Abstract
Human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells represent a potential alternative source for red blood cell transfusion. However, when using traditional methods with embryoid bodies, ES cell-derived erythroid cells predominantly express embryonic type ε-globin, with lesser fetal type γ-globin and very little adult type β-globin. Furthermore, no β-globin expression is detected in iPS cell-derived erythroid cells. ES cell-derived sacs (ES sacs) have been recently used to generate functional platelets. Due to its unique structure, we hypothesized that ES sacs serve as hemangioblast-like progenitors capable to generate definitive erythroid cells that express β-globin. With our ES sac-derived erythroid differentiation protocol, we obtained ~120 erythroid cells per single ES cell. Both primitive (ε-globin expressing) and definitive (γ- and β-globin expressing) erythroid cells were generated from not only ES cells but also iPS cells. Primitive erythropoiesis is gradually switched to definitive erythropoiesis during prolonged ES sac maturation, concurrent with the emergence of hematopoietic progenitor cells. Primitive and definitive erythroid progenitor cells were selected on the basis of GPA or CD34 expression from cells within the ES sacs before erythroid differentiation. This selection and differentiation strategy represents an important step toward the development of in vitro erythroid cell production systems from pluripotent stem cells. Further optimization to improve expansion should be required for clinical application.
Keywords: Pluripotent stem cells, Erythroid differentiation, Primitive and definitive hematopoiesis, Globin expression
Introduction
Red blood cell (RBC) transfusion is central to the management of a number of severe congenital and acquired anemias, including that due to hematological malignancies, aplastic anemia, pure red blood cell aplasia, thalassemias, and hemoglobinopathies. The risk factors associated with RBC transfusion have been significantly reduced over the past decades. However, despite improved infectious screening and blood group matching, every transfusion represents the associated risks of alloimmunization, transmitting infectious disease, and immediate transfusion reactions. Limitations of current blood substitutes such as artificial oxygen carriers have hindered their general clinical application. Efforts to develop a potential alternative source for RBC transfusion have been pursued by using in vitro erythroid differentiation techniques from human CD34+ cells, peripheral blood mononuclear cells, and embryonic stem/induced pluripotent stem (ES/iPS) cells [1]. The combination of modern reprogramming methods with state of the art genome editing techniques may allow for the creation of identical and genetically corrected RBCs for transfusion [2–4]. Autologous iPS cell-derived RBC circumvents the significant problem of alloimmunization seen in hemoglobinopathy or bone marrow failure patients.
Unfortunately, when erythroid cells are derived from ES/iPS cells with traditional in vitro differentiation protocols using embryoid body (EB) formation and co-culture system, the erythroid cells mainly express embryonic type ε-globin, some fetal type γ-globin, and very little adult type β-globin [5–11]. The predominant production of ε- and γ-globin without β-globin by iPS cell-derived erythroid cells also encumbers their use as an alternative RBC source and a model system to develop genome editing tools for the hemoglobinopathies. Therefore, we sought to generate ES/iPS cell-derived erythroid cells that express high levels of β-globin as means to provide a more useful alternative source for RBC transfusion and as a disease model for new therapy development.
In mammalian development, primitive hematopoiesis begins in the yolk sac (YS), which directly generates primitive RBCs expressing ε-globin (with ζ-globin). Subsequently, definitive hematopoiesis commences in the aorta-gonad-mesonephros (AGM) region and forms definitive RBCs expressing γ- or β-globin (with α-globin). Definitive RBCs are subsequently differentiated from hematopoietic stem cells (HSCs)/hematopoietic progenitor cells (HPCs) in the fetal liver, and finally the bone marrow (BM) [12–17]. HSCs/HPCs are generated from hemangioblasts which produce both hematopoietic cells and endothelium [18–22]. Therefore, hemangioblast formation during in vitro differentiation of ES/iPS cells might be crucial for the derivation of definitive erythroid cells. Recently, β-globin-expressing erythroid cells were generated after induction of hemangioblast-like blast colonies from EBs [23]. In this report, primitive erythroid cells emerged in the early phase of erythroid cell generation, while definitive erythroid cells emerged in the late phase of hemangioblast-like blast colonies [23]. Although high efficiency of erythroid cell generation when using direct co-culture and EB formation has also been described [5–7, 11], the method based on hemangioblast-like colonies seems to have lower efficiency for erythroid cell generation [23, 24]. It has also recently been shown that hemangioblast-like ES cell sacs (ES sacs) can be derived from ES cells to generate functional platelets [25]. We thus hypothesized that the ES sac method would be useful to generate β-globin-expressing definitive erythroid cells that are derived from ES/iPS cells in vitro.
In this study, we generated erythroid cells through ES sacs and evaluated the production efficiency of erythroid cells. Primitive erythroid progenitor cells emerged prior to the emergence of definitive erythroid progenitor cells when HPCs emerge in ES sacs, and these primitive and definitive progenitor cells were identified by the surface markers CD34 or glycophorin A (GPA). For the first time, β-globin-expressing erythroid cells were generated from fibroblast-derived iPS cells as well as ES cells.
Materials and Methods
Cell culture conditions for cell lines, ES cells, and iPS cells
We used the human H1 ES cell line and human iPS cell lines under a protocol approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute (07-H-0113). The H1 ES cell line was used between passages 40 and 60. Two human iPS cell lines (iPS-1; CTRL-2 c1 and iPS-2; CTRL-1 c3) were established, as previously described [26]. The iPS-1 cell clones were derived from BJ1 neonatal foreskin fibroblasts (American Type Culture Collection; ATCC, Manassas, VA, USA), and iPS-2 cell clones were derived from adult dermal fibroblasts from a healthy donor. They were used between passages 40 and 50. Both ES and iPS cells were maintained as undifferentiated cells on irradiated primary murine embryonic feeder cells (MEF cells; Global Stem, Rockville, MD, USA) in Dulbecco modified Eagle medium (DMEM)/F12 media (Life Technologies, Grand Island, NY, USA) containing 20% Knockout Serum replacement (Life Technologies), 1% MEM-nonessential amino acid (Life Technologies), 2 mM L-glutamine (Life Technologies), 0.1 mM 2-mercaptoethanol (Life Technologies), and 10 ng/ml basic fibroblast growth factor (bFGF; Perpro Tech, Rockey hill, NJ, USA). Media were changed daily, and the cells were passaged weekly using dissociation with 1 mg/ml collagenase IV (Life Technologies). The mouse mesenchymal C3H10T1/2 cell line (ATCC) was maintained in Eagle basal medium (Life Technologies) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) and 2 mM L-glutamine. The OP9 mouse bone marrow stromal cell line (ATCC) was maintained in α-minimum essential medium (α-MEM; Life Technologies) containing 20% FBS and 2 mM L-glutamine. C3H10T1/2 and OP9 cells were irradiated (50 Gy) before used as feeder cells.
