Abstract
Heterotopic ossification (HO), the formation of extra-skeletal bone in soft tissues, is a pathologic process occurring after substantial burns or trauma, or in patients with type I bone morphogenetic protein (BMP) receptor hyperactivating mutations. Identifying the cells responsible for de novo bone formation during adulthood is of critical importance for therapeutic and regenerative purposes. Using a model of trauma-induced HO with hindlimb Achilles’ tenotomy and dorsal burn injury and a genetic non-trauma HO model (Nfatc1-Cre/caAcvr1fl/wt), we demonstrate enrichment of previously defined bone-cartilage-stromal progenitor cells (BCSP: AlphaV+/CD105+/Tie2-/CD45-/Thy1-/6C3-) at the site of HO formation when compared with marrow isolated from the ipsilateral hindlimb, or from tissue of the contralateral, uninjured hindlimb. Upon transplantation into tenotomy sites soon after injury, BCSPs isolated from neonatal mice or developing HO incorporate into the developing lesion in cartilage and bone and express chondrogenic and osteogenic transcription factors. Additionally, BCSPs isolated from developing HO similarly incorporate into new HO lesions upon transplantation. Finally, adventitial cells, but not pericytes, appear to play a supportive role in HO formation. Our findings indicate that BCSPs contribute to de novo bone formation during adulthood and may hold substantial regenerative potential.
INTRODUCTION
Heterotopic ossification (HO), the formation of extra-skeletal bone in soft tissues, is a pathologic process that occurs in patients following major burns, musculoskeletal trauma, and orthopaedic surgical procedures. A second group of patients with a mutation in the type I bone morphogenetic protein (BMP) receptor ACVR1 develop fibrodysplasia ossificans progressiva (FOP), which results in progressive, systemic soft tissue ossification.1 Identification of the cells responsible for de novo bone formation as observed in HO is of critical importance for developing therapeutic avenues to prevent or treat HO, and for harnessing these cells for regenerative purposes.2–4
A major challenge in the identification of HO “progenitor cells” is the diverse array of injuries that cause HO. HO may form in patients after blast or crush injuries directly at the site of trauma; in spinal cord injury (SCI) or traumatic brain injury (TBI) patients, HO may develop at distant sites such as the hips. 3,5–7,8–13 Finally, patients with large cutaneous burn injuries have been reported to develop HO within the elbow joint even in the absence of injury to the upper extremity.14–20 Patients with FOP develop ectopic bone lesions within soft tissues after only minimal injury such as needle sticks.1,21–23
Recently, several different cell lineages have been identified within the developing heterotopic bone. Lineage-tracing studies using the Tie2-cre reporter have demonstrated that Tie2-cre cells are present within developing HO but are absent in the final mature ectopic bone3,4. Other studies have demonstrated that Glast-cre cells may contribute to HO throughout its development2,24. However, the contribution of osteoprogenitor cells involved in normal bone to HO development has never been explored. Furthermore, pericytes, which also contribute to normal bone development have not been studied in the context of HO. Importantly, the models utilized to study HO progenitor cells have generally required introduction of bone morphogenetic protein (BMP), either through exogenous administration or genetic up-regulation. These conditions are not representative of HO caused by trauma or even that caused by dysfunctional BMP receptor signaling in the absence of supplied ligand.
Here we evaluate the contribution of known bone-chondro-stromal progenitor (BCSP) cell and pericyte populations to the development of HO in the setting of trauma or constitutive BMP receptor activity (Nfatc1-cre/caACVR1fl/wt)25. BCSPs (AlphaV+/CD105+/Tie2-/CD45-/Thy1-/6C3-) present in normal developing bone have been shown to give rise to de novo endochondral ossification upon transplantation into kidney capsules26–28. However, it is unclear whether these same cells are present and capable of contributing to HO following trauma. We hypothesized that HO, in both the trauma and genetic settings, is characterized by enrichment of BCSP and pericyte populations traditionally observed in normal, developing bone.
