Abstract
Although the support for the use of antioxidants, such as coenzyme Q10 (CoQ10), to treat Parkinson’s disease (PD) comes from the extensive scientific evidence, the results of conducted thus far clinical trials are inconclusive. It is assumed that the efficacy of CoQ10 is hindered by insolubility, poor bioavailability, and lack of brain penetration. We have developed a nanomicellar formulation of CoQ10 (Ubisol-Q10) with improved properties, including the brain penetration, and tested its effectiveness in mouse MPTP (1-methyl-4-phenyl- 1, 2, 3, 6-tetrahydropyridine) model with the objectives to assess its potential use as an adjuvant therapy for PD. We used a subchronic MPTP model (5-daily MPTP injections), characterized by 50% loss of dopamine neurons over a period of 28 days. Ubisol-Q10 was delivered in drinking water. Prophylactic application of Ubisol-Q10, started 2 weeks before the MPTP exposure, significantly offset the neurotoxicity (approximately 50% neurons died in MPTP group vs. 17% in MPTP+ Ubisol-Q10 group by day 28). Therapeutic application of Ubisol-Q10, given after the last MPTP injection, was equally effective. At the time of intervention on day 5 nearly 25% of dopamine neurons were already lost, but the treatment saved the remaining 25% of cells, which otherwise would have died by day 28. This was confirmed by cell counts, analyses of striatal dopamine levels, and improved animals’ motor skill on a beam walk test. Similar levels of neuroprotection were obtained with 3 different Ubisol-Q10 concentrations tested, that is, 30 mg, 6 mg, or 3 mg CoQ10/kg body weight/day, showing clearly that high doses of CoQ10 were not required to deliver these effects. Furthermore, the Ubisol-Q10 treatments brought about a robust astrocytic activation in the brain parenchyma, indicating that astroglia played an active role in this neuroprotection. Thus, we have shown for the first time that Ubisol-Q10 was capable of halting the neurodegeneration already in progress; however, to maintain it a continuous supplementation of Ubisol-Q10 was required. The pathologic processes initiated by MPTP resumed if supplementation was withdrawn. We suggest that in addition to brain delivery of powerful antioxidants, Ubisol-Q10 might have also supported subcellular oxidoreductase systems allowing them to maintain a favorable cellular redox status, especially in astroglia, facilitating their role in neuroprotection. Based on this data further clinical testing of this formulation in PD patients might be justifiable.
Keywords: Antioxidant, Astroglia activation, Motor function, Neuroprotection, Oxidative stress, Vitamin E
1. Introduction
Multiple lines of evidence point to the role of mitochondrial damage and oxidative stress as the major contributing factors to the pathogenesis of age-related neurodegenerative diseases, such as Parkinson’s disease (PD) (Beal, 2003; Exner et al., 2012; Gille et al., 2004; Hauser and Hastings, 2012; Jenner, 2003; Mattson et al., 1999; Schapira, 2007). For example, a significant decrease in complex I activity of the mitochondrial respiratory chain, accompanied by reduced levels of coenzyme Q10 (CoQ10), is found in the brain and platelets of PD patients (Hargreaves et al., 2008; Shults et al., 1998, 1999). In experimental settings, several chemicals (i.e., MPTP [1- methyl-4-phenyl-1,2,3,6-tetrahydropyridine], paraquat, and rotenone) that inactivate complex I of the oxidative phosphorylation pathway also induce degeneration of dopaminergic neurotransmission in rodents (Cannon et al., 2009; Cicchetti et al., 2005; Jackson-Lewis and Przedborski, 2007; McCarthy et al., 2004). All this leads to the conclusions that the excessive reactive oxygen species (ROS) burden, some of it generated by dysfunctional mitochondria, and the inability of dopamine (DA) neurons to neutralize them are at the center of PD pathophysiology. Accordingly, it is envisioned that compounds capable of reducing the levels of free radicals in the central nervous system (CNS) might be able to interfere with the progression of neurodegeneration and serve as a therapeutic strategy for PD (Koppula et al., 2012a). However, numerous antioxidant compounds, some directly targeting mitochondria, have been investigated, but none of them have been used yet as the effective disease therapy (Hart et al., 2009; Lew, 2011).
One potential candidate for PD therapy is CoQ10 because of its fundamental role in cellular energy production and antioxidant properties (Crane, 2001). CoQ10 (2,3-dimethoxy, 5-methyl, 6- polyisoprene para-benzoquinone) or ubiquinone 50 is a lipophilic, redox active molecule located in all cellular membranes. Its 50- carbon polyisoprene chain ensures the insertion in cellular membranes and the quinone ring, which undergoes reduction and/or oxidation transitions, becomes a carrier of protons and electrons. In the mitochondrial membrane, CoQ10 is an essential component of the mitochondrial respiratory chain where it transfers electrons from complex I and II to complex III and is an inhibitor of the mitochondrial permeability transition pore (Crane, 2001). CoQ10 undergoes oxidation and/or reduction in other cell membranes, such as Golgi vesicles, lysosomes, or plasma membrane, where it modulates vesicles acidification, subcellular redox state and is responsible for the generation of superoxide anion and hydrogen peroxide, which constitute a major regulatory signaling system essential for normal cell function and metabolism (Crane, 2001; Gille and Nohl, 2000). In most membranes CoQ10 exists in the reduced form of quinol and acts as a powerful antioxidant protecting cells from ROS-induced damage either by direct reaction with ROS or by regenerating α-tocopherol and ascorbate (Crane, 2001; Turunen et al., 2004).
Thus far, it has been established that CoQ10 delivers therapeutic benefits in some heart conditions, including heart failure and coronary artery disease (Lee et al., 2012a, 2012b; Littarru and Tiano, 2010; Singh et al., 1998; Smith and Murphy, 2010). A human clinical study investigating the relation between CoQ10 and inflammation in patients with coronary artery disease reports that oral supplementation of CoQ10 at 150 mg/day results in decreased expression of inflammatory markers, that is, C-reactive protein, IL-6, and homocysteine (Lee et al., 2012b). Furthermore, data collected from human and rodent cellular models show that CoQ10 affects gene expression and inhibits the release of IL-6 and TNF-α, consistent with its anti-inflammatory effects (Schmelzer et al., 2007, 2008). Far less convincing benefits are reported for neurodegenerative diseases, such as PD (Shults and Haas, 2005; Shults et al., 2002; Villalba et al., 2010). However, CoQ10 supplementation of pediatric patients with Down syndrome has produced promising results, pointing out to a potential role of CoQ10 in a modulation of DNA repair mechanisms (Miles et al., 2007; Tiano et al., 2012).
Although numerous beneficial outcomes, both experimental and clinical, have been reported in response to CoQ10 supplementation, its full therapeutic potential and clinical use seems to be limited by poor solubility, low absorption in the body, and insufficient brain penetration. This necessitates a development of more efficacious CoQ10 formulations addressing some of these drawbacks. One such formulation has been developed in our laboratory (Borowy-Borowski et al., 2004; Sikorska et al., 2003). The solubilization of CoQ10 is achieved due to the self-emulsifying properties of PEG-derivatized α-tocopherol (PTS), allowing the formation of nanomicelles. The water-dispersible CoQ10/PTS formulation (Ubisol-Q10) is easily delivered and can be tested in any experimental paradigms, both in vitro and in vivo. Accordingly, we show that Ubisol-Q10 stabilizes mitochondrial membranes and protects human NT2 and SH-SY5Y cells against apoptotic cell death triggered by either oxidative stress (McCarthy et al., 2004; Somayajulu et al., 2005) or glutamate excitotoxicity (Sandhu et al., 2003). It prevents the release of pro-apoptotic protein Bax as well as cytochrome c from the mitochondria (Naderi et al., 2006). In cybrid cells harboring mitochondrial DNA mutations, Ubisol-Q10 reduces the generation of ROS and prevents the tertiarybutyl hydroperoxide-induced adenosine triphosphate depletion (Sikorska et al., 2009). In vivo, it protects hippocampal pyramidal neurons from ischemia and/or reperfusion injury when delivered systemically within 30 minutes, post a 10-minute ischemic episode (Sikorska et al., 2003) and in rats protects DA neurons of substantia nigra pars compacta (SNpc) from paraquat toxicity when provided prophylactically in drinking water (Somayajulu-Nitu et al., 2009).
This study was designed to evaluate the effectiveness of Ubisol- Q10 in halting the progression of the ongoing neurodegenerative processes; hence, to assess its potential for a therapeutic use in the management of PD. To date, despite enormous efforts, there is no disease modifying treatment that halt or slow down the progression of PD. The study was performed using mouse subchronic MPTP model (5 daily intraperitoneal injections of MPTP) and Ubisol-Q10 was delivered in drinking water at different time points. The efficacy assessment included stereological DA cell counts in SNpc, striatal dopamine measurement, motor skills evaluation of a beam walk test, as well as brain bioavailability analyses of CoQ10 and vitamin E. We report here that orally administered Ubisol-Q10 into mice, in which MPTP had already initiated the neurodegeneration, blocked the neuronal death pathway allowing the DA neurons to survive as long as the supplementation was continued (for at least 8 weeks post-MPTP treatment). However, when the supplementation was withdrawn the neurodegeneration resumed and neurons begun to die. The neuroprotective Ubisol-Q10 treatment brought about a robust astrocytic response (activation) suggesting that these cells played a significant role in protecting the neurons.
2. Methods
2.1. Animals
Male C57BL/6 mice, 8 to 10-week-old, weighing 22–25 g (Charles River, St. Constant, Quebec, Canada) were on arrival individually housed in a temperature and humidity controlled room under a 12-hour light and/or dark cycle. All animals were provided ad libitum access to food and water and allowed to acclimatize for at least 1 week before the start of the experiments. All animal protocols were approved by the Institutional Animal Care Committee in accordance with the guidelines from the Canadian Council on Animal Care.
2.2. MPTP treatments
Mice received 5 intraperitoneal injections of MPTP-HCl (25 mg/ kg body weight [BW]/injection; Sigma Aldrich), once a day for 5 days. MPTP handling and safety measures were in accordance with the published guidelines (Jackson-Lewis and Przedborski, 2007).
2.3. Ubisol-Q10 supplementation
Stock solution of Ubisol-Q10 in water (200 mg/mL containing 50 mg CoQ10/mL), provided by Zymes LLC (Hasbrouck, NJ, USA) was stored at 4 ºC and a drinking water supplemented with required concentration of Ubisol-Q10 was prepared twice weekly. It was estimated that on average mice were drinking approximately 5 mL of solution per day, therefore the daily intake of Ubisol-Q10 was contained in this volume. For a prophylactic use, supplementation with Ubisol-Q10 begun 2 weeks before the MPTP treatment and was continued throughout the duration of the experiments. For a therapeutic use, supplementations with Ubisol-Q10 begun on day 5 after the last MPTP injection and continued till the conclusion of the experiments.
2.4. Bioavailability of Ubisol-Q10
C57BL/6 male mice were deprived of food for 16 hours before the delivery (by gavage) of a single Ubisol-Q10 dose (6 mg CoQ10/kg BW given in 400 μL). Animals were sacrificed at 1, 3, 6, and 24 hours after the gavage, brain tissue was snap frozen in liquid nitrogen, stored at −80 ºC and, subsequently, CoQ10 and vitamin E levels were analyzed by High-performance liquid chromatography (HPLC) as described in the following section.
2.5. Motor behavior testing
Motor skills were assessed on the beam walk test by measuring the ability of mice to traverse a 5-mm square and 100-cm long beam to a dark goal box (Carter et al., 2001). Before any experimental treatments mice were handled for 3 consecutive days and trained to traverse an elevated 12-mm square beam to reach a goal box. Mice able to traverse the full length of the beam to the “goal box” were considered fully trained. Typically, mice would require 4 trials on the beam to become fully trained and the training took place over 2 consecutive days. Subsequently, the motor skills of experimental mice were tested. The latency to traverse the 5-mm square beam as well as the number of forelimb and hindlimb faults were recorded, with both sides of the animal being videotaped during 2 trials using a Sony Handycam (HDR-XR550V). Videotapes were viewed and scored in slow motion for the number of foot faults made by each mouse by an investigator blind to the experimental groups using the Vegas Movie Studio HD, version 11.0 (Sony Creative Software Inc, 2011). A foot fault was recorded when, during the forward movement, a limb slipped off the horizontal surface of the beam. An individual animal could make a maximum of 4 slips per step.
2.6. Tissue preparation
Mice were anesthetized with isofluorane and perfused transcardially with 10 mL ice-cold 1× phosphate-buffered saline (PBS) followed by 10% neutral buffered formalin (Fischer Scientific). The brains were rapidly removed and dissected into 2 parts to separate the substantia nigra and the striatum. The mesencephalon was postfixed overnight in 10% neutral buffered formalin, washed once with 0.04 M sodium phosphate buffer (pH 7.4), transferred to 30% sucrose for 48 hours, embedded in Optimal Cutting Temperature compound and frozen in liquid nitrogen. Coronal brain sections across the whole SNpc region were cut on a cryotome at 50 μm and stored at −20 ºC in a buffer consisting of 30% glycerol/30% ethylene glycol in 0.04 M sodium phosphate buffer. After all tissue processing, the mean section thickness in the z-axis was 20.3 ± 0.18 μm.
In some experiments, mice were perfused with 1× PBS only, cortex and striatal tissues were dissected, frozen by immersion in liquid nitrogen and stored at −80 ºC for subsequent determinations of dopamine, CoQ10 and vitamin E levels and as well as for Western blotting.
