Abstract
Charged multivesicular body protein 5 (CHMP5) has a key role in multivesicular body biogenesis and a critical role in the downregulation of signaling pathways through receptor degradation. However, the role of CHMP5 in T-cell receptor (TCR)–mediated signaling has not been previously investigated. In this study, we utilized a short hairpin RNA-based RNA interference approach to investigate the functional role of CHMP5. Upon TCR stimulation, CHMP5-knockdown (CHMP5KD) Jurkat T cells exhibited activation of TCR downstream signaling molecules, such as PKCθ and IKKαβ, and resulted in the activation of nuclear factor-κB and the marked upregulation of TCR-induced gene expression. Moreover, we found that activator protein-1 and nuclear factor of activated T-cells transcriptional factors were markedly activated in CHMP5KD Jurkat cells in response to TCR stimulation, which led to a significant increase in interleukin-2 secretion. Biochemical studies revealed that CHMP5 endogenously forms high-molecular-weight complexes, including TCR molecules, and specifically interacts with TCRβ. Interestingly, flow cytometry analysis also revealed that CHMP5KD Jurkat T cells exhibit upregulation of TCR expression on the cell surface compared with control Jurkat T cells. Taken together, these findings demonstrated that CHMP5 might be involved in the homeostatic regulation of TCR on the cell surface, presumably through TCR recycling or degradation. Thus CHMP5 is implicated in TCR-mediated signaling.
Introduction
Charged multivesicular body protein 5 (CHMP5) is a coiled protein homologous to the yeast Vps60/Mos10 gene and other endosomal sorting complexes required for transport (ESCRT)-III complex members, which are responsible for the final conversion of late endosomal multivesicular body (MVB) to lysosomes.1, 2 MVB is a special type of late endosome and a crucial intermediate in the internalization of nutrients, ligands and receptors via the endolysosomal system; therefore, MVB has a crucial role in sorting membrane proteins destined for degradation or routing to the lysosome.3, 4, 5, 6, 7 It is well established that the degradation of cell surface receptors through endocytosis is a common mechanism for the downregulation of growth factor and TGFβ (transforming growth factor β) receptor signaling.3 A previous study has shown that CHMP5 is required for the downregulation of TGFβ signaling pathways via the lysosomal degradation of internalized receptors.2 These results suggest that CHMP5 may have a key role in the regulation of signaling pathways via receptor downregulation.
Recently, several reports have illustrated novel functions of CHMP5, which include cooperating with the ESCRT-III complex in programmed cell death, antiviral mechanisms, the maintenance of centrosomes and cellular cytokinesis.8, 9, 10, 11, 12 CHMP5-knockdown leukemic cells exhibited activation of two programmed cell death pathways: the Granzyme B/Perforin apoptotic pathway and the AIF (apoptosis-inducing factor)-mediated caspase-independent necrosis pathway.9 Moreover, anti-CHMP5 single chain variable fragment antibody retrovirus infection induces programmed cell death in leukemic cells via AIF-mediated caspase-independent necrosis and apoptosis13; this result suggests that CHMP5 may be involved in cellular apoptotic processes. In addition, CHMP5 is involved in the primary switch that initiates the antiviral mechanism via the regulation of the ISGylation of CHMP2A and CHMP6 and in the availability of the co-activator protein LIP5 to the ESCRT-III-Vps4 complex.10, 14 These results suggest that CHMP5 is a multi-functional protein with other potential functions in cellular signaling and maintenance.
CHMP5 downregulates signaling pathways through receptor degradation.1, 2 Therefore, we determined whether CHMP5 is involved in TCR-mediated signaling via TCR modulation. In this study, we utilized a short hairpin RNA (shRNA)-based RNA interference approach to generate CHMP5-knockdown (CHMP5KD) Jurkat T cells. Our data demonstrated that, upon TCR stimulation, CHMP5KD Jurkat T cells exhibit a marked augmentation of TCR-mediated signaling with regard to the activation of three transcriptional factors (nuclear factor (NF)-κB, activator protein 1 (AP-1) and nuclear factor of activated T-cells (NFAT)), leading to the upregulation of TCR-induced genes and interleukin (IL)-2 secretion. Furthermore, biochemical studies revealed that CHMP5 endogenously forms high-molecular-weight complexes, including TCR molecules, and specifically interacted with TCRβ. These results suggested that CHMP5 regulates cell surface TCR expression and is thereby implicated in TCR-mediated signaling.
Materials and methods
Cells and reagents
Jurkat cells were grown in RPMI 1640 media supplemented with 10% fetal bovine serum (Sigma-Aldrich, St Louis, MO, USA), 50 U ml−1 penicillin and 50 μg ml−1 streptomycin at 37 °C in an atmosphere of 5% CO2/95% air. Jurkat T cells were infected with control shRNA lentiviral particles (sc-108080, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or CHMP5 shRNA (h) lentiviral particles (sc-60374-V, Santa Cruz) and selected based on the manufacturer's protocols. Control (Ctrl) Jurkat and CHMP5KD Jurkat cells were maintained and grown in RPMI 1640 media supplemented with 10% fetal bovine serum (Sigma-Aldrich), 50 U ml−1 penicillin, 4–8 μg ml−1 puromycin and 50 μg ml−1 streptomycin at 37 °C in an atmosphere of 5% CO2/95% air. The antibodies used were anti-CHMP5 (Abcam, Cambridge, CO, USA), anti-GAPDH (Santa Cruz Biotechnology), anti-TCRαβ (BD Biosciences, San Jose, CA, USA), anti-CD3 (BioLegend, San Diego, CA, USA), anti-CD28 (BioLegend), anti-TCRβ (Abcam), anti-pho-PKCθ (Cell Signaling Technology, Danvers, MA, USA), anti-PKCθ (Cell Signaling Technology), anti-pho-IKKαβ (Cell Signaling Technology), anti-IKKα (Cell Signaling Technology), anti-pho-ZAP-70 (Santa Cruz Biotechnology), anti-ZAP-70 (Santa Cruz Biotechnology), anti-pho-Lck (Santa Cruz Biotechnology) and anti-Lck (Santa Cruz Biotechnology).
