Abstract
Background
Therapeutic human papillomavirus (HPV) vaccines are currently being developed. However, no therapeutic efficacy has been achieved in clinical trials for the treatment of cervical intraepithelial neoplasia or cancer. One of the important issues in increasing vaccine efficacy is determining the best way to enhance tumor antigen-specific cellular immune responses. This study aimed to explore the virus-like particles (VLPs) of hepatitis B core antigen (HBcAg) as potential therapeutic vaccine carriers and to assess its immunological characteristics.
Methods
Chimeric VLPs presenting a HPV 16 cytotoxic T lymphocytes epitope E749–57 (amino acid 49–57 of the E7 protein) were prepared using recombinant genes. C57BL/6 mice were immunized with VLPs and grafted with tumor cells TC-1 which is an E7-expressing tumorigenic cell line. The dynamic tumor growth was monitored and anti-tumor immune responses were investigated.
Results
Using a preventive strategy, immunization with VLPs resulted in nearly complete suppression of tumor growth. In treatment studies, VLP immunization significantly suppressed the tumor progression in mice carrying 2–3 mm tumors and in those bearing even larger tumors with diameters up to 8–9 mm. The VLP structure was shown to be important to induce vigorous antitumor immunity and effects. In immunized mice, enhanced E749–57-specific cellular immune responses were evidenced by increased interferon (IFN)-γ expression and decreased interleukin (IL)-4 expression in splenic lymphocytes, as well as an elevated number of effector cells expressing IFN-γ in response to the in vitro stimulation of the specific peptide E749–57. In addition, effective immune memory after VLP immunization was maintained for at least 16 weeks, preventing significant tumor growth after subsequent TC-1 challenge.
Conclusion
While VLPs were highly immunogenic in stimulating humoral immunity, our results strongly indicated that VLPs, such as HBcAg particles, might also be potent therapeutic vaccine carriers to elicit robust cellular immune responses, even in the immunosuppressive microenvironment of a tumor.
Keywords: cellular immune responses, human papillomavirus, tumor, therapeutic vaccine, virus-like particles
Introduction
Persistent infection with high-risk types of human papillomavirus (HPV) causes human malignancies, including cervical, anal, vulvar, vaginal, and penile cancers, as well as no-genital head and neck cancers.1,2 Cervical cancer is the second most common cause of cancer death in women worldwide, and more than 70% of cases are attributed to HPV types 16 and 18. Recently, three commercially available prophylactic HPV vaccines, which are virus-like particles (VLPs) composed of major capsid protein L1, have been shown to induce effective antibody responses and prevent infection with the most prevalent high-risk types of HPV, such as HPV 16 and 18. However, these vaccines do not exert any therapeutic effects on established infection and neoplasia.3
Therapeutic HPV vaccines, which aim to generate specific cellular immune responses and kill virus-infected cells directly, are urgently needed for the treatment of HPV-associated diseases. HPV E6 and E7 oncoproteins encoded by early genes appear to be the most ideal target molecules for developing effective therapeutic vaccines because they are constitutively expressed in HPV-infected and cancerous cells.4 Numerous strategies to effectively enhance antitumor immunity have been employed in the development of therapeutic vaccines,5 including the identification and modification of potent antigens;6,7 the improvement of antigen uptake, processing, and presentation;8 the use of different vaccine forms, such as peptide/protein, nucleic acids, virus or bacterial vectors;3 the optimization of the vaccine delivery and immunization procedure;9,10 and the development and application of adjuvants.11,12 However, to date, no therapeutic efficacy has been achieved in clinical trials for the treatment of cervical intraepithelial neoplasia or cancer.3
The induction of vigorous tumor-specific cellular immunity, especially under the immunosuppressive microenvironment of an established tumor, is the fundamental requirement for a potential vaccine against tumors. Employing potent carriers to enhance the immunogenicity of a specific antigen is an important vaccinology approach. Hepatitis B virus core antigen (HBcAg) has the ability to efficiently self-assemble into VLPs in Escherichia coli cells,13 and the inherent features of the VLP structure make it highly immunogenic and well equipped to generate high titers of a specific antibody.14 HBcAg VLPs have been broadly used as powerful vaccine carriers for heterogeneous antigens to induce desired humoral immune responses.15 Furthermore, HBcAg VLPs presenting self molecules and peptides could evade immune tolerance, induce specific autoantibodies, and successfully intervene with the pathological effects of dysfunctional target molecules in disease models.16,17 However, whether HBcAg VLPs are likely to be used as a therapeutic vaccine carrier and whether they facilitate antigen-specific cellular immune responses have not yet been well identified.
In this study, we sought to investigate whether HBcAg VLPs carrying a model cytotoxic T lymphocyte (CTL) epitope of HPV16 E7 would elicit potent tumor-specific cellular immune responses and effectively suppress the development of tumors in mice, thus demonstrating the potential of HBcAg VLPs to serve as a vaccine carrier for the development of effective therapeutic vaccines.
Materials and methods
Mice and cell line
Female C57BL/6 mice (6–8 weeks; 16–18 g; SCXK [Beijing] 2012–0001) were purchased from Vital River Laboratory Animal Technology Ltd. (Beijing, People’s Republic of China). All mice were kept under specific pathogen-free conditions in the Central Animal Care Services of the Institute of Medical Biology, Chinese Academy of Medical Sciences, and Peking Union Medical College, People’s Republic of China. All animal experiments were approved by the Animal Ethics Committee of Institute of Medical Biology.
A previously reported HPV16 CTLs epitope E749–57 (49RAHYNIVTF57) was synthesized by GL Biochem Ltd. (Shanghai, People’s Republic of China). The TC-1 tumor cell line, which was derived from primary lung epithelial cells of a C57BL/6 mouse cotransformed with HPV-16 oncoproteins E6 and E7 and c-Ha-ras oncogene, was purchased from Tumor Center of the Chinese Academy of Medical Sciences. The cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 with 10% fetal bovine serum.
