Abstract
IMPORTANCE
Isolated dystonia and Parkinson disease (PD) are disorders of the basal gangliothalamocortical network. They have largely distinct clinical profiles, but both disorders respond to deep brain stimulation (DBS) in the same subcortical targets using similar stimulation paradigms, suggesting pathophysiologic overlap. We hypothesized that, similar to PD, isolated dystonia is associated with elevated cortical neuronal synchronization.
OBJECTIVE
To investigate the electrophysiologic characteristics of the sensorimotor cortex arm-related area using a temporary subdural electrode strip in patients with isolated dystonia and PD undergoing DBS implantation in the awake state.
DESIGN, SETTING, AND PARTICIPANTS
An observational study recruited patients scheduled for DBS at the University of California, San Francisco and the San Francisco Veterans Affairs Medical Center. Data were collected from May 1, 2008, through April 1, 2015. Findings are reported for 22 patients with isolated cervical or segmental dystonia (8 with [DYST-ARM] and 14 without [DYST] arm symptoms] and 14 patients with akinetic rigid PD. Data were analyzed from November 1, 2014, through May 1, 2015.
MAIN OUTCOMES AND MEASURES
Cortical local field potentials, power spectral density, and phase-amplitude coupling (PAC).
RESULTS
Among our 3 groups that together included 36 patients, cortical PAC was present in primary motor and premotor arm-related areas for all groups, but the DYST group was less likely to exhibit increased PAC (P = .008). Similar to what has been shown for patients with PD, subthalamic DBS reversibly decreased PAC in a subset of patients with dystonia who were studied before and during intraoperative test stimulation (n = 4). At rest, broadband gamma (50–200 Hz) power in the primary motor cortex was greater in the DYST-ARM and PD groups compared with the DYST group, whereas alpha (8–13 Hz) and beta (13–30 Hz) power was comparable in all 3 groups. During movement, the DYST-ARM group had impaired beta and low gamma desynchronization in the primary motor cortex.
CONCLUSIONS AND RELEVANCE
Isolated dystonia and PD have physiologic overlap with respect to high levels of motor cortex synchronization and reduction of cortical synchronization by subthalamic DBS, providing an explanation for their similar therapeutic response to basal ganglia stimulation.
Isolated dystonia and Parkinson disease (PD) are both disorders of the basal gangliothalamocortical network. Increasing evidence suggests that the motor signs of PD are related to excessive synchronized oscillatory activity in the basal gangliothalamocortical network, manifested primarily by increased synchronization of population spiking to the motor beta rhythm.1–5 Isolated dystonia also may be associated with abnormalities in synchronized oscillations.6,7 Both disorders can be ameliorated by the same deep brain stimulation (DBS) paradigms, such as 100- to 200-Hz constant stimulation of the globus pallidus internus or subthalamic nucleus (STN).8,9 This empirical finding suggests similarities in the underlying brain circuit abnormalities in both disorders.
Previous studies5,10 used intraoperative electrocorticography (ECOG) in humans undergoing DBS implantation in the awake state to characterize specific patterns of neuronal synchronization in the arm-related area of the primary motor cortex (M1). Compared with other forms of invasive human recordings, such as basal ganglia local field potentials, ECOG potentials can compare the spectral characteristics of low-frequency rhythms, such as the dominant motor beta rhythm, across multiple diseases and extract a surrogate measure of population spiking from the ECOG power spectrum (ie, the amplitude of broadband high-frequency activity in the gamma range of 50–200 Hz).11,12 The most striking abnormality in patients with rigid akinetic PD compared with patients without PD is the excessive coupling of M1 broadband gamma activity to the phase of the beta rhythm. This coupling implies excessive phase locking of M1 neurons to the beta rhythm and may constrain the motor cortex into an inflexible pattern of activity such that initiation and execution of movement is impaired. This abnormality is reversed by acute therapeutic STN DBS.13 Furthermore, M1 broadband gamma activity at rest is elevated in PD,10 which is indicative of increased resting-state activity and consistent with functional imaging studies.14
Herein we test the hypothesis that patients with isolated generalized dystonia show patterns in the M1 that are similar to patterns in patients with PD, with similar reversal by therapeutic DBS. Patients with dystonia may have physiologic endophenotypes that are also present in asymptomatic carriers of dystonia-predisposing genes or in the brain hemisphere contralateral to an asymptomatic body side. To resolve whether observed physiologic motifs reflect such an endophenotype (vs overtly manifesting motor signs), we separated patients with dystonia into those with clinically apparent arm involvement and those without arm involvement on the side contralateral to the ECOG recording in the arm area of the M1 (DYST-ARM and DYST groups, respectively). Although a prior study included a cohort with dystonia, that cohort only included patients with craniocervical dystonias without involvement of the arm contralateral to the ECOG recording.5
Methods
Patient Selection
We recruited patients with isolated dystonia or idiopathic PD scheduled to undergo DBS surgery at the University of California, San Francisco or the San Francisco Veterans Affairs Medical Center for the study. Inclusion criteria for the PD group were rigidity and akinesia as the most prominent symptoms and a lack of dystonic symptoms in the upper body while not receiving medications. Written informed consent was obtained before surgery under a protocol approved by the institutional review boards of the University of California, San Francisco and the San Francisco Veterans Affairs Medical Center, and all patients were aware that the temporary subdural strip was used strictly for research purposes. Motor impairment was quantified for the DYST groups using the Burke Fahn Marsden Dystonia Rating Scale movement score15 or, for the PD group while not receiving medications, part III of the Unified Parkinson’s Disease Rating Scale.16 Forty-eight patients were initially included in the study, but 12 were excluded for reasons listed below; therefore, results are reported for 36 patients. Data were collected from May 1, 2008, through April 1, 2015.
