Abstract
Striatal-enriched protein tyrosine phosphatase (STEP) is abundantly expressed in the striatum, which strongly expresses dopamine and opioid receptors and mediates the effects of many drugs of abuse. However, little is known about the role of STEP in opioid receptor function. In the present study, we generated STEP-targeted mice carrying a nonsense mutation (C230X) in the kinase interaction domain of STEP by screening the N-ethyl-N-nitrosourea (ENU)-driven mutant mouse genomic DNA library and subsequent in vitro fertilization. It was confirmed that the C230X nonsense mutation completely abolished functional STEP protein expression in the brain. STEPC230X−/− mice showed attenuated acute morphine-induced psychomotor activity and withdrawal symptoms, whereas morphine-induced analgesia, tolerance and reward behaviors were unaffected. STEPC230X−/− mice displayed reduced hyperlocomotion in response to intrastriatal injection of the μ-opioid receptor agonist DAMGO, but the behavioral responses to δ- and κ-opioid receptor agonists remained intact. These results suggest that STEP has a key role in the regulation of psychomotor action and physical dependency to morphine. These data suggest that STEP inhibition may be a critical target for the treatment of withdrawal symptoms associated with morphine.
Introduction
Morphine and other μ-opioid agonists elicit beneficial analgesic effects, but they have high tolerance and addiction potential. Many drugs of abuse, including morphine, stimulate dopamine transmission in the nucleus accumbens (NAc) and dorsal striatum,1 areas that regulate reward behavior and motor activity.2, 3 Morphine binds to μ-opioid receptors (MORs), which exert their cellular effects via GTP-binding proteins Gi/o.4 Opioid and dopamine receptors are abundantly expressed in medium spiny neurons, the main cell type in the striatum.5 However, the molecular mechanisms operating downstream of opioid and dopamine receptors, which regulate morphine-induced reward behavior, psychomotor activity, tolerance and physical dependency, have not yet been fully elucidated.
Striatum-enriched protein tyrosine phosphatase (STEP, also called protein tyrosine phosphatase non-receptor type 5) is preferentially expressed in the basal ganglia and related structures.6, 7 Among four splice isoforms, STEP46 and STEP61 are functional and contain a kinase interaction domain (KIM) and a phosphatase domain.8 STEP is highly expressed in the medium spiny neurons, where it is phosphorylated when dopamine D1 receptors are activated or by protein kinase A.9 The medium spiny neurons receive two major synaptic inputs: glutamatergic afferents from the cerebral cortex10 and dopaminergic afferents from the midbrain synapse.11 Several target proteins of STEP have been identified, including the mitogen-activated protein kinases (MAPKs), tyrosine kinase Fyn and N-methyl-D-aspartate receptor (NMDAR).12, 13, 14 ERKs are vital regulators of drug addiction.15 Administration of either acute or chronic morphine phosphorylates ERK in the dorsal striatum and NAc.16 MAPK phosphorylation is also critical for opioid receptor desensitization.17 A direct association between μ-opioid receptors and NMDA glutamate receptors, as well as sustained potentiation of NMDAR-mediated glutamate responses by MOR signaling, has been reported.18, 19 NR2B-containing NMDAR in the NAc regulates morphine-associated contextual memory.20 STEP inhibition by a substrate-trapping mutant form of STEP prevented the neuroadaptive changes in synaptic efficacy that occurred during drug sensitization and drug-seeking behaviors.21 However, the precise role of STEP in the opioid receptor signaling system is yet to be determined.
To understand the role of STEP in behavioral and pharmacological responses to morphine, mice with a nonsense mutation in the STEP gene were generated after screening of the RIKEN N-ethyl-N-nitrosourea (ENU) mutant mouse library and subsequent in vitro fertilization. Using the newly generated STEP mutant mice, we demonstrated the role of STEP in MOR signaling and morphine-dependent psychostimulatory and addictive behaviors.
Materials and methods
Mice
The G1 mouse, Rgsc1947 screened from the RIKEN ENU mutant mouse library22, 23 and carrying the C230X mutation on the STEP target gene, was revived from frozen sperm by in vitro fertilization into fresh C57BL/6J oocytes and subsequent transfer of two-cell-staged embryos to pseudopregnant females. All offspring were subjected to genotyping to search for C230X, and identified heterozygous carriers were used for subsequent phenotype analyses. Frozen embryos from the G2 heterozygous carriers were transferred to pseudopregnant females, and offspring carrying the C230X mutations were backcrossed with C57BL/6J for more than 11 generations. Homozygote (STEPC230X−/− or STEP−/−), heterozygote (+/−) and wild-type (STEP+/+) littermates were used in this study unless otherwise indicated.
The genotyping for identifying STEP mutants was performed by PCR using the following primer sets (Figure 2b); STEP wild-type (a, 5′-CCTTGACCCTGGACATGTGT-3′ and 5′-GCCCTTTGTCTGACTTTCCA-3′) and STEP mutant type (b, 5′-CCTTGACCCTGGACATGTGA-3′and 5′-GCCCTTTGTCTGACTTTCCA-3′). The PCR reaction for wild-type and mutant alleles generated 384-bp PCR products. Therefore, to determine the sequence of a point mutation, we performed DNA-sequencing analysis after obtaining additional PCR reaction product using the primer sets 5′-GCCCTTTGTCTGACTTTCCA-3′ and 5′-TCCACTGACCACCTTTCTCC-3′.
All the mice were housed in regular polycarbonate plastic cages, with two to four mice per cage, in a temperature- and humidity-controlled environment (20–22 °C, 50–60% humidity) under a 12-h light/dark cycle (lights on at 0700 h). Mice had access to lab chow and water ad libitum. The mice were maintained in a specific-pathogen-free condition. The animals used in the experimental group were obtained from three to four litters. Unless otherwise indicated, both males and females (weight, 22–27 g) were used. All animal experiments were approved and conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Korea Research Institute of Bioscience and Biotechnology (KRIBB, Daejeon, Korea).
Western blot analysis
Western blots were performed as described in a previous report.24 Nine-week-old male mice of wild type and STEPC230X−/− were used in this assay. Briefly, the brain was quickly removed and homogenized in lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS and 0.1% sodium deoxycholate) containing a cocktail of protease inhibitors (Roche, Mannheim, Germany). Protein samples were resolved on 10% SDS-polyacrylamide gel electrophoresis; the transferred blots were incubated with primary antibodies, followed by secondary antibody, and the signals were visualized using an ECL kit (Amersham Bioscience, Little Chalfont, UK). Antibodies used were monoclonal anti-STEP (23E5, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-phospho-STEP (Ser221/Ser49, Millipore, Billerica, MA, USA), anti-ERK1/2 (Cell Signaling Technology, MA, USA), anti-phospho-ERK1/2 (Thr202/Tyr204, Cell Signaling Technology), anti-μ-opioid receptor (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-δ-opioid receptor (Santa Cruz Biotechnology), anti-κ-opioid receptor (GeneTex, Irvine, CA, USA) and anti-actin (Millipore). The secondary antibodies used were peroxidase-conjugated anti-rabbit-IgG and anti-mouse-IgG (Santa Cruz Biotechnology).