Expression and purification of chimeric VLPs presenting the E749–57 peptide
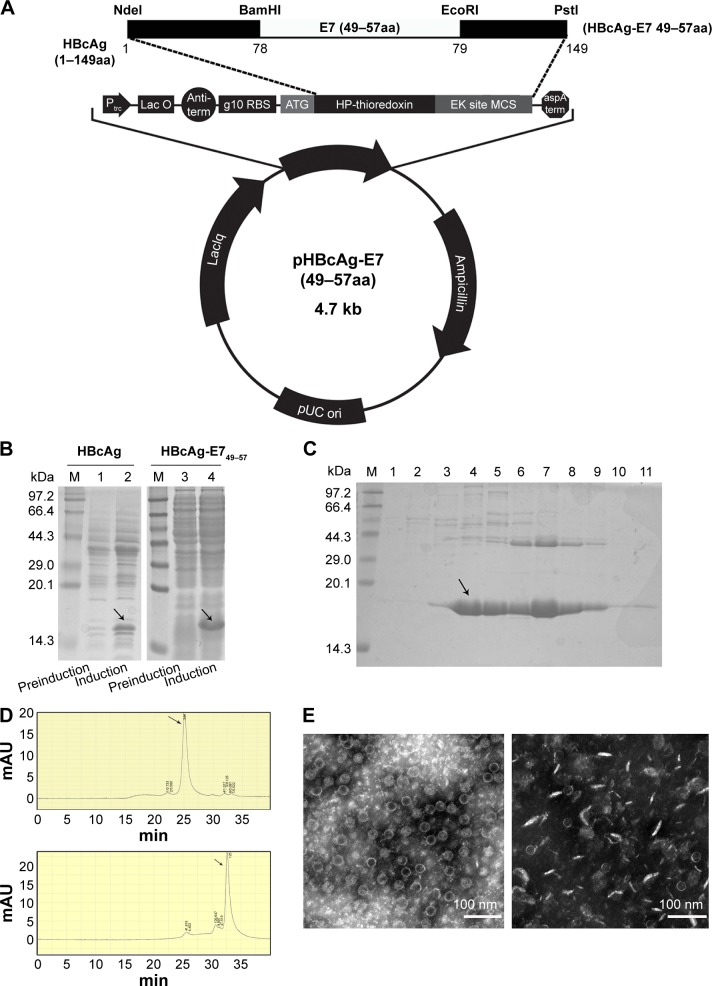
The oligonucleotides encoding E749–57 (plus strand: 5′-gatct CGTGCTCACTACAACATCGTTACCTTCggatccggtg-3′; minus strand: 5′-aattcaccggatccGAAGGTAACGATGTTGT AGTGAGCACGa-3′) were synthesized by Sangon Biotech Ltd. (Shanghai, People’s Republic of China). The coding region is shown in capitals and spanned by adaptor sequences for insertion into a vector plasmid. The vector was previously constructed by introducing BamHI and EcoRI recognition sequences between codes for amino acids 78 and 79 of truncated HBcAg (1–149 amino acid, GenBank accession number: GQ 377581) (Figure 1A) in plasmid pThioHisA (Thermo Fisher Scientific, Waltham, MA, USA).18 The mixture of equal amounts of two synthesized oligonucleotides were subjected to denaturation and renaturation treatment, and acquired duplex chains of DNA fragments were ligated with vector digested with BamHI and EcoRI. The constructed recombinant plasmids were identified and transformed into DH5α cells for the induction of chimeric HBcAg-E749–57. The recombinant proteins were purified with a previously established procedure consisting of ammonium sulfate precipitation and 10%–50% sucrose density gradient ultracentrifugation.19 Eleven 1 mL fractions were collected from the top to bottom of the ultracentrifugation tube. The purified recombinant proteins were analyzed with high-performance liquid chromatography with size-exclusion chromatography (HPLC-SEC) (SRT SEC-1000, 7.8×300 mm 5 μm; Sepax Technologies, Inc., Newark, DE, USA) at a flow rate of 0.2 mL/minute to identify the purity and VLP structure of HBcAg-E749–57. Furthermore, negative-stain electron microscopy was used to visualize the morphology of VLPs.
Figure 1.
The preparation and identification of chimeric HBcAg VLPs that present HPV 16 E749–57.
Notes: (A) Plasmid mapping of a recombinant plasmid expressing HBcAg-E749–57. (B) Expression of recombinant proteins analyzed by SDS-PAGE using total cell lysates. M, Protein Marker; lanes 1 and 3, without induction; lanes 2 and 4, induction with IPTG for 4 hours; arrows point to the expressed recombinant proteins. (C) SDS-PAGE analysis of samples collected from the sucrose density gradient ultracentrifugation of HBcAg-E749–57; the sample loaded onto the density gradient centrifugation was previously subjected to precipitation with ammonium sulfate; lanes 1–11, eleven 1 mL fractions collected from ultracentrifugation from top to bottom. (D)The purity and morphology of the recombinant protein were analyzed by HPLC-SEC; upper figure, fraction 7 from density gradient centrifugation, and the arrows point to the peak of the eluted protein; lower figure, fraction 4. (E) Electronic microscopy of HBcAg-E749–57 VLPs from fraction 7 of sucrose density gradient centrifugation (left) and non-VLPs of HBcAg-E749–57 from fraction 4 (right).
Abbreviations: HBcAg, hepatitis B core antigen; VLPs, virus-like particles; HPLC-SEC, high-performance liquid chromatography with size-exclusion chromatography; IPTG, isopropyl β-d-1-thiogalactopyranoside; min, minutes; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Tumor challenge and mouse immunization
To establish the grafted tumor model, 1×105 TC-1 cells mixed with Basement Membrane Matrix (BD Biosciences, San Jose, CA, USA) were injected subcutaneously (sc) into the right flank of the mice.
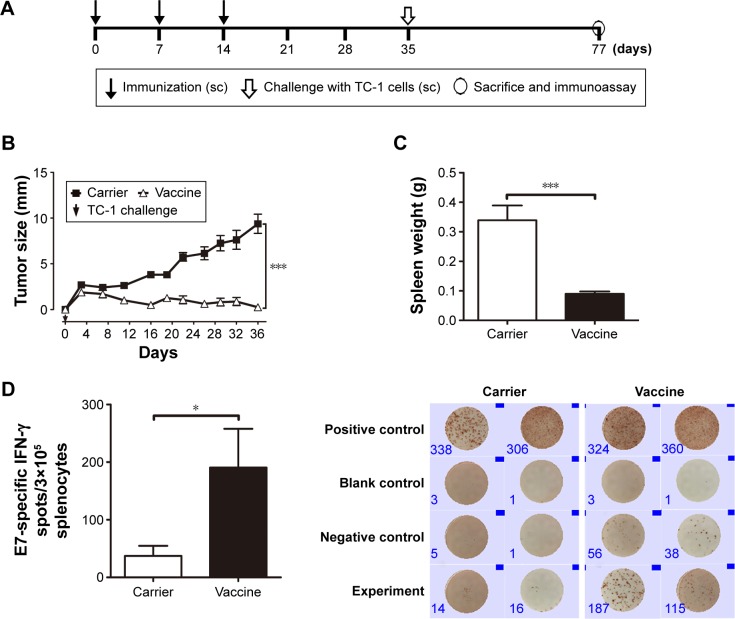
To evaluate the immunological characteristics of HBcAg-E749–57 VLPs without the influence of the tumor microenvironment, a preventive experiment was performed (Figure 2A). Mice were first immunized sc with 50 μg of either the carrier HBcAg or the vaccine HBcAg-E749–57 VLPs three times at an interval of 7 days; then the mice were challenged with TC-1 cells 3 weeks after the last immunization (n=8 mice per group).
Figure 2.
Preventive immunization with HBcAg-E749–57 VLPs significantly prevents the development of grafted TC-1 tumors in mice.
Notes: (A) Protocol – mice were immunized with the vaccine first and then challenged with TC-1 tumor cells; (B) changes in tumor size; one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test, ***P<0.001, n=8 per group; (C) spleen weight; unpaired Student’s t-test, *P<0.05, n=8 per group; (D) E749–57-specific IFN-γ-expressing lymphocytes detected by ELISPOT, n=5 per group; representative picture is shown on the right.
Abbreviations: HBcAg, hepatitis B core antigen; sc, subcutaneous; IFN-γ, interferon-γ; ELISOPT, enzyme-linked immunospot assay; VLPs, virus-like particles.
