Abstract
G-protein–coupled receptors (GPCRs) are one of the most attractive therapeutic target classes because of their critical roles in intracellular signaling and their clinical relevance to a variety of diseases, including cancer, infection and inflammation. However, high conformational variability, the small exposed area of extracellular epitopes and difficulty in the preparation of GPCR antigens have delayed both the isolation of therapeutic anti-GPCR antibodies as well as studies on the structure, function and biochemical mechanisms of GPCRs. To overcome the challenges in generating highly specific anti-GPCR antibodies with enhanced efficacy and safety, various forms of antigens have been successfully designed and employed for screening with newly emerged systems based on laboratory animal immunization and high-throughput-directed evolution.
Introduction
Since the beginning of the monoclonal antibody era led by the success of hybridoma technology in the middle of the 1970s, a variety of platform technologies, including antibody humanization, antibody display techniques and the use of humanized animals for the generation of fully human antibodies have been used to convert immunoglobulin G (IgG) molecules into remarkable therapeutics with outstanding efficacy and significantly decreased side effects. For the treatment of patients with acute or chronic diseases such as cancer, autoimmune disease, infection and inflammation, conventional small-molecule drugs have been replaced with target-specific and highly serum-stable monoclonal antibody therapeutics in the clinic. Many pharmaceutical companies have expanded their monoclonal antibody business to keep pace with the growing market for therapeutic monoclonal antibodies.1
G-Protein–coupled receptors (GPCRs) are cell-surface signal transmission receptor proteins that transfer messages regarding the extracellular conditions to intracellular effector molecules for signaling or cellular physiological changes. They interact with various extracellular ligands, including peptides, nucleotides, lipids, small organic compounds, ions and photons, and influence a broad array of key biological processes such as homeostasis, proliferation and migration of cells as well as the sensing of olfaction, taste and vision. Upon binding of ligands to the extracellular domain of a GPCR, the conformation of the transmembrane region is altered, changing the interaction between intracellular molecules, G-proteins and arrestins.2 Because of the functional diversity of GPCRs, abnormal expression and activity of GPCRs are involved in many types of diseases, including cancer,3 infection,4 inflammation5 and cardiovascular disease.6 Therefore, GPCRs are considered key target molecules for drug discovery, and 30–50% of the targets of currently marketed therapeutic drugs are GPCRs.7, 8, 9
Most drugs directed at GPCRs in the clinic are analogs of natural ligands that function as an agonist or an antagonist. To regulate the diverse conformation of GPCRs for the desired intracellular signaling, monoclonal antibodies exhibiting superior specificity, which is not possible with conventional small molecules, show great potential. In addition, monoclonal antibodies show prolonged serum half-lives and therapeutic effector functions for the clearance of defective cells such as tumor cells and infected cells, indicating their high druggability compared with small-molecule chemical drugs.
Despite the success of monoclonal therapeutic antibodies in the clinic and in the market, substantial bottlenecks exist in the development of anti-GPCR antibodies, and no GPCR targeting antibody has been approved by the United States Food and Drug Administration and European Medicines Agency. A glycoengineered antibody, mogamulizumab (Poteligeo) targeting CCR4, was approved for use in Japan in 2012.10 Overall, there are four major reasons for the delay in the development of anti-GPCR antibodies. (1) Preparing homogeneous functional GPCR antigens is difficult; (2) it is not easy to develop efficient antibody screening tools; (3) the conformation of the GPCR extracellular region is highly variable and (4) the exposed area of the GPCR extracellular epitopes is limited.
However, recent advances in the development of antibody isolation technologies as well as in the understanding of GPCR structure, function and clinical relevance has shown some promise to overcome these hurdles. This review focuses on the techniques used to prepare functional GPCR antigens and the isolation of highly challenging anti-GPCR therapeutic antibodies. In addition, therapeutic anti-GPCR antibodies under clinical trials are discussed.
Human GPCR structure and relevance in diseases
GPCRs belong to the largest membrane receptor family and share common heptahelical transmembrane receptor structure. Nearly 4% of all genes in the human genome encode GPCRs,11 and more than 800 members have been identified.3 Based on sequence, structural and functional similarities, GPCRs are generally divided into five major classes: rhodopsin, adhesion, secretin, metabotropic glutamate and frizzled.9, 12, 13
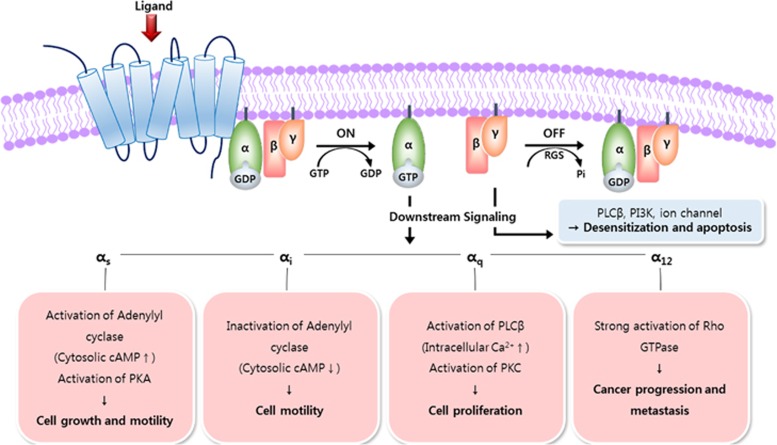
Although GPCRs are indispensable for numerous critical cellular physiological functions as well as for the sensing of vision, taste and olfaction, low expression levels and instability after extraction from the native membrane have delayed the crystallization of GPCRs and the determination of their structure and function. After the first report of the crystal structure of bovine rhodopsin in 200014 and the second crystal structure of GPCR, the human β2 adrenergic receptor 7 years later,15, 16 significant progress has been made in understanding the structure and function relationship of GPCRs. The intracellular C-terminal region of a GPCR interacts with the heterotrimeric G-protein α, β and γ subunits. The binding of agonist ligands to the extracellular region of a GPCR changes the conformation of the transmembrane and intracellular regions and induces GDP exchange, which is bound to Gα, with GTP. Next, Gα is released from the Gβγ dimer coupled to GPCR, and a subsequent interaction of Gα and Gβγ with effector molecules inside the cells triggers intracellular downstream signal transduction processes. Gα, which possesses GTPase activity, hydrolyzes the GTP, and then subsequent reassociation of GDP-bound Gα to Gβγ restores the GPCR to the inactive state. By recruiting β-arrestin, GPCR signaling is turned off or becomes independent of G-proteins (Figure 1).
