Abstract
Pediculosis is a prevalent parasitic infestation of humans, which is increasing due, in part, to the selection of lice resistant to either the pyrethrins or pyrethroid insecticides by the knockdown resistance (kdr) mechanism. To determine the extent and magnitude of the kdr-type mutations responsible for this resistance, lice were collected from 138 collection sites in 48 U.S. states from 22 July 2013 to 11 May 2015 and analyzed by quantitative sequencing. Previously published data were used for comparisons of the changes in the frequency of the kdr-type mutations over time. Mean percent resistance allele frequency (mean % RAF) values across the three mutation loci were determined from each collection site. The overall mean % RAF (±SD) for all analyzed lice was 98.3 ± 10%. 132/138 sites (95.6%) had a mean % RAF of 100%, five sites (3.7%) had intermediate values, and only a single site had no mutations (0.0%). Forty-two states (88%) had a mean % RAF of 100%. The frequencies of kdr-type mutations did not differ regardless of the human population size that the lice were collected from, indicating a uniformly high level of resistant alleles. The loss of efficacy of the Nix formulation (Prestige Brand, Tarrytown, NY) from 1998 to 2013 was correlated to the increase in kdr-type mutations. These data provide a plausible reason for the decrease in the effectiveness of permethrin in the Nix formulation, which is the parallel increase of kdr-type mutations in lice over time.
Keywords: Pediculus humanus capitis, human head louse, knockdown resistance frequency, quantitative sequencing
Pediculus humanus capitis (De Geer), the human head louse, causes one of the most prevalent human parasitic infestations, pediculosis (Gratz 1997, Raoult and Roux 1999). Although head lice are not known to vector life-threatening human diseases (Pittendrigh et al. 2015), most people find louse infestation intolerable and choose to apply pediculicides (Clark et al. 2013). Treatment is largely dependent upon topically applied insecticides found in commercially available formulations including the natural pyrethrin esters (pyrethrum) and the synthetic “pyrethroids” (permethrin, phenothrin; Durand et al. 2012). Recently, three new treatments, Ulesfia (Concordia Pharmaceuticals Inc., St. Michael, Barbados), Natroba (ParaPRO, LLC, Carmel, IN), and Sklice (Arbor Pharmaceuticals, LLC, Atlanta, GA), were registered for use in the United States (Stough et al. 2009, Meinking et al. 2010, Pariser et al. 2012). Nevertheless, the pyrethrins or pyrethroids continue to dominate the louse treatment market as inexpensive and easily obtainable over-the-counter (OTC) formulations (Clark 2009).
The natural pyrethrins were formulated as a pediculicide in 1945 (Durand et al. 2012). Permethrin was first used in the Nix formulation in the 1980s, an OTC product sold worldwide (Durand et al. 2012) that has been used extensively and intensely for over 20 year.
Pyrethrins, pyrethroids, and DDT share a common target site and mode of action in the nervous system. They bind to voltage-sensitive sodium channels (VSSC), and act as agonistic neuroexcitants by prolonging inward sodium current, leading to nerve depolarization and hyperexcitation, followed by muscle paralysis and death (Clark 2009). Because of their widespread and intense use except DDT in the United States, the lack of suitable alternatives (until recently), and the fact that they share their common site of action with the extensively used insecticide DDT, resistance to the pyrethrins or pyrethroids is common in lice (Yoon et al. 2014).
Resistance to the pyrethrins or pyrethroids in insects is caused by multiple mechanisms, one of which is target site insensitivity, which is known as knockdown resistance or kdr. The kdr phenotype (recalcitrant to knockdown), a heritable trait associated with nerve insensitivity to DDT, the pyrethrins, and pyrethroids, was first discovered in the house fly, Musca domestica L. (Farnham 1977), and is one of the most common mechanisms of resistance in insects against these insecticides. Point mutations within the α-subunit gene of voltage-sensitive sodium channel (VSSC) are functionally responsible for the nerve insensitivity and result in the kdr, kdr-type, and super kdr traits (Williamson et al. 1993, Dong and Scott 1994, Knipple et al. 1994). Lee et al. (2000) first determined that lice from Florida and Massachusetts were resistant to permethrin and showed in vivo behavioral responses consistent with kdr. Subsequently, three point mutations (M815I, T917I, and L920F) in the VSSC α-subunit gene of permethrin-resistant lice were found (Lee et al. 2000, 2003) and validated as kdr-type mutations (Yoon et al. 2008).