RT-PCR analyses
Total RNA was purified from the tissue samples of four to five animals for each group using TRI reagent (Sigma-Aldrich, St Louis, MO, USA). RNA from each group was treated with DNase I to avoid genomic contamination. The PCR analysis shown in Figure 6 was carried out using the following primer sets: μ-opioid receptor (5′-CTACCTGATGGGAACGTGGC-3′ and 5′-GGAGGGGTGTTCCCTAGTGT-3′), δ-opioid receptor (5′-TCAATCTGGCTTTGGCTGAT-3′ and 5′-TCCAGACGATGACGAAGATG-3′), κ-opioid receptor (5′-CAGCACTCTGAAAGGGCATA-3′ and 5′-AAAGCTGACGGTGACTTGG-3′) and glyceraldehyde-3-phosphate dehydrogenase (5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′). Expression levels were quantified using a gel documentation system (Bio-Rad, Hercules, CA, USA) and determined relative to the expression levels in wild-type mice.
Histology
The mice were anesthetized with a mixture of ketamine hydrochloride and xylazine hydrochloride, as described previously.24 The mice were transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were isolated and post-fixed in the same solution at 4 °C overnight. Brain sections of 40-μm thickness were prepared using a vibratome, as previously described.25 Free-floating sections were incubated with primary antibody at 4 °C overnight. The primary antibody for STEP (1:1000) and MOR-1 (1:200) were obtained from Santa Cruz Biotechnology. For Nissl staining, brain sections were stained using 0.25% cresyl violet.
Open-field test
The mice were habituated in the test room for 30 min, and horizontal locomotion was measured in a white chamber (45 cm × 45 cm × 40 cm) using a computerized video-tracking system SMART (Panlab, Barcelona, Spain), as described previously.26
Hot-plate test
The mice were placed individually onto a temperature-controlled glass plate maintained at 52 °C, and the time required to show a pain response, such as paw licking or jumping, was recorded. The cutoff time for this test was 60 s. Analgesic responses were tested 30 min after the administration of morphine HCl (10 mg kg−1, intraperitoneal). Pain responses were presented as the percentage of maximum possible effect (%MPE), which was calculated using the following formula: 100% × [(drug response time−basal response time)/(60 s-basal response time)]=%MPE.
Conditioned place preference test
Psychological dependence on morphine was tested using a morphine-conditioned place preference (CPP) test, as previously described.26 CPP was tested using an apparatus consisting of two compartments (15 × 16 × 16 cm each) separated by a door. One compartment has black walls and a black plastic floor (vehicle-paired compartment), whereas the other compartment has white walls and a metal grill floor (morphine-paired compartment). Each mouse was familiarized with the apparatus for 20 min per day for 2 days. On day 3, the amount of time the mouse spent in each compartment was recorded over a 20-min period. On days 4, 6, 8 and 10, the mice were injected with morphine (10 mg kg−1, subcutaneous) and placed in the white compartment. On days 5, 7, 9 and 11, the mice were injected with saline and placed in the black compartment. On day 12, each mouse was allowed to freely explore the compartments with the door open, and the time spent in each of the two compartments was recorded over 20 min. The change of preference was calculated as the difference between the time spent in the morphine-receiving (white) chamber on days 12 and 3.
Morphine-withdrawal test
Physical dependence on morphine was induced by repeated injection of morphine, as previously described.26 The morphine dose was given twice daily at 12-h intervals for 5 days and was progressively increased as follows: first day, 20 mg kg−1 (intraperitoneal); second day, 40 mg kg−1; third day, 60 mg kg−1; fourth day, 80 mg kg−1; fifth day, 100 mg kg−1. On day 6, mice were injected with 100 mg kg−1 morphine (intraperitoneal) and withdrawal symptoms were precipitated 2 h later by naloxone injection (1 mg kg−1, subcutaneous). The mice were habituated for 15 min to an observation chamber made of a large transparent plastic cylinder (30 cm diameter, 30 cm high) before the naloxone injection. Naloxone-precipitated morphine-withdrawal symptoms were observed for 30 min. The number of instances in which the mice showed jumping, paw tremor, sniffing and wet-dog shaking were recorded separately. Ptosis, teeth chattering, body tremor and diarrhea were evaluated over a 5-min period, with a point given for each withdrawal sign, and the number of periods in which the sign was observed was counted (maximum score, 6). A global withdrawal score was calculated for each mouse using the following scoring system; jumping × 0.8; paw tremor × 0.35; sniffing × 0.5; wet-dog shake × 1; ptosis × 1.5; teeth chattering × 1.5; body tremor × 1.5; and diarrhea × 1.5.27, 28
Drug administration
DAMGO (μ-opioid agonist, [D-Ala2, N-MePhe4, Gly-ol]-enkephalin), DPDPE (δ-opioid agonist, [D-Pen(2),D-Pen(5)]-enkephalin) and U69593 (κ-opioid agonist) were purchased from Sigma-Aldrich, and morphine HCl was purchased from MyungMoon Pharmaceuticals (Hwasung, Kyunggido, Korea). Drugs were dissolved in 0.9% saline. For direct injections into the dorsal striatum, animals were anesthetized using isoflurane, as described previously.26 The stereotaxic injection coordinates used to deliver drugs to the dorsal striatum were anteroposterior, +1.0; mediolateral, ±1.8; and dorsoventral, −3.6 mm, and the injection volume was 2 μl (1 μl min−1) per injection.24, 26 In the present study, the dorsal striatum refers to the caudate and putamen.
Statistical analysis
Two-sample comparisons were performed usingStudent's t-test. Multiple comparisons were performed by one-way analysis of variance followed by Newman–Keuls multiple comparison test or two-way (genotype × drug) analysis of variance followed by Bonferroni post hoc test. All data are presented as the mean±s.e.m., and differences were considered significant at the 5% level.
