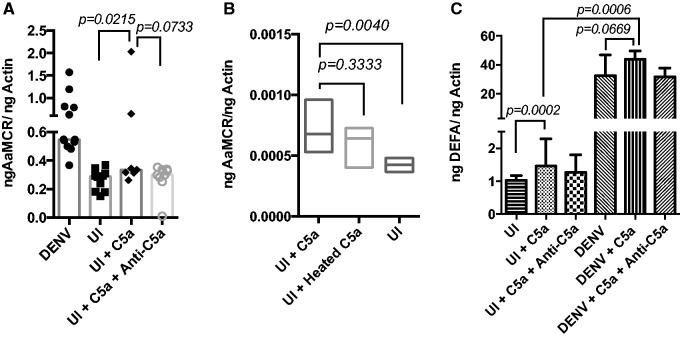
The C5a effect on Aag2 cells was also tested in the absence of DENV infection. Our results show that AaMCR expression in Aag2 cells was significantly increased in uninfected cells after treatment with C5a alone compared with the C5a-untreated uninfected cells (P = 0.0215; Fig. 3A). As observed with infected cells, there were no significant differences in the AaMCR expression between uninfected cells treated with heated and non-heated C5a (P = 0.3333; Fig. 3B). In addition, DEFA gene expression was significantly higher in uninfected cells treated with C5a than the non-C5a treated (P = 0.0002; Fig. 3C).
Fig. 3.
C5a protein induces AaMCR gene expression in uninfected AaG2 cells. (A) AaMCR expression in UI cells in comparison with DENV-infected cells and treated with either C5a or anti-C5a antibodies, (B) AaMCR expression levels in UI cells exposed to heat-treated C5a and (C) DEFA expression in Aag2 infected and uninfected cells. P-values are indicated on each panel. Samples were tested in sextuplets. Bars show median – interquartile range and boxes show line at median.
Human Complement C5a Interacts With Mosquito Proteins
As C5a showed an increase in the expression of immune-related mosquito genes along with a decrease in DENV infection, we tested the ability of C5a to bind mosquito cell proteins. Standard ELISA, using Aag2 cell lysate as antigen (Fig. 4A), and an in-cell ELISA with Aag2 fixed cells (Fig. 4B) showed that human C5a did bind to mosquito proteins and that this binding was C5a protein concentration-dependent. Our results indicated that at 10 μg/mL of C5a, the amount of bound protein to either 10 or 1 μg/mL of Aag2 lysate was three times higher than the amount bound when using 1 or 0.1 μg/mL of C5a. This difference was statistically significant (ANOVA, P < 0.0001). In addition, a Far-Western assay showed that C5a was able to bind Aag2 proteins with molecular weights between 50 and 80 kDa (Fig. 4C). Finally, the localization of bound C5a was tested in Aag2 cells by IFA. Our results showed that C5a was located at both membrane and peri-nuclear regions of Aag2 cells (Fig. 4D). To identify the mosquito proteins that bound C5a, we used anti-C5a antibodies to precipitate these proteins and sent the solution for MS analysis. The MS/MS analysis showed that four proteins were able to bind Aag2 lysate and 28 bound to mosquito midgut tissue. Two of these proteins were predicted to be located on the cell membrane. The list of these two proteins is found in Table 1. These are designated as AAEL006844 and AAEL003776.
Table 1.
List of proteins able to bind human C5a
| Tissue | Binding protein | Protein name | Predicted function | Citation |
|---|---|---|---|---|
| Mosquito midgut | AAEL006844-PA | Uncharacterized | Seven transmembrane receptor (rhodopsin family) G protein-coupled receptors | Nene et al. (2007), Dissanayake et al. (2010) |
| Aag2 lysate | AAEL003776-PA | Uncharacterized | Putative transmembrane protein precursor | Nene et al. (2007), Choi et al. (2012) |
10 μg/mL of Aag2 cell lysate and Ae. aegypti midgut tissue was incubated with 1 μg/mL of human C5a protein, bound proteins were then harvested with anti-human C5a coated beads, and sent for mass spectrometry analysis.