While the presence of kdr-type mutations alone may not directly predict clinical failure, their increasing frequency in louse populations coincides with reports of product failures in controlled studies. Early reports of permethrin use from 1984 through 1995 consistently showed 96% to 100% effectiveness (Brandenburg et al. 1986, Taplin et al. 1986, Bainbridge et al. 1998, Carson et al. 1988, DiNapoli et al. 1988). In 2001, a report described a reduced effectiveness of only 80%, and subsequent reports of effectiveness have ranged from 28% to 55%, even where treatments have been augmented by nit combing (Burkhart and Burkhart 2000; Hipolito et al. 2001; Meinking et al. 2004, 2007, 2010; Stough et al. 2009; Kim 2011). In 2003, Gao et al. reported that increased frequency of the kdr-type mutations in permethrin-resistant louse populations from California, Florida, and Texas were highly correlated with increased survivorship, and that kdr resistance was completely recessive. Thus, even in populations (SCA-HL collected from southern California and TMS-HL collected from Mathis, Texas) that did not show high levels of permethrin resistance, the high proportion of heterozygous individuals (50 and 41%) indicated that these populations would rapidly become resistant if continual permethrin selection was maintained (Gao et al. 2003). It therefore seems likely that the prevalence of the kdr-type mutations can be an indicator of permethrin resistance and the probable cause of failure of products containing pyrethrins or pyrethroids in providing effective control of louse infestations.
Several genotyping techniques based on these mutations have been developed (Lee et al. 2010), and their use established that although widespread, kdr-type mutations were not yet uniform in U.S. lice as far back as 2001–2007 (Hodgdon et al. 2010). More recently, Yoon et al. (2014) examined the extent and frequency of kdr-type mutations in North American lice from 1999 to 2009. Genotyping results substantiated that the T917I mutation occurred at high levels in lice by 2009 (88.4%). These authors cautioned, however, that their results were preliminary and perhaps biased due to the small number of lice analyzed and because most of the lice were collected from metropolitan and urban collection sites. In view of these limitations, the actual level of susceptibility could be higher than what was reported. It was concluded that additional studies should be carried out from more geographical locations in more states and to increase sampling in suburban and rural areas.
In this study, we utilized three quantitative sequencing (QS) reactions to determine the kdr-type mutation frequency at each allele. Lice from 138 geographical collection sites, ranging from rural to metropolitan areas, were collected from 48 U.S. states. This expanded dataset was then compared with previous collected data to show changes in the kdr-type mutation frequencies over time. This genotyping information was correlated to existing clinical findings that have shown a substantial decrease in the ability of the permethrin-based OTC products in providing effective control of louse infestations over time.
Materials and Methods
Collections
Lice were collected from 7 July 2013 to 11 May 2015 by 71 volunteers (school nurses and professional lice combers) from 138 collection sites in 48 states. Four of these states (AZ, CA, FL, and TX) had collection sites that had been sampled twice before (1999–2006 and 2006–2008) and an additional eight states (OH, MA, MI, MN, NY, SC, TN, and WI) had been sampled only once before (2007–2009), allowing the determination of kdr-type mutation frequency changes in those locations over time. Prospective volunteers were contacted by Lice Solutions Resource Network, Inc., West Palm Beach, FL, and provided an information sheet, which explained the study’s objectives, method for louse collection, sample preparation, labeling instructions, storage requirements, collection log form, and shipping instructions. Interested individuals were then contacted directly by researchers at the Pesticide Toxicology Laboratory at the University of Massachusetts-Amherst who discussed the study and answered any questions. Individuals who were still interested in collecting lice were sent a consent form. Upon receiving the signed consent form, they were sent a collection kit with sufficient materials to collect the required louse samples and an addressed return box with prepaid postage. The protocols for collections were approved by the University of Massachusetts Internal Review Board (number 104-1423 and number 109-1792) and the National Institutes of Health (number 06-003).
Collection kits were fabricated in house and contained 15 sequentially numbered 2-ml plastic vials, prefilled with 95% ethanol, contained within a small shipping box. Collection log forms were provided to record the patient’s town of residence, sex, age, the number of lice collected, and the collection date. Once the volunteered collector had filled all 15 vials (using one vial per one infested person) with a minimum of five lice per vial, the sample kit was returned to the University of Massachusetts-Amherst. Upon arrival, samples were pooled by their collection site (based upon the town of residence of the infested patient). Once pooled, 10 or more lice of the same life stage (and sex in the case of adults) were randomly selected and transferred to a new vial for genomic DNA extraction.