Human ES/iPS cell-derived erythroid cell differentiation through the ES/iPS sacs
Human ES/iPS cell-derived erythroid cells were generated through ES/iPS sacs using a four-step culture method (Figure 1A). Step 1 (ES sac culture phase) was performed as previously described [25], in which ES/iPS cells were harvested by collagenase treatment, and small clumps of ES/iPS cells (1.0×10e5 cells per 100-mm dish) were cultured on irradiated C3H10T1/2 feeder cells (1.0×10e6 cells per 100-mm dish) for 15 days in Iscove modified Dulbecco medium (IMDM) (Sigma Aldrich, Saint Louis, MO, USA) supplemented with 10 μg/ml insulin, 5.5 μg/ml transferrin, 5 ng/ml sodium selenite (Sigma Aldrich), 0.45 mM α-mono-thioglycerol (Sigma Aldrich), 50 μg/ml ascorbic acid (Sigma Aldrich), 20 ng/ml human vascular endothelial growth factor (VEGF; Perpro Tech), 2 mM l-glutamine, and 15% FBS. The ES sac culture media were replaced on days 3, 6, 9, 11, and 13. Step 2 (Transfer phase) was performed on day 15 of culture, on which ES/iPS sacs were harvested, gently crushed using a 1000-μl pipette, and passed through a 40-μm cell strainer. These cells were cultured on irradiated OP9 feeder cells for 2 days in IMDM media containing 20 ng/ml VEGF, 50 ng/ml stem cell factor (SCF; R&D systems, Minneapolis, MN, USA), 50 ng/ml fms-related tyrosine kinase 3 ligand (FL; R&D systems), 50 ng/ml thrombopoietin (TPO; R&D systems), 5 μg/ml interleukin-3 (IL3; R&D systems), 10 ng/ml bone morphogenetic protein 4 (BMP4; R&D systems), erythropoietin (EPO; AMGEN, Thousand Oaks, CA, USA), and 15% FBS. We prepared this culture to separate adherent and suspension cells, and induce hematopoietic cell differentiation. Step 3 (Erythroid differentiation phase) was performed according to the human erythroid massive amplification (HEMA) protocol [27] on day 17 of total culture, on which only suspension cells were collected and transferred onto fresh OP9 feeder cells for 5 days in IMDM media containing 10 ng/ml SCF, 1.0 ng/ml IL3, 2.0 U/ml EPO, 1.0 μM dexamethasone (DEX; VETone, Boise, ID, USA), 1.0 μM estradiol (E2; Pfizer, NY, NY, USA), and 20% FBS. Step 4 (Erythroid maturation phase) was performed according to the HEMA protocol at day 22 of culture, on which the erythroid differentiation media were replaced with IMDM media containing 2.0 U/ml EPO, 10 ng/ml insulin (Lilly, Indianapolis, IN, USA), 0.56 mg/ml transferrin (Sigma Aldrich), 2% bovine serum albumin (BSA; Roche, Indianapolis, IN, USA), 2 mM l-glutamine, and 20% FBS. The cells were cultured for 8 days, and the erythroid maturation media were replaced on days 25 and 28.
Figure 1. Erythroid cells differentiated from human embryonic stem cell sacs (ES sacs).
(A). Schematic diagrams of the procedure to establish human erythroid cells through human ES sacs. We generated ES sacs from embryonic stem (ES) cells (1×10e5) using Iscove’s Modified Dulbecco’s Medium (IMDM) containing vascular endothelial growth factor (VEGF) on irradiated C3H10T1/2 feeder cells for 15 days, as previously described [25]. We harvested spherical cells that emerged within the ES sacs, and at 2 days after culture of the spherical cells on irradiated OP9 feeder cells, the suspension cells were collected and differentiated into erythroid cells using stem cell factor (SCF) and erythropoietin (EPO) on OP9 feeder cells for 5 days, and EPO alone for the following 8 days. (B). After 15 days of culture, sac-like structures were generated from ES cells (Left image: 40×) and the ES sacs contained spherical cells (right image: 100×). (C). CD31+CD34+ hematopoietic endothelial cells comprised 22.2 ± 3.3 % of the spherical cells that were harvested from the ES sacs on day 15 (n=6), consistent with a hemangioblast-like phenotype. (D). After 13 days of culture for erythroid differentiation using the spherical cells (day 30), we observed small eosinophilic cells with high density chromatin by Wright-Giemsa staining consistent with erythroblasts. (E). We observed a high percentage of glycophorin A (GPA)+ in the differentiated erythroid cells (n=8). FL, fms-related tyrosine kinase 3 ligand; TPO, thrombopoietin; IL3, interleukin-3; BMP4, bone morphogenetic protein 4.
Cell separation using magnetic beads
To separate ES sac-derived spherical cells between primitive erythroid progenitor cells and definitive erythroid progenitor cells, ES sac-derived spherical cells were separated using corresponding direct microbeads of anti-CD34 antibody or anti-GPA antibody (Miltenyi Biotech, Auburn, CA, USA) by magnetic cell sorting on day 15 of ES sac culture according to the manufacturer’s instructions.
Flow cytometry analysis
We performed cell surface analysis using a FACSCalibur flow cytometer (Becton Dickinson, East Rutherford, NJ, USA). The following monoclonal antibodies were used for identifying and characterizing cell subsets: CD31 (clone WM59), CD34 (clone 581 or 563), CD41a (clone HIP8), CD45 (clone HI30), CD71 (clone M-A712), and GPA (clone GA-R2) (Becton Dickinson). The positive and negative fractions were defined by a negative control without antibody staining.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
After 13-day erythroid differentiation culture (day 30), erythroid cells were collected and evaluated to determine RNA expression of levels of ε-globin, γ-globin, β-globin, ζ-globin, α-globin, BCL11a, cMYB, GATA1, GATA2, and KLF1. Total RNA was isolated using the RNeasy mini kit (Qiagen, Valencia, CA, USA), and the Superscript 3 First-strand for RT-PCR Synthesis (Life Technologies) was used to obtain complementary DNA (cDNA). Quantitative PCR assay was performed using gene-specific primers and probes in the Mx3000P (Agilent Technologies, Santa Clara, CA, USA). The following primer and probe sequences were used [28–31]: ε-globin forward primer, 5′-TGG CAA GGA GTT CAC CCC T -3′; ε-globin reverse primer, 5′-AAT GGC GAC AGC AGA CAC C -3′; ε-globin probe, 5-ROX- TGC AGG CTG CCT GGC AGA AGC -IBRQ-3′; γ-globin forward primer, 5′-GGC AAC CTG TCC TCT GCC TC -3′; γ-globin reverse primer, 5′-GAA ATG GAT TGC CAA AAC GG -3′; γ-globin probe, 5′-Cy5- CAA GCT CCT GGG AAA TGT GCT GGT G -IBRQ-3′; β-globin forward primer, 5′-CTC ATG GCA AGA AAG TGC TCG -3′; β-globin reverse primer, 5′-AAT TCT TTG CCA AAG TGA TGG G -3′; β-globin probe, 5′-FAM- CGT GGA TCC TGA GAA CTT CAG GCT CCT -IBRQ-3′; ζ-globin forward primer, 5′-GTG TCC ATG TGG GCC AAG -3′, ζ-globin reverse primer, 5′-GAA GTG CGG GAA GTA GGT CTT -3′, ζ-globin probe, Roche universal probe #83 (Roche); α-globin forward primer, 5′-TCC CCA CCA CCA AGA CCT AC -3′, α-globin reverse primer, 5′-CCT TAA CCT GGG CAG AGC C -3′, α-globin probe, 5′-HEX- TCC CGC ACT TCG ACC TGA GCC A -IBRQ-3′; BCL11a forward primer, 5′-CCC AAA CAG GAA CAC ATA GCA -3′; BCL11a reverse primer, 5′-GAG CTC CAT GTG CAG AAC G -3′; and BCL11a probe, Roche universal probe #52 (Roche). We used TaqMan® Gene Expression Assays (Life Technologies) to evaluate RNA expression levels for cMYB (Hs00920556_m1), GATA1 (Hs01085823_m1), GATA2 (Hs00231119_m1), and KLF1 (Hs00610592_m1).
Hemoglobin electrophoresis analysis
After 13-day erythroid differentiation culture (day 30), erythroid cells were collected and evaluated to determine the hemoglobin types by hemoglobin electrophoresis using cellulose acetate membranes in alkaline buffer according to the manufacturer’s instruction (HELENA LABORATORIES, Beaumount, TX, USA).
Statistical analysis
Statistical analyses were performed using JMP 9 (SAS Institute Inc., Cary, NC, USA). The averages in various conditions were evaluated by Dunnett’s test (one-way analysis of variance for one control). Two averages were evaluated using Student’s t-test. Standard errors of the mean are shown as error bars in all figures. The sample sizes (n) were defined by biological replicates.