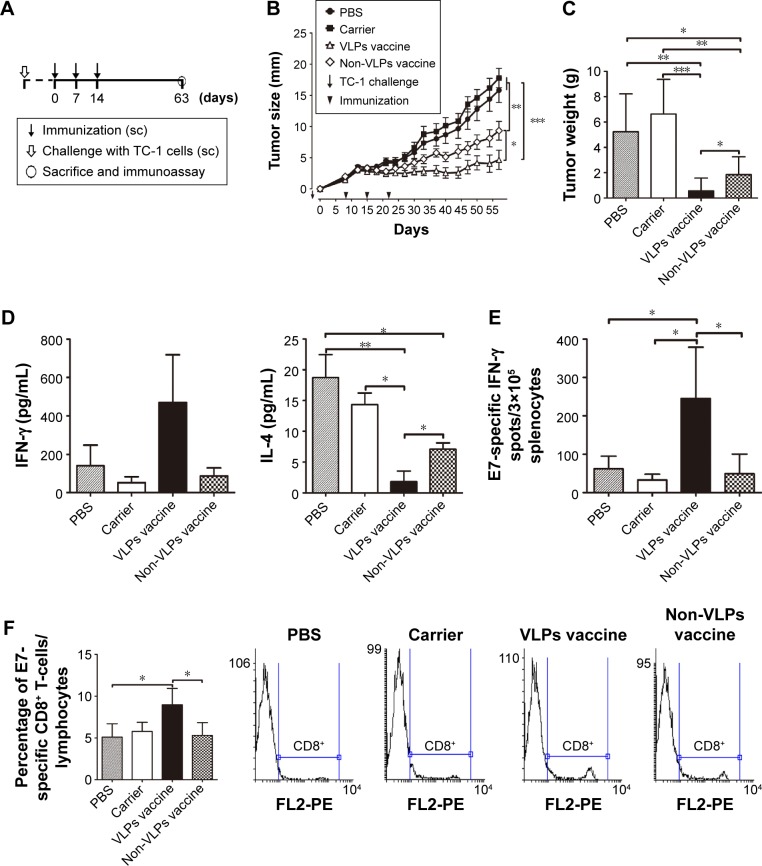
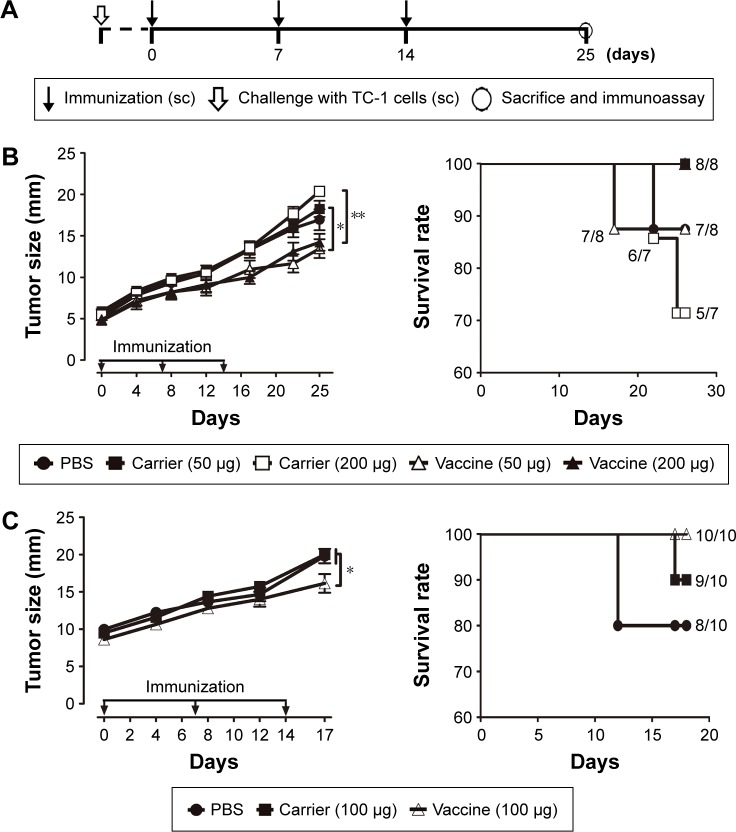
To assess the potential of VLP-based vaccines to intervene with the growth of established tumors, treatment studies were performed. Mice were inoculated with TC-1 cells, and immunization with the vaccine was initiated when the tumor diameter reached 2–3, 5–6, or 8–9 mm in our three separate experiments. Mice that underwent treatment for tumor of size 2–3 mm (Figure 3A) were divided into four groups and received 50 μg VLP vaccine, 50 μg non-VLP vaccine, 50 μg carrier, or phosphate-buffered saline (PBS) (n=9 per group). Both the VLP vaccine and the non-VLP vaccine were recombinant chimeric HBcAg with the E749–57 peptide inserted between amino acid 79 and 80, showing different conformations (VLPs versus non-VLPs). Theoretically, equal amounts of VLPs or non-VLPs proteins, as determined by protein concentration measured by the Bradford method, contained equal amounts of E749–57 peptide. Mice that underwent treatment for tumor of size 5–6 mm (Figure 4A) were divided into five groups and received PBS, 50 μg vaccine, 50 μg carrier, 200 μg vaccine, or 200 μg carrier (n=7–8 per group). Mice that underwent treatment for tumor of size 8–9 mm (Figure 4A) were divided into three groups and received PBS, 100 μg vaccine, or 100 μg carrier (n=10 per group).
Figure 3.
Therapeutic immunization with HBcAg-E749–57 significantly prevents the development of grafted TC-1 tumors with a diameter of 2–3 mm in mice.
Notes: (A) Protocol – the vaccine was administered when the tumors grew to a diameter of 2–3 mm. (B) Dynamic measurement of tumor size (n=9 per group). (C) Tumor weight (n=9 per group). (D) ELISA analysis of cytokines in the culture supernatant of isolated splenocytes simulated with E749–57 (n=5 per group). (E) IFN-γ-expressing splenocytes detected by ELISPOT after stimulation by E749–57 (n=5 per group). (F) CD8+ lymphocytes detected by flow cytometry (n=4 per group). One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test, *P<0.05, **P<0.01, ***P<0.01.
Abbreviations: HBcAg, hepatitis B core antigen; IFN-γ, interferon-γ; sc, subcutaneous, PBS, phosphate-buffered saline; VLPs, virus-like particles; ELISA, enzyme-linked immunosorbent assay; ELISOPT, enzyme-linked immunospot assay.
Figure 4.
Therapeutic immunization with HBcAg-E749–57 VLPs suppresses the growth of grafted TC-1 tumors in mice, even with a larger tumor mass.
Notes: (A) Protocol – the vaccine was administered when the tumors grew to a diameter of 5–6 or 8–9 mm. (B) tumor size after treating TC-1 tumors with a diameter of up to 5–6 mm (n=7–8 per group). (C) Tumor size after treating TC-1 tumors with a diameter of up to 8–9 mm (n=10 per group). The numbers presented with the symbol “/” in the panels of survival rate of (B) and (C) denote how many mice showing no signs of suffering and less than 20 mm of tumor size in total mice in each group. One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test, *P<0.05, **P<0.01. TC-1 is an E7-expressing tumorigenic cell line.
Abbreviations: sc, subcutaneous; HBcAg, hepatitis B core antigen; VLPs, virus-like particles; PBS, phosphate-buffered saline.