Results
Morphine increased STEP phosphorylation in the striatum
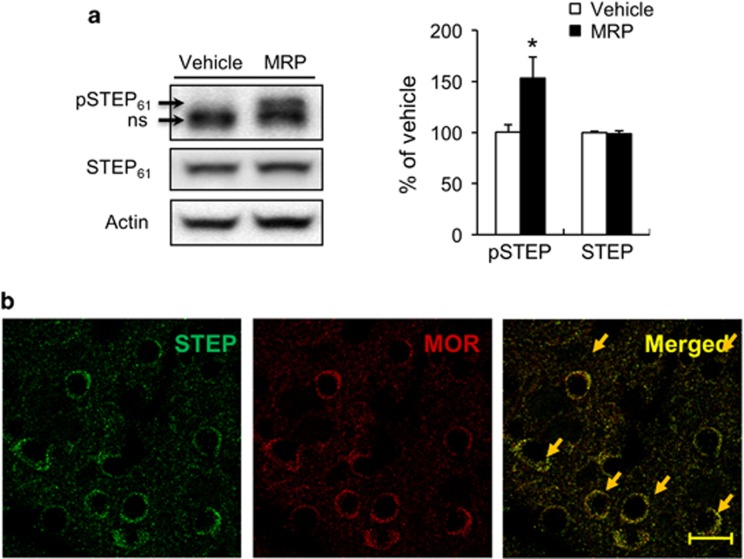
To investigate whether morphine affected the STEP signaling pathway, the phosphorylation level of STEP was determined in morphine-treated C57BL/6J mice (Figure 1). Administration of morphine increased STEP phosphorylation compared with saline-treated animals in dorsal striatum of wild-type mice (Figure 1a, P<0.05), but the expression of STEP did not change in the morphine-treated samples compared with the saline-treated samples. In the immunohistochemistry, STEP and MOR1 signals were abundantly co-localized in the dorsal striatum of wild-type mice (Figure 1b).
Figure 1.
Effects of administration of morphine on STEP phosphorylation in dorsal striatum. Mice were injected with 20 mg kg−1 morphine (intraperitoneal). After 30 min, the dorsal striatum was obtained for western blot analysis of phospho-STEP and STEP (a). Significant differences between saline-treated groups and indicated groups at *P<0.05, (n=4–6 animals per group, Student's t-test). Representative examples of neurons showing colocalization of STEP and MOR immunosignals in the dorsal striatum (b). Scale bar, 20 μm. MOR, μ-opioid receptor; NS, nonspecific.
STEP C230X−/− mice carrying the ENU-induced C230X point mutation within the STEP gene
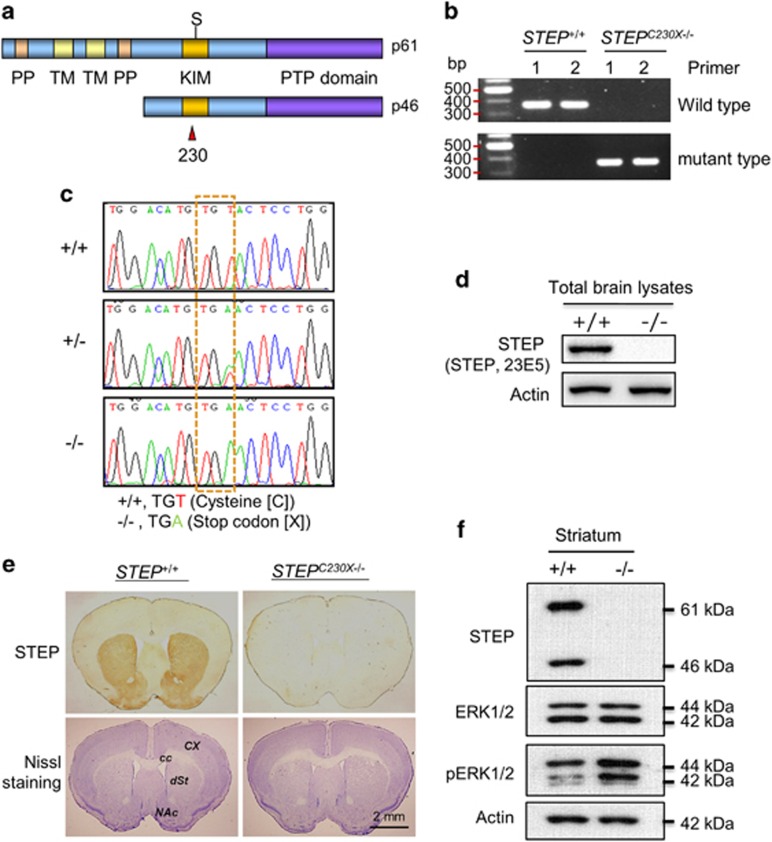
To examine the role of STEP on morphine effects, we generated mutant mice carrying the nonsense mutation at Cys230 within the KIM domain (C230X; Figure 2a).
Figure 2.
Characterization of mice carrying the ENU-induced C230X point mutation in the STEP gene. (a) The position of Cys230 within the KIM domain of STEP-61 and -46 isoforms. (b, c) The ENU-induced nonsense mutation at Cys230 within the KIM domain was detected by genomic PCR (b) and by DNA sequencing (c) from the genomic DNA of ENU-mutant mice. KIM, kinase interacting motif; PP, polyproline; PTP domains, protein tyrosine phosphatase; TM, transmembrane domain. (d, e) Western blotting (d) and immunohistochemical (e) analyses using anti-STEP antibody (anti-STEP, 23E5) showing the complete absence of functional STEP isoforms in the brain. (e, lower panel) Nissl-stained brain sections showing the cortex, dorsal and ventral striatum of wild-type and STEPC230X−/− mice. (f) Western blot analysis of STEP-dependent regulation of phospho-ERK levels in the striatum. STEP proteins of 61-kDa and 46-kDa isoforms were detected in the striatum of wild-type mice but not in STEPC230X−/− mice. Phosphorylation of ERK was increased in the striatum of STEPC230X−/− mice. Actin was used as a loading control. +/+, STEP wild-type mice; +/−, STEP heterozygous mice; −/−, STEPC230X−/− mice; cc, corpus callosum; cx, cortex; dSt, dorsal striatum; NAc, nucleus accumbens.
