Abstract
IMPORTANCE
Severe neuropsychiatric conditions, such as schizophrenia, affect distributed neural computations. One candidate system profoundly altered in chronic schizophrenia involves the thalamocortical networks. It is widely acknowledged that schizophrenia is a neurodevelopmental disorder that likely affects the brain before onset of clinical symptoms. However, no investigation has tested whether thalamocortical connectivity is altered in individuals at risk for psychosis or whether this pattern is more severe in individuals who later develop full-blown illness.
OBJECTIVES
To determine whether baseline thalamocortical connectivity differs between individuals at clinical high risk for psychosis and healthy controls, whether this pattern is more severe in those who later convert to full-blown illness, and whether magnitude of thalamocortical dysconnectivity is associated with baseline prodromal symptom severity.
DESIGN, SETTING, AND PARTICIPANTS
In this multicenter, 2-year follow-up, case-control study, we examined 397 participants aged 12–35 years of age (243 individuals at clinical high risk of psychosis, of whom 21 converted to full-blown illness, and 154 healthy controls). The baseline scan dates were January 15, 2010, to April 30, 2012.
MAIN OUTCOMES AND MEASURES
Whole-brain thalamic functional connectivity maps were generated using individuals’ anatomically defined thalamic seeds, measured using resting-state functional connectivity magnetic resonance imaging.
RESULTS
Using baseline magnetic resonance images, we identified thalamocortical dysconnectivity in the 243 individuals at clinical high risk for psychosis, which was particularly pronounced in the 21 participants who converted to full-blown illness. The pattern involved widespread hypoconnectivity between the thalamus and prefrontal and cerebellar areas, which was more prominent in those who converted to full-blown illness (t173 = 3.77, P < .001, Hedge g = 0.88). Conversely, there was marked thalamic hyperconnectivity with sensory motor areas, again most pronounced in those who converted to full-blown illness (t173 = 2.85, P < .001, Hedge g = 0.66). Both patterns were significantly correlated with concurrent prodromal symptom severity (r = 0.27, P < 3.6 × 10−8, Spearman ρ = 0.27, P < 4.75 × 10−5, 2-tailed).
CONCLUSIONS AND RELEVANCE
Thalamic dysconnectivity, resembling that seen in schizophrenia, was evident in individuals at clinical high risk for psychosis and more prominently in those who later converted to psychosis. Dysconnectivity correlated with symptom severity, supporting the idea that thalamic connectivity may have prognostic implications for risk of conversion to full-blown illness.
Schizophrenia is characterized as a neurodevelopmental disorder1 of distributed brain dysconnectivity,2 emerging from complex biological alterations that affect large-scale neural systems.3 Its symptoms are correspondingly pervasive,4 leading to lifelong disability for most patients5 and profound economic consequences. Understanding neural disturbances in schizophrenia constitutes a critical research goal that necessitates identification of pathophysiologic mechanisms and biomarkers that aid risk prediction. Growing sophistication in non-invasive neuroimaging offers away to characterize large-scale neural system disturbances in psychiatric illness6–8 by studying low-frequency fluctuations in the blood oxygenation level–dependent (BOLD) signal at rest7 (ie, via resting-state functional connectivity magnetic resonance imaging [rs-fcMRI]). This technique is increasingly applied to the study of neuropsychiatric conditions given its brief acquisition time, cost-effectiveness, and reproducibility6,9 based on the hypothesis that conditions such as schizophrenia are brain disorders that affect exchange of information across large-scale neural networks.
One such neural system, repeatedly implicated in schizophrenia,10–12 involves thalamocortical loops13 through which most neural computations flow. Thalamocortical systems have been studied extensively in humans using noninvasive neuroimaging.14 Both rs-fcMRI14 and structural diffusion studies in humans15,16 revealed that the thalamus is organized into parallel pathways that form information routes with the neocortex. This property makes the thalamus an ideal starting point and a possible lens into large-scale neural system disruptions in schizophrenia.12,17
Indeed, several groups have recently reported disrupted thalamocortical functional connectivity in chronic schizophrenia.10–12 However, schizophrenia is a neurodevelopmental illness associated with brain abnormalities that likely occur before onset of all clinical symptoms.18 Currently, it is unknown whether thalamocortical dysconnectivity emerges exclusively in association with chronic illness or whether high clinical risk (CHR) and subsequent longitudinal conversion to full-blown illness (CHR-C), as opposed to nonconversion (CHR-NC), are also associated with functional thalamocortical disruptions. It is vital to address this question for 3 reasons: (1) to elucidate how incipient pathophysiologic stages of putative schizophrenia affect large-scale neural systems before full-blown symptoms emerge; (2) to establish whether disruptions in thalamocortical connectivity could provide viable neural markers associated with clinical risk before conversion; and (3) to extend recent discoveries while bypassing typical confounds associated with chronic illness (eg, years of medication exposure).
In this study, we examined resting-state thalamocortical connectivity in the North American Prodromal Longitudinal Study (NAPLS) clinical high-risk sample. After obtaining baseline images, we longitudinally studied 243 CHR patients (21 CHR-C patients and 222 CHR-NC patients) and 154 healthy controls who were demographically similar to the clinical group. We tested the following 3 questions: (1) “Is CHR associated with thalamocortical dysconnectivity?” (2) “Is thalamocortical dysconnectivity more severe in CHR-C compared with CHR-NC patients?” and (3) “Is thalamocortical dysconnectivity associated with severity of psychotic symptoms at baseline?”
Methods
Participants
Our final sample included 243 CHR individuals (21 in the CHR-C group and 222 in the CHR-NC group) and 154 controls. All participants were recruited as part of the NAPLS 2 cohort19 and underwent rs-fcMRI at their baseline evaluations (eTable 1 in the Supplement). The study protocol and consent form were reviewed and approved by the institutional review boards at each of the 8 participating data collection sites (University of California, Los Angeles, Emory University, Harvard Medical School, Zucker Hillside Hospital, University of North Carolina, University of California, San Diego, University of Calgary, and Yale University). All participants provided written informed consent. All recruitment, symptom assessment, and longitudinal evaluation details are presented in eTable 1 in the Supplement.