Immunofluorescence microscopy and flow cytometry analyses
Control or CHMP5KD Jurkat cells were stained with control Ig or phycoerythrin (PE)-conjugated TCR antibody, washed with phosphate-buffered saline and analyzed by flow cytometry (FACSCalibur, BD).15 For immunofluorescence microscopy analysis, control or CHMP5KD Jurkat cells were stained with PE-conjugated TCR antibody and washed with phosphate-buffered saline. Cells were imaged on a Zeiss LSM 710 laser-scanning confocal microscope (Carl Zeiss, Jena, Germany).16 For intracellular IL-2 staining, control or CHMP5KD Jurkat cells were either treated or not treated with anti-CD3 (10 μg ml−1) and anti-CD28 (5 μg ml−1) antibodies, together with 5 μg of goat anti-mouse immunoglobulin G (IgG) antibody per ml (Signal Transduction Laboratories, Lexington, KY, USA) for 18 h. Intracellular staining for IL-2 with anti-PE-conjugated IL-2 antibody was performed with reagents from Pharmingen Inc. (San Diego, CA, USA) according to the manufacturer's protocol. Cells were analyzed with flow cytometry (FACSCalibur, BD). For the analysis, cell debris was excluded on the forward scatter (FSC) and side scatter (SSC) dot plot panels by using the gating method; IL-2-expressing cells stained with PE-conjugated IL-2 antibodies were then further gated on the FL-2 (PE-IL-2) and FL-1 (auto-fluorescence) dot plot panels.
Gel filtration chromatography
The S-100 fraction from Jurkat T cells was loaded onto a Superose6 10/300 GL column pre-equilibrated with buffer A plus 0.1% Chaps and 0.01% Brij35. The proteins were eluted at 0.3 ml min−1 as previously described.16, 17, 18, 19 Samples were analyzed by western blotting using antibodies specific for CHMP5 and TCRβ.
Immunoblotting (IB) and immunoprecipitation (IP)
Control and CHMP5KD Jurkat cells were either treated or not treated with anti-CD3 (10 μg ml−1) and anti-CD28 (5 μg ml−1) antibodies, together with 5 μg of goat anti-mouse IgG antibody per ml at different times. Samples were analyzed by western blotting using the indicated antibodies as previously described.16, 17, 18, 19 For the endogenous IP assay, cell lysates from Jurkat T cells were treated with control IgG or anti-CHMP5 antibodies; the IP assay was then performed, as previously described.16, 17, 18, 19 Samples were analyzed by western blotting using antibodies specific for CHMP5 and TCRβ.
Luciferase reporter assay
Control and CHMP5KD Jurkat cells were transiently transfected with the pBIIx-luc nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-dependent reporter or AP-1 constructs, together with the Renilla luciferase vector, using the Neon transfection system (Invitrogen, Carlsbad, CA, USA). Cells pretreated in the presence or absence of anti-CD3 (10 μg ml−1) and anti-CD28 (5 μg ml−1) antibodies at different times were treated with 5 μg of goat anti-mouse IgG antibody per ml at different times. Luciferase activities were measured using a dual Luciferase Assay Kit (Promega, Southampton, UK).
p65/p50/c-fos/pho-c-Jun/NFAT DNA-binding assay
Control and CHMP5KD Jurkat cells were treated in the presence or absence of anti-CD3 (10 μg ml−1) and anti-CD28 (5 μg ml−1) antibodies together with 5 μg of goat anti-mouse IgG antibody per ml at different times. Nuclear proteins were prepared with a CelLytic NuCLEAR Extraction Kit (Sigma-Aldrich) in accordance with the manufacturer's protocol. P65/p50/c-fos/pho-c-Jun/NFAT transcription factor activities were determined using transcription factor assay kits (Active Motif, Carlsbad, CA, USA) according to the manufacturer's instructions.
Enzyme-linked immunosorbent assay (ELISA)
Control and CHMP5KD Jurkat cells were stimulated with or without anti-CD3 (10 μg ml−1) and anti-CD28 (5 μg ml−1) antibodies, together with 5 μg of goat anti-mouse IgG antibody per ml for 24 h. IL-2 secreted in the culture supernatant was measured using ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Quantitative reverse trnscriptase-PCR (qRT-PCR) analysis
Total RNA was isolated and cDNA was synthesized following the manufacturer's protocol (Qiagen, Valencia, CA, USA). All primers were purchased from Qiagen: CD69 (PPH00831F-200), HLA-B (PPH05982B-200), NFKB2 (PPH00782F-200), LTA (PPH00337F-200), NFKB1 (PPH00204F-200), RELB (PPH00287A-200), FBLN2 (PPH14234A-200), and SPOCK2 (PPH20551B-200). The qRT-PCR analysis was performed using Roter-GeneQ (Qiagen) according to the manufacturer's protocol.
Microarray analysis
Control and CHMP5KD Jurkat cells were treated in the presence or absence of anti-CD3 (10 μg ml−1) and anti-CD28 (5 μg ml−1) antibodies together with 5 μg of goat anti-mouse IgG antibody per ml at different times. Total RNA was extracted using TRIzol (Invitrogen) and purified using RNeasy columns (Qiagen,) according to the manufacturer's protocol. Microarray analysis, raw data preparation and statistical analysis was performed as previously described.20, 21, 22
Statistical analysis
In vitro data are presented as the mean±s.d. of the mean from triplicate samples. Comparisons were statistically tested using Student's t-test. P values <0.05 or <0.01 were considered to be statistically significant.