Systematic genetic and molecular analyses led us to generate hetero- and homozygous mutant STEP mice. The ENU-induced nonsense mutation (TGT to TGA) at Cys230 within the KIM domain was identified by the genomic PCR method using the primer sets that detected exclusively either wild-type or mutant types. The wild-type primer set produced a band only in wild-type mice, whereas the mutant primer set produced a band only in mutant mice (Figure 2b). We further confirmed the genotypes of mice by DNA sequencing after obtaining additional PCR reaction product using other primer sets (Figure 2c). Western blotting and immunohistological analyses revealed that the mutant mice carrying the C230X nonsense mutation expressed no functional STEP protein in the brain (Figures 2d and e, upper panel). Together, these data suggest that STEPC230X−/− mice were generated by a point mutation, but they were functionally null. The homozygous mutant mice carrying the C230X point mutation have now been renamed STEPC230X−/−. Cresyl violet staining of brain sections showed no distinct abnormalities in cell density in the striatum and cerebral cortex of STEPC230X−/− mice (Figure 2e, lower panel). Activated STEP in the striatum dephosphorylates its target substrates, and the ability of STEP to regulate ERK has been reported in previous studies.9, 12, 13, 14 STEP KO mice exhibit increased ERK1/2 phosphorylation in the striatum.8 Western blot analysis revealed that STEPC230X−/− had enhanced phospho-ERK levels in the striatum compared with wild-type mice (Figure 2f). These results indicate that mice carrying the C230X point mutation within the STEP gene express no functional STEP protein in the brain.
Morphine-induced analgesia and tolerance are induced in STEP C230X−/− mice
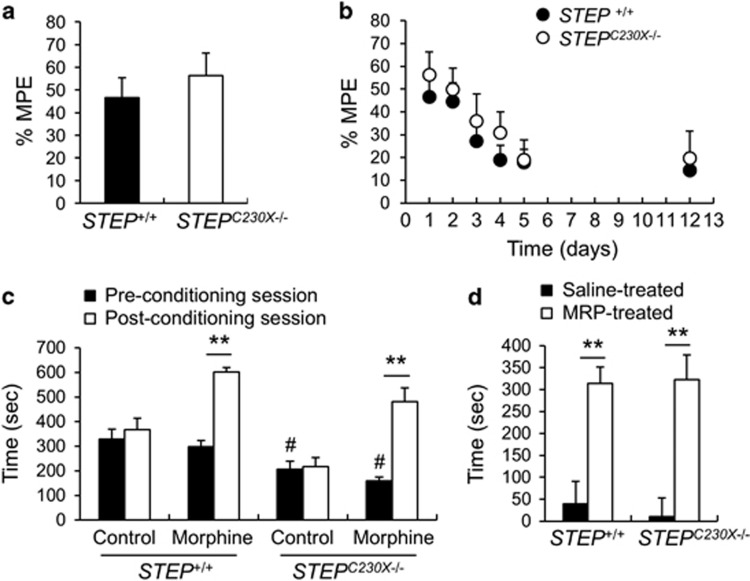
Using the newly generated STEPC230X−/−, we investigated behavioral responses to morphine. To understand the role of STEP in morphine analgesia and tolerance, the hot plate antinociception test was examined in STEPC230X−/− mice. The time required for STEP+/+ or STEPC230X−/− mice to display a pain response on the hot plate at 52 °C was 23.76 s±1.58 and 20.07 s±1.11, respectively (P>0.05). These results show that wild-type and mutant mice respond similarly to the nociceptive stimulus. Administration of morphine (10 mg kg−1, intraperitoneal) increased the %MPE in both genotypes (Figure 3a). We examined the effects of repeated administration of morphine to STEPC230X−/− mice because this procedure is known to result in the development of a substantial tolerance to pain.26 Injection of morphine (10 mg kg−1) in STEP+/+ and STEPC230X−/− mice induced 46 and 56% of the MPE, respectively, on day 1 (Figures 3a and b), whereas repeated injection produced 17.8 and 19% of MPE on day 5 (Figure 3b). After 7 days of abstinence, the induced tolerance was maintained in both the genotypes. These data suggest that morphine is analgesic in both genotypes, and morphine tolerance on the basis of analgesic responses on the hot plate test was also detected in STEPC230X−/− mice.
Figure 3.
Morphine-induced analgesia, tolerance and psychological dependency in STEP+/+ and STEPC230X−/− mice. (a) The pain response on a 52 °C hot plate. Mice received morphine (10 mg kg−1, intraperitoneal) 30 min before the behavioral assay. (b) Repeated morphine treatment decreased the %MPE in both STEP+/+ and STEPC230X−/− mice. (c) The dwelling time spent in the white compartment before (pre-conditioning session) and after (post-conditioning session) morphine injection in the morphine conditioned place preference test. (d) The preference for each compartment was calculated by deducting the time of the pre-conditioning session in the saline–saline and saline–morphine-treated groups.Significant difference compared with wild type at #P<0.05 and significant difference between the indicated groups at **P<0.01 (n=6–10, Student's t-test). MPE, maximum possible effect.
Morphine-induced CPP
To understand the role of STEP in the reward system activated by morphine, we carried out a morphine-CPP test. During the pre-conditioning session, each mouse was allowed to freely explore both white and dark compartments of the apparatus for 20 min. The wild-type mice spent 312±30 s in the non-preferred white compartment, whereas STEPC230X−/− mice stayed in the white compartment for 182±18 s (Figure 3c; P<0.01). The STEP null mutation is likely to have increased anxiety in this context. When morphine administration was paired for 4 days with placement in the white chamber, the morphine-treated mice showed preference for the morphine-paired compartment, but saline-treated mice did not. The time spent in the morphine-paired compartment was markedly increased on the test day in both the genotypes (Figures 3c and d; P<0.01). These results indicate that the rewarding effects of morphine are preserved in STEPC230X−/− mice.