Discussion
Dengue fever is the main cause of pediatric death in Asia and Latin America. There is no specific antiviral treatment available; consequently, decreasing mosquito–human contact is the main transmission control method currently used. The DENV is transmitted via mosquito bite from an infected female Aedes spp. These mosquitoes depend on a bloodmeal to produce eggs, a characteristic that has been exploited by many vector-transmitted pathogens to continue their natural cycle. In Anopheles spp., cell and host factors transferred during blood feeding have been shown to clear pathogens, such as malaria parasites, thereby lowering mosquito infection and subsequent transmission (Tempelis 1975, Washino and Tempelis 1983, Margos et al. 2001, Stowers and Carter 2001, Simon et al. 2013)
Recent research has shown that the human complement system can be active in the mosquito for up to 3 h after blood feeding (Khattab et al. 2015). In our studies, we examined the effect of complement proteins in human serum on DENV infection of Ae. aegypti midguts at 3 h post feeding. Mosquitoes fed with normal human serum had lowered viral titers than mosquitoes fed with inactivated serum. Although the difference of the medians in the viral titers was not statistically significant, it was interesting to find an 89% reduction of the viral titer in mosquitoes fed with normal serum as compared with inactivated serum. This discovery suggests that active complement proteins have an effect on virus concentration in the early hours of mosquito midgut infection. AaMCR is a complement-like mosquito protein that has been associated with immune defense against DENV infection in vivo. Our results showed that the expression of AaMCR between normal serum-fed and inactivated serum-fed mosquitoes was significantly different. In fact, we found that mosquitoes fed with normal human serum presented significantly higher expression of AaMCR, which suggests a relationship between the presence of active human complement proteins and mosquito complement-like responses. Furthermore, the AaMCR protein has been previously found to participate in the immune response against DENV by interacting with scavenger receptors that later modulate the expression of AMPs. This relationship was further tested in our study in a correlation analysis where we found a significant positive correlation between DEFA and AaMCR expression in both inactivated and normal serum-fed infected mosquitoes. In previous studies, the knockdown of the AaMCR gene resulted in considerably higher DENV titers 6 d post infection (Cheng et al. 2011, Xiao et al. 2014). Further research is needed to determine whether feeding mosquitoes with normal versus inactivated serum results in differing viral loads once the virus has migrated from the midgut to the salivary glands (i.e., day 3 and day 7 post infection).
Using an in vitro approach, we investigated the effects of human complement proteins on dengue infection beyond the first few hours of infection. Using Aag2 cells, we found that, after 48 h of incubation, the level of DENV infection was significantly lower and AaMCR expression significantly higher in infected Aag2 cells treated with human complement protein C5a in comparison with cells without C5a. Interestingly, disabling C5a through the addition of anti-C5a antibodies, but not by heat inactivation, reverses the effect of C5a on DENV infection in concordance with previous studies showing that C5a is a heat-stable molecule (Clark and Klebanoff 1976, Craddock et al. 1977). This finding suggests an involvement of C5a in the antiviral response against DENV. In addition, uninfected Aag2 cells expressed appreciably higher levels of AaMCR in the presence of C5a, implying that the presence of human complement protein C5a is able to signal mosquito cells. Our results are in accordance with preceding studies demonstrating that human factors can affect mosquito gene expression and modify DENV infection of mosquitoes (Kang et al. 2008, Surachetpong et al. 2011, Drexler et al. 2014). Specifically, human insulin and transforming growth factor beta have been shown to interact with and signal mosquito cells in Anopheles spp., regulating the immune response against Plasmodium spp. parasites (Lieber and Luckhart 2004, Pakpour et al. 2012). However, further investigation is needed to determine the exact mechanism by which C5a participates in Aedes gene expression, cell signaling, and, specifically, AaMCR expression. In addition, in the mosquito experiments, we found that heat inactivation impacts virus titers and AaMCR expression; it is important to clarify that mosquitoes receiving inactivated serum may have some remains of active C5a. However, new C5a resulting from complement activation in midgut should be inhibited or significantly reduced due to the heat-susceptibility of C3 and C4 (Mankovich et al. 2013). It is possible that the nonsignificant difference in virus infection between mosquitoes receiving inactivated or non-inactivated serum may be influenced by the presence of a small amount of active C5a in the heat-inactivated serum.
Xiao et al. (2014) previously showed that silencing DEFA gene expression did not have a significant effect on DENV infection in Ae. aegypti mosquitoes. In concurrence with those results, we showed that in both in vivo and in vitro assays, there were no significant differences between DEFA gene expression levels in the presence or absence of active human complement or C5a on infected Aag2 cells or mosquitoes. However, we found significant increase of DEFA expression in the absence of DENV infection in cells treated with C5a. This suggests that human complement proteins may interact with factors involved in the expression of AMP independently of infection. Another possibility is that the human-complement-induced antiviral effect is used without going through AMP pathways in the presence of high AaMCR expression. Previous studies have shown that defensins, such as defensin D and defensin C, are associated with anti-DENV responses in Ae. aegypti (Xiao et al. 2014, Wasinpiyamongkol et al. 2015). Unfortunately, we did not test the gene expression of these proteins in the current study. Our laboratory is currently performing additional experiments to evaluate if there is a direct relationship between Ae. aegypti defensin expression and human complement factors.