Figure 1.
G-protein–coupled receptor (GPCR) signaling pathway. GPCR signaling is initiated by ligand binding that triggers a conformational change in the receptor. GPCR in the inactive state interacts with a heterotrimeric G-protein consisting of a GDP-bound Gα subunit and Gβγ subunit. Ligand binding induces the exchange of GDP with GTP and the dissociation of the Gβγ subunit, leading to a wide range of biological regulation. Depending on the subclasses of Gα, Gαs, Gαi, Gαq and Gα12, various downstream signaling molecules, such as adenylyl cyclase, protein kinase A (PKA), phospholipase Cβ (PLCβ), protein kinase C (PKC) and Rho GTPase are involved in the change of cellular physiology and the progression of diseases. The dissociated Gβγ subunit is related to desensitization and apoptosis.
Given the relevance of GPCRs to the progression of diseases such as cancer, infection, inflammation and neurological disorder, they are excellent therapeutic targets. Aberrant expression and activity of GPCRs is closely related to all stages of tumor development.11, 17 Tumor cells hijack GPCR function for angiogenesis, proliferation and metastasis to evade normal immune functions. It has been well documented that numerous types of GPCRs are overexpressed in a variety of tumor cells.11 Particularly, chemokine GPCRs, including CXCR4, are highly involved in angiogenesis and the movement of tumor cells in the tumor microenvironment.18, 19 In addition, GPCRs directed at lipid ligands, lysophosphatidic acid and sphingosine-1-phosphate, are highly expressed on the surface of tumor cells and induce the growth, proliferation and metastasis of aberrant cancerous cells.11 GPCRs targeting neuropeptides, including gastrin-releasing peptide, endothelin, bradykinin, neuromedin B, cholecystokinin and angiotensin II are activated in many types of cancers such as small-cell lung cancer,20 pancreatic cancer,21 prostate cancer22 and head and neck cancer.23 The association between prostaglandins derived from cyclooxygenases (COX1 and COX2) and GPCRs are known to trigger pro-inflammatory functions, leading to the tumorigenesis of common cancers.11
Various GPCRs have crucial roles in infection processes. For example, CCR5 and CXCR4 are co-receptors for the entry of HIV and are considered to be excellent drug targets against HIV infection.4 Persons with a deficiency in the CCR5 gene were shown to be resistant to HIV infection,24 and the transplantation of stem cells from a donor who carried the homozygous genetic mutation of CCR5 gene, known as CCR5-delta32, into an HIV patient was effective for recovering normal T-cell immune functions.25
Preparation of GPCR antigens
Purified GPCR antigens
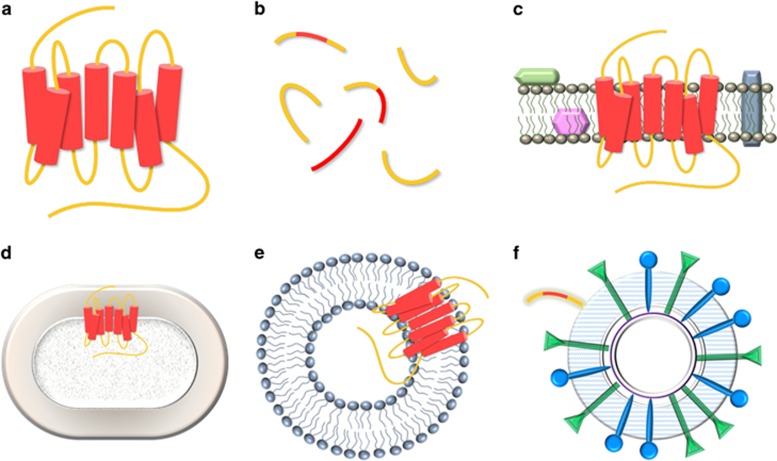
Anti-GPCR antibodies can be isolated via animal immunization and high-throughput antibody-directed evolution techniques. However, one of the biggest hurdles is the difficulty in preparing homogeneous and functional GPCR antigens. For the purification of functional GPCRs (Figure 2a), various cellular and cell-free expression systems have been employed. In most cases, the GPCR expression level is typically very low compared with the expression level of other soluble proteins. Considering features such as inexpensive cultivation, fast growth and excellent scalability, the Escherichia coli expression system has been used to express a variety of GPCRs. However, many types of GPCRs are localized in the inclusion body fraction, requiring complex and inefficient refolding steps to induce GPCR activity.26 Lundstrom et al. successfully expressed 103 types of GPCRs in three expression hosts, E. coli, Pichia pastoris and mammalian cells. In the comprehensive expression trial, about half of the tested GPCRs were expressed in the inclusion body form in the E. coli expression system.26 Lee et al. fused the P9 protein, one of the envelope proteins in bacteriophage phi6, to the N-terminus of the human endothelin receptor, which is associated with multiple diseases including cardiovascular disease and cancer. The GPCR proteins were expressed mostly in the membrane fraction of E. coli and could be purified with excellent yield (over 1 mg l−1). The resulting purified proteins exhibited high-binding affinity to the ligand (ET-1) and Gα, suggesting the formation of a near native structure of the endothelin receptor.27
Figure 2.
Preparation of G-protein–coupled receptor (GPCR) antigens. (a) GPCRs purified and solubilized in detergent. (b) Linear or cyclic constrained peptide made up of N-terminus or extracellular loops. (c) Membrane fraction containing diverse membrane proteins. (d) Whole cells expressing GPCR on the surface. (e) GPCR surrounded by a liposome-like lipid bilayer. (f) Virus-like particle (VLP) coupled with GPCR peptide.