Morphine-withdrawal symptoms
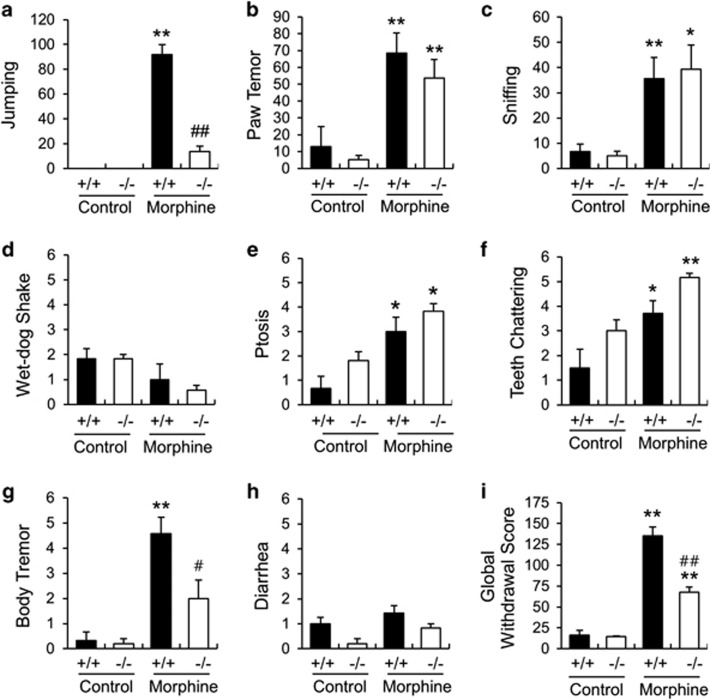
Next, we investigated the role of STEP in morphine-withdrawal responses. Withdrawal was induced by repeated morphine exposure and subsequent administration of naloxone. The withdrawal syndrome was assessed by quantifying eight distinct withdrawal signs (Figures 4a–h). Jumping and body tremor were significantly lower in STEPC230X−/− mice compared with the wild-type controls (Figures 4a and g; P<0.01 and P<0.05). In contrast, teeth chattering was high in STEPC230X−/− mice compared with wild-type mice (Fig. 4f; P<0.05). Other behavioral responses were not significantly altered in the STEPC230X−/− mice (Figures 4b–e and h). The global morphine-withdrawal score, which reflects overall morphine-withdrawal symptoms, was significantly lower in the STEPC230X−/− mice compared with wild-type controls (Figure 4i; P<0.01). These data suggest that a deficiency in STEP protein at least partially suppressed morphine-induced physical dependency and withdrawal symptoms.
Figure 4.
Naloxone-precipitated morphine-withdrawal responses in STEP+/+ and STEPC230X−/− mice. (a–h) Physical withdrawal symptoms in STEP+/+ and STEPC230X−/− mice. The number of jumps (a), paw tremors (b), sniffs (c) and wet-dog shakes (d) displayed were recorded during a 30-min period. Ptosis (e), teeth chattering (f), body tremor (g) and diarrhea (h) were evaluated over six 5-min periods. The number of periods in which the signs were observed were counted, and the maximum score was 6. (i) The global withdrawal score was calculated for each animal by giving each sign a relative weight. Data are expressed as the mean±s.e.m. Significant differences between saline-treated control and indicated groups at *P<0.05 and **P<0.01, respectively; Significant differences between morphine-treated STEP+/+ mice and morphine-treated STEP−/− mice at #P<0.05 and ##P<0.01, respectively (n=6–8 animals per group, one-way analysis of variance followed by Newman–Keuls multiple comparison test).
Morphine-induced hyperlocomotion
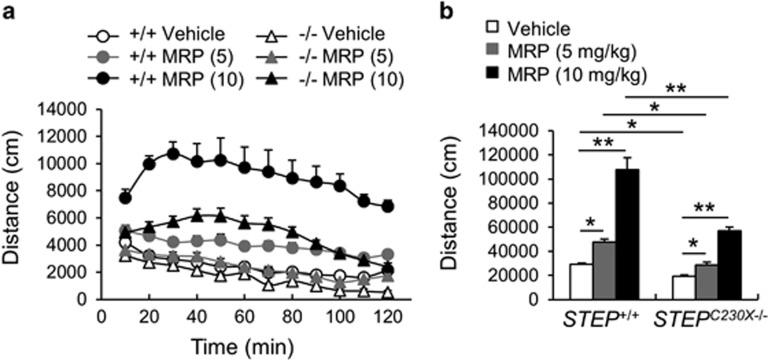
To examine morphine-induced behavioral responses of mice, the hyperlocomotion induced by morphine was investigated in wild-type and STEPC230X−/− mice. The administration of morphine (5 and 10 mg kg−1) in wild-type mice increased locomotion in a dose-dependent manner (Figures 5a and b). Administration of morphine in STEPC230X−/− mice also increased locomotion, but to a lesser extent than that in wild-type mice (Figures 5a and b; genotype effect, F(1, 39)=75.01, P<0.0001; morphine effect, F(2, 39)=125.17, P<0.0001; and genotype and morphine interaction, F(2, 39)=15.52, P<0.0001; two-way analysis of variance). In this test, STEPC230X−/− mice exhibited reduced locomotion in the novel environment of the open-field test (Figure 5b, P<0.01; Student's t-test).
Figure 5.
Morphine-induced hyperlocomotion in STEP+/+ and STEPC230X−/− mice. (a) Time-course changes of morphine-induced hyperlocomotion in the open field in STEP+/+ and STEPC230X−/− mice. Each point represents the distance that mice moved in a 10-min block. (b) Summed locomotor activity for the 120-min period after morphine administration in STEP+/+ and STEPC230X−/− mice. Significant difference between indicated groups at *P<0.05 and **P<0.01, respectively (n=6–8 animals per group, two-way analysis of variance followed by Bonferroni post hoc test).
Opioid receptor subtype-specific behavioral changes in STEP C230X−/− mice
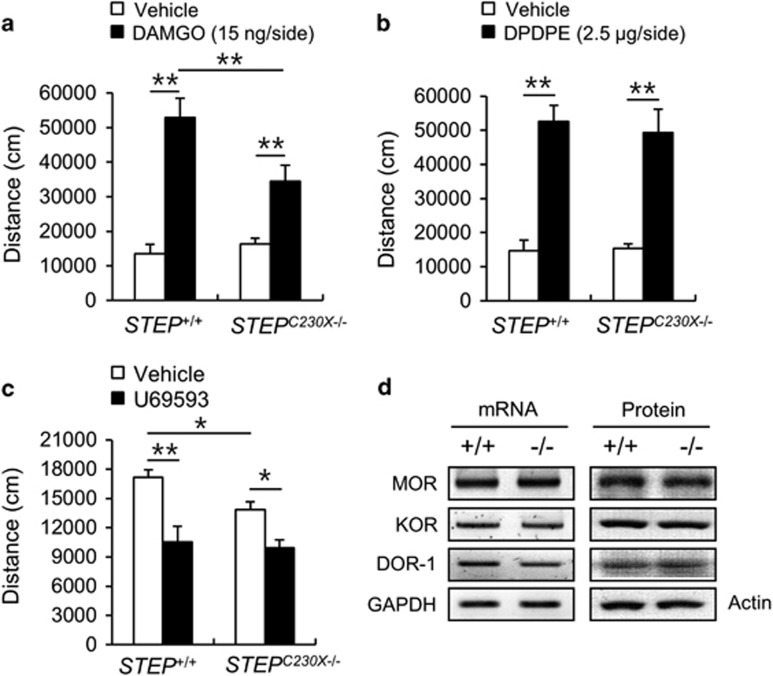
Next, we examined the opioid receptor subtypes linked to STEP and their roles in morphine-induced hyperlocomotion. To clarify the effects of the STEP null mutation on specific opioid receptor subtypes, we examined the behavioral responses of mice to specific agonists of opioid receptor subtypes in the open-field test. Injection of DAMGO (15 ng per side), a μ-opioid receptor agonist, into the dorsal striatum bilaterally in wild-type mice increased their locomotor activity (Figure 6a). Similarly, injection of DAMGO into the dorsal striatum in STEPC230X−/− mice increased their locomotor activity, but their locomotion was significantly decreased compared with that of the wild-type mice (Figure 6a, P<0.01). Injection of D-Pen2,D-Pen5 enkephalin (DPDPE, 2.5 μg per side), a δ-opioid receptor agonist, into the dorsal striatum bilaterally significantly enhanced the locomotor activity in both STEP+/+ and STEPC230X−/−/− mice (Figure 6b, P<0.05). No significant difference was observed between the two genotypes. The administration of the κ-opioid receptor agonist, U69593 (3 mg kg−1, i.p.), decreased the locomotor activity at a level comparable to that in both genotypes (Figure 6c; STEP+/+, P<0.01; STEPC230X−/−, P<0.05), but there was no difference between the wild-type and mutant mice. These data suggest that the STEP null mutation in the striatum reduced the acute responses to μ-opioid receptor signaling, but left δ- and κ-opioid receptor signaling largely unaffected. There was a slight difference in the basal locomotor activity between normal mice and mice that received intrastriatal injections. We speculate that this difference was owing to differences in sensitivity to the intrastriatal injection between the two genotypes. The intrastriatal injection of vehicle in STEPC230X−/− mice influenced general locomotion slightly less than that in wild-type mice. We investigated whether the reduced hyperlocomotive response to morphine and DAMGO observed in STEPC230X−/− mice was because of low expression of the μ-opioid receptor. RT-PCR and western blot analysis revealed that the levels of μ-, δ- and κ-opioid receptors in the striatum of STEPC230X−/− mice did not differ from those in the wild-type mice (Figure 6d).