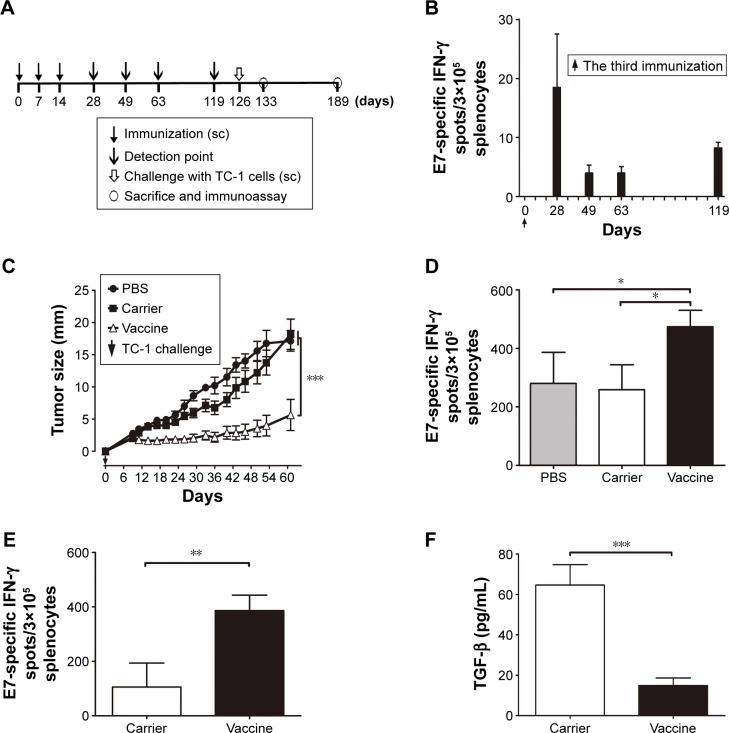
To investigate the effective antitumor immune memory induced by VLP-based vaccines, mice were immunized sc with HBcAg-E749–57 VLPs (50 μg per mouse, n=26) three times with an interval of 1 week, followed by a 4-month rest, and then challenged sc with TC-1 tumor cells. Carrier and PBS were administered instead of the vaccine as controls (n=10 per group). At designated time points, four mice that received the vaccine were sacrificed and E749–57-specific cellular immunity was assessed by ELISPOT assay (Figure 5A).
Figure 5.
Immunization with HBcAg-E749–57 VLPs elicits potent antitumor cellular immune memory in mice.
Notes: (A) Protocol – mice were immunized with the vaccine first, followed by challenge with TC-1; carrier and PBS were administered instead of the vaccine as controls. (B) Dynamic detection of IFN-γ-expressing lymphocytes by ELISPOT after stimulation by E749–57; n=4 per each time point. (C) Tumor size; one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparisons test; ***P<0.001; n=10. (D) IFN-γ-expressing lymphocytes determined by ELISPOT 1 week after TC-1 challenge; one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test; *P<0.05; n=4. (E) IFN-γ-expressing lymphocytes determined by ELISPOT at 9 weeks after TC-1 challenge; unpaired Student’s t-test; **P<0.01; n=6. (F) TGF-β1 concentration in the supernatant of E749–57 stimulated splenocytes was measured with ELISA 9 weeks after TC-1 challenge; unpaired Student’s t-test; ***P<0.001, n=10.
Abbreviations: PBS, phosphate-buffered saline; HBcAg, hepatitis B core antigen; IFN-γ, interferon-γ; VLPs, virus-like particles; sc, subcutaneous; TGF, transforming growth factor; ELISA, enzyme-linked immunosorbent assay; ELISOPT, enzyme-linked immunospot assay.
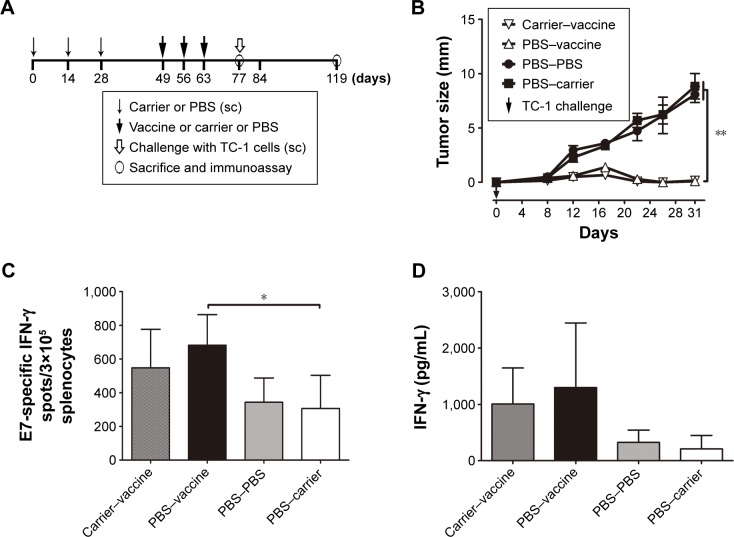
To investigate whether preexisting anti-HBcAg antibodies affect the efficacy of therapeutic vaccines employing HBcAg VLPs as carriers, mice were divided into four groups (n=7 per group) and they received different treatments as shown in Table 1 and Figure 6A.
Table 1.
The groups of mice and the administered treatments
| Groups | Day
|
|||
|---|---|---|---|---|
| 0, 14, and 28 | 49, 56, and 63 | 77 | 119 | |
| Carrier–vaccine | Carriera | Vaccineb | TC-1 | Sacrifice and immunoassay |
| PBS–vacci ne | PBSa | Vaccineb | TC-1 | |
| PBS–PBS | PBSa | PBSb | TC-1 | |
| PBS–carrier | PBSa | Carrierb | TC-1 | |
Notes:
The carrier was immunized sc to induce the anti-HBcAg antibody, and PBS was administered as a control.
HBcAg-E749–57 was immunized sc to induce E749–57-specific antitumor immunity, and carrier and PBS were administered as controls.
Abbreviations: sc, subcutaneous; HBcAg, hepatitis B core antigen; PBS, phosphate-buffered saline.
Figure 6.
Preexisting anti-HBcAg antibodies did not exert a significant influence on HBcAg-E749–57-elicited suppressive effects on tumor growth in TC-1 cell-grafted mice.
Notes: (A) Protocol; (B) tumor size; one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test; **P<0.01; n=7; (C) IFN-γ-expressing lymphocytes detected by ELISPOT after stimulation by E749–57; ANOVA followed by Tukey’s multiple comparisons test; *P<0.05; n=4; (D) IFN-γ concentration in the supernatant of E749–57 stimulated splenocytes measured with ELISA.
Abbreviations: HBcAg, hepatitis B core antigen; IFN-γ, interferon-γ; PBS, phosphate-buffered saline; sc, subcutaneous; ELISA, enzyme-linked immunosorbent assay; ELISOPT, enzyme-linked immunospot assay.
Tumor growth was monitored periodically (twice a week) using a slide caliper. Mice were euthanized when the tumor diameter reached ~20 mm or sooner if the animals showed any signs of suffering, at which times the humane endpoints of the experiments were met based on animal care regulations. At the end of each experiment, the tumor mass and spleen were carefully isolated and weighed.
