Abstract
The pulvinar, the largest thalamus nucleus, has rich anatomical connections with several different cortical and subcortical regions suggesting its important involvement in high-level cognitive and emotional functions. Unfortunately, pulvinar dysfunction in psychiatric disorders particularly major depression disorder has not been thoroughly examined to date. In this study we explored the alterations in the baseline regional and network activities of the pulvinar in MDD by applying spectral analysis of resting-state oscillatory activity, functional connectivity and directed (effective) connectivity on resting-state fMRI data acquired from 20 healthy controls and 19 participants with MDD. Furthermore, we tested how pharmacological treatment with duloxetine can modulate the measured local and network variables in ten participants who completed treatment. Our results revealed a frequency-band dependent modulation of power spectrum characteristics of pulvinar regional oscillatory activity. At the network level, we found MDD is associated with aberrant causal interactions between pulvinar and several systems including default-mode and posterior insular networks. It was also shown that duloxetine treatment can correct or overcompensate the pathologic network behavior of the pulvinar. In conclusion, we suggest that pulvinar regional baseline oscillatory activity and its resting-state network dynamics are compromised in MDD and can be modulated therapeutically by pharmacological treatment.
1. Introduction
The pulvinar, located in the posterior part of the thalamus, is the largest primate thalamic nucleus. The pulvinar has extensive bidirectional connections with temporal, parietal, cingulate, frontal and insular cortices reported in primate tracing and human diffusion tensor imaging (DTI) studies (Jones and Burton, 1976; Kumar et al., 2014; Shipp, 2003). Pharmacological imaging studies of thalamic neurotransmission have detected the presence of active serotonergic and noradrenergic but little to no dopaminergic activity in the pulvinar part of thalamus (Oke et al., 1997; Takano et al., 2008).
The pulvinar was once considered a simple “relay center” for information transmission between thalamus and cortex (Pessoa and Adolphs, 2010). That simplistic model of pulvinar function has been revised by recent evidence showing an intricate computational role of the pulvinar in modulating cortical synchrony needed for perceptual attention and emotional processing (Padmala et al., 2010; Pessoa and Adolphs, 2010; Saalmann et al., 2012).
Pulvinar anatomical and physiological abnormalities have also been found to be involved in the pathophysiology of different neuropsychiatric disorders like visual neglect syndrome, attentional deficits, anxiety disorders and attention deficit hyperactivity disorder (ADHD) (Arend et al., 2008; Ipser et al., 2013; Ivanov et al., 2010; Snow et al., 2009). In addition, results of a recent meta-analysis of resting-state PET and SPECT studies in MDD found a significant increase in pulvinar baseline activity in participants with MDD suggesting pulvinar hyperactivity as a potential mechanism of “heightened negative stimuli processing and awareness” (Hamilton et al., 2012). Prior studies also show abnormal functional connectivity of thalamic regions, if not the pulvinar specifically, in MDD (Greicius et al., 2007). Furthermore, μ-opioid availability in the pulvinar was predictive of degree of treatment response in MDD (Kennedy et al., 2006). Abnormalities in serotonin transporter system and pulvinar volume size via neuron counts were also reported in a postmortem study of individuals with history of MDD (Young et al., 2007).
In the last decade, resting-state fMRI (rsfMRI) method has been used extensively to investigate regional and network dysfunction in neuropsychiatric disorders including major depressive disorder (Biswal et al., 1995; Fox and Raichle, 2007). Spectral analysis based approaches of Amplitude of Low-Frequency Fluctuation (ALFF) or Fractional ALFF (fALFF) are applied for measuring characteristics of regional activity in different brain regions in normal and pathologic conditions (Zang et al., 2007; Zou et al., 2008). Using ALFF/fALFF methods, several studies have shown alterations in resting state baseline activity in several cortical (frontal, temporal and cingulate) and subcortical (thalamus, striatum) regions (Lai and Wu, 2015; Tadayonnejad et al., 2014). More than looking at resting-state regional activity of brain, rsfMRI data has been used to explore network dysfunction in depression. For that purpose, two main approaches of functional connectivity (FC) and effective connectivity (EC) are practiced to measure interregional correlation (synchronicity) or causal influence between interacting brain regions respectively (Friston, 2011). Findings of a recent meta-analysis of FC studies in major depressive disorder showed the frontoparietal network hypoconnectivity, hyperconnectivity within the default mode network, believed to be involved in internally oriented and self-referential thought processing, and hypoconnectivity in neural networks mediating top-down emotion regulation in subjects with depression compared to healthy controls (Kaiser et al., 2015). EC studies in depression have also found impairment in causal dynamics behavior of areas involve in neurocircuitry of depression particularly medial frontal and anterior cingulate cortex (Feng et al., 2016; Hamilton et al., 2011).
Despite intriguing evidence for a role of the pulvinar nucleus in the pathology and expression of MDD, there are still many important gaps in our knowledge. By applying above-mentioned fALFF, FC and EC analysis methods on resting-state fMRI data, we want to explore pulvinar regional and network dysfunction in depression. We hypothesize that MDD is associated with alterations in pulvinar regional resting-state oscillatory activity and also its network dynamics. Furthermore, we hypothesize that pharmacological treatment with the serotonin-norepinephrine reuptake inhibitor duloxetin can change (correct or overcompensate) MDD related changes in regional activity and network behavior of pulvinar.
2. Methods
2.1. Participants
Twenty-four subjects with diagnosis of major depressive disorder and 26 sex- and age-matched health control subjects were recruited in this study. Nineteen MDD who completed the first part of the study and had data at free of movement and distortion artifacts were included in the analyses and reported in this paper, along with 20 healthy control subjects with usable data at baseline. Healthy control subjects were only studied at baseline. Clinical assessment was conducted using the structured clinical interview for DSM-IV and the Hamilton Rating Scales for Depression and Anxiety. BJM or JKZ provided treatment for participants through the Translational Neuroimaging Laboratory at the University of Michigan Medical Center, supported by services and clinic space provided by the Michigan Clinical Research Unit. Prior to enrollment in the study, MDD participants were unmedicated and had been medication-free for at least 90 days for any SSRIs or SNRIs and for at least 30 days for all other medications (including birth control) to eliminate medication effects on functional neural activation. Individuals who smoked cigarettes, met criteria for alcohol abuse, or had used any illegal drugs in the past 2 years were excluded. All MDD participants underwent fMRI and completed several measures including the Hamilton Depression Rating Scale (HDRS) at baseline. Ten of nineteen MDD participants completed open-label treatment with duloxetine for 10 weeks with second scanning and psychological assessment after treatment. Participants were given 30 mg during weeks 1-2 and then 60 mg during weeks 3-6. If participants' symptoms had not improved by at least 50%, participants' dose was titrated up to 90 mg during weeks 7-8 and if symptoms had still not improved by at least 50%, their dose was titrated up to 120 mg during weeks 9-10, and then either continued on a 60 mg dose or were given 90 mg if symptoms had not improved by at least 50%. Repeat fMRI and HDRS measurements took place upon completion of treatment.