DNA Extraction
Genomic DNA (gDNA) was extracted and purified using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA) following the manufacturer’s instructions and using an automated homogenizer (Geno Grinder 2010, SPEX Sample Prep, Metuchen, NJ) at 1,250 strokes per min for 1 min. gDNA was diluted to a concentration of 10 ng µl−1 in AE (elution) buffer and stored at −20°C for PCR amplification (Kwon et al. 2008).
PCR Amplification
A 908 bp gDNA fragment of the VSSC α-subunit gene (insecticide-susceptible P. h. capitis from Ecuador [EC-HL], complete cds, Accession: AY191156), containing the three kdr-type mutation sites, was PCR amplified using the method of Kwon et al. (2008).
PCR Product Purification
The QIAquick PCR Purification Kit (Qiagen) was used for PCR product purification following the manufacturer’s instructions.
Sequencing and Analysis
Triplicates for each sample containing sequencing primers for either the M815I mutation or the T917I and L920F mutations (Kwon et al. 2008) were mixed with 10 µl of purified PCR product, loaded into a 96-well plate, and sent to GeneWiz (South Plainfield, NJ). Sequence chromatograms were analyzed using Chromas lite software (Technelysium Pty Ltd., Tewantin, Australia) to determine the nucleotide signal intensity at the respective mutation sites, and the nucleotide signal ratios (NSRs) were calculated as ([Resistant Nucleotide Signal] / [Resistant Nucleotide Signal + Susceptible Nucleotide Signal]). The NSR was entered into the standard curve equation to predict the kdr-type resistance allele frequencies (RAFs) at all three mutations.
Standard Curves
Standard curves were generated using authenticated permethrin-resistant FL-HL and the insecticide-susceptible EC-HL strains of lice for all three kdr-type mutations following the protocol of Kwon et al. (2008). Resulting NSRs were plotted against the known molar RAFs to generate standard curve equations as well as lower and upper 95% prediction band equations using Sigma Plot 10.0 (Systat Software Inc., San Jose, CA). The standard curves were then used to predict the RAFs for the collected louse samples at the 95% confidence level.
Results
QS Standard Curves
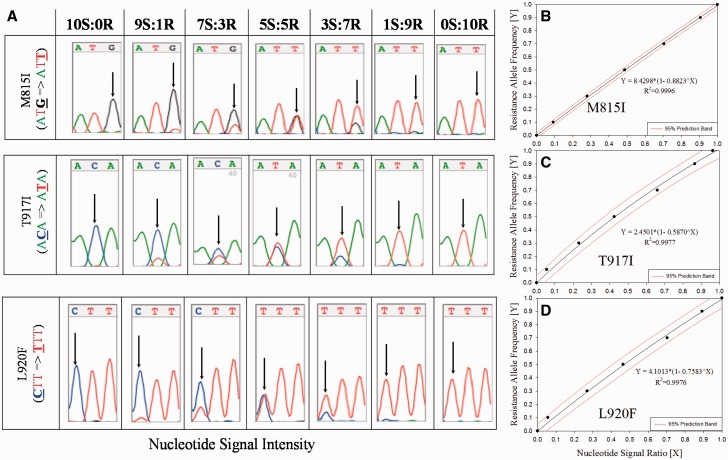
Nucleotide signal intensities for both susceptible and resistant alleles at each mutation site (see arrows, Panel A, Fig. 1) were determined from the sequence chromatograms, and standard regression equations were generated by plotting the calculated NSR versus RAF (Panel B–D, Fig. 1). Using the upper and lower 95% prediction intervals, the M815I, T917I, and L920F RAFs could be accurately predicted within the range of 2.0–97, 5.4–94, and 5.3–92%, respectively. The lower detection limits for the three resistance mutations ranged from 2.0–5.4% (4.2 ± 1.9%), which is not significantly different (Student’s t-test, P > 0.05) from that determined initially by Kwon et al. (2008) (7.4 ± 2.7%). Once established, these regression equations can be used repetitively for the prediction of the RAF of unknown samples.
Fig. 1.
Generation of standard curves using quantitative sequencing for the determination of resistance allele frequencies (RAF) from collected lice. Nucleotide signal intensities for susceptible and resistant alleles at each mutation site (see arrows, Panel A, Fig. 1) were determined from the chromatograms, ratios calculated, and plotted versus RAF to yield standard regression equations (Panels B–D, Fig. 1) and used to calculate RAF from collected samples.