2.7. Tyrosine hydroxylase immunohistochemistry
For stereological analysis of tyrosine hydroxylase (TH)-immunopositive neurons, every second section through the entire substantia nigra was collected (10–12 sections per region per brain). Sections were transferred to a 24-well dish, rinsed twice with Tris-buffered saline (TBS; 50 mM Tris-HCl, 130 mM NaCl, pH 7.6) and incubated in 1% hydrogen peroxide on a shaker for 30 minutes at room temperature to inactivate endogenous peroxidase activity. Following 2× washes with 50 mM TBS, nonspecific immunoglobulins were blocked with 5% normal goat serum containing 0.3% TritonX-100 on a shaker for 60 minutes at room temperature. Sections were then incubated with 1:6000 rabbit anti-tyrosine hydroxylase (Pel-Freeze Biologicals, Rogers, AR, USA) or rabbit anti-glial fibrillary acidic protein (GFAP) (Dako Canada Inc., Mississauga, Ontario, Canada) on a shaker overnight at 4 ºC. Following incubation, sections were washed thrice with 50 mM TBS and incubated with biotinylated anti-rabbit IgG (1:300; Vector Laboratories, Burlingame, CA, USA) on a shaker for 90 minutes at room temperature. Sections were washed thrice with 50 mM TBS and the biotinylated conjugate was detected by incubating sections with avidin-peroxidase (Vectastain Elite ABC kit; Vector Laboratories) on a shaker for 90 minutes at room temperature. Following 3× washes with 50 mM TBS, immunolabeling was detected using freshly prepared 3-diaminobenzidine (DAB, 0.025%) and hydrogen peroxide (0.006%) on a shaker for 10 minutes at room temperature. Negative controls included the omission of the primary antibody. Finally, sections were mounted on superfrost Plus slides (Fischer Scientific, Ottawa, Ontario, Canada), counterstained with 0.1% cresyl violet, and coverslipped with permount.
2.8. Stereological cell counts
The total number of TH-immunopositive neurons in the SNpc were quantified using the computerized stereologer system (Stereology Resource Center, Chester, MD, USA) as described previously (Maswood et al., 2004; Mouton et al., 2012). The identification of the SNpc region to be counted on each section was outlined at 2.5× magnification and TH-immunopositive neurons were estimated using the dissector probe at 63× oil immersion with a guard volume of 2 μm to avoid sectioning artifacts. Cells exhibiting neuronal phenotype, that is, a clear nuclear membrane, distinct nucleolus, and TH-immunoreactivity in the cytoplasm, were counted. The coefficient of error for all samples was ≤0.10.
2.9. Western blot assay
Brains were homogenized in a buffer containing 50 mM Tris pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.1% SDS, 1% deoxycholate, 1% TritonX-100 (RIPA buffer), and proteins separated by 10% SDS-PAGE. Samples were transferred to nitrocellulose and probed with 1:1000 rabbit polyclonal anti-tyrosine hydroxylase (Pel-Freeze Biologicals) or 1:5000 mouse monoclonal anti-β actin (Sigma-Aldrich Canada Co. Oakville, Ontario, Canada), respectively followed by HRP-conjugated anti-rabbit or anti-mouse secondary antibody. ECL plus (Amersham Piscataway, NJ, USA) was used for detection.
2.10. Measurement of CoQ10 levels
CoQ10 was measured as previously described (Graves et al., 1998; Souchet and Laplante, 2007; Tang et al., 2004). Briefly, samples (100 mg of solid tissue or 100 μL of plasma) were homogenized in 1 mL cold PBS and subjected to repeated freezing and/or thawing steps to disrupt protein and/or lipid complexes. CoQ10 was extracted with 2mL anhydrous ethanol and 5 mL n-hexane solvents. Some samples were spiked with a known amount of CoQ10 to establish efficiency of the extraction procedure, which was found to be >95%. The n-hexane phase was collected and dried under a gentle stream of nitrogen. Dry residue was dissolved in isopropanol, treated with hydrogen peroxide to oxidize ubiquinol and analyzed by HPLC following separation on a TSK-GEL ODS-100S column (4.6 mm × 150 mm, 7 μ particle size, TOSOH Biosep LLC, Montgomeryville), equipped with a 1 mm C18 guard column (Optimize Technologies Inc, Oregon City, OR, USA) and isocratic elution with isopropanol, ethanol and methanol (35:24:41) at room temperature at a flow rate of 0.7mL/min. Absorbance at 275 nm was monitored and recorded using Beckman-Coulter’s System Gold 32 Karat software version 7.0 (Beckman-Coulter Canada Inc.)
2.11. Measurement of vitamin E levels
Tissue contents of α-tocopherol (vitamin E) were determined by the methods previously described (Itoh et al., 2006; Ren et al., 2006). Briefly, samples (100 mg of solid tissue or 100 μL of plasma) were homogenized in cold PBS and vitamin E was extracted with chloroform/methanol (2:1 vol/vol). Some samples were spiked with a known amount of α-tocopherol to establish efficiency of the extraction procedure, which was found to be >95%. The chloroform phase was dried under a gentle stream of nitrogen. Dry residue was then dissolved in methanol and analyzed by HPLC with ultraviolet (UV) detection at 293 nm (System Gold, Beckman- Coulter) with a TSK-GEL ODS-100S column and eluted with methanol at a flow rate of 1 mL/min at room temperature.
2.12. Measurement of MPP+ levels
Striatal and liver MPP+ levels were determined by HPLC as previously described (Ren et al., 2006) with few modifications. Briefly, dissected tissues were immediately frozen in liquid nitrogen and stored at −80 ºC. Tissue samples were weighed and homogenized in 10 volumes of ice-cold 0.1 M perchloric acid and 0.1 mM EDTA containing 10 μM 4-phenylpiridine (Sigma-Aldrich, Oakville, Ontario, Canada) as an internal standard. The homogenized samples were centrifuged at 20,000× g for 10 minutes at 4 ºC and the supernatants were filtered and injected onto a reverse-phase C18 HPLC column (4.6 × 150 mm; TSK-GEL ODS-100S, 7 μ particle size; Tosoh Biosep LLC, Montgomereyville) equipped with a 1 mm C18 OPTI-GUARD column (Optimize Technologies). The mobile phase was delivered at a flow rate of 1.0 mL/min at ambient temperature and consisted of 0.02 M NaH2PO4, 3 mM tetrabutylammonium bisulfate (Sigma-Aldrich), 0.5 mM 1-heptanesulfonic acid sodium salt (Sigma-Aldrich) and 10% isopropanol adjusted to pH 2.5 with orthophosphoric acid. Eluting peaks were detected by UV at 293 nm (System Gold HPLC, model 166 Programmable UV Detector Module; Beckman-Coulter Canada Inc, Mississauga, Ontario, Canada). Detector response was assessed daily by injecting known amounts of freshly prepared MPP+ standards (Sigma-Aldrich) in the previously mentioned homogenization solution. The chromatographic data was processed using Beckman-Coulter’s System Gold 32 Karat software and was reported as pmoles of MPP+ per mg of tissue.
2.13. Measurement of dopamine levels
Striatal tissue samples were weighed and homogenized in 10 volumes of ice-cold 0.1 M perchloric acid containing 0.2 μg/mL 3,4- dihydroxybenzylamine (DHBA, Sigma-Aldrich) as an internal standard. The homogenized samples were centrifuged at 20,000× g for 10 minutes at 4 ºC and the supernatants were analyzed using reverse-phase C18 HPLC column (4.6 × 150 mm; Synergi 4 μ particle size Hydro-RP 80Å; Phenomenex, Torrance, CA, USA) equipped with a 3 mm C18 Aqua guard cartridge (Phenomenex) as described previously (Tremblay et al., 2010).
2.14. Statistical analysis
Data is shown as mean ±standard error of the mean. Differences among means were analyzed using 1-way analysis of variance (ANOVA). When ANOVA showed significant differences, pairwise comparisons between means were analyzed by post hoc Bonferroni multiple comparison test. Significance is shown as *or # p < 0.05, **or ## p < 0.01 and *** p < 0.001.
3. Results
3.1. Properties and bioavailability of Ubisol-Q10
Ubisol-Q10, or water-dispersible formulation of CoQ10, actually consists of 2 components CoQ10 and polyoxyethanyl-α-tocopheryl sebacate (PTS), combined at a ratio 1:2 mol/mol. The PTS molecule is an amphiphile, possessing both hydrophilic (PEG-600) and lipophilic (alpha-tocopherol) properties, separated by an aliphatic spacer sebacic acid, and has self-emulsifying properties (Fig. 1A). Based on the chemical structure, α-tocopherol constitutes 35.6% or one-third of the PTS molecule (Borowy-Borowski et al., 2004; Sikorska et al., 2003). When combined with CoQ10 and water PTS facilitates the formation of nanomicelles (Fig. 1C). It is reported (www.Zymesllc.com), based on transmission electron microscopy analyses that a single PTS-CoQ10 micelle measures 22 ± 7 nm in diameter. Suspensions of CoQ10 in water and CoQ10/PTS complex (Ubisol-Q10) in water are shown in Fig. 1B and C. Thus, CoQ10 added directly to water floated on the surface as insoluble material (Fig. 1B); whereas Ubisol-Q10 was fully dispersed in water and remained as a stable clear solution for ≥2 years even at room temperature (Fig. 1C).
Fig. 1.
Brain bioavailability of Ubisol-Q10 components. (A) Structure of polyoxyethanyl α-tocopheryl sebacate (PTS). The tripartite PTS molecule is amphiphilic with hydrophobic and hydrophilic parts of α-tocopherol and PEG 600 derivatives, respectively, separated by 8 carbon aliphatic spacer of sebacate. The chemical formula of PTS is C65H118O18 when n = 12 (in PEG) and the estimated molecular mass is 1210. Arrows indicate potential cleavage sites for nonspecific esterases. (B) Suspension of CoQ10 in water. CoQ10 is insoluble in water and floats on the surface. (C) Ubisol-Q10 formulation in water. CoQ10 was co-melted with PTS at a ratio 1:3 wt/wt (or 1:2 mol/mol) and subsequently diluted with water to 50 mg CoQ10/mL, as previously described (Borowy-Borowski et al., 2004). The clear yellow solution of Ubisol-Q10 is stable at room temperature for ≥2 years and could be diluted further to any required concentration. (D–G) Mice were given Ubisol-Q10 (6mg CoQ10/kg BW)by gavage and were sacrificed 1, 3, 6 and 24 hours later. CoQ10 (E) and vitamin E (G) were extracted from the brains and analyzed by HPLC (D and F, respectively) as described in Section 2. (D) Representative chromatogram of CoQ10 separation by HPLC. Black arrow shows CoQ10 peak and gray arrow shows CoQ9 peak with appropriate retention times written above the arrows. (E) Brain contents of CoQ10 following Ubisol-Q10 ingestion. Results are shown as mean ± SEM (n=5–10 per group, from 2 separate experiments). Statistically significant differences between control versus 1, 3, 6, and 24 hours groups are indicated as *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05. (F) Representative chromatogram of vitamin E (VE) separation by HPLC. Black arrow indicates peak of α-tocopherol with retention time written above the arrow. (G) Brain contents of vitamin E following Ubisol-Q10 ingestion. Results are shown as mean ± SEM(n=6–8 per group, from 2 separate experiments). Statistically significant differences between control versus 1, 3, and 6 hours groups are indicated as *** p ≤ 0.001 and * p ≤ 0.05. Abbreviations: CoQ10, coenzyme Q10; SEM, standard error of the mean.
Brain bioavailability of the Ubisol-Q10 components was tested in intact mice following oral intake (by gavage) of the formulation. Brain tissue was collected at different time points; both CoQ10 and α-tocopherol were extracted and analyzed by HPLC (Fig. 1D–G). Because rodents have a high content of CoQ9, we applied chromatographic conditions allowing its separation from CoQ10. As shown in Fig. 1D, CoQ9 was eluted from the column ahead of CoQ10 with elution time of 7.217 minutes versus 8.850 minutes for CoQ10, making the quantitation of CoQ10 possible. The data showed transiently elevated brain contents of CoQ10 following a single Ubisol- Q10 dose of 6 mg CoQ10/kg BW. As shown in Fig. 1E, an increased content of CoQ10 was evident within 1 hour post-gavage (3-fold over the basal level); it peaked at 3 hours (5-fold over the basal level), and remained elevated for up to 24 hours. We have also established that the other component of Ubisol-Q10 formulation, α-tocopherol (vitamin E) was systemically released as revealed by the HPLC analyses of its content (Fig. 1F). To solubilize 6 mg of CoQ10 3 times more PTS was required, that is, 18 mg, and at this dose the highest brain content of vitamin E, approximately 4-fold increase over the control value, was measured 1 hour post-delivery (p < 0.01, Fig. 1G). Thus, pharmacokinetic distribution of the 2 Ubisol- Q10 components followed separate paths, especially because the release of α-tocopherol required the hydrolysis of PTS to its primary components (Fig. 1A). The measured molar concentration of CoQ10 in control brains was 4-fold higher than vitamin E (approximately 154 pmol/mg protein vs. 43 pmol/mg protein) and a similar ratio of the 2 antioxidants seemed to be maintained following Ubisol-Q10 intake as the maximal tissue increases for both compounds ranged between 4- and 5-fold. Similar to the brain levels, plasma levels of both CoQ10 and vitamin E were also elevated within 1 hour (data not shown).