2.2. Data acquisition
Whole brain, eyes-open resting state scans were performed with a 3.0 Tesla GE Signa scanner (Milwaukee, Wisconsin) over 8 minutes using a standard ratio frequency coil and T2*-weighted pulse sequence with a gradient-echo axial forward-reverse spiral sequence at UM (2). The following parameters were used: echo time= 3.4 ms, TR= 2 sec, degree flip= 27, field of view= 22cm, matrix size= 64 × 64, slice thickness= 5mm, slices= 30. High-resolution anatomical T1 scans were obtained for spatial normalization. During scanning, participants were asked to look at a fixation cross on the display and the importance of staying still was conveyed to each participant.
2.3. fMRI preprocessing
All preprocessing were conducted using statistical parametric mapping software (SPM8, http//www.fil.ion.uvl.ac.uk/spm). The first 10 volumes of the functional images were discarded for obtaining signal equilibrium and allowing participants adaptation to scanning noise. The artifact detection tool (ART: http://www.nitrc.org/projects/artifact_detect) was used to measure motion artifacts in all subjects. None of the subjects used in this study had more than 1 mm maximum displacement in x, y or z axis or 1 angular motion during fMRI scanning. Furthermore, there was no significant difference in composite motion between groups (HC: 0.13 ± 0.01; pre-treatment MDD: 0.16 ± 0.03; post treatment MDD: 0.17 ± 0.04). Raw EPI images were subsequently realigned, coregistered, normalized, and smoothed with a smoothing kernel of 8 mm before analyses. Confound effects from motion artifact, white matter, and CSF were regressed out of the signal. We did not regress out whole-brain noise effect. Finally, BOLD signal data were passed through two band-pass filters (lower frequency band (LF): 0.01 to 0.1 Hz and higher frequency band (HF): 0.1 to 0.25 Hz) for further fALFF and functional connectivity analyses.
2.4. Power spectrum distribution analysis
Lower and higher frequency bands power spectrum distribution were calculated in terms of Lower and Higher Frequency fractional Amplitude of Low-Frequency Fluctuation (LF/HF fALFF) values. fALFF analyses were performed using Resting-State fMRI Data Analysis Toolkit (REST, http://www.rest.restfmri.net). For each voxel, the filtered time series was transformed to the frequency domain using a fast Fourier transformation (FFT) analysis, and the power spectrum was then measured. The average square root of power in the 0.01–0.1 Hz (LF) or 0.1– 0.25 Hz (HF) bands was calculated. Then, the average square root of power in the 0.01– 0.1 Hz or 0.1–0.25 Hz bands for each voxel was normalized by total power across all available frequencies for that voxel (LF-fALFF and HF-fALFF). We applied a brain mask on subject-level voxel-wise fALFF maps for removing non-brain tissues. Finally, all fALFF maps were standardized into subject-level Z-score maps for improving statistical analyses and test-retest reliability (Chen et al., 2013; Zuo et al., 2010).
2.5. Functional connectivity analysis
Functional connectivity (FC) analysis was performed with REST software. We used a 5 mm radius sphere centered on the peak coordinates based on the Hamilton et al. meta-analysis of depression associated with increased activity in the pulvinar bilaterally. The right side pulvinar cluster (20 -25 4) was used to generate our pulvinar seed for FC analysis (Hamilton et al., 2012). After 0.01–0.1 Hz (LF) or 0.1-0.25 Hz (HF) band pass filtering and linear regression removal of ventricular, white matter, the time series of voxels within each seed region was averaged as the seed reference time course. For each subject, LF and HF based FC of each seed reference time course with the rest of the brain gray matter voxels (extracted by using a mask) were calculated separately for obtaining correlation coefficient maps. Finally, all correlation maps were transformed to Z-value FC maps by applying Fisher's r-to-z conversion for performing subsequent FC group comparison.
2.6. Granger Causality Analysis
We used Granger Causality Analysis (GCA) to examine the causal influence of pulvinar on brain (pulvinar causal outflow to brain or Pulvinar-to-Whole-Brain effective connectivity) as well as the causal influence of the rest of the brain on the pulvinar (pulvinar causal inflow from brain or Whole-Brain-to-Pulvinar effective connectivity). We used signed-path coefficients method (Hamilton et al., 2011) implemented in REST-GCA toolkit (Zang et al., 2012) with a time lag order of 1 (1 TR, 2 second) to determine the probable strength and sign (inhibitory versus excitatory) of causal effect. Similar to the FC analysis, a 5 mm radius sphere centered on (20 -25 2) coordinate was generated as the pulvinar seed for measuring GCA based seed-to-voxel and voxel-to-seed effective connectivity. For each subject, effective connectivity maps of pulvinar seed causal influence on the rest of the brain gray matter voxels (Pulvinar-to-Whole-Brain effective connectivity map) and the whole brain gray matter voxels causal influence on pulvinar seed (Whole-Brain-to-Pulvinar effective connectivity map) were generated. At the end, all effective connectivity maps were transformed to Z-value effective connectivity maps for more reliable statistical analysis(Zang et al., 2012).
2.7. Statistical analysis
Demographic and clinical variables were analyzed for between-group differences using an independent sample t-test for continuous variables and chi-squared test for categorical variables. fALFF, functional connectivity and GCA effective connectivity group differences were also analyzed using independent sample t-test. Pearson's correlations were used to analyze the relationship between LF/HF-fALFF, HF/LF functional connectivity and effective connectivity values and depression severity in terms of HDRS. For all of the above analyses, Monte Carlo simulation was applied for multiple comparisons correction using the REST AlphaSim program (Ledberg et al., 1998). In this study, a corrected significant level of p<0.05 was obtained by using a combination of individual voxel probability threshold p < 0.01 and a minimum cluster size of 64 voxels (or 1728 mm3).