QS of Collected Lice
In total, 14,281 lice from 479 human subjects from 138 collection sites in 48 states (no lice from Alaska and West Virginia) were returned to UMASS for the determination of their RAFs (see Supp. Table 1 [online only]). There was a total of 1,925 lice analyzed by QS from all sites (1,925/14,281 = 13.55%), resulting in an average of 13.95 lice per site with a standard deviation of 4.10. Randomly selected lice within the most prevalent life stage from all subjects at a site were included for analysis. Females were used for 86 (62%), males for 18 (13%), third instars for 20 (15%), and second for 14 (10%) sites. Average numbers (±SD) of females, males, third and second instars used per site was 14.8 ± 4.6, 12.6 ± 2.3, 12.0 ± 2.2, and 12.5 ± 1.7, respectively. These values were not significantly different from one another (Student’s t-test, P > 0.05). The average % RAF (±SD) for M815I, T917I, or L920F was 98.1 ± 10.4%, 98.5 ± 9.4%, or 98.3 ± 9.9%, respectively. Given that these values are not significantly different from one another (Student’s t-test, P > 0.05), we averaged the % RAF for each of the three kdr-type mutations for each collection site in order to graphically display this data set as mean % RAF.
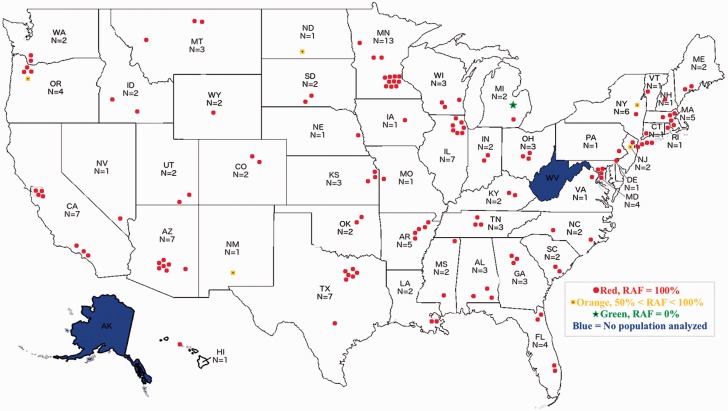
Figure 2 presents the mean % RAF (average of the RAF for the three kdr-type mutations × 100) for each of the 138 collection sites from 2013 to 2015. Of the 138 sites, 132 (95.6%) had lice that had a mean % RAF of 100% (all lice had the three kdr-type mutations; red dots, Fig. 2). Five sites (3.7%) had intermediate mean % RAF ranging for 60–87% (orange dots, Fig. 2). Only a single site had no mutations (0.0 % RAF; green dot, Fig. 2). The overall mean % RAF for all sites was 98.3% with standard deviation of 10.0%.
Fig. 2.
The kdr-type allele frequency map using mean % RAF values from head lice collected in the United States from 2013–2015. Each collection site is color coordinated based on the mean % RAF of kdr-type mutations found, where red is fully resistant (RAF = 100%), orange (50% ≤ RAF < 99%) is intermediate, and green is fully susceptible (RAF = 0%).
As indicated by red dots, 42 of the 48 states sampled (88%) showed a mean % RAF of 100% (Fig. 2). The remaining six states (12%), represented as a mixture of orange and green dots (MI, NJ, NM, NY, ND, and OR), had intermediate mean % RAF that ranged from 50 to 98% (see Supp. Table 1 [online only]). In each of these six states, however, only a single site had lice that were determined to have either a mean % RAF of 0.0% (Frankenmuth, MI; no mutations) or had intermediate mean % RAF (North Haledon, NJ, Alamogordo, NM; Saratoga County, NY; Fort Yates, North Dakota; and Wilsonville, OR; Fig. 3).
Fig. 3.
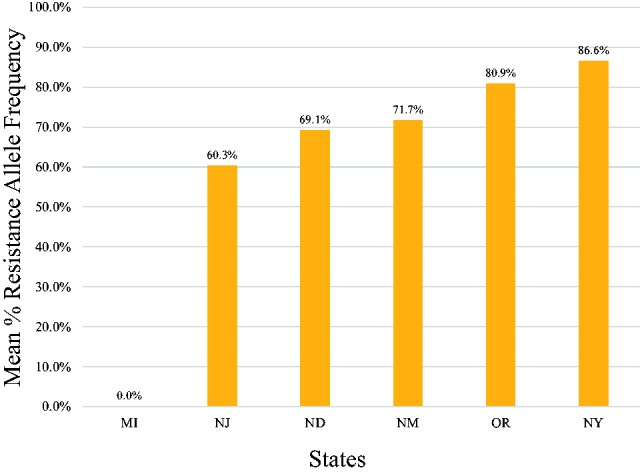
Mean % RAF of kdr-type mutations found in lice from collection sites that were not 100% resistant.