The unicellular yeast strains have many advantages such as ease of genetic manipulation, fast growth and inexpensive cultivation compared with other eukaryotic hosts. The yeast strains Pichia pastoris26, 28 and Saccharomyces cerevisiae29 have been successfully utilized for the expression of various functional GPCRs. To produce functional GPCRs with improved quality, an insect cell culture system in combination with baculoviruses has been previously used.30, 31 Researchers at GlaxoSmithKline comparatively analyzed the expression of 16 types of human GPCRs in three different insect cell lines, Sf9, Sf21 and High Five. Over half of the GPCRs were successfully expressed and the yield of individual GPCRs varied greatly with the type of cell line.30 In addition, in spite of the high cost and limited scalability, mammalian cells combined with transient transfection or viral infection have been employed for the production of high-quality functional GPCRs.26, 32
Cell-free protein synthesis is an excellent alternative to cellular expression for the production of proteins exhibiting very low expression levels and stability in heterologous hosts,33 despite disadvantages such as high reagent cost, low production rates and short reaction duration.34 Takeda et al. used a wheat cell-free system combined with a bilayer-dialysis method and prepared 26 types of GPCRs on a milligram scale, which were suitable for animal immunizations. They were able to isolate anti-GPCR antibodies against four types of GPCRs, DRD1, GHSR, PTGER and T1R1.35
GPCRs expressed in heterologous hosts should be solubilized before purification. For extraction from cell membranes, mild long-chain detergents such as dodecylmaltoside have been widely used.36 However, GPCRs extracted from the native membrane are generally unstable and prone to degradation. In addition, plasticity of the GPCR structure results in conformational differences between the detergent-solubilized form and the native membrane-bound form. Therefore, it is essential to check whether the GPCRs solubilized in detergents have native-like conformations before use as functional GPCR antigens.
To improve the stability and expression level of GPCRs, significant efforts in protein engineering and high-throughput screening have been made. Alanine scanning is one of the protein engineering strategies to identify stabilizing mutations.37, 38 In this method, each amino acid, except alanine itself, which is mutated to leucine, is exchanged with alanine to confer relatively lower steric hindrance and structural disruption.39 Based on this technique, Heptares Therapeutics generated hundreds of variants containing point mutations for a target GPCR, and conformationally thermostabilized mutants could be successfully isolated.40 As an alternative to laborious mutagenesis and screening methods, a more efficient strategy to improve GPCR expression levels and stability in detergents is directed evolution in conjunction with high-throughput screening tools. Plückthun and colleagues constructed GPCR libraries via error-prone PCR or chimerization techniques, and they permeabilized the outer membranes to bind to fluorescently labeled GPCR ligands. Using flow cytometric screening to interrogate individual cells, which express a GPCR variant at a different level, they could isolate functional GPCR mutants exhibiting significantly improved expression. The stability in detergent was then analyzed for the selected GPCR mutants.41, 42, 43, 44 To isolate genes conferring enhanced expression in E. coli, Georgiou and colleagues screened a genomic library composed of chromosomal fragments by either expressing GFP-fused GPCRs or displaying GPCR on the inner membrane of E. coli to bind fluorescent ligands following spheroplast formation. Using flow cytometric sorting of highly fluorescent E. coli resulting from the co-expression of a beneficial genetic component of the genomic fragments library, they could identify three genes that allowed for 3- to 10-fold improved expression of three model mammalian GPCRs.45
Peptides, whole cells, virus-like particles, proteoliposomes and denatured GPCRs
Linear peptides or cyclic constrained peptides derived from the N-terminus or extracellular loops can mimic the extracellular domains of GPCRs on human cells, and they have been utilized as antigens to elicit anti-GPCR antibodies (Figure 2b). However, there is a possibility that the surrogate peptides may not resemble the structural features and conformation of the original extracellular loops of the GPCRs in the native membrane. By immunizing an N-terminal domain peptide, Kirin Co. prepared hybridoma cells and isolated an antibody (KM4056) exhibiting very-high-specific binding affinity to LGR5 (KD<34 pM), which is a leucine-rich repeat-containing orphan GPCR, but without significant binding to the structurally similar LGR4 and LGR6. The antibody showed high complement-mediated cytotoxicity (CDC) activity, and the effectiveness was validated using a LGR5-overexpressing xenograft tumor