Abstract
The MC1R gene, suggested to be involved in Parkinson disease (PD) and melanoma, was sequenced in PD patients (n=539) and controls (n=265) from New-York, and PD patients (n=551), rapid eye movement sleep behavior disorder (RBD) patients (n=351) and controls (n=956) of European ancestry. Sixty-eight MC1R variants were identified, including 7 common variants with frequency>0.01. None of the common variants was associated with PD or RBD in the different regression models. In a meta-analysis with fixed-effect model, the p.R160W variant was associated with an increased risk for PD (OR=1.22, 95%CI 1.02-1.47, p=0.03) but with significant heterogeneity (p=0.048). Removing one study that introduced the heterogeneity resulted in non-significant association (OR=1.11, 95%CI 0.92-1.35, p=0.27, heterogeneity p=0.57). Rare variants had similar frequencies in patients and controls (10.54% and 10.15%, respectively, p=0.75), and no cumulative effect of carrying more than one MC1R variant was found. The current study does not support a role for the MC1R p.R160W and other variants in susceptibility for PD or RBD.
Introduction
There is an unexplained yet well-validated association between Parkinson disease (PD, MIM no. 168600) and melanoma; patients with PD have an increased risk for developing melanoma, and melanoma patients have an increased risk for PD.(Liu, et al., 2011,Pan, et al., 2011) Several hypotheses were proposed in an attempt to explain the mechanism underlying this association; however, the exact causative factors are yet to be determined. One of the hypotheses is the existence of genetic pleotropy, i.e. the same genetic risk factors for both diseases.
Large genetic studies have identified various risk factors for PD, including genome wide associated loci(Do, et al., 2011,International Parkinson Disease Genomics, et al., 2011,Nalls, et al., 2014,Satake, et al., 2009,Simon-Sanchez, et al., 2009) or mutations in specific genes such as GBA, LRRK2, SNCA, VPS35, SMPD1, PARK2, PINK1, PARK7 and others (reviewed in (Gan-Or, et al., 2015,Trinh and Farrer, 2013)). However, none of these genetic loci or genes can currently explain the association between PD and melanoma. Recently, it was suggested that the melanoma-related MC1R gene, encoding the melanocortin 1 receptor, is associated with PD,(Tell-Marti, et al., 2015b) but this association is currently controversial.(Dong, et al., 2014,Elincx-Benizri, et al., 2014,Lubbe, et al., 2015,Tell-Marti, et al., 2015a) In a Spanish cohort of PD patients and controls, the melanoma-associated MC1R p.R160W variant was associated with a 2-fold increased risk for PD.(Tell-Marti, et al., 2015b) However, a previous, larger case-control study that included the MC1R p.R160W variant did not identify this association.(Dong, et al., 2014) Moreover, in a smaller study that included patients with PD alone, PD with melanoma, and melanoma alone, neither this variant nor other MC1R variants were associated with PD.(Elincx-Benizri, et al., 2014) In a Chinese cohort, in which the MC1R p.R160W was absent, other MC1R variants were also not associated with an increased risk for PD.(Foo, et al., 2015) Furthermore, the MC1R gene was not identified in any of the large genome wide association studies (GWAS) (Do, et al., 2011,International Parkinson Disease Genomics, et al., 2011,Nalls, et al., 2014,Satake, et al., 2009,Simon-Sanchez, et al., 2009), and no single nucleotide polymorphism (SNP) in the MC1R locus was associated with PD, even without correction for multiple comparisons in the PDGene database (www.pdgene.org).
In the current study, we aimed to examine the role of the MC1R gene in PD. Furthermore, since rapid eye movement (REM) sleep behavior disorder (RBD), a parasomnia that is a prodromal disorder for PD and other synucleinopathies, (Postuma, 2014,Postuma, et al., 2015b) the association between MC1R variants and RBD was also studied.
Methods
Study populations
Three populations were included in the current study: 1) a cohort of unrelated, consecutively recruited PD patients (n=539) and controls (n=265) from Columbia University, New-York, NY, USA (more details on the recruitment of cohort 1, termed “SPOT” cohort, were previously published(Alcalay, et al., 2015)). 2) a cohort of unrelated, consecutively recruited PD patients (n=551) and controls (n=956) of European ancestry, mainly French-Canadian and French, and 3) a cohort of unrelated, consecutively recruited RBD patients (n=351) of European ancestry, also mainly French-Canadian and French. Cohort 2 was collected through a network of collaborators from France and Quebec, including the Quebec Parkinson’s Network (http://rpq-qpn.ca/). Cohort 3 was collected at the Montreal Neurological Institute (MNI), Montreal, Canada, by collaborators from the International RBD Study Group (Postuma, et al., 2015b, Schenck, et al., 2013) from Europe and Canada. Basic demographic characteristics of these cohorts are detailed in Table 1. The control population from the MNI was composed of ethnicity-matched elderly controls (n=553, average age 51.8 ± 13.2 years) and young controls (n=403, average age 31.9 ± 4.9 years). Since the frequencies of the tested variants were nearly identical in these two control populations (see results), they could be reliably combined for the analysis, after adjusting for the age differences in the association analyses (see methods). Lack of relatedness and the ancestry in cohorts 2 and 3 were ascertained by unpublished genome-wide association study data, and in cohort 1 it was ascertained by the clinician who recruited and routinely treating the patients. PD patients were diagnosed according to the UK Brain Bank Criteria (but without excluding patients who reported family history of PD) by neurologists specialized in movement disorders. RBD was diagnosed according to the International Classification of Sleep Disorders criteria (ICSD-2) by neurologists specialized in sleep disorders, based on both history and polysomnography showing REM sleep without atonia. All patients and controls signed an informed consent form before entering the study, and the protocols were approved by the respective institutional review boards.