Surgical Process and ECOG Strip Localization
To record cortical ECOG potentials, a 6-contact subdural ECOG strip was placed on the surface of the brain through the same burr hole used for the DBS implantation.4,5,10,13 The intended target location was the arm area of the M1, 3 cm from the mid-line and slightly medial to the hand knob. Recordings were performed at least 12 hours after stopping all antiparkinsonian and antidystonic medication therapies and at least 30 minutes after stopping propofol therapy. Data were collected before the DBS electrode implantation except for STN stimulation-related data. The DBS electrodes were placed in the STN as previously described.17
The ECOG contact locations were confirmed anatomically using lateral fluoroscopy or intraoperative computed tomography fused to the preoperative planning magnetic resonance imaging with standard surgical planning software (Framelink, version 5.1; Medtronic, Inc). Median nerve somatosensory-evoked potentials (SSEPs) were recorded, and reversal of the first negative component of the cortical SSEP (N20) waveform indicated the M1 location.10 We found high concordance between anatomical and physiologic determinations of contact position with respect to the central sulcus (CS) (Figure 1A), but in case of discrepancy (10 patients), the SSEP location was used. Patients whose SSEPs did not show clear N20 reversal and who did not have computed tomography–confirmed ECOG strip location within 30 ± 5 mm from the mid-line were excluded from analysis (5 patients with dystonia and 3 with PD).
Figure 1. Study Methods.
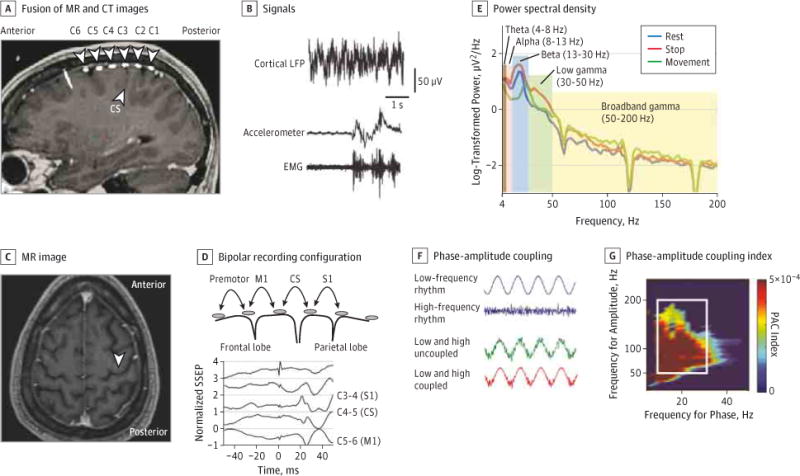
A, Images show placement of subdural 6-contact strip with 1-cm spacing between contacts, spanning the central sulcus (CS). B, Example of the electrocorticography (ECOG) signal, wrist accelerometer, and biceps electromyographic (EMG) signals. C, Placement of the ECOG strip is targeted approximately 3 cm from the midline, medial to the hand knob (unlabeled white arrowhead). D, Median nerve somatosensory evoked potential (SSEP) shows reversal of the N20 potential at the electrode contact pair spanning the primary motor cortex (C5–C6). E, Example of power spectral density from 1 patient obtained by transforming the cortical ECOG signal into the frequency domain during rest and the movement and stop phases of the elbow flexion-extension task. F, Interaction between low- and high-frequency rhythms in a schematic representation of phase-amplitude coupling (PAC). G, Each color data point is the phase-amplitude coupling index between the phase of the low-frequency band defined on the x-axis and the amplitude of the high-frequency band defined on the y-axis, extracted from the same ECOG potential. Mean beta-broadband gamma PAC was calculated by averaging the indices within the white box. CT indicates computed tomographic; LFP, local field potential; M1, primary motor cortex; MR, magnetic resonance; and S1, primary sensory cortex.