The enzyme-linked immunosorbent assay (ELISA)
Spleens were removed from mice and dispersed using a 70 μm cell strainer (Becton, Dickinson and Company FalconTM, USA). Splenocytes were plated into 96-microwell plates at 3×105 cells per well and incubated with RPMI-1640 containing 5 μg/mL E749–57 peptide at 37°C and 5% CO2 for 3 days. The culture supernatant was collected and the concentrations of interferon (IFN)-γ and interleukin (IL)-4 were detected using ELISA. The paired capture antibody and biotinylated detection antibody were purchased from (Affymetrix eBioscience, Inc., San Diego, CA, USA), and the assay was performed according to the manufacturer’s instruction. Briefly, capture antibodies of IFN-γ or IL-4 were coated onto microplates at 4°C overnight, and then the cell supernatants were added and incubated for 1 hour at room temperature, followed by the incubation of biotinylated detection antibodies of IFN-γ or IL-4 for another 1 hour. Alkaline phosphatase–conjugated avidin was then added and incubated for half an hour, and the reaction was developed with the substrate tablet (Sigma-Aldrich Co., St Louis, MO, USA).
Enzyme-linked Immunospot Assay (ELISPOT) assay
Antigen-specific IFN-γ-producing splenocytes were detected using a commercial ELISPOT kit (cat#: DKW22-2000-048) (DAKEWE Biotechnology Co., Ltd., Shenzhen, People’s Republic of China) with a pulse of E749–57 stimulation. The assay was performed following the manufacturer’s instructions. Briefly, splenocytes were plated into an anti-mouse IFN-γ capture antibody-precoated 96-well ELISPOT plate at 3×105 cells per well with 5 μg/mL E749–57 peptide, phorbol myristate acetate (PMA)/ionomycine, or media only, respectively, and then incubated for 16–20 hours at 37°C and 5% CO2. After the cells were washed, a biotinylated anti-mIFN-γ detection antibody was added and incubated for 1 hour, followed by incubation with horseradish peroxidase (HRP)-conjugated avidin. Finally, the AEC substrate was used to develop the plate. Spots were counted using an ELISPOT reader system (AID Diagnostika GmbH, Straßberg, Germany), and the results were calculated as the mean number of IFN-γ positive cells per 3×105 splenocytes ± standard error of mean (SEM).
Flow cytometry
Splenocytes were plated into a 48-microwell plate at 1×106 cells per well. The cell suspensions were incubated with fluorochrome-conjugated anti-CD8a monoclonal antibody (eBioscience) and analyzed by FACS Arial (BD Bioscience). The data were analyzed using Flowing Software 2.5.1, Turku, Finland.
Statistical analysis
The significance of the differences between experimental groups was analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test or unpaired Student’s t-test (GraphPad Prism 5.0, GraphPad Software, Inc., La Jolla, CA, USA). Values were reported as the mean ± SEM.
Results
Expression and purification of chimeric VLPs
The recombinant proteins–truncated HBcAg (named carrier) and chimeric HBcAg-E749–57 (named vaccine) were expressed efficiently in E. coli cells, analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Figure 1B). The electrophoresis analysis of collected fractions from sucrose density gradient ultracentrifugation showed that the vaccine HBcAg-E749–57 and the carrier HBcAg had identical patterns, which is that the target proteins appeared mainly in fractions 4 to 9 (HBcAg-E749–57 shown in Figure 1C). The purity and morphology of recombinant proteins were identified by HPLC-SEC (Figure 1D). A difference between VLP and non-VLP fractions was observed: VLPs mainly were present in the fractions 7–9 collected by ultracentrifugation, which were eluted at 25 minutes, and non-VLP components were mainly present in fractions 4–6 that were eluted at 32.5 minutes. The VLP structure was further identified by electron microscopy (Figure 1E). The recombinant vaccine protein HBcAg-E749–57 was assembled into VLPs similar to the carrier protein HBcAg, which are hollow particles with a diameter of ~22–27 nm.
Preventive immunization with HBcAg-E749–57 VLPs significantly prevents the development of grafted TC-1 tumors in mice
Preventive immunization analysis was conducted before TC-1 challenge was performed (Figure 2A). Continuous measurement of tumor size showed that mice immunized with HBcAg-E749–57 VLPs exhibited significantly suppressed tumor growth compared to mice that received the carrier (HBcAg VLPs) (Figure 2B). At the experimental endpoint, the tumor grew to ~10 mm in diameter in control mice, while tumor growth was hardly detectable in vaccinated mice. Spleens were collected and weighed, and the results showed that the vaccinated mice had significantly smaller spleens than the control mice (0.09±0.008 g versus 0.34±0.05 g; P<0.001; n=8) (Figure 2C), indicating the dramatically suppressed proliferation of immune cells stimulated by tumor growth. ELISPOT was performed to reflect tumor-specific in vivo cellular immune responses. Splenocytes that received polyclonal stimulation with PMA/ionomycine served as positive controls, which showed spots of IFN-γ-expressing lymphocytes; wells without the addition of cells served as controls and showed no spots. Without any stimulation, few splenocytes isolated from the vaccinated or carrier-immunized mice still showed IFN-γ-expression; however, the vaccinated mice showed clearly more spots than the carrier-vaccinated mice. Responding to the in vitro stimulation of the specific peptide E749–57, the numbers of IFN-γ-expressing lymphocytes were elevated dramatically, and the cells from the vaccinated mice showed more spots than those from the control mice that received the carrier (190.6±67.3 versus 37.17±17.70; P<0.05, n=5) (Figure 2D). This indicated that immunization with HBcAg-E749–57 VLPs elicited more robust tumor-specific cellular immune responses in vivo than administration of the carrier as a control.
Therapeutic immunization with HBcAg-E749–57 significantly prevents the development of grafted TC-1 tumors 2–3 mm in diameter
Because a preventive study proved that HBcAg-E749–57 elicited effective tumor-specific immune responses, a tumor size of 2–3 mm was further treated to assess the potential of VLP immunization to intervene with tumor growth (Figure 3A). Two-month monitoring of changes in tumor size showed that HBcAg-E749–57 VLPs significantly suppressed tumor growth in the vaccinated mice compared to the control mice that received carrier or PBS (Figure 3B). After mice were sacrificed, the tumors were isolated and weighed, and the data obtained were consistent with those obtained from the measurement of tumor size. The vaccinated mice (0.56±0.34 for VLP vaccines, n=9 and 1.85±0.47 for non-VLPs vaccines, n=9) showed a significantly smaller tumor mass (grams) than the control mice (5.24±0.99 for PBS, n=9 and 6.63±0.97 for carrier, n=9) and P<0.001 and P<0.01 for the VLP vaccine versus carrier or PBS, respectively (Figure 3C). Splenocytes isolated from the vaccinated mice showed higher production of the Th1/CTLs cytokine IFN-γ and a lower level of the Th2 cytokine IL-4 in the culture supernatants than those isolated from the control mice (P<0.05 and P<0.01 for the VLPs vaccine versus carrier or PBS, respectively, and P<0.05 for non-VLPs vaccine versus PBS, n=5), responding to in vitro stimulation of a specific peptide E749–57 (Figure 3D). Consistent with increased IFN-γ levels in the supernatant, the vaccinated mice exhibited more IFN-γ-expressing lymphocytes than the control mice (P<0.05, n=5; 245.2±59.63, 49.2±22.84, 32.75±7.58, and 62±16.38 for the VLPs vaccine, non-VLPs vaccine, carrier, and PBS, respectively), as detected by ELISPOT with stimulation by E749–57. Additionally, spots of IFN-γ-expressing lymphocytes were found in the cells isolated from mice that received carrier or PBS, and the numbers were similar, which indicates that E7-specific cellular immunity was elicited in response to the inoculation of tumor cells (Figure 3E). Further, flow cytometry assay indicated that immunization with HBcAg-E749–57 VLPs elicited more vigorous tumor-specific CD8+ immune cell responses than administration with carrier or PBS (P<0.05 for the VLPs vaccine versus PBS or non-VLPs vaccine, n=4; 8.97±0.98, 5.3±0.77, 5.78±0.55, and 5.1±0.8 for the VLPs vaccine, non-VLPs vaccine, carrier, and PBS, respectively).