Table 1.
Demographic characteristics of the study populations
| MNIa | New York | |||||
|---|---|---|---|---|---|---|
| PD | RBD | Total PD + RBD | Controls | PD | Controls | |
| Number | 551 | 351 | 902 | 956 | 539 | 265 |
|
Men, n
(%) b |
346 (63.7%) |
264 (78.6%) |
610 (69.4%) |
480 (50.3%) |
346 (64.2%) |
92 (34.7%) |
|
Age
(± SD)c |
65.7 (± 9.7) |
67.5 (± 8.7) |
66.4 (± 9.4) |
43.5 (± 14.4) |
65.9 (± 10.6) |
64.8 (± 10.4) |
PD, Parkinson disease; RBD, rapid eye movement sleep behavior disorder; SD, standard deviation
MNI, Montreal Neurological Institute, Montreal, Canada, is the center where samples of European ancestry where collected through international collaborations as detailed in the methods.
Data on gender was not available for 8 PD, 15 RBD and one control from Montreal.
Data on age was not available for 14 PD, 41 RBD, 10 control from Montreal, and for one PD and 3 controls from Columbia, NY.
Sequencing of the MC1R gene
DNA was extracted by using a standard salting-out protocol. The entire coding sequence of the MC1R gene (NM_002386) was amplified by using the forward primer 5’ GCAGCACCATGAACTAAGCA 3’ and the reverse primer 5’ CAGGGTCACACAGGAACCA 3’ with the AmpliTaq Gold DNA Polymerase (Applied Biosystems, CA, USA) or the Taq DNA polymerase (Qiagen, Maryland, USA). The amplified products were then sequenced using the forward primer 5’ AACCTGCACTCACCCATGTA 3’ and the reverse primer 5’ TTTAAGGCCAAAGCCCTGGT 3’ at the Genome Quebec Innovation Centre (Montréal, QC, Canada) using a 3730XL DNAnalyzer (Applied Biosystems, CA, USA). The sequences were analyzed using the Genalys 3.3b software. All variants that were identified in the forward sequencing were also identified in the reverse sequencing in the overlapping region. Furthermore, forward and reverse sequencing of 40 samples was repeated from another tube of DNA that was taken in another visit of the patient, with a 100% match. Only samples with both forward and reverse sequencing were included in the analysis.
Statistical analysis
To compare single categorical variables, χ2 or Fisher’s exact test was used, and to compare continuous variables, Student’s t-test or ANOVA was used. χ2 with one degree of freedom was used to determine whether the observed genotype frequencies of the common MC1R variants deviate from the expected frequencies based on Hardy-Weinberg Equilibrium (HWE). To estimate the association between the detected common MC1R variants and PD or RBD, binary logistic regression with the status of the individual (patient or control) as the dependent variable was used. When patients and controls did not match for sex or age, these variables were added as covariates for the analysis to adjust for their effects. When a regression model for all patients from both centers (NY and Montreal) was performed, the site was also added as covariate to adjust for the differences in the genetic background of the two populations from the two centers. Power analysis demonstrated that our population had a power of >98% to detect the originally reported association between the MC1R p.R160W and PD (Tell-Marti, et al., 2015b). Furthermore, our PD population (1090 patients and 1221 controls) had a power of >80% to detect a much lower odds ratio of 1.4. All the statistical analysis, except for the meta-analysis, was performed using the SPSS v.21 software (IBM, Ltd.). The meta-analysis was performed by using an R package (Metafor). Data for the meta-analysis were collected and weighted at the individual level, and heterozygous and homozygous carriers were considered as carriers for the analysis. The Cochran-Mantel-Haenszel test was used to pool the studies and calculate the odds ratios (OR) using either the fixed-or random-effect models. Tarone's test was applied to estimate heterogeneity, and in case of significant heterogeneity, the source of heterogeneity was identified by excluding studies one by one, and re-calculating the Tarone's test for heterogeneity. The online tools SIFT (Kumar, et al., 2009) and PolyPhen-2 (Adzhubei, et al., 2010) were used to predict the effects of the MC1R variants.
Results
Common and rare MC1R variants have no or minimal association with the risk for PD or RBD
A total of 68 MC1R variants were identified in patients and controls. Supplementary Table 1 details these variants, their predicted effect on the protein, and their distribution among patients and controls. Seven common variants with allele frequency > 0.01 were included in logistic regression models to determine their association with the risk for PD and RBD (Table 2). None of these variants deviated from Hardy-Weinberg equilibrium (HWE). First, PD patients and RBD patients were separately compared to their respective control populations from each center. Subsequently, a combined analysis of the PD and RBD patients from Montreal vs. their controls, and a combined analysis of all PD and RBD patients and controls from both centers were performed. The elderly and young controls from Montreal had nearly identical frequencies for the seven common variants (0.29 and 0.30 for the p.V60L variant, respectively, 0.13 and 0.13 for p.V92M, 0.11 and 0.10 for p.R151C, 0.08 and 0.08 for p.R160W, 0.07 and 0.08 for p.R163Q, 0.04 and 0.04 for p.D294H, and 0.17 and 0.17 for p.T314T) and could therefore be combined reliably as one control group. None of the seven common MC1R variants was associated with risk for PD or RBD in any of the models (Table 2). The MC1R p.R160W, which was suggested to be associated with PD,(Tell-Marti, et al., 2015b) had non-significant odd ratios (ORs) ranging between 0.76-1.13 in the different analyses (uncorrected p value > 0.45 in all the analyses, Table 2). Of the seven common variants, six were nonsynonymous, and similar analysis of these six variants alone resulted in very similar, non-significant results (data not shown).