All participants were between 12–35 years of age with IQ >70, no history of central nervous system disorders and no substance dependence in the past 6 months. The CHR sample met the Criteria of Prodromal Syndromes (COPS) following assessment with the Structured Interview for Prodromal Syndromes (SIPS) by experienced MA/PhD level clinicians. Participants were excluded for current or past diagnosis with Axis I psychotic disorders, including affective psychoses, as determined by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders-IV (SCID). Other co-morbid Axis I diagnoses, such as mood or anxiety disorders, were not exclusionary provided they did not account for the subject’s prodromal symptoms. There were no significant differences in substance use, anxiety, or age between CHR subjects who converted and those who did not. HCS were excluded if they met criteria for any prodromal syndrome, current or past psychotic disorder, or Cluster A personality disorder. HCS were also excluded for family history (first-degree relatives) of any disorder involving psychotic symptoms and for current use of psychotropic medication.
Neuroimaging Data Acquisition
Neuroimaging was performed at 8 sites. Five sites (University of California, Los Angeles, Emory University, Harvard University, University of North Carolina, and Yale University) used Siemens-Trio 3-T scanners (Siemens), 2 sites (Zucker Hillside Hospital and University of California, San Diego) used General Electric HDx Signa scanners (General Electric), and 1 site (University of Calgary) used a General Electric Discovery scanner (General Electric). All neuroimaging and functional connectivity analyses followed prior work and best practices in the clinical connectivity literature,20,21 with details presented in the eAppendix in the Supplement.
Seed-Based Functional Connectivity Analysis Based on Thalamic Anatomy
Our seed-based approach followed a prior study22 using anatomically defined thalamic nuclei. In-house Matlab tools23,24 were used to examine thalamus connectivity with all gray matter voxels. We computed a seed-based thalamus correlation map by extracting mean time series across all voxels in each participant’s bilateral thalamus (anatomically defined through Freesurfer-based segmentation25,26). This entire thalamic signal was then correlated with each gray matter voxel, and the computed Pearson correlation values were transformed to Fisher z values using a Fisher r-to-z transformation, providing a map for each participant that was entered into second-level analyses in which each voxel’s value represented its connectivity with the whole thalamus (eAppendix in the Supplement). To examine between-group differences, all individual-subject maps were entered into appropriate second-level tests (either independent samples t-test or 1-way ANOVA with three between-group levels [CHR-NC, CHR-C, HCS]), which was computed within FSL’s Randomize tool with 10,000 permutations. Type I error correction was determined via threshold-free cluster enhancement (TFCE) using a P <.01 as the statistical threshold.
Results
Association of Psychosis Risk and Thalamic Dysconnectivity
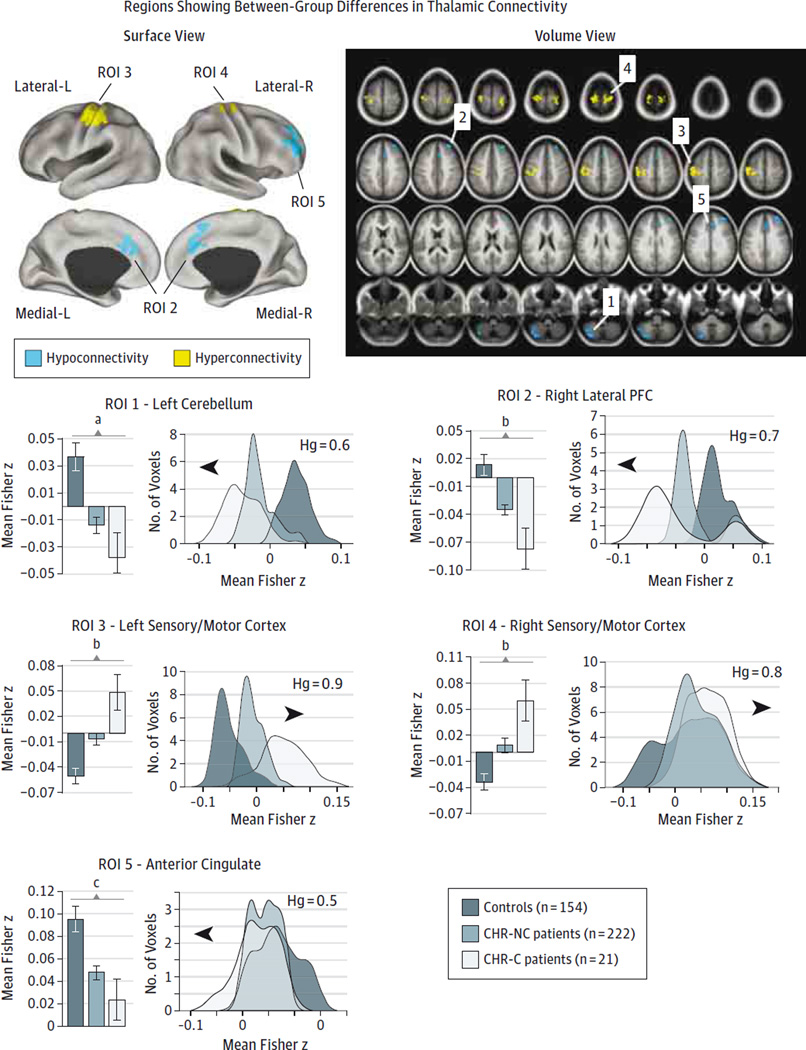
We tested whether the risk of psychosis was associated with thalamocortical dysconnectivity across the 21 participants in the CHR-C group, 222 participants in the CHR-NC group, and the 154 participants in the control group (computed via 1-way analysis of variance with one between-group factor). We took 2 approaches to testing this hypothesis: (1) at the whole-brain level without regional constraints (eFigure 1 in the Supplement) and (2) within regions known to exhibit robust thalamocortical dysconnectivity in chronic schizophrenia10–12 (eFigure 2 in the Supplement). Although similar effects were apparent in both cases, the whole-brain results did not survive type I error correction (eFigure 1 in the Supplement). The a priori constrained analyses, however, revealed robust between-group differences best described as increased connectivity between thalamus and bilateral sensory motor cortices but decreased connectivity among the thalamus, prefrontal cortex (PFC), and cerebellum, similar to prior qualitative and quantitative observations in chronic schizophrenia.10–12 This effect was more prominent in CHR-C patients (Figure 1 and Table). To further characterize the effects, we extracted the mean thalamic functional connectivity from the identified regions of interest, as well as the connectivity distributions across all voxels (Figure 1). Results indicated a marked shift for the CHR-C patients across all regions of interest, confirmed via formal effect sizes (ie, overall hyperconnectivity: t173 = 3.77, P < .001, Hedge g = 0.88;overall hypoconnectivity: t173 = 2.85, P < .001, Hedge g = 0.66).28 Across areas, the CHR-NC group was associated with an intermediate level of dysconnectivity between the individuals in the CHR-C and control groups.