To clarify whether VLP structure is critical for HBcAg-E749–57 to elicit effective antitumor cellular immunity, a purified HBcAg-E749–57 fraction with a non-VLPs structure was also used to immunize mice. The results demonstrated that although immunization with the non-VLPs vaccine significantly suppressed tumor growth compared to the carrier (P<0.01) and PBS (P<0.05), the effect was less efficient than that of VLPs immunization (P<0.05) (Figure 3B and C). The elevation of IFN-γ and the reduction of IL-4 levels in the culture supernatant of splenocytes (Figure 3D), an increase in the number of IFN-γ-expressing splenocytes (Figure 3E), and the enhancement of CD8+ immune cell responses (Figure 3F) in response to stimulation with E749–57 were less significant in non-VLP-immunized mice than in VLP-immunized mice.
Therapeutic immunization with HBcAg-E749–57 VLPs significantly suppresses the growth of grafted TC-1 tumor in mice with a larger tumor mass
Immunization with HBcAg-E749–57 VLPs showed almost complete suppression of the growth of tumors in TC-1 grafted mice with a tumor diameter of 2–3 mm. To investigate whether the VLP vaccine had the potential to evade more severe immune suppression in the tumor microenvironment and induce an effective antitumor immune response, TC-1 grafted models with even larger tumor masses were employed. In one of our experiments, when the tumor grew up to a diameter of 5–6 mm, the tumor-bearing mice were divided randomly into five groups (n=7–8 per group) and administered different doses of HBcAg-E749–57 VLPs and the carrier HBcAg VLPs (50 or 200 μg per mouse) and PBS, respectively. The tumor-bearing mice that received HBcAg-E749–57 immunization showed significantly suppressed tumor growth (Figure 4B) as compared to the control mice. There was no significant difference between mice immunized with 50 and 200 μg of HBcAg-E749–57. There was one mouse in the group immunized with 50 μg HBcAg-E749–57 VLPs and the group receiving PBS respectively euthanized at day 17 and 22. In our another experiment, when the tumor grew to an even larger mass with a diameter of up to 8–9 mm, the tumor-bearing mice were divided randomly into three groups (n=10 per group) and administered HBcAg-E749–57 VLPs (100 μg per mouse), carrier (100 μg per mouse), and PBS, respectively. Mice immunized with HBcAg-E749–57 VLPs showed significantly suppressed tumor growth (Figure 4C) compared to those control mice that received either carrier or PBS. Two mice were euthanized at day 12 in the PBS group, and one mouse was euthanized at day 17 in the carrier group.
Immunization with HBcAg-E749–57 VLPs elicits potent antitumor cellular immune memory in mice
HBcAg-E749–57 VLP immunization prevented TC-1 tumor growth in both preventive and treatment studies, indicating that VLPs effectively elicit specific antitumor cellular immune responses. To further demonstrate the potential of VLPs to serve as a promising carrier platform for the development of therapeutic vaccines, the duration of effective anti-tumor immune memory induced by VLPs was investigated. Mice were immunized sc with HBcAg-E749–57 VLPs (50 μg per mouse) three times at an interval of 1 week, followed by a 4-month rest, and then challenged sc with TC-1 tumor cells (Figure 5A). During the rest period, the tumor-specific cellular immune response was checked by detecting E749–57-specific IFN-γ-expressing lymphocytes at designated time points by ELISPOT assay. The results showed that the response of IFN-γ-expressing lymphocytes to E749–57 stimulation was maintained for at least 4 months (Figure 5B). Immunization with HBcAg-E749–57 VLPs significantly prevented the development of grafted TC-1 tumors, as evidenced by a significantly smaller tumor mass in vaccinated mice compared to the control mice (Figure 5C). One week (day 133) after tumor administration (day 126), the splenocytes were isolated and analyzed by ELISPOT assay. The inoculation of tumor cells elicited antitumor cellular immunity, presented by significantly increased numbers of reactive spots representing E749–57-specific IFN-γ-secreting lymphocytes in mice that received carrier and PBS. However, the results strongly indicated that the vaccinated mice had more robust tumor-specific cellular immune responses (474±28.1, 258.3±43.1, and 280±53.26 for vaccine, carrier, and PBS, respectively; P<0.05 for the vaccine versus carrier and PBS; n=4) (Figure 5D). Even 9 weeks (day 189) after the administration of tumor cells, the splenocytes from vaccinated mice still showed more spots of IFN-γ-secreting lymphocytes than the carrier-immunized mice (386±56.9 for vaccine and 105.8±87.7 for carrier; P<0.01; n=6) (Figure 5E). In addition, transforming growth factor (TGF)-β1, which is generally a potent immunosuppressive cytokine, was significantly reduced in the culture supernatant of splenocytes stimulated with E749–57 (14.9±3.7 for vaccine and 64.7±10.1 for carrier; P<0.001; n=10) (Figure 5F). The results indicated that immunization with VLPs could elicit potent cellular immune memory, and the effectiveness of antitumor immunity lasted at least 16 weeks.
Preexisting anti-HBcAg antibodies did not produce significant influences on HBcAg-E749–57-elicited suppressive effects on tumor growth in TC-1 cell-grafted mice
Mice were immunized with HBcAg VLPs to induce fully established anti-carrier antibody responses, followed by HBcAg-E749–57 immunization and TC-1 cell challenge (Figure 6A). The results showed that in mice that received PBS or carrier, subsequent vaccine immunization significantly protected mice from TC-1 cell challenge, which was evidenced by almost completely suppressed tumor growth in both groups compared to mice that received PBS or carrier (Figure 6B). Further, ELISPOT assay showed that the number of E749–57-specific IFN-γ-producing cells in mice that received PBS–vaccine (682±90.8; n=4) was greater than the control mice that received PBS–PBS (343.3±71.8; n=4) or PBS–carrier (306±98.9; P<0.05; n=4) and showed no significant difference compared to the mice that received carrier–vaccine (547.8±114.2; n=4) (Figure 6C). It was consistent with the results of IFN-γ concentration detected with ELISA in the culture supernatant of E749–57-stimulated splenocytes (1,008±318.5, 1,299±573, 326.6±109, and 209.6±119.8 for carrier–vaccine, PBS–vaccine, PBS–PBS, and PBS–carrier, respectively) (Figure 6D). All the mice developed significant anti-HBcAg IgG response, and the titers ranged from 16,000 to 64,000. The results indicated that preexisting anti-HBcAg antibodies did not exert significant influences on HBcAg-E749–57-elicited antitumor immunity in mice.