Signal Recordings
The ECOG potentials were recorded using an intraoperative electrophysiology recording system (Guideline 4000 [FHC] or MicroGuide Pro [Alpha Omega]). Recordings were obtained from 5 contacts differentially referenced to the most anterior sixth contact, with a scalp needle electrode as ground. Signals were band-pass filtered at 1 to 500 Hz, amplified, and sampled at a minimum 1000 Hz (Guideline 4000) or 3000 Hz (MicroGuide Pro), with additional notch filtering to remove power line artifacts. The Guideline 4000 system had slight attenuation up to 20 Hz owing to the slow rolloff characteristics of an intrinsic 1-Hz high-pass filter, which was compensated for using an empirically determined correction factor.10 Intraoperative STN stimulation was performed through the DBS lead (model 3389; Medtronic, Inc) using an analog neurostimulator (model 3625; Medtronic, Inc) and a bipolar configuration (contact 0 or 1 was negative; contact 2 or 3 was positive; amplitude, 4 V; pulse width, 60 microseconds; and frequency, 180–200 Hz).
Behavioral Tasks
The ECOG potentials were recorded in 2 behavioral conditions. During rest, patients were instructed to relax with their eyes open for at least 30 seconds. During the movement task, patients performed flexion-extension of the elbow (3- to 5-second movement phase and 3- to 5-second stop phase, repeated 5 times) with slow deliberate movements to reduce the effect of velocity as a confounding variable. All patients underwent testing at rest, and all but 3 patients with dystonia and 5 patients with PD underwent the movement task. Muscle activity was recorded using surface electromyography from the contralateral anterior deltoid, biceps brachii, extensor carpi radialis (band-pass filtered at 20–1000 Hz, amplified, and sampled at 1 kHz), and a wrist accelerometer.
Signal Processing
Data analysis was performed using custom scripts in MATLAB (version R2014a; MathWorks). All recordings were downsampled to 1 kHz and visually inspected for electrical noise or signal loss; those portions were excluded. Patients whose recordings were excessively contaminated by noise or whose M1 signal root mean square was less than 10 μV were excluded from analysis (2 patients with PD were excluded for noise; 1 patient with dystonia and 1 patient with PD were excluded for low signal amplitude). The electromyographic and accelerometer recordings were analyzed using a semiautomated threshold detection method with visual confirmation to determine movement start and stop times. Movement and stop segments had to be at least 3 seconds long to be included in the analysis.
The ECOG potentials were rereferenced in a bipolar montage and assigned to one of the following 4 anatomical locations: M1 or precentral gyrus, CS, postcentral gyrus (S1), and premotor (11 patients did not have premotor coverage; Figure 1). Data were analyzed in the frequency domain and log transformed for statistical comparison (Figure 1E). We calculated mean log-transformed power spectral density for multiple-frequency bands (4–8, 8–13, 13–30, 30–50, and 50–200 Hz) and compared them among the patient groups.
We calculated phase-amplitude coupling (PAC) between the phase of low frequencies (4–50 Hz) and the amplitude of high frequencies (50–200 Hz) from the same cortical signal. Specifically, the PAC index was computed for multiple narrow-frequency band combinations that yielded a colored plot (Figure 1G). To obtain a single beta-gamma interaction value for each plot (mean PAC), we calculated mean PAC indices from 13 to 30 Hz on the x-axis and from 50 to 200 Hz on the y-axis (details are given in the eMethods in the Supplement).
Statistical Analysis
Data were analyzed from November 1, 2014, through May 1, 2015. We performed 1-way analysis of variance on log power in each frequency band for each cortical contact pair. We used Bonferroni correction to account for multiple comparisons. For the mean PAC, nonparametric Kruskal-Wallis and rank sum tests were used to evaluate statistical differences among the patient groups.
Results
Patient Characteristics and Signal Amplitudes
We included 14 patients in the DYST group, 8 patients in the DYST-ARM group, and 14 patients in the PD group. Clinical characteristics are summarized in the Table, with further details given in the eTable in the Supplement. The mean (SD) resting M1 ECOG amplitude (root mean square) was comparable among the groups at 40.1 (22.8) μV for the DYST group, 39.4 (16.9) μV for the DYST-ARM group, and 44.6 (30.7) μV for the PD group.
Table.
Patient Groups
| Patient Group | No. of Patients | Age, Mean (SD), y | Sex, No. Female/Male | Side of ECOG Strip | Motor Score, Mean (SD)a | Arm Subscore, Mean (SD)b |
|---|---|---|---|---|---|---|
| DYST | 14 | 55.9 (10.8) | 8/6 | 4L/10R | 12.8 (6.2) | 0 |
| DYST-ARM | 8 | 50.4 (8.7) | 5/3 | 4L/4R | 29.5 (14.9) | 5.1 (2.9) |
| PD | 14 | 56.7 (10.0) | 3/11 | 7L/7R | 35.4 (17.7) | 12.4 (5.7) |
Abbreviations: DYST-ARM, isolated dystonia with arm symptoms contralateral to the ECOG side; DYST, isolated dystonia without arm symptoms contralateral to the ECOG side; ECOG, electrocorticography; L, left; PD, Parkinson disease; R, right.