Table 2.
Association of common MC1R variants with risk for PD or RBD
| Minor allele frequency | OR (95% CI), p value a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variant | PD MNI (n=551) |
RBD MNI (n=351) |
Control MNI (n=956) |
PD NY (n=539) |
Control NY (n=265) |
A b | B c | C d | D e | E f |
| p.V60L | 0.142 | 0.121 | 0.156 | 0.195 | 0.200 | 0.83 (0.60-1.15), p=0.26 |
0.72 (0.47-1.09), p=0.12 |
0.80 (0.59-1.09), p=0.15 |
0.91 (0.65-1.28), p=0.61 |
0.83 (0.67-1.03), p=0.10 |
| p.V92M | 0.066 | 0.068 | 0.067 | 0.070 | 0.072 | 1.01 (0.47-2.15), p=0.99 |
1.15 (0.47-2.80), p=0.77 |
1.03 (0.52-2.05), p=0.93 |
0.90 (0.45-1.78), p=0.75 |
0.88 (0.55-1.41), p=0.60 |
| p.R151C | 0.054 | 0.064 | 0.054 | 0.065 | 0.070 | 0.92 (0.57-1.47), p=0.72 |
1.14 (0.67-1.96), p=0.63 |
1.05 (0.69-1.60), p=0.81 |
0.77 (0.48-1.23), p=0.27 |
0.90 (0.67-1.23), p=0.52 |
| p.R160W | 0.048 | 0.037 | 0.039 | 0.045 | 0.053 | 1.13 (0.68-1.90), p=0.63 |
0.76 (0.38-1.54), p=0.45 |
1.10 (0.67-1.80), p=0.72 |
0.92 (0.53-1.57), p=0.76 |
0.96 (0.68-1.35), p=0.80 |
| p.R163Q | 0.024 | 0.036 | 0.036 | 0.064 | 0.047 | 0.80 (0.43-1.50), p=0.49 |
1.26 (0.61-2.63), p=0.53 |
0.92 (0.52-1.62), p=0.77 |
1.30 (0.77-2.21), p=0.33 |
1.09 (0.76-1.58), p=0.63 |
| p.D294H | 0.018 | 0.014 | 0.021 | 0.011 | 0.019 | 0.64 (0.32-1.29), p=0.21 |
0.46 (0.18-1.21), p=0.12 |
0.60 (0.31-1.17), p=0.13 |
0.68 (0.28-1.68), p=0.41 |
0.67 (0.40-1.11), p=0.12 |
| p.T314T | 0.085 | 0.093 | 0.091 | 0.102 | 0.102 | 0.92 (0.46-1.82), p=0.80 |
0.83 (0.37-1.88), p=0.65 |
0.90 (0.48-1.67), p=0.73 |
0.99 (0.54-1.81), p=0.98 |
1.03 (0.68-1.56), p=0.90 |
OR, odds ratio; CI, confidence interval, PD MNI, Parkinson disease samples collected at the Montreal Neurological Institute; RBD MNI, REM sleep Behavior Disorder patients collected at the Montreal Neurological Institute, through the International RBD study group; PD NY, Parkinson disease patients collected at Columbia University, New-York; Control MNI, controls collected at the Montreal Neurological Institute; Control NY, controls collected at Columbia University, New-York. Variants were called according to NM_002386.
Bonferroni correction for multiple comparisons set the cut-of p value for statistical significance at p<0.0071.
p value comparing PD MNI to Control MNI using a regression model, adjusted for gender and age
p value comparing RBD MNI to Control MNI using a regression model, adjusted for gender and age
p value comparing PD MNI + RBD MNI to Control MNI using a regression model, adjusted for gender and age
p value comparing PD NY to Control NY using a regression model, adjusted for gender and age
p value comparing all patients (PD MNI + RBD MNI + PD NY) to all controls (Control MNI + Control NY) using a regression model, adjusted for site, gender and age
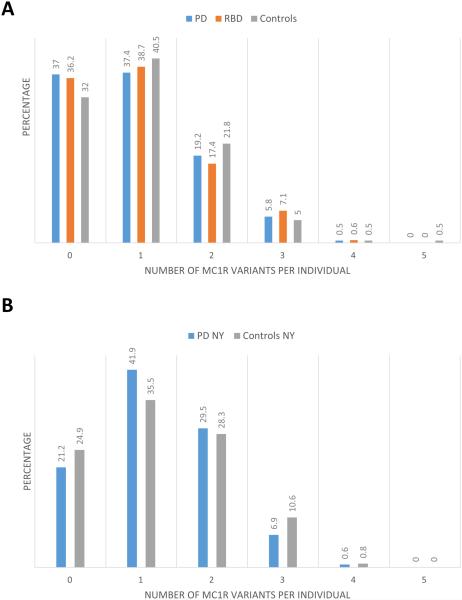
The combined frequency of all the rare variants (allele frequency < 0.01) was nearly identical among patients and controls (10.54% and 10.15%, respectively, p=0.75, Fischer exact test). The combined frequency of rare nonsynonymous, frameshift or stop mutations was also very similar among patients and controls (8.53% and 8.68%, respectively, p=0.44, Fisher’s exact test). Furthermore, no cumulative effect of carrying more than one MC1R variant was found (Figure 1). Several rare variants had higher frequencies in patients compared to controls (Supplementary Table 1), but none of these variants reached statistical significance after correction for multiple comparisons.
