Abstract
Objective
To test whether a relatively low single dose or week-long dosing of dietary inorganic nitrate can improve exercise tolerance in patients with Heart Failure with preserved ejection fraction (HFPEF).
Background
Exercise intolerance is the primary manifestation of HFPEF and is largely due to non-cardiac factors that reduce oxygen delivery to active skeletal muscles. A recent study showed improved exercise capacity in patients with HFPEF after a single, acute dose of beetroot juice (BRJ, 12.9 mmol inorganic nitrate) while another recent study showed neutral and negative effects of an organic nitrate.
Methods
Twenty HFPEF patients (age: 69 ± 7 years) were enrolled in an initial cross-over design comparing a single, acute dose of BRJ (6.1 mmol nitrate) to a nitrate-depleted, placebo BRJ. A second, one week of daily dosing, phase employed an all-treated design in which patients consumed BRJ for an average of 7 days. The primary outcome of the study was submaximal aerobic endurance, measured as cycling time to exhaustion at 75% of measured maximal power output.
Results
No adverse events were associated with the intervention. Submaximal aerobic endurance improved 24% after one week of daily BRJ dosing (p =0.02), but was not affected by the single, acute dose of the BRJ compared to placebo. Consumption of BRJ significantly reduced resting systolic blood pressure and increased plasma nitrate and nitrite in both dosing schemes.
Conclusions
One week of daily dosing with BRJ (6.1 mmol inorganic nitrate) significantly improves submaximal aerobic endurance and blood pressure in elderly HFPEF patients.
Keywords: heart failure with preserved ejection fraction, nitric oxide, exercise, nitrite, nitrate
INTRODUCTION
Heart failure with preserved ejection fraction (HFPEF) is the most common form of HF, is nearly unique to the older population, particularly older women, and is increasing in prevalence (1,2). HFPEF has a distinct pathophysiology compared to heart failure with reduced ejection fraction and thus warrants distinct targeted treatment (3). Exercise intolerance is the primary clinical feature in chronic HFPEF and is a major determinant of these patients’ severely reduced quality of life (4). Endurance exercise training is currently the only proven therapy to improve aerobic capacity in older HFPEF patients (5–7). Medications tested to date, most of which have primarily targeted cardiac mechanisms, have been unsuccessful (8–10).
We have reported that in older HFPEF patients, non-cardiac factors contribute significantly to their exercise intolerance and are the major contributor to its improvement following endurance exercise training (7,11,12). Specifically, skeletal muscle abnormalities, including reduced capillary density, percent type I oxidative fibers, and mitochondrial mass and function coupled with impaired skeletal muscle perfusion may contribute significantly to reduced exercise tolerance in older HFPEF patients (5,13–15). Moreover, it has been suggested that impaired perfusion results are due, at least partially, to low availability of the vasodilator nitric oxide (NO) (16).
Emerging evidence suggests that dietary inorganic nitrate (NO3−) supplementation has beneficial effects on blood pressure control, vascular health, exercise capacity, and oxygen metabolism though targeted NO production (17–19). It is important to discern inorganic nitrate from organic nitrates such as nitroglycerine and isosorbide mononitrate. The latter was recently shown to decrease daily activity and not effect exercise capacity in patients with HFPEF (20). Both are thought to produce NO or one of its active congeners, but the pharmacokinetics of the two differ considerably. Organic nitrates rapidly release relatively large amounts of NO, while inorganic nitrate slowly produces NO and thereby produces more mild but sustained vasodilation (21,22). Perhaps most importantly, inorganic nitrate, through its conversion to inorganic nitrite, targets NO delivery to areas of low oxygen and low pH, such as occur in skeletal muscle during exercise (21–23).
Dietary NO3− is particularly abundant in beetroot juice (BRJ). Indeed, BRJ supplementation has been shown in multiple studies to improve exercise performance and oxygen metabolism in younger healthy individuals (24–31) and in older patients with peripheral arterial disease (32). Specifically, BRJ supplementation has been shown to increase time to exhaustion during high-intensity exercise and to reduce oxygen consumption (VO2) during submaximal exercise (i.e. reduce oxygen cost at a given submaximal work rate) (25,28,29,32).
In healthy older adults, BRJ supplementation improved VO2 kinetics but did not alter overall exercise performance or the oxygen cost of exercise (33), and not all studies in younger adults have shown a positive effect (34–36). In addition, some positive effects are seen after one week of daily dosing that are not seen after a single, acute dose (29,31). In a recent study in patients with HFPEF, a single, acute BRJ dose (12.9 mmoles) increased total work performed and cardiac output while decreasing systemic vascular resistance during a maximal exercise test, but did not affect exercise efficiency (as defined by total work/total oxygen consumed) compared to placebo (37). As the optimal BRJ supplementation strategy (single, acute versus loading over several days) remains uncertain,(29) we conducted a pilot study to assess the feasibility (recruitment, retention, safety) and the preliminary efficacy of either a smaller single, acute dose (6.1 mmol) or short-term (1 dose per day of 6.1 mmol for 6–8 days) BRJ supplementation in older HFPEF patients. We hypothesized that one week of daily dosing would be more efficacious than a single dose in improving submaximal aerobic endurance.