As mosquito and human complement proteins belong to the same protein superfamily (Blandin and Levashina 2004, Nonaka and Kimura 2006, Ricklin et al. 2010), we speculate that C5a may be able to interact with receptors in mosquito cells and modulate immune responses. To evaluate if there was direct interaction between C5a and specific mosquito proteins, we tested the binding of C5a protein to Aag2 cells and cell lysate. Our results showed that C5a was able to bind mosquito proteins with molecular weights between ∼50 and 80 kDa. C5a was also able to bind proteins located in the membrane and peri-nuclear space of Aag2 cells.
Human complement C5a receptors belong to the G protein-coupled receptors (GPCR) family and the rhodopsin family of seven transmembrane-containing GPCRs (Monk et al. 2007). We tested the hypothesis that mosquito midgut proteins interact with recombinant C5a. MS results revealed several mosquito proteins that were able to bind Aag2 cells and mosquito midgut tissue. We selected the two proteins with the highest functional relevance for this report. First, the protein AAEL006844 (∼56.6 kDa), a hypothetical GPCR, was found to bind C5a in midgut tissue. The second protein, AAEL003776, is a putative transmembrane protein precursor. AAEL003776 has a molecular weight of 44 kDa and our experiments show that it was able to bind C5a in Aag2 cells; thus, this could be the protein we identified by the in-cell ELISA that was around 50 kDa. The gene expression of these two proteins was previously found to be upregulated in Aedes hemocytes after bacterial infections when compared with uninfected controls (Dissanayake et al. 2010, Choi et al. 2012). Further exploration is needed to characterize the function of the mosquito proteins found to interact with human C5a.
In summary, our research indicates a potential immune crosstalk between insect and mammalian complement factors in response to DENV infection. Considerable additional research is required to uncover the mechanisms by which these two systems interplay. The current data support the hypothesis of a receptor-mediated response to human C5a by Ae. aegypti cells that is able to regulate antiviral immunity.
Acknowledgments
We would like to thank Mir Bear-Johnson, Erin Cloherty, Samuel Jameson, Dawn Wesson, and Patricia Scaraffia for providing mosquitoes and adding their insights to this project.
Funding
NIH NIAD K22 AI103067-01
References Cited
- Barletta A. B., Silva M. C., Sorgine M. H. 2012. Validation of Aedes aegypti Aag-2 cells as a model for insect immune studies. Parasites Vectors 5: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin S., Levashina E. A. 2004. Thioester-containing proteins and insect immunity. Mol. Immunol. 40: 903–908. [DOI] [PubMed] [Google Scholar]
- Blandin S. A., Levashina E. A. 2007. Phagocytosis in mosquito immune responses. Immunol. Rev. 219: 8–16. [DOI] [PubMed] [Google Scholar]
- Bou Aoun R., Hetru C., Troxler L., Doucet D., Ferrandon D., Matt N. 2011. Analysis of thioester-containing proteins during the innate immune response of Drosophila melanogaster. J. Innate Immun. 3: 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buresova V., Hajdusek O., Franta Z., Loosova G., Grunclova L., Levashina E. A., Kopacek P. 2011. Functional genomics of tick thioester-containing proteins reveal the ancient origin of the complement system. J. Innate Immun. 3: 623–630. [DOI] [PubMed] [Google Scholar]
- Cheng G., Liu L., Wang P., Zhang Y., Zhao Y. O., Colpitts T. M., Feitosa F., Anderson J. F., Fikrig E. 2011. An in vivo transfection approach elucidates a role for Aedes aegypti thioester-containing proteins in flaviviral infection. PLoS ONE 6: e22786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. J., Fuchs J. F., Mayhew G. F., Yu H. E., Christensen B. M. 2012. Tissue-enriched expression profiles in Aedes aegypti identify hemocyte-specific transcriptome responses to infection. Insect Biochem. Mol. Biol. 42: 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. 1976. Generation of a neutrophil chemotactic agent by spermatozoa: Role of complement and regulation by seminal plasma factors. J. Immunol. 117: 1378–1386. [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. 1977. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J. Clin. Invest. 60: 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings K. L., Waggoner S. N., Tacke R., Hahn Y. S. 2007. Role of complement in immune regulation and its exploitation by virus. Viral Immunol. 20: 505–524. [DOI] [PubMed] [Google Scholar]