Figure 1. Cumulative carriage of MC1R variants in the different study populations.
A. The carriage frequencies of one or more MC1R variants were similar among PD patients, RBD patients and controls collected at the MNI (p>0.05 for all comparisons) B. The carriage frequencies of one or more MC1R variants were similar among PD patients and controls collected at Columbia, NY (p>0.05 for all comparisons).
Meta-analysis of the effect of the MC1R p.R160W variant on PD risk demonstrates minimal or no effect
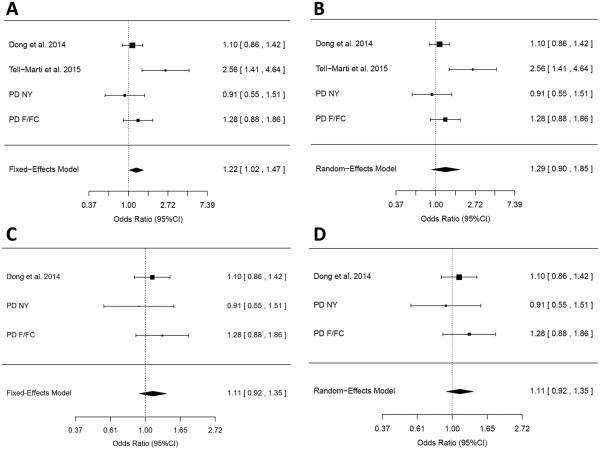
In order to further determine whether the MC1R p.R160W is associated with the risk for PD as was previously suggested,(Tell-Marti, et al., 2015b) a meta-analysis of four populations where this variant was specifically analyzed (two that were previously published and two from the current study, Table 3) was performed. Both fixed-and random-effect models were used (Figure 2A and 2B, respectively). In the fixed-effect model, the MC1R p.R160W was associated with an increased risk for PD (OR = 1.22, 95% CI 1.02-1.47, p=0.03, Figure 2A). However, there was significant heterogeneity in this model (p=0.048), which was introduced by the only study that had previously demonstrated an association between this variant and PD,(Tell-Marti, et al., 2015b) rendering the meta-analysis results less reliable. After the exclusion of this study, there was no significant association (OR = 1.11, 95% CI 0.92-1.35, p=0.27), and the heterogeneity was not significant (p=0.57, Figure 2C). In the random-effect model, the MC1R p.R160W was not associated with an increased risk for PD (OR = 1.29, 95% CI 0.90-1.85, p=0.17, p for heterogeneity = 0.048, Figure 2B). In this model too, the exclusion of the same study resulted in non-significant association with non-significant heterogeneity (Figure 2D). When other studies rather than the original study describing this suggested association are being removed from the meta-analysis, the heterogeneity remains significant, demonstrating that this study (Tell-Marti, et al., 2015b) is the source of heterogeneity.
Table 3.
Populations included in meta-analysis of the MC1R p.R160W variant in PD.
| Study | population | Number of PD patients, (% carriers of the MC1R p.R160W variant)a |
Number of controls (% carriers of the MC1R p.R160W variant) |
|---|---|---|---|
| Dong et al.13 | Non-Hispanic whites |
777 (13.6%) | 1550 (12.5%) |
| Tell-Marti et al.11 |
Caucasians from Spain |
870 (5.0%) | 736 (2.0%) |
| Current study | Mainly French- Canadian / French |
551 (9.6%) | 956 (7.8%) |
| Current study | North-American from NY |
539 (8.9%) | 265 (9.8%) |
| Current study total |
1090 (9.3%) | 1221 (8.2%) |
Since RBD patients may convert to other synucleinopathies, such as Dementia with Lewy Bodies and Multiple System Atrophy, they were not included in the meta-analysis for the effect of the MC1R p.R160W variant on PD risk.
Figure 2. Meta-analyses of the MC1R p.R160W variant.
A. Meta-analysis of the MC1R p.R160W in four studies under the fixed-effect model. While three studies had non-significant ORs of 0.91 – 1.28, one study alone (Tell-Marti et al.) drove the association. However, these results cannot be considered statistically significant due to the significant heterogeneity (p=0.048). B. Meta-analysis of the MC1R p.R160W in four studies under the random-effect model. Here too, one study introduced a significant heterogeneity to the model (Heterogeneity p=0.048) C. Meta-analysis of the MC1R p.R160W in three studies, excluding the one study that introduced heterogeneity, under the fixed-effect model (OR=1.11, 95% CI 0.92-1.35, p=0.27, heterogeneity p=0.57). D. Meta-analysis of the MC1R p.R160W in three studies, excluding the one study that introduced heterogeneity, under the random-effect model, resulting in identical OR estimates and statistics as the fixed-effect model.