Figure 1. Regions With Between-Group Differences in Thalamic Connectivity.
Significant between-group effects were found for 5 regions of interest (ROIs) after a 1-way analysis of variance F test using cluster protection after 10 000 permutations27 (see Methods). Results were visualized using surface-based and volume maps. All displayed foci revealed significant between-group effects within the thalamocortical masks identified in our prior work12 (in which patients with chronic illness exhibited robust thalamocortical disruptions; eAppendix and eTables 3 and 4 in the Supplement). Blue and yellow areas indicate regions where the F test is driven by a reduction or increase, respectively, in thalamic connectivity in the clinical high risk for psychosis (CHR) groups. Magnitudes (left) and distributions (right) across groups for each of the identified regions qualitatively illustrate the direction of the effect. Effect sizes (Hedge g [Hg]) reflect the shift for the CHR converted to full-blown illness (CHR-C) group relative to controls. For a complete list of regions and statistics, see the Table. We used the Hg as a measure of effect size to account for differences in sample size between the CHR-C and CHR-nonconversion (CHR-NC) groups.28 Error bars indicate ±1 SEM. The histograms are based on the data extracted from the F map presented in the surface view panel. We present reduced-threshold pairwise effects in the eAppendix in the Supplement. L indicates left; and R, right.
a P < .01.
b P < .001.
c P < .05.
Table.
Pairwise Comparisons of Region Coordinates
| Region Coordinate | Hemisphere | Landmark | Size, mm3 | Comparison Groups | Hedge g | t | P Value | ||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||||
| −31 | −67 | −43 | Left | Cerebellum | 4428 | Controls vs CHR-NC | −0.47 | 4.47 | <.001 |
| Controls vs CHR-C | −0.60 | 2.56 | .01 | ||||||
| CHR-C vs CHR-NC | −0.27 | 1.17 | .24 | ||||||
| 31 | 42 | 31 | Right | Lateral prefrontal cortex/Brodmann 9 | 3429 | Controls vs CHR-NC | −0.44 | 4.19 | <.001 |
| Controls vs CHR-C | −0.69 | 2.96 | .003 | ||||||
| CHR-C vs CHR-NC | −0.46 | 2.03 | .04 | ||||||
| 2 | 21 | 36 | Midline | Anterior cingulate gyrus/Brodmann 32 | 2403 | Controls vs CHR-NC | −0.41 | 3.89 | <.001 |
| Controls vs CHR-C | −0.53 | 2.27 | .02 | ||||||
| CHR-C vs CHR-NC | −0.26 | 1.14 | .26 | ||||||
| −35 | −23 | 58 | Left | Sensory motor cortex/Brodmann 4 | 7074 | Controls vs CHR-NC | 0.39 | 3.69 | <.001 |
| Controls vs CHR-C | 0.87 | 3.73 | <.001 | ||||||
| CHR-C vs CHR-NC | 0.50 | 2.21 | .03 | ||||||
| 19 | −27 | 70 | Right | Sensory motor cortex/Brodmann 4 | 2403 | Controls vs CHR-NC | 0.36 | 3.43 | <.001 |
| Controls vs CHR-C | 0.80 | 3.45 | <.001 | ||||||
| CHR-C vs CHR-NC | 0.42 | 1.85 | .07 | ||||||
Abbreviations: CHR-C, clinical high risk and conversion to psychosis; CHR-NC, clinical high risk and nonconversion to psychosis.
These results, however, were restricted within the a priori regions previously identified in chronic schizophrenia, guaranteeing effects across similar regions. There may be attenuated effects elsewhere that do not resemble prior schizophrenia results. To examine this possibility, we computed 2 post hoc pairwise comparisons presented at lower thresholds, allowing qualitative inspection (eFigure 5 in the Supplement). Results revealed consistent patterns of increased connectivity around sensory motor cortices but reductions around PFC, striatum, and cerebellum, which were also more prominent in the CHR-C group.
Finally, there is increasing emphasis on processing procedures in rs-fcMRI research because certain procedures, specifically, global signal regression (GSR) (eAppendix in the Supplement), may complicate between-group comparisons.29 The GSR is associated with the removal of a mean brain signal from each voxel’s time series, the magnitude of which may differ among clinical conditions.30 To rule out GSR confounds, we repeated analyses without GSR (eFigure 3 and eFigure 4 in the Supplement), which did not alter results.