METHODS
Participants
As previously described in studies from our laboratory (4,6,11,12,38) and in accord with the ACCF/AHA 2013 HF management guidelines (39), HFPEF was defined as symptoms and signs of HF according to the National Health and Nutrition Examination Survey HF clinical score of ≥3 and the criteria of Rich et al. (40,41), preserved resting left ventricular systolic function (ejection fraction ≥50%, and no segmental wall motion abnormalities), and no significant ischemic or valvular heart disease, pulmonary disease, anemia, or other disorder that could explain the patients’ symptoms (4,6,11). All patients were required to have significant exercise intolerance (peak VO2 ≤ 16.0 ml/kg/min for women and ≤ 20.0 ml/kg/min for men, a reduction of >25% compared to age-matched healthy controls in our laboratory). Further, patients could not be on prescription nitroglycerine, other nitrate preparations used for angina, phosphodiesterase type 5 inhibitors, or medication regimens to alter stomach pH (antacids, proton pump inhibitors, H2 antagonists). The protocol was approved by the institutional review board, and all participants provided written, informed consent.
BRJ and Placebo
The BRJ and placebo (70 ml; Beet It Sport Shot; James White Drinks, Ipswich, UK) were identical in appearance, taste, smell, and nutrient composition except that the NO3− was removed by the manufacturer to make the placebo juice. As measured in our lab, the BRJ contained 0.38 g (6.1 mmol) NO3− and the placebo contained 0.0003 g (4.8 mmole) NO3−. The person responsible for dispensing the juice was not involved in study testing or analysis.
Study Design
In total, participants completed 5 clinic visits over approximately a 4-week period (Supplementary Figure 1). We initially screened 252 patients from past study participant lists, electronic medical records, and community advertisements. Fifty seven appeared to initially qualify and were scheduled for a screening visit. Of those, 32 signed informed consent and completed the screening visit. Ultimately, 20 participants met all inclusion/exclusion criteria and were enrolled into the study. Following the initial screening visit and a second visit to refine the exercise testing parameters (as described below), participants were assigned using a double-blinded, randomized crossover design to receive BRJ and placebo. A single bottle of the assigned juice was consumed for the crossover visits (single, acute dosing), which were separated by a 3–7 day washout period. Following the last crossover visit, all participants consumed 1 bottle of the BRJ once per day for 6–8 days (one week of daily dosing) and returned for the final clinic visit. Importantly, participants were unaware whether they were consuming BRJ or placebo during the one week of daily dosing period. For all visits, the participant consumed the juice ~ 45 minutes before arriving at the clinic.
Nitrite and Nitrate
After 10 minutes of supine rest (~ 1 hour after the participant consumed the juice), venous blood samples were drawn into 4 ml lithium heparin tubes and centrifuged at 4,000 rpm at 20°C for 3 minutes within 1 minute of collection. Plasma was transferred in 400 μl volumes to sterile 500 μl Sarstedt polypropylene microtubes containing no additives and frozen at −70°C for later analysis. Nitrite (NO2−) and nitrate were measured as described previously (42) using an ENO-20 nitric oxide analyzer (EICOM, San Diego, CA USA).
Exercise testing
As previously described (8,38), all exercise tests were performed with the participant in an upright position on an electronically braked cycle ergometer with the pedal rate at ~ 60 rpm. At the initial screening visit, a maximal, graded (10 W per minute) exercise test was performed to assess peak capacity (43). The maximal work rate was defined as the greatest work rate that could be maintained for ≥ 30 seconds. At all subsequent visits, a submaximal constant work rate exercise test at ~75% of maximal work rate was performed. During all tests, heart rate and rhythm was monitored continuously using an electrocardiogram and blood pressure were taken at rest (following 2 minutes of quiet breathing) and every two minutes during the test. Breath-by-breath gas exchange data (Medgraphics Ultima; Minneapolis, Minnesota) were measured continuously at rest and during exercise. All submaximal constant work rate tests were performed ~ 1.5–2 hours after the participant consumed the juice.
Submaximal aerobic endurance
The submaximal constant work rate exercise test began with a 2-minute period of unloaded pedaling followed by an immediate increase to the specified submaximal work rate, which was maintained until volitional exhaustion. In addition, the test was terminated if the pedal rate fell below 50 rpm for ~ 10 seconds. The submaximal work rate was initially prescribed at 75% of maximal power output. At the second visit (pre-randomization), the adequacy of this work rate was confirmed. If the participant was not able to maintain the work rate for ≥ 4 minutes, the work rate was decreased by ~ 10 W for subsequent tests. Likewise, if the participant was able to maintain the work rate for ≥ 10 minutes, the work rate was increased by ~ 10 W for subsequent tests. Importantly, the submaximal work rate was the same for all post-randomization efficacy visits.
Study Outcomes
Feasibility was assessed by examining recruitment yield, retention and adherence rates, and safety events. The predetermined primary efficacy outcome was aerobic endurance defined as the exercise time to volitional exhaustion during submaximal cycling at 75% of maximal power output.