3. Results
3.1. Demographics
Demographic and clinical data of both healthy and MDD participants are summarized in Table 1. There were no significant differences between the two groups in age, gender or years of education. As expected, MDD participants scored significantly higher on depression severity in terms of Hamilton Depression Rating Scale (HDRS) score.
Table 1.
Demographic, clinical and cognitive characteristics for those with valid and usable data.
| HC (n=20) | MDD; all participants (n=19) | p-value | |
|---|---|---|---|
| Age | 31.8 ± 10.2 | 27.4 ± 7.8 | 0.14a |
| Sex (M/F) | 7/13 | 7/12 | 0.91b |
| Education | 15.6 ± 1.5 | 15.3 ± 1.3 | 0.90a |
| HDRS | 0.4 ± 0.08 | 19.1 ± 3.2 | < 0.001a |
| HC (n=20) | MDD; treatment group (n=10) | p-value | |
| Age | 31.8 ± 10.2 | 30.5 ± 9.3 | 0.09a |
| Sex (M/F) | 7/13 | 3/7 | 0.07b |
| Education | 15.6 ± 1.5 | 15.3 ± 1.4 | 0.90a |
| HDRS | 0.4 ± 0.08 | 20.4 ±5.3 | < 0.001a |
The P value were obtained by simple t-test.
The P value was obtained by chi-square root.
Abbreviation: HC: healthy control; MDD: major depressive disorder; HDRS: Hamilton Depression Rating Scale.
3.2. Pharmacological alteration of regional power spectrum characteristics of pulvinar resting-state oscillations
A whole brain data driven approach was used to explore how specific lower and higher frequency bands power spectrum distributions in terms of LF and HF fALFF values were altered in MDD patients and how duloxetine treatment in that group affected those variables. We found no significant LF or HF fALFF differences between healthy and pre-treatment MDD groups but our results showed significant post-treatment increase in LF fALFF and decrease in HF fALFF in the posterior part of right thalamus which mainly includes pulvinar (Fig. 1A). The reported coordinate of hyperactive pulvinar cluster peak reported in the Hamilton et al, meta-analysis (20 -25 2) was found to be included in our fALFF altered pulvinar clusters and was used as the center of a sphere covering the right pulvinar area that we chose for our next seed-to-brain functional and effective connectivity analyses (Green sphere in Fig 1A). Consistent with the abovementioned whole brain LF and HF fALFF analysis results, the right pulvinar sphere seed fALFF measurement showed higher LF fALFF and lower HF fALFF values in post-treatment participants compared to healthy group (Fig. 1B).
Figure 1.
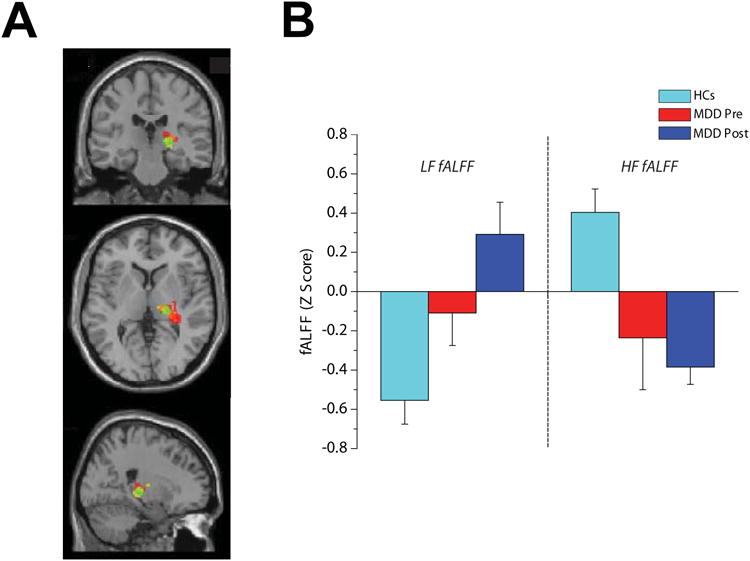
Effect of duloxetine treatment on spectral power distribution of the right pulvinar resting-state oscillations expressed by the Lower and Higher fractional Amplitude of the Low-Frequency Fluctuations (LF and HF fALFF). (A) Overlapping clusters of increased LF fALFF (in red) and decreased HF fALFF (in yellow) and sphere seed (in green) used for connectivity analyses. (B) Graph bar showing averages of LF and HF fALFF values in HCs, MDD Pre and MDD post subjects. Note the opposite direction of change in LF and HF fALFF after treatment.
3.3. Pharmacological manipulation of pulvinar resting-state Functional Connectivity
We measured pulvinar functional connectivity mediated by two lower (0.01-0.1 Hz) and higher frequency (0.1-0.25) bands. Compared to healthy controls, MDD participants (n = 19) have higher LF functional connectivity between the right pulvinar and right precuneus, higher right pulvinar-right precentral HF functional connectivity but lower right pulvinar-right putamen and right pulvinar-left thalamus LF functional connectivity (Table. 2). Pulvinar hyperconnectivity with the right rectal gyrus/medial orbitofrontal cortex was found for both LF and HF functional connectivity analyses in post-treatment participants compared to healthy controls (Fig 2 A,B ; Table. 2). Increased LF functional connectivity between pulvinar seed and the right anterior cingulate cortex, left middle temporal gyrus, left lingual and left dorsomedial prefrontal cortex (DMPFC) were also found in post-treatment MDD participants compared to healthy controls (Fig 2 A; Table. 2).
Table 2.