Patterns of Thalamocortical Dysconnectivity and Shared Disturbances
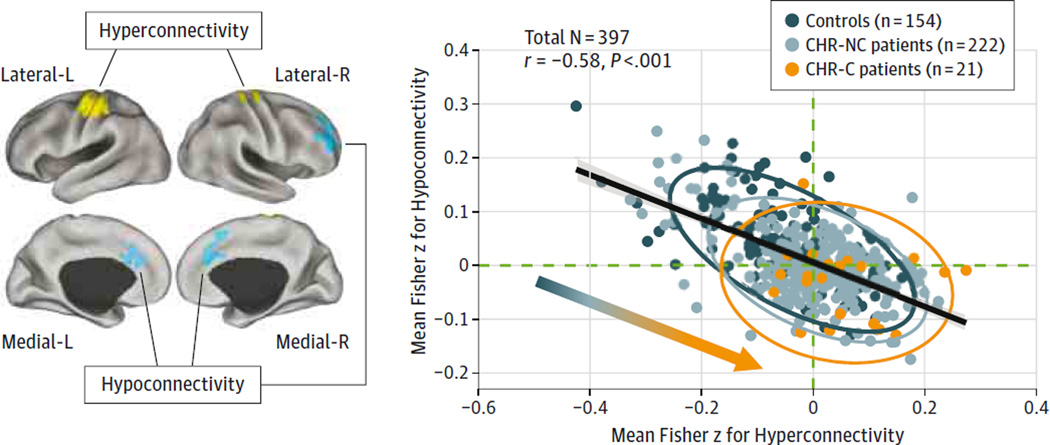
The present effects highlight 2 dissociable patterns: hyperconnectivity centered on sensory motor cortices and reductions in thalamo-prefrontal-cerebellar connectivity. These effects may reflect separable alterations.12 Alternatively, as found in chronic schizophrenia,12 these findings could constitute shared disturbances whereby individuals with most severe thalamic-sensory perturbations also have the greatest thalamo-prefrontal-cerebellar dysconnectivity. To test this hypothesis, we correlated the strength of connectivity from regions with reduced thalamic connectivity with regions with increased thalamic connectivity, explicitly following approaches in our prior work (Figure 2).12
Figure 2. Association Between Thalamic Hyperconnectivity and Hypoconnectivity Across Study Participants.
Regions with reduced (blue) and increased (yellow) thalamic connectivity (Figure 1). A significant negative association is evident across all participants, collapsing across all 3 samples (r = −0.58, P < 4.1 × 10−38). Vertical/horizontal green dashed lines mark the zero points. Patients with clinical high risk of psychosis (CHR) who converted to full-blown illness (CHR-C) had a shift across the zero lines, indicative of weaker prefrontal-cerebellar-thalamic connectivity but stronger sensory-motor-thalamic connectivity. Patients with CHR who did not convert (CHR-NC) had a more intermediate degree of disruption, suggesting a gradient (inset arrow for qualitative illustration). Ovals for each group mark the 95%CI. L indicates left; and R, right.
Across all participants, there was a highly significant negative relationship (N = 397, r = −0.58, P < 4.1 × 10−38) between the 2 findings, which held for the CHR group (N = 242, r = −0.53, P < 1.2 × 10−19): individuals with the most severe thalamic-sensory hyperconnectivity had the greatest thalamo-prefrontal-cerebellar hypoconnectivity. This pattern indicates that the 2 disruptions represent shared thalamocortical alterations, consistent with schizophrenia findings.12
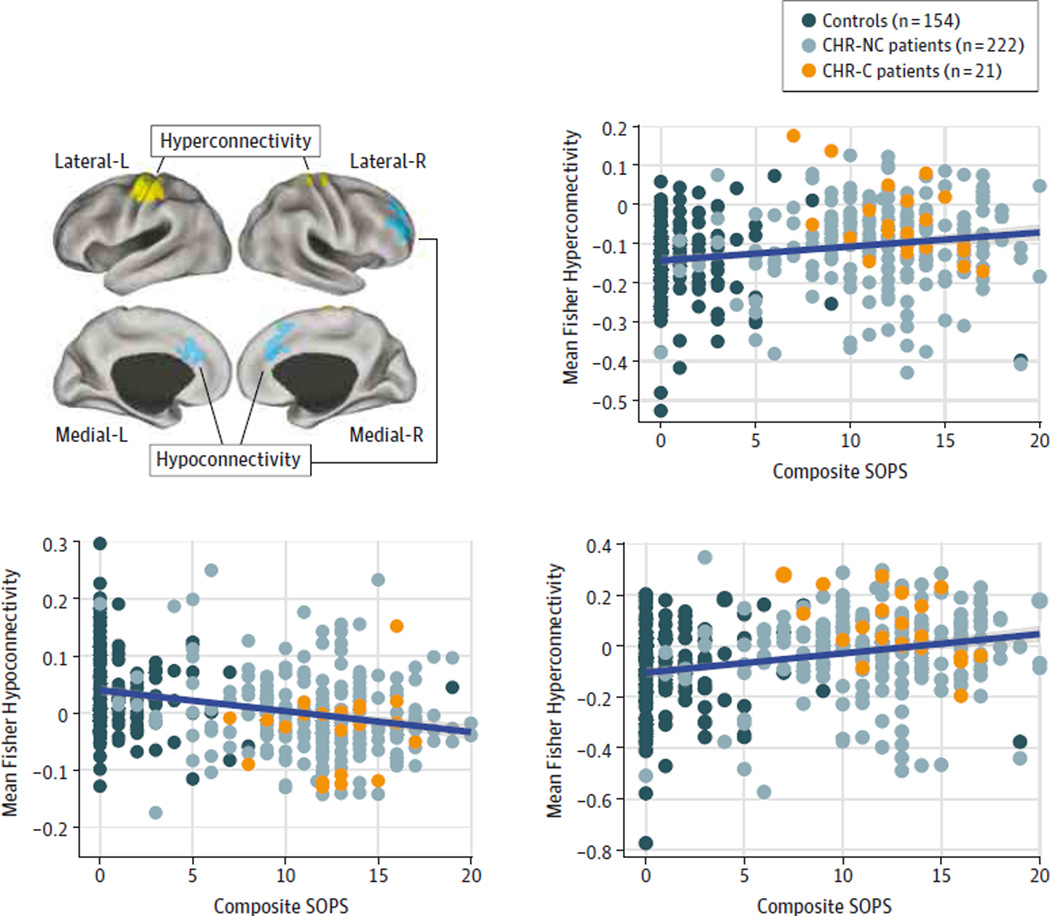
Association Between Baseline Symptoms and Thalamic Dysconnectivity
Next, to provisionally test the clinical significance of thalamic dysconnectivity, we examined its association with symptoms. We examined the composite score using the Scale of Prodromal Symptoms (SOPS; composite positive symptoms31) for 2 reasons. First, prior work12 in chronic schizophrenia identified a significant association between positive symptoms and thalamic-sensory-motor hyperconnectivity, providing strong a priori predictions. Second, we sought to circumvent stringent type I error correction arising from many exploratory comparisons. We correlated symptoms with connectivity measures separately for areas with reduced vs increased thalamic connectivity (ie, aggregate signal across yellow vs blue foci in Figure 1). We examined this association across all participants to remain maximally powered and motivated by a Research Domain Criteria32 strategy because low SOPS scores may exist even in the general population (eTable 1 in the Supplement). We identified a significant negative correlation between regions with thalamic hypoconnectivity and symptoms (r = −0.31, P < 9.54 × 10−10, Spearman ρ = −0.32, P < 1.36 × 10−9, 2-tailed), which also held when we restricted analyses to those individuals who presented with symptoms (r = −0.21, P < .001, Spearman ρ = −0.22, P < .001, 2-tailed) and only to CHR patients (r = −0.14, P = .03, Spearman ρ = −0.32, P = .04, 2-tailed; eFigures 6 and 7 in the Supplement). There was also a significant positive correlation between SOPS scores and hyperconnectivity across participants (r = 0.21, P < 4.1 × 10−5, Spearman ρ = 0.21, P < 4.8 × 10−5 ; Figure 3), which did not survive when we restricted the analysis to symptomatic participants or CHR patients (eFigure 7 in the Supplement). To confirm that the 2 disturbances