Secondary efficacy outcomes included plasma NO3− and NO2− levels, VO2, and blood pressure at rest, after unloaded cycling, at 2 and 4 minutes, and at volitional exhaustion, and heart rate and other gas exchange measures at volitional exhaustion. Values for VO2 were averaged from the entire resting period and from the final two 15-second averaged values prior to the specified time-point during exercise.
Statistical Methods
Using preliminary data from another study at our institution, the study was designed to have 80% power to detect a 25% difference in the primary efficacy outcome, submaximal exercise time. To determine the single, acute dosing effect of BRJ using the randomized crossover design, comparison of outcome measures were made by repeated measures analysis of variance, controlling for visit. To determine the one week of daily dosing effect of BRJ, comparison of outcome measures were made by a paired t-test, with the placebo visit during the cross-over design as the baseline value and the visit following one week of daily dosing as the final value. A two-tailed p-value of <0.05 was required for significance.
RESULTS
Participants
Twenty older HFPEF patients (age 69 ± 7 years) with typical characteristics of HFPEF were enrolled into the study (Table 1). All participants were New York Heart Association class II (70%) or class III (30%) and 100% had a history of hypertension.
Table 1.
Participant Characteristics
| Characteristic | HFPEF (N=20) |
|---|---|
| Age (years) | 69.0 ± 6.8 |
| Women | 17 (85%) |
| White | 12 (60%) |
| Body Weight (kg) | 89 ± 16 |
| BMI (kg/m2) | 32.9 ± 5.6 |
| NYHA class | |
| II | 14 (70%) |
| III | 6 (30%) |
| Diastolic Function | |
| Normal | 3 (15%) |
| Impaired Relaxation | 17 (85%) |
| Pseudonormal | 0 (0%) |
| Restrictive | 0 (0%) |
| e′ (cm/s) | 6.5 ± 2.0 |
| E/e′ ratio | 13.2 ± 6.6 |
| BNP* | 21 ± 19 |
| Diabetes mellitus | 7 (35%) |
| Hx hypertension | 20 (100%) |
| Systolic BP (mmHg) | 145 ± 15 |
| Diastolic BP (mmHg) | 70 ± 10 |
| Current medication | |
| ACE-inhibitors | 7 (35%) |
| Diuretics (all) | 13 (65%) |
| Loop diuretics | 3 (15%) |
| Beta-blockers | 5 (25%) |
| Calcium channel blockers | 7 (35%) |
| ARB’s | 6 (30%) |
| Peak VO2 (ml/kg/min) | 12.0 ± 2.1 |
| Peak workload (watts) | 58 ± 21 |
Data presented as mean ± SD or number (%).
Abbreviations- BMI: body mass index; NYHA: New York Heart Association; e′; mitral annulus velocity; E; early mitral velocity; BNP, B-type natriuretic peptide; Hx; history; BP: blood pressure; ACE; angiotensin converting enzyme; ARB; angiotensin receptor blocker
Adherence, Retention, Safety
Adherence to the supplementation, as measured by returned bottle count, was 100%. One participant did not complete the study due to personal time conflicts; therefore 19 participants completed the study. In addition, 1 participant was excluded from the analysis due to knee pain that resulted in failure to complete the final exercise test. There were no adverse events related to the supplementation.
Plasma NO3− and NO2−
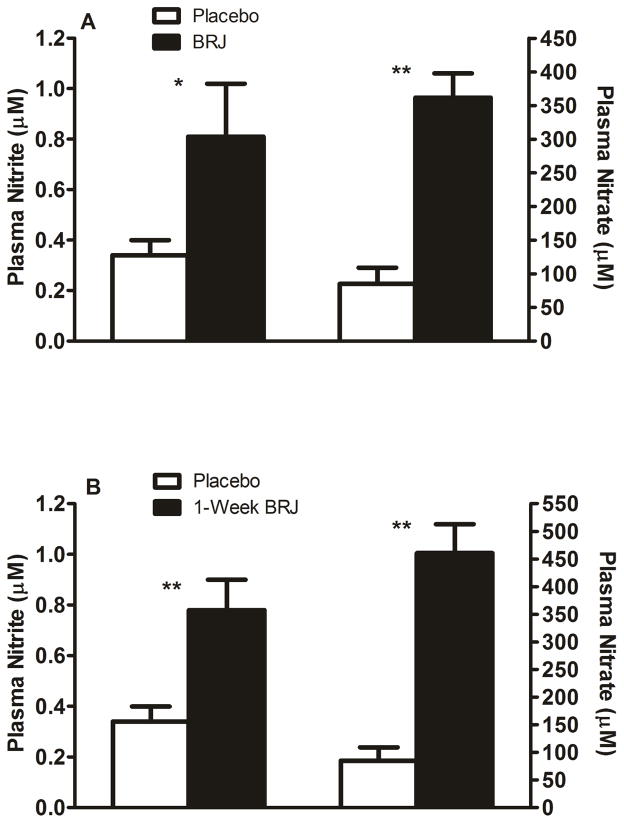
With single, acute dosing, plasma NO3− (362 ± 158 vs. 85 ± 104 μM; p<0.001) and NO2− (0.81 ± 0.91 vs. 0.34 ± 0.26 μM; p=0.01) were significantly increased. Similarly, with one week of daily dosing, plasma NO3− (461 ± 229 vs. 85 ± 104 μM; p<0.001) and NO2− (0.78 ± 0.52 vs. 0.34 ± 0.26 μM; p<0.001) were significantly increased (Figure 1).