Figure 6.
Opioid receptor agonist-induced locomotor activity in STEP+/+ and STEPC230X−/− mice. (a–c) Total locomotor activity of STEP+/+ and STEPC230X−/− mice in the open field. The μ-opioid selective agonist, DAMGO (15 ng per side, intrastriatal injection, (a); the δ-opioid agonist, DPDPE (2.5 μg per side, intrastriatal injection, b; and the κ-opioid receptor agonist, U69593 (3 mg kg−1, intraperitoneal; c) were used. Significant differences between indicated groups at *P<0.05 and **P<0.01, respectively (n=6–7 animals per group, one-way analysis of variance followed by Newman–Keuls multiple comparison test). (d) Expression of opioid receptor subtypes in the dorsal striatum was performed. RT-PCR analysis and western blot analysis results are presented. DOR-1, δ-opioid receptor-1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; KOR, κ-opioid receptor; MOR, μ-opioid receptor (n=4).
Discussion
In the current study, we successfully identified an array of point mutations in STEP. Identification of the C230X mutation of STEP led us to generate a novel mutant mouse line, STEPC230X−/−, which does not express functional STEP protein in the brain. Our STEPC230X−/− mouse line is comparable to the recently introduced STEP knockout mouse line that was generated by a classical homologous recombination at the 1.3 kb genomic DNA region of the STEP gene, encompassing a portion of the phosphatase domain with the catalytic site, by replacing it with the 1.9 kb neomycin gene.29 We believe that these independently generated STEP mutant mouse lines, along with the other STEP mutations identified in the present study, will be useful tools for future studies of the in vivo function of STEP. Both STEPC230X−/− mice and STEPKO mice29 were healthy with no apparent gross anatomical abnormality, which further confirms that the genomic region at STEP does not contain other genes or genomic regions crucial to life or brain development. STEP KO mice were reported to exhibit anxiety-like behaviors in the elevated zero maze and the light/dark test8, which are similar to those of STEPC230X−/− mice. In the open-field test, STEPC230X−/− mice showed increased locomotion, whereas STEP KO mice showed control levels of locomotion and rearing behaviors in the open-field test.29, 30 STEP KO mice in these studies carry the genetic background of C57BL/6NCRI, whereas STEPC230X−/− mice had the genetic background of C57BL/6J. Therefore, further studies are needed to understand whether the difference in the genetic background influences locomotor activity and other behaviors.
Tyrosine phosphatases have important roles in both normal physiological processes and the manifestation of disease.31 STEP has been suggested to be a therapeutic target for the treatment of neuropsychiatric disorders.8 Administration of morphine in vivo increased the phosphorylation of STEP (Ser221/Ser49) in the dorsal striatum (Figure 1a). STEPC230X−/− mice, which do not express functional STEP proteins in the brain, showed hyperlocomotor activity and withdrawal signs in response to morphine administration (Figures 4 and 5). In contrast, STEP was not required for morphine-induced analgesia, tolerance or psychological dependency. These results suggest that STEP mediates the behavioral responses to morphine in only limited aspects. STEP was found to be involved in the μ-opioid receptor signaling pathway in the dorsal striatum. To our knowledge, this is the first evidence that STEP has a role in the opioid receptor system.
Morphine increases the firing rate of dopaminergic neurons in the substantia nigra and ventral tegmental area, and it increases synaptic dopamine concentrations in the striatum and NAc.1, 32, 33, 34, 35, 36 The morphine-induced response disinhibits GABAergic interneurons in the substantia nigra and ventral tegmental area34, 35 and increases dopaminergic neuronal firing, which subsequently increases dopamine release in the striatum and NAc.37 On the contrary, DAMGO microinjection into the NAc increased the motor activity in both 6-OHDA-lesioned and sham-lesioned rats, and hyperlocomotion was higher in the lesioned group than in the sham group.37 Although levels of dopamine or its metabolites were not altered in the NAc, D-Ala2-Met5-enkephalinamide injection into the NAc elicited hyperlocomotion in rodents.38 These results suggest that hyperlocomotion induced by the local infusion of opioid receptor agonist into the NAc /striatum does not depend entirely on dopamine release. Motor activity is increased in mice by local striatal infusion of DAMGO and DPDPE.26 Consistently, the DAMGO infusion into the striatum increased locomotor activity in the open field (Figure 6a).