Brain regions showing statistically significant altered LF or HF functional connectivity with right Pulvinar seed in MDD subjects before and after treatment with duloxetine compared to healthy group.
| Brain regions (Brodmann areas) | Cluster size | MNI coordinates | t values | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| LF-based FC: Healthy controls (n = 20) compared to all MDD participants (n = 19) | ||||||
| Right precuneus (BA7,19) | 205 | 30 | -80 | 40 | HC< MDD | 3.8 |
| HF-based FC: Healthy controls (n = 20) compared to all MDD participants (n = 19) | ||||||
| Right precentral (BA3,4) | 112 | 40 | -26 | 74 | HC< MDD | 3.74 |
| Right putamen | 239 | 19 | 9 | 11 | HC >MDD | 16.60 |
| Left thalamus | 135 | -22 | -36 | 4 | HC > MDD | 6.15 |
| LF-based FC: Healthy controls (n = 20) compared to post treatment MDD participants (n = 10) | ||||||
| Right Rectal gyrus (BA11,47) | 240 | 72 | -10 | 4 | HC< Post Treatment MDD | 11.31 |
| Right Anterior Cingulate cortex (BA24) | 113 | 2 | 26 | 2 | HC< Post Treatment MDD | 6.53 |
| Left Middle Temporal gyrus (BA21) | 93 | -60 | 16 | -8 | HC< Post Treatment MDD | 13.60 |
| Left Lingual (BA 17,18) | 83 | -30 | -96 | -22 | HC< Post Treatment MDD | 11.53 |
| Left Dorsomedial Prefrontal cortex (BA 10) | 183 | -4 | 72 | 4 | HC< Post Treatment MDD | 10.38 |
| HF-Based FC: Healthy controls (n = 20) compared to post treatment MDD participants (n = 10) | ||||||
| Right Rectal gyrus (BA11,47) | 129 | 22 | -24 | -36 | HC< Post Treatment MDD | 8.61 |
Abbreviations: BA: Brodmann area; HC: healthy controls; MDD: major depressive disorder; LF- and HF-based FC: Lower and Higher Frequency based Functional Connectivity MNI: Montreal Neurologic Institute.
Figure 2.
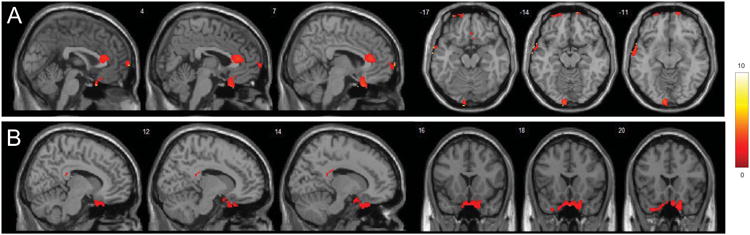
Alteration of pulvinar functional connectivity after duloxetine treatment. T-statistical maps demonstrating clusters with different functional connectivity mediated by lower and higher frequency bands (A and B respectively) with the right pulvinar seed in MDD participants after treatment compared to controls. Significant clusters greater than 64 voxels were shown after correction for multiple comparison (p < 0.05). The color bar on the left side refers to the range of t values.
3.4. Pharmacological modulation of pulvinar resting-state Effective Connectivity
Pulvinar-to-Whole-Brain effective connectivity (EC) comparison between healthy controls and all MDD participants (n = 19) showed significant higher positive causal influence of the right pulvinar on bilateral DMPFC, bilateral precuneus, the right middle frontal gyrus and left anterior cingulate in MDD group (Table. 3). When we compared healthy subjects with selective pre-treatment MDD participants (n = 10), the results of Pulvinar-to-Whole-Brain effective connectivity (EC) analysis were relatively similar: significant increase in excitatory causal outflow from the right pulvinar to the right precentral, right superior parietal and bilateral DMPFC and also significant decrease in inhibitory causal outflow from the right pulvinar to the right angular and left precentral in pre-treatment MDD participants compared to controls (Fig. 3A, B). Abnormal higher excitatory influence of the right pulvinar on the bilateral DMPFC, right precentral and superior parietal as well as abnormal lower inhibitory influence of the right pulvinar on the right angular and left precentral gyri were found to be corrected back to the normal range of healthy controls in MDD group after treatment with duloxetine. We also found that pulvinar positive causal influence on the left inferior frontal and left posterior insula in MDD participants decreased after treatment to even a significantly lower level relative to healthy controls, potentially in an compensatory manner (Fig. 3A,C; Table. 3).
Table 3.
Brain arears with statistically significant altered Pulvinar-to-Whole-Brain or Whole-Brain-to-Pulvinar effective connectivity in MDD subjects before or after treatment with Duloxetine compared to healthy controls.
| Brain regions (Brodmann areas) | Cluster size | MNI coordinates | t values | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Pulvinar-to-Whole-Brain EC comparison between HC (n = 20) and all MDD subjects (n = 19) | ||||||
| Right precuneus (BA7) | 263 | 12 | -68 | 52 | HC:+CO < Pre-treatment:+CO | 7.71 |
| Right middle frontal gyrus (BA9) | 138 | 36 | 28 | 40 | HC:+CO < Pre-treatment:+CO | 3.77 |
| Right dorsomedial prefrontal cortex (BA10) | 394 | 6 | 42 | 21 | HC:+CO < Pre-treatment:+CO | 4.09 |
| Left dorsomedial prefrontal cortex (BA10) | 625 | -7 | 52 | 30 | HC:+CO < Pre-treatment:+CO | 7.34 |
| Left precuneus (BA7) | 95 | -28 | -64 | 42 | HC:+CO < Pre-treatment:+CO | 4.09 |
| Left anterior cingulate (BA32) | 578 | -5 | 49 | 2 | HC:+CO < Pre-treatment:+CO | 6.51 |
| Whole-Brain-to-Pulvinar EC comparison between HC (n = 20) and all MDD subjects (n = 19) | ||||||
| No group difference | ||||||
| Pulvinar-to-Whole-Brain EC comparison between HC (n = 20) and pre-treatment MDD subjects (n = 10) | ||||||
| Right dorsomedial prefrontal cortex (BA 9,10) | 207 | 16 | 54 | 8 | HC:+CO < Pre-treatment:+CO | 7.50 |
| Right angular gyrus (BA39) | 107 | 60 | -64 | 32 | HC:-CO > Pre-treatment:-CO | 4.24 |
| Right precentral (B9) | 221 | 40 | 8 | 52 | HC:+CO < Pre-treatment:+CO | 4.32 |
| Right superior Parietal (BA7) | 127 | 20 | -74 | 48 | HC:+CO < Pre-treatment:+CO | 3.98 |
| Left dorsomedial prefrontal cortex (BA 10) | 145 | -20 | 48 | 21 | HC:+CO <Pre-treatment:+CO | 7.44 |
| Left precentral (B44,9) | 69 | -46 | -4 | 20 | HC:-CO > Pre-treatment:-CO | 4.18 |
| Pulvinar-to-Whole-Brain EC comparison between HC (n = 20) and post treatment MDD subjects (n = 10) | ||||||
| Left inferior frontal gyrus (BA 47) | 104 | -42 | 42 | -24 | HC:+CO > Post treatment:-CO | 5.50 |
| Left insula (BA13) | 92 | -16 | -4 | 14 | HC:+CO > Post treatment:-CO | 6.30 |
| Whole-Brain-to-Pulvinar EC comparison between HC (n = 20) and pre-treatment MDD subjects (n = 10) | ||||||
| No group difference | ||||||
| Whole-Brain-to-Pulvinar EC comparison between HC (n = 20) and post treatment MDD subjects (n = 10) | ||||||
| Right lingual gyrus (BA17) | 165 | 2 | -98 | 2 | HC:-CI > Post treatment:-CI | 5.31 |
| Right mid cingulate cortex (BA24) | 190 | 16 | -4 | 50 | HC:+CI < Post treatment:+CI | 7.21 |
| Right dorsomedial prefrontal cortex (BA 10) | 84 | -32 | 62 | 18 | HC:-CI > Post treatment:-CI | 4.45 |
| Left insula (BA13) | 200 | -30 | 24 | 18 | HC:+CI > Post treatment:+CI | 10.83 |
| Left superior frontal gyrus (BA 6,8) | 170 | -36 | 6 | 46 | HC:+CI > Post treatment:+CI | 4.69 |
Abbreviations: BA: Brodmann area; HC: healthy controls; MDD: major depressive disorder; EC: Effective Functional; +CO: pulvinar positive (excitatory) Causal Outflow to brain; -CO: pulvinar negative (inhibitory) Causal Outflow to brain; +CI: pulvinar positive (excitatory) Causal Inflow from brain ; -CI: pulvinar negative (inhibitory) Causal Inflow from brain; MNI: Montreal Neurologic Institute.