3.2. Kinetics of nigrostriatal degeneration in mouse subchronic MPTP model
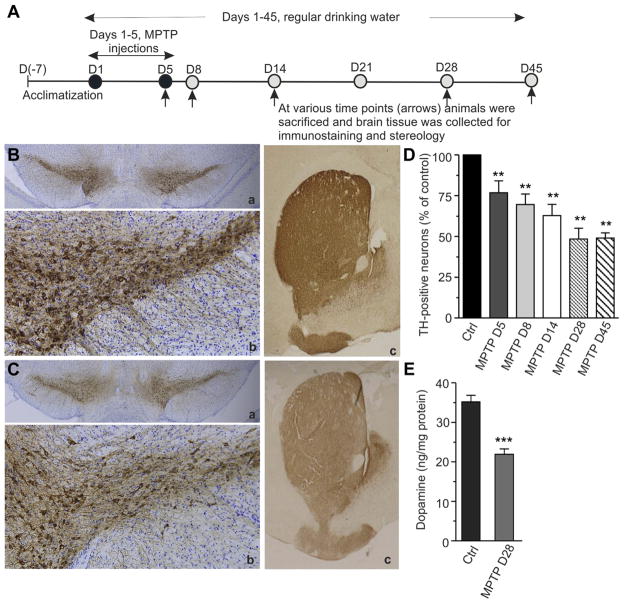
To induce the dopaminergic neurodegeneration, male C57BL/6 mice were subjected to 5 daily intraperitoneal injections of MPTP (25 mg/kg BW/injection). Although this subchronic MPTP model has been previously described (Jackson-Lewis and Przedborski, 2007; Schober, 2004), we standardized it here to create a picture of progressive neurodegeneration, which we would subsequently attempt to inhibit. The experimental outlines are presented in Fig. 2A. The brains were sampled every week over a period of 45 days (i.e., the first MPTP injection was counted as day 1). Brains were sectioned, stained with anti-tyrosine hydroxylase antibody, and the TH-positive cells were counted using a computerized stereologer system. The images of immunostained midbrain sections examined at day 28 (MPTP-D28) of the experiment revealed a significantly reduced TH-immunostaining and decreased number of SNpc neurons in the MPTP-injected mice (Fig. 2C a and b) in comparison to control animals (Fig. 2B a and b). Similarly, the density of TH-positive fibers in striatum at the same time point (MPTP-D28) was much lower in the MPTP-treated group (Fig. 2C c) than in the controls (Fig. 2B c). Accordingly, the TH-positive cell counts revealed that the neurons were progressively dying over a period of the first 28 days of the experiment, with overall cell loss of 51.6% at day 28 (Fig. 2D). No further cell loss was observed during the subsequent days of the experiment as the same neuronal counts (i.e., 50% survival) were found on day 45 (MPTP-D45). The striatal dopamine content also decreased by close to 50% in MPTP-treated mice during that time period (Fig. 2E, MPTP-D28), indicative of extensive degeneration of the nigrostriatal pathway.
Fig. 2.
Progressive loss of TH-positive neurons in subchronic MPTP model. (A) Schematic outline of the experimental design. Mice were acclimatized to the new environment for 7 days before the start of the experiment. Animals were randomly divided into experimental groups and given 5 daily injections of MPTP (25 mg/kg/injection). Control mice were injected with saline. On days 5, 8, 14, 28, and 45 after the MPTP injections (arrows), mice were sacrificed and brain tissue was collected for immunohistochemistry (B and C), stereology (D), and biochemical (E) analyses. Other details as described in Section 2. (B and C) Brains were fixed and immunostained with rabbit polyclonal anti-tyrosine hydroxylase antibody (brown) and counterstained with cresyl violet (blue) for anatomic reference. Images were captured on an Olympus microscope equipped with Microcast 3CCD 1080p HD color camera. (B) Representative photomicrographs of midbrain sections from control mouse showing TH-immunoreactivity. In B (a) coronal section of mouse midbrain across both hemispheres showing intense brown staining of SNpc region; B (b) shows dense network of TH-immunopositive cell bodies and fibers in the SNpc; B (c) shows strong TH-immunoreactive dopamine terminals in the striatum. (C) Representative photomicrographs of midbrain sections from MPTP-injected mouse showing reduced TH-immunoreactivity (pale brown) on D28. In C (a) reduced TH staining of SNpc (pale brown); C (b) decreased density of TH-immunopositive cell bodies and fibers in SNpc; C (c) reduction in TH-immunoreactive dopamine terminals in the striatum. Magnifications: B (a), B (c), C (a), and C (c) = 2.5×; B (b) and C (b) = 10×. (D) Progression of dopaminergic neurodegeneration over a period of 45 days. TH-positive cells were counted using an unbiased stereology method and cell survival was plotted as percentage of control. Results are shown as mean ± SEM (n = 5–6 per group from a single experiment). Statistically significant differences between control and MPTP-injected groups are shown as ** p ≤ 0.01, for all groups. (E) Reduction in the striatal dopamine level during the course of neurodegeneration. Brain tissue was snap-frozen in liquid nitrogen and dopamine levels were analyzed by HPLC. Results are shown as mean ± SEM (n = 8–10 per group from 5 separate experiments). Statistically significant difference between control and MPTP-injected group is indicated as *** p ≤ 0.001. Abbreviations: MPTP, 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; SNpc, substantia nigra pars compacta; TH, tyrosine hydroxylase.
Thus, in the experimental paradigm used here, that is, 5- intraperitoneal injections of MPTP (Fig. 2A), the dopaminergic degeneration occurred over a period of 28 days, with approximately 25% of TH-positive neurons being killed after 5 days of MPTP injections (MPTP-D5), and further 25% between the days 5–28 of the experiment, resulting also in the reduction of striatal dopamine by 50% (MPTP-D28). These markers of neurodegeneration plateaued at day 28 as neither the number of TH-positive neurons nor dopamine level declined further at day 45-time point. Therefore, day 28 was chosen as the end point in the subsequent experiments (Figs. 3,5, and 6).
Fig. 3.
Effects of prophylactic supplementation of Ubisol-Q10 at 30 mg CoQ10/kg/day. (A) Schematic outline of the experimental design. Mice were acclimatized for 7 days before the start of the experiment and were randomly divided into 3 experimental groups (I–III). Groups I and II were drinking regular water throughout the duration of the experiment. Ubisol-Q10 supplementation of drinking water (30 mg CoQ10/kg/day) in group III begun 2 weeks before the MPTP injections (D[−14]) and was maintained till the termination of experiment on day 28 (D28). Groups II and III received 5 daily intraperitoneal injections of MPTP (25 mg/kg/injection); whereas, group I was injected with saline (dark circles, D1–D5). At the conclusion of the experiment on D28 all animals were sacrificed and brain tissue was collected for immunohistochemistry (B–D), sterology (E), and Western blot (F) analyses as described in Section 2. (B–D) Representative photomicrographs of mouse midbrain sections showing TH-immunopositive neurons in group I (B; saline-injected controls), group II (C; MPTP-injected receiving regular drinking water), and group III (D; MPTP-injected receiving Ubisol-Q10 supplemented water). Images were captured on an Olympus microscope equipped with Microcast 3CCD 1080p HD color camera system. Cresyl violet (blue) counterstain was used for anatomic reference. Magnification = 10×. (E) Survival of TH-positive neurons in the SNpc. The TH-positive cells were counted using an unbiased stereology method as described in Section 2. Results are shown as mean ± SEM (n = 10–12 per group, from 2 separate experiments). Statistically significant differences between group I versus II (control vs. MPTP, *** p ≤ 0.01) and group II versus III (MPTP vs. MPTP + Ubisol-Q10; ## p ≤ 0.01) are shown. (F) Western blot analysis of TH protein in mouse midbrain tissue. Brains were snap-frozen in liquid nitrogen, extracted proteins were separated on 10% SDS-PAGE and blotted onto nitrocellulose membranes. Membranes were probed with anti-tyrosine hydroxylase antibody and re-probed with anti-β-actin. Marked are: 62-kDa band of tyrosine hydroxylase and 38-kDa band of β-actin. Shown is a representative blot of 2 independent experiments. Abbreviations: MPTP, 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; SNpc, substantia nigra pars compacta; TH, tyrosine hydroxylase.
Fig. 5.
Effects of therapeutic Ubisol-Q10 supplementation at high dose (30 mg CoQ10/kg/day). (A) Schematic outline of the experimental design. Mice were acclimatized to the new environment for 7 days before the start of the experiment and were randomly divided into 3 experimental groups (I–III). Control group I was injected with saline and groups II and III received 5 daily MPTP injections (25 mg/kg/injection; dark circles, D1–D5). Mice in groups I and II were given regular drinking water throughout the duration of the experiment; whereas, mice in group III were placed on Ubisol-Q10 supplemented water starting on day 5 (D5), immediately after the last MPTP injection. At the conclusion of the experiment on day 28 (D28), all mice were sacrificed and brains were collected for immunohistochemistry (B–D) and stereology (E). (B–D) Brains were fixed and immunostained with rabbit polyclonal anti-tyrosine hydroxylase antibody (brown) and counterstained with cresyl violet (blue) for anatomic reference. Images were captured on an Olympus microscope equipped with Microcast 3CCD 1080p HD color camera system. Representative photomicrographs of mouse midbrain sections shown in: (B) strong TH-immunostaining of neurons and fibers in SNpc of control mice (group I); (C) reduced cell number in SNpc of MPTP-injected mice drinking regular water (group II), and (D) preservation of both TH-positive neurons and fibers in MPTP-injected mice receiving Ubisol-Q10 supplemented water (group III). Magnification = 10×. (E) Quantitative analysis of cell survival in SNpc in response to Ubisol-Q10 supplementation. Results are expressed as percentage of control and are shown as mean ± SEM (n = 7–8 per group, from 2 separate experiments). For comparison, a bar indicating cell viability on the day when the Ubisol-Q10 supplementation begun, that is, the last day of MPTP injection (MPTP D5), is shown. Statistically significant differences between groups I versus II (*** p ≤ 0.001; control vs. MPTP) and groups II versus III (# p ≤ 0.05, MPTP alone vs. MPTP + Ubisol-Q10) are indicated. Abbreviations: MPTP, 1- methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; SEM, standard error of the mean; SNpc, substantia nigra pars compacta; TH, tyrosine hydroxylase.
Fig. 6.
Effects of therapeutic Ubisol-Q10 supplementation at 5× and 10× reduced dosages. (A) Schematic outline of the experiment. Mice were acclimatized for 7 days before the start of the experiment and were randomly divided into 4 experimental groups (I–IV). Control group I was injected with saline and groups II–IV with MPTP (5 × 25 mg/kg; dark circles, D1–D5). Mice in groups I and II were drinking regular water throughout the duration of the experiment. Mice in groups III and IV received Ubisol-Q10 supplemented water at 3 mg and 6 mg CoQ10/kg/day, respectively, starting immediately after the last injection (D5). At the conclusion of the experiment (D28), all mice were sacrificed and brains were collected for immunohistochemistry (B–D), stereology (E), and biochemistry (F and G). (B–D) Brains were fixed and immunostained with rabbit polyclonal anti-tyrosine hydroxylase antibody and counterstained with cresyl violet. Images were captured on an Olympus microscope equipped with Microcast 3CCD 1080p HD color camera. In B and B′-representative photomicrographs of mouse midbrain sections showing normal distribution of TH-immunopositive neurons in control mice of group I; C and C′-MPTP-injected mice receiving regular water of group II, showing significantly reduced TH-positive cell number and density of the fibers and, D and D′-MPTP-injected mice receiving 6 mg/kg/day Ubisol-Q10 water starting immediately after the last injection on D5 showing nearly complete neuroprotection. Magnification in B, C, D; 10×, and in B′, C′, D′; 40× E). Survival of TH-positive neurons in the SNpc. TH-positive cells were counted and analyzed using an unbiased stereology method. Results are presented as an average cell number per SNpc region and plotted as mean ±SEM (n = 8–10 per group, from a single experiment). For comparison, a bar indicating cell viability on the day when the Ubisol-Q10 supplementation begun, that is, the last day of MPTP injection (MPTP D5), is shown. Statistically significant differences between group I versus II (*** p < 0.001); group II versus III (# p < 0.05); and group II versus IV (* p < 0.05) are indicated. (F) Striatal dopamine levels at the conclusion of the experiment on D28. Brain samples from mice of groups I–III were snap-frozen in liquid nitrogen, stored at −80 ºC and subsequently analyzed by HPLC for the dopamine contents, as described in Section 2. Results are shown as mean ±SEM (n = 6–9 per group, from 2 independent experiments). Statistically significant differences between control group I versus group II (*** p < 0.001) and group II versus group III (** p < 0.01) are shown. (G) Brain CoQ10 contents at the conclusion of the experiment on D28. Brain samples from mice of groups I–III were snap-frozen in liquid nitrogen, stored at −80 ºC, CoQ10 was extracted and analyzed by HPLC as described in the Section 2. Results are shown as mean ± SEM (n = 8 per group, from 2 independent experiments). No statistically significant differences were found. Abbreviations: CoQ10, coenzyme Q10; MPTP, 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; SEM, standard error of the mean; SNpc, substantia nigra pars compacta; TH, tyrosine hydroxylase.
3.3. Prophylactic application of Ubisol-Q10 prevented the MPTP damage to nigrostriatal pathway
As outlined in Fig. 3A, Ubisol-Q10 supplementation begun 14 days [D(−14)] before the initiation of the MPTP injections on day 1 (D1) and was maintained until the termination of the experiment. Evaluation of the outcomes was performed on day 28 (D28) when the neurodegenerative processes in the brain already plateaued (Fig. 2D). Indeed, the microscopic examination of midbrain sections revealed a clear reduction in the number of TH stained DA neurons in the MPTP-injected mice (group II, Fig. 3C) in comparison to saline-injected controls (group I, Fig. 3B). This observation was confirmed by cell counts, consistently showing 50% loss of TH neurons in SNpc at day 28-time point (Fig. 3E, compare groups I and II, p < 0.001). By contrast, the TH-stained midbrain sections of MPTP-treated mice receiving the prophylactic supplementation of Ubisol-Q10 (group III) were significantly different from those without the supplementation of group II (compare, Fig. 3C and D). The density of TH-positive neurons in the SNpc and DA fibers in striatum were nearly indistinguishable from those seen in the saline-injected control mice of group I (compare Fig. 3B and D). The cell counting established a greater than 80% survival of TH-positive neurons at D28 in this group of mice (Fig. 3E, p < 0.01). Western blot analysis further confirmed the immunostaining and counting data, showing much stronger TH-immunoreactive band in the brain of MPTP mice receiving prophylactically Ubisol-Q10 rather than regular water (Fig. 3F). Therefore, the prophylactic application of Ubisol-Q10 at a dose of 30 mg CoQ10/kg BW effectively protected mouse dopaminergic pathway against MPTP-induced neurodegeneration. This was consistent with previous reports (Beal et al., 1998; Ferrante et al., 2002; Matthews et al., 1998).