A concern of the study published in 2014 by Yoon et al. was that too few collecting sites (18) from only 12 states were examined and most of the sites were metropolitan or urban in nature. This allowed the possibility that more louse susceptibility (as judged by the absence or the lower frequency of the kdr-type mutations) could exist in more suburban and rural areas. In the current study, care was given to collect lice from sites that varied widely in their human population size (Table 1). Collection sites were subjectively grouped into four population sizes: high, large, moderate, or low, depending on the human population (see Supp. Table 1 [online only]). As seen in Table 1, the means (±SD) of all four population sizes were significantly different from each other. However, the average numbers of lice analyzed per collection site within a population size were not significantly different and ranged from 13.17 to 14.12 lice. When the average % RAF of each of the three mutation sites were compared, there was no significant difference amongst the four different population sizes.
Table 1.
Comparison of human population size versus head louse RAF values
| Population size | No. of collection sites | Mean population (±SD) | Total no. of lice analyzed | Avg. no. of lice analyzed (±SD) | Mutation site | Avg. % RAF per allele (±SD) |
|---|---|---|---|---|---|---|
| High (H) | 20 | 1,042,000 (1,083,00)a | 299 | 14.95e (5.13) | M815I T917I L920F | 99% (0.0)f 100% (0.0)f 99% (0.0)f |
| Large (L) | 45 | 120,116 (71,845)b | 624 | 13.87e (4.1) | M815I T917I L920F | 99% (3.0)f 100% (2.0)f 99% (2.0)f |
| Moderate (M) | 43 | 29,749 (12,141)c | 607 | 14.12e (4.14) | M815I T917I L920F | 98% (6.0)f 100% (5.0)f 98% (6.0)f |
| Low (l) | 30 | 3,548 (2,562)d | 395 | 13.17e (3.31) | M815I T917I L920F | 93% (21.0)f 96% (19.0)f 93% (20.0)f |
Abbreviations: RAF, resistance allele frequency; Avg, average number of lice per site.
a–d Mean population sizes are significantly different from one another (unpaired Student’s t-test, P < 0.0001).
e Average number of lice analyzed per site are not significantly different from one another (unpaired Student’s t-test, P > 0.05).
f Mean % RAF values are not significantly different either in an intrapopulation or an interpopulation comparison (unpaired Student’s t-test, P > 0.05 [0.5–0.8 range]).
Discussion
QS-Based Population Genotyping
QS analyzes gDNA from multiple louse specimens and is suitable for processing large numbers of lice from many sites necessary for monitoring insecticide resistance (Kwon et al. 2008). This approach greatly reduces the overall cost and effort compared with other population genotyping methods, such as rtPASA-TaqMan and rtPASA (Lee et al. 2010). Additionally, the use of automated homogenization greatly reduced the analysis time, and our current results were found to be not significantly different from those initially reported by Kwon et al. (2008). Lastly, the genotyping results obtained using either the serial invasive signal amplification reaction (SISAR) or QS were shown to be highly correlated (Yoon et al. 2014), allowing intermethod comparisons.
Genotyping of Lice (1999–2015)
Using SISAR, Hodgdon et al. (2010) reported that lice collected from a single site in Mathis, TX, in 2001 had an overall mean % RAF of 37%, indicating the presence of the three kdr-type mutations. This finding also showed, however, that these mutations were not uniform, and that susceptible (wild type) alleles were still present. In the same study, they also collected lice from seven collection sites in five states (AZ, FL, MI, MN, and TX) from 2006 to 2007 and found that the overall mean % RAF had increased to 74% (a 37% increase over six years).
In a related study (Yoon et al. 2014), lice were analyzed from 23 collection sites in 12 states from 1999–2009 (AZ, CA, FL, MA, MI, MN, NY, OH, SC, TN, TX, and WI) and the overall mean % RAF had increased to 84.4%. Four states sampled initially in 1999–2006 (AZ, CA, FL, and TX) were resampled in 2006–2008 and showed an overall increase in their mean % RAF of 37.1% ± 25.2%, indicating that the prevalence of kdr-type mutations was increasing over time. Using the data from lice collected only from 2007–2009, the overall mean % RAF increased to 99.6 ± 0.9%.