Results
CHMP5 knockdown enhances TCR-induced signaling for the activation of NF-κB
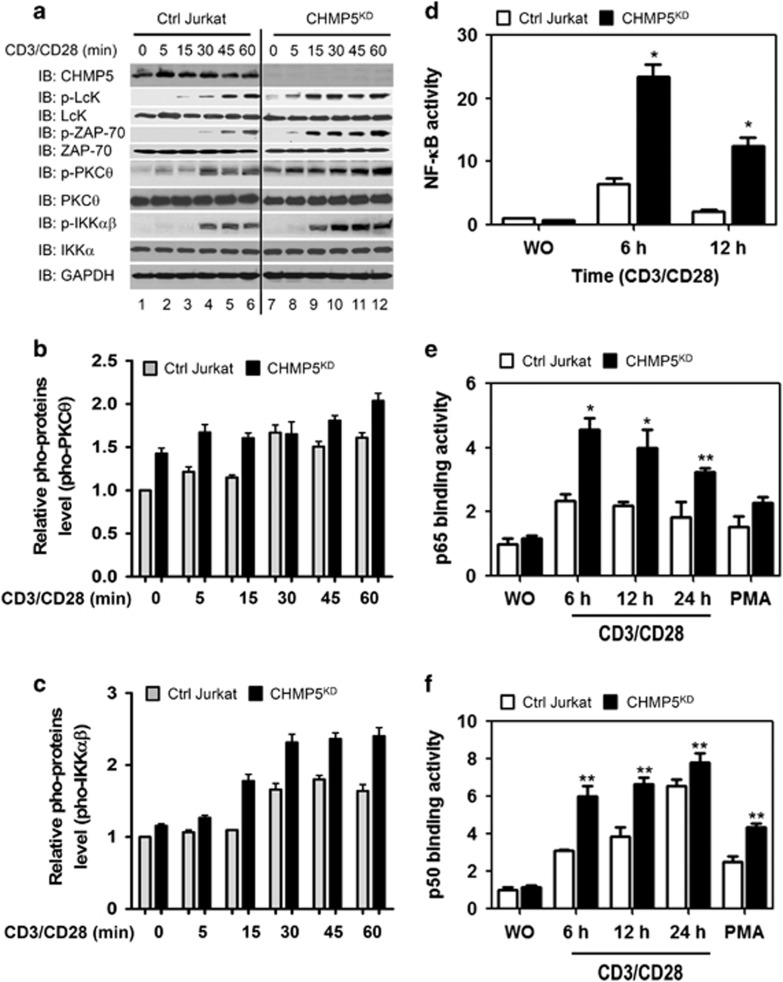
To examine the role of CHMP5 in TCR-mediated signaling, we generated CHMP5-knockdown (CHMP5KD) Jurkat T cells using a short hairpin RNA-based RNA interference method. Jurkat T cells were transduced with lentiviral particles containing shRNA targeted to human CHMP5 or control shRNA sequences, as described in Materials and Methods section (Figure 1a, IB: CHMP5). To determine whether CHMP5 is functionally implicated in TCR-mediated signaling for the activation of NF-κB, control (Ctrl) and CHMP5KD Jurkat cells were stimulated at different times with anti-CD3 and anti-CD28 antibodies, and the activation of TCR downstream molecules was evaluated. Upon TCR stimulation, phosphorylation of p-Lck, p-ZAP-70, PKCθ and IKKαβ were significantly increased in control Jurkat cells (Figure 1a: IB: p-Lck, p-ZAP-70, p-PKCθ, and p-IKKαβ). The phosphorylated PKCθ and IKKαβ were also significant when compared with non-stimulated cells (Figures 1b and c, Ctrl Jurkat cells). Interestingly, phosphorylation of Lck, ZAP-70, PKCθ and IKKαβ was markedly enhanced in CHMP5KD Jurkat cells compared with the control Jurkat cells (CHMP5KD in Figures 1a–c). In TCR-mediated signaling, activation of PKCθ and IKKαβ led to activation of NF-κB.23, 24 Therefore, we further examined whether CHMP5 deficiency leads to activation of NF-κB in response to TCR stimulation. NF-κB reporter activities were significantly higher in CHMP5KD Jurkat cells than in control Jurkat cells (Figure 1d, closed bars versus open bars). After treatment with 100 ng ml−1 PMA, which is known to induce the activation of NF-κB,25 marginal increases in p65- and p50-DNA-binding activities were observed (Figures 1e and f, closed bars versus open bars in PMA). Interestingly, p65- and p50-DNA-binding activities were markedly higher in CHMP5KD Jurkat cells than control Jurkat cells (Figures 1e and f, closed bars versus open bars), suggesting that CHMP5 deficiency is implicated in the TCR-induced activation of NF-κB.
Figure 1.
CHMP5 knockdown enhances TCR-induced signaling, activating NF-κB. (a) Control (Ctrl) and CHMP5KD Jurkat cells generated by control shRNA lentiviral particles and CHMP5 shRNA lentiviral particles, respectively, were stimulated with anti-CD3/anti-CD28 for different times, as described in Materials and methods section. Cell extracts were analyzed by western blotting for CHMP5, pho-Lck, Lck, pho-ZAP-70, ZAP-70, pho-PKCθ, PKCθ, pho-IKKαβ, IKKα and GAPDH as a loading control. (b, c) Relative pho-PKCθ (b) and pho-IKKαβ (c) levels were evaluated by Image J (lower panels). All error bars represent ±s.d. of the mean from triplicate samples. (d) Control (Ctrl) and CHMP5KD Jurkat cells were transfected with pBIIx-luc NF-κB-dependent reporter together with the Renilla luciferase vector. After 36 h, cells were stimulated with anti-CD3/anti-CD28 at different times and a luciferase assay was performed. All error bars represent ±s.d. of the mean from triplicate samples. *P<0.01. (e, f) Ctrl and CHMP5KD Jurkat cells were stimulated with anti-CD3/anti-CD28 for different times or PMA as a positive control. Nuclear extracts were prepared and analyzed for p65 (e) and p50 (f) binding to an NF-κB consensus oligonucleotide. All error bars represent ±s.d. of the mean from triplicate samples. *P<0.01 and **P<0.05.