- Das S., Garver L., Ramirez J. R., Xi Z., Dimopoulos G. 2007. Protocol for dengue infections in mosquitoes (A. aegypti) and infection phenotype determination. J. Vis. Exp. JoVE: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos G. 2003. Insect immunity and its implication in mosquito-malaria interactions. Cell. Microbiol. 5: 3–14. [DOI] [PubMed] [Google Scholar]
- Dissanayake S. N., Ribeiro J. M., Wang M. H., Dunn W. A., Yan G., James A. A., Marinotti O. 2010. aeGEPUCI: A database of gene expression in the dengue vector mosquito, Aedes aegypti. BMC Res. Notes 3: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler A. L., Pietri J. E., Pakpour N., Hauck E., Wang B., Glennon E. K., Georgis M., Riehle M. A., Luckhart S. 2014. Human IGF1 regulates midgut oxidative stress and epithelial homeostasis to balance lifespan and Plasmodium falciparum resistance in Anopheles stephensi. PLoS Pathogens 10: e1004231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkelberger J. R., Song W. C. 2010. Complement and its role in innate and adaptive immune responses. Cell Res. 20: 34–50. [DOI] [PubMed] [Google Scholar]
- Fujita T. 2002. Evolution of the lectin-complement pathway and its role in innate immunity. Nat. Rev. Immunol. 2: 346–353. [DOI] [PubMed] [Google Scholar]
- Gubler D. J. 2011. Dengue, urbanization and globalization: The unholy trinity of the 21(st) century. Trop. Med. Health 39: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R. F., Ward P. A. 2005. Role of C5a in inflammatory responses. Ann. Rev. Immunol. 23: 821–852. [DOI] [PubMed] [Google Scholar]
- Hagenlocher M., Delmelle E., Casas I., Kienberger S. 2013. Assessing socioeconomic vulnerability to dengue fever in Cali, Colombia: Statistical vs expert-based modeling. Int. J. Health Geogr. 12: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John D. V., Lin Y. S., Perng G. C. 2015. Biomarkers of severe dengue disease - a review. J. Biomed. Sci. 22: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M. A., Mott T. M., Tapley E. C., Lewis E. E., Luckhart S. 2008. Insulin regulates aging and oxidative stress in Anopheles stephensi. J. Exp. Biol. 211: 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattab A., Barroso M., Miettinen T., Meri S. 2015. Anopheles midgut epithelium evades human complement activity by capturing factor H from the blood meal. PLoS neglected tropical diseases 9: e0003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levashina E. A., Moita L. F., Blandin S., Vriend G., Lagueux M., Kafatos F. C. 2001. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell 104: 709–718. [DOI] [PubMed] [Google Scholar]
- Lieber M. J., Luckhart S. 2004. Transforming growth factor-betas and related gene products in mosquito vectors of human malaria parasites: Signaling architecture for immunological crosstalk. Mol. Immunol. 41: 965–977. [DOI] [PubMed] [Google Scholar]
- Malasit P. 1987. Complement and dengue haemorrhagic fever/shock syndrome. Southeast Asian J. Trop. Med. Public Health 18: 316–320. [PubMed] [Google Scholar]
- Mankovich A. R., Lee C. Y., Heinrich V. 2013. Differential effects of serum heat treatment on chemotaxis and phagocytosis by human neutrophils. PLoS ONE 8: e54735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G., Navarette S., Butcher G., Davies A., Willers C., Sinden R. E., Lachmann P. J. 2001. Interaction between host complement and mosquito-midgut-stage Plasmodium berghei. Infect. Immunity 69: 5064–5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk P. N., Scola A. M., Madala P., Fairlie D. P. 2007. Function, structure and therapeutic potential of complement C5a receptors. Br. J. Pharmacol. 152: 429–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvizhskii A. I., Keller A., Kolker E., Aebersold R. 2003. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75: 4646–4658. [DOI] [PubMed] [Google Scholar]