Figure 3.
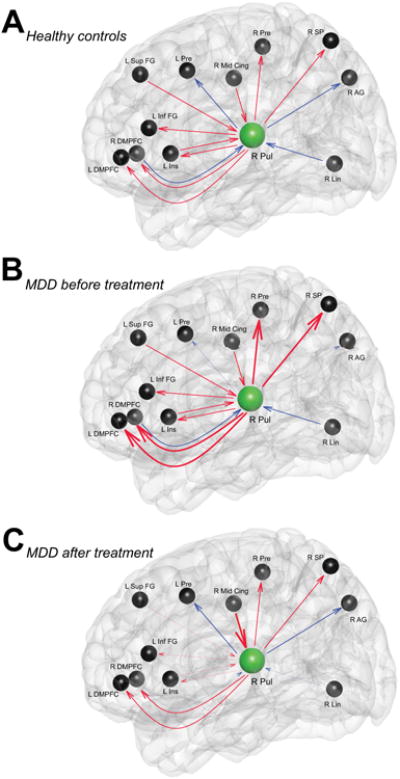
Schematic illustration of pulvinar causal outflow and inflow in healthy controls (A), participants with MDD before (B) and after treatment (C). Red and blue lines indicate excitatory and inhibitory influences between nodes respectively. Thick and thin lines show stronger and weaker strengths of connectivity in MDD participants compared to healthy controls respectively. R Pul, right pulvinar; DMPFC, dorsomedial prefrontal cortex; Pre, Precentral; AG, Angular gyrus; SP, Superior Parietal; Sup FG, Superior Frontal gyrus; Ins, Insula; Inf FG, Inferior Frontal gyrus ; Lin, Lingual gyrus; Mid Cing, Middle Cingulate cortex.
Whole-Brain-to-Pulvinar EC analysis did not show any significant difference between MDD participants and healthy control subjects. Medication treatment in MDD participants resulted in significant decrease in negative influence of the right lingual to the right pulvinar, significant decrease in negative influence of the right DMPFC to the right pulvinar and significant increase in positive influence of the right mid cingulate to the right pulvinar. Positive causal outflow of the left insula and left superior frontal gyrus on pulvinar were also detected to be reduced in post-treatment MDD group compared to healthy subjects. (Fig. 3A,C ; Table. 3)
3.5. Clinical correlation of pulvinar regional and network function
Here, we test to see if change in regional and network activity of pulvinar (fLAFF, FC and EC) in pretreatment and posttreatment condition has any correlation with change in clinical symptomology. We did two sets of analysis. First, we checked to see if there is any correlation between doluxetine induced changes in fALFF/FC/EC values and change in clinical depression symptom severity. Second, we analyzed to see if change in symptom severity has any correlation with change in above hemodynamic measures when we compare first and second scans in treatment MDD group (n=10). The first analysis did not reveal any result but in the second analysis, we found significant negative correlation between (delta) change in HF functional connectivity and (delta) change in depression symptom severity (HDRS pretreatment minus posttreatment) in the right and left inferior temporal gyrus and right superior frontal gyrus (figure 4; Table 4).
Figure 4.
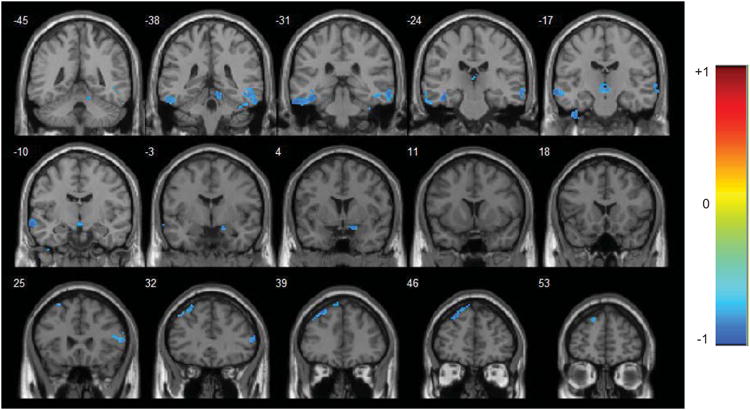
R-statistical maps illustrating correlation between change in HF functional connectivity and change in symptom severity in terms of HAMD score (pretreatment minus posttreatment values). Corrected for multiple comparison (p < 0.05). The color bar on the left side refers to the range of r values.
Table 4.