Figure 1. Plasma nitrite and nitrate.
Mean ± SE of plasma nitrite and plasma nitrate after a single, acute dose (panel A) and after 1-week of daily dosing (panel B). White columns represent placebo and black columns represent BRJ. **=p<0.001; *=p<0.05.
Submaximal aerobic endurance
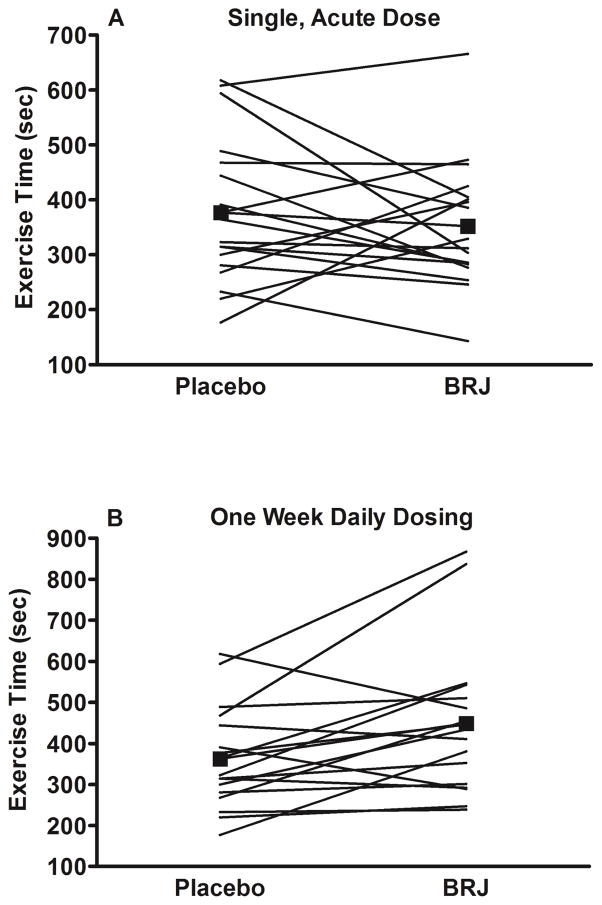
With single, acute dosing, there was no difference in the primary outcome of submaximal aerobic endurance (BRJ: 352 ± 116 vs. placebo: 377 ± 134 sec.; p=0.47), however with one week of daily dosing, there was a significant increase (449 ± 180 vs. 363 ± 125 sec.; 24% increase; p=0.02) (Figure 2 and Tables 2 and 3).
Figure 2. Exercise Time.
Submaximal exercise time (sec.) with placebo and single, acute dose of BRJ (panel A) and with placebo and 1-week of daily dosing of BRJ (panel B). Black squares represent mean values. Circles and lines represent individual values.
Table 2.
Acute Effect of Beetroot Juice versus Placebo
| Placebo | Beetroot Juice | P-value | |
|---|---|---|---|
| Exercise time at constant work rate, sec | 377 ± 134 | 352 ± 116 | 0.47 |
| At exhaustion: | |||
| VO2, ml/kg/min | 11.9 ± 2.2 | 11.8 ± 2.4 | 0.63 |
| VO2, ml/min | 1054 ± 315 | 1040 ± 312 | 0.43 |
| VCO2, ml/min | 1239 ± 418 | 1211 ± 383 | 0.28 |
| Respiratory exchange ratio | 1.17 ± 0.09 | 1.16 ± 0.08 | 0.69 |
| Heart rate, bpm | 120 ± 20 | 121 ± 21 | 0.69 |
| Systolic blood pressure, mmHg | 167 ± 16 | 163 ± 21 | 0.15 |
| Diastolic blood pressure, mmHg | 78 ± 10 | 78 ± 10 | 0.73 |
| Rate pressure product, bpm*mmHg | 20245 ± 4491 | 19997 ± 5009 | 0.66 |
Data is presented as mean ± SD. Mean constant work rate = 45 W. Exercise time excludes the 2-minute unloaded pedaling period.
Table 3.
Short term daily dosing effect- 1 dose placebo versus 1 week beetroot juice
| 1 Dose Placebo | 1 week Beetroot Juice | P-value | |
|---|---|---|---|
| Exercise time at constant work rate, sec | 363 ± 125 | 449 ± 180 | 0.02 |
| At exhaustion: | |||
| VO2, ml/kg/min | 12.0 ± 2.2 | 12.0 ± 2.6 | 0.79 |
| VO2, ml/min | 1065 ± 321 | 1069 ± 341 | 0.82 |
| VCO2, ml/min | 1259 ± 421 | 1239 ± 422 | 0.46 |
| Respiratory exchange ratio | 1.18 ± 0.08 | 1.16 ± 0.09 | 0.32 |
| Heart rate, bpm | 121 ± 21 | 122 ± 23 | 0.59 |
| Systolic blood pressure, mmHg | 166 ± 16 | 159 ± 17 | 0.054 |
| Diastolic blood pressure, mmHg | 77 ± 9 | 75 ± 6 | 0.38 |
| Rate pressure product, bpm*mmHg | 20187 ± 4622 | 19616 ± 5182 | 0.43 |
Data is presented as mean ± SD. Mean constant work rate = 45 W. Exercise time excludes the 2-minute unloaded pedaling period.