model.46 The activity of cholecystokinin-B receptor (CCK-BR) is highly relevant to gastric or gastrointestinal cancers. To isolate antibodies targeting CCK-BR, Tohidkia et al. synthesized biotinylated peptides corresponding to the second extracellular loop (ECL2) of CCK-BR and screened a scFv phage library. Major portions of the scFvs isolated from the library could recognize the native conformation of CCK-BR.47 Huang et al. synthesized a biotinylated peptide comprising N-terminal 31 amino acids of the C5aR, a receptor for complement anaphylatoxin C5a, which is highly involved in inflammatory disorders. A Fab phage library was screened against immobilized C5aR, and several full-length IgG antibodies converted from the isolated Fab showed excellent binding to differentiated U-937 cells expressing C5aR.48
Generally, the GPCR expression level on the native cell membrane is not very high, and there are many other proteins on the cell surface. In addition, the extracellular region is relatively small, and the extracellular epitopes of interest may not be accessible for antibody binding. Therefore, animal immunization and standard phage library panning against whole cells (Figure 2c) or membrane fractions (Figure 2d) result in high levels of nonspecific antibody background; antibodies not relevant to target GPCRs are amplified because of steric hindrance and the low abundance of antigens on the surface. To isolate antibodies targeting CCR5, a chemokine receptor and one of the co-receptors for HIV infection, Osbourn et al. screened a human naïve library. They used biotin tyramine-based proximity selection (step-back selection) to isolate antibodies inhibiting the association of a CCR5 ligand, macrophage inflammatory protein, to CD4 because it shared the same binding site as macrophage inflammatory protein-α.49 Using the same technique,49 Sui et al. screened a human nonimmune antibody phage display library to isolate antibodies against CXCR4, which is a co-receptor in addition to CCR5 that has a central role in HIV infection. Although antibodies specific to CD4 and transferrin were isolated, they were unable to isolate CXCR4-specific antibodies.50
To prepare paramagnetic CCR5 proteoliposomes (Figure 2e), Mirzabekow et al. immobilized streptavidin and antibodies recognizing the C-terminus tag of CCR5, followed by capturing CCR5 from the native membrane. The paramagnetic beads were then reconstituted with detergent-solubilized lipids, biotinylated-DOPE 2000 and dialyzed to remove detergents for lipid bilayer self-assembly. Using the prepared CCR5 proteoliposomes, antibodies specific to CCR5 were isolated from the human scFv antibody library. After the 5th round of panning, more than 50% of the clones were found to show positive signals, with significant CCR5-binding affinities in the phage ELISA and in the analysis with native cells.51
Virus-like particles are non-infectious particles that are more stable to handle than intact GPCR-expressing cells for antibody generation (Figure 2f). A peptide sequence of the extracellular region was synthesized and coupled to a virus-like particle derived from the RNA bacteriophage Qβ to immunize rabbits and mice. The resulting antibody could bind to CCR5 on the cells and exhibited in vitro HIV-neutralizing activity.52
Talmont et al. found that GPCRs denatured in SDS and urea could be used to generate GPCR-specific polyclonal antibodies, which would be useful for the analysis of GPCRs. This approach was evaluated in three types of human GPCRs: neuropeptide FF receptor type 2, m-opioid receptor and k-opioid receptor. They produced the GPCRs in Pichia pastoris, solubilized in SDS and urea, and subsequently immunized mice with the denatured GPCRs. The resulting polyclonal antibodies could recognize the native conformation of GPCRs on the cell surfaces, suggesting partial folding of the GPCRs during denaturing or restoration of the native conformation in Freund's adjuvant, which might be similar to the lipid bilayer of cells.53
Techniques to isolate anti-GPCR antibodies
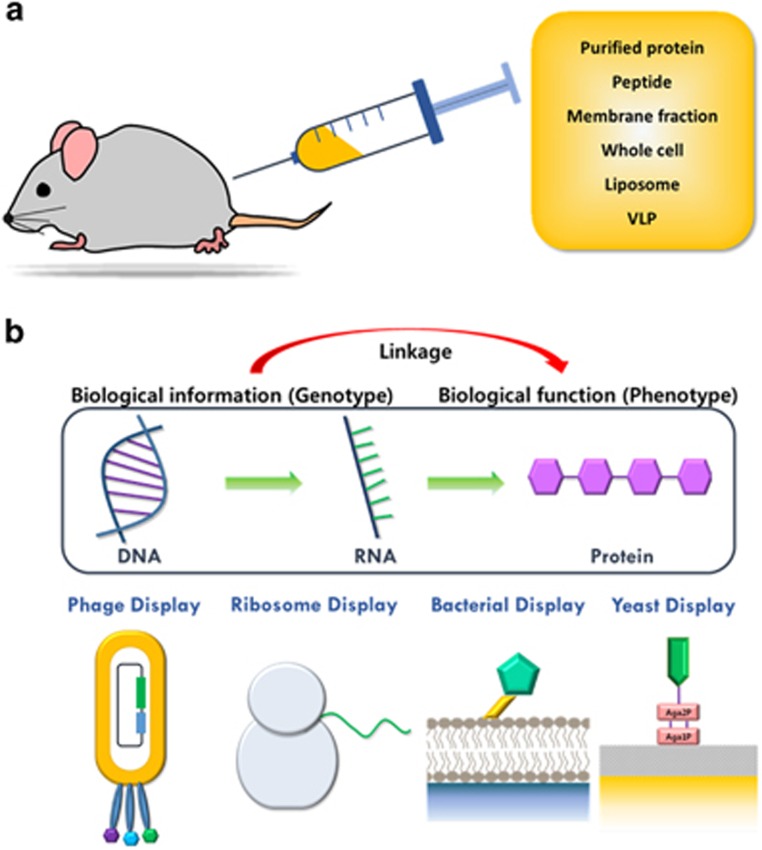
Owing to the high conformational variability of the extracellular region of GPCRs and the limited exposed area of the extracellular epitopes of interest, it is highly challenging to isolate anti-GPCR therapeutic antibodies. To isolate antibodies targeting GPCRs, commonly used methods include animal immunization (Figure 3a), high-throughput-directed evolution (Figure 3b) and the combination of immunization and high-throughput-directed evolution for affinity and functional maturation.
Figure 3.
Screening technologies for the isolation of anti-G-protein–coupled receptor (GPCR) antibodies. (a) Immunization of laboratory animals with various types of GPCR antigens such as purified protein, peptide, membrane fraction, virus-like particle and proteoliposome. (b) High-throughput-directed evolution combined with antibody display technologies such as phage, ribosome, bacterial and yeast display for the linkage of genotype and phenotype.