Indicates the Motor score of the Burke Fahn Marsden (BFM) Dystonia Rating Scale (DYST groups) or the Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) while not receiving medication (PD group).
Indicates the arm subscore of the BFM (DYST groups) or items 20 through 25 of the UPDRS III (PD group) on the arm contralateral to the side of the ECOG recording.
PAC in the M1
At rest, PAC was increased in the M1 and premotor regions for all 3 patient groups compared with the primary sensory cortex (for M1 vs S1, P = .002, P = .004, and P = .02 for the DYST, DYST-ARM, and PD groups, respectively) (Figure 2 and eFigure 1 in the Supplement). The median values for the mean PAC indices at M1 were 1.8 × 10−4 (range, 0 to 80.7 × 10−4) for the DYST group, 3.9 × 10−4 (range, 0.01 × 10−4 to 18.4×10−4) for the DYST-ARM group, and 3.3 × 10−4 (range, 0 to 31.1 × 10−4) for the PD group. Differences in mean PAC between the groups did not reach the threshold of significance at any location. However, review of individual patient results (eFigure 2 in the Supplement) revealed that the DYST group included 5 patients without an elevated PAC (empirically defined as the first quartile of all mean PAC values, 1.2 × 10−4), whereas the DYST-ARM and PD groups included only 1 patient each without an elevated PAC (P = .008, χ2 test). The PAC is generally stable over time (eFigure 3 in the Supplement), but we did not explore this finding systematically in this study.
Figure 2. Phase-Amplitude Coupling (PAC) at Multiple Cortical Recording Locations for the 3 Patient Groups.
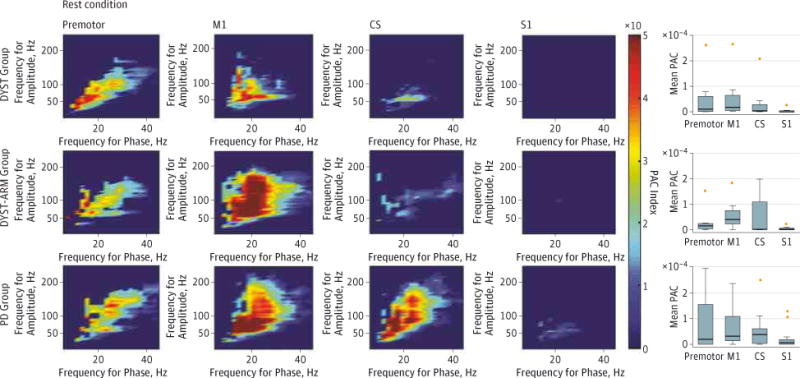
Patient groups include those with isolated dystonia with no arm symptoms contralateral to the electrocorticography (ECOG) side (DYST), with isolated dystonia with arm symptoms contralateral to the ECOG side (DYST-ARM), and with Parkinson disease (PD). Color indicates the value of the median PAC index for group data. The box plots show mean beta-broadband gamma PAC. For S1 vs M1, P = .002 for the DYST group, P = .004 for the DYST-ARM group, and P = .02 for the PD group (rank sum test for all comparisons). Horizontal lines represent medians of the individual patients’ mean PAC; boxes, the 25th and 75th percentiles; limit lines, the most extreme data points not considered outliers; and crosses, outliers (2 M1 outliers were not plotted, 80.7 × 10−4 for the DYST group and 31.1 × 10−4 for the PD group). Eleven patients did not have premotor contact. CS indicates central sulcus; M1, primary motor cortex; and S1, primary sensory cortex.
The mean PAC decreased approximately 80% during the movement phase (compared with the stop phase) for all 3 groups. This decrease was significant for the DYST group (P = .01) but was not significant for the PD group (P = .07). During the movement and stop phases of the movement task, we found no significant differences in mean PAC among the patient groups (eFigure 4 in the Supplement).
Effect of Acute DBS on PAC in Dystonia
In 4 patients with dystonia, we evaluated the effect of DBS on cortical activity. A previous study5 showed that, in PD, therapeutic STN DBS reduces the PAC without a consistent effect on beta or gamma power (an example appears in Figure 3). In the present study, STN DBS also reduced the PAC in 2 patients in the DYST group (by 100% and 30%) and 2 patients in the DYST-ARM group (by 89% and 61%) (Figure 3). We had an insufficient number of cases to determine the effects of STN DBS on beta and gamma power (eFigure 5 in the Supplement), but prior analyses of DBS in PD13 showed a reduction in PAC without a consistent change in power. Owing to intraoperative constraints, patients were not assessed for therapeutic benefit, but dystonia can demonstrate a very quick clinical response to STN DBS.18
Figure 3. Phase-Amplitude Coupling (PAC) in the Primary Motor Cortex in Deep Brain Stimulation of the Subthalamic Nucleus (STN DBS).