Results
Erythroid cells can be differentiated through human ES sacs
We began our studies by first generating human ES sacs, which were differentiated to erythroid cells (Figure 1A). Human ES sacs were generated from H1 ES cells (1.0×10e5) using IMDM containing VEGF on irradiated C3H10T1/2 feeder cells for 15 days, as previously described [25]. The ES sac consists of a sac-like external layer and hematopoietic cell-like spherical cells (covered by the external layer). The spherical cells were separated by a 40μm cell strainer and cultured for 2 days using IMDM containing VEGF, SCF, FL, TPO, IL3, BMP4, and EPO on irradiated OP9 cells to remove attached cells (probably including some cells from the external layer). The hematopoietic cell-like suspension cells were collected and differentiated into erythroid cells using IMDM containing SCF, IL3, EPO, DEX, and E2 on OP9 cells for 5 days, and the media were changed to IMDM containing EPO and insulin for 8 days (Figure 1A).
During 15 days of culture, sac-like structures were generated from ES cells (Figure 1B left panel). The ES sacs contained spherical cells which were covered by an external layer (Figure 1B right panel), and were connected with the feeder cells by a stem-like tissue (unlike cystic EBs). We observed 22.2 ± 3.3 % of CD31+CD34+ cells which express both CD34 hematopoietic marker and CD31 endothelial marker, suggesting that these cells contain the hematopoietic endothelial cell population (n=6) (Figure 1C). After 13 days of culture for erythroid differentiation (day 30), we observed small eosinophilic cells (11.2 ± 1.3 μm, n=20) with a high density of chromatin, evaluated by Wright-Giemsa stain (Figure 1D), which were not enucleated (~100%) and highly GPA+ (98.4 ± 0.1 %) (n=8) (Figure 1E). These data suggest that hemangioblast-like ES sacs were generated as previously described [25], and the spherical cells in ES sacs contain hematopoietic (or erythroid) progenitor cells.
A greater number of erythroid cells were obtained by cytokine supplementation during ES sac generation
When ES sac-derived erythroid cells were generated using our protocol, 1.0×10e5 ES cells routinely generate 1.2 ± 0.7×10e7 GPA+ erythroid cells during 30-day culture (n=18). We hypothesized that cytokine supplementation to stimulate immature hematopoietic progenitor cells during ES sac generation could lead to greater expansion of HPCs, which might thus improve in vitro erythroid cell production. We added hematopoiesis-stimulating cytokines (SCF, FL, TPO, IL3, EPO, and BMP4) into ES sac culture media on days 9–15, and spherical cells in the ES sacs were differentiated into erythroid cells in the same manner (Supplementary figure 1A). In 15-day ES sac culture, cytokine supplementation slightly increased the absolute number of CD34+CD45+ HPCs (Supplementary figure 1B) and CD31+CD34+ hematopoietic endothelial cells (Supplementary figure 1C); however, no significant difference was observed. During erythroid differentiation culture, we enumerated the GPA+ erythroid cells on days 15, 27, 29, 32, and 34. Cytokine supplementation resulted in a maximum 1.4-fold higher number of GPA+ erythroid cells compared to the control (p < 0.05 on days 32 and 34) (Supplementary figure 1D). Interestingly, after 13-day erythroid differentiation (day 30), a 1.3-fold lower level of ε-globin RNA expression was observed in the cytokine supplementation group compared to the control group (p < 0.05), while no significant difference in γ- and β-globin levels was observed between the two groups (Supplementary figure 1E). We mainly detected α-globin RNA expression in erythroid cells for both groups with small amounts of ζ-globin (Supplementary figure 1F). These data suggest that cytokine supplementation decreases the population of primitive erythroid cells (expressing ε-globin) during ES sac generation, maybe due to an increase of HPCs.
Definitive erythroid progenitor generation was induced through ES sac maturation
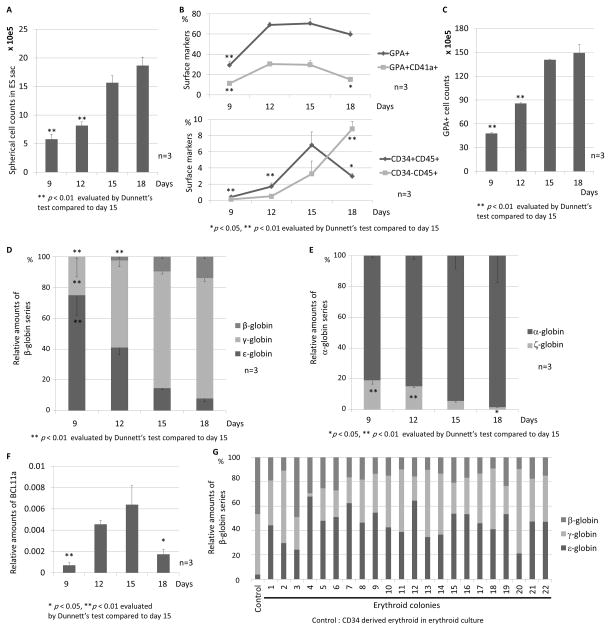
We hypothesized that during ES sac maturation, primitive erythroid progenitor generation (resulting in expression of ε-globin) in ES sacs could be switched to definitive erythroid progenitor generation (resulting in expression of γ- or β-globin). We analyzed the spherical cells from ES sacs on days 9, 12, 15, and 18 after ES sac maturation culture for expression of CD34, CD45, GPA, and CD41a (before erythroid differentiation). The number of spherical cells that were harvested from the ES sacs increased over time during ES sac maturation (p < 0.01 days 9 and 12, compared to day 15) (Figure 2A). In the early time point (day 9) during ES sac maturation, we observed a low percentage of CD34+CD45+ HPCs (0.4 ± 0.1%) and CD34−CD45+ hematopoietic cells (0.1 ± 0.1%), contrasting with a high percentage of GPA+ erythroid cells (29.2 ± 3.7%) (Figure 2B upper panel). The CD34+CD45+ HPCs gradually increased until day 15 (6.8 ± 1.6%), while we observed an increasing percentage of CD34−CD45+ hematopoietic cells until day 18 (8.8 ± 0.9%) (Figure 2B lower panel). In addition, small amounts of GPA+CD41a+ megakaryo-erythroid progenitor cells were observed in 9-day ES sacs, and this percentage increased in 12- and 15-day ES sacs (Figure 2B upper panel). To evaluate the production efficiency of erythroid cells within each group, we evaluated the number of GPA+ erythroid cells after 13-day erythroid differentiation culture. In more mature ES sacs, we observed a higher number of GPA+ cells (p < 0.01 days 9 and 12 after ES sac culture, compared to day 15; Figure 2C). When using more mature ES sacs, we observed lower ε-globin RNA expression (p < 0.01 day 9, compared to day 15) and higher expression of both γ-globin (p < 0.01 day 9, compared to day 15) and β-globin (p < 0.01 day 9 and 12, compared to day 15) in the differentiated erythroid cells (Figure 2D). In addition, lower ζ-globin (p < 0.05, compared to day 15) and relatively higher α-globin were detected in erythroid cells derived from more mature ES sacs (Figure 2E). These data suggest that primitive erythroid cells (expressing ε-globin) are initially generated from ES sacs, while definitive erythroid cells (expressing ε-globin) are differentiated from HPCs that emerge in ES sacs. Maturation of ES sacs thus results in lower primitive erythroid progenitor generation and higher definitive erythroid progenitor generation from ES sacs.
Figure 2. Definitive erythroid progenitor generation induced by prolonged ES sac maturation.