Discussion
The current study demonstrates that variants in the MC1R gene have minimal or no association with the risk for PD or RBD. More specifically, the MC1R p.R160W variant, which was suggested to be a risk factor for PD,(Tell-Marti, et al., 2015b) was not associated with PD or RBD when comparing specific populations or in the meta-analysis. The discrepancies between the single study that suggested an association between the MC1R p.R160W variant and the current study could be explained by the different populations used. It is possible, for example, that in different populations, other genetic or environmental factors exist that differentially modify the association of MC1R with PD. However, if this variant is hypothesized to be the functional variant that increases the risk for PD, by affecting the function of the melanocortin 1 receptor, it should have relatively similar effects in each population. Further support for the lack of association of MC1R with PD is provided by the meta-OR previously reported for this variant (OR=0.98, 95% CI 0.89-1.07, p=0.62) in the International PD Genomic Consortium data,(Dong, et al., 2014) and the lack of signal in this locus in the PDGene database (www.pdgene.org). Although we cannot decisively rule out that this variant has a minor role in PD susceptibility that can only be detected in a much larger meta-analysis, the current data suggest that the MC1R gene, and specifically the p.R160W variant, are probably not associated with PD, or have a very small effect. A previous observation from a cohort of 272 PD patients and 1185 controls, which suggested that the p.R151C variant is associated with PD,(Gao, et al., 2009b) was also not supported by our results.
From a purely genetic perspective, there is not enough evidence that currently points to a specific shared genetic background; however, a few interesting observations were made. The largest GWASs from both diseases showed no overlap between the associated loci,(Law, et al., 2015,Nalls, et al., 2014) and a study that specifically targeted known PD loci in a large melanoma cohort failed to identify an association.(Meng, et al., 2012) However, one of the GWAS loci that was identified in melanoma(Meng, et al., 2012) and melanocytic cutaneous nevi(Falchi, et al., 2009) cohorts is the PLA2G6 locus. Interestingly, mutations in PLA2G6 may cause PD or Parkinsonism-dystonia syndrome,(Gui, et al., 2013,Kauther, et al., 2011,Malaguti, et al., 2015,Paisan-Ruiz, et al., 2009) suggesting a potential genetic link between the two conditions.(Paisan-Ruiz and Houlden, 2010) Another intriguing locus that was identified in PD GWAS is around the GPNMB gene,(Nalls, et al., 2014) which codes for the glycoprotein non-metastatic melanoma protein B, which may have an important role in melanoma.(Maric, et al., 2013,Tomihari, et al., 2010) Additional studies are needed to determine if these genes may contribute to the co-occurrence of PD and melanoma. In the current study, data on melanoma occurrence in the PD and RBD cohorts were not available.
In order to determine whether genetics, environmental factors, or their interaction leads to the co-occurrence of PD and melanoma, large genetic-environmental studies are needed. To reach statistical power that will allow drawing strong conclusions, large collaborations between different centers are needed to reach the number of patients required for such analysis. Understanding the underlying causes of this co-morbidity is of great importance, since it may allow specific interventions in PD patients or in prodromal PD to prevent melanoma.
Supplementary Material
Highlights.
The melanoma variant MC1R p.R160W was suggested to be involved in Parkinson disease
MC1R was sequenced in a total of 2662 individuals with PD, RBD and controls
No MC1R variant, including p.R160W, was associated with PD
The MC1R gene has no major role in PD
Acknowledgements
We thank the patients and controls for their participation in this study. This work was financially supported by the Canadian Institutes of Health Research (CIHR) and by the Michael J. Fox Foundation. The cohort from Columbia, NY, was funded by Parkinson's Disease Foundation and the NIH (K02NS080915, and UL1 TR000040). ZGO is supported by a postdoctoral fellowship from the Canadian Institutes for Health Research (CIHR). JFG holds a Canada Research Chair in Cognitive Decline in Pathological Aging. GAR holds a Canada Research Chair in Genetics of the Nervous System and the Wilder Penfield Chair in Neurosciences. We thank Daniel Rochefort, Pascale Hince, Helene Catoire, Cathy Mirarchi and Vessela Zaharieva for their assistance. We thank the Quebec Parkinson’s Network and its members (http://rpq-qpn.ca/) for their collaboration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors report no conflict of interests.