In the current study using QS, the overall mean % RAF was 98.3% with a standard deviation of 10.0%, indicating that the kdr-type mutations are almost uniformly present at high levels in lice collected recently in the United States. It is also interesting to note that the overall mean % RAF determined for 2007–2009 (99.6%, Yoon et al. 2014) has remained uniformly high (98.3%) in the present study, indicating the kdr-type resistance levels are not decreasing with time. This finding is expected, as the OTC products containing the pyrethrins or pyrethroids are still being used. Furthermore, the level of kdr-type mutations in lice recently collected (2013–2015) are uniformly high and not significantly different regardless of the human population size from which they were collected, indicating that there are few susceptible alleles remaining in U.S. lice.
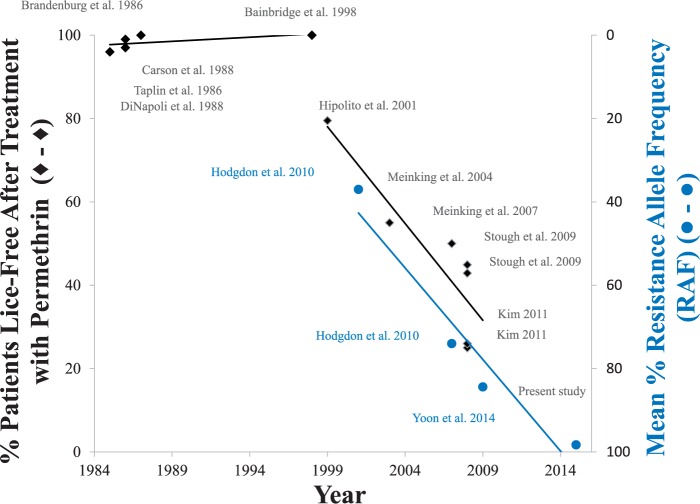
Lastly, eight different clinical studies carried out from the late 1990s until recently show that the effectiveness of the permethrin-containing Nix formulation has gradually decreased over time and no longer provides the level of effective control that it had when it was first introduced into the marketplace as an OTC formulation. As indicated in Fig. 4, the percent of lice-free patients following treatment by the OTC Nix formulation was essentially 100% from 1986 to 1998 (solid diamonds 1–8). From 1998, the overall effectiveness of the Nix formulation steadily declined and was only ∼25% effective by 2009, a ∼75% decline in overall effectiveness in clinical trials over 10 years. Over this same time, the relative frequency of the three kdr-type mutations, as judged by the mean % RAF, increased from 37% in 2001 (solid circles 9–12, Fig. 4) to 98% in 2015, a ∼61% increase over 14 years.
Fig. 4.
Increasing frequency of kdr-type mutations parallels the decreasing effectiveness of the OTC formulations containing permethrin. Each data point was determined from referenced articles. Data obtained from the present study were also used for preparing this figure.
In conclusion, QS was used as a population genotyping method to determine the relative frequency of the three kdr-type mutations that are responsible for nerve insensitivity and ultimately pyrethrin or pyrethroid resistance by the knockdown resistance mechanism or phenotype. This method was found to perform in a manner not significantly different from that of the previously used SISAR method, allowing for intermethod comparisons. Our current and previously published data sets provide a plausible reason, in part, for the decrease in the overall effectiveness of the Nix formulation that is apparent from clinical studies published from 1998 to 2009. This reason is the parallel increase of kdr-type mutations in louse population in the United States over this same time interval. Given the extensive sampling done in the current study, it now appears that kdr-type mutations are widely and uniformly present in U.S. lice, and susceptible alleles at these loci are rare.
Supplementary Data
Supplementary data are available at Journal of Medical Entomology online.
Acknowledgments
This work was supported, in part, by a cooperative agreement (SKL01/PO#4700516733) from Sanofi Pasteur, Swiftwater, PA 18370. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The project could not have been completed without the help and organization of Kathleen McManus at The Shepherd Institute for Lice Solutions, West Palm Beach, FL, and the 71 volunteers, who collected the lice used in this research. We wholeheartedly thank each of them for their interest and efforts. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the sponsor.