(A). We hypothesized that during ES sac maturation, primitive erythropoiesis (to express ε-globin) in ES sacs is switched to definitive erythropoiesis (to express γ- or β-globin). We counted the spherical cells that were harvested in ES sacs on days 9–18, and the spherical cells expanded according to ES sac maturation. (B). We harvested spherical cells in ES sacs on days 9, 12, 15, and 18. We observed a small percentage of CD34+CD45+ HPCs and a high percentage (29.2 ± 3.7%) of GPA+ erythroid cells in the ES sacs on day 9. The CD34+CD45+ HPCs gradually increased until day 15. (C). We evaluated the number of GPA+ erythroid cells after 13-day erythroid differentiation using the spherical cells in the ES sacs on days 9–18. More mature ES sacs lead to greater amounts of GPA+ cells. (D). We analyzed relative RNA expression of ε-, γ-, and β-globin. More mature ES sacs resulted in lower ε-globin expression, higher γ-globin expression, and higher β-globin expression. (E). We observed lower ζ-globin expression and higher α-globin expression in erythroid cells derived from more mature ES sacs. (F). We evaluated BCL11a expression after 13 days of erythroid differentiation, compared to human CD34+ cell-derived erythroblasts. We observed higher expression of BCL11a according to ES sac maturation until day 15. (G). We evaluated globin expression patterns in each erythroid clone by colony forming unit (CFU) assay using spherical cells in the ES sacs on day 15. All 22 erythroid colonies, which were picked up after 14–16 days, expressed ε-, γ-, and β-globin.
In addition, we evaluated BCL11a expression using RT-qPCR after 13 days of erythroid differentiation culture (Figure 2F), since BCL11a is an important factor in definitive erythropoiesis, especially for hemoglobin switching from γ-globin to β-globin [24, 31–33]. As a control, we used human mobilized CD34+ cell-derived erythroblasts on day 8 after erythroid differentiation culture, and compared BCL11a expression in ES sac-derived erythroid cells to the control cells. We confirmed increasing expression of BCL11a during ES sac maturation until day 15 (p < 0.01 day 9, compared to day 15).
We had two hypotheses: in ES sac-derived erythroid cells, (1) single cells produce all ε-, γ-, and β-globins, and (2) single cells produce either ε-, γ-, or β-globin. To investigate the hypothesis, we performed colony forming unit (CFU) assays using spherical cells that were harvested from 15-day ES sacs. We analyzed RNA expression of ε-, γ-, and β-globin in 22 erythroid colonies (BFU-E), which represented all three types of globin expression in all colonies (Figure 2G). These data suggest that ES sac-derived erythroid cells produce ε-globin, γ-globin, and β-globin.
Surface marker CD 34 or GPA distinguishes between primitive and definitive erythroid progenitors during ES sac culture
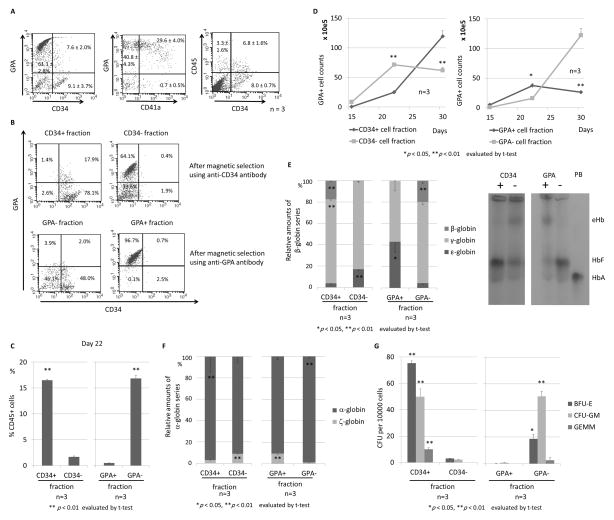
To evaluate whether both primitive and definitive erythroid progenitor cells are contained in ES sacs, we analyzed GPA, CD41a, CD34, and CD45 surface markers among spherical cells that were harvested from ES sacs on day 15. A high percentage of GPA+ erythroid cells (68.7 ± 4.0%), GPA+CD41a− differentiated erythroid progenitor cells (40.8 ± 4.3%), and GPA+CD41a+ undifferentiated megakaryo-erythroid progenitor cells (29.6 ± 4.0%), while only a low percentage of CD34+CD45+ HPCs (6.8 ± 1.6%) was observed (Figure 3A) at this time point. We hypothesized that primitive erythroid progenitor cells are mainly included in CD34− cells or GPA+ cells (an erythroid progenitor phenotype) and that definitive erythroid progenitor cells are mainly derived from CD34+ cells or GPA− cells (an HPC phenotype) in ES sacs after the 15-day ES sac culture, since we expected that primitive erythroid cells were directly generated from ES sacs and definitive erythroid cells were differentiated from ES sac-derived HPCs. We separated spherical cells using magnetic beads with an anti-CD34 or anti-GPA antibody, and we obtained a high purity of the CD34+ (96.0%) and CD34− (97.7%) cell fractions as well as the GPA− (94.1%) and GPA+ (97.4%) cell fractions (Figure 3B). After 5-day erythroid differentiation (day 22) using these 4 fractions of cells, we analyzed the CD45 hematopoietic marker, which demonstrated a higher percentage of CD45+ cells in the CD34+ (16.5 ± 0.1%) and GPA− (16.8 ± 0.6%) cell fractions than the CD34− (1.7 ± 0.2%) or GPA+ (0.5 ± 0.0%) cell fractions (p < 0.01) (Figure 3C). These data suggest that cells in the CD34+ or GPA− cell fraction were differentiated to erythroid cells through CD45+ hematopoietic cells, while cells in the CD34− or GPA+ cell fraction were differentiated to erythroid cells without the differentiation step to CD45+ cells. During 13-day erythroid differentiation culture after the bead selection, the number of GPA+ cells quickly increased in the CD34− or GPA+ cell fractions until day 22 and decreased on day 30, while the number of GPA+ cells slowly increased in the CD34+ or GPA− cell fractions until day 22 and more efficiently increased on day 30 (p < 0.01) (Figure 3D). In RT-qPCR evaluation of the globin expression among erythroid cells in each fraction (day 30), we observed higher β-globin expression (p < 0.01) and lower ε-globin expression (p < 0.05) among erythroid cells derived from the CD34+ or GPA− cell fraction (Figure 3E left panels), compared to the CD34− or GPA+ cell fraction. We confirmed the same trend using hemoglobin electrophoresis, through which we observed lower embryonic hemoglobin (eHb) and higher adult hemoglobin (HbA) among erythroid cells derived from the CD34+ or GPA− cell fraction (Figure 3E right panels). In addition, higher β-globin expression (p < 0.01) and lower ε-globin expression (p < 0.01) were observed among erythroid cells derived from the CD34+ or GPA− cell fraction (Figure 3F), compared to the CD34− or GPA+ cell fraction. These data suggest that definitive erythroid progenitor cells were included in the CD34+ or GPA− cell fraction, while primitive progenitor erythroid cells were included in the CD34− or GPA+ cell fraction.
Figure 3. CD34 or GPA selection to distinguish between primitive and definitive erythroid progenitors in ES sacs.
(A). We hypothesized that primitive and definitive erythroid progenitors can be separated by a CD34 or GPA surface marker in ES sacs before erythroid differentiation. We analyzed spherical cells from 15-day ES sacs, in which we observed mainly two populations of GPA+(CD34−) erythroid cells and CD34+(GPA−) HPCs. (B). We separated spherical cells using CD34 or GPA magnetic beads, and obtained a high purity of the CD34+ or − cell fractions as well as the GPA+ or − cell fractions, which were differentiated into erythroid cells. (C). After 5-day erythroid differentiation (day 22), a higher percentage of CD45+ cells was observed in CD34+ or GPA− cell fractions than CD34− or GPA+ cell fractions. (D). During erythroid differentiation (days 17–30), GPA+ erythroid cells efficiently increased in the CD34− or GPA+ cell fractions until day 22 and decreased on day 30, while the erythroid cells slowly increased in the CD34+ or GPA− cell fractions until day 22 and robustly increased on day 30. (E). After 13-day erythroid differentiation (day 30), we observed higher β-globin expression (p < 0.01) and lower ε-globin expression (p < 0.05) in the CD34+ or GPA− cell fraction, compared to the CD34− or GPA+ cell fraction. We confirmed the same trend using hemoglobin electrophoresis. (F). After erythroid differentiation (day 30), higher α-globin expression and lower ζ-globin expression were observed in the CD34+ or GPA− cell fraction, compared to the CD34− or GPA+ cell fraction. (G). In CFU assay, greater CFUs were observed in the CD34+ or GPA− cell fraction. PB, peripheral blood (expressing HbA); eHb, embryonic hemoglobin; HbF, fetal hemoglobin, HbA, adult hemoglobin.