3.4. Ubisol-Q10 did not interfere with MPTP to MPP+ conversion
The results of the previously mentioned study posed a question whether the bioactive components of Ubisol-Q10,CoQ10, and vitamin E, neutralized MPP+ and prevented it from penetrating the brain? To answer this question, the MPP+ levels in the brain and liver samples were compared between MPTP-treated mice drinking regular water and water supplemented Ubisol-Q10 (Fig. 4). The Ubisol-Q10 supplementation at 30 mg CoQ10/kg BW started 2 weeks before the MPTP injection (the same as in Fig. 3A), but the mice received only a single intraperitoneal MPTP injection (25 mg/kg BW) and the samples were collected 90 minutes and 4 hours after the injection. Using the HPLC based analysis of MPP+ (Fig. 4A), the results revealed the presence of the neurotoxin in both liver and brain samples at the analyzed time points (Fig. 4B). For both tissues the higher content of MPP+ was detected at 90 minutes than at 4 hours post-MPTP injection. Importantly, no statistically significant differences in the MPP+ levels between groups drinking regular water and Ubisol-Q10 supplemented water were identified (Fig. 4B) showing, clearly that Ubisol-Q10 supplementation did not interfere with the MPTP metabolism and the generation of the neurotoxic MPP+.
Fig. 4.

MPP+ levels in brain and liver of mice receiving Ubisol-Q10 supplementation. Mice were given Ubisol-Q10 (30 mg CoQ10/kg/day) supplemented water for 2 weeks and then received a single intraperitoneal injection of MPTP (25 mg/kg). Brain (striatum–ST) and liver samples were collected 90 minutes and 4 hours after the MPTP injection. MPP+ levels were measured by HPLC as described in Section 2. (A) Representative HPLC chromatogram of MPP+ obtained from mouse striatum. Black arrow indicates peak of MPP+ with retention time written above the arrow. (B) MPP+ levels in striatum and liver. Results are shown as mean ± SD (n = 5–6 per group, from a single experiment). No statistically significant differences were detected between the groups (p ≥ 0.05, MPTP ST vs. MPTP + Ubisol-Q10 ST at 90 minutes and 4 hours; p ≥ 0.05, MPTP liver vs. MPTP + Ubisol-Q10 liver at 90 minutes and 4 hours). Abbreviations: MPTP, 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; MPP+, 1-methyl-4- phenylpyridinium; SD, standard deviation.
3.5. Ubisol-Q10 blocked the ongoing neurodegeneration in MPTP-treated mice
Encouraged by the generated thus far positive results, a question now was whether any neuroprotection could be achieved by delaying the Ubisol-Q10 supplementation past the MPTP injections? The experimental paradigm addressing this question is depicted in Fig. 5A. The mice were challenged with 5-daily MPTP injections (days 1–5) and on day 5 and after the last MPTP injection they were given the Ubisol-Q10 supplemented water containing 30 mg CoQ10/ kg BW/day. The supplementation continued until the conclusion of the experiment on D28. Thus, the Ubisol-Q10 intervention begun when the neurodegeneration was already well under way as the trigger of neuronal killing, MPP+, was reaching the brain within 90 minutes of the MPTP injection (Fig. 4B) and by D5 of the experiment, over 25% of neurons were already lost (Fig. 2D). Therefore, the question was whether the Ubisol-Q10 treatment could save the remaining 25%, which, without any intervention, would be lost by D28 of the experiment. Thus, at the conclusion of the experiment on D28, brains were collected, processed and immunostained, and TH-positive DA neurons were sterologically counted (Fig. 5B–D). Consistent with the earlier experiments (Figs. 2 and 3), microscopic examination of midbrain sections showed a similar reduction in TH immunostaining of both the cell bodies and the fibers in the MPTP-injected animals of group II (Fig. 5C) in comparison to saline-injected control animals of group I (Fig. 5B). In striking contrast, the midbrain sections from MPTP-treated mice receiving Ubisol-Q10 from D5 onward (group III), showed very well preserved TH-immunostained cell bodies and as well as fibers and could hardly be distinguished from the control group I (Fig. 5B and D). These observations were also corroborated by the counts of surviving neurons (Fig. 5E). Clearly, the number of TH neurons dropped by nearly 50% in the MPTP-treated animals of group II; however, much higher number of surviving TH neurons (approximately 70%) were found in mice of group III receiving Ubisol-Q10. The Ubisol-Q10 treatment was initiated on D5 with approximately 75% of alive neurons and it culminated at D28 with nearly the same percentage of viable cells, that is, 70% ± 6.4%, indicating that once the Ubisol-Q10 intervention begun the neuronal death pathway was blocked.
3.6. Therapeutic neuroprotection could be achieved at much lower dose of Ubisol-Q10
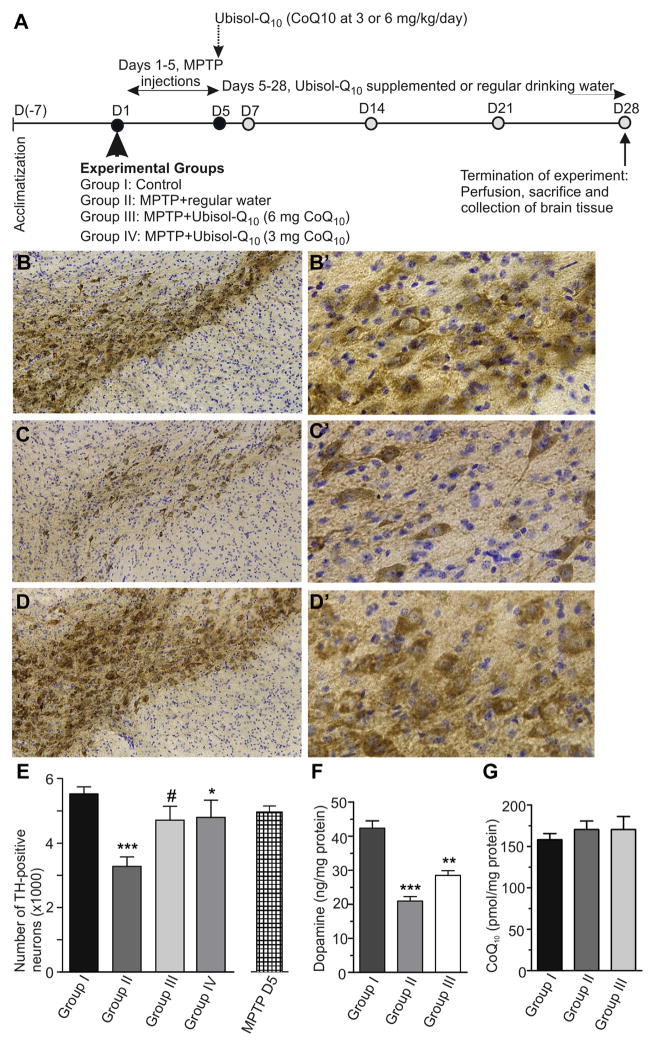
In all the experiments described previously, Ubisol-Q10 was used at a dose equivalent to 30 mg CoQ10/kg BW/day. This concentration was consistent with previous report on in vivo CoQ10 testing (Beal et al., 1998; Cleren et al., 2008) as well as with CoQ10 dosing of PD patients in clinical trial (Shults et al., 1998, 2002). However, although at this concentration Ubisol-Q10 was well tolerated, the delivered dose of CoQ10 was well above the acceptable daily intake (ADI) of 12 mg/kg/day (Hidaka et al., 2008). Therefore, in the next set of experiments, we proceeded to test Ubisol-Q10 at 5× and 10× lower doses, that is, 6 mg/kg/day and 3 mg/kg/day, well within the ADI value. The experimental design is outlined in Fig. 6A. Mice were challenged with MPTP and then were given Ubisol-Q10 supplementation from D5 until D28. Here again, visual examination of midbrain sections showed a dramatic reduction of neurons in the SNpc of the MPTP-treated animals without supplementation (group II, Fig. 6C) and saving of the neurons in the MPTP-treated mice on the Ubisol-Q10 supplementation (group III, Fig. 6D). Higher magnification photomicrographs of SNpc from MPTP-treated animals (group II, Fig. 6C′) revealed reduced TH-staining and pyknotic cells with shortened neuronal processes, displaying distinct neuritic beading as compared with the control group I (Fig. 6B′). Significantly, such pathology was not evident in the Ubisol-Q10 treated mice of groups III and IV. Instead, a greater number of TH-stained cells with round soma and well-preserved neuronal processes were seen (Fig. 6D′). This was true for both Ubisol-Q10 concentrations tested, that is, 6 mg CoQ10/kg/day (Fig. 6D′) and 3 mg CoQ10/kg/day (data not shown). Consistent with these observations, a quantification of TH-positive neurons, using a computerized stereologer system, showed much higher numbers of surviving dopamine neurons in the MPTP-Ubisol-Q10 treated groups III and IV than in MPTP alone of group II (Fig. 6E). Approximately 20% more TH-positive neurons in SNpc were found in groups III and IV compared with group II (p < 0.05). As shown previously (Fig. 2E), the MPTP treatment (group II) reduced striatal dopamine level to 50% by D28 (Fig. 6F). The drop in dopamine level was less severe in mice given the Ubisol supplementation (group III), consistent with higher number of surviving TH-positive neurons in these animals (Fig. 6E). Taking into consideration the fact that the Ubisol-Q10 treatment begun in the midst of ongoing neurodegeneration and after 20%–25% of neurons were already gone, the therapeutic supplementation of Ubisol-Q10 offered an unprecedented neuroprotection against MPTP-induced neuronal killing, it stopped completely its further progression. The formulation was equally effective at CoQ10 dose of 3 mg/kg/day as it was at a dose 10 times higher of 30 mg/kg/day, meaning that a 70 kg patient would require only 210 mg/day, well within the accepted ADI value. Similar data has been obtained in the rat paraquat model of neurodegeneration (Muthukumaran et al., (2014), in press).
At the conclusion of the experiment on D28 we also measured brain (cortex) CoQ10 content to establish whether the 3 weeks Ubisol-Q10 supplementation altered its brain levels. As shown in Fig. 6G, there was no statistically significant difference in CoQ10 content between mice receiving Ubisol-Q10 supplementation (group III) and mice drinking regular water (groups I and II), indicating clearly that the delivered CoQ10 must have been used in processes supporting the neuroprotection and did not accumulate in the brain. This was in contrast to transient elevation of brain CoQ10 in intact mice following a single bolus dose of Ubisol-Q10 (Fig. 1E). No change in the content of alpha-tocopherol was seen at day 28.
3.7. Ongoing Ubisol-Q10 supplementation was required to maintain the neuroprotection
According to the data presented in Fig. 2D, the neurodegenerative processes initiated by MPTP and responsible for 50% loss of SNpc neurons, were completed by day 28 of the experiment and no further cell loss occurred after this time point. Therefore, we asked a question whether Ubisol-Q10 supplementation was still required past that time point. This was addressed in the experiment outlined in Fig. 7A. Two groups of MPTP-injected mice, group V and VI, were placed on the therapeutic Ubisol-Q10 supplementation (6 mg CoQ10/kg BW/day) on day 5 (D5). In group V, the supplementation was withdrawn after 3 weeks (on D28) and the mice were given regular drinking water for another 4 weeks (until D56). The animals in group VI were kept on the supplement for the full 7 weeks (from D5 till D56). The outcomes were assessed based on TH-immunochemistry (Fig. 7B–E) and stereological counting of TH-positive neurons in the SNpc region (Fig. 7F). As shown in Fig. 7B–E, and consistent with the data from previous experiments (Fig. 6), the neuroprotection delivered by the 7 weeks Ubisol-Q10 supplementation (group VI, Fig. 7E) was the same as after an uninterrupted 3 weeks supplementation in group III in the experiment depicted in Fig. 6D and D′. Furthermore, the number of surviving neurons after 7 weeks of treatment (group VI) was nearly the same as it was at the start of the treatment on D5 (Fig. 7F), suggesting, clearly, that this treatment continued to block the progression of neurodegeneration. However, the data also revealed that if the supplementation was withdrawn after 3 weeks and the animals were given regular drinking water for the subsequent 4 weeks period (group V), the neurodegeneration resumed (Fig. 7D). This resulted in fewer viable neurons being accounted for in group V at the termination of the experiment (white bar, Fig. 7F). Taken together, these results showed that the bioactive components of Ubisol-Q10 were capable of penetrating and blocking the molecular pathway(s) activated by MPTP and responsible for the death of DA neurons, but their ongoing supply was needed to maintain that block.
Fig. 7.