CHMP5-knockdown Jurkat T cells exhibit upregulation of TCR-induced gene expression
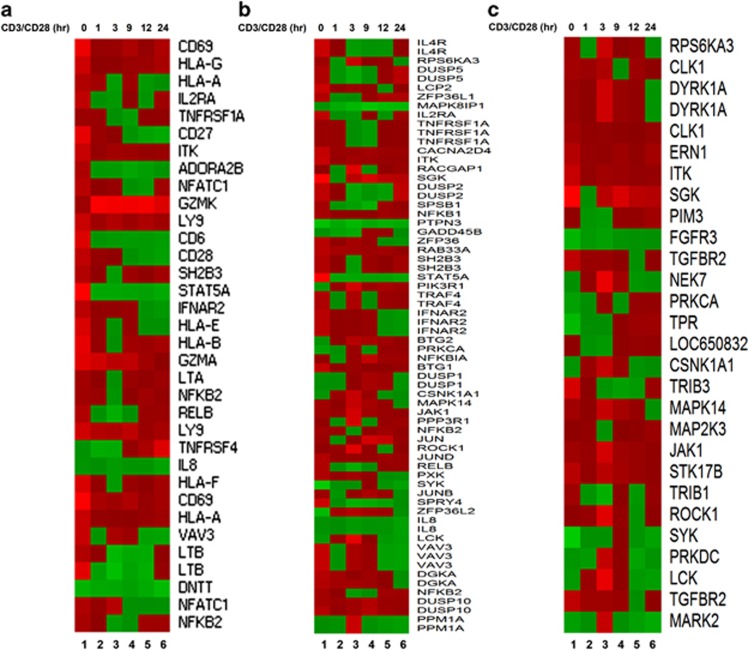
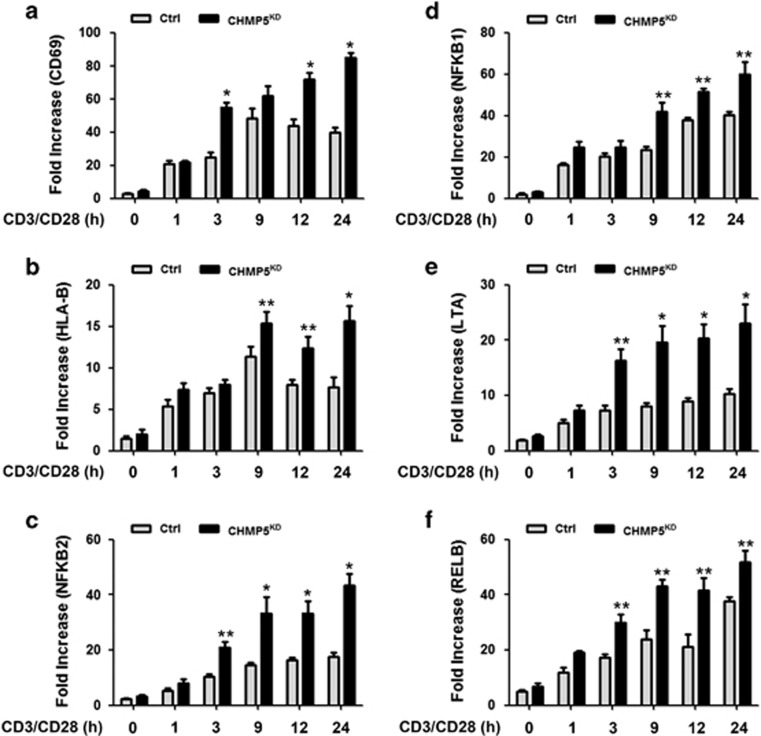
Because the results indicated that CHMP5 knockdown results in the enhancement of TCR-induced signaling, activating NF-κB, we next examined whether the results are functionally linked to TCR-mediated gene expression. Control and CHMP5KD Jurkat cells were stimulated at different times with anti-CD3/CD28 antibodies, followed by the isolation and preparation of RNA for array analysis. Stimulation with TCR in CHMP5KD Jurkat cells led to the marked upregulation of a number of genes represented on the array compared with TCR-stimulated control Jurkat cells (Supplementary Figure S1). Genes represented on the array were those associated with T-cell immunity, intracellular signaling or intracellular kinases, and the expression patterns of these genes were compared between control and CHMP5KD Jurkat cells; it was found that the expression profiles were markedly upregulated in CHMP5KD Jurkat cells compared with that in control Jurkat T cells (Figure 2a, T-cell immunity–related genes; Figure 2b, intracellular signaling–related genes; Figure 2c, kinase-related genes). To validate the microarray results, the expression of 6 NF-κB-dependent genes, that is, CD69, HLA-B, NFKB2, NFKB1, LTA and RELB, was examined by qRT-PCR. Upon TCR stimulation with anti-CD3/CD28 antibodies, the expression of these genes was significantly higher in CHMP5KD Jurkat cells than in control Jurkat cells (Figure 3a, CD69; Figure 3b, HLA-B; Figure 3c, NFKB2; Figure 3d, NFKB1; Figure 3e, LTA; Figure 3f, RELB: closed bars versus open bars). Although the precise mechanism remains to be determined, these results suggest a role for CHMP5 in TCR-induced NF-κB activation.
Figure 2.
Selective effects of CHMP5 knockdown on TCR-induced genes. (a–c) RNA was extracted from Ctrl and CHMP5KD Jurkat cells treated with anti-CD3/anti-CD28 for 1, 3, 9, 12 and 24 h, followed by an analysis of the expression of T-cell immunity–related genes (a), intracellular signaling–related genes (b), and kinase-related genes (c) in Ctrl and CHMP5KD Jurkat cells (CHMP5KD versus Ctrl Jurkat cells). Lane 1, Unstimulated cells; Lane 2, TCR stimulation for 1 h; Lane 3, TCR stimulation for 3 h; Lane 4, TCR stimulation for 9 h; Lane 5, TCR stimulation for 12 h; Lane 6, TCR stimulation for 24 h.
Figure 3.
NF-κB-dependent genes induced by TCR stimulation are enhanced in CHMP5-knockdown Jurkat cells. (a–f) RNA was extracted from Ctrl and CHMP5KD Jurkat cells stimulated with anti-CD3/anti-CD28 for 1, 3, 9, 12 and 24 h, followed by quantitative RT-PCR analysis of the effects of CHMP5 knockdown (a, CD69; b, HLA-B; c, NFKB2; d, NFKB1; e, LTA; f, RELB). Data represent the average of data from two independent experiments, each conducted in triplicate. Error bars represent the mean±s.d. of six samples. *P<0.01 and **P<0.05.