are related, we calculated the difference in magnitude of hyperconnectivity vs hypoconnectivity for each participant (ie, difference in connectivity for yellow vs blue foci in Figure 1). We correlated the magnitude of this connectivity difference with symptoms, which also revealed a significant association (r = 0.27, P < 3.6 × 10−8, Spearman ρ = 0.27, P < 4.75 × 10−5, 2-tailed), which held when we restricted analyses to those individuals who presented with symptoms (r = 0.17, P < .001, Spearman ρ = 0.14, P < .001, 2-tailed) but did not survive when restricted to CHR patients (eFigure 7 in the Supplement). Of note, we did not identify a significant association between identified thalamic dysconnectivity and SOPS scores at the 2-year follow-up.
Figure 3. Association Between Prodromal Schizophrenia Symptoms and Thalamic Dysconnectivity.
Regions showing reduced (blue) and increased (yellow) thalamic connectivity. Significant positive association was found between thalamic connectivity across all areas showing increased connectivity (yellow regions) and composite positive symptoms on the Scale of Prodromal Symptoms (SOPS) across all participants (r = 0.21, P < 4.1 × 10−5, Spearman ρ = 0.21, P < 4.8 × 10−5). A significant negative association was found between thalamic connectivity across all areas showing reduced connectivity (blue regions) and composite positive symptoms on the SOPS across all participants (r = −0.31, P < 9.54 × 10−10, Spearman ρ = −0.32, P < 1.36 × 10−9, 2-tailed). We computed a difference score between the regions showing hyperconnectivity (yellow) and hypoconnectivity (blue). The purpose of this calculation was to establish that the total magnitude of connectivity disruptions in either direction still relates to psychotic symptoms as opposed to these 2 patterns capturing independent sources of variability. We found a significant association between thalamic connectivity difference score and composite positive symptoms on the SOPS across all participants (r = 0.27, P < 3.6 × 10−8, Spearman ρ = 0.27, P < 4.75 × 10−5, 2-tailed). For a figure presenting clinical high risk (CHR) patients only, see eFigure 7 in the Supplement. CHR-C indicates clinical high risk of psychosis converted to full-blown illness; CHR-NC, clinical high risk of psychosis not converted to full-blown illness; L indicates left; and R, right.
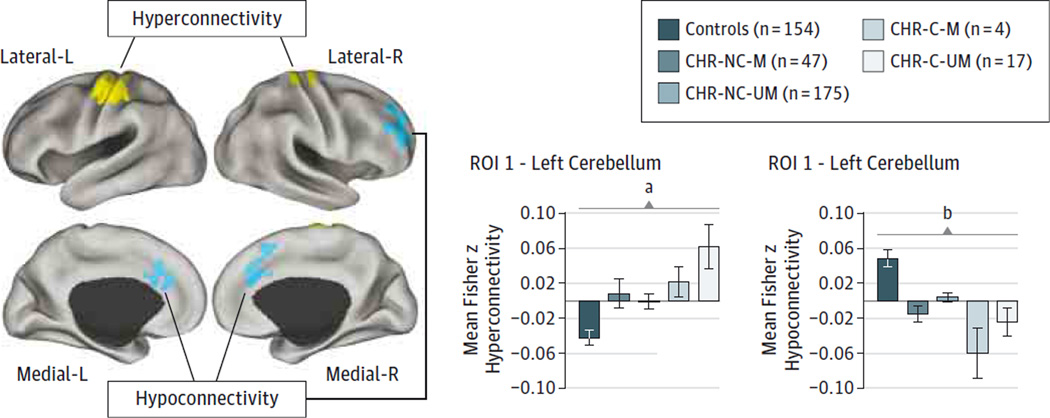
Association Between Medication and Thalamic Dysconnectivity
Last, we tested whether identified thalamic dysconnectivity was related to medication dose and/or status. We first correlated medication level (calculated via chlorpromazine equivalents33) with magnitude of thalamic hyperconnectivity and hypoconnectivity. This analysis revealed no significant association, although there were modest trends (thalamic hyperconnectivity: r = 0.19, P = .19; thalamic hypoconnectivity: r = −0.2, P = .16; n = 50 medicated patients). These trends imply that individuals who received higher doses of medication had greater thalamocortical dysconnectivity at baseline. Next, we tested whether effects differed as a function of medication status (because some individuals were medicated even at their baseline scans34) (Figure 4). This analysis revealed that unmedicated patients in the CHR-Cgroup (n = 17) exhibited the most profound thalamic-sensory-motor hyperconnectivity (Figure 4), which significantly differed from the controls (t169 = 3.7, P < .001, 2-tailed, Hedge g = 0.81). This effect is the opposite of what one would expect if a medication confound were driving thalamic hyperconnectivity because medicated individuals had a milder profile. Medicated and unmedicated patients in the CHR-C group exhibited thalamic hypoconnectivity of similar magnitude (Figure 4). Again, the unmedicated patients in the CHR-C group significantly differed from the controls (t169 = 2.34, P = .02, 2-tailed, Hedge g = 0.31). These secondary medication analyses rule out the possibility that medication is driving the observed patterns.