Effects of Ubisol-Q10 supplementation withdrawal. (A) Schematic outline of the experiment. Mice were acclimatized to the new environment and were randomly divided into 4 experimental groups (I, II, V, and VI). Group I was injected with saline and groups II, V, and VI were given 5-daily (D1–D5) injections of MPTP (25 mg/kg/injection). Mice of groups I and II were drinking regular water until the termination of the experiment on day 56 (D56). Mice in group V were given Ubisol-Q10 supplemented water (6 mg CoQ10/kg/day) from D5 till D28 (3 weeks), and then they were switched to regular water for the rest of the experimental period (till D56 or for additional 4 weeks). Mice in group VI were placed on the same Ubisol-Q10 supplementation from D5 till D56 (total 7 weeks). At the conclusion of the experiment on D56 mice were sacrificed and brain tissue was collected for immunohistochemistry (B–E) and stereology (F). (B–F) Brains were fixed and immunostained with rabbit polyclonal anti-tyrosine hydroxylase antibody (brown) and counterstained with cresyl violet (blue). Images were captured on an Olympus microscope equipped with Microcast 3CCD 1080p HD color camera as described in Section 2. Representative photomicrographs of mouse midbrain sections shown in: (B) intense TH-immunostaining and normal density of TH-positive neurons in control (group I); (C) profoundly reduced TH-staining in SNpc region of group II; (D) lower than in (B) or (E) TH-staining of cells and fibers in SNpc of group V (receiving Ubisol-Q10 supplementation for 3 weeks and, subsequently, regular water for 4 weeks); (E) strong TH immunostaining of SNpc in group VI (receiving Ubisol-Q10 supplementation for full 7 weeks), nearly indistinguishable from group I (B). (F) Survival of TH-positive neurons in the SNpc. Cells were counted using the stereology method as described in Section 2. Results are presented as mean ± SEM (n = 8–10, from 2 separate experiments). Statistically significant differences are indicated as *** p < 0.001, group I versus II and ## p < 0.01, group II versus IV. Abbreviations: MPTP, 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; SEM, standard error of the mean; SNpc, substantia nigra pars compacta; TH, tyrosine hydroxylase.
3.8. Assessments of motor skills
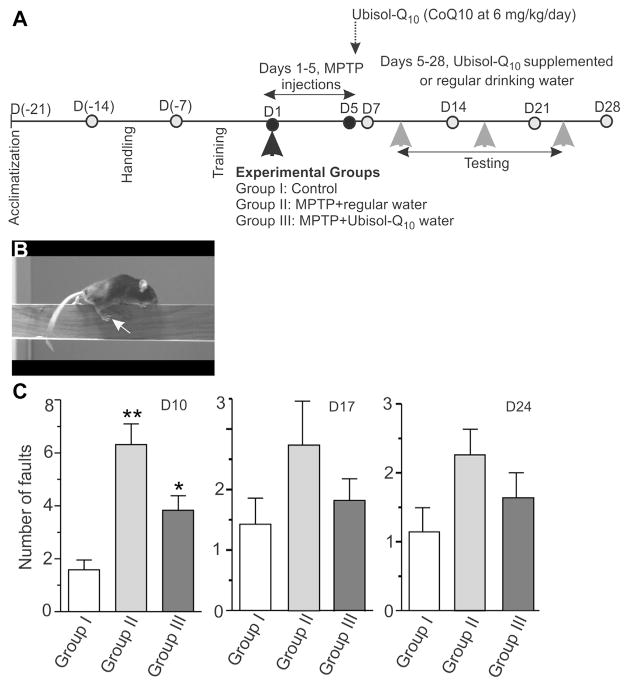
As an additional step in the assessment of Ubisol-Q10 neuroprotective efficacy, we applied a simple behavioral test, the beam walk test, used by others to examine motor skills (Carter et al., 2001). This experiment is outlined in Fig. 8A. The handling and training of mice on the test apparatus were performed before the MPTP injections. Therapeutic Ubisol-Q10 supplementation at 6 mg CoQ10/kg/day commenced on D5 after the last MPTP injection. Mice were tested on D10 (5 days on Ubisol-Q10), D17 (12 days on Ubisol-Q10), and on D24 (after 19 days on Ubisol-Q10). Animals were allowed to cross a 5-mm square and 100-cm long beam and the number of faults, as well as, time taken to walk the beam were recorded (Fig. 8B). On D10 of the experiment, the MPTP-treated mice of group II exhibited a hindlimb weakness resulting in an increased number of hindlimb faults, on average 6 slips as compared with less than 2 in controls (p < 0.001, Fig. 8B). Significantly fewer faults were recorded in mice receiving Ubisol- Q10 for only 5 days (group III), less than 4 faults on average in group III as compared with nearly 7 in group II (p < 0.05, Fig. 8C), proving further evidence for the neuroprotective effects of Ubisol- Q10 supplementation. However, in the subsequent tests on D17 and D24, the differences between the groups were less obvious, although the same trend toward the improvement of motor skills in the Ubisol-Q10 treated mice was seen; the data did not reach statistical significance (Fig. 8D and E). This was consistent with the abilities of mice to adapt and learn (Ansah et al., 2011; Quinn et al., 2008). No differences in the time taken to traverse the beam or forelimb faults between the experimental groups were found (data not shown).
Fig. 8.
Results of the beam walk test. (A) Schematic outline of the experimental design. Mice were acclimatized to the new environment for 7 days (D[−21] – D[−14]) before the handling (D[−14] – D[−7]) and training (D[−7] – D1) as described in Section 2. Animals were randomly divided into 3 experimental groups (I–III). Group I (control) was injected with saline and groups II and III received 5 daily MPTP injections (25 mg/kg/injection; D1–D5). Mice in groups I and II were given regular drinking water throughout the duration of the experiment; whereas, mice in group III were placed on Ubisol-Q10 supplemented water (6 mg CoQ10/kg/day) starting on day 5 immediately after the last MPTP injection (D5). Motor performance was evaluated by measuring the ability of mice to traverse a 5 mm square and 100-cm long beam as described in Section 2. (B) A mouse showing a right hindlimb fault, which were counted on days 10, 17, and 24 [gray arrowheads in (A)]. (C) Quantitation of the right hindlimb faults number. Results are shown as mean ± SEM (n = 16 per group, from 2 separate experiments). Statistically significant differences are indicated as ** p < 0.01, group I versus II; * p < 0.05, group II versus III. Abbreviations: MPTP, 1- methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; SEM, standard error of the mean.
3.9. Midbrain astroglia responses
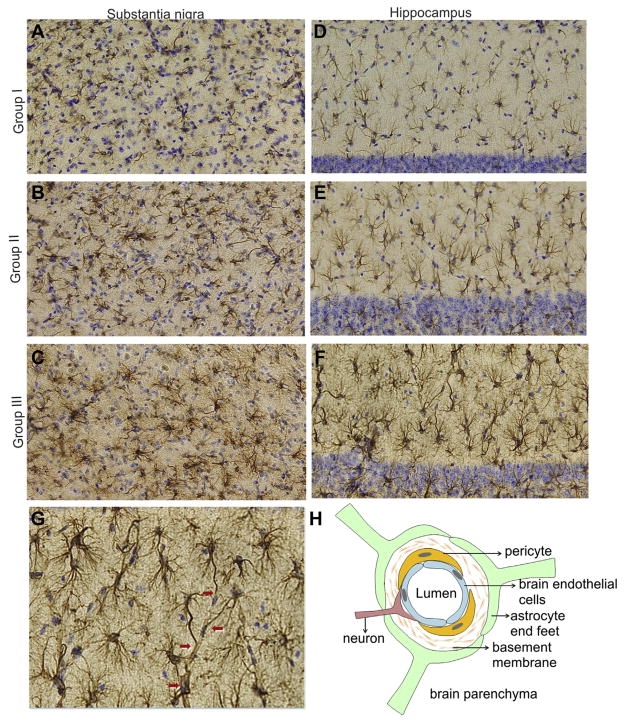
Several studies point to a role of activated glia and inflammation in the pathogenesis of neurodegenerative diseases, especially in PD (Hirsch et al., 2005; Liu et al., 2003; Mena et al., 2002; Mirza et al., 2000). Activation of both, microglia and astroglia is seen in animal models of PD and they are considered active participants in the nigrostriatal degeneration (Halliday and Stevens, 2011; Mena and Garcia de, 2008; Teismann and Schulz, 2004; Wu et al., 2002). Yet, in the brain parenchyma astrocytes are responsible for creating and maintaining an environment that optimizes neuronal function (Barres, 2008; Episcopo et al., 2013; Hertz and Zielke, 2004; Hirrlinger and Dringen, 2010; Kimelberg, 2010; Volterra and Meldolesi, 2005). Here, we examined a response of midbrain astroglia to Ubisol-Q10 supplementation in MPTP-treated mice. Anti-GFAP immunostaining was used to identify brain astrocytes. GFAP is highly expressed by the CNS astrocytes and is widely used as a marker of astrocytic activation, although, it only reveals the changes in cellular intermediate filaments, but not in the morphology of the activated cells (Eng et al., 2000; Wilhelmsson et al., 2006). The collected brain tissue from the experiment outlined in Fig. 6A (groups I, II, and III) was sectioned and immunostained with anti-GFAP antibody (Fig. 9). Consistent with previous reports (Reinhard et al., 1988; Middeldorp and Hol, 2011), an increased anti-GFAP staining was seen at D28 of the experimental treatment (Fig. 6A) in all MPTP-injected animals of group II as compared with the control animals in group I. In group II mice, astrocytes throughout the brain parenchyma, that is, substantia nigra (Fig. 9B), striatum (not shown), hippocampus (Fig. 9E) and cortex (not shown), were decorated with thick bundles of GFAP-positive (hypertrophic) intermediate filaments, characteristic of the activated phenotype. The strongest GFAP response, however, was seen in the brains of MPTP-treated mice supplemented with Ubisol-Q10 (Fig. 9C and F). Under a higher magnification, the astrocyte processes appeared to be more numerous, thicker, and extensively branched, longer, extending further distances from the cell center (Fig. 9G). Furthermore, numerous GFAP-positive processes terminating in glial end-feet surrounding microvessels were clearly seen in Ubisol-Q10 treated mice, but not in controls (Fig. 9G, arrows). As depicted in Fig. 9H, these astrocytes might have been the components of neurovascular unit responsible for controlling cerebral blood flow and maintaining the integrity of blood–brain barrier (Koehler et al., 2009).
Fig. 9.
Astrocytic response to Ubisol-Q10 supplementation. At the conclusion of the experiment depicted in Fig. 6A (D28), brain slides of substantia nigra (A–C) and hippocampus (D–G) from groups I–III were prepared, stained with anti-GFAP antibody (brown) and counterstained with cresyl violet (blue) as described in Section 2. Images were captured on an Olympus microscope equipped with Microcast 3CCD 1080p HD color camera. A and D shows SN and hippocampal sections, respectively, from group I (control), showing a pale filamentous GFAP-immunostaining of resting astrocytes; B and E shows SN and hippocampal sections, respectively, from group II (MPTP-injected receiving regular drinking water), showing enhanced anti-GFAP staining of IF indicative of astrocytic activation; C and F shows SN and hippocampal sections, respectively, from group III (MPTP-injected mice receiving Ubisol-Q10 supplemented water), showing hypertrophic, highly branched GFAP-positive IF, extending further from cell center toward plasma membranes indicative of a strong response to the Ubisol-Q10 treatment; G shows hippocampal section from a mouse of group III at higher magnification, showing elongated GFAP-positive filaments, extending toward blood microvessels and forming astrocytic end-feet (red arrows); H represents the diagram of neurovascular unit, consisting of brain endothelial cells, pericytes, astrocytic end feet, and innervated by neurons, which controls cerebral blood flow and regulates properties of blood–brain barrier. Abbreviations: GFAP, glial fibrillary acidic protein; IF, intermediate filaments; MPTP, 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; SN, substantia nigra.
Quantitative analysis of GFAP-positive cells at D28 in substantia nigra, hippocampus, striatum, and cortex of Ubisol-Q10 treated animals did not show any significant increase in astrocytic numbers, as compared with controls (data not shown). This indicated further that the neuroprotective treatment with Ubisol-Q10 facilitated astrocytic activation, but not reactive gliosis, which is characterized by cell proliferation (Pekny and Nilsson, 2005). It should be noted that such enhanced hypertrophy of GFAP-positive intermediate filaments (IF) in response to Ubisol-Q10 was only seen in MPTP-treated mice, but not in intact controls given the same concentration of Ubisol-Q10 (data not shown) indicating that astroglia activation was initiated by the MPTP inflicted injury. However, Ubisol-Q10 provided an additional signal to which these cells metabolically responded, suggesting that they played an active role in the observed neuroprotection.
No change in microglial response at the end of the observation time on day 28 (Fig. 6A) was seen in any group of animals as assessed by Iba1 immunohistochemistry (not shown).
4. Discussion
We presented in this manuscript an extensive evaluation of neuroprotective efficacy of Ubisol-Q10 against MPTP-induced degeneration of nigrostriatal pathway in mice. MPTP, a precursor of neurotoxin MPP+, causes a preferential loss of neurons in the ventral and lateral segments of the SNpc and damages the dopaminergic pathway in a pattern similar to that seen in PD (Muthane et al., 1994; Seniuk et al., 1990) hence, the MPTP model is commonly used to study the Parkinsonian neurodegeneration. For the purpose of this study, we standardized the subchronic MPTP model (5-daily injections) and established that the degeneration of nigrostriatal pathway occurred over a period of 28 days, creating an opportunity to test the Ubisol-Q10 intervention in several experimental paradigms. Ubisol-Q10 was easily and quantitatively delivered as a daily drinking solution, allowing testing dose range and timing of the intervention, from a prophylactic dosing starting ahead of the MPTP exposure to a therapeutic intervention delivered in the midst of ongoing neurodegeneration. In all the paradigms tested, the Ubisol- Q10 effectively blocked the neurodegenerative processes. When applied prophylactically, it prevented the destruction of nigrostriatal pathway, although it did not interfere with the conversion of MPTP to neurotoxic MPP+. When applied therapeutically, it inhibited the pathologic processes leading to neuronal death and blocked further progression of neurodegeneration from the time it was delivered and for as long as it was delivered. If, however, the Ubisol-Q10 intervention was withdrawn, the neurodegeneration resumed, meaning that Ubisol-Q10 could not reverse the damage initiated by MPTP death pathway, but it blocked it at some yet unknown step, allowing neurons to stay alive. Higher contents of striatal dopamine, measured following Ubisol-Q10 supplementation, were also indicative of its efficacy in preserving the dopaminergic neurotransmission. This unprecedented neuroprotection was achieved with the Ubisol-Q10 dose of 3mg CoQ10/kg/day, which was well within the ADI value of 12 mg/kg/day. The higher Ubisol-Q10 doses, that is, 10× higher and equivalent to 30 mg CoQ10/kg/day, seemed unnecessary, as they did not improve the outcomes. No such efficacy has ever been reported for any known CoQ10 formulations, although they have been extensively studied, including animal models of neurodegeneration and human clinical studies (Beal, 1999; Ihara et al., 1989; Shults et al., 2002). The results of trials are, at best, inconclusive. Some benefits, evaluated using the Unified Parkinson’s Disease Rating Scale, were reported in a pilot clinical trial (QE2 study) involving a group of 80 patients with early stage PD who were treated with increasing doses of CoQ10 from 300 mg to 1200 mg/day (Shults et al., 2002). The trial concludes that patients receiving the highest dose of CoQ10 (1.2 g/day) develop less disability in comparison to patients receiving placebo. It remains unclear from these reports whether CoQ10 had a symptomatic benefit or it was actually neuroprotective because the true assessment of neuroprotection would require quantification of the dopamine neuron survival, which is not presently possible in vivo (Hart et al., 2009). The investigators of this trial, encouraged by the results, conducted a much larger clinical trial to test the effectiveness of CoQ10 at 2.4 g per day. The trial involved 600 patients with early PD at 67 sites throughout North America. Unfortunately, the NIH in conjunction with the NINDS stopped the QE3 Phase III of this trial in 2011 for the lack of benefits. The investigators report no beneficial differences between treated and placebo groups, although there was no safety concern related to such a high dose of CoQ10 taken for up to 16 months. The final results of this study have not been published yet. Similarly, disappointing results are obtained in a phase II trial evaluating the efficacy of 1.8 g/day and 2.7 g/day of CoQ10 for amyotrophic lateral sclerosis (Kaufmann et al., 2009).