References Cited
- Bainbridge C. V., Klein G. L., Neibart S. I., Hassman H., Ellis K., Manring D., Goodyear R., Newman J., Micik S., Hoehler F., et al. 1998. Comparative study of the clinical effectiveness of a pyrethrin-based pediculicide with combing versus a permethrin-based pediculicide with combing. Clin. Pediatr. 37: 17–22. [DOI] [PubMed] [Google Scholar]
- Brandenburg K., Deinard A. S., DiNapoli J., Englender S. J., Orthoefer J., Wagner. D. 1986. 1% Permethrin cream rinse vs 1% lindane shampoo in treating Pediculosis capitis. Am. J. Dis. Child. 140: 894–896. [DOI] [PubMed] [Google Scholar]
- Burkhart C. G., Burkhart. C. N. 2000. Clinical evidence of lice resistance to over-the-counter products. J. Cutan. Med. Surg. 4: 199–201. [DOI] [PubMed] [Google Scholar]
- Carson D. S., Tribble P. W., Weart. C. W. 1988. Pyrethrins combined with piperonyl butoxide (RID) vs 1% permethrin (NIX) in the treatment of head lice. Am. J. Dis. Child. 142: 768–769. [DOI] [PubMed] [Google Scholar]
- Clark J. M. 2009. Determination, mechanism and monitoring of knockdown resistance in permethrin-resistant human head lice, Pediculus humanus capitis. J. Asia Pac. Entomol. 12: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. M., Yoon K. S., Lee S. H., Pittendrigh. B. R. 2013. Human lice: Past, present and future control. Pectic. Biochem. Physiol. 106: 162–171. [Google Scholar]
- DiNapoli J. B., Austin R. D., Englender S. J., Gomez M. P., Barrett. J. F. 1988. Eradication of head lice with a single treatment. Am. J. Public Health 78: 978–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong K., Scott. J. G. 1994. Linkage of kdr-type resistance and the para-homologous sodium channel gene in German cockroaches (Blattella germanica). Insect Biochem. Mol. Biol. 24: 647–654. [DOI] [PubMed] [Google Scholar]
- Durand R., Bouvresse S., Berdjane Z., Izri A., Chosidow O., Clark. J. M. 2012. Insecticide resistance in head lice: clinical, parasitological and genetic aspects. Clin. Microbiol. Infect. 18: 338–344. [DOI] [PubMed] [Google Scholar]
- Farnham A. W. 1977. Genetics of resistance of house fly (Musca domestica) to pyrethroids. I. Knock-down resistance. Pestic. Sci. 8: 631–636. [Google Scholar]
- Gao J. -R., Yoon K. S., Lee S. H., Takano-Lee M., Edman J. D., Meinking T. L., Taplin D., Clark. J. M. 2003. Increased frequency of the T929I and L932F mutations associated with knockdown resistance in permethrin-resistant populations of the human head louse, Pediculus capitis, from California, Florida, and Texas. Pestic. Biochem. Physiol. 77: 115–124. [Google Scholar]
- Gratz N. G. 1997. Human Lice: Their Prevalence, Control and Resistance to Insecticides, vol. WHO/CTD/WHOPES/97.8. World Health Organization, Switzerland. [Google Scholar]
- Hipolito R. B., Mallorca F. G., Zuniga-Macaraig Z. O., Apolinario P. C., Wheeler-Sherman. J. 2001. Head lice infestation: single drug versus combination therapy with one percent permethrin and trimethoprim/sulfamethoxazole. Pediatrics 107: E30. [DOI] [PubMed] [Google Scholar]
- Hodgdon H. E., Yoon K. S., Previte D. J., Kim H. J., Aboelghar G. E., Lee S. H., Clark. J. M. 2010. Determination of knockdown resistance allele frequencies in global human head louse populations using the serial invasive signal amplification reaction. Pest Manag Sci 66: 1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. 2011. Biostatistics team leader memorandum. US Department of Health and Human Services. Center for Drug Evaluation and Research., (http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022408Orig1s000StatR.pdf) last accessed March 05, 2016. [Google Scholar]