CHMP5 knockdown enhances TCR-induced AP-1 and NFAT activations
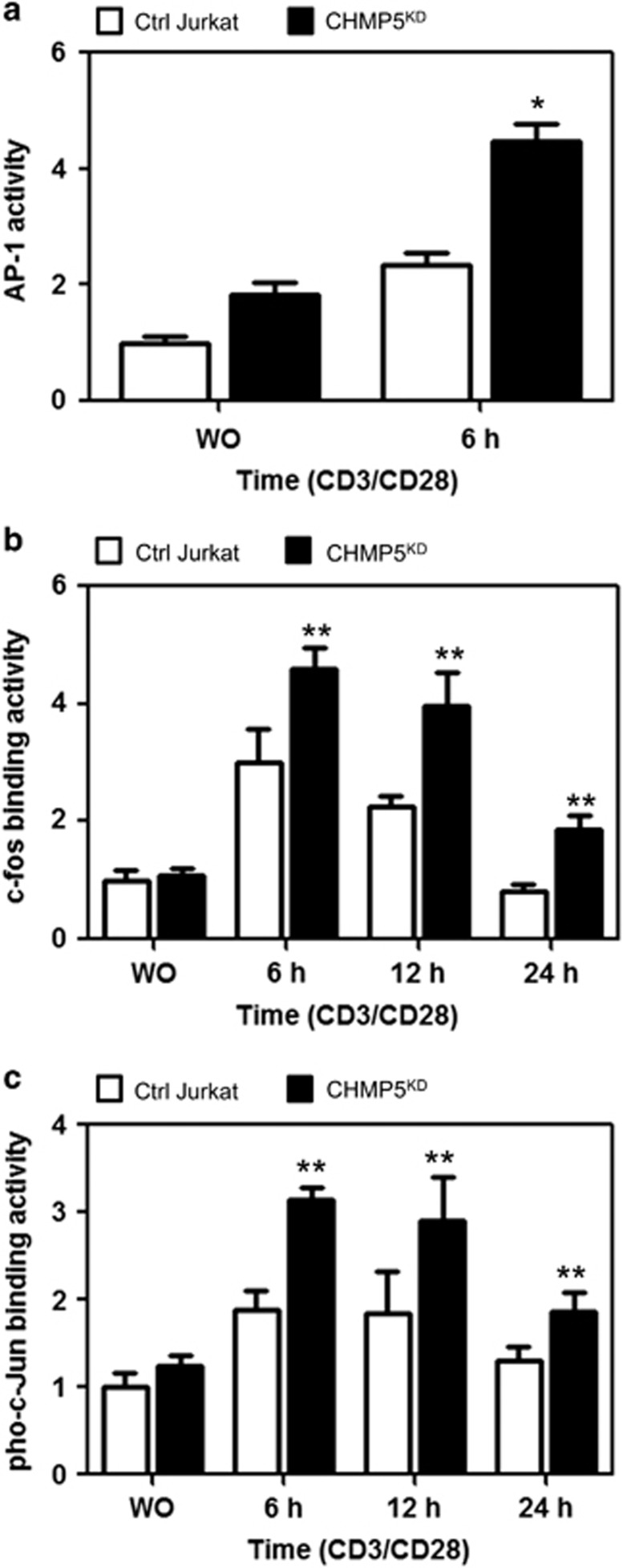
TCR-mediated signaling leads to activation of transcriptional factors, such as NF-κB, NFAT and AP-1, which eventually links to the expression of IL-2.23, 24, 26, 27, 28 Because our results indicated that CHMP5 is functionally involved in the TCR-mediated activation of NF-κB, we examined whether TCR stimulation of CHMP5KD Jurkat cells induces AP-1 and NFAT transcription factor activation and resultant IL-2 expression. Following TCR stimulation, AP-1 reporter activities were significantly enhanced in CHMP5KD Jurkat cells compared with Ctrl Jurkat cells (Figure 4a). Furthermore, the c-fos- and pho-c-junbinding activities were also significantly higher in CHMP5KD Jurkat cells than in control Jurkat cells (Figure 4b, c-fos; Figure 4c, pho-c-jun), indicating that AP-1 activation is enhanced in CHMP5KD Jurkat cells in response to TCR stimulation.
Figure 4.
CHMP5-knockdown Jurkat cells enhance the activation of AP-1 in response to TCR stimulation. (a) Control (Ctrl) and CHMP5KD Jurkat cells were transfected with AP-1 reporter together with the Renilla luciferase vector. After 36 h, cells were stimulated for 6 h with or without anti-CD3/anti-CD28 antibodies, and a luciferase assay was performed. All error bars represent ±s.d. of the mean from triplicate samples. *P<0.05. (b, c) Ctrl and CHMP5KD Jurkat cells were stimulated with anti-CD3/anti-CD28 for different times. Nuclear extracts were prepared and analyzed for DNA-binding activities of c-fos (b) and pho-c-Jun (c). All error bars represent ±s.d. of the mean from triplicate samples. **P<0.05.