Why did these clinical trials fail then? At present there is no clear explanation for these failures. Most of them put the blame on the lack of solubility and poor bioavailability of CoQ10. Numerous reports show elevated plasma content of CoQ10 in rodents and human following injection, reaching a maximal plasma concentration 4–6 hours after administration (Bhagavan and Chopra, 2006, 2007; Miles, 2007). There is no solid experimental evidence yet demonstrating that CoQ10 efficiently penetrates the brain. Our results showed that delivered orally as Ubisol-Q10, CoQ10 reached the brain within 1 hour where it remained elevated for up to 24 hours (Fig. 1). However, it did not accumulate in the brain as a result of long periods of supplementation and no statistically significant differences in its contents between treated and untreated group of mice were found at the conclusion of the experiments (Fig. 6G). This might explain, in part, a lack of data in the literature, showing elevated brain contents of CoQ10 in experimental animals following supplementation. Furthermore, Zhang et al. (1995) report that only 2%–3% of ingested CoQ10 is absorbed by the body, indicating that the absorption is not dose-dependent, but it plateaus at certain concentrations. Here, we have tested CoQ10 doses in 10-fold range (3–30 mg/kg/day) and found that the neuroprotection achieved with the lowest tested dose was the same as with the highest one (Figs. 6 and 7). Our unpublished data also indicated that higher doses of Ubisol-Q10 did not deliver more CoQ10 to the brain; to the contrary, they seemed to have inhibitory effects on the CoQ10 absorption (data not shown). Thus, the expectations that better therapeutic outcomes could be achieved with higher CoQ10 doses may not have been based on a solid physiological premise.
Why was Ubisol-Q10 so effective in these pre-clinical assessments? There is also no obvious explanation why Ubisol-Q10 was so effective in halting the on-going neurodegeneration. Some explanations might be derived from its unique properties. First, water-dispersible Ubisol-Q10 allowed easy and quantitative delivery of CoQ10. Second, CoQ10 was delivered in the form of nanomicelles (a single micelle measures 22.7 nm), which most likely facilitated its uptake by enterocytes and subsequent passage to lymphatic system, resulting the elevated plasma and brain contents within 1 hour of the Ubisol-Q10 ingestion. Such efficient tissue penetration brought about nearly immediate effects of neuroprotection against MPTP. As shown in Figs. 5 and 6, the neuronal death pathway was blocked from the day the intervention begun (D5). In a pilot experiment (not shown here)we delayed the Ubisol-Q10 intervention even further, until day 8 and, again, the ongoing neurodegeneration seized at that stage and no more cell death occurred during the supplementation period. And, third, Ubisol-Q10 delivered to the brain 2 bioactive molecules, CoQ10, and vitamin E (α-tocopherol). These 2 antioxidants are present in all cellular membranes, in close proximity to unsaturated lipids and act as primary scavengers of free radicals, preventing oxidative damage to the constituents of the cell membranes. Furthermore, the quinol form of CoQ10 can transform α-tocopheryl radicals generated during lipid peroxidation back to α-tocopherol and restore its biological antioxidant activity (Wang and Quinn, 1999). We have established here that mouse brain content of CoQ10 was at least 4 times higher than vitamin E. This ratio between the 2 compounds was maintained following the supplementation suggesting that the physiological mechanisms must exist determining their optimal tissue penetration. It is reported that in most cellular membrane CoQ10 content is 3 times higher than vitamin E, with the exception of mitochondrial cristae in which CoQ10 exceeds vitamin E content by 35-fold (Crane, 2001). However, the classical molecular function of CoQ10 as antioxidant and electron carrier in mitochondrial respiratory chain complexes might not be sufficient to provide a satisfactory explanation for the results of our study. Others have shown that targeting CoQ10 to mitochondria by mitoquinone, designed based on the premises that offsetting mitochondria-generated oxidative stress will protect dopaminergic neurons did not achieve this goal. A clinical trial involving 128 newly diagnosed PD patients receiving either 40 or 80 mg/day mitoquinone for over 12 months failed to slow the progression of PD as measured by the Unified Parkinson’s Disease Rating Scale (Snow et al., 2010).
Several articles, published in the recent years, draw attention to the role CoQ10 as cellular prooxidant, which plays a role in ROS signaling (Hyun et al., 2006; Linnane and Eastwood, 2006; Wang and Quinn, 1999). Therefore, in the context of results described here, that is, a relatively low dose of CoQ10 producing full neuroprotection and causing the upregulation of astrocytic GFAP-immunoreactivity, other facets of CoQ10 function should be also considered. Thus, CoQ10 which is present in most cellular membranes as a fundamental component of oxidoreductase systems can also be a source of superoxide and, consequently, hydrogen peroxide. They are generated by auto-oxidation of ubisemiquinone, an intermediate radical of ubiquinone and/or quinol oxidoreduction cycle (Abramov et al., 2005; Hyun et al., 2006; Liu et al., 2005; Ushio-Fukai, 2006; Wright and Kuhn, 2002). Until recently, superoxide and hydrogen peroxide have been regarded, mainly, as harmful by-products of mitochondrial respiration and targeted by various antioxidant-based strategies to control neurodegeneration. However, it is now apparent that the molecule of hydrogen peroxide is an important second messenger in ROS controlled signal transduction pathway implicated in such diverse processes as the regulation of cell proliferation, differentiation, migration, and apoptosis (Stone and Yang, 2006; Veal et al., 2007). Therefore, we could not exclude a possibility that Ubisol-Q10, used at relatively low dose, delivered sufficient amounts of CoQ10 to optimize the function of oxidoreductase systems and, thus, facilitated the ROS signaling.
Among the targets of hydrogen peroxide are the effectors of other signaling systems, namely, protein kinases and phosphatases (Thannickal and Fanburg, 2000; Wright and Kuhn, 2002). Zhu et al. (2005) report that hydrogen peroxide alters membrane and cytoskeleton properties of astrocytes through the activation of p38 MAPK pathway. According to these authors, hydrogen peroxide promotes actin polymerization and induces the formation of intercellular connections (tunneling nanotubes), essential for the astrocytic communication. Therefore, it is possible that such effects played a role in the formation of GFAP-positive IFs in the Ubisol-Q10 treated mouse brains.
It is well known that the expression of GFAP is markedly upregulated in the context of reactive response of astrocytes to nearly all types of injury and disease in the CNS; although, the exact molecular mechanisms responsible for this upregulation are not fully understood (Lepinoux-Chambaud and Eyer, 2013; Middeldorp and Hol, 2011). Nevertheless, we could not exclude a possibility that the observed changes in GFAP-immunoreactivity reflected also an altered gene expression. Since growth factors and/or cytokines signaling, which undoubtedly contribute to the activation of astrocytes, are associated with generation of ROS via NADPH oxidoreductase system, the contribution of CoQ10 to the modulation of GFAP gene expression could not be ruled out either.
Earlier reports suggest that the increase of GFAP expression correlates with the severity of astroglial response and negative predictions for tissue survival (Eng, 1985; Eng et al., 2000). Furthermore, the hypertrophy of GFAP-positive filaments has often been interpreted as evidence of cellular hypertrophy creating hostile environment for neurons (Brahmachari et al., 2006). Recently, these concepts are being re-evaluated due to the advancements of research tools and improved understanding of astrocyte physiology. Thus, it has been revealed in the elegant study from Pekny and Nilsson, 2005 that, in the context of neurotrauma, reactive astrocytes exhibit prominent hypertrophy of GFAP-positive processes, but not cellular hypertrophy. Using dye-filling method and 3D reconstruction technique these authors demonstrate that despite seemingly enlarged IF the overall size of reactive astrocyte is the same as the nonreactive ones. Furthermore, there is no overlapping of astrocytic domains, consistent with the maintenance of the “tiling” phenomenon, as the cells show very little interdigitation (Wilhelmsson et al., 2006). The morphologic features of astrocytic GFAP-positive IF in Ubisol-Q10 treated mice, showing the prominent hypertrophy and no evidence of overlap (Fig. 8), were consistent with this data. This would indicate that the neuroprotective Ubisol-Q10 treatment, via its effects on cellular IFs, might have been able to enhance astrocyte metabolic activity, strengthen their intercellular connections allowing them to function as a syncytium of interconnected cells and, thus, to create favorable, pro-survival environment in the brain parenchyma.
Several studies published in recent years show protective role of reactive astrocytes in traumatic brain injury, spinal cord injury, brain ischemia, and cortical lesion innervation dentate gyrus in mice, highlighting the facts that their function is critical for the survival of neurons (Eng et al., 2000; Hertz and Zielke, 2004; Li et al., 2008; Nedergaard and Dirnagl, 2005; Wilhelmsson et al., 2006). This is because reactive astrocytes produce energy substrates and trophic factors, control neuroinflammation, act as free radical and glutamate scavengers, restore CNS ionic homeostasis, promote remyelination and actively restore the blood–brain barrier (Prat et al., 2001). We have clearly observed significantly enhanced interactions of astrocytic end-feet with brain microvessels in response to the Ubisol-Q10 treatment (Fig. 9G), which might be considered as the additional evidence that activated actrocytes played an active role in the observed neuroprotection. Indeed, astrocytes can produce and release vasodilatory mediators, such as prostaglandins, nitric oxide, and arachidonic acid, which controls various aspects of the blood–brain barrier, including blood flow, metabolic trafficking, and water homeostasis (Kimelberg and Nedergaard, 2010; Simard et al., 2003; Takano et al., 2006).
A large number of studies carried out in cellular and animal models of neurodegeneration have convincingly shown the neuroprotective effects of antioxidants (like vitamin E, reduced glutathione, polyphenols, lipoic acid etc.) for midbrain dopaminergic neurons and other neuronal and nonneuronal cell populations (Dumont and Beal, 2011; Ebadi et al., 1996; Fariss and Zhang, 2003; Koppula et al., 2012a, 2012b; Mayo et al., 2005; Sharma and Nehru, 2013). However, the results of human clinical trials are inconsistent in slowing down disease progression, but there is still hope that improved antioxidant mimetics that better target ROS production pathways might prove beneficial. Glutathione (GSH) is considered the main regulator of redox balance in the cellular milieu due to its capacity for detoxifying noxious molecules (Byrd et al., 2004; Dringen, 2000; Dringen and Hirrlinger, 2003). Upregulation of GSH levels has been achieved using oral administration of N-acetyl- L-cysteine (NAC), which results in increased brain GSH level, brain synaptic mitochondrial complex I activity (Banaclocha, 2000), and also increased neuronal survival and behavioral read-outs in MPTP-induced parkinsonian degeneration in mice (Berman et al., 2011; Clark et al., 2010). NAC is currently approved for use in the United States, Europe, and Canada for the treatment of acetaminophen-induced hepatotoxicity (Prescott, 2005) and can be used for treatment of neurodegenerative diseases. A brain-penetrable version of NAC, N-acetylcysteine amide (also known as AD4), has recently been developed (Offen et al., 2004) for efficient delivery to the brain. Despite these promising results, there have been only 2 clinical trials involving PD patients where GSH was delivered intravenously (Hauser et al., 2009; Sechi et al., 1996). Although the open label study by Sechi et al. (1996), showed a significant improvement, with a 42% decline in disability, the randomized, placebo-controlled, double blind by Hauser et al. (2009), showed only a mild symptomatic effect. At present there is no approved GSH therapy for treatment of PD patients.
Vitamin E is a naturally occurring chain breaking antioxidant in biological membranes that can react with free radicals, and possibly with other oxidizing intermediates, and prevent the chain of events (Wang and Quinn, 1999). Vitamin E family consists of tocopherols and tocotrienols (each form has several isomers), which have shown to be neuroprotective and anti-inflammatory (Bardhan et al., 2011). Although the results of some studies suggest that diets rich in vitamin E are protective against PD, other studies show no such benefits (Ricciarelli et al., 2007). An earlier clinical trial reported that PD patients taking vitamin E had significantly less severe disease than those not taking vitamin E (Factor et al., 1990). However, the deprenyl and tocopherol antioxidative therapy of parkinsonism clinical trial after a follow-up of 14 ± 6 months failed to show any beneficial effect of alpha-tocopherol or any interaction between alpha-tocopherol and deprenyl (Shoulson, 1998). The reason for the conflicting findings on vitamin E protection in vivo is unknown.