We performed CFU assays for 14–16 days using 1.0×10e4 cells in each fraction from 15-day ES sacs. The number of BFU-E, CFU-GM, and GEMM cells increased in the CD34+ or GPA− cell fraction (p < 0.05 except for GEMM in the GPA fraction) (Figure 3G), suggesting that multi-lineage HPCs were mainly included in the CD34+ or GPA− cell fractions.
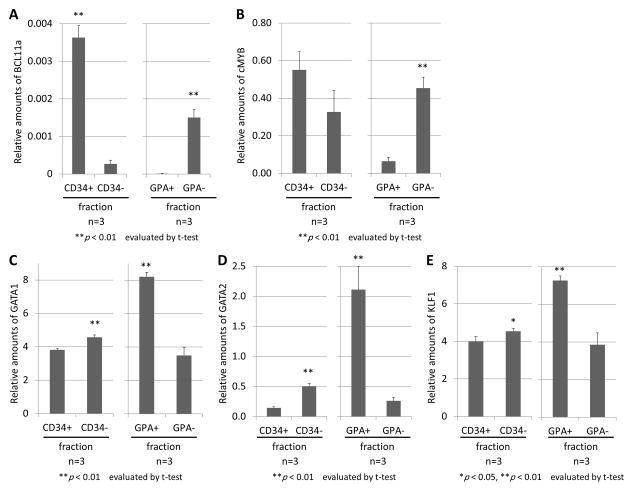
In addition, we analyzed RNA expression of erythroid-related genes including BCL11a, cMYB, GATA1, GATA2, and KLF1 among erythroid cells derived from both primitive (CD34− or GPA+) and definitive (CD34+ or GPA−) fractions [34, 35]. We detected higher BCL11a expression (p < 0.01), higher cMYB expression (p < 0.01 in GPA fractions), lower GATA1 expression (p < 0.01), lower GATA2 expression (p < 0.01), and lower KLF1 expression (p < 0.05) in the CD34+ or GPA− cell fraction, compared to the CD34− or GPA+ cell fraction, after 13-day erythroid differentiation (day 30) following CD34 or GPA selection from ES sac at day 15 (Figures 4A–E).
Figure 4. Gene expression analysis between primitive and definitive erythroid cells following CD34 or GPA selection from ES sacs.
(A–E). We analyzed RNA expression of several erythroid-related genes (BCL11a (A), cMYB (B), GATA1 (C), GATA2 (D), and KLF1 (E)) after 13-day erythroid differentiation (day 30) following CD34 or GPA selection from ES sac at day 15. We observed higher BCL11a expression, higher cMYB expression, lower GATA1 expression, lower GATA2 expression, and lower KLF1 expression in the CD34+ or GPA− cell fraction, compared to the CD34− or GPA+ cell fraction.
Definitive erythroid cells that express β-globin were obtained from fibroblast-derived iPS cells through the iPS cell-derived sacs (iPS sacs)
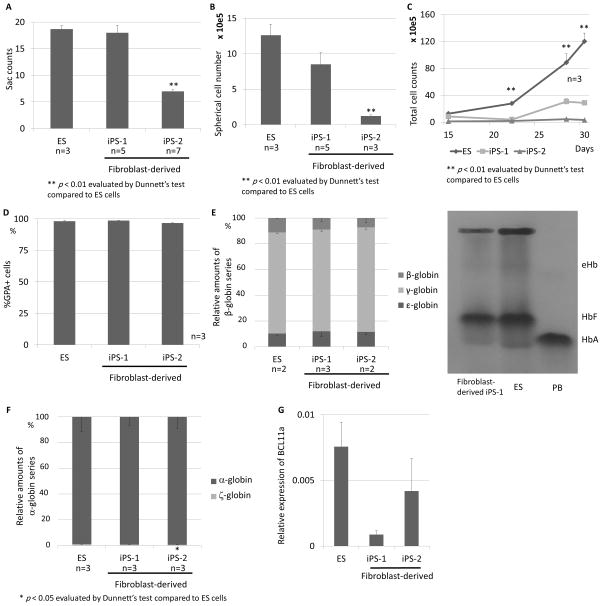
We generated erythroid cells from two types of human fibroblast-derived iPS cells (iPS-1 and iPS-2) using the same method (Figure 1A), in which we generated iPS sacs with VEGF for 15 days (without additional cytokines), the spherical cells in iPS sacs were cultured on irradiated OP9 feeder cells for 2 days (without CD34 or GPA selection), and the suspension cells were differentiated into erythroid cells for 13 days. The number of ES/iPS sacs was lower in iPS-2 cells compared with ES cells (p < 0.01), while there was no difference in the sac number between ES cells and iPS-1 cells (Figure 5A). Before erythroid differentiation (day 15), the number of spherical cells within the iPS sacs was reduced in both iPS cells (p < 0.01 in iPS-2 cells) (Figure 5B). During erythroid differentiation culture, the total cell numbers in both iPS sac-derived cells were still lower than that of ES sac-derived cells on days 22, 28 and 30 (p < 0.01) (Figure 5C). However, efficient erythroid differentiation was obtained from all three sources (ES, iPS-1 and iPS-2 sacs) after 13-day erythroid differentiation (day 30), with more than 95% of GPA+ (Figure 5D). In RT-q PCR evaluation of the globin expression in iPS sac-derived erythroid cells (day 30), we observed β-globin expression in both iPS sac-derived erythroid cells (iPS-1 cells: 9.0±2.6%, iPS-2 cells 7.3±3.7%), that was comparable to ES sac-derived cells (Figure 5E left panel). We confirmed the same trend using hemoglobin electrophoresis for iPS-1 cells (Figure 5E right panel), while hemoglobin electrophoresis could not be performed for iPS-2 cells due to the low erythroid production. Both iPS sac-derived erythroid cells mostly produced α-globin with small amounts of ζ-globin, comparable to ES sac-derived erythroid cells (Figure 5F). We also observed slightly lower BCL11a expression levels in iPS sac-derived erythroid cells (day30), compared to ES sac-derived cells; however, no significant difference was observed (Figure 5G). These data suggest that iPS sacs as well as ES sacs contain definitive erythroid progenitor cells that express β-globin after differentiation.
Figure 5. Generation of definitive erythroid cells that express β-globin derived from induced pluripotent stem (iPS) cells using the iPS cell-derived sacs (iPS sacs).
(A). We generated iPS sac-derived erythroid cells from two clones of human fibroblast-derived iPS cells (iPS-1 and iPS-2) with the same method. The ES/iPS sac number was equivalent in iPS-1 cells and lower in iPS-2 cells, compared with ES cells. (B). We observed lower spherical cell counts in both iPS sacs on day 15 (p < 0.01 in iPS-2 cells). (C). During 13-day erythroid differentiation (days 15, 22 and 30), total numbers of both iPS sac-derived cells were lower than ES sac-derived cells (p < 0.01 days 22 and 30). (D). We observed more than 95% of GPA+ erythroid cells among all types of cells after 13-day erythroid differentiation (day 30). (E). After 13-day erythroid differentiation (day 30), we observed β-globin RNA expression in both iPS sac-derived erythroid cells (iPS-1 cells: 9.0±2.6%, iPS-2 cells 7.3±3.7%), comparable to ES sac-derived cells. We confirmed HbA expression using hemoglobin electrophoresis. (F). In both iPS sac-derived erythroid cells, we mainly detected α-globin expression with small amounts of ζ-globin production, comparable to ES sac-derived erythroid cells. (G). We evaluated relative BCL11a RNA expression after 13-day erythroid differentiation (day 30), which resulted in relatively lower BCL11a expression in both iPS sac-derived erythroid cells compared to ES sac-derived cells; however, no significant difference was observed.