Phosphorylation of opioid receptors leads to receptor desensitization and internalization.39 The MAPK family has been suggested to be involved in the specific behavioral effects of morphine or MOR agonists.16, 17 MOR internalization, desensitization and phosphorylation were prevented by ERK1/2 inhibition,17, 40 and the MAPK cascade regulates phosphorylation and desensitization of MOR.40 Naloxone-precipitated morphine withdrawal increases the phosphorylation of ERK1/2 in limbic areas.41 Opioid receptor-mediated activation of p38 MAPK is essential for MOR endocytosis in vitro.42 These results suggest that the reduction in morphine- and μ-agonist-induced psychomotor behavior and withdrawal symptoms in STEPC230X−/− mice might be related to the enhanced levels of phospho-ERK (Figure 2f). Furthermore, phosphorylation of the μ-opioid receptor at tyrosine 166 was increased by DAMGO or EGF, and co-treatment with DAMGO and EGF, which results in tyrosine phosphorylation at residue 166, decreases the efficacy of the agonist.43, 44 Further studies using inhibitors of the ERK pathway will be useful for characterizing the direct or indirect regulation of MOR signaling by STEP.
In spite of their decreased motor responses and withdrawal signs to morphine, STEPC230X−/− mice were fully capable of developing morphine-induced reward behaviors in the CPP test and morphine-induced analgesia and tolerance in the hot-plate test (Figure 3). Synergistic interactions between spinal and supraspinal pathways are believed to be important for morphine potency.45, 46 The supraspinal mechanisms originate in structures within the brain stem, including the periaqueductal gray matter, locus coeruleus and nuclei of the medulla.45 In comparison, STEP is highly expressed in the hippocampus, cortex and striatal brain regions6, 7 (Figure 2e). Furthermore, because morphine exerts its effects via MORs at multiple sites, including medium spiny neurons in the striatum and spinal cord, our results suggest that the STEP-mediated effects of morphine are not involved in reward behavior or analgesia, despite their likely involvement in psychomotor effects and withdrawal symptoms. In addition, different types of PTPs expressed in various brain regions may be involved in the psychological dependency and analgesic responses induced by morphine.
An involvement of STEP in neuropsychiatric disorders, such as Alzheimer's disease, schizophrenia, alcohol-induced memory loss, epileptogenesis, drug abuse and Fragile X syndrome, has been reported.8, 21, 47, 48, 49, 50, 51, 52 It will be interesting to test a strategy that could help downregulate the high STEP activity that occurs in neuropsychiatric disorders, which might result in beneficial outcomes in these diseases.8 The physical dependency induced by repeated administration of high doses of morphine resulted in severe withdrawal signs in wild-type mice, whereas the morphine-withdrawal signs were markedly attenuated in STEPC230X−/− mice, suggesting that STEP suppression is also beneficial for morphine withdrawal. Moreover, it was shown that STEP acts on the μ-opioid receptor signaling pathway in the striatum. These results could aid in the development of a new strategy for region-specific regulation of the μ-opioid system to prevent physical dependence on morphine.
Acknowledgments
This work was supported by a grant from the KRIBB Research Initiative Program and the Basic Science Research Program through the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (2013M3A9D5072559 and 2015M3C7A1029113), JSPS KAKENHI Grant Numbers 21240043 (to TM and YG), 25241016 (to YG) and the joint research collaboration program for Chinese-Korean-Japanese research (to D-YY). We thank Dong-Hee Choi for the technical assistance.
The authors declare no conflict of interest.
References
- Di-Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 1988; 85: 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev 1987; 94: 469–492. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci USA 2011; 108: 15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol 2000; 40: 389–430. [DOI] [PubMed] [Google Scholar]
- Wang H, Moriwaki A, Wang JB, Uhl GR, Pickel VM. Ultrastructural immunocytochemical localization of μ-opioid receptors in dendritic targets of dopaminergic terminals in the rat caudate-putamen nucleus. Neuroscience 1997; 81: 757–771. [DOI] [PubMed] [Google Scholar]
- Lombroso PJ, Murdoch G, Lerner M. Molecular characterization of a protein-tyrosine-phosphatase enriched in striatum. Proc Natl Acad Sci USA 1991; 88: 7242–7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombroso PJ, Naegele JR, Sharma E, Lerner M. A brain-enriched protein tyrosine phosphatase is present in dopaminoceptive neurons. J Neurosci 1993; 13: 3064–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P et al. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev 2012; 64: 65–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Snyder GL, Yokakura H, Picciotto MR, Nairn AC, Lombroso PJ. The Dopamine/D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J Neurosci 2000; 20: 5630–5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F, Storm-Mathisen J, Divac I. Biochemical evidence for glutamate as neurotransmitter in corticostriatal and corticothalamic fibers in rat brain. Neuroscience 1981; 6: 863–873. [DOI] [PubMed] [Google Scholar]
- Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience 1984; 13: 1189–1215. [DOI] [PubMed] [Google Scholar]
- Pulido R, Zúñiga A, Ullrich A. PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. EMBO J 1998; 17: 7337–7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem 2002; 277: 24274–24279. [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Adkisson M, Leung J, Nava A, Masterson B, Urfer R et al. Regulation of NMDA receptor trafficking and function by striatal-enriched tyrosine phosphatase (STEP). Eur J Neurosci 2006; 23: 2847–2856. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W et al. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron 2002; 34: 807–820. [DOI] [PubMed] [Google Scholar]
- Muller DL, Unterwald EM. In vivo regulation of extracellular signal-regulated protein kinase (ERK) and protein kinase B (Akt) phosphorylation by acute and chronic morphine. J Pharmacol Exp Ther 2004; 310: 774–782. [DOI] [PubMed] [Google Scholar]
- Polakiewicz RD, Schieferl SM, Dorner LF, Kansra V, Comb MJ. A mitogen-activated protein kinase pathway is required for mu-opioid receptor desensitization. J Biol Chem 1998; 273: 12402–12406. [DOI] [PubMed] [Google Scholar]
- Chen L, Huang LY. Sustained potentiation of NMDA receptor-mediated glutamate responses through activation of protein kinase C by a mu opioid. Neuron 1991; 7: 319–326. [DOI] [PubMed] [Google Scholar]
- Garzón J, Rodríguez-Muñoz M, Sánchez-Blázquez P. Direct association of Mu-opioid and NMDA glutamate receptors supports their cross-regulation: molecular implications for opioid tolerance. Curr Drug Abuse Rev 2012; 5: 199–226. [DOI] [PubMed] [Google Scholar]