Brain regions showing statistically significant correlation between changes in HF FC and depression symptom severity (HDRS) when comparing first and second scans in MDD treatment group (n=10).
| Brain regions (Brodmann areas) | Cluster size | MNI coordinates | Peak r values | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Right inferior temporal gyrus (B21) | 101 | 66 | -28 | -12 | -0.91 |
| Left inferior temporal gyrus (B20) | 130 | -40 | -26 | -18 | -0.84 |
| Left superior frontal gyrus (BA8) | 159 | -28 | 42 | 48 | -0.90 |
Abbreviations: BA: Brodmann area; HF FC: High frequency Functional connectivity; MDD: major depressive disorder; HDRS: Hamilton depression rating scale; MNI: Montreal Neurologic Institute; r: Peak correlation value
4. Discussion
In this work, we expand the understanding of pulvinar dysfunction in a major and common psychiatric disorder, MDD, by investigating disease-related changes in pulvinar resting-state regional activity and its network dynamics. Furthermore, we also test to see how pharmacological treatment with duloxetine can affect pulvinar baseline local activity and its network behavior. Our findings revealed alterations in characteristics of pulvinar baseline oscillatory activity and its network interaction with important affective neurocircuitries involving in self-referral processing (default-mode network), psychological pain processing (posterior Insula) and emotion regulation (orbitofrontal cortex). We also found that duloxetine treatment can correct or perhaps overcompensate those depression-related changes in pulvinar resting-state activity and network dynamics.
4.1. Pharmacological modulation of pulvinar resting-state oscillations spectral features
Resting-state BOLD oscillation frequency-dependent power spectrum has been shown to vary across different brain anatomical regions (Baria et al., 2011; Salvador et al., 2008; Tadayonnejad et al., 2014; Zuo et al., 2010). Studies that measured the spatial distribution of brain oscillatory activity found that lower frequency oscillations have more power and are more dominant in frontal, temporal, parietal and occipital areas compared to higher frequency oscillations with greater dominance and power in subcortical regions like amygdala, thalamus and basal ganglia (Baria et al., 2011; Zuo et al., 2010). In terms of their neural functions, it has been speculated that lower frequency oscillations mediate cortical long-distance and “simple/unimodal” neural transmission compared to higher frequency oscillations which are more involved in short distance and “complex/transmodal” neural processing in subcortical regions like the limbic system (Baria et al., 2011; Buzsaki and Draguhn, 2004).
Regional frequency-specific power spectrums were reported to be altered in different neuropsychiatric disorders including MDD (Esposito et al., 2013; Han et al., 2012; Tadayonnejad et al., 2014; Wang et al., 2014; Yu et al., 2014). In this work, we found a relative decrease in LF power spectrum and increase in HF power spectrum of regional pulvinar resting-state oscillations. This opposite direction of difference in lower and higher frequency power is very similar to the results of Malinen et al. spectral analysis in patients with chronic back pain showing attenuation of lower frequency power at 0-0.05 Hz and accentuation of higher frequency power at 0.12-0.25 Hz in anterior cingulate cortex and insula (Malinen et al., 2010). Furthermore, our results are in line with Hamilton et al. recent meta-analysis of resting-state SPECT and PET studies in MDD showed baseline pulvinar hyperactivity in MDD participants (Hamilton et al., 2012), but now extended into a more temporally and spatially specific analysis here with fALFF.
Few studies reported the effect of medications treatment on regional spectral power of resting-state brain oscillations (Esposito et al., 2013; Lai, 2012). Interestingly, in our study we found that not only can duloxetine treatment affect the spectral characteristics of pulvinar resting-state oscillatory activity, but it also alters this activity in a frequency-dependent manner in the favor of enhancing lower frequency and reducing higher frequency oscillations. Furthermore, these changes are not always in the direction of normalization toward the healthy comparison pattern.
4.2. Pharmacological modulation of pulvinar functional connectivity with medial orbitofrontal cortex
Converging lines of evidence from rodent and primate electrophysiology to human neuroimaging studies propose a principal function of orbitofrontal cortex (OFC) in goal directed behavior, flexible behavior controlling and inhibition, reward processing and emotional regulation (Kringelbach, 2005; Rudebeck and Murray, 2014). Capability of OFC to do those complex cognitive and affective functions to a great extent relies on the OFC's rich pattern of connectivity to several cortical and subcortical regions (Rudebeck and Murray, 2014). Particularly related to our discussion, direct anatomical connections between the pulvinar and the OFC have been reported reliably in several primate tracing and human DTI studies (Catani and Thiebaut de Schotten, 2008; Kumar et al., 2014; Romanski et al., 1997). In the current study, we found treatment with duloxetine resulted in a significant strong increase in functional connectivity mediated by both LF and HF oscillations between the right pulvinar and right medial OFC (rectal gyrus) as well as increased LF-based connectivity of the right pulvinar with limbic regions including the right rostal anterior cingulate cortex (ACC), left DMPFC and left temporal gyrus. Based on these findings, we suggest a network-based treatment mechanism by which duloxetine can enhance emotional regulation by giving OFC more access and control on affective regions like the rostral ACC and DMPFC through the pulvinar. A potential interesting future study will be to test the validity of that speculated model by applying multivariate functional and effective connectivity analysis between the related regions before and after treatment.
4.3. Pharmacological modulation of pulvinar causal interaction with default-mode and salient networks
Recent system neuroscience studies of pulvinar function in visual processing demonstrated a complicated role of the pulvinar in mediating synchrony between spatially distributed cortical areas needed for visual attention (Fischer and Whitney, 2012; Pessoa and Adolphs, 2010; Saalmann et al., 2012). This complex computational ability of the pulvinar in regulation cortical synchronization is supported by its rich bidirectional anatomical connections with almost all areas of visual cortex (Pessoa and Adolphs, 2010; Shipp, 2003). Despite the similar pattern of anatomical connections of the pulvinar with several cortical and subcortical structures (Kumar et al., 2014; Romanski et al., 1997), beside the case of amygdala (Pessoa and Adolphs, 2010), pulvinar function in dynamic regulating of other networks has not been explored yet. Results of our study point to a potential pathological causal/dynamic interaction of the pulvinar with at least two emotionally important networks: the default mode network (DMN) and the posterior insular network.