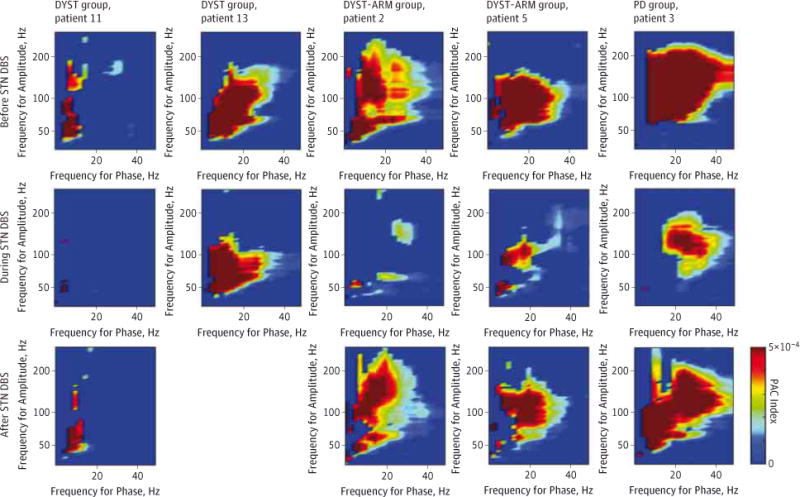
Color indicates PAC index for individual patients. Columns represent individual patients (all data were acquired after electrode implantation; poststimulation data for patient 13 in the DYST group were not obtained). DYST indicates isolated dystonia with no arm symptoms contralateral to the electrocorticography (ECOG) side; DYST-ARM, isolated dystonia with arm symptoms contralateral to the ECOG side; and PD, Parkinson disease.
Resting Gamma Power– and Movement-Related Beta Power Decrease
A previous study10 reported the M1 arm area ECOG spectral power characteristics in a series of patients with PD and dystonia, but insufficient data were available to separate the patients with dystonia into those with and without arm involvement. We therefore present similar analyses in this expanded data set to evaluate whether arm-area cortical function that is characteristic of dystonia represents an endophenotype vs a correlate of symptom expression. In the awake resting state, we found no difference in the mean log-transformed spectral theta, alpha, or beta power among the 3 groups in any contact. Low and broadband gamma power was higher in the DYST-ARM group than in the DYST group, and this difference was specific to the M1 and CS, both of which included an electrode contact over the precentral gyrus (Figure 4A). Gamma power in the DYST-ARM group was similar to that in the PD group.
Figure 4. Power Spectral Density (PSD) Analysis at Multiple Cortical Recording Locations at Rest and During Movement.
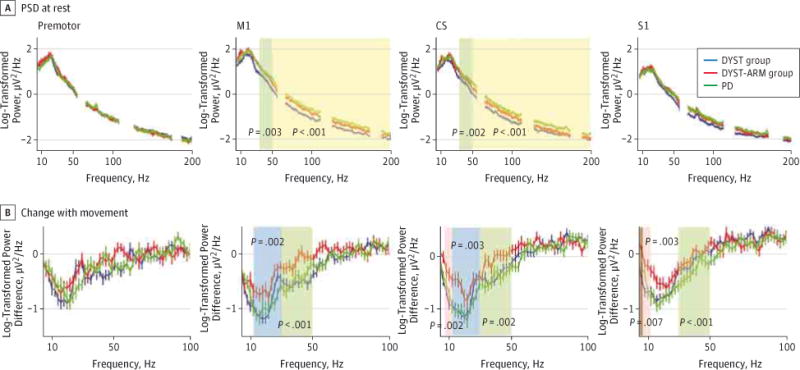
Change that occurs with movement is shown as the difference in log-transformed power spectra between the movement and stop phases of the movement task. Shaded areas are statistically different by 1-way analysis of variance (post hoc comparisons are described in text); the significant P value accounting for multiple comparisons is less than .003. We found no significant difference in broadband gamma modulation between groups. Eight patients did not have movement data. CS indicates central sulcus; DYST, isolated dystonia with no arm symptoms contralateral to the electrocorticography (ECOG) side; DYST-ARM, isolated dystonia with arm symptoms contralateral to the ECOG side; M1, primary motor cortex; PD, Parkinson disease; and S1, primary sensory cortex.
We analyzed a total of 131 movement epochs (mean, 4.8, 4.7, and 4.6 per patient for the DYST, DYST-ARM, and PD groups, respectively). Movement-related alpha, beta, and low gamma power desynchronization (decrease) was reduced in the DYST-ARM group compared with the DYST and PD groups in the M1 and CS (Figure 4B). In the premotor region, all patient groups were comparable. Coherence between the M1 and S1 did not differ among the groups in any frequency band, but peak coherence frequency increased with movement in all 3 groups from middle to high beta power (eFigure 6 in the Supplement). In summary, elevated cortical resting-state gamma power and reduced movement-related alpha-beta power desynchronization are present in dystonia only when the contralateral arm is symptomatic and only in contacts covering the M1 area. Elevated M1 resting gamma power appears to be a feature common to dystonia and PD.