Immunization of laboratory animals
The success of hybridoma technology in the mid-1970s promoted laboratory animal immunization with prepared antigens as a powerful method for the generation of antigen-specific monoclonal antibodies. This approach uses the natural immune selection system of the immunized animals. Although ethical issues concerning the use of laboratory animals should be fully addressed and sometimes additional in vitro affinity or functional maturation is required, various forms of GPCR antigens such as purified protein, peptide, virus-like particle and proteolipsomes have been widely used to isolate target GPCR-specific antibodies. For therapeutic use without potential immunogenicity, the antibodies isolated from laboratory animals should be humanized by grafting the GPCR-binding complementarity determining regions (CDRs) to the human antibody framework. Alternatively, humanized transgenic animals that produce the complete human antibody sequence can be used.54 To develop therapeutic antibodies effective for patients with type 2 diabetes, researchers in Amgen immunized humanized mice with whole cells or N-terminal extracellular region peptides fused to Fc, and they could isolate fully human antibodies against hGCGR, a human glucagon receptor. The generated monoclonal antibody exhibited higher antagonizing binding affinity (KD=35 pM) to the hGCGR compared with the natural hGCGR ligand, glucagon (KD=100 pM), and long-term efficacy in mice and the cynomolgus monkey model, which is in sharp contrast to the previously developed anti-glucagon antibodies, which resulted in the secretion of a large amount of endogenous glucagon and requires a high dosage for efficacy.55
High-throughput-directed evolution
Various combinatorial approaches have been developed to isolate antigen-specific antibodies, and these high-throughput-directed evolution techniques include phage, bacterial, yeast surface, mammalian cell, mRNA and ribosome displays.56 Of these, phage display, a robust technique for presenting antibody fragments on the surface of bacteriophages by the fusion of a gene encoding a coat protein is the most widely used.57 Screening of an antibody library, such as an immune or synthetic library, has been performed against different types of antigens, purified proteins, peptides,47, 48, 58 whole cells50, 59 and proteoliposomes.52
Ravn et al. purified the extracellular domain of the receptor for glucose-dependent insulinotropic polypeptide (GIP), which regulates insulin secretion. Using phage display and ribosome display, they isolated antagonizing antibodies with a high-binding affinity to the GIPr and decreased cyclic adenosine monophosphate (cAMP) production mediated by inhibition of the GIP–GIPr interaction.60 The C–C chemokine receptor (CCR4), which is overexpressed in cancerous T cells, regulates the migration of immune cells and is critical for tumor development. Although anti-CCR4 antibodies were isolated by animal immunization in the previous study, Hagemann et al. used a cell-based panning method and screened the naïve human antibody phage library on CCR4-expressing cells. After affinity maturation, the resulting antagonizing anti-CCR4 antibodies inhibited intracellular signaling as measured by a calcium release assay and showed dose-dependent inhibition of cell migration. Furthermore, the antibodies exhibited excellent antibody-dependent cell-mediated cytotoxicity (ADCC) activities, which could be enhanced by defucosylation.61 Antibody candidates generated by initial screening of the phage display library could be characterized by the SHM-XEL platform, a mammalian cell surface display system developed by AnaptysBio.62
pH-dependent antigen binding is useful for improving the efficacy of therapeutic antibodies. Antigen binding at a neutral pH in serum and the release of bound antigens at a slightly acidic pH in endosomes facilitates the removal of target antigens in the internalized compartment and the regeneration of antibodies by reducing antigen-mediated antibody clearance.63 Bonvin et al. engineered antibodies targeting CXCR4 for pH-dependent binding. By randomizing the third complementarity determining region of the antibody heavy chain (CDRH3) region with selected codons, which were biased for the high abundance of histidine, they used phage display techniques to generate antagonizing antibodies to the CXCR4 receptor at neutral pH with no significant binding at pH 6.0.64
Combination of animal immunization and high-throughput-directed evolution
In numerous cases, the affinity of antibodies isolated by either animal immunization or the screening of a naïve antibody library using various display techniques is insufficient and affinity maturation is often required. Antibody display techniques can be employed with purified GPCR proteins, such as extracellular loop mimicking peptides and GPCR-expressing whole cells. Douthwaite et al. immunized transgenic mice possessing the human immunoglobulin variable genes (VelocImmune) with human embryonic kidney cells overexpressing human anti-formyl peptide receptor 1 (FPR1), which regulates the movement of leukocytes during inflammation and is also highly involved in the progression of malignant human glioblastoma. The isolated antibody had a long CDRH3 consisting of 24 amino acids, which is distinguishable from normal CDRH3 (length 8–18 residues), suggesting a critical role of CDRH3 and the long length of the CDRH3 in binding to the extracellular loop of formyl peptide receptor 1. For functional maturation, 19 individual phage libraries incorporating 4–6 randomized amino acids in the 6 CDRs were constructed and screened on human embryonic kidney cells transfected with formyl peptide receptor 1. The resulting affinity-matured anti-formyl peptide receptor 1 antibody showed ~40-fold improved IC50 in the calcium release assay compared with the antibody isolated in the initial screening from immunized transgenic mice.65
Anti-GPCR antibodies in the clinic
Despite the significance of GPCRs as therapeutic targets and the excellent druggability of monoclonal antibodies, the use of anti-GPCR therapeutic antibodies in the clinical setting is limited and no anti-GPCR antibodies has been approved by the United States Food and Drug Administration and European Medicines Agency. The only commercialized antibody is mogamulizumab (KW-0761), a humanized IgG1 antibody, developed exclusively by Kyowa Hakko Kirin and approved in Japan in 2012 for the treatment of relapsed or refractory adult T-cell leukemia-lymphoma. This antibody is a humanized version of KW-2160 isolated from mice immunized with an N-terminal peptide consisting of 18 amino acids of human CCR4. To enhance the binding affinity to FcγRIIIa expressed on the surface of natural killer cells and effecting natural killer cell-mediated ADCC effector function against CCR4 positive cells, the N-linked glycan appended at Asn297 of the IgG Fc is defucosylated.66, 67, 68 In the United States, clinical trials for adult T-cell leukemia-lymphoma and cutaneous T-cell lymphoma are being evaluated under clinical phase II and phase III trials, respectively. For various other GPCR targets, anti-GPCR antibodies are under development. The detailed clinical trial status is summarized in the Table 1.