Accumulating neuroimaging evidence points to hyperactivity, hyperconnectivity and improper deactivation of DMN as the core network dysfunctions in MDD (Broyd et al., 2009; Whitfield-Gabrieli and Ford, 2012). However, the mechanisms of DMN dysregulation have not been extensively studied or explained yet. Our findings demonstrating abnormally higher excitatory causal influence of the pulvinar on the bilateral DMPFC and precuneus and increased in causal excitatory influence of pulvinar on the left anterior cingulate area in participants with MDD can be interpreted as a potential mechanism mediating DMN dysfunction. In other words, our results suggest an aberrant higher pulvinar stimulating effect on DMN as a possible pathologic source in disturbing DMN normal functioning. Furthermore, we found that abnormal causal positive influence of the pulvinar on these DMN nodes could be corrected or even overcompensated after treatment with duloxetine.
The other network implicated by our results has been shown to be pulvinar-posterior insular network. In a recent emotional pain processing meta-analysis in MDD, Mutschler et al, reported a shift in pain-related emotional processing toward dorsal insula in patients with MDD (Mutschler et al., 2012). They suggested abnormally higher involvement and activity of dorsal insula as a mechanism of exaggerated pain processing (allodynia) and psychological pain feeling in MDD. Consistent with Mutschler et al, report, our findings illustrated that treatment with duloxetine reduces the positive causal influence of pulvinar on dorsal insula suggesting a treatment mechanism by which duloxetine modulates pulvinar-posterior Insula dynamics.
In a resting-state effective connectivity analysis of the anterior insula in MDD, Iwabuchi et al. reported a significant decrease in causal influence between the left pulvinar and bilateral striatum/insula. Compared to our results showing mainly increase in right pulvinar causal influence on different regions, Iwabuchi et al, reported a decrease in effective connectivity between the left pulvinar and several areas including the striatum/insula, calcarine/cuneus and cerebellum (Iwabuchi et al., 2014) indicating a different patterns of causal influence abnormalities between the right and left pulvinar. Taken together, the findings from Iwabuchi et al. and the current study, demonstrate bilateral pulvinar neurodynamics pathology in MDD.
4.4. Methodological considerations
This current work has some limitations that need to be discussed here. First, our sample size for this study is relatively small. Although all the presented results are statistically significant after correction for multiple comparisons, our findings need to be replicated and validated, especially with larger sample sizes. Second, the exact underlying neural source of fMRI BOLD-based power spectrum signal has not been comprehensively investigated. Simultaneous EEG/fMRI technique will be an advantageous way to elucidate the exact relation between power spectrum values extracted from BOLD versus neural signals. Third, the healthy control group in our study was only scanned once compared to treatment MDD participants with two scans before and after duloxetine medication. This could be a potential confound and the effect of time on connectivity measures cannot be ruled out completely. As the work was funded only by small grants (K and NARSAD), we had to be selective about where our costs were allocated. We do note, however, that the majority of our results are not of ‘regression to the mean’, or even to the pattern observed in the healthy control group. With treatment and symptom resolution, the MDD group moved further away from the control group in many measurements. Forth, the sluggish nature of hemodynamic BOLD signal and variability of hemodynamic delay between brain areas may confound a reliable detection of underlying neural-based causal influences between different regions (Smith et al., 2011). Although, it has been shown that applying GCA method on real fMRI data results in accurate identification of causal influences with high level of sensitivity and specificity (Schippers et al., 2011), but replication of our results by performing GCA on higher temporal resolution fMRI data (shorter TR) or using other techniques like magnetoencephalography with its significant higher temporal resolution are good confirmatory approaches.
4.5. Clinical Implication
Current study findings have some clinical implications that need to be discussed. We showed aberrant pulvinar causal network interaction with well-known areas/networks involved in the pathophysiology of depression including DMN nodes, insula and dmPFC. We speculate then that pulvinar might be considered as a potential source for “causing” dysfunction in those MDD relevant regions suggesting pulvinar as a new neuroimaging network-based biomarker for diagnosis, treatment follow up and disease prognosis in depression. It was also demonstrated how pharmacological treatment with a serotonergic/noradrenergic agent like duloxetine can modulate pulvinar local and network normal and pathologic (dys)functions. These results suggest pulvinar as a potential novel target in depression treatment. More specific and individualized pharmacological manipulation of serotonin and norepinephrine systems/receptors in pulvinar as well as application of neurostimulation techniques for modulating pulvinar electrophysiological activity would be novel treatment options for depression.
4.5. Conclusion
In summary, we applied spectral analysis, functional connectivity and GCA-based effective connectivity methods on resting state fMRI data to explore the alterations of pulvinar regional properties and its dynamic interaction with other nodes of involved networks in MDD. We also examined the effect of pharmacological treatment on those measures. Our findings indicated that MDD is related with changes in regional baseline activity and network behavior of the pulvinar that can be modulated (corrected or overcompensated) by pharmacological intervention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arend I, Rafal R, Ward R. Spatial and temporal deficits are regionally dissociable in patients with pulvinar lesions. Brain. 2008;131(Pt 8):2140–2152. doi: 10.1093/brain/awn135. [DOI] [PubMed] [Google Scholar]
- Baria AT, Baliki MN, Parrish T, Apkarian AV. Anatomical and functional assemblies of brain BOLD oscillations. J Neurosci. 2011;31(21):7910–7919. doi: 10.1523/JNEUROSCI.1296-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Esposito F, Tessitore A, Giordano A, De Micco R, Paccone A, Conforti R, Pignataro G, Annunziato L, Tedeschi G. Rhythm-specific modulation of the sensorimotor network in drug-naive patients with Parkinson's disease by levodopa. Brain. 2013;136(Pt 3):710–725. doi: 10.1093/brain/awt007. [DOI] [PubMed] [Google Scholar]