Discussion
We analyzed intraoperative ECOG of the arm region of the sensorimotor cortex in patients with dystonia and PD who underwent DBS implantation surgery in the awake state. We found that M1 PAC is prominent in dystonia and PD, is more likely to occur in patients with generalized dystonia than in those with focal or segmental dystonia without arm involvement, and is reversibly decreased by acute STN DBS. We also identified several features of the arm-area ECOG power spectrum that distinguish patients with and without arm symptoms in the population with dystonia, including resting-state broadband gamma power and the magnitude of the movement-related decrease of beta power.
Elevated Cortical Synchronization in Dystonia and PD
Phase-amplitude coupling is a physiologic phenomenon important for information processing between and within cortical regions and is dynamically modulated during task performance.19 Motor cortex PAC can be detected in interictal recordings from patients with epilepsy undergoing long-term monitoring, suggesting a role in normal motor function.20,21 Previous studies5,13 showed that M1 PAC is exaggerated in patients with PD compared with patients without a movement disorder (epilepsy cohort) and is reduced by acute therapeutic DBS. These studies proposed that elevated cortical PAC is related to the expression of parkinsonian motor signs. Herein, we extend this model to isolated dystonia, offering an explanation for the fact that identical stimulation paradigms ameliorate both disorders. Because broadband gamma amplitude is a surrogate measure of population spiking, we interpret elevated coupling between the beta phase and broadband gamma amplitude as a manifestation of excessive synchronization of population spiking to the motor beta rhythm, a phenomenon also observed in the basal ganglia from single-unit and local field potential recording studies in PD.1–3
Similarities in the pathophysiologic signatures of PD and dystonia are not surprising given the phenotypic overlap in motor symptoms observed clinically. Both disorders are characterized by slowness of movement (although bradykinesia is much more pronounced in PD), with a tendency for development of dystonia in PD (as a presenting sign or later as part of the clinical spectrum of motor fluctuations), and both disorders can coexist in rare dystonia-parkinsonism syndromes.22,23 Furthermore, dopaminergic deficiency can result in PD or dystonia (consider dopa-responsive dystonia and neuroleptic-induced dystonia), and striatal dopaminergic signaling pathways, fundamental to the pathophysiologic features of PD, have been strongly implicated in the development of dystonia.24 Although both disorders involve dysfunction in the basal gangliothalamocortical loop, additional undefined perturbations may bias the system toward a more parkinsonian or more dystonic phenotype.
Cortical Function in Dystonia
We differentiated dystonia based on the presence or absence of arm symptoms in the arm contralateral to cortical recordings to address whether electrophysiologic findings are endophenotypic (present in the brain of patients with dystonia independent of symptom expression) or if they correspond to symptom expression in the homologous body part. Prominent motor cortex PAC was observed in both dystonia cohorts, but it tended to be lower in the DYST group. A previous study5 reported low PAC in patients with craniocervical disease; however, in the present enlarged cohort, we detected several patients without arm symptoms who had increased PAC, in some cases of similar magnitude as PAC in PD and dystonia with arm symptoms. Elevated PAC may therefore be an endophenotype of the disease similar to excessive motor cortex plasticity shown in studies of transcranial magnetic stimulation.25 Alternatively, elevated PAC may have been caused by abnormalities in the neck region of the cortex (medial to the arm region), or some patients without overt arm dystonia may have had subclinical arm symptoms. For example, 1 patient in the DYST group had strong M1 PAC and reported that DBS resolved his purported arthritic hand pain and improved his handwriting, which suggests the presence of subclinical arm symptoms before surgery. This result highlights the difficulty of accurate symptom identification based only on visual observation.
Movement-related desynchronization of alpha-beta activity is a normal physiologic mechanism of motor function26–28 and was previously shown to be reduced in dystonia.7,10,29,30 Our results suggest that impaired desynchronization relates specifically to symptomatic expression. Beta activity in the motor system has been proposed to function in maintaining the existing motor state, requiring reduction to accomplish fluid movement.31,32 An inability to reduce beta activity at the onset of movement could thus produce action dystonia by inappropriately maintaining previous postures during the attempted movement.
Gamma Band Activity as a Measure of Cortical Activation State
In ECOG recordings, broadband gamma band power (approximately 50–200 Hz) tracks local cortical function by increasing in amplitude in a highly topographic, task-specific manner when the relevant functional region is engaged.27 Gamma power is positively correlated with the functional magnetic resonance imaging blood oxygenation–level dependent signal.33 We may therefore interpret the finding of elevated gamma power in PD and dystonia with arm symptoms as evidence of increased resting-state activation. Consistent with this finding, resting-state cortical metabolism in the M1 area has been found to be elevated in positron emission tomography imaging in PD.14 In isolated dystonia, the results of functional imaging studies on resting-state metabolic activity in the M1 area are not consistent,34 but our findings suggest further pathophysiologic overlap between PD and isolated dystonia with respect to resting-state activation.