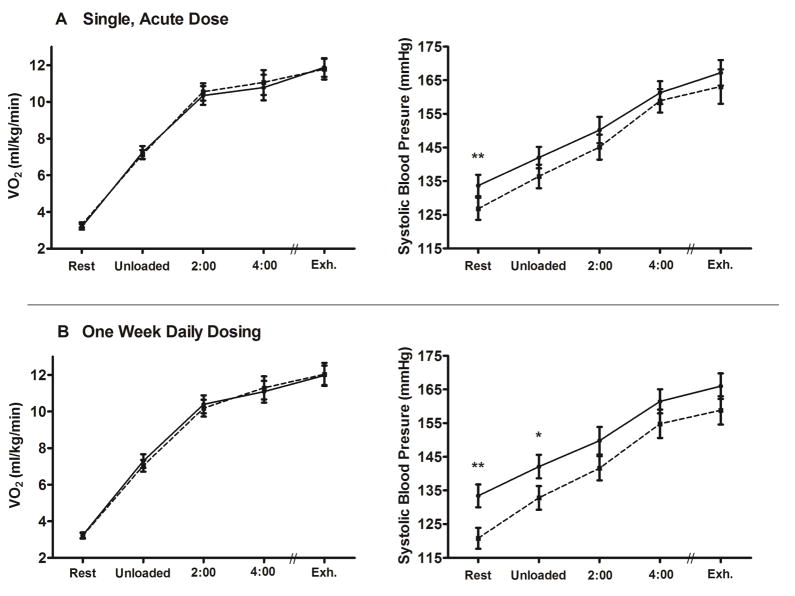
With both single, acute and one week of daily dosing, there were no differences in VO2 between BRJ vs placebo at rest or at any time-point measured during exercise, including at volitional exhaustion (Figure 3). Notably, mean respiratory exchange ratio at exhaustion was > 1.15 with all treatments; indicating that although a work rate of ~75% of maximal was used, a similar, exhaustive, severe-intensity level was reached at the end of the test. Finally, with both single, acute and one week of daily dosing, there were no differences in heart rate or any other gas exchange measure at volitional exhaustion (Tables 2 and 3).
Figure 3. Oxygen Consumption and Blood Pressure.
VO2 (ml/kg/min) and systolic blood pressure (mmHg) at rest, after unloaded cycling, after 2 and 4 minutes of cycling, and at volitional exhaustion with single, acute dosing (panel A) and with 1-week of daily dosing (panel B). The solid line represents placebo and the dotted line represents BRJ. **=p<0.001; *=p<0.05.
Blood Pressure
With single, acute dosing, resting systolic blood pressure was significantly reduced (127 ± 14 vs. 134 ± 14 mmHg; p=0.008) and there was a strong trend to reduced systolic blood pressure after unloaded cycling (p=0.06). Further, there were trends to reduced systolic blood pressure at 2 minutes and volitional exhaustion (p=0.15 for both, Figure 3). There were no differences in diastolic blood pressure or rate pressure product at any time-point.
With one week of daily dosing, systolic blood pressure was significantly reduced at rest (120 ± 13 vs. 134 ± 14 mmHg; p<0.001) and after unloaded cycling (p=0.03). There was a strong trend to reduced systolic blood pressure at volitional exhaustion (159 ± 17 vs. 166 ± 16 mmHg; p=0.054) and there were trends to reduced systolic blood pressure at 2 and 4 minutes (p=0.08 and 0.11, respectively); Figure 3. Further, there was a strong trend to reduced rate pressure product at rest (p=0.051), with no differences at any other time-point. There were no differences in diastolic blood pressure at any time-point.
DISCUSSION
The primary symptom in patients with chronic HFPEF, even when well compensated, is severe exercise intolerance; this symptom is associated with reduced quality of life (4). Only endurance exercise training has been shown to improve exercise capacity in these patients, as standard medications tested to date have proven ineffective (5–8,10). Increased NO bioavailability has been suggested to partially account for the efficacy of exercise training (16). Our study is the first to examine the safety and efficacy of one week of daily dosing with dietary nitrate in older patients with HFPEF. The major new findings of this study are that: 1) BRJ supplementation was feasible and safe, 2) one week of daily dosing improved submaximal aerobic endurance, and 3) both single, acute and one week of daily dosing reduced resting systolic blood pressure, tended to decrease exercise systolic blood pressure, and increased circulating plasma NO3− and NO2− levels. These results suggest that one week of daily BRJ supplementation could be a potential therapeutic option to improve submaximal aerobic endurance (as occurs during instrumental and recreational activities of daily living) with a reduction in blood pressure (and thus afterload) in the growing population of older HFPEF patients.