References
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–9. doi: 10.1038/nmeth0410-248. doi:10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcalay RN, Levy OA, Waters CC, Fahn S, Ford B, Kuo SH, Mazzoni P, Pauciulo MW, Nichols WC, Gan-Or Z, Rouleau GA, Chung WK, Wolf P, Oliva P, Keutzer J, Marder K, Zhang X. Glucocerebrosidase activity in Parkinson's disease with and without GBA mutations. Brain. 2015 doi: 10.1093/brain/awv179. doi:10.1093/brain/awv179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorfa A, Betemps D, Morignat E, Lazizzera C, Hogeveen K, Andrieu T, Baron T. Specific pesticide-dependent increases in alpha-synuclein levels in human neuroblastoma (SH-SY5Y) and melanoma (SK-MEL-2) cell lines. Toxicol Sci. 2013;133(2):289–97. doi: 10.1093/toxsci/kft076. doi:10.1093/toxsci/kft076. [DOI] [PubMed] [Google Scholar]
- Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, Francke U, Mountain JL, Goldman SM, Tanner CM, Langston JW, Wojcicki A, Eriksson N. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson's disease. PLoS Genet. 2011;7(6):e1002141. doi: 10.1371/journal.pgen.1002141. doi:10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Gao J, Nalls M, Gao X, Huang X, Han J, Singleton AB, Chen H, International Parkinson's Disease Genomics, C Susceptibility loci for pigmentation and melanoma in relation to Parkinson's disease. Neurobiol Aging. 2014;35(6):1512–e5. 10. doi: 10.1016/j.neurobiolaging.2013.12.020. doi:10.1016/j.neurobiolaging.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elincx-Benizri S, Inzelberg R, Greenbaum L, Cohen OS, Yahalom G, Laitman Y, Djaldetti R, Orlev Y, Scope A, Azizi E, Friedman E, Hassin-Baer S. The melanocortin 1 receptor (Mc1r) variants do not account for the co-occurrence of Parkinson's disease and malignant melanoma. J Mol Neurosci. 2014;54(4):820–5. doi: 10.1007/s12031-014-0425-1. doi:10.1007/s12031-014-0425-1. [DOI] [PubMed] [Google Scholar]
- Falchi M, Bataille V, Hayward NK, Duffy DL, Bishop JA, Pastinen T, Cervino A, Zhao ZZ, Deloukas P, Soranzo N, Elder DE, Barrett JH, Martin NG, Bishop DT, Montgomery GW, Spector TD. Genome-wide association study identifies variants at 9p21 and 22q13 associated with development of cutaneous nevi. Nat Genet. 2009;41(8):915–9. doi: 10.1038/ng.410. doi:10.1038/ng.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo JN, Zhao Y, Liu J, Tan EK. Nonsynonymous variants in MC1R are rare in Chinese Parkinson disease cases. Ann Neurol. 2015;78(1):152–3. doi: 10.1002/ana.24419. doi:10.1002/ana.24419. [DOI] [PubMed] [Google Scholar]
- Frauscher B, Jennum P, Ju YE, Postuma RB, Arnulf I, Cochen De Cock V, Dauvilliers Y, Fantini ML, Ferini-Strambi L, Gabelia D, Iranzo A, Leu-Semenescu S, Mitterling T, Miyamoto M, Miyamoto T, Montplaisir JY, Oertel W, Pelletier A, Prunetti P, Puligheddu M, Santamaria J, Sonka K, Unger M, Wolfson C, Zucconi M, Terzaghi M, Hogl B, Mayer G, Manni R. Comorbidity and medication in REM sleep behavior disorder: a multicenter case-control study. Neurology. 2014;82(12):1076–9. doi: 10.1212/WNL.0000000000000247. doi:10.1212/WNL.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan-Or Z, Dion PA, Rouleau GA. Genetic perspective on the role of the Autophagy-Lysosome Pathway in Parkinson disease. Autophagy. 2015:0. doi: 10.1080/15548627.2015.1067364. doi:10.1080/15548627.2015.1067364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Simon KC, Han J, Schwarzschild MA, Ascherio A. Family history of melanoma and Parkinson disease risk. Neurology. 2009a;73(16):1286–91. doi: 10.1212/WNL.0b013e3181bd13a1. doi:10.1212/WNL.0b013e3181bd13a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Simon KC, Han J, Schwarzschild MA, Ascherio A. Genetic determinants of hair color and Parkinson's disease risk. Ann Neurol. 2009b;65(1):76–82. doi: 10.1002/ana.21535. doi:10.1002/ana.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui YX, Xu ZP, Wen L, Liu HM, Zhao JJ, Hu XY. Four novel rare mutations of PLA2G6 in Chinese population with Parkinson's disease. Parkinsonism Relat Disord. 2013;19(1):21–6. doi: 10.1016/j.parkreldis.2012.07.016. doi:10.1016/j.parkreldis.2012.07.016. [DOI] [PubMed] [Google Scholar]
- International Parkinson Disease Genomics, C. Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simon-Sanchez J, Schulte C, Lesage S, Sveinbjornsdottir S, Stefansson K, Martinez M, Hardy J, Heutink P, Brice A, Gasser T, Singleton AB, Wood NW. Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377(9766):641–9. doi: 10.1016/S0140-6736(10)62345-8. doi:10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauther KM, Hoft C, Rissling I, Oertel WH, Moller JC. The PLA2G6 gene in early-onset Parkinson's disease. Mov Disord. 2011;26(13):2415–7. doi: 10.1002/mds.23851. doi:10.1002/mds.23851. [DOI] [PubMed] [Google Scholar]
- Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4(7):1073–81. doi: 10.1038/nprot.2009.86. doi:10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- Law MH, Bishop DT, Lee JE, Brossard M, Martin NG, Moses EK, Song F, Barrett JH, Kumar R, Easton DF, Pharoah PD, Swerdlow AJ, Kypreou KP, Taylor JC, Harland M, Randerson-Moor J, Akslen LA, Andresen PA, Avril MF, Azizi E, Scarra GB, Brown KM, Debniak T, Duffy DL, Elder DE, Fang S, Friedman E, Galan P, Ghiorzo P, Gillanders EM, Goldstein AM, Gruis NA, Hansson J, Helsing P, Hocevar M, Hoiom V, Ingvar C, Kanetsky PA, Chen WV, Geno MELC, Essen-Heidelberg I, Group SDHS, Q M, Investigators Q, Investigators A, Group AMS, Landi MT, Lang J, Lathrop GM, Lubinski J, Mackie RM, Mann GJ, Molven A, Montgomery GW, Novakovic S, Olsson H, Puig S, Puig-Butille JA, Qureshi AA, Radford-Smith GL, van der Stoep N, van Doorn R, Whiteman DC, Craig JE, Schadendorf D, Simms LA, Burdon KP, Nyholt DR, Pooley KA, Orr N, Stratigos AJ, Cust AE, Ward SV, Hayward NK, Han J, Schulze HJ, Dunning AM, Bishop JA, Demenais F, Amos CI, MacGregor S, Iles MM. Genome-wide meta-analysis identifies five new susceptibility loci for cutaneous malignant melanoma. Nat Genet. 2015 doi: 10.1038/ng.3373. doi:10.1038/ng.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Gao X, Lu Y, Chen H. Meta-analysis of the relationship between Parkinson disease and melanoma. Neurology. 2011;76(23):2002–9. doi: 10.1212/WNL.0b013e31821e554e. doi:10.1212/WNL.0b013e31821e554e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbe SJ, Escott-Price V, Brice A, Gasser T, Hardy J, Heutink P, Sharma M, Wood NW, Nalls M, Singleton AB, Williams NM, Morris HR, International Parkinson's Disease Genomics, C Is the MC1R variant p.R160W associated with Parkinson's? Ann Neurol. 2015 doi: 10.1002/ana.24527. doi:10.1002/ana.24527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaguti MC, Melzi V, Di Giacopo R, Monfrini E, Di Biase E, Franco G, Borellini L, Trezzi I, Monzio Compagnoni G, Fortis P, Feraco P, Orrico D, Cucurachi L, Donner D, Rizzuti M, Ronchi D, Bonato S, Bresolin N, Corti S, Comi GP, Di Fonzo A. A novel homozygous PLA2G6 mutation causes dystonia-parkinsonism. Parkinsonism Relat Disord. 2015;21(3):337–9. doi: 10.1016/j.parkreldis.2015.01.001. doi:10.1016/j.parkreldis.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Maric G, Rose AA, Annis MG, Siegel PM. Glycoprotein non-metastatic b (GPNMB): A metastatic mediator and emerging therapeutic target in cancer. Onco Targets Ther. 2013;6:839–52. doi: 10.2147/OTT.S44906. doi:10.2147/OTT.S44906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng S, Song F, Chen H, Gao X, Amos CI, Lee JE, Wei Q, Qureshi AA, Han J. No association between Parkinson disease alleles and the risk of melanoma. Cancer Epidemiol Biomarkers Prev. 2012;21(1):243–5. doi: 10.1158/1055-9965.EPI-11-0905. doi:10.1158/1055-9965.EPI-11-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, DeStefano AL, Kara E, Bras J, Sharma M, Schulte C, Keller MF, Arepalli S, Letson C, Edsall C, Stefansson H, Liu X, Pliner H, Lee JH, Cheng R, International Parkinson's Disease Genomics, C., Parkinson's Study Group Parkinson's Research: The Organized, G.I., andMe, GenePd, NeuroGenetics Research, C., Hussman Institute of Human, G., Ashkenazi Jewish Dataset, I., Cohorts for, H., Aging Research in Genetic, E., North American Brain Expression, C., United Kingdom Brain Expression, C., Greek Parkinson's Disease, C., Alzheimer Genetic Analysis, G. Ikram MA, Ioannidis JP, Hadjigeorgiou GM, Bis JC, Martinez M, Perlmutter JS, Goate A, Marder K, Fiske B, Sutherland M, Xiromerisiou G, Myers RH, Clark LN, Stefansson K, Hardy JA, Heutink P, Chen H, Wood NW, Houlden H, Payami H, Brice A, Scott WK, Gasser T, Bertram L, Eriksson N, Foroud T, Singleton AB. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat Genet. 2014;46(9):989–93. doi: 10.1038/ng.3043. doi:10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JH, Friis S, Frederiksen K. Malignant melanoma and other types of cancer preceding Parkinson disease. Epidemiology. 2006;17(5):582–7. doi: 10.1097/01.ede.0000229445.90471.5e. doi:10.1097/01.ede.0000229445.90471.5e. [DOI] [PubMed] [Google Scholar]
- Olsen JH, Jorgensen TL, Rugbjerg K, Friis S. Parkinson disease and malignant melanoma in first-degree relatives of patients with early-onset melanoma. Epidemiology. 2011;22(1):109–12. doi: 10.1097/EDE.0b013e3181fe21a8. doi:10.1097/EDE.0b013e3181fe21a8. [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Bhatia KP, Li A, Hernandez D, Davis M, Wood NW, Hardy J, Houlden H, Singleton A, Schneider SA. Characterization of PLA2G6 as a locus for dystonia-parkinsonism. Ann Neurol. 2009;65(1):19–23. doi: 10.1002/ana.21415. doi:10.1002/ana.21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Houlden H. Common pathogenic pathways in melanoma and Parkinson disease. Neurology. 2010;75(18):1653–5. doi: 10.1212/WNL.0b013e3181fb4466. doi:10.1212/WNL.0b013e3181fb4466. [DOI] [PubMed] [Google Scholar]