Discussion
In our previous studies, HBcAg was successfully shown to be a potent carrier for the presentation of self molecules and antigenic peptides.17,19,20 The chimeric VLPs induced high titers and sustained specific autoantibodies to intervene in the pathological effects of dysfunctional cytokines in mouse models of asthma and inflammatory bowel diseases. It has been proven that as a vaccine carrier, HBcAg can be extremely effective in helping self antigens evade B-cell tolerance, even without the use of conventional adjuvants.16,17 The highly immunogenic features of HBcAg are attributed to the following characteristics: the structure of its nanoparticle, which facilitates their uptake by antigen-presenting cells (APCs),21 their ability to activate naive B-cells to function as APCs with 105-fold higher efficiency than typical dendritic cell and macrophage interactions,14 the unique highly ordered and repetitive arrays of inserted self-peptides (up to 240 copies for each VLP),13,22 and the presence of well-defined T-helper cell epitopes in the primary sequence.14,15 Furthermore, it was reported that nucleic acids packed into VLPs also significantly contribute to the immunogenicity of HBcAg VLPs as well as induced immune response types.23 In our previous study, the HBcAg carrier facilitated Th1-biased responses, which may be attributed to the nucleic acid content of VLPs. The nucleic acid immunostimulators were delivered directly into APCs by VLPs and efficiently contacted Toll-like receptors 7/8 and 9, enhancing major histocompatibility complex (MHC)-I-type antigen processing and presentation. Therefore, we are encouraged to investigate the immune characteristics of HBcAg VLPs in stimulating cellular immune responses and the potential to serve as a novel therapeutic vaccine carrier.
In this study, using the effective CTL epitope E749–57 as a model antigen,11 a HBcAg VLP-based vaccine was successfully prepared. In preventive and treatment studies, the VLPs immunization effectively stimulated E749–57-specific cellular immune responses, which was evidenced by elevated IFN-γ expression and increased numbers of IFN-γ-expressing lymphocytes in splenocytes stimulated specifically with E749–57 in vitro.
The grafted tumor in mice was used as a model to assess the efficacy of a therapeutic vaccine, and in most relevant studies, a tumor size of 2–3 mm was targeted.9,24 In the current study, immunization with HBcAg-E749–57 VLPs elicited almost complete suppression of the growth of tumors of diameter 2–3 mm. Considering that larger tumors might have a more severely immunosuppressive microenvironment, which may affect vaccine efficacy, we performed vaccinations when the tumor grew to ~5–6 or even 8–9 mm to further assess the potential of a VLP-based vaccine. Although the differences between the vaccine group and carrier group were not as significant as those in the study of small tumors (2–3 mm), the tumor growth was still suppressed significantly in the vaccinated mice. It is worth noting that the immunization of VLPs was conducted even without the use of any conventional adjuvant. The results cannot be simply extrapolated to clinical applications; however, they strongly indicated that HBcAg VLPs do exhibit potential as a promising carrier to facilitate antigen-specific cellular immunity. The results also implied the importance of regulating the tumor microenvironment for treating established tumors.
Whether assembly into VLPs is necessary for recombinant HBcAg-E749–57 to stimulate specific cellular immunity was assessed in the study. First, sucrose density centrifugation was employed to purify the recombinant proteins, and the results showed that recombinant HBcAg-E749–57 mainly appeared in the collected fractions 5–9, suggesting that the recombinant proteins formed polymer structures with larger buoyant density than the other proteins with even larger molecular weights. Using electron microscopy and HPLC, we further found that fractions 7–9 had exhibited the structure of VLPs as opposed to fractions 5–6. In the therapeutic study, tumor growth was more efficiently suppressed in mice immunized with VLPs than in those immunized with the non-VLP vaccine but polymerized HBcAg E749–57. Therefore, the structure of VLPs endowed the vaccine with a more powerful capacity to elicit antitumor immunity, and the possible causes may be attributed to the enhanced efficiency of VLP uptake by APCs as well as the simultaneous delivery of antigens and the immune stimulators in VLPs to APCs.14,15
In the current study, we evaluated IFN-γ, IL-4, and TGF-β1 expression in splenocytes stimulated by E749–57 and showed significantly elevated expression of IFN-γ and reduced levels of IL-4 and TGF-β1. IFN-γ is produced by activated Th1/CTLs cells and natural killer cells, which promotes dendritic Cells maturation as well as its interaction with T-cells. It activates macrophages, promotes the expression of MHC molecules and antigen presentation, induces the differentiation and proliferation of Th1 cells, and suppresses the differentiation and function of Th2 cells.25,26 IL-4 is a Th2-type cytokine produced by Th2 cells, macrophages, dendritic cells, mast cells, NKT, and natural killer cells. IL-4 activates transcription factor STAT6, stimulates the differentiation of Th0 into Th2 cells, and directly promotes the growth of tumor cells by suppressing cell apoptosis.27 TGF-β1 is a potent immune regulatory factor; it suppresses the proliferation, differentiation, and activation of immune cells; reduces the expression of MHC; and suppresses cytokine production. In the tumor, TGF-β1 promotes the migration and invasion of tumor cells and maintains tumor stem cells.28 In addition, TGF-β1 induces Th0 cells to differentiate into Tregs and even converts effector T-cells into Tregs, which suppress the activation of effector T-cells by direct interaction or the secretion of mediators such as TGF-β1 and IL-10.28,29 In summary, IFN-γ, IL-4, and TGF-β1 are closely associated with the effects of Th1/CTLs, Th2, and Tregs, respectively. Our results strongly indicated that immunization with a HBcAg VLP-based vaccine produced robust antitumor Th1/CTL responses and might reduce Th2 and Tregs-related immunosuppressive responses.
The duration of antitumor immunity elicited by VLPs is an important indicator of the success of VLPs as carriers of therapeutic vaccines. This study demonstrated that at least 16 weeks after the last immunization with VLPs, IFN-γ-expressing lymphocytes responding to E749–57 stimulation were still present in splenocytes isolated from the vaccinated mice. Once they underwent challenge with TC-1 tumor cells, the numbers of IFN-γ-expressing lymphocytes and the IFN-γ expression level in splenocytes were dramatically elevated, accompanied by significantly suppressed tumor growth. Therefore, VLP vaccines can not only stimulate the E7-specific cellular immune response but also produce sustained effective immune memory of antitumor immunity.
For a carrier vaccine to be potent, the induced antibody responses to the carrier itself should be mild to avoid disrupting the desired antigen-specific responses or preventing the repeated use of the vaccine. It was reported that preexisting antibodies may mediate antibody-dependent phagocytosis and promote antigen clearance, thus reducing antigen uptake by APCs and weakening subsequent immune responses. In contrast, it was also reported that preexisting antibodies enhanced immune responses by promoting the antigen uptake of APCs. To evaluate whether preexisting anti-HBcAg antibodies will influence the application of an HBcAg-based vaccine, in this study, three doses of immunization with carrier HBcAg VLPs were performed to induce anti-carrier immune responses before the administration of the HBcAg-E749–57 vaccine. Our results in the current study showed that preexisting anti-HBcAg antibodies did not produce significant influences on tumor-specific immune responses and suppressive effects on tumor growth, implying that the clinical application of HBcAg VLP-based vaccines is promising. Even if preexisting anti-HBcAg antibodies have adverse influences on HBcAg VLPs-based vaccines, it is still optional and valuable to employ this vaccine in the prime–boost strategy of vaccination.
Conclusion
In summary, while VLPs are highly immunogenic in stimulating humoral immunity, our results strongly indicated that VLPs, such as HBcAg particles, might also be potent therapeutic vaccine carriers to elicit cellular immune responses.