Compared to placebo, either a single, acute dose or one week of daily dosing of BRJ significantly increased plasma NO2− similarly, by 138% and 129% compared to placebo, respectively. The blood samples were collected ~ 1 hour after juice consumption and recent evidence suggests that in healthy older adults, plasma NO2− levels peak 2–3 hours after BRJ consumption.(44) Thus, the NO2− levels were likely even higher when the exercise test was performed, 1.5–2 hours after BRJ consumption. The increase in plasma NO2− is similar to other studies in younger and older adults without HF that have reported a significant increase in plasma NO2− following dietary NO3− supplementation (24,27,32,33).
One week of daily dosing with BRJ improved submaximal aerobic endurance; no significant effect was found for this outcome with a single, acute BRJ dose. However, a recent study by Zamani and colleagues observed an increase in total work performed after a single, acute dose of BRJ dose in HFPEF patients compared to placebo in a cross-over study (37). These investigators also observed increased cardiac output, decreased systemic vascular resistance and reduced aortic augmentation index due to BRJ compared to placebo, but no improvement in exercise efficiency (total work/total oxygen consumed) was observed (37), a finding confirmed in our study (Figure 3).
It is important to note that our study used a 6.1 mmol dose, whereas Zamani and colleagues administered a 12.9 mmol NO3− dose (37). A larger nitrate dose results in increased plasma nitrite (45) which is the essential factor in producing physiological changes. The dose we administered resulted in significantly higher plasma nitrite compared to placebo (Figure 1), but the response was also variable. Comparison of the two studies suggests that a higher nitrate dose may be more effective, at least for acute, single dose effects. The fact that we observed positive effects at the smaller dose we used with chronic but not with the acute dosing schedule is consistent with some previous observations (29,31). That we did not see an improvement in VO2 suggests that intrinsic mitochondrial function was probably not primarily improved. An increase in perfusion could be dependent on increasing functional capillary density that is achievable after a large single, acute nitrate dose, but needs chronic administration to be achieved with a smaller dose. This is consistent with the notion that microvasccular function may be important in oxygen delivery in HFPEF patients (46).
Taken together with the previous study on BRJ and patients with HFPEF (37), the overall data suggest that a single, acute higher dose of BRJ or smaller dose administered over one week can improve aerobic endurance in patients with HFPEF. The mechanism for this effect is likely due to targeted delivery of NO which decreases systemic vascular resistance. Ingested dietary NO3− is reduced to bioactive NO2− by bacteria found in the oral cavity; the NO2− is then taken up by the plasma from the digestive system and can be converted to nitric oxide (NO), particularly under hypoxic or acidic conditions (23), which can occur in HFPEF and during exercise. In the case of HFPEF, as previously discussed (37,47), improved oxygen cost during exercise is not observed, and increases in exercise capacity are likely due to lower systemic vascular resistance with increased cardiac output (observed in the study published by Zamani and colleagues) (37) and/or more effective shunting of blood flow resulting in increased perfusion of exercising muscles due to the actions of plasma nitrite. Variability in response may be largely due to individual oral nitrate to plasma nitrite conversion which could be improved at larger doses.
A recent study examined the effect of an organic nitrate, isosorbide mononitrate, on improving daily activity level, quality of life score, and 6-minute walk test in 110 patients with HFPEF (20). There were no significant effects of isosorbide mononitrate on quality of life or the 6-miute walk test and activity was actually less compared to placebo (20). Another study examined whether a phosphodiesterase inhibitor, which increases the efficacy of a given amount of NO by increasing levels of its downstream signaling agent cyclic guanosine monophosphate, can improve peak oxygen consumption, exercise capacity and clinical status in patients with HFPEF (48). This study also found no improvement in any of the test-outcomes compared to placebo (48). Differences in the pharmacokinetics and especially the ability of inorganic nitrite derived from inorganic nitrate to target delivery of NO to hypoxic areas (21–23), a feature organic nitrates and oral phosphodiesterae inhibitors lack, is most likely the main reason that positive effects have been seen with use of inorganic nitrate but not these other agents. This notion is also reinforced by a recent study where infused inorganic nitrite improved exercise hemodynamics (particularly pulmonary capillary wedge pressure, cardiac output reserve, and left ventricular stroke work) compared to placebo (49).
Limitations of this study include the small number of participants and lack of mechanistic measures including cardiac output and skeletal muscle perfusion. Another possible limitation would be a learning effect in exercise testing, but previous work in our lab showed no learning effect with maximal exercise testing (38). Further work, with larger sample size, is required to fully evaluate the benefits of oral inorganic nitrate on improving exercise tolerance in patients with HFPEF.
Our study found positive effects of week-long dietary inorganic nitrate intake using a lower dose of nitrate while the previous study by Zamani et al demonstrated positive effects of a single, acute larger dose (37). There are potential benefits to both chronic and acute dosing. For example, if a single, acute dose has an effect, a patient may consume BRJ (probably at a higher dose) a couple of hours before an anticipated activity that may be physically challenging. On the other hand, a patient may chronically consume BRJ (probably at a lower dose) to improve everyday activity and quality of life. However, both studies examining efficacy of inorganic nitrate in treating HFPEF had small sample sizes. Thus, a much larger study should be undertaken examining both acute (single dose) and chronic (over several weeks) dosing of BRJ at several NO3− levels.