- Pan T, Li X, Jankovic J. The association between Parkinson's disease and melanoma. Int J Cancer. 2011;128(10):2251–60. doi: 10.1002/ijc.25912. doi:10.1002/ijc.25912. [DOI] [PubMed] [Google Scholar]
- Pan T, Zhu J, Hwu WJ, Jankovic J. The role of alpha-synuclein in melanin synthesis in melanoma and dopaminergic neuronal cells. PLoS One. 2012;7(9):e45183. doi: 10.1371/journal.pone.0045183. doi:10.1371/journal.pone.0045183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB. Prodromal Parkinson's disease--using REM sleep behavior disorder as a window. Parkinsonism Relat Disord. 2014;20(Suppl 1):S1–4. doi: 10.1016/S1353-8020(13)00400-8. doi:10.1016/S1353-8020(13)00400-8. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Gagnon JF, Bertrand JA, Genier Marchand D, Montplaisir JY. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology. 2015a;84(11):1104–13. doi: 10.1212/WNL.0000000000001364. doi:10.1212/WNL.0000000000001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Iranzo A, Hogl B, Arnulf I, Ferini-Strambi L, Manni R, Miyamoto T, Oertel W, Dauvilliers Y, Ju YE, Puligheddu M, Sonka K, Pelletier A, Santamaria J, Frauscher B, Leu-Semenescu S, Zucconi M, Terzaghi M, Miyamoto M, Unger MM, Carlander B, Fantini ML, Montplaisir JY. Risk factors for neurodegeneration in idiopathic rapid eye movement sleep behavior disorder: a multicenter study. Ann Neurol. 2015b;77(5):830–9. doi: 10.1002/ana.24385. doi:10.1002/ana.24385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Montplaisir J. Predicting Parkinson's disease - why, when, and how? Parkinsonism Relat Disord. 2009;15(Suppl 3):S105–9. doi: 10.1016/S1353-8020(09)70793-X. doi:10.1016/S1353-8020(09)70793-X. [DOI] [PubMed] [Google Scholar]
- Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami H, Sakoda S, Yamamoto M, Hattori N, Murata M, Nakamura Y, Toda T. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat Genet. 2009;41(12):1303–7. doi: 10.1038/ng.485. doi:10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- Schenck CH, Montplaisir JY, Frauscher B, Hogl B, Gagnon JF, Postuma R, Sonka K, Jennum P, Partinen M, Arnulf I, Cochen de Cock V, Dauvilliers Y, Luppi PH, Heidbreder A, Mayer G, Sixel-Doring F, Trenkwalder C, Unger M, Young P, Wing YK, Ferini-Strambi L, Ferri R, Plazzi G, Zucconi M, Inoue Y, Iranzo A, Santamaria J, Bassetti C, Moller JC, Boeve BF, Lai YY, Pavlova M, Saper C, Schmidt P, Siegel JM, Singer C, St Louis E, Videnovic A, Oertel W. Rapid eye movement sleep behavior disorder: devising controlled active treatment studies for symptomatic and neuroprotective therapy--a consensus statement from the International Rapid Eye Movement Sleep Behavior Disorder Study Group. Sleep Med. 2013;14(8):795–806. doi: 10.1016/j.sleep.2013.02.016. doi:10.1016/j.sleep.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet. 2009;41(12):1308–12. doi: 10.1038/ng.487. doi:10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tell-Marti G, Puig-Butille JA, Potrony M, Badenas C, Malvehy J, Marti MJ, Ezquerra M, Fernandez-Santiago R, Puig S. Lubbe SJ, Escott-Price V, Brice A, et al., editors. Reply to Letter to Editor ANA-15-1051 'Is the MC1R variant p.R160W associated with Parkinson's?'. Ann Neurol. 2015a doi: 10.1002/ana.24373. M, M. doi:10.1002/ana.24526. [DOI] [PubMed] [Google Scholar]
- Tell-Marti G, Puig-Butille JA, Potrony M, Badenas C, Mila M, Malvehy J, Marti MJ, Ezquerra M, Fernandez-Santiago R, Puig S. The MC1R melanoma risk variant p.R160W is associated with Parkinson disease. Ann Neurol. 2015b;77(5):889–94. doi: 10.1002/ana.24373. doi:10.1002/ana.24373. [DOI] [PubMed] [Google Scholar]
- Tomihari M, Chung JS, Akiyoshi H, Cruz PD, Jr., Ariizumi K. DC-HIL/glycoprotein Nmb promotes growth of melanoma in mice by inhibiting the activation of tumor-reactive T cells. Cancer Res. 2010;70(14):5778–87. doi: 10.1158/0008-5472.CAN-09-2538. doi:10.1158/0008-5472.CAN-09-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh J, Farrer M. Advances in the genetics of Parkinson disease. Nat Rev Neurol. 2013;9(8):445–54. doi: 10.1038/nrneurol.2013.132. doi:10.1038/nrneurol.2013.132. [DOI] [PubMed] [Google Scholar]
- Vermeij JD, Winogrodzka A, Trip J, Weber WE. Parkinson's disease, levodopa-use and the risk of melanoma. Parkinsonism Relat Disord. 2009;15(8):551–3. doi: 10.1016/j.parkreldis.2009.05.002. doi:10.1016/j.parkreldis.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Walter U, Heilmann E, Voss J, Riedel K, Zhivov A, Schad SG, Gross GE, Benecke R, Trcka J. Frequency and profile of Parkinson's disease prodromi in patients with malignant melanoma. J Neurol Neurosurg Psychiatry. 2015 doi: 10.1136/jnnp-2014-310239. doi:10.1136/jnnp-2014-310239. [DOI] [PubMed] [Google Scholar]
- Wirdefeldt K, Weibull CE, Chen H, Kamel F, Lundholm C, Fang F, Ye W. Parkinson's disease and cancer: A register-based family study. Am J Epidemiol. 2014;179(1):85–94. doi: 10.1093/aje/kwt232. doi:10.1093/aje/kwt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.