RESULTS
Human HO tissue and surrounding muscle are enriched for BCSPs when compared with human skeletal derived osteoblasts and uninjured tissue
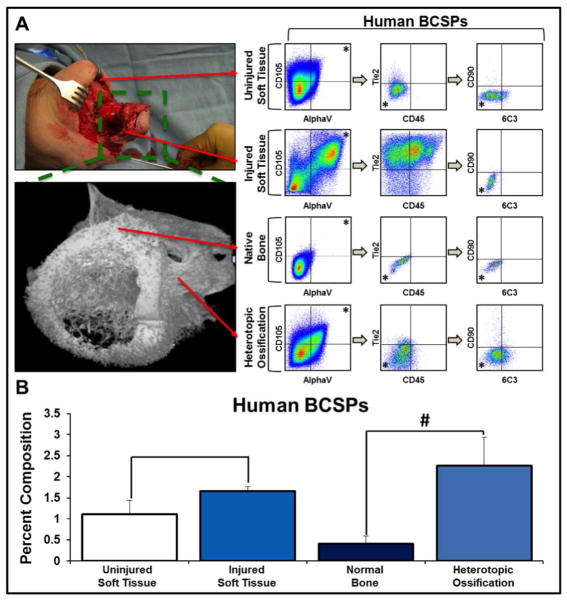
We first set out to analyze the number of BCSPs (AlphaV+/CD105+/Tie2-/CD45-/Thy1-/6C3-) that are present in human HO and normal bone osteoblasts (Fig. 1A). Using flow cytometry, we noted an over four-fold increase in BCSPs in human HO when compared with normal bone osteoblasts (2.26 +/− 0.68% v. 0.41 +/− 0.18%, p<0.05) (Fig. 1B). Though earlier stages of HO would allow for a more representative analysis of HO development, surgical excision of ectopic bone lesions is not indicated until growth has stopped. Therefore, we analyzed BCSP cells in soft tissues of extremities at risk of HO early after trauma and found a similar trend in BCSP enrichment, though to a lesser extent (1.66 +/− 0.11 % v. 1.12 +/− 0.33%) (Fig. 1A, B). Together, these findings indicate that BCSP cells are present in both traumatized soft tissues as well as heterotopic bone, suggesting their involvement in HO subsequent to trauma.
Figure 1. Human HO and injured tissues are enriched for BCSPs when compared to uninjured soft tissue and native bone.
A) FACS schematic demonstrating the identification of BCSPs (AlphaV+/CD105+/CD45-/Tie2-/CD90-/6C3-) from human tissues at the following sites: uninjured soft tissue, injured soft tissue, native bone, and heterotopic ossification; B) Quantification of BCSPs in human tissue at the site of injury as a percent of viable cells (Uninjured soft tissue (n=3): 1.12% (s.d. 0.33%); injured soft tissue (n=2): 1.66% (s.d. 0.11%); normal bone (n=2): 0.41% (s.d. 0.18%); heterotopic ossification (n=2): 2.26% (s.d. 0.68%)). Error bars demonstrate standard deviation. # represents p<0.05.