- Niederbichler A. D., Hoesel L. M., Westfall M. V., Gao H., Ipaktchi K. R., Sun L., Zetoune F. S., Su G. L., Arbabi S., Sarma J. V., et al. 2006. An essential role for complement C5a in the pathogenesis of septic cardiac dysfunction. J. Exp. Med. 203: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka M. 2000. Origin and evolution of the complement system. Curr. Topics Microbiol. Immunol. 248: 37–50. [DOI] [PubMed] [Google Scholar]
- Nonaka M., Kimura A. 2006. Genomic view of the evolution of the complement system. Immunogenetics 58: 701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osta M. A., Christophides G. K., Vlachou D., Kafatos F. C. 2004. Innate immunity in the malaria vector Anopheles gambiae: Comparative and functional genomics. J. Exp. Biol. 207: 2551–2563. [DOI] [PubMed] [Google Scholar]
- Pakpour N., Corby-Harris V., Green G. P., Smithers H. M., Cheung K. W., Riehle M. A., Luckhart S. 2012. Ingested human insulin inhibits the mosquito NF-kappaB-dependent immune response to Plasmodium falciparum. Infect. Immunity 80: 2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Rawal N. 2002. Structure and function of complement C5 convertase enzymes. Biochem. Soc. Trans. 30: 1006–1010. [DOI] [PubMed] [Google Scholar]
- Ricklin D., Hajishengallis G., Yang K., Lambris J. D. 2010. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 11: 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi R., Fujito N. T., Nonaka M. 2012. Evolution of the thioester-containing proteins (TEPs) of the arthropoda, revealed by molecular cloning of TEP genes from a spider, Hasarius adansoni. Dev. Comp. Immunol. 36: 483–489. [DOI] [PubMed] [Google Scholar]
- Simon N., Lasonder E., Scheuermayer M., Kuehn A., Tews S., Fischer R., Zipfel P. F., Skerka C., Pradel G. 2013. Malaria parasites co-opt human factor H to prevent complement-mediated lysis in the mosquito midgut. Cell Host Microbe 13: 29–41. [DOI] [PubMed] [Google Scholar]
- Stoermer K. A., Morrison T. E. 2011. Complement and viral pathogenesis. Virology 411: 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers A., Carter R. 2001. Current developments in malaria transmission-blocking vaccines. Expert Opin. Biol. Ther. 1: 619–628. [DOI] [PubMed] [Google Scholar]
- Surachetpong W., Pakpour N., Cheung K. W., Luckhart S. 2011. Reactive oxygen species-dependent cell signaling regulates the mosquito immune response to Plasmodium falciparum. Antioxid. Redox Signal. 14: 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempelis C. H. 1975. Host-feeding patterns of mosquitoes, with a review of advances in analysis of blood meals by serology. J. Med. Entomol. 11: 635–653. [DOI] [PubMed] [Google Scholar]
- Washino R. K., Tempelis C. H. 1983. Mosquito host bloodmeal identification: Methodology and data analysis. Ann. Rev. Entomol. 28: 179–201. [DOI] [PubMed] [Google Scholar]
- Wasinpiyamongkol L., Missé D., Luplertlop N. 2015. Induction of defensin response to dengue infection in Aedes aegypti. Entomol. Sci. 18: 199–206. [Google Scholar]
- Waterhouse R. M., Kriventseva E. V., Meister S., Xi Z., Alvarez K. S., Bartholomay L. C., Barillas-Mury C., Bian G., Blandin S., Christensen B. M., et al. 2007. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science 316: 1738–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D. M., Burke D. S., Harrison B. A., Whitmire R. E., Nisalak A. 1987. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am. J. Trop. Med. Hyg. 36: 143–152. [DOI] [PubMed] [Google Scholar]
- Xiao X., Liu Y., Zhang X., Wang J., Li Z., Pang X., Wang P., Cheng G. 2014. Complement-related proteins control the flavivirus infection of Aedes aegypti by inducing antimicrobial peptides. PLoS Pathogens 10: e1004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z. Q., Qian W., Suzuki K., McNamara J. O. 2003. Formation of complement membrane attack complex in mammalian cerebral cortex evokes seizures and neurodegeneration. The J. Neurosci.: Official J. Soc. Neurosci. 23: 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro T., Disla M., Petit A., Taveras D., Castro-Bello M., Lora-Orste M., Vardez S., Cesin A. J., Garcia B., Nishizono A. 2004. Seroprevalence of IgG specific for dengue virus among adults and children in Santo Domingo, Dominican Republic. Am. J. Trop. Med. Hyg. 71: 138–143. [PubMed] [Google Scholar]