Table 1. Anti-GPCR antibodies under clinical trials.
| Receptor target | Antibody drug | Disease indication | Company/institute | Status |
| CCR2 | MNL1202 | Bone metastases | Southwest Oncology group | Phase II |
| Arterial inflammation | Takeda | Phase II | ||
| Atherosclerosis | Millennium Pharmaceuticals | Phase II | ||
| CCR4 | KW-0761 (Mogamulizumab) | Adult T-cell leukemia and lymphoma | Kyowa Hakko Kirin | Phase II |
| Peripheral T/NK-cell lymphoma | Kyowa Hakko Kirin | Phase II | ||
| Adult PTCL | Kyowa Hakko Kirin | Phase II | ||
| CTCL | Kyowa Hakko Kirin | Phase III | ||
| AMG761 | Asthma | Amgen | Phase I | |
| CCR5 | PRO140 | HIV infection | CytoDyn | Phase III |
| CCR5 mAb004 | HIV infection | Human Genome Science | Phase I | |
| HGS1025 | Ulcerative colitis | Human Genome Science | Phase I | |
| HGS004 | HIV infection | Human Genome Science | Phase I | |
| CCR9 | MLN3126 | Inflammation | Takeda | Phase I |
| CXCR4 | BMS-936564 | Multiple myeloma | Bristol-Myers Squibb | Phase I |
| ALX-0651 | Stem cell mobilization | Ablynx | Phase I | |
| LY-2624587 | Metastatic cancer | Eli Lilly | Phase I | |
| CXCR5 | SAR113244 | Systemic lupus erythematosus | Sanofi | Phase I |
| C5aR | NNC0151-0000-0000 (NN-8209) | Rheumatoid arthritis | Novo Nordisk | Phase II |
| NNC0215-0384 (NN-8210) | Rheumatoid arthritis, inflammation | Novo Nordisk | Phase I | |
| CGRP-R | AMG-334 | Migraine | Amgen | Phase II |
| Hot flashes | Amgen | Phase I | ||
| GCG-R | AMG-477 | Type 2 diabetes | Amgen | Phase I |
| FZD1, 2, 5, 7, 8 | OMP-18R5 (Vantictumab) | Solid tumor (NSCLC) | Oncomed | Phase I (combined with Docetaxel) |
| Metastatic breast cancer | Oncomed | Phase I (combined with paclitaxel) |
Abbreviations: CTCL, cutaneous T-cell lymphoma; GPCR, G-protein–coupled receptor; NK, natural killer; NSCLC, non-small-cell lung cancer; PTCL, peripheral T-cell lymphoma.
Conclusion
GPCRs are one of the most attractive drug targets in the pharmaceutical industry, and monoclonal antibodies are an essential agent for the functional evaluation and therapeutic application of GPCRs. Conventionally, major therapeutic drugs in the pharmaceutical industry directed at GPCRs have been small molecules, even though monoclonal antibodies have been one of the most effective therapeutics for the treatment of cancer, infection and inflammatory disease. Mining for therapeutic anti-GPCR antibodies has been slow. A major reason for this delay in anti-GPCR antibody development is the preparation of functional and conformationally active GPCR antigens, the expression and purification of which are notoriously difficult to achieve, owing to poor stability in detergents. Moreover, a small extracellular region and conformational variability depending on the activation status have hindered the isolation of excellent anti-GPCR agonists and antagonists.
However, many researchers are making efforts to understand GPCR structures and functions. The accumulated knowledge will provide insights for anti-GPCR drug discovery, and the emerging techniques for preparation of functional GPCR antigens and novel screening tools will accelerate the isolation of clinically crucial anti-GPCR antibody therapeutics. Although only one anti-GPCR antibody, mogamulizumab, has been commercialized for clinical use so far, more anti-GPCR antibodies will enter clinical evaluation and will be commercialized for use in the clinic in the near future. Accurate control of GPCR functions mediated by anti-GPCR therapeutic antibodies will be used to treat cancer, infection and inflammation and to meet the medical needs of patients.
Acknowledgments
This work was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1420160), the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2013R1A1A1004576), and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIP; 2014M3A9D9069609).
The authors declare no conflict of interest.
References
- Walsh G. Biopharmaceutical benchmarks 2014. Nat Biotechnol 2014; 32: 992–1000. [DOI] [PubMed] [Google Scholar]
- Kobilka BK. G protein coupled receptor structure and activation. Biochem Biophys Acta 2007; 1768: 794–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hayre M, Vazquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S et al. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer 2013; 13: 412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib G. The biology of CCR5 and CXCR4. Curr Opin HIV AIDS 2009; 4: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Ye RD. Role of G protein-coupled receptors in inflammation. Acta Pharmacol Sin 2012; 33: 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte SL, Blaxall BC. Conducting the G-protein coupled receptor (GPCR) signaling symphony in cardiovascular diseases: new therapeutic approaches. Drug Discov Today Dis Models 2012; 9: e85–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Stevens RC, Xu F. The importance of ligands for G protein-coupled receptor stability. Trends Biochem Sci 2015; 40: 79–87. [DOI] [PubMed] [Google Scholar]
- Schlyer S, Horuk R. I want a new drug: G-protein-coupled receptors in drug development. Drug Discov Today 2006; 11: 481–493. [DOI] [PubMed] [Google Scholar]
- Salon JA, Lodowski DT, Palczewski K. The significance of G protein-coupled receptor crystallography for drug discovery. Pharmacol Rev 2011; 63: 901–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R, Satoh M. The current status and prospects of antibody engineering for therapeutic use: focus on glycoengineering technology. J Pharm Sci 2015; 104: 930–941. [DOI] [PubMed] [Google Scholar]
- Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer 2007; 7: 79–94. [DOI] [PubMed] [Google Scholar]