- Feng Z, Xu S, Huang M, Shi Y, Xiong B, Yang H. Disrupted causal connectivity anchored on the anterior cingulate cortex in first-episode medication-naive major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:124–130. doi: 10.1016/j.pnpbp.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Fischer J, Whitney D. Attention gates visual coding in the human pulvinar. Nat Commun. 2012;3:1051. doi: 10.1038/ncomms2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1(1):13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in Major Depressive Disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Mol Psychiatry. 2011;16(7):763–772. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Lui S, Kuang W, Lang Q, Zou L, Jia J. Anatomical and functional deficits in patients with amnestic mild cognitive impairment. PLoS One. 2012;7(2):e28664. doi: 10.1371/journal.pone.0028664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser JC, Singh L, Stein DJ. Meta-analysis of functional brain imaging in specific phobia. Psychiatry Clin Neurosci. 2013;67(5):311–322. doi: 10.1111/pcn.12055. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Bansal R, Hao X, Zhu H, Kellendonk C, Miller L, Sanchez-Pena J, Miller AM, Chakravarty MM, Klahr K, Durkin K, Greenhill LL, Peterson BS. Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am J Psychiatry. 2010;167(4):397–408. doi: 10.1176/appi.ajp.2009.09030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi SJ, Peng D, Fang Y, Jiang K, Liddle EB, Liddle PF, Palaniyappan L. Alterations in effective connectivity anchored on the insula in major depressive disorder. Eur Neuropsychopharmacol. 2014;24(11):1784–1792. doi: 10.1016/j.euroneuro.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Jones EG, Burton H. A projection from the medial pulvinar to the amygdala in primates. Brain Res. 1976;104(1):142–147. doi: 10.1016/0006-8993(76)90654-5. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63(11):1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kumar V, Mang S, Grodd W. Direct diffusion-based parcellation of the human thalamus. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0748-2. [DOI] [PubMed] [Google Scholar]
- Lai CH. Increases in amplitude of low-frequency fluctuations in left fronto-parietal area after duloxetine therapy in first-episode, drug-naive, major depressive disorder with panic disorder patients. J Neuropsychiatry Clin Neurosci. 2012;24(3):E24–25. doi: 10.1176/appi.neuropsych.11070157. [DOI] [PubMed] [Google Scholar]
- Lai CH, Wu YT. The patterns of fractional amplitude of low-frequency fluctuations in depression patients: the dissociation between temporal regions and frontoparietal regions. J Affect Disord. 2015;175:441–445. doi: 10.1016/j.jad.2015.01.054. [DOI] [PubMed] [Google Scholar]
- Ledberg A, Akerman S, Roland PE. Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage. 1998;8(2):113–128. doi: 10.1006/nimg.1998.0336. [DOI] [PubMed] [Google Scholar]
- Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, Kalso E, Hari R. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci U S A. 2010;107(14):6493–6497. doi: 10.1073/pnas.1001504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler I, Ball T, Wankerl J, Strigo IA. Pain and emotion in the insular cortex: evidence for functional reorganization in major depression. Neurosci Lett. 2012;520(2):204–209. doi: 10.1016/j.neulet.2012.03.095. [DOI] [PubMed] [Google Scholar]
- Oke AF, Carver LA, Gouvion CM, Adams RN. Three-dimensional mapping of norepinephrine and serotonin in human thalamus. Brain Res. 1997;763(1):69–78. doi: 10.1016/s0006-8993(97)00404-6. [DOI] [PubMed] [Google Scholar]
- Padmala S, Lim SL, Pessoa L. Pulvinar and Affective Significance: Responses Track Moment-to-Moment Stimulus Visibility. Front Hum Neurosci. 2010;4 doi: 10.3389/fnhum.2010.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010;11(11):773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS. Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1997;379(3):313–332. [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. The Orbitofrontal Oracle: Cortical Mechanisms for the Prediction and Evaluation of Specific Behavioral Outcomes. Neuron. 2014;84(6):1143–1156. doi: 10.1016/j.neuron.2014.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337(6095):753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Martinez A, Pomarol-Clotet E, Gomar J, Vila F, Sarro S, Capdevila A, Bullmore E. A simple view of the brain through a frequency-specific functional connectivity measure. Neuroimage. 2008;39(1):279–289. doi: 10.1016/j.neuroimage.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Schippers MB, Renken R, Keysers C. The effect of intra- and inter-subject variability of hemodynamic responses on group level Granger causality analyses. Neuroimage. 2011;57(1):22–36. doi: 10.1016/j.neuroimage.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Shipp S. The functional logic of cortico-pulvinar connections. Philos Trans R Soc Lond B Biol Sci. 2003;358(1438):1605–1624. doi: 10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann CF, Nichols TE, Ramsey JD, Woolrich MW. Network modelling methods for FMRI. Neuroimage. 2011;54(2):875–891. doi: 10.1016/j.neuroimage.2010.08.063. [DOI] [PubMed] [Google Scholar]
- Snow JC, Allen HA, Rafal RD, Humphreys GW. Impaired attentional selection following lesions to human pulvinar: evidence for homology between human and monkey. Proc Natl Acad Sci U S A. 2009;106(10):4054–4059. doi: 10.1073/pnas.0810086106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayonnejad R, Yang S, Kumar A, Ajilore O. Clinical, cognitive, and functional connectivity correlations of resting-state intrinsic brain activity alterations in unmedicated depression. J Affect Disord. 2014;172C:241–250. doi: 10.1016/j.jad.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano A, Varrone A, Gulyas B, Karlsson P, Tauscher J, Halldin C. Mapping of the norepinephrine transporter in the human brain using PET with (S,S)-[18F]FMeNER-D2. Neuroimage. 2008;42(2):474–482. doi: 10.1016/j.neuroimage.2008.05.040. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang Z, Liao W, Xu Q, Zhang J, Lu W, Jiao Q, Chen G, Feng J, Lu G. Frequency-dependent amplitude alterations of resting-state spontaneous fluctuations in idiopathic generalized epilepsy. Epilepsy Res. 2014;108(5):853–860. doi: 10.1016/j.eplepsyres.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Young KA, Holcomb LA, Bonkale WL, Hicks PB, Yazdani U, German DC. 5HTTLPR polymorphism and enlargement of the pulvinar: unlocking the backdoor to the limbic system. Biol Psychiatry. 2007;61(6):813–818. doi: 10.1016/j.biopsych.2006.08.047. [DOI] [PubMed] [Google Scholar]
- Yu R, Chien YL, Wang HL, Liu CM, Liu CC, Hwang TJ, Hsieh MH, Hwu HG, Tseng WY. Frequency-specific alternations in the amplitude of low-frequency fluctuations in schizophrenia. Hum Brain Mapp. 2014;35(2):627–637. doi: 10.1002/hbm.22203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zang ZX, Yan CG, Dong ZY, Huang J, Zang YF. Granger causality analysis implementation on MATLAB: a graphic user interface toolkit for fMRI data processing. J Neurosci Methods. 2012;203(2):418–426. doi: 10.1016/j.jneumeth.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172(1):137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, Castellanos FX, Biswal BB, Milham MP. The oscillating brain: complex and reliable. Neuroimage. 2010;49(2):1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]