Limitations
Lack of a healthy control group, a difficulty inherent to invasive brain recordings in humans, precludes definitive labeling of a physiologic variant as abnormal. Previous ECOG work5 showed that patients without a movement disorder (eg, those with epilepsy undergoing interictal monitoring) have a lower PAC than patients with PD, so our finding of a similarly elevated PAC in dystonia is likely related to pathophysiologic mechanisms, but further studies are needed to confirm this finding in different cohorts. The range of clinical severity scores within each patient group was relatively narrow, and we did not find a correlation between clinical severity and any of the physiologic features that distinguished disease groups. Medication effects may not have fully washed out after overnight withdrawal and may have affected cortical activity, specifically in terms of modulating beta power. However, both dystonia groups received similar levels of benzodiazepines, so this effect is unlikely to be responsible for the observed differences.
Conclusions
Dystonia and PD are both disorders of high synchronization in the M1 area as assessed by coupling between broadband gamma amplitude and the phase of the motor beta rhythm; in both cases, this measure of neuronal synchronization is reduced by therapeutic DBS. This finding may account for the coexistence of dystonia and parkinsonism in several clinical syndromes and for the similarity in stimulation targets and stimulation settings that alleviate isolated dystonia and PD.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by a research training fellowship from the American Brain Foundation, a clinical fellowship from the Dystonia Medical Research Foundation, a grant from the Bachmann-Strauss Dystonia & Parkinson Foundation, and grant R01-NS069779 from the National Institutes of Health.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplemental content at jamaneurology.com
Author Contributions: Drs Miocinovic and de Hemptinne had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Miocinovic, de Hemptinne, Starr.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Miocinovic.
Critical revision of the manuscript for important intellectual content: de Hemptinne, Qasim, Ostrem, Starr.
Statistical analysis: Miocinovic, de Hemptinne. Obtained funding: de Hemptinne, Starr.
Administrative, technical, or material support: de Hemptinne, Qasim, Ostrem.
Study supervision: Ostrem, Starr.
Conflict of Interest Disclosures: None reported.
Additional Contributions: Nicole Swann, PhD, Department of Neurosurgery, University of California, San Francisco (UCSF) provided discussion and critical review of the manuscript. Nathan Ziman, BS, Department of Neurology, UCSF, helped with clinical data retrieval. Neither contributor received any compensation for their contributions.
References
- 1.Moran A, Bergman H, Israel Z, Bar-Gad I. Subthalamic nucleus functional organization revealed by parkinsonian neuronal oscillations and synchrony. Brain. 2008;131(pt 12):3395–3409. doi: 10.1093/brain/awn270. [DOI] [PubMed] [Google Scholar]
- 2.Kühn AA, Brücke C, Schneider GH, et al. Increased beta activity in dystonia patients after drug-induced dopamine deficiency. Exp Neurol. 2008;214(1):140–143. doi: 10.1016/j.expneurol.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger M, Hutchison WD, Dostrovsky JO. Pathological subthalamic nucleus oscillations in PD: can they be the cause of bradykinesia and akinesia? Exp Neurol. 2009;219(1):58–61. doi: 10.1016/j.expneurol.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Shimamoto SA, Ryapolova-Webb ES, Ostrem JL, Galifianakis NB, Miller KJ, Starr PA. Subthalamic nucleus neurons are synchronized to primary motor cortex local field potentials in Parkinson’s disease. J Neurosci. 2013;33(17):7220–7233. doi: 10.1523/JNEUROSCI.4676-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Hemptinne C, Ryapolova-Webb ES, Air EL, et al. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110(12):4780–4785. doi: 10.1073/pnas.1214546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CC, Kühn AA, Trottenberg T, Kupsch A, Schneider GH, Brown P. Neuronal activity in globus pallidus interna can be synchronized to local field potential activity over 3−12 Hz in patients with dystonia. Exp Neurol. 2006;202(2):480–486. doi: 10.1016/j.expneurol.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Wang S, Yianni J, et al. The sensory and motor representation of synchronized oscillations in the globus pallidus in patients with primary dystonia. Brain. 2008;131(pt 6):1562–1573. doi: 10.1093/brain/awn083. [DOI] [PubMed] [Google Scholar]
- 8.Mills KA, Starr PA, Ostrem JL. Neuromodulation for dystonia: target and patient selection. Neurosurg Clin N Am. 2014;25(1):59–75. doi: 10.1016/j.nec.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Vitek JL, Delong MR, Starr PA, Hariz MI, Metman LV. Intraoperative neurophysiology in DBS for dystonia. Mov Disord. 2011;26(suppl 1):S31–S36. doi: 10.1002/mds.23619. [DOI] [PubMed] [Google Scholar]