Figure 4. Thalamic Dysconnectivity as a Function of Medication Status.
Regions with reduced (blue) and increased (yellow) thalamic connectivity, driven by the clinical high risk of psychosis (CHR) converted to full-blown illness (CHR-C). The association between medication status and thalamic hyperconnectivity indicated that CHR-C patients who remained unmedicated (CHR-C-UM) exhibited the most severe hyperconnectivity, significantly differing from controls (t169 = 3.7, P < .001, 2-tailed, Hedge g = 0.81) and CHR patients who did not convert (CHR-NC) without medication (CHR-NC-UM) (t190 = 2.27, P = .02, 2-tailed, Hedge g = 0.53). However, no significant difference was found between medicated (CHR-C-M and CHR-NC-M) and CHR-C-UM and CHR-NC-UM patients (t19 = 0.46, P = .76). Again, CHR-C-UM patients exhibited significant reductions in thalamic connectivity with prefrontal cortex and cerebellar nodes relative to controls (t169 = 2.34, P = .02, 2-tailed, Hedge g = 0.31) but not the other clinical groups. We used Hedge g as a measure of effect size to account for differences in sample sizes.28 Error bars indicate ±1 SEM. L indicates left; and R, right.
a P < .001.
b P < .05.
Discussion
Thalamic circuits feature prominently in theoretical models of schizophrenia17,35 and are implicated in empirical schizophrenia studies.10–12 However, patterns of thalamic dysconnectivity before full-blown illness conversion remain unknown. To address this knowledge gap, we used rs-fcMRI to characterize thalamocortical dysconnectivity in association with clinical risk and future conversion to full-blown psychosis. We identified patterns consistent with recent discoveries in schizophrenia10–12: increased thalamic connectivity with sensory motor cortices but reduced thalamic connectivity with PFC and cerebellum, a pattern more prominent in CHR-C patients. Both patterns were modestly correlated with symptoms and were observed in unmedicated individuals in the CHR group. Results were observed during baseline imaging before psychosis onset, indicating that these disturbances predate full-blown illness.
Risk for Psychosis and Thalamocortical Dysconnectivity
Schizophrenia is a neurodevelopmental disorder associated with genetic risk factors.36,37 However, indicators of its emerging pathophysiologic conditions remain poorly understood, with few viable neural markers that predict illness development and subsequent frank onset. This lack of at-risk endophenotypes for schizophrenia is compounded by the scarcity of well-established neural markers of chronic illness. The field has made progress in defining neural markers of cortical and subcortical dysfunction in schizophrenia through a combination of postmortem38–40 and neuroimaging studies examining structure,41 task-based activation,42–46 and functional and structural connectivity.21,47 This work has been enriched by resting-state studies mapping large-scale network disturbances in schizophrenia48: during the past year, several groups independently replicated prominent thalamocortical disturbances in chronic schizophrenia.10–12 Although promising, this work has not addressed 2 vital questions. First, “Are the observed thalamocortical connectivity disturbances in part related to long-term medication effects?” Second, from a clinical standpoint, “Are these thalamocortical connectivity disturbances an exclusive feature of long-standing illness, or do they emerge during early illness stages?” The second question is particularly important for identifying neural markers that appear before progression to chronic illness.
We found that thalamocortical dysconnectivity was present in the CHR state before full-blown psychosis. The effects followed previously reported hyperconnectivity with sensory motor areas and hypoconnectivity with PFC and cerebellum (note that hyperconnectivity with other auditory and visual sensory regions was evident, although these regions did not survive more stringent thresholding). Both patterns were more severe for CHR-C patients, suggesting pathophysiologic relevance to the pathogenic cascade that culminates in psychosis. These effects address the limitations of prior long-term studies: because thalamic dysconnectivity in CHR patients closely mirrors effects reported in patients with chronic illness and these patterns were observed in unmedicated patients, it is unlikely that these alterations are driven by long-term pharmacotherapy. However, our analyses provisionally imply that medication may exert a beneficial effect by reducing thalamocortical hyperconnectivity.
Follow-up analyses revealed similar effects even at lower statistical thresholds (Figure 2). Furthermore, thalamic hyperconnectivity and hypoconnectivity were highly related across participants, suggesting a common system-wide disturbance (as reported in chronic schizophrenia10–12). Finally, the magnitude of thalamic dysconnectivity in both hyperconnected and hypoconnected regions predicted symptoms. The difference in magnitude between hyperconnectivity and hypoconnectivity also correlated with symptoms, supporting the hypothesis that the 2 effects constitute a shared, system-wide thalamic abnormality. By linking thalamic dysconnectivity with symptom levels before illness onset, these results raise the question of whether thalamic dysconnectivity relates to genetic risk and/or appears in unaffected relatives or whether it only emerges in association with symptom expression (because patients in the CHR group were seeking help).
Although this study focused on the thalamus, other groups documented abnormalities across striatal and cortical networks during CHR states49 or in patients with chronic illness.9,50–52 Therefore, thalamocortical dysconnectivity cannot be construed as the only disturbance that occurs during this at-risk period. For instance, Fornito and colleagues49 reported changes in frontostriatal circuits that were strikingly similar to reported thalamic effects: a prominent dorsal-to-ventral gradient of hypoconnectivity to hyperconnectivity between the striatal and PFC regions, which correlated with symptoms. Baker and colleagues50 reported functional connectivity disruptions in associative cortices in patients with chronic illness, which may be related to abnormal BOLD signal variance in these same associative networks.30 It is unclear whether these disparate effects possibly reflect common upstream mechanisms3 (eg, disrupted glutamate signaling or microglial alterations) or whether they constitute separable abnormalities that emerge at distinct illness stages.53,54 Longitudinal and genetic studies are needed to map risk- and disease-related connectivity changes in relation to these emerging neuroimaging markers. Such efforts will help disambiguate which markers relate to genetic risk, early-course prodromal symptoms, frank illness onset, and, ultimately, chronic illness effects, informing therapeutic design for specific illness phases. Finally, although these effects are potentially compelling, the relatively small sample of converters limits firm conclusions about whether this particular imaging phenotype is a true clinical predictor of conversion.