- Knipple D. C., Doyle K. E., Marsella-Herrick P. A., Soderlund. D. M. 1994. Tight genetic linkage between the kdr insecticide resistance trait and a voltage-sensitive sodium channel gene in the house fly. Proc. Natl. Acad. Sci. USA 91: 2483–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon D. H., Yoon K. S., Strycharz J. P., Clark J. M., Lee. S. H. 2008. Determination of permethrin resistance allele frequency of human head louse population by quantitative sequencing. J. Med. Entomol. 45: 912–920. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Yoon K. S., Williamson M. S., Goodson S. J., Takano-Lee M., Edman J. D., Devonshire A. L., Clark. J. M. 2000. Molecular analysis of kdr-like resistance in permethrin-resistant strains of head lice, Pediculus capitis. Pestic. Biochem. Physiol. 66: 130–143. [Google Scholar]
- Lee S. H., -. Gao J., Yoon K. S., Mumcuoglu K. Y., Taplin D., Edman J. D., Takano-Lee M., Clark. J. M. 2003. Sodium channel mutations associated with knockdown resistance in the human head louse, Pediculus capitis (De Geer). Pestic. Biochem. Physiol. 75: 79–91. [Google Scholar]
- Lee S. H., Clark J. M., Ahn Y. J., Lee W. J., Yoon K. S., Kwon D. H., Seong. K. M. 2010. Molecular mechanisms and monitoring of permethrin resistance in human head lice. Pectic. Biochem. Physiol. 97: 109–114. [Google Scholar]
- Meinking T. L., Vicaria M., Eyerdam D. H., Villar M. E., Reyna S., Suarez. G. 2004. Efficacy of a reduced application time of Ovide lotion (0.5% malathion) compared to Nix creme rinse (1% permethrin) for the treatment of head lice. Pediatr Dermatol. 21: 670–674. [DOI] [PubMed] [Google Scholar]
- Meinking T. L., Vicaria M., Eyerdam D. H., Villar M. E., Reyna S., Suarez. G. 2007. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Creme Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr. Dermatol. 24: 405–411. [DOI] [PubMed] [Google Scholar]
- Meinking T. L., Villar M. E., Vicaria M., Eyerdam D. H., Paquet D., Mertz-Rivera K., Rivera H. F., Hiriart J., Reyna. S. 2010. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (Pediculosis humanus capitis). Pediatr. Dermatol. 27: 19–24. [DOI] [PubMed] [Google Scholar]
- Pariser D. M., Meinking T. L., Bell M., Ryan. W. G. 2012. Topical 0.5% ivermectin lotion for treatment of head lice. N. Engl. J. Med. 367: 1687–1693. [DOI] [PubMed] [Google Scholar]
- Pittendrigh B. R., Clark J. M., Lee S. H., Yoon K. S., Steele L., Seong K. M. 2015. Body lice: From the genome project to functional genomics and reverse genetics, pp. 1–18Raman C., Goldsmith M. R., Agunbiade T. A. (eds.), Short views on insect genomics and proteomics, vol. 1 Springer International Publishing; Switzerland. [Google Scholar]
- Raoult D., Roux. V. 1999. The body louse as a vector of reemerging human diseases. Clin. Infect. Dis. 29: 888–911. [DOI] [PubMed] [Google Scholar]
- Stough D., Shellabarger S., Quiring J., Gabrielsen A. A. 2009. Efficacy and safety of spinosad and permethrin creme rinses for Pediculosis capitis (head lice). Pediatrics 124: e389–e395. [DOI] [PubMed] [Google Scholar]
- Taplin D., Meinking T. L., Castillero P. M., Sanchez. R. 1986. Permethrin 1% creme rinse for the treatment of Pediculus humanus var capitis infestation. Pediatr. Dermatol. 3: 344–348. [DOI] [PubMed] [Google Scholar]
- Williamson M. S., Denholm I., Bell C. A., Devonshire. A. L. 1993. Knockdown resistance (kdr) to DDT and pyrethroid insecticides maps to a sodium channel gene locus in the housefly (Musca domestica). Mol. Gen. Genet. 240: 17–22. [DOI] [PubMed] [Google Scholar]
- Yoon K. S., Symington S. B., Lee S. H., Soderlund D. M., Clark. J. M. 2008. Three mutations identified in the voltage-sensitive sodium channel a-subunit gene of permethrin-resistant human head lice reduce the permethrin sensitivity of house fly Vssc1 sodium channel expressed in Xenopus oocytes. Insect Biochem. Mol. Biol. 38: 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. S., Previte D. J., Hodgdon H. E., Poole B. C., Kwon D. H., El-Ghar G. E., Lee S. H., Clark. J. M. 2014. Knockdown resistance allele frequencies in North American head louse (Anoplura: Pediculidae) populations. J. Med. Entomol. 51: 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.