- Xu Y, Lv XF, Cui CL, Ge FF, Li YJ, Zhang HL. Essential role of NR2B-containing NMDA receptor-ERK pathway in nucleus accumbens shell in morphine-associated contextual memory. Brain Res Bull 2012; 89: 22–30. [DOI] [PubMed] [Google Scholar]
- Tashev R, Moura PJ, Venkitaramani DV, Prosperetti C, Centonze D, Paul S et al. A substrate trapping mutant form of striatal-enriched protein tyrosine phosphatase prevents amphetamine-induced stereotypies and long-term potentiation in the striatum. Biol Psychiatry 2009; 65: 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba Y, Sezutsu H, Takahasi KR, Tsuchihashi K, Ichikawa R, Fujimoto N et al. Molecular characterization of ENU mouse mutagenesis and archives. Biochem Biophys Res Commun 2005; 336: 609–616. [DOI] [PubMed] [Google Scholar]
- Gondo Y. Trends in large-scale mouse mutagenesis: from genetics to functional genomics. Nat Rev Genet 2008; 9: 803–810. [DOI] [PubMed] [Google Scholar]
- Park HY, Kang YM, Kang Y, Park TS, Ryu YK, Hwang JH et al. Inhibition of adenylyl cyclase type 5 prevents L-DOPA-induced dyskinesia in an animal model of Parkinson's disease. J Neurosci 2014; 34: 11744–11753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Kang YM, Kang Y, Park TS, Park HY, Kim YJ et al. Pitx3 deficient mice as a genetic animal model of co-morbid depressive disorder and parkinsonism. Brain Res 2014; 1552: 72–81. [DOI] [PubMed] [Google Scholar]
- Kim KS, Lee KW, Lee KW, Im JY, Yoo JY, Kim SW et al. Adenylyl cyclase type 5 (AC5) is an essential mediator of morphine action. Proc Natl Acad Sci USA 2006; 103: 3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Blendy JA, Tzavara E, Gass P, Roques BP, Hanoune J et al. Reduction of morphine abstinence in mice with a mutation in the gene encoding CREB. Science 1996; 273: 657–659. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Castañé A, Ledent C, Parmentier M, Maldonado R, Valverde O. Increase of morphine withdrawal in mice lacking A2a receptors and no changes in CB1/A2a double knockout mice. Eur J Neurosci 2003; 17: 315–324. [DOI] [PubMed] [Google Scholar]
- Venkitaramani DV, Paul S, Zhang Y, Kurup P, Ding L, Tressler L et al. Knockout of striatal enriched protein tyrosine phosphatase in mice results in increased ERK1/2 phosphorylation. Synapse 2009; 63: 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukoff Rizzo SJ, Lotarski SM, Stolyar P, McNally T, Arturi C, Roos M et al. Behavioral characterization of striatal-enriched protein tyrosine phosphatase (STEP) knockout mice. Genes Brain Behav 2014; 13: 643–652. [DOI] [PubMed] [Google Scholar]
- Zhang ZY. Protein tyrosine phosphatases: structure and function, substrate specificity, and inhibitor development. Annu Rev Pharmacol Toxicol 2002; 42: 209–234. [DOI] [PubMed] [Google Scholar]
- Matthews RT, German DC. Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience 1984; 11: 617–625. [DOI] [PubMed] [Google Scholar]
- Spangel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem 1990; 55: 1734–1740. [DOI] [PubMed] [Google Scholar]
- Di-Chiara G, North RA. Neurobiology of opiate abuse. Trends Pharmacol Sci 1992; 13: 185–193. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 1992; 12: 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 2006; 29: 565–598. [DOI] [PubMed] [Google Scholar]
- Churchill L, Klitenick MA, Kalivas PW. Dopamine depletion reorganizes projections from the nucleus accumbens and ventral pallidum that mediate opioid-induced motor activity. J Neurosci 1998; 18: 8074–8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Widerlov E, Stanley D, Breese G, Prange AJ. Enkephalin action on the mesolimbic system: a dopamine-dependent and a dopamine-independent increase in locomotor activity. J Pharmacol Exp Ther 1983; 227: 229–237. [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von-Zastrow M, Schulz S et al. Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 2013; 65: 223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Schulz S, Klutzny M, Koch T, Händel M, Höllt V. Involvement of mitogen-activated protein kinase in agonist-induced phosphorylation of the mu-opioid receptor in HEK 293 cells. J Neurochem 2000; 74: 414–422. [DOI] [PubMed] [Google Scholar]
- Hofford RS, Hodgson SR, Roberts KW, Bryant CD, Evans CJ, Eitan S. Extracellular signal-regulated kinase activation in the amygdala mediates elevated plus maze behavior during opioid withdrawal. Behav Pharmacol 2009; 20: 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macé G, Miaczynska M, Zerial M, Nebreda AR. Phosphorylation of EEA1 by p38 MAP kinase regulates mu opioid receptor endocytosis. EMBO J 2005; 24: 3235–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Chavkin C. Tyrosine phosphorylation of the mu-opioid receptor regulates agonist intrinsic efficacy. Mol Pharmacol 2001; 59: 1360–1368. [DOI] [PubMed] [Google Scholar]
- Clayton CC, Bruchas MR, Lee ML, Chavkin C. Phosphorylation of the mu-opioid receptor at tyrosine 166 (Tyr3.51) in the DRY motif reduces agonist efficacy. Mol Pharmacol 2010; 77: 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp J. Possible mechanisms of morphine analgesia. Clin Neuropharmacol 1991; 14: 131–147. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Suenaga NM, Ossipov MH, Malan TP Jr, Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci 2001; 21: 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci 2005; 8: 1051–1058. [DOI] [PubMed] [Google Scholar]
- Choi YS, Lin SL, Lee B, Kurup P, Cho HY, Naegele JR. Status epilepticus-induced somatostatinergic hilar interneuron degeneration is regulated by striatal enriched protein tyrosine phosphatase. J Neurosci 2007; 27: 2999–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kurup P, Xu J, Carty N, Fernandez SM, Nygaard HB. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer's disease mouse model. Proc Natl Acad Sci USA 2010; 107: 19014–19019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicklin TR, Wu PH, Radcliffe RA, Freund RK, Goebel-Goody SM, Correa PR et al. Alcohol inhibition of the NMDA receptor function, long-term potentiation, and fear learning requires striatal-enriched protein tyrosine phosphatase. Proc Natl Acad Sci USA 2011; 108: 6650–6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty NC, Xu J, Kurup P, Brouillette J, Goebel-Goody SM, Austin DR et al. The tyrosine phosphatase STEP: implications in schizophrenia and the molecular mechanism underlying antipsychotic medications. Transl Psychiatry 2012; 2: e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel-Goody SM, Wilson-Wallis ED, Royston S, Tagliatela SM, Naegele JR, Lombroso PJ. Genetic manipulation of STEP reverses behavioral abnormalities in a fragile X syndrome mouse model. Genes Brain Behav 2012; 11: 586–600. [DOI] [PMC free article] [PubMed] [Google Scholar]