- Hutchings CJ, Koglin M, Marshall FH. Therapeutic antibodies directed at G protein-coupled receptors. MAbs 2010; 2: 594–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SGF, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature 2009; 459: 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science 2000; 289: 739–745. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SGF, Thian FS, Kobilka TS et al. GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science 2007; 318: 1266–1273. [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SGF, Thian FS, Kobilka TS et al. High resolution crystal structure of an engineered human β(2)-adrenergic G protein-coupled receptor. Science (New York, N.Y.) 2007; 318: 1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Xie N, Zhao X, Nice EC, Huang C. Dissection of aberrant GPCR signaling in tumorigenesis – a systems biology approach. Cancer Genomics Proteomics 2012; 9: 37–50. [PubMed] [Google Scholar]
- Balkwill FR. The chemokine system and cancer. J Pathol 2012; 226(2): 148–157. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410: 50–56. [DOI] [PubMed] [Google Scholar]
- Heasley LE. Autocrine and paracrine signaling through neuropeptide receptors in human cancer. Oncogene 2001; 20: 1563–1569. [DOI] [PubMed] [Google Scholar]
- Szepeshazi K, Schally AV, Nagy A, Halmos G. Inhibition of growth of experimental human and hamster pancreatic cancers in vivo by a targeted cytotoxic bombesin analog. Pancreas 2005; 31: 275–282. [DOI] [PubMed] [Google Scholar]
- Daaka Y. G proteins in cancer: the prostate cancer paradigm Sci Signal 2004; 2004: re2. [DOI] [PubMed] [Google Scholar]
- Lango MN, Dyer KF, Lui VWY, Gooding WE, Gubish C, Siegfried JM et al. Gastrin-releasing peptide receptor-mediated autocrine growth in squamous cell carcinoma of the head and neck. J Natl Cancer Inst 2002; 94: 375–383. [DOI] [PubMed] [Google Scholar]
- O'Brien TR, Winkler C, Dean M, Nelson JAE, Carrington M, Michael NL et al. HIV-1 infection in a man homozygous for CCR5▵32. Lancet 1997; 349: 1219. [DOI] [PubMed] [Google Scholar]
- Hütter G, Zaia JA. Allogeneic haematopoietic stem cell transplantation in patients with human immunodeficiency virus: the experiences of more than 25 years. Clin Exp Immunol 2011; 163: 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom K, Wagner R, Reinhart C, Desmyter A, Cherouati N, Magnin T et al. Structural genomics on membrane proteins: comparison of more than 100 GPCRs in 3 expression systems. J Struct Funct Genomics 2006; 7: 77–91. [DOI] [PubMed] [Google Scholar]
- Lee K, Jung Y, Lee JY, Lee W-K, Lim D, Yu YG. Purification and characterization of recombinant human endothelin receptor type A. Protein Expr Purif 2012; 84: 14–18. [DOI] [PubMed] [Google Scholar]
- Shimamura T, Shiroishi M, Weyand S, Tsujimoto H, Winter G, Katritch V et al. Structure of the human histamine H1 receptor complex with doxepin. Nature 2011; 475: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley MA, Mancini JD, Young CL, McCusker EC, Raden D, Robinson AS. Progress toward heterologous expression of active G-protein-coupled receptors in Saccharomyces cerevisiae: linking cellular stress response with translocation and trafficking. Protein Sci 2009; 18: 2356–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akermoun M, Koglin M, Zvalova-Iooss D, Folschweiller N, Dowell SJ, Gearing KL. Characterization of 16 human G protein-coupled receptors expressed in baculovirus-infected insect cells. Protein Expr Purif 2005; 44: 65–74. [DOI] [PubMed] [Google Scholar]
- Wu B, Chien EYT, Mol CD, Fenalti G, Liu W, Katritch V et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 2010; 330(6007): 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deupi X, Edwards P, Singhal A, Nickle B, Oprian D, Schertler G et al. Stabilized G protein binding site in the structure of constitutively active metarhodopsin-II. Proc Natl Acad Sci USA 2012; 109: 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin AS. High-throughput cell-free systems for synthesis of functionally active proteins. Trends Biotechnol 2004; 22: 538–545. [DOI] [PubMed] [Google Scholar]
- Zheng X, Dong S, Zheng J, Li D, Li F, Luo Z. Expression, stabilization and purification of membrane proteins via diverse protein synthesis systems and detergents involving cell-free associated with self-assembly peptide surfactants. Biotech Adv 2014; 32: 564–574. [DOI] [PubMed] [Google Scholar]
- Takeda H, Ogasawara T, Ozawa T, Muraguchi A, Jih P-J, Morishita R et al. Production of monoclonal antibodies against GPCR using cell-free synthesized GPCR antigen and biotinylated liposome-based interaction assay. Sci Rep 2015; 5: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke RM, Koglin M, Errey JC, Marshall FH. Preparation of purified GPCRs for structural studies. Biochem Soc Trans 2013; 41: 185–190. [DOI] [PubMed] [Google Scholar]
- Dore AS, Okrasa K, Patel JC, Serrano-Vega M, Bennett K, Cooke RM et al. Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Nature 2014; 511: 557–562. [DOI] [PubMed] [Google Scholar]
- Lebon G, Bennett K, Jazayeri A, Tate CG. Thermostabilisation of an agonist-bound conformation of the human adenosine A(2A) receptor. J Mol Biol 2011; 409: 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaber M, Zhang X, Matthews B. Structural basis of amino acid alpha helix propensity. Science 1993; 260: 1637–1640. [DOI] [PubMed] [Google Scholar]
- Robertson N, Jazayeri A, Errey J, Baig A, Hurrell E, Zhukov A et al. The properties of thermostabilised G protein-coupled receptors (StaRs) and their use in drug discovery. Neuropharmacology 2011; 60: 36–44. [DOI] [PubMed] [Google Scholar]
- Sarkar CA, Dodevski I, Kenig M, Dudli S, Mohr A, Hermans E et al. Directed evolution of a G protein-coupled receptor for expression, stability, and binding selectivity. Proc Natl Acad Sci USA 2008; 105: 14808–14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodevski I, Plückthun A. Evolution of three human GPCRs for higher expression and stability. J Mol Biol 2011; 408: 599–615. [DOI] [PubMed] [Google Scholar]