In conclusion, our study revealed that Ubisol-Q10 intervention could stop, but not reverse, the on-going neurodegeneration in MPTP-treated mouse brain. For this to occur, the astrocytic surveillance of brain parenchyma needed to be mobilized. This was achieved with relatively low concentrations of Ubisol-Q10, that is, 3 mg CoQ10/kg/day, well within ADI value of 12 mg/kg/day. Ubisol- Q10 delivered to the brain 2 bioactive compounds, CoQ10 and vitamin E, both powerful scavengers of free radicals and antioxidants. However, the data also suggested that CoQ10, via cellular oxidoreductase systems, might have played a role in the generation of second messenger hydrogen peroxide. This, in turn, would have engaged cellular ROS signaling and altered the metabolic activity of astroglia required to support the neuronal survival. Thus, Ubisol- Q10 with its safety and ease of application (as a supplement in drinking water) should prove beneficial for the management of PD.
Acknowledgments
This work was supported, in part, by grants from the Michael J Fox Foundation for Parkinson’s disease under the “Therapeutics Development Initiative Fall 2010 Program” to Zymes LLC and the National Research Council Canada and a grant from Canadian Institute for Health Research to Siyaram Pandey, Marianna Sikorska, and Jagdeep K Sandhu. The authors are not part of Zymes LLC and have no conflict of interest in the commercial development of Ubisol-Q10.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansah TA, Ferguson MC, Nayyar T. The 5-HT(2A) receptor antagonist M100907 produces antiparkinsonian effects and decreases striatal glutamate. Front Syst Neurosci. 2011;5:48. doi: 10.3389/fnsys.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaclocha MM. N-Acetylcysteine elicited increase in complex I activity in synaptic mitochondria from aged mice: implications for treatment of Parkinson’s disease. Brain Res. 2000;859:173–175. doi: 10.1016/s0006-8993(00)02005-9. [DOI] [PubMed] [Google Scholar]
- Bardhan J, Chakraborty R, Raychaudhuri U. The 21st century form of vitamin E–tocotrienol. Curr Pharm Des. 2011;17:2196–2205. doi: 10.2174/138161211796957472. [DOI] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Beal MF. Coenzyme Q10 administration and its potential for treatment of neurodegenerative diseases. Biofactors. 1999;9:261–266. doi: 10.1002/biof.5520090222. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- Beal MF, Matthews RT, Tieleman A, Shults CW. Coenzyme Q10 attenuates the 1-methyl-4-phenyl-1,2,3, tetrahydropyridine (MPTP) induced loss of striatal dopamine and dopaminergic axons in aged mice. Brain Res. 1998;783:109–114. doi: 10.1016/s0006-8993(97)01192-x. [DOI] [PubMed] [Google Scholar]
- Berman AE, Chan WY, Brennan AM, Reyes RC, Adler BL, Suh SW, Kauppinen TM, Edling Y, Swanson RA. N-acetylcysteine prevents loss of dopaminergic neurons in the EAAC1-/- mouse. Ann Neurol. 2011;69:509–520. doi: 10.1002/ana.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagavan HN, Chopra RK. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40:445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- Bhagavan HN, Chopra RK. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion. 2007;(7 Suppl):S78–S88. doi: 10.1016/j.mito.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Borowy-Borowski H, Sodja C, Docherty J, Walker PR, Sikorska M. Unique technology for solubilization and delivery of highly lipophilic bioactive molecules. J Drug Target. 2004;12:415–424. doi: 10.1080/10611860412331285233. [DOI] [PubMed] [Google Scholar]
- Brahmachari S, Fung YK, Pahan K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci. 2006;26:4930–4939. doi: 10.1523/JNEUROSCI.5480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd AS, Sikorska M, Walker PR, Sandhu JK. Effects of glutathione depletion on the viability of human NT2-derived neuronal and astroglial cultures. Neuron Glia Biol. 2004;1:317–326. doi: 10.1017/S1740925X05000207. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol Dis. 2009;34:279–290. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RJ, Morton J, Dunnett SB. Motor coordination and balance in rodents. Curr Protoc Neurosci Unit. 2001;8:12. doi: 10.1002/0471142301.ns0812s15. [DOI] [PubMed] [Google Scholar]
- Cicchetti F, Lapointe N, Roberge-Tremblay A, Saint-Pierre M, Jimenez L, Ficke BW, Gross RE. Systemic exposure to paraquat and maneb models early Parkinson’s disease in young adult rats. Neurobiol Dis. 2005;20:360–371. doi: 10.1016/j.nbd.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Clark J, Clore EL, Zheng K, Adame A, Masliah E, Simon DK. Oral N-acetyl- cysteine attenuates loss of dopaminergic terminals in alpha-synuclein overexpressing mice. PLoS One. 2010;5:e12333. doi: 10.1371/journal.pone.0012333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleren C, Yang L, Lorenzo B, Calingasan NY, Schomer A, Sireci A, Wille EJ, Beal MF. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J Neurochem. 2008;104:1613–1621. doi: 10.1111/j.1471-4159.2007.05097.x. [DOI] [PubMed] [Google Scholar]
- Crane FL. Biochemical functions of coenzyme Q10. J Am Coll Nutr. 2001;20:591–598. doi: 10.1080/07315724.2001.10719063. [DOI] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003;384:505–516. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- Dumont M, Beal MF. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic Biol Med. 2011;51:1014–1026. doi: 10.1016/j.freeradbiomed.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebadi M, Srinivasan SK, Baxi MD. Oxidative stress and antioxidant therapy in Parkinson’s disease. Prog Neurobiol. 1996;48:1–19. doi: 10.1016/0301-0082(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Eng LF. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol. 1985;8:203–214. doi: 10.1016/s0165-5728(85)80063-1. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Episcopo FL, Tirolo C, Testa N, Caniglia S, Morale MC, Marchetti B. Reactive astrocytes are key players in nigrostriatal dopaminergic neurorepair in the MPTP mouse model of Parkinson’s disease: focus on endogenous neurorestoration. Curr Aging Sci. 2013;6:45–55. doi: 10.2174/1874609811306010007. [DOI] [PubMed] [Google Scholar]
- Exner N, Lutz AK, Haass C, Winklhofer KF. Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences. EMBO J. 2012;31:3038–3062. doi: 10.1038/emboj.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor SA, Sanchez-Ramos JR, Weiner WJ. Vitamin E therapy in Parkinson’s disease. Adv Neurol. 1990;53:457–461. [PubMed] [Google Scholar]
- Fariss MW, Zhang JG. Vitamin E therapy in Parkinson’s disease. Toxicology. 2003;189:129–146. doi: 10.1016/s0300-483x(03)00158-6. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, Dedeoglu A, Ferrante KL, Jenkins BG, Hersch SM, Beal MF. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington’s disease. J Neurosci. 2002;22:1592–1599. doi: 10.1523/JNEUROSCI.22-05-01592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille G, Hung ST, Reichmann H, Rausch WD. Oxidative stress to dopaminergic neurons as models of Parkinson’s disease. Ann N Y Acad Sci. 2004;1018:533–540. doi: 10.1196/annals.1296.066. [DOI] [PubMed] [Google Scholar]
- Gille L, Nohl H. The existence of a lysosomal redox chain and the role of ubiquinone. Arch Biochem Biophys. 2000;375:347–354. doi: 10.1006/abbi.1999.1649. [DOI] [PubMed] [Google Scholar]
- Graves S, Sikorska M, Borowy-Borowski H, Ho RJ, Bui T, Woodhouse C. Analysis of coenzyme Q10 content in human plasma and other biological samples. Methods Mol Biol. 1998;108:353–365. doi: 10.1385/0-89603-472-0:353. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson’s disease. Mov Disord. 2011;26:6–17. doi: 10.1002/mds.23455. [DOI] [PubMed] [Google Scholar]
- Hargreaves IP, Lane A, Sleiman PM. The coenzyme Q10 status of the brain regions of Parkinson’s disease patients. Neurosci Lett. 2008;447:17–19. doi: 10.1016/j.neulet.2008.09.069. [DOI] [PubMed] [Google Scholar]
- Hart RG, Pearce LA, Ravina BM, Yaltho TC, Marler JR. Neuroprotection trials in Parkinson’s disease: systematic review. Mov Disord. 2009;24:647–654. doi: 10.1002/mds.22432. [DOI] [PubMed] [Google Scholar]
- Hauser DN, Hastings TG. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease and monogenic parkinsonism. Neurobiol Dis. 2012;51:35–42. doi: 10.1016/j.nbd.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser RA, Lyons KE, McClain T, Carter S, Perlmutter D. Randomized, double-blind, pilot evaluation of intravenous glutathione in Parkinson’s disease. Mov Disord. 2009;24:979–983. doi: 10.1002/mds.22401. [DOI] [PubMed] [Google Scholar]
- Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27:735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Hidaka T, Fujii K, Funahashi I, Fukutomi N, Hosoe K. Safety assessment of coenzyme Q10 (CoQ10) Biofactors. 2008;32:199–208. doi: 10.1002/biof.5520320124. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Dringen R. The cytosolic redox state of astrocytes: maintenance, regulation and functional implications for metabolite trafficking. Brain Res Rev. 2010;63:177–188. doi: 10.1016/j.brainresrev.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S, Hartmann A. Neuroinflammatory processes in Parkinson’s disease. Parkinsonism Relat Disord. 2005;11(Suppl 1):S9–S15. doi: 10.1016/j.parkreldis.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Hyun DH, Emerson SS, Jo DG, Mattson MP, de CR. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci US A. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara Y, Namba R, Kuroda S, Sato T, Shirabe T. Mitochondrial encephalomyopathy (MELAS): pathological study and successful therapy with coenzyme Q10 and idebenone. J Neurol Sci. 1989;90:263–271. doi: 10.1016/0022-510x(89)90112-3. [DOI] [PubMed] [Google Scholar]
- Itoh N, Masuo Y, Yoshida Y, Cynshi O, Jishage K, Niki E. gamma- Tocopherol attenuates MPTP-induced dopamine loss more efficiently than alpha-tocopherol in mouse brain. Neurosci Lett. 2006;403:136–140. doi: 10.1016/j.neulet.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S26–S36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Thompson JL, Levy G, Buchsbaum R, Shefner J, Krivickas LS, Katz J, Rollins Y, Barohn RJ, Jackson CE, Tiryaki E, Lomen-Hoerth C, Armon C, Tandan R, Rudnicki SA, Rezania K, Sufit R, Pestronk A, Novella SP, Heiman-Patterson T, Kasarskis EJ, Pioro EP, Montes J, Arbing R, Vecchio D, Barsdorf A, Mitsumoto H, Levin B QALS Study Group. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann Neurol. 2009;66:235–244. doi: 10.1002/ana.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK. Functions of mature mammalian astrocytes: a current view. Neuroscientist. 2010;16:79–106. doi: 10.1177/1073858409342593. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7:338–353. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Koppula S, Kumar H, More SV, Kim BW, Kim IS, Choi DK. Recent advances on the neuroprotective potential of antioxidants in experimental models of Parkinson’s disease. Int J Mol Sci. 2012a;13:10608–10629. doi: 10.3390/ijms130810608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppula S, Kumar H, More SV, Lim HW, Hong SM, Choi DK. Recent updates in redox regulation and free radical scavenging effects by herbal products in experimental models of Parkinson’s disease. Molecules. 2012b;17:11391–11420. doi: 10.3390/molecules171011391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BJ, Huang YC, Chen SJ, Lin PT. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with coronary artery disease. Nutrition. 2012a;28:250–255. doi: 10.1016/j.nut.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Lee BJ, Huang YC, Chen SJ, Lin PT. Effects of coenzyme Q10 supplementation on inflammatory markers (high-sensitivity C-reactive protein, interleukin-6, and homocysteine) in patients with coronary artery disease. Nutrition. 2012b;28:767–772. doi: 10.1016/j.nut.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Lepinoux-Chambaud C, Eyer J. Review on intermediate filaments of the nervous system and their pathological alterations. Histochem Cell Biol. 2013;140:13–22. doi: 10.1007/s00418-013-1101-1. [DOI] [PubMed] [Google Scholar]
- Lew MF. The evidence for disease modification in Parkinson’s disease. Int J Neurosci. 2011;121(Suppl 2):18–26. doi: 10.3109/00207454.2011.620194. [DOI] [PubMed] [Google Scholar]
- Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Ståhlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- Linnane AW, Eastwood H. Cellular redox regulation and prooxidant signaling systems: a new perspective on the free radical theory of aging. Ann N Y Acad Sci. 2006;1067:47–55. doi: 10.1196/annals.1354.008. [DOI] [PubMed] [Google Scholar]
- Littarru GP, Tiano L. Clinical aspects of coenzyme Q10: an update. Nutrition. 2010;26:250–254. doi: 10.1016/j.nut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Liu B, Gao HM, Hong JS. Parkinson’s disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ Health Perspect. 2003;111:1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kang JH, Zheng RL. NADPH oxidase produces reactive oxygen species and maintains survival of rat astrocytes. Cell Biochem Funct. 2005;23:93–100. doi: 10.1002/cbf.1171. [DOI] [PubMed] [Google Scholar]
- Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MA, Carson RE, Cohen RM, Mouton PR, Quigley C, Mattson MP, Ingram DK. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc Natl Acad Sci US A. 2004;101:18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, Yang L, Browne S, Baik M, Beal MF. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci US A. 1998;95:8892–8897. doi: 10.1073/pnas.95.15.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer’s and Parkinson’s diseases. Ann N Y Acad Sci. 1999;893:154–175. doi: 10.1111/j.1749-6632.1999.tb07824.x. [DOI] [PubMed] [Google Scholar]
- Mayo JC, Sainz RM, Tan DX, Antolin I, Rodriguez C, Reiter RJ. Melatonin and Parkinson’s disease. Endocrine. 2005;27:169–178. doi: 10.1385/ENDO:27:2:169. [DOI] [PubMed] [Google Scholar]
- McCarthy S, Somayajulu M, Sikorska M, Borowy-Borowski H, Pandey S. Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble coenzyme Q10. Toxicol Appl Pharmacol. 2004;201:21–31. doi: 10.1016/j.taap.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Mena MA, de Bernardo S, Casarejos MJ, Canals S, Rodriguez-Martin E. The role of astroglia on the survival of dopamine neurons. Mol Neurobiol. 2002;25:245–263. doi: 10.1385/MN:25:3:245. [DOI] [PubMed] [Google Scholar]
- Mena MA, Garcia de YJ. Glial cells as players in parkinsonism: the “good,” the “bad,” and the “mysterious” glia. Neuroscientist. 2008;14:544–560. doi: 10.1177/1073858408322839. [DOI] [PubMed] [Google Scholar]
- Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Miles MV. The uptake and distribution of coenzyme Q10. Mitochondrion. 2007;(7 Suppl):S72–S77. doi: 10.1016/j.mito.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Miles MV, Patterson BJ, Chalfonte-Evans ML, Horn PS, Hickey FJ, Schapiro MB, Steele PE, Tang PH, Hotze SL. Coenzyme Q10 (ubiquinol- 10) supplementation improves oxidative imbalance in children with trisomy 21. Pediatr Neurol. 2007;37:398–403. doi: 10.1016/j.pediatrneurol.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Mirza B, Hadberg H, Thomsen P, Moos T. The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson’s disease. Neuroscience. 2000;95:425–432. doi: 10.1016/s0306-4522(99)00455-8. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Kelley-Bell B, Tweedie D, Spangler EL, Perez E, Carlson OD, Short RG, deCabo R, Chang J, Ingram DK, Li Y, Greig NH. The effects of age and lipopolysaccharide (LPS)-mediated peripheral inflammation on numbers of central catecholaminergic neurons. Neurobiol Aging. 2012;33:423–436. doi: 10.1016/j.neurobiolaging.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthane U, Ramsay KA, Jiang H, Jackson-Lewis V, Donaldson D, Fernando S, Ferreira M, Przedborski S. Differences in nigral neuron number and sensitivity to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57/bl and CD-1 mice. Exp Neurol. 1994;126:195–204. doi: 10.1006/exnr.1994.1058. [DOI] [PubMed] [Google Scholar]
- Muthukumaran K, Leahy S, Harrison K, Sikorska M, Sandhu JK, Cohen J, Keshan C, Lopatin D, Miller H, Borowy-Borowski H, Lanthier P, Weinstock S, Pandey S. Orally delivered water soluble Coenzyme Q10 (Ubisol-Q10) blocks on-going neurodegeneration in rats exposed to paraquat: potential for therapeutic application in Parkinson's disease. BMC Neurosc. 2014 doi: 10.1186/1471-2202-15-21. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi J, Somayajulu-Nitu M, Mukerji A, Sharda P, Sikorska M, Borowy-Borowski H, Antonsson B, Pandey S. Water-soluble formulation of coenzyme Q10 inhibits Bax-induced destabilization of mitochondria in mammalian cells. Apoptosis. 2006;11:1359–1369. doi: 10.1007/s10495-006-8417-4. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Dirnagl U. Role of glial cells in cerebral ischemia. Glia. 2005;50:281–286. doi: 10.1002/glia.20205. [DOI] [PubMed] [Google Scholar]
- Offen D, Gilgun-Sherki Y, Barhum Y, Benhar M, Grinberg L, Reich R, Melamed E, Atlas D. A low molecular weight copper chelator crosses the blood-brain barrier and attenuates experimental autoimmune encephalomyelitis. J Neurochem. 2004;89:1241–1251. doi: 10.1111/j.1471-4159.2004.02428.x. [DOI] [PubMed] [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood-brain barrier. Glia. 2001;36:145–155. doi: 10.1002/glia.1104. [DOI] [PubMed] [Google Scholar]
- Prescott L. Oral or intravenous N-acetylcysteine for acetaminophen poisoning? Ann. Emerg Med. 2005;45:409–413. doi: 10.1016/j.annemergmed.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Quinn LP, Crook B, Hows ME, Vidgeon-Hart M, Chapman H, Upton N, Medhurst AD, Virley DJ. The PPARgamma agonist pioglitazone is effective in the MPTP mouse model of Parkinson’s disease through inhibition of monoamine oxidase B. Br J Pharmacol. 2008;154:226–233. doi: 10.1038/bjp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard JF, Jr, Miller DB, O’Callaghan JP. The neurotoxicant MPTP (1- methyl-4-phenyl-1,2,3,6-tetrahydropyridine) increases glial fibrillary acidic protein and decreases dopamine levels of the mouse striatum: evidence for glial response to injury. Neurosci Lett. 1988;95:246–251. doi: 10.1016/0304-3940(88)90665-9. [DOI] [PubMed] [Google Scholar]
- Ren YR, Nishida Y, Yoshimi K, Yasuda T, Jishage K, Uchihara T, Yokota T, Mizuno Y, Mochizuki H. Genetic vitamin E deficiency does not affect MPTP susceptibility in the mouse brain. J Neurochem. 2006;98:1810–1816. doi: 10.1111/j.1471-4159.2006.03994.x. [DOI] [PubMed] [Google Scholar]
- Ricciarelli R, Argellati F, Pronzato MA, Domenicotti C. Vitamin E and neurodegenerative diseases. Mol Aspects Med. 2007;28:591–606. doi: 10.1016/j.mam.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Sandhu JK, Pandey S, Ribecco M, Monette R, Borowy-Borowski H, Walker PR, Sikorska M. Molecular mechanisms of glutamate neurotoxicity in mixed cultures of NT2-derived neurons and astrocytes: protective effects of coenzyme Q10. J Neurosci Res. 2003;72:691–703. doi: 10.1002/jnr.10579. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Mitochondrial dysfunction in Parkinson’s disease. Cell Death Differ. 2007;14:1261–1266. doi: 10.1038/sj.cdd.4402160. [DOI] [PubMed] [Google Scholar]
- Schmelzer C, Lindner I, Rimbach G, Niklowitz P, Menke T, Doring F. Functions of coenzyme Q10 in inflammation and gene expression. Biofactors. 2008;32:179–183. doi: 10.1002/biof.5520320121. [DOI] [PubMed] [Google Scholar]
- Schmelzer C, Lorenz G, Lindner I, Rimbach G, Niklowitz P, Menke T, Doring F. Effects of coenzyme Q10 on TNF-alpha secretion in human and murine monocytic cell lines. Biofactors. 2007;31:35–41. doi: 10.1002/biof.5520310104. [DOI] [PubMed] [Google Scholar]
- Schober A. Classic toxin-induced animal models of Parkinson’s disease: 6- OHDA and MPTP. Cell Tissue Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- Sechi G, Deledda MG, Bua G, Satta WM, Deiana GA, Pes GM, Rosati G. Reduced intravenous glutathione in the treatment of early Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:1159–1170. doi: 10.1016/s0278-5846(96)00103-0. [DOI] [PubMed] [Google Scholar]
- Seniuk NA, Tatton WG, Greenwood CE. Dose-dependent destruction of the coeruleus-cortical and nigral-striatal projections by MPTP. Brain Res. 1990;527:7–20. doi: 10.1016/0006-8993(90)91055-l. [DOI] [PubMed] [Google Scholar]
- Sharma N, Nehru B. Beneficial effect of vitamin E in rotenone induced model of PD: behavioural, neurochemical and biochemical study. Exp Neurobiol. 2013;22:214–223. doi: 10.5607/en.2013.22.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulson I. DATATOP: a decade of neuroprotective inquiry. Parkinson study group. Deprenyl and tocopherol antioxidative therapy of parkinsonism. Ann Neurol. 1998;44:S160–S166. [PubMed] [Google Scholar]
- Shults CW, Beal MF, Fontaine D, Nakano K, Haas RH. Absorption, tolerability, and effects on mitochondrial activity of oral coenzyme Q10 in parkinsonian patients. Neurology. 1998;50:793–795. doi: 10.1212/wnl.50.3.793. [DOI] [PubMed] [Google Scholar]
- Shults CW, Haas R. Clinical trials of coenzyme Q10 in neurological disorders. Biofactors. 2005;25:117–126. doi: 10.1002/biof.5520250113. [DOI] [PubMed] [Google Scholar]
- Shults CW, Haas RH, Beal MF. A possible role of coenzyme Q10 in the etiology and treatment of Parkinson’s disease. Biofactors. 1999;9:267–272. doi: 10.1002/biof.5520090223. [DOI] [PubMed] [Google Scholar]
- Shults CW, Oakes D, Kieburtz K, Beal MF, Haas R, Plumb S, Juncos JL, Nutt J, Shoulson I, Carter J, Kompoliti K, Perlmutter JS, Reich S, Stern M, Watts RL, Kurlan R, Molho E, Harrison M, Lew M. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- Sikorska M, Borowy-Borowski H, Zurakowski B, Walker PR. Derivatised alpha-tocopherol as a CoQ10 carrier in a novel water-soluble formulation. Biofactors. 2003;18:173–183. doi: 10.1002/biof.5520180220. [DOI] [PubMed] [Google Scholar]
- Sikorska M, Sandhu JK, Simon DK, Pathiraja V, Sodja C, Li Y, Ribecco-Lutkiewicz M, Lanthier P, Borowy-Borowski H, Upton A, Raha S, Pulst SM, Tarnopolsky MA. Identification of ataxia-associated mtDNA mutations (m. 052T>C and m.9035T>C) and evaluation of their pathogenicity in trans-mitochondrial cybrids. Muscle Nerve. 2009;40:381–394. doi: 10.1002/mus.21355. [DOI] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RB, Wander GS, Rastogi A, Shukla PK, Mittal A, Sharma JP, Mehrotra SK, Kapoor R, Chopra RK. Randomized, double-blind placebo- controlled trial of coenzyme Q10 in patients with acute myocardial infarction. Cardiovasc Drugs Ther. 1998;12:347–353. doi: 10.1023/a:1007764616025. [DOI] [PubMed] [Google Scholar]
- Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord. 2010;25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- Somayajulu M, McCarthy S, Hung M, Sikorska M, Borowy-Borowski H, Pandey S. Role of mitochondria in neuronal cell death induced by oxidative stress; neuroprotection by coenzyme Q10. Neurobiol Dis. 2005;18:618–627. doi: 10.1016/j.nbd.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Somayajulu-Nitu M, Sandhu JK, Cohen J, Sikorska M, Sridhar TS, Matei A, Borowy-Borowski H, Pandey S. Paraquat induces oxidative stress, neuronal loss in substantia nigra region and parkinsonism in adult rats: neuroprotection and amelioration of symptoms by water-soluble formulation of coenzyme Q10. BMC Neurosci. 2009;10:88. doi: 10.1186/1471-2202-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souchet N, Laplante S. Seasonal variation of Co-enzyme Q10 contents in pelagic fish tissues from Eastern Quebec. J Food Compost Anal. 2007;20:403–410. [Google Scholar]
- Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Tang PH, Miles MV, Miles L, Quinlan J, Wong B, Wenisch A, Bove K. Measurement of reduced and oxidized coenzyme Q9 and coenzyme Q10 levels in mouse tissues by HPLC with coulometric detection. Clin Chim Acta. 2004;341:173–184. doi: 10.1016/j.cccn.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Teismann P, Schulz JB. Cellular pathology of Parkinson’s disease: astrocytes, microglia and inflammation. Cell Tissue Res. 2004;318:149–161. doi: 10.1007/s00441-004-0944-0. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Tiano L, Padella L, Santoro L, Carnevali P, Principi F, Bruge F, Gabrielli O, Littarru GP. Prolonged coenzyme Q10 treatment in Down syndrome patients: effect on DNA oxidation. Neurobiol Aging. 2012;33:626–628. doi: 10.1016/j.neurobiolaging.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Tremblay RG, Sikorska M, Sandhu JK, Lanthier P, Ribecco-Lutkiewicz M, Bani-Yaghoub M. Differentiation of mouse Neuro 2A cells into dopamine neurons. J Neurosci Methods. 2010;186:60–67. doi: 10.1016/j.jneumeth.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Villalba JM, Parrado C, Santos-Gonzalez M, Alcain FJ. Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opin Investig Drugs. 2010;19:535–554. doi: 10.1517/13543781003727495. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Wang X, Quinn PJ. Vitamin E and its function in membranes. Prog Lipid Res. 1999;38:309–336. doi: 10.1016/s0163-7827(99)00008-9. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci US A. 2006;103:17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MV, Kuhn TB. CNS neurons express two distinct plasma membrane electron transport systems implicated in neuronal viability. J Neurochem. 2002;83:655–664. doi: 10.1046/j.1471-4159.2002.01176.x. [DOI] [PubMed] [Google Scholar]
- Wu dC, Tieu K, Cohen O, Choi DK, Vila M, Jackson-Lewis V, Teismann P, Przedborski S. Glial cell response: a pathogenic factor in Parkinson’s disease. J Neurovirol. 2002;8:551–558. doi: 10.1080/13550280290100905. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Aberg F, Appelkvist EL, Dallner G, Ernster L. Uptake of dietary coenzyme Q supplement is limited in rats. J Nutr. 1995;125:446–453. doi: 10.1093/jn/125.3.446. [DOI] [PubMed] [Google Scholar]
- Zhu D, Tan KS, Zhang X, Sun AY, Sun GY, Lee JC. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J Cell Sci. 2005;118:3695–3703. doi: 10.1242/jcs.02507. [DOI] [PubMed] [Google Scholar]