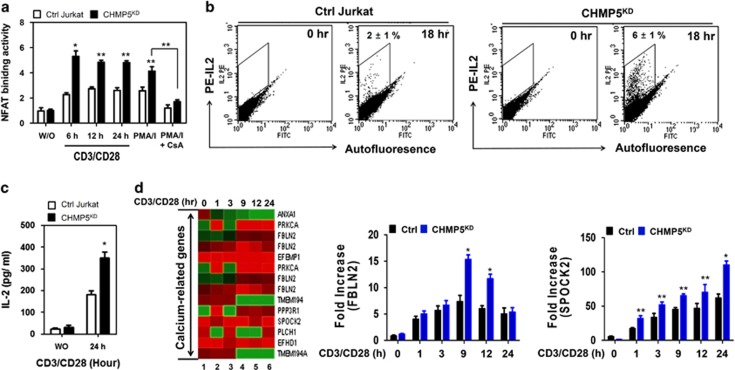
Next we examined NFAT activation in response to TCR stimulation. As expected, NFAT DNA-binding activity was markedly enhanced in the PMA/ionomycin (PMA/I)-treated cells, whereas this activity was markedly abolished in PMA/I plus CsA-treated cells (Figure 5a, PMA/I and PMA/I+CsA). Upon TCR stimulation, NFAT DNA-binding activity was significantly increased in control Jurkat cells (Figure 5a, open bars); interestingly, this activity was markedly enhanced in CHMP5KD Jurkat cells (Figure 5a, closed bars). The activation of NFAT, along with NF-κB and AP-1 transcription factors, is critical for IL-2 expression through Ca2+/calcineurin signaling.23, 24, 26, 27, 28 Therefore, we assessed whether CHMP5KD Jurkat cells have enhanced IL-2 secretion in response to TCR stimulation. Accordingly, control and CHMP5KD Jurkat cells were stimulated for 18 h with or without anti-CD3 and anti-CD28 antibodies, and the intracellular IL-2 levels were measured by flow cytometry. The level of intracellular IL-2 was significantly higher in CHMP5KD Jurkat cells than in control Jurkat cells (Figure 5b, 6±1% versus 2±1%, respectively). Furthermore, ELISA assay revealed that the marked increase of IL-2 secretion could be observed in CHMP5KD Jurkat cells in response to TCR stimulation compared with that in control Jurkat cells (Figure 5c, closed bars versus open bars). To determine whether the expression of calcium-related genes is associated with CHMP5 knockdown in response to TCR stimulation, we performed microarray analysis. Control and CHMP5KD Jurkat cells were treated with anti-CD3/anti-CD28 at different times, as indicated in Figure 5d. Ten different calcium-related genes were identified from the microarray results, and their expression levels were then compared (Figure 5d, CHMP5KD versus control Jurkat cells). Seven of these calcium-related genes, including FBLN2 and SPOCK2, were significantly upregulated in CHMP5KD Jurkat cells in response to TCR stimulation (Figure 5d); in addition, the results were partly confirmed by qRT-PCR analysis (Figure 5d, right panels). These results suggest that CHMP5 deficiency leads to activation of NF-kB, NFAT and AP-1 transcriptional factors, thereby increasing IL-2 expression in response to TCR stimulation.
Figure 5.
CHMP5-knockdown Jurkat cells enhance the activation of NFAT in response to TCR. (a) Ctrl and CHMP5KD Jurkat cells were stimulated with anti-CD3/anti-CD28 for different times or 10 ng ml−1 PMA and 1.5 μM ionomycin in the presence or absence of 100 nM CsA, as a positive control. Nuclear extracts were prepared and analyzed for DNA-binding activities of NFAT. All error bars represent ±s.d. of the mean from triplicate samples. *P<0.01 and **P<0.05. (b) Ctrl and CHMP5KD Jurkat cells were treated with or without anti-CD3/anti-CD28 at different times, as indicated. The levels of intracellular IL-2 were measured by flow cytometry. The results represent ±s.d. of the mean from triplicate samples. (c) Ctrl and CHMP5KD Jurkat cells were stimulated with or without anti-CD3/anti-CD28 for 24 h, and the amount of IL-2 in the supernatant was measured by ELISA. All error bars represent ±s.d. of the mean from triplicate samples. *P<0.05. (d) RNA was extracted from Ctrl and CHMP5KD Jurkat cells treated with anti-CD3/anti-CD28 for 1, 3, 9, 12 and 24 h, followed by analysis of calcium-related gene expression in Ctrl and CHMP5KD Jurkat cells (CHMP5KD versus Ctrl Jurkat cells). Lane 1, Unstimulated cells; Lane 2, TCR stimulation for 1 h; Lane 3, TCR stimulation for 3 h; Lane 4, TCR stimulation for 9 h; Lane 5, TCR stimulation for 12 h; Lane 6, TCR stimulation for 24 h. RNA was extracted from cells, and then q-RT-PCR analysis was performed with specific primers targeted to FBLN2 and SPOCK2, respectively. Data represent the average of data from two independent experiments, each conducted in triplicate. Error bars represented the mean±s.d. of six samples. *P<0.01 and **P<0.05.
CHMP5 is associated with TCR expression on the cell surface
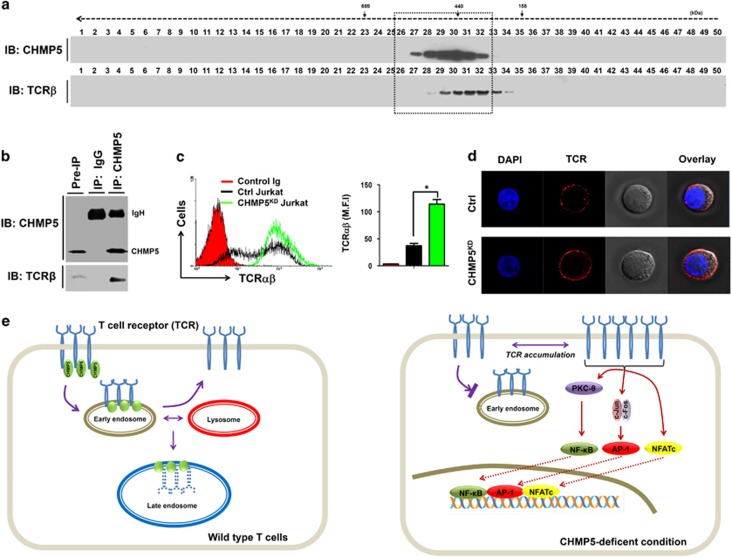
Based on the above results, we explored the cellular mechanism in which CHMP5 deficiency is implicated in TCR-mediated signaling. Previous reports showed that CHMP5 is required for ESCRT-III complex members that are responsible for the final conversion of late endosomal MVB to lysosomes and also has a critical role in the downregulation of signaling pathways through receptor degradation.1, 2, 3 We evaluated the role of CHMP5 in the downregulation of cell surface TCR. We first tested whether CHMP5 protein is associated with the TCR molecule. Cellular complexes were isolated from wild Jurkat T cells by gel filtration. Western blotting analysis was subsequently performed to identify the co-localization of CHMP5 and TCR molecules. Interestingly, several fractions containing CHMP5 were significantly localized with TCRβ (Figure 6a, fractions 29–32), suggesting that CHMP5 is endogenously associated with TCR molecules. To verify whether this association results in the molecular interaction between CHMP5 and TCR, we further performed an endogenous IP assay. CHMP5 proteins were pulled down in cell lysates derived from wild-type Jurkat cells with anti-CHMP5 or IgG control antibody. As shown in Figure 6b, endogenous CHMP5 proteins significantly co-precipitated with TCRβ, whereas no significant bands could be observed for the control IgG antibody, suggesting that CHMP5 is endogenously associated with TCR molecules through molecular interactions. It has been shown that a putative CHMP5 dominant-negative mutant leads to accumulation of ligand-bound epidermal growth factor receptor and results in a delay in epidermal growth factor receptor degradation.28 Therefore, we examined whether CHMP5 knockdown in Jurkat cells affects the expression of TCR on the cell surface. Control or CHMP5KD Jurkat cells were stained with anti-TCRαβ antibody followed by flow cytometry analysis. Interestingly, TCR expression was markedly higher in CHMP5KD Jurkat cells than in control Jurkat cells (Figure 6c, black line versus green line; black bar versus green bar in right). The results were further confirmed by immunofluorescence microscopic analysis (Figure 6d). These results demonstrated that CHMP5 is endogenously associated with TCR, meaning its deficiency affects cell surface TCR expression.