Acknowledgments
This work was supported by Fundamental Research Funds for the Central Universities of China (2012N08) and Applied Basic Research Projects of Yunnan Province, People’s Republic of China, from Yunnan Provincial Science and Technology Department (2010ZC232 and 2013FZ137), Fundamental Research Funds for Institute of Pathogen Biology of Peking Union Medical College (2014IPB107), the Peking Union Medical College (PUMC) Youth Fund, the Fundamental Research Funds for the Central Universities of China (3332015197 and 3332015147). The authors thank Mr Jingxian Zhou for excellent assistance in electron microscopy.
Footnotes
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Bolhassani A, Mohit E, Rafati S. Different spectra of therapeutic vaccine development against HPV infections. Hum Vaccin. 2009;5(10):671–689. doi: 10.4161/hv.5.10.9370. [DOI] [PubMed] [Google Scholar]
- 3.Hung CF, Ma B, Monie A, Tsen SW, Wu TC. Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Expert Opin Biol Ther. 2008;8(4):421–439. doi: 10.1517/14712598.8.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 5.Berzofsky JA, Ahlers JD, Janik J, et al. Progress on new vaccine strategies against chronic viral infections. J Clin Invest. 2004;114(4):450–462. doi: 10.1172/JCI22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melief CJ, van Hall T, Arens R, Ossendorp F, van der Burg SH. Therapeutic cancer vaccines. J Clin Invest. 2015;125(9):3401–3412. doi: 10.1172/JCI80009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wieking BG, Vermeer DW, Spanos WC, et al. A non-oncogenic HPV 16 E6/E7 vaccine enhances treatment of HPV expressing tumors. Cancer Gene Ther. 2012;19(10):667–674. doi: 10.1038/cgt.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung ID, Shin SJ, Lee MG, et al. Enhancement of tumor-specific T cell-mediated immunity in dendritic cell-based vaccines by Mycobacterium tuberculosis heat shock protein X. J Immunol. 2014;193(3):1233–1245. doi: 10.4049/jimmunol.1400656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith KA, Meisenburg BL, Tam VL, et al. Lymph node-targeted immunotherapy mediates potent immunity resulting in regression of isolated or metastatic human papillomavirus-transformed tumors. Clin Cancer Res. 2009;15(19):6167–6176. doi: 10.1158/1078-0432.CCR-09-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manuri PR, Nehete B, Nehete PN, et al. Intranasal immunization with synthetic peptides corresponding to the E6 and E7 oncoproteins of human papillomavirus type 16 induces systemic and mucosal cellular immune responses and tumor protection. Vaccine. 2007;25(17):3302–3310. doi: 10.1016/j.vaccine.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song YC, Cheng HY, Leng CH, et al. A novel emulsion-type adjuvant containing CpG oligodeoxynucleotides enhances CD8+ T-cell-mediated anti-tumor immunity. J Control Release. 2014;173:158–165. doi: 10.1016/j.jconrel.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 12.Lin YC, Lou PJ, Young TH. Chitosan as an adjuvant-like substrate for dendritic cell culture to enhance antitumor effects. Biomaterials. 2014;35(31):8867–8875. doi: 10.1016/j.biomaterials.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Birnbaum F, Nassal M. Hepatitis B virus nucleocapsid assembly: primary structure requirements in the core protein. J Virol. 1990;64(7):3319–3330. doi: 10.1128/jvi.64.7.3319-3330.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee BO, Tucker A, Frelin L, et al. Interaction of the hepatitis B core antigen and the innate immune system. J Immunol. 2009;182(11):6670–6681. doi: 10.4049/jimmunol.0803683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pumpens P, Grens E. HBV core particles as a carrier for B cell/T cell epitopes. Intervirology. 2001;44(2–3):98–114. doi: 10.1159/000050037. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Halayko AJ, Basu S, et al. Sustained suppression of IL-13 by a vaccine attenuates airway inflammation and remodeling in mice. Am J Respir Cell Mol Biol. 2013;48(5):540–549. doi: 10.1165/rcmb.2012-0060OC. [DOI] [PubMed] [Google Scholar]
- 17.Long Q, Huang W, Yao Y, et al. Virus-like particles presenting interleukin-33 molecules: immunization characteristics and potentials of blocking IL-33/ST2 pathway in allergic airway inflammation. Hum Vaccin Immunother. 2014;10(8):2303–2311. doi: 10.4161/hv.29425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Y, HayGlass KT, Becker AB, et al. Novel recombinant interleukin-13 peptide-based vaccine reduces airway allergic inflammatory responses in mice. Am J Respir Crit Care Med. 2007;176(5):439–445. doi: 10.1164/rccm.200610-1405OC. [DOI] [PubMed] [Google Scholar]
- 19.Guan Q, Ma Y, Aboud L, et al. Targeting IL-23 by employing a p40 peptide-based vaccine ameliorates murine allergic skin and airway inflammation. Clin Exp Allergy. 2012;42(9):1397–1405. doi: 10.1111/j.1365-2222.2012.04022.x. [DOI] [PubMed] [Google Scholar]
- 20.Guan Q, Ma Y, Hillman CL, et al. Development of recombinant vaccines against IL-12/IL-23 p40 and in vivo evaluation of their effects in the downregulation of intestinal inflammation in murine colitis. Vaccine. 2009;27(50):7096–7104. doi: 10.1016/j.vaccine.2009.09.058. [DOI] [PubMed] [Google Scholar]
- 21.Conniot J, Silva JM, Fernandes JG, et al. Cancer immunotherapy: nanodelivery approaches for immune cell targeting and tracking. Front Chem. 2014;2:105. doi: 10.3389/fchem.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wynne SA, Crowther RA, Leslie AG. The crystal structure of the human hepatitis B virus capsid. Mol Cell. 1999;3(6):771–780. doi: 10.1016/s1097-2765(01)80009-5. [DOI] [PubMed] [Google Scholar]
- 23.Sominskaya I, Skrastina D, Petrovskis I, et al. A VLP library of C-terminally truncated hepatitis B core proteins: correlation of RNA encapsidation with a Th1/Th2 switch in the immune responses of mice. PLoS One. 2013;8(9):e75938. doi: 10.1371/journal.pone.0075938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grasso F, Negri DR, Mochi S, et al. Successful therapeutic vaccination with integrase defective lentiviral vector expressing nononcogenic human papillomavirus E7 protein. Int J Cancer. 2013;132(2):335–344. doi: 10.1002/ijc.27676. [DOI] [PubMed] [Google Scholar]
- 25.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 26.Tau GZ, von der Weid T, Lu B, et al. Interferon gamma signaling alters the function of T helper type 1 cells. J Exp Med. 2000;192(7):977–986. doi: 10.1084/jem.192.7.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallett MA, Venmar KT, Fingleton B. Cytokine stimulation of epithelial cancer cells: the similar and divergent functions of IL-4 and IL-13. Cancer Res. 2012;72(24):6338–6343. doi: 10.1158/0008-5472.CAN-12-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drabsch Y, ten Dijke P. TGF-beta signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev. 2012;31(3–4):553–568. doi: 10.1007/s10555-012-9375-7. [DOI] [PubMed] [Google Scholar]
- 29.Moustakas A, Heldin CH. Induction of epithelial-mesenchymal transition by transforming growth factor beta. Semin Cancer Biol. 2012;22(5–6):446–454. doi: 10.1016/j.semcancer.2012.04.002. [DOI] [PubMed] [Google Scholar]