Discussion
In this study, we developed an ES/iPS sac-based method to generate erythroid cells that express β-globin. Unlike other methods, the advantages of this method include the following: (1) ES/iPS sacs have a hemangioblast-like function which allows definitive hematopoiesis, (2) β-globin-expressing erythroid cells are obtained from not only ES cells but also iPS cells, and (3) definitive erythroid progenitor cells are simply selected by a CD34 or GPA maker in ES sacs before erythroid differentiation.
Human ES sacs can be generated with VEGF on irradiated C3H10T1/2 feeder cells for 15 days (Figure 1A). During 15 days of culture, sac-like structures were generated from ES cells (Figure 1B left panel). The ES sacs contained spherical cells which were covered by an external layer (Figure 1B right panel), and were connected with the feeder cells by a stem-like tissue (unlike cystic EBs). The ES sacs contain hematopoietic endothelial cells (Figure 1C) and hematopoietic progenitor cells (Supplementary figure 1B). The ES sacs were recently reported to produce functional platelets [25]. Since erythroid differentiation procedure is close to platelet differentiation (both erythrocyte and platelet productions are mediated by megakaryoerythroid progenitors), we hypothesized that the ES sac method would allow us to generate definitive erythroid cells. In the beginning, we simply generated erythroid cells using 15-day ES sacs (Figures 1D and E), which should contain both primitive and definitive erythroid cells (Figure 2D).
In human development, embryological hematopoiesis begins in the YS and transiently produces ε-globin-expressing primitive RBCs before HSCs/HPCs are generated, while fetal hematopoiesis begins in AGM and produces definitive RBCs that express γ- or β-globin which are differentiated from HSCs/HPCs. Therefore, we hypothesized that hemangioblast-like ES/iPS sac formation is crucial for generation of β-globin-expressing definitive erythroid cells, which might be mediated by HPC emergence. This hypothesis was supported by our data in hematopoiesis-stimulating cytokine supplementation during ES sac generation (day 9–15), in which CD34+CD45+ HPCs increased (Supplementary figure 1B) and primitive erythropoiesis (ε-globin production) decreased (Supplementary figure 1E). Our data suggest that ES sacs contain both primitive and definitive erythroid progenitor cells, which is consistent with previous data in erythroid cell generation from ES cells [10, 23, 29]. The cytokine supplementation might improve definitive erythropoiesis by stimulating definitive erythroid progenitor cells and/or HPCs.
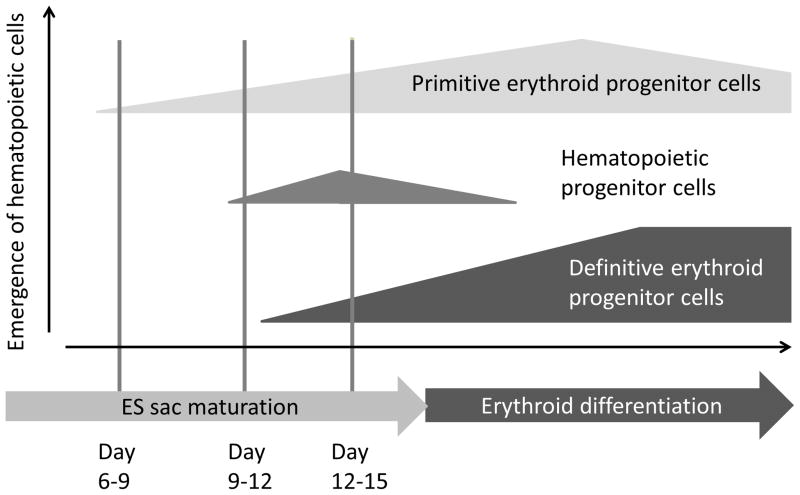
Interestingly, in this study, we observed mimic erythropoiesis development during ES sac culture, in which primitive erythropoiesis gradually switched to definitive erythropoiesis. In an early phase of ES sac culture (day 9), a high percentage of GPA+ cells and small amounts of GPA+CD41a+ megakaryo-erythroid progenitor cells were detected before CD34+CD45+ HPCs emerged (Figure 2B), and these cells produce more than 70% of ε-globin and very low levels of β-globin after erythroid differentiation (Figure 2D), suggesting that primitive erythroid progenitor cells initially emerge in ES sacs and might be partially mediated by megakaryo-erythroid progenitor cells.[36] After day 12 of ES sac culture, the number of CD34+CD45+ HPCs gradually increased (Figure 2B), and higher β-globin expression was detected among the ES sac-derived erythroid cells according to the degree of ES sac maturation (Figure 2D). In addition, more mature ES sacs resulted in lower ζ-globin expression and higher α-globin expression in erythroid cells (Figure 2E). These results suggest that HPCs emerge in ES sacs after day 9 of ES sac culture, and that the ES sac-derived HPCs can be differentiated to definitive erythroid progenitor cells in ES sacs. Collectively, these data demonstrate for the first time that the primitive erythropoiesis initially observed in ES sacs can be switched to definitive erythropoiesis maybe due to HPC emergence during ES sac maturation (Figure 6).
Figure 6. Primitive erythropoiesis is switched to definitive erythropoiesis in ES sacs during ES sac maturation.
We demonstrated schematic diagrams in which primitive erythropoiesis is switched to definitive erythropoiesis in ES sacs during ES sac maturation. Primitive erythroid progenitor cells initially emerge in ES sacs after day 6–9 of ES sac culture, and hematopoietic progenitor cells emerge in ES sacs after day 9–12, which might be differentiated to definitive erythroid progenitor cells.
To confirm whether ES sacs contain the two distinct types of erythroid progenitor cell populations, we separated primitive and definitive erythroid progenitor cells using the CD34 or GPA surface marker on day 15 of ES sac culture before performing erythroid differentiation culture. In addition, we hypothesized that primitive erythroid cells are directly derived from ES sacs, and that definitive erythroid cells are differentiated from ES sac-derived HPCs. In the current study, higher CD45+ rates during erythroid differentiation (Figure 3C), higher cell expansion capacity (Figure 3D), higher β-globin expression and lower ε-globin expression (Figure 3E), higher α-globin expression and lower ζ-globin expression (Figure 3F), and greater amounts of CFUs (Figure 3G) were observed in the definitive erythroid progenitor cells (CD34+ or GPA− cell fraction), as compared to the primitive erythroid progenitor cells (CD34− or GPA+ cell fraction). In a previous ES cell experiment, primitive and definitive erythroid cells were separately detected by more complicated methods, such as colony morphology in CFU assays [23] and various markers at several timepoints [10]. However, in the current study, we demonstrated that a simple, one-time separation with a CD34 or GPA surface marker which allows for discriminating between primitive and definitive erythroid progenitor cells from ES sacs before erythroid differentiation. The CD34 and GPA selection markers should be useful for separation between primitive and definitive erythroid progenitor cells in ES sacs. In addition, when we compared the primitive and definitive fractions regarding the ratio of CD45+ cells, we observed a higher purity of primitive erythroid cells by GPA+ selection, as compared to by CD34− selection. GPA positive-selection might be more suitable for separating primitive erythroid progenitor cells from ES sacs.