Figure 6.
CHMP5 regulates the TCR expression on the surface. (a) Cell lysates from Jurkat T cells were prepared and fractionated through a Superose6 10/300 GL column, as described in Materials and methods section. Each fraction (40 μl) was analyzed by immunoblotting (IB) with the indicated antibody to anti-CHMP5 or anti-TCRβ. (b) Cell lysates from Jurkat T cells were prepared and an immunoprecipitation assay was performed with anti-CHMP5 antibody or IgG antibody as a negative control. The interaction between CHMP5 and TCRβ was evaluated by western blotting. (c) Control (Ctrl) and CHMP5KD Jurkat cells were stained with control Ig or PE-conjugated TCRαβ antibody and then analyzed by flow cytometry. The TCR expression was represented by mean fluorescence intensity (MFI). *P<0.01. (d) Ctrl and CHMP5KD Jurkat cells were stained with PE-conjugated TCRαβ antibody and immunofluorescence microscopy analysis was performed. (e) A mechanistic model of the role of CHMP5 in TCR expression. Left: In wild-type cells, CHMP5 is associated with TCR and involved in the regulation of constitutive T-cell antigen receptor (TCR) recycling to the cell surface. Right: TCR recycling is impaired in deficiency of CHMP5 and TCR expression increases on the cell surface. Upon TCR stimulation, the accumulated TCRs are functionally induced to activate TCR-induced transcriptional factors, such as NF-κB, AP-1 and NFAT, leading to gene expression.
Discussion
In this study, we investigated the role of CHMP5 in TCR-mediated signaling. To overcome CHMP5−/− homozygote mice showing embryonic lethality at day 10 (E10),2 we utilized a shRNA-based RNA interference approach and generated CHMP5-knockdown Jurkat cells to investigate its functional role in T cells. Consistent with previous reports,1, 2 CHMP5 knockdown was accompanied by an accumulation of structures that appeared to be early endosomes and lysosomes compared with control (Supplementary Figure S2), supporting the concept that CHMP5 is functionally involved in the formation of early endosomes and lysosomes. We also found that CHMP5KD Jurkat cells exhibited a marked enhancement of TCR-induced transcriptional factors (NF-κB, AP-1 and NFAT), consequently leading to an increase in TCR-dependent gene expression. Moreover, CHMP5 is endogenously associated and interacts with TCR. Interestingly, TCR expression in CHMP5KD Jurkat T cells accumulated on the surface, strongly demonstrating a role for CHMP5 in the homeostatic regulation of cell surface TCR.
In addition to the previous findings that CHMP5, one of components of the ESCRT-III, is involved in the final conversion of late endosomal MVB to lysosomes and the downregulation of signaling pathways through receptor degradation,1, 2, 6, 7 recent evidence indicates that CHMP5 modulates apoptosis in neurodegenerative diseases and cancer through changes in ECSRT-III complex.29, 30 In line with these results, a previous report showed that CHMP5 is involved in programmed cell death in leukemic cells.9 Furthermore, CHMP5 is implicated in the antiviral mechanism through the prevention of ISGylation of CHMP2A and CHMP6 and in the retroviral release process via the prevention of LIP5 binding to Vps4.10, 14 These results suggest that CHMP5 has multicellular functions.
On the basis of these results, we propose a current model, shown in Figure 6e. Briefly, CHMP5 is associated with TCR, and the complex may go to either a recycling pathway through early endosomes or to a degradation pathway through late endosomes fused with lysosomes to maintain homeostatic levels of TCR in normal T cells (Figure 6e, left). However, the TCR recycling process for the maintenance of homeostatic cell surface TCR levels may be affected in CHMP5KD cells, leading to increased TCR expression on the cell surface. In this study, we found that, upon TCR stimulation, the accumulated TCRs are functionally active and induce the activation of three transcriptional factors (NF-κB, AP-1 and NFAT), resulting in the regulation of target gene expression, including IL-2 (Figure 6e, right). In conclusion, these results improve our understanding of the precise role of CHMP5 in different cell types.
Acknowledgments
This work was supported by the Mid-career Researcher Program through an NRF grant (NRF-2014R1A2A1A11053221).