In summary, our finding that submaximal aerobic endurance was increased at a lower blood pressure suggests that HFPEF patients could potentially perform submaximal exercise at a lower intensity and for longer periods which has implications for everyday activities. Given that a hallmark feature of HFPEF is exercise intolerance even while performing submaximal exercise, our findings may have important functional and therapeutic implications.
Supplementary Material
Clinical Perspective.
Reduced quality of life in patients with HFPEF is exacerbated by exercise intolerance. Our study suggests that targeted chronic delivery of NO through dietary consumption of oral inorganic nitrate could improve submaximal exercise tolerance. More, larger clinical trials are needed to determine whether dietary inorganic nitrate can truly help improve quality of life in patients with HFPEF.
Translational Outlook.
A large variety of clinical trials have recently been completed or are underway exploring the use of inorganic nitrite as a therapeutic for a variety of conditions including hypertension, pulmonary hypertension, myocardial infarction, COPD, PAD and (relevant to the current study) HFPEF. These clinical studies illustrate translation of basic science studies demonstrating how nitrite can be reduced to NO. Whereas some clinical studies employ inhaled or infused nitrite, others (such as this one) deliver nitrite through administration of oral nitrate. The efficacy of this method of administration, which is attractive due to its potential use of natural products and applicability to long-term dosing, needs further exploration.
Acknowledgments
Financial Support: This work was partially supported by NIH grants R01AG18915, R01AG045551, P30AG021332, HL058091, The Kermit Glenn Phillips II Chair in Cardiovascular Medicine, Wake Forest School of Medicine, and the Moritz Chair in Geriatrics in the College of Nursing and Health Innovation at the University of Texas at Arlington. It was also partially supported by the Translational Science Center of the Reynolda Campus of Wake Forest University.
We thank Thomas Becton for technical help in preparing the manuscript.
Abbreviations
- BRJ
beetroot juice
- HFPEF
heart failure with preserved ejection fraction
- NO
nitrite oxide
- NO2−
inorganic nitrite
- NO3−
inorganic nitrate
- VO2
oxygen consumption
Footnotes
Disclosures: Dr. Kim-Shapiro is listed as a co-inventor on a patent related to use of nitrite in cardiovascular conditions, and owns stock in and serves on the scientific advisory board for Beverage Operations LLC which has licensed Wake Forest University intellectual properties and thus has a financial interest in Beverage Operations LLC. Dr. Kitzman declares the following relationships: Consultant for Abbvie, GSK, Relypsa, Regeneron, Merck, Corvia Medical, and Actavis, grant funding from Novartis, and stock ownership in Gilead Sciences and Relypsa.
No other members of the writing group have conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients > or = 65 Years of Age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of Vasodilation in Heart Failure With Preserved or Reduced Ejection Fraction: Implications of Distinct Pathophysiologies on Response to Therapy. J Am Coll Cardiol. 2012;59:442–451. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 4.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 5.Haykowsky MJ, Kitzman DW. Exercise physiology in heart failure and preserved ejection fraction. Heart Failure Clin. 2014;10:445–452. doi: 10.1016/j.hfc.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitzman D, Brubaker P, Morgan T, Stewart K, Little W. Exercise training in older patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–592. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitzman DW, Hundley WG, Brubaker P, Stewart K, Little WC. A randomized, controlled, double-blinded trial of enalapril in older patients with heart failure and preserved ejection fraction; effects on exercise tolerance, and arterial distensibility. Circ Heart Fail. 2010;3:477–485. doi: 10.1161/CIRCHEARTFAILURE.109.898916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitzman D, Upadhya B. Heart failure with preserved ejection fraction: a heterogenous disorder with multifactorial pathophysiology. J Am Coll Cardiol. 2014;63:457–459. doi: 10.1016/j.jacc.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redfield M, Chen H, Borlaug B, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of Exercise Intolerance in Elderly Heart Failure Patients With Preserved Ejection Fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haykowsky M, Brubaker P, Morgan T, Kritchevsky S, Eggebeen J, Kitzman D. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68:968–975. doi: 10.1093/gerona/glt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitzman DW, Nicklas B, Kraus WE, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal Muscle Composition and Its Relation to Exercise Intolerance in Older Patients With Heart Failure and Preserved Ejection Fraction. Am J Cardiol. 2014;113:1211–1216. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirai DM, Musch TI, Poole DC. Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. Am J Physiol Heart Circ Physiol. 2015 doi: 10.1152/ajpheart.00469.2015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapil V, Weitzberg E, Lundberg JO, Ahluwalia A. Clinical evidence demonstrating the utility of inorganic nitrate in cardiovascular health. Nitric Oxide. 2014;38:45–57. doi: 10.1016/j.niox.2014.03.162. [DOI] [PubMed] [Google Scholar]