- Schlinkmann KM, Hillenbrand M, Rittner A, Künz M, Strohner R, Plückthun A. Maximizing detergent stability and functional expression of a GPCR by exhaustive recombination and evolution. J Mol Biol 2012; 422: 414–428. [DOI] [PubMed] [Google Scholar]
- Schlinkmann KM, Plückthun A. Directed evolution of G-protein-coupled receptors for high functional expression and detergent stability. Methods Enzymol 520: 67–97. [DOI] [PubMed] [Google Scholar]
- Skretas G, Makino T, Varadarajan N, Pogson M, Georgiou G. Multi-copy genes that enhance the yield of mammalian G protein-coupled receptors in Escherichia coli. Metab Eng 2012; 14: 591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Kosaka H, Usami K, Toki H, Kawai H, Shiraishi N et al. Establishment of a novel monoclonal antibody against LGR5. Biochem Biophys Res Commun 2010; 394: 498–502. [DOI] [PubMed] [Google Scholar]
- Tohidkia M, Asadi F, Barar J, Omidi Y. Selection of Potential therapeutic human single-chain Fv antibodies against cholecystokinin-B/gastrin receptor by phage display technology. BioDrugs 2013; 27: 55–67. [DOI] [PubMed] [Google Scholar]
- Huang L, Sato AK, Sachdeva M, Fleming T, Townsend S, Dransfield DT. Discovery of human antibodies against the C5aR target using phage display technology. J Mol Recognit 2005; 18: 327–333. [DOI] [PubMed] [Google Scholar]
- Osbourn JK, Earnshaw JC, Johnson KS, Parmentier M, Timmermans V, McCafferty J. Directed selection of MIP-1[alpha] neutralizing CCR5 antibodies from a phage display human antibody library. Nat Biotechnol 1998; 16: 778–781. [DOI] [PubMed] [Google Scholar]
- Sui J, Bai J, St. Clair Tallarico A, Xu C, Marasco WA. Identification of CD4 and transferrin receptor antibodies by CXCR4 antibody-guided Pathfinder selection. Eur J Biochem 2003; 270: 4497–4506. [DOI] [PubMed] [Google Scholar]
- Mirzabekov T, Kontos H, Farzan M, Marasco W, Sodroski J. Paramagnetic proteoliposomes containing a pure, native, and oriented seven-transmembrane segment protein, CCR5. Nat Biotechnol 2000; 18: 649–654. [DOI] [PubMed] [Google Scholar]
- Sommerfelt MA. Circular CCR5 peptide conjugates and uses thereof (WO2008074895). Expert Opin Ther Pat 2009; 19: 1323–1328. [DOI] [PubMed] [Google Scholar]
- Talmont F, Moulédous L, Boué J, Mollereau C, Dietrich G. Denatured G-protein coupled receptors as immunogens to generate highly specific antibodies. PLoS ONE 2012; 7: e46348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg N. Human antibodies from transgenic animals. Nat Biotechnol 2005; 23: 1117–1125. [DOI] [PubMed] [Google Scholar]
- Yan H, Gu W, Yang J, Bi V, Shen Y, Lee E et al. Fully human monoclonal antibodies antagonizing the glucagon receptor improve glucose homeostasis in mice and monkeys. J Pharmacol Exp Ther 2009; 329: 102–111. [DOI] [PubMed] [Google Scholar]
- Hoogenboom HR. Selecting and screening recombinant antibody libraries. Nat Biotechnol 2005; 23: 1105–1116. [DOI] [PubMed] [Google Scholar]
- Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 1985; 228: 1315–1317. [DOI] [PubMed] [Google Scholar]
- Hawlisch H, Frank R, Hennecke M, Baensch M, Sohns B, Arseniev L et al. Site-directed C3a receptor antibodies from phage display libraries. J Immunol 1998; 160: 2947–2958. [PubMed] [Google Scholar]
- Hoogenboom HR, Lutgerink JT, Pelsers MMAL, Rousch MJMM, Coote J, van Neer N et al. Selection-dominant and nonaccessible epitopes on cell-surface receptors revealed by cell-panning with a large phage antibody library. Eur J Biochem 1999; 260: 774–784. [DOI] [PubMed] [Google Scholar]
- Ravn P, Madhurantakam C, Kunze S, Matthews E, Priest C, O'Brien S et al. Structural and pharmacological characterization of novel potent and selective monoclonal antibody antagonists of glucose-dependent insulinotropic polypeptide receptor. J Biol Chem 2013; 288: 19760–19772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann UB, Gunnarsson L, Géraudie S, Scheffler U, Griep RA, Reiersen H et al. Fully human antagonistic antibodies against CCR4 potently inhibit cell signaling and chemotaxis. PLoS ONE 2014; 9: e103776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers PM, Horlick RA, Neben TY, Toobian RM, Tomlinson GL, Dalton JL et al. Coupling mammalian cell surface display with somatic hypermutation for the discovery and maturation of human antibodies. Proc Natl Acad Sci USA 2011; 108: 20455–20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igawa T, Mimoto F, Hattori K. pH-dependent antigen-binding antibodies as a novel therapeutic modality. Biochecm Biophys Acta 2014; 1844: 1943–1950. [DOI] [PubMed] [Google Scholar]
- Bonvin P, Venet S, Fontaine G, Ravn U, Gueneau F, Kosco-Vilbois M et al. De novo isolation of antibodies with pH-dependent binding properties. MAbs 2015; 7: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douthwaite JA, Sridharan S, Huntington C, Hammersley J, Marwood R, Hakulinen JK et al. Affinity maturation of a novel antagonistic human monoclonal antibody with a long VH CDR3 targeting the Class A GPCR formyl-peptide receptor 1. MAbs 2014; 7: 152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R, Shoji-Hosaka E, Sakurada M, Shinkawa T, Uchida K, Nakamura K et al. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res 2004; 64: 2127–2133. [DOI] [PubMed] [Google Scholar]
- Ishii T, Ishida T, Utsunomiya A, Inagaki A, Yano H, Komatsu H et al. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin Cancer Res 2010; 16: 1520–1531. [DOI] [PubMed] [Google Scholar]
- Beck A, Reichert JM. Marketing approval of mogamulizumab: a triumph for glyco-engineering. MAbs 2012; 4: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]