Author contributions
SMW and YM performed experiments and data analysis. K-YL designed the study and drafted the manuscript. All authors read and approved the final manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Experimental & Molecular Medicine website (http://www.nature.com/emm)
Supplementary Material
References
- Kranz A, Kinner A, Kolling R. A family of small coiled-coil-forming proteins functioning at the late endosome in yeast. Mol Biol Cell 2001; 12: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JH, Xiao C, Hayden MS, Lee KY, Trombetta ES, Pypaert M et al. CHMP5 is essential for late endosome function and down-regulation of receptor signaling during mouse embryogenesis. J Cell Biol 2006; 172: 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol 2002; 3: 893–905. [DOI] [PubMed] [Google Scholar]
- Raiborg C, Rusten TE, Stenmark H. Protein sorting into multivesicular endosomes. Curr Opin Cell Biol 2003; 15: 446–455. [DOI] [PubMed] [Google Scholar]
- Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature 2010; 464: 864–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature 2009; 458: 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH, Ren X. The circuitry of cargo flux in the ESCRT pathway. J Cell Biol 2009; 185: 185–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vild CJ, Li Y, Guo EZ, Liu Y, Xu Z. A novel mechanism of regulating the ATPase VPS4 by its cofactor LIP5 and the endosomal sorting complex required for transport (ESCRT)-III protein CHMP5. J Biol Chem 2015; 290: 7291–7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu J, Wang F, Chen M, Xiao Z, Ouyang R et al. The role of charged multivesicular body protein 5 in programmed cell death in leukemic cells. Acta Biochim Biophys Sin (Shanghai) 2013; 45: 383–390. [DOI] [PubMed] [Google Scholar]
- Kuang Z, Seo EJ, Leis J. Mechanism of inhibition of retrovirus release from cells by interferon-induced gene ISG15. J Virol 2011; 85: 7153–7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita E, Colf LA, Karren MA, Sandrin V, Rodesch CK, Sundquist WI. Human ESCRT-III and VPS4 proteins are required for centrosome and spindle maintenance. Proc Natl Acad Sci USA 2010; 107: 12889–12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajorek M, Morita E, Skalicky JJ, Morham SG, Babst M, Sundquist WI. Biochemical analyses of human IST1 and its function in cytokinesis. Mol Biol Cell 2009; 20: 1360–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HR, Xiao ZY, Chen M, Wang FL, Liu J, Zhong H et al. Anti-CHMP5 single chain variable fragment antibody retrovirus infection induces programmed cell death of AML leukemic cells in vitro. Acta Pharmacol Sin 2012; 33: 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincetic A, Kuang Z, Seo EJ, Leis J. The interferon-induced gene ISG15 blocks retrovirus release from cells late in the budding process. J Virol 2010; 84: 4725–4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wi SM, Lee KY. 5-Aminoimidazole-4-carboxamide riboside induces apoptosis through AMP-activated protein kinase-independent and NADPH oxidase-dependent pathways. Immune Netw 2014; 14: 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wi SM, Park J, Shim JH, Chun E, Lee KY. Ubiquitination of ECSIT is crucial for the activation of p65/p50 NF-κBs in Toll-like receptor 4 signaling. Mol Biol Cell 2015; 26: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Matsumoto R, You Y, Che T, Lin XY, Gaffen SL et al. CD3/CD28 costimulation-induced NF-kappaB activation is mediated by recruitment of protein kinase C-theta, Bcl10, and IkappaB kinase beta to the immunological synapse through CARMA1. Mol Cell Biol 2004; 24: 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Takeda K, Matsuzawa A, Saegusa K, Nakano H, Gohda J et al. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J Biol Chem 2005; 280: 37033–37040. [DOI] [PubMed] [Google Scholar]
- Wi SM, Moon G, Kim J, Kim ST, Shim JH, Chun E et al. TAK1-ECSIT-TRAF6 complex plays a key role in the TLR4 signal to activate NF-κB. J Biol Chem 2014; 289: 35205–35214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Baik KH, Baek KH, Chah KH, Kim KA, Moon G et al. S6K1 negatively regulates TAK1 activity in the toll-like receptor signaling pathway. Mol Cell Biol 2014; 34: 510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Chun E, Lee KY. Phospholipase A (2) of peroxiredoxin 6 has a critical role in tumor necrosis factor-induced apoptosis. Cell Death Differ 2011; 18: 1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Jeong S, Jung E, Baik KH, Chang MH, Kim SA et al. AMP-activated protein kinase-α1 as an activating kinase of TGF-β-activated kinase 1 has a key role in inflammatory signals. Cell Death Dis 2012; 3: e357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudronniere N, Villalba M, Englund N, Altman A. NF-kappa B activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-theta. Proc Natl Acad Sci USA 2000; 97: 3394–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, O'Mahony A, Mu Y, Geleziunas R, Greene WC. Protein kinase C-theta participates in NF-kappaB activation induced by CD3-CD28 costimulation through selective activation of IkappaB kinase beta. Mol Cell Biol 2000; 20: 2933–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie GG, Carrasquedo F, Delfino JM, Keen CL, Fraga CG, Oteiza PI. Epicatechin, catechin, and dimeric procyanidins inhibit PMA-induced NF-kappaB activation at multiple steps in Jurkat T cells. FASEB J 2004; 18: 167–169. [DOI] [PubMed] [Google Scholar]
- Frantz B, Nordby EC, Bren G, Steffan N, Paya CV, Kincaid RL et al. Calcineurin acts in synergy with PMA to inactivate I kappa B/MAD3, an inhibitor of NF-kappa B. EMBO J 1994; 13: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshnan A, Kempiak SJ, Bennett BL, Bae D, Xu W, Manning AM et al. Primary human CD4+ T cells contain heterogeneous I kappa B kinase complexes: role in activation of the IL-2 promoter. J Immunol 1999; 163: 5444–5452. [PubMed] [Google Scholar]
- Ward DM, Vaughn MB, Shiflett SL, White PL, Pollock AL, Hill J et al. The role of LIP5 and CHMP5 in multivesicular body formation and HIV-1 budding in mammalian cells. J Biol Chem 2005; 280: 10548–10555. [DOI] [PubMed] [Google Scholar]
- Wu W, Hodges E, Hoog C. Thorough validation of siRNA-induced cell death phenotypes defines new anti-apoptotic protein. Nucleic Acids Res 2006; 34: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksena S, Emr SD. ESCRTs and human disease. Biochem Soc Trans 2009; 37: 167–172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.