- 18.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 19.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, Phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redfield MM, Anstrom KJ, Levine JA, et al. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. New England Journal of Medicine. 2015 doi: 10.1056/NEJMoa1510774. In Press:null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundberg JO, Carlstrom M, Larsen FJ, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovascular Research. 2011;89:525–532. doi: 10.1093/cvr/cvq325. [DOI] [PubMed] [Google Scholar]
- 22.Omar SA, Artime E, Webb AJ. A comparison of organic and inorganic nitrates/nitrites. Nitric Oxide-Biol Chem. 2012;26:229–240. doi: 10.1016/j.niox.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 24.Bailey SJ, Fulford J, Vanhatalo A, et al. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol. 2010;109:135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 25.Lansley K, Winyard P, Fulford J, et al. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol. 2011;110:591–600. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- 26.Lansley KE, Winyard PG, Bailey SJ, et al. Acute dietary nitrate supplementation improves cycling time trial performance. Med Sci Sports Exerc. 2011:1125–1131. doi: 10.1249/MSS.0b013e31821597b4. [DOI] [PubMed] [Google Scholar]
- 27.Vanhatalo A, Fulford J, Bailey SJ, Blackwell JR, Winyard PG, Jones AM. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. The Journal of Physiology. 2011;589:5517–5528. doi: 10.1113/jphysiol.2011.216341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey SJ, Winyard P, Vanhatalo A, et al. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 29.Hoon MW, Johnson NA, Chapman PG, Burke LM. The effect of nitrate supplementation on exercise performance in healthy individuals: a systematic review and meta-analysis. Int J Sport Nutr Exerc Metab. 2014;23:522–532. doi: 10.1123/ijsnem.23.5.522. [DOI] [PubMed] [Google Scholar]
- 30.Cermak N, Gibala MJ, van Loon LJC. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int J Sport Nutr Exer Metab. 2012;22:64–71. doi: 10.1123/ijsnem.22.1.64. [DOI] [PubMed] [Google Scholar]
- 31.Vanhatalo A, Bailey SJ, Blackwell JR, et al. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2010;299:R1121–R1131. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 32.Kenjale AA, Ham KL, Stabler T, et al. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol. 2011;110:1582–1591. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly J, Fulford J, Vanhatalo A, et al. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am J Physiol Regul Integr Comp Physiol. 2013;304:R73–R83. doi: 10.1152/ajpregu.00406.2012. [DOI] [PubMed] [Google Scholar]
- 34.Cermak N, Res P, Stinkens R, Lundberg JO, Gibala MJ, van Loon LJC. No improvement in endurance performance following a single dose of beetroot juice. Int J Sport Nutr Exer Metab. 2012 doi: 10.1123/ijsnem.22.6.470. In Press. [DOI] [PubMed] [Google Scholar]
- 35.Glaister M, Pattison J, Muniz-Pumares D, Patterson S, Foley P. Effects of dietary nitrate, caffeine, and their combination on 20 km cycling time-trial performance. J Strength Cond Res. 2014;29:165–174. doi: 10.1519/JSC.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 36.Boorsma R, Whitfield J, Spriet L. Beetroot juice supplementation does not improve performance in elite 1500-m runners. Med Sci Sport Exerc. 2014;46:2326–2334. doi: 10.1249/MSS.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 37.Zamani P, Rawat D, Shiva-Kumar P, et al. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380. doi: 10.1161/CIRCULATIONAHA.114.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott JM, Haykowsky MJ, Eggebeen J, Morgan TM, Brubaker PH, Kitzman DW. Reliability of peak exercise testing in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:1809–1813. doi: 10.1016/j.amjcard.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the management of heart-failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 40.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney R. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 41.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 42.Berry M, Justus N, Hauser J, et al. Dietary nitrate supplementation improves exercise performance and decreases blood pressure in COPD patients. Nitric Oxide. 2015;48:22–30. doi: 10.1016/j.niox.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ACSM. ACSM’s Guidelines for Exercise Testing and Prescription. 7. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 44.Miller GD, Marsh AP, Dove RW, et al. Plasma nitrate and nitrite are increased by a high-nitrate supplement but not by high-nitrate foods in older adults. Nutrition Research. 2012;32:160–168. doi: 10.1016/j.nutres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wylie L, Kelly J, Bailey S, et al. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol. 2013;115:325–336. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 46.Haykowsky M, Tomczak C, Scott J, Patterson D, Kitzman D. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol. 2015 doi: 10.1152/japplphysiol.00049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanderpool R, Gladwin MT. Harnessing the nitrate-nitrite-nitric oxide pathway for therapy of heart failure with preserved ejection fraction. Circulation. 2015;131:334–336. doi: 10.1161/CIRCULATIONAHA.114.014149. [DOI] [PubMed] [Google Scholar]
- 48.Redfield MM, Chen HH, Borlaug BA, et al. Effect of Phosphodiesterase-5 Inhibition on Exercise Capacity and Clinical Status in Heart Failure With Preserved Ejection Fraction A Randomized Clinical Trial. Jama-Journal of the American Medical Association. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borlaug BA, Koepp KE, Melenovsky V. Sodium Nitrite Improves Exercise Hemodynamics and Ventricular Performance in Heart Failure With Preserved Ejection Fraction. Journal of the American College of Cardiology. 2015;66:1672–1682. doi: 10.1016/j.jacc.2015.07.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.