Implications for the Neurobiology of Schizophrenia Onset
Present findings are correlational given the indirect neuroimaging measure (BOLD fcMRI) and therefore cannot address upstream causal mechanisms. To map such upstream cellular mechanisms, translational research from animal experiments,55 experimental pharmacologic neuroimaging studies,56 and computational models can generate mechanistic and testable predictions.17,30,57,58 Schizophrenia likely involves alterations in glutamatergic, dopaminergic, and inhibitory gamma-aminobutyric acid (GABA) neurotransmission.3,38,39,59–62 Theoretical models of the illness repeatedly implicate these neurotransmitter systems in thalamo-striatal-cortical circuits, which may contribute to the observed alterations. It currently remains unknown which upstream mechanisms and at which locus (subcortical or cortical) produce such widespread thalamic dysconnectivity. One possibility may involve dysfunction of the N-methyl-d-aspartate glutamate receptor,63 hypothesized to occur in inhibitory interneurons,38,39,62 which affects the balance of neural excitation and inhibition64 in cortical circuits, producing brain-wide disturbances in thalamocortical information flow.
Limitations
Although medication effects were largely ruled out, some CHR patients were medicated. Thus, we correlated medication levels with magnitude of thalamocortical dysconnectivity, which did not reveal significant effects. Furthermore, we compared medicated and unmedicated CHR patients: although hypoconnectivity did not vary as a function of medication status, the hyperconnectivity profile was actually worse for unmedicated CHR patients, suggesting that medication did not drive primary effects. Another concern, present in all clinical connectivity studies, relates to head movement. We used careful movement-censoring methods for all data and used movement (percentage of frames scrubbed) as a covariate in the analysis, which did not alter effects (eAppendix in the Supplement). Movement levels did not significantly differ between CHR-NC and CHR-C patients (Table), increasing confidence that head movement did not drive effects. The data were pooled from multiple sites and scanners, introducing possible scanner bias and other site-specific effects on cohort recruitment and assessment. To mitigate this concern, we conducted 2 analyses: we confirmed that results remained despite using site as a covariate in the reported analysis (of note, there was a slight deviation from expected proportions revealed by a χ2 test; eTable 2 in the Supplement). Another issue relates to the definition of risk; here, we studied individuals who were clinically symptomatic and seeking help and therefore presented with clinical elevated risk for psychosis conversion. Consequently, these findings cannot directly address whether genetic liability for schizophrenia is associated with the same pattern of thalamic dysconnectivity. We observed reported effects within a priori masks exhibiting thalamocortical dysconnectivity in our prior work12 but not at the whole-brain level. This finding may reflect restricted power because of the relatively smaller CHR-C group but also the possibility that the CHR state may be associated with weaker effects relative to the more robust patterns found in patients with chronic illness.12 In addition, although starting from the entire thalamus may be a well-justified first-pass approach (to remain sensitive to pan-thalamic disruptions), there may be important discrepancies across thalamic subnuclei that the current analysis did not consider.10,65
Conclusions
This study establishes that thalamocortical dysconnectivity is present in CHR states before psychosis onset. We found that sensory motor and prefrontal-cerebellar thalamic dysconnectivity was more severe in the CHR-C patients. These effects are congruent with recent discoveries in patients with chronic illness, suggesting that brain-wide thalamocortical dysconnectivity is apparent even during incipient illness stages and may provide a sensitive marker for elevated clinical risk of full illness onset.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by grants MH081902 (Dr Cannon), MH081857 (Dr Cornblatt), MH081988 (Dr Walker), MH081928 (Dr Seidman), MH082004 (Dr Perkins), MH082022 (Dr Cadenhead), MH081984 (Dr Addington), and MH066160 (Dr Woods) from the National Institute of Mental Health, grants DP5OD012109-02 (principal investigator: Dr Anticevic) and T32GM 007205 (Ms Yang) from the National Institutes of Health, a Brain and Behavior Research Foundation Young Investigator Award (principal investigator: Dr Anticevic), and grant SCDMH82101008006 from the Commonwealth Research Center (principal investigator: Dr Seidman).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Anticevic had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Anticevic and Haut contributed equally to this work.
Study concept and design: Anticevic, Haut, Murray, Addington, Cadenhead, McGlashan, Perkins, Belger, Tsuang, van Erp, Walker, Cannon.
Acquisition, analysis, or interpretation of data: Anticevic, Haut, Repovs, Yang, Diehl, McEwen, Bearden, Goodyear, Mirzakhanian, Cornblatt, Olvet, Mathalon, McGlashan, Perkins, Belger, Seidman, van Erp, Walker, Hamann, Woods, Qiu, Cannon.
Drafting of the manuscript: Anticevic, Haut, Mathalon, McGlashan, Seidman, Cannon.
Critical revision of the manuscript for important intellectual content: Anticevic, Murray, Repovs, Yang, Diehl, McEwen, Bearden, Addington, Goodyear, Cadenhead, Mirzakhanian, Cornblatt, Olvet, Mathalon, McGlashan, Perkins, Belger, Seidman, Tsuang, van Erp, Walker, Hamann, Woods, Qiu, Cannon.
Statistical analysis: Anticevic, Haut, Repovs, Mathalon, Qiu, Cannon.
Obtained funding: Bearden, Addington, Cadenhead, Mirzakhanian, Cornblatt, McGlashan, Perkins, Belger, Seidman, Walker, Cannon.
Administrative, technical, or material support: Haut, McEwen, Addington, Goodyear, Cadenhead, Mirzakhanian, McGlashan, Perkins, van Erp, Walker, Qiu.
Study supervision: Anticevic, Bearden, Cadenhead, McGlashan, Tsuang, van Erp, Hamann, Cannon.