We also evaluated erythroid-related gene expression in erythroid cells (day 30) derived from CD34 or GPA selection from ES sacs. In the cell fraction resembling definitive erythroid progenitor cells (CD34+ or GPA− cell fraction), we observed higher levels of BCL11a (Figure 4A) and cMYB (Figure 4B) and lower levels of GATA1 (Figure 4C), GATA2 (Figure 4D), and KLF1 (Figure 4E), as compared to the primitive erythroid progenitor cells (CD34− or GPA+ cell fraction). These data suggest that BCL11a and cMYB are definitive markers, while GATA1, GATA2, and KLF1 are primitive markers in our ES sac-derived erythroid differentiation culture when evaluated at day 30. In the ES sac-derived erythroid differentiation, BCL11a expression peaked at day 22 (Supplementary figure 2). However, BCL11a expression was detected among all time points (days 15, 22, 26, and 30), and significant difference of BCL11a expression was observed between primitive and definitive erythroid cells at day 30 (Figure 4A).
To evaluate whether the ES sac-derived definitive erythroid cells express only γ- or β-globin, we performed CFU assays using ES sac-derived spherical cells on day 15 of ES sac culture. We picked up 22 erythroid colonies, which expressed not only γ- or β-globin but also ε-globin (Figure 2G), which is in agreement with previous studies [10, 23]. In nature, we cannot detect ε-globin in definitive RBCs in human adult peripheral blood nor β-globin in primitive RBCs in human YS. However, a low level of ε-globin can be detected in in vitro differentiated erythroid cells that were derived from cord blood (CB) cells [7]. The ε-globin expression in the GPA- fraction-derived erythroid cells may be occurring via mechanisms similar to that observed among CB-derived erythroid cells [7]. Conversely, ES cell-derived primitive erythroid cells expressed not only ε-globin but also γ-globin in our study, similar to that previously reported [10, 23]. Similarly, YS-derived erythroid cells express mainly γ-globin and but also express a low level of ε-globin during in vitro culture [7]. In addition, higher BCL11a expression was detected in ES sac-derived definitive erythroid cells with greater amounts of β-globin, such as erythroid cells differentiated from in the CD34+ or GPA− cell fraction (Figures 3E and 4A). These data are consistent with the BCL11a function of γ- to β-globin switching in definitive erythrocytes [24, 31–33]. It is not clear why γ-globin expression was not reduced by BCL11a expression; however, a much lower BCL11a expression level (<1%) was observed as compared to mobilized CD34+ cell-derived erythroid cells.
When we generated erythroid cells through ES sacs using H1 ES cells, we obtained 120 (90–200) erythroid cells per single ES cell, which is comparable to in vitro erythroid differentiation from mobilized CD34+ cells and 10-fold less than EB-derived erythroid cell generation as previously described [6, 11]. In a previous report, the EB-based method resulted in 1500–3500 erythroid cells per single ES cells and 210–440 erythroid cells per single iPS cell (with little β-globin expressing erythroid cells) [6]. In our laboratory, EB-derived erythroid cells were generated using a similar protocol except human plasma was switched to FBS and it was performed in a total of 3 times, which resulted in 0.25 (0.0045–0.67) erythroid cells per single ES cell, suggesting that the EB-based method might be technically more difficult to produce erythroid cells. Regarding the erythroid differentiation and maturation protocol, ~1000 erythroid cells per single human CD34+ cell was demonstrated in a previous study [37], which is 10-fold more efficient than in our erythroid cell production in the same protocol (maybe due to the lot of FBS), suggesting a possibility to improve our expansion efficiency of ES sac-derived erythroid cells during the erythroid differentiation and maturation phases. Taken together, the ES sac method would be acceptable amplification efficiency for in vitro erythroid cell generation at present. However, further optimization to improve expansion should be required for clinical application.
In the current study, for the first time, we generated iPS sac-derived erythroid cells with β-globin expression using two clones of fibroblast-derived iPS cells (neonatal foreskin-derived and adult dermal skin-derived) (Figure 5E). As previously described, no β-globin expression was detected among iPS cell-derived erythroid cells using traditional methods, such as co-culture on feeder cells or EB formation [5, 6, 8]. Recently, another group also confirmed β-globin expression in iPS sac-derived erythroid cells using hematopoietic cell-derived iPS cells [24]; however, no detection of β-globin expression was reported when using fibroblast-derived iPS cells. When comparing their iPS sac-derived erythroid generation methods to our methods, different feeder cells were used for erythroid differentiation: OP9 feeder cells in our group (Figure 1A) as opposed to C3H10T1/2 feeder cells [24]. When generating erythroid cells on OP9 feeder cells, a higher expression of β-globin was reported in ES cell-derived erythroid cells [23]. The difference of feeder cells might affect successful generation of β-globin-expressing erythroid cells from fibroblast-derived iPS cells.
In this study, lower erythroid cell production was observed from both iPS cell lines compared to ES cells (Figure 5C), which is consistent with a previously report [6]. Between iPS-1 cells and ES cells, there was no significant difference in ES/iPS sac production (Figure 5A), spherical cell number within ES/iPS sacs (Figure 5B), and GPA positivity in differentiated erythroid cells (Figure 5D), yet lower erythroid cell production was observed (Figure 5C). Interestingly, we observed a significant difference between iPS-1 and iPS-2 cells regarding iPS sac production (Figure 5A), spherical cell number within iPS sacs (Figure 5B), and erythroid cell production (Figure 5C). However, comparing erythroid cell production per spherical cell, the production efficiency is similar between the two iPS cells (iPS-1 cell: 2.6±0.1 cells, iPS-2 cell: 2.7±0.1 cells). These data suggest that the variation of iPS cell clones might affect the ability to be differentiated to erythroid cells from iPS sacs.
In summary, we generated β-globin-expressing definitive erythroid cells through both ES and iPS sac generation. We demonstrated that primitive erythroid progenitor cells emerge before definitive erythroid progenitor cells emerge in ES sacs and that these populations can be discriminated using either CD34 or GPA surface markers. The ability to generate erythroid progenitors through ES/iPS sacs should be valuable for comparing the globin regulation between primitive and definitive cells, the development of genetic strategies aimed at the correction of hemoglobinopathies, and generating erythroid cells that express higher β-globin and lower ε-globin in the future.
Supplementary Material
(A). We hypothesized that hematopoietic stem cells (HSCs)/hematopoietic progenitor cells (HPCs) could be differentiated and expanded in ES sacs by supplementation of hematopoiesis-stimulating cytokines. We added cytokine mixtures (SCF, FL, TPO, IL3, EPO, and BMP4) into media during ES sac culture at days 9–15, followed by erythroid differentiation in the same manner. (B and C). We harvested and analyzed the spherical cells from the ES sac on day 15. Cytokine supplementation slightly increased the absolute number of CD34+CD45+ HPCs and CD31+CD34+ hematopoietic endothelial cells; however, no significant difference was observed. (D). During erythroid differentiation culture, we counted the number of GPA+ erythroid cells on days 15, 27, 29, 32, and 34. Cytokine supplementation resulted in a higher number of GPA+ erythroid cells compared to the control (p < 0.05 on days 32 and 34). (E). After 13-day erythroid differentiation (day 30), we analyzed relative RNA expression of ε-, γ-, and β-globin using reverse transcription quantitative polymerase chain reaction (RT-qPCR). The percentage of ε-globin was reduced in the cytokine supplementation group compared to the control (p < 0.05), while no significant difference in γ- and β-globin was observed between the two groups. (F). We mainly detected α-globin expression with small amounts of ζ-globin among erythroid cells in both groups.
We evaluated BCL11a RNA expression during erythroid differentiation from ES sacs at day 15. We observed a peak of BCL11a expression after 5 days of erythroid differentiation (day 22); however, BCL11a expression was detected among all time points (days 15, 22, 26, and 30).
Acknowledgments
This work was supported by the intramural research program of the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) at the National Institutes of Health (NIH).
Footnotes
Disclosure of potential conflicts of interest
The authors indicate no potential conflicts of interest.