- 10.Crowell AL, Ryapolova-Webb ES, Ostrem JL, et al. Oscillations in sensorimotor cortex in movement disorders: an electrocorticography study. Brain. 2012;135(pt 2):615–630. doi: 10.1093/brain/awr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29(43):13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller KJ, Sorensen LB, Ojemann JG, den Nijs M. Power-law scaling in the brain surface electric potential. PLoS Comput Biol. 2009;5(12):e1000609. doi: 10.1371/journal.pcbi.1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Hemptinne C, Swann NC, Ostrem JL, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci. 2015;18(5):779–786. doi: 10.1038/nn.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko JH, Mure H, Tang CC, et al. Parkinson’s disease: increased motor network activity in the absence of movement. J Neurosci. 2013;33(10):4540–4549. doi: 10.1523/JNEUROSCI.5024-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35(1):73–77. doi: 10.1212/wnl.35.1.73. [DOI] [PubMed] [Google Scholar]
- 16.Fahn S, Elton RL. UPDRS program members. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinson’s Disease. Vol. 2. Florham Park, NJ: Macmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- 17.Starr PA, Christine CW, Theodosopoulos PV, et al. Implantation of deep brain stimulators into the subthalamic nucleus: technical approach and magnetic resonance imaging–verified lead locations. J Neurosurg. 2002;97(2):370–387. doi: 10.3171/jns.2002.97.2.0370. [DOI] [PubMed] [Google Scholar]
- 18.Ostrem JL, Racine CA, Glass GA, et al. Subthalamic nucleus deep brain stimulation in primary cervical dystonia. Neurology. 2011;76(10):870–878. doi: 10.1212/WNL.0b013e31820f2e4f. [DOI] [PubMed] [Google Scholar]
- 19.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14(11):506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller KJ, Hermes D, Honey CJ, et al. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Comput Biol. 2012;8(9):e1002655. doi: 10.1371/journal.pcbi.1002655.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanagisawa T, Yamashita O, Hirata M, et al. Regulation of motor representation by phase-amplitude coupling in the sensorimotor cortex. J Neurosci. 2012;32(44):15467–15475. doi: 10.1523/JNEUROSCI.2929-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121(pt 7):1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- 23.Balint B, Bhatia KP. Isolated and combined dystonia syndromes: an update on new genes and their phenotypes. Eur J Neurol. 2015;22(4):610–617. doi: 10.1111/ene.12650. [DOI] [PubMed] [Google Scholar]
- 24.Goodchild RE, Grundmann K, Pisani A. New genetic insights highlight “old” ideas on motor dysfunction in dystonia. Trends Neurosci. 2013;36(12):717–725. doi: 10.1016/j.tins.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Quartarone A, Morgante F, Sant’angelo A, et al. Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry. 2008;79(9):985–990. doi: 10.1136/jnnp.2007.121632. [DOI] [PubMed] [Google Scholar]
- 26.Crone NE, Miglioretti DL, Gordon B, et al. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis, I: alpha and beta event-related desynchronization. Brain. 1998;121(pt 12):2271–2299. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- 27.Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis, II: event-related synchronization in the gamma band. Brain. 1998;121(pt 12):2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- 28.Pfurtscheller G. Central beta rhythm during sensorimotor activities in man. Electroencephalogr Clin Neurophysiol. 1981;51(3):253–264. doi: 10.1016/0013-4694(81)90139-5. [DOI] [PubMed] [Google Scholar]
- 29.Toro C, Deuschl G, Hallett M. Movement-related electroencephalographic desynchronization in patients with hand cramps: evidence for motor cortical involvement in focal dystonia. Ann Neurol. 2000;47(4):456–461. [PubMed] [Google Scholar]
- 30.Kristeva R, Chakarov V, Losch F, Hummel S, Popa T, Schulte-Mönting J. Electroencephalographic spectral power in writer’s cramp patients: evidence for motor cortex malfunctioning during the cramp. Neuroimage. 2005;27(3):706–714. doi: 10.1016/j.neuroimage.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Gilbertson T, Lalo E, Doyle L, Di Lazzaro V, Cioni B, Brown P. Existing motor state is favored at the expense of new movement during 13–35 Hz oscillatory synchrony in the human corticospinal system. J Neurosci. 2005;25(34):7771–7779. doi: 10.1523/JNEUROSCI.1762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engel AK, Fries P. Beta-band oscillations: signalling the status quo? Curr Opin Neurobiol. 2010;20(2):156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 34.Poston KL, Eidelberg D. Functional brain networks and abnormal connectivity in the movement disorders. Neuroimage. 2012;62(4):2261–2270. doi: 10.1016/j.neuroimage.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