Additional Contributions: Michael Cole, PhD, and Heidi Thermenos, PhD, assisted with this article.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 2.Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59(10):929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26(4–6):365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker E, Kestler L, Bollini A, Hochman KM. Schizophrenia: etiology and course. Annu Rev Psychol. 2004;55:401–430. doi: 10.1146/annurev.psych.55.090902.141950. [DOI] [PubMed] [Google Scholar]
- 5.Murray CJL, Lopez AD. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability From Diseases, Injuries and Risk Factors in 1990 and Projected to 2020. Vol. 1. Cambridge, MA: Harvard University Press; 1996. [Google Scholar]
- 6.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswal BB, Mennes M, Zuo X-N, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79(4):814–828. doi: 10.1016/j.neuron.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169(10):1092–1099. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klingner CM, Langbein K, Dietzek M, et al. Thalamocortical connectivity during resting state in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2014;264(2):111–119. doi: 10.1007/s00406-013-0417-0. [DOI] [PubMed] [Google Scholar]
- 12.Anticevic A, Cole MW, Repovs G, et al. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24(12):3116–3130. doi: 10.1093/cercor/bht165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20(5):1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6(7):750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 16.Johansen-Berg H, Behrens TE, Sillery E, et al. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005;15(1):31–39. doi: 10.1093/cercor/bhh105. [DOI] [PubMed] [Google Scholar]
- 17.Lisman JE, Pi HJ, Zhang Y, Otmakhova NA. A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biol Psychiatry. 2010;68(1):17–24. doi: 10.1016/j.biopsych.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Insel TR. Rethinking schizophrenia. Nature. 2010;468(7321):187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 19.Addington J, Cadenhead KS, Cornblatt BA, et al. North American Prodrome Longitudinal Study (NAPLS-2): overview and recruitment. Schizophr Res. 2012;142(1–3):77–82. doi: 10.1016/j.schres.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anticevic A, Repovs G, Barch DM. Emotion effects on attention, amygdala activation, and functional connectivity in schizophrenia. Schizophr Bull. 2012;38(5):967–980. doi: 10.1093/schbul/sbq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole MW, Anticevic A, Repovs G, Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry. 2011;70(1):43–50. doi: 10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anticevic A, Repovs G, Barch DM. Resisting emotional interference: brain regions facilitating working memory performance during negative distraction. Cogn Affect Behav Neurosci. 2010;10(2):159–173. doi: 10.3758/CABN.10.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anticevic A, Repovs G, Krystal JH, Barch DM. A broken filter: prefrontal functional connectivity abnormalities in schizophrenia during working memory interference. Schizophr Res. 2012;141(1):8–14. doi: 10.1016/j.schres.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. 2011;69(10):967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 26.Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 29.Saad ZS, Gotts SJ, Murphy K, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2(1):25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang GJ, Murray JD, Repovs G, et al. Altered global brain signal in schizophrenia. Proc Natl Acad Sci U S A. 2014;111(20):7438–7443. doi: 10.1073/pnas.1405289111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the Structured Interview for Prodromal Syndromes and the Scale of Prodromal Symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 32.Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull. 2010;36(6):1061–1062. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull. 2014;40(2):314–326. doi: 10.1093/schbul/sbu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods SW, Addington J, Bearden CE, et al. Psychotropic medication use in youth at high risk for psychosis: comparison of baseline data from two research cohorts 1998–2005 and 2008–2011. Schizophr Res. 2013;148(1–3):99–104. doi: 10.1016/j.schres.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andreasen NC. The role of the thalamus in schizophrenia. Can J Psychiatry. 1997;42(1):27–33. doi: 10.1177/070674379704200104. [DOI] [PubMed] [Google Scholar]
- 36.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cannon TD, Huttunen MO, Lonnqvist J, et al. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet. 2000;67(2):369–382. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull. 2012;38(5):950–957. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of γ-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63(10):1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 41.Cannon TD, Thompson PM, van Erp TG, et al. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci U S A. 2002;99(5):3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carter CS, MacDonald AW, III, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158(9):1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- 43.Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155(9):1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- 44.Barch DM. The cognitive neuroscience of schizophrenia. In: Cannon T, Mineka S, editors. Annual Review of Clinical Psychology. Vol. 1. Washington, DC: American Psychological Association; 2005. pp. 321–353. [DOI] [PubMed] [Google Scholar]
- 45.Glahn DC, Therman S, Manninen M, et al. Spatial working memory as an endophenotype for schizophrenia. Biol Psychiatry. 2003;53(7):624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- 46.Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16(1):27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62(4):2296–2314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- 48.Anticevic A, Cole MW, Repovs G, et al. Connectivity, pharmacology, and computation: toward a mechanistic understanding of neural system dysfunction in schizophrenia. Front Psychiatry. 2013;4:169. doi: 10.3389/fpsyt.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fornito A, Harrison BJ, Goodby E, et al. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 2013;70(11):1143–1151. doi: 10.1001/jamapsychiatry.2013.1976. [DOI] [PubMed] [Google Scholar]
- 50.Baker JT, Holmes AJ, Masters GA, et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71(2):109–118. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 52.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loh M, Rolls ET, Deco G. A dynamical systems hypothesis of schizophrenia. PLoS Comput Biol. 2007;3(11):e228. doi: 10.1371/journal.pcbi.0030228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uhlhaas PJ. Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Curr Opin Neurobiol. 2013;23(2):283–290. doi: 10.1016/j.conb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Schobel SA, Chaudhury NH, Khan UA, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78(1):81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anticevic A, Gancsos M, Murray JD, et al. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci U S A. 2012;109(41):16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr Opin Neurobiol. 2012;22(3):537–544. doi: 10.1016/j.conb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murray JD, Anticevic A, Gancsos M, et al. Linking microcircuit dysfunction to cognitive impairment: effects of disinhibition associated with schizophrenia in a cortical working memory model. Cereb Cortex. 2014;24(4):859–872. doi: 10.1093/cercor/bhs370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67(3):231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 61.Kegeles LS, Mao X, Stanford AD, et al. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69(5):449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12(4):335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169(3–4):215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 64.Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 65.Anticevic A, Yang G, Savic A, et al. Medio-dorsal and visual thalamic connectivity differ in schizophrenia and bipolar disorder with and without psychosis history. Schizophr Bull. 2014;40(6):1227–1243. doi: 10.1093/schbul/sbu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.