Author contributions
A.F.: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing; N.U.: Conception and design, Data analysis and interpretation, Manuscript writing; J.H.: Data analysis and interpretation; T.W.: Provision of study material or patients, Manuscript writing; J.T.: Conception and design, Financial support, Final approval of manuscript.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science (New York, NY. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science (New York, NY. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 4.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 5.Dias J, Gumenyuk M, Kang H, et al. Generation of red blood cells from human induced pluripotent stem cells. Stem cells and development. 2011;20:1639–1647. doi: 10.1089/scd.2011.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lapillonne H, Kobari L, Mazurier C, et al. Red blood cell generation from human induced pluripotent stem cells: perspectives for transfusion medicine. Haematologica. 2010;95:1651–1659. doi: 10.3324/haematol.2010.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olivier E, Qiu C, Bouhassira EE. Novel, high-yield red blood cell production methods from CD34-positive cells derived from human embryonic stem, yolk sac, fetal liver, cord blood, and peripheral blood. Stem cells translational medicine. 2012;1:604–614. doi: 10.5966/sctm.2012-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu C, Olivier EN, Velho M, et al. Globin switches in yolk sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells. Blood. 2008;111:2400–2408. doi: 10.1182/blood-2007-07-102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou BK, Mali P, Huang X, et al. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell research. 2011;21:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi KD, Vodyanik MA, Togarrati PP, et al. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell reports. 2012;2:553–567. doi: 10.1016/j.celrep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma F, Ebihara Y, Umeda K, et al. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13087–13092. doi: 10.1073/pnas.0802220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore MA, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. British journal of haematology. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 13.Tavian M, Hallais MF, Peault B. Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo. Development (Cambridge, England) 1999;126:793–803. doi: 10.1242/dev.126.4.793. [DOI] [PubMed] [Google Scholar]
- 14.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 15.de Bruijn MF, Ma X, Robin C, et al. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16:673–683. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- 16.Taoudi S, Medvinsky A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9399–9403. doi: 10.1073/pnas.0700984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanovs A, Rybtsov S, Welch L, et al. Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. The Journal of experimental medicine. 2011;208:2417–2427. doi: 10.1084/jem.20111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortes F, Debacker C, Peault B, et al. Differential expression of KDR/VEGFR-2 and CD34 during mesoderm development of the early human embryo. Mechanisms of development. 1999;83:161–164. doi: 10.1016/s0925-4773(99)00030-1. [DOI] [PubMed] [Google Scholar]
- 19.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa SI, Nishikawa S, Kawamoto H, et al. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8:761–769. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- 21.Pardanaud L, Luton D, Prigent M, et al. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development (Cambridge, England) 1996;122:1363–1371. doi: 10.1242/dev.122.5.1363. [DOI] [PubMed] [Google Scholar]
- 22.Oberlin E, Tavian M, Blazsek I, et al. Blood-forming potential of vascular endothelium in the human embryo. Development (Cambridge, England) 2002;129:4147–4157. doi: 10.1242/dev.129.17.4147. [DOI] [PubMed] [Google Scholar]
- 23.Zambidis ET, Park TS, Yu W, et al. Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 2008;112:3601–3614. doi: 10.1182/blood-2008-03-144766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochi K, Takayama N, Hirose S, et al. Multicolor staining of globin subtypes reveals impaired globin switching during erythropoiesis in human pluripotent stem cells. Stem cells translational medicine. 2014;3:792–800. doi: 10.5966/sctm.2013-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takayama N, Nishikii H, Usui J, et al. Generation of functional platelets from human embryonic stem cells in vitro via ES-sacs, VEGF-promoted structures that concentrate hematopoietic progenitors. Blood. 2008;111:5298–5306. doi: 10.1182/blood-2007-10-117622. [DOI] [PubMed] [Google Scholar]
- 26.Winkler T, Hong SG, Decker JE, et al. Defective telomere elongation and hematopoiesis from telomerase-mutant aplastic anemia iPSCs. The Journal of clinical investigation. 2013;123:1952–1963. doi: 10.1172/JCI67146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.di Giacomo V, Matteucci A, Stellacci E, et al. Expression of signal transduction proteins during the differentiation of primary human erythroblasts. Journal of cellular physiology. 2005;202:831–838. doi: 10.1002/jcp.20179. [DOI] [PubMed] [Google Scholar]
- 28.Smith RD, Li J, Noguchi CT, et al. Quantitative PCR analysis of HbF inducers in primary human adult erythroid cells. Blood. 2000;95:863–869. [PubMed] [Google Scholar]
- 29.Umeda K, Heike T, Nakata-Hizume M, et al. Sequential analysis of alpha- and beta-globin gene expression during erythropoietic differentiation from primate embryonic stem cells. Stem cells (Dayton, Ohio) 2006;24:2627–2636. doi: 10.1634/stemcells.2006-0199. [DOI] [PubMed] [Google Scholar]
- 30.Fibach E, Bianchi N, Borgatti M, et al. Mithramycin induces fetal hemoglobin production in normal and thalassemic human erythroid precursor cells. Blood. 2003;102:1276–1281. doi: 10.1182/blood-2002-10-3096. [DOI] [PubMed] [Google Scholar]
- 31.Kurita R, Suda N, Sudo K, et al. Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PloS one. 2013;8:e59890. doi: 10.1371/journal.pone.0059890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sankaran VG, Xu J, Ragoczy T, et al. Developmental and species-divergent globin switching are driven by BCL11A. Nature. 2009;460:1093–1097. doi: 10.1038/nature08243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science (New York, NY. 2008;322:1839–1842. doi: 10.1126/science.1165409. [DOI] [PubMed] [Google Scholar]
- 34.Sankaran VG, Xu J, Orkin SH. Advances in the understanding of haemoglobin switching. British journal of haematology. 2010;149:181–194. doi: 10.1111/j.1365-2141.2010.08105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang J, Best S, Menzel S, et al. cMYB is involved in the regulation of fetal hemoglobin production in adults. Blood. 2006;108:1077–1083. doi: 10.1182/blood-2006-01-008912. [DOI] [PubMed] [Google Scholar]
- 36.Clarke RL, Robitaille AM, Moon RT, et al. A Quantitative Proteomic Analysis of Hemogenic Endothelium Reveals Differential Regulation of Hematopoiesis by SOX17. Stem Cell Reports. 2015;5:291–304. doi: 10.1016/j.stemcr.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilber A, Tschulena U, Hargrove PW, et al. A zinc-finger transcriptional activator designed to interact with the gamma-globin gene promoters enhances fetal hemoglobin production in primary human adult erythroblasts. Blood. 2010;115:3033–3041. doi: 10.1182/blood-2009-08-240556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A). We hypothesized that hematopoietic stem cells (HSCs)/hematopoietic progenitor cells (HPCs) could be differentiated and expanded in ES sacs by supplementation of hematopoiesis-stimulating cytokines. We added cytokine mixtures (SCF, FL, TPO, IL3, EPO, and BMP4) into media during ES sac culture at days 9–15, followed by erythroid differentiation in the same manner. (B and C). We harvested and analyzed the spherical cells from the ES sac on day 15. Cytokine supplementation slightly increased the absolute number of CD34+CD45+ HPCs and CD31+CD34+ hematopoietic endothelial cells; however, no significant difference was observed. (D). During erythroid differentiation culture, we counted the number of GPA+ erythroid cells on days 15, 27, 29, 32, and 34. Cytokine supplementation resulted in a higher number of GPA+ erythroid cells compared to the control (p < 0.05 on days 32 and 34). (E). After 13-day erythroid differentiation (day 30), we analyzed relative RNA expression of ε-, γ-, and β-globin using reverse transcription quantitative polymerase chain reaction (RT-qPCR). The percentage of ε-globin was reduced in the cytokine supplementation group compared to the control (p < 0.05), while no significant difference in γ- and β-globin was observed between the two groups. (F). We mainly detected α-globin expression with small amounts of ζ-globin among erythroid cells in both groups.
We evaluated BCL11a RNA expression during erythroid differentiation from ES sacs at day 15. We observed a peak of BCL11a expression after 5 days of erythroid differentiation (day 22); however, BCL11a expression was detected among all time points (days 15, 22, 26, and 30).