BCSPs are enriched at the site of future HO in a mouse trauma model
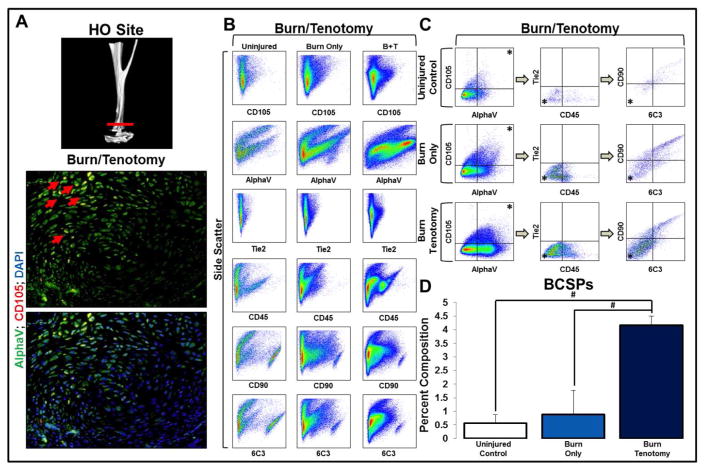
Given that human HO is generally excised after formation of mature osteoid, human samples are unable to elucidate the presence of BCSPs during HO development. Thus, we utilized a mouse trauma model of dorsal burn with Achilles’ tendon transection (burn/tenotomy) to localize and quantify BCSPs in the developing HO lesions7. Tissue was harvested from the tenotomy site 3 weeks after injury and compared with the tissue from uninjured hindlimbs. Immunostaining demonstrated the presence of AlphaV+/CD105+ cells at the tendon transection site (Fig 2A). These tissues were then compared with the tissue from uninjured hindlimbs to quantify BCSP enrichment (Fig. 2B). We found a 4.7-fold enrichment of BCSPs in the tissues harvested from the tenotomy site when compared with the contralateral, uninjured hindlimb (4.17 +/− 0.34% v. 0.88 +/− 0.88%, p<0.05) and a 7-fold enrichment when compared with the uninjured hindlimb of unburned mice (4.17 +/− 0.34% v. 0.57 +/− 0.31%, p<0.05) (Fig 2C, D). Therefore, our findings indicate that developing de novo bone is characterized by enrichment of BCSPs.
Figure 2. Bone progenitor cells are enriched at the site of future HO formation in a trauma model of HO.
A) Micro-CT demonstrating tenotomy site and immunostaining demonstrating presence of BCSPs in tendon transection site three weeks after injury; B) FACS analysis characteristics of murine hindlimb soft tissue in: uninjured, burn only, and burn/tenotomy mice; C) FACS analysis demonstrating the identification of proposed BCSPs in murine hindlimb soft tissue in: uninjured, burn only, and burn/tenotomy mice; D) Quantification of BCSPs in hindlimb soft tissue in uninjured hindlimb, unburned mouse (0.57% +/− 0.31%), uninjured hindlimb, burned mouse: (0.88% +/− 0.88%), and injured hindlimb of burned mouse (4.17% +/− 0.34%). Error bars demonstrate standard deviation. * represents population analyzed in successive panels; # represents p<0.05; N=3 for all samples.
BCSPs are enriched at the site of future HO formation in genetic HO
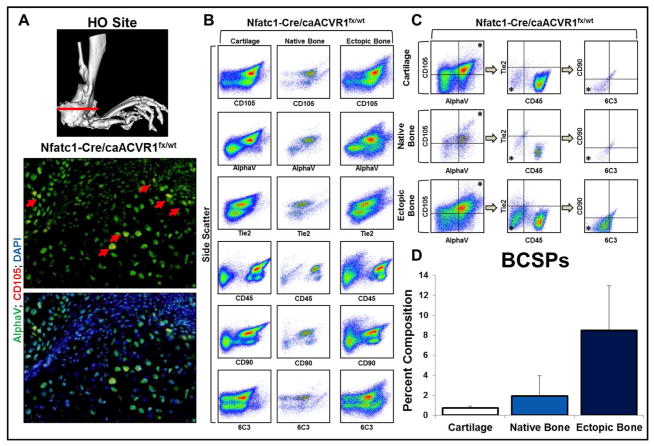
To determine whether genetic HO and trauma-induced HO share similar osteoprogenitor populations, we used our genetic HO model (Nfatc1-cre/caACVR1fl/wt) (Fig. 3A).25 Ectopic bone isolated from mutants demonstrated 4.4-fold enrichment for BCSPs when compared with native bone from littermate control mice (8.48 +/− 4.49% v. 1.93 +/− 2.02%) (Fig. 3B–D). Additionally, BCSPs appeared to be enriched in ectopic bone when compared with cartilage suggesting a defined role for BCSPs specific to endochondral ossification. Overall, these findings suggest that genetic and trauma-induced HO share a common osteoprogenitor cell involved in bone formation.
Figure 3. Bone progenitor cells are enriched at the site of future HO formation in a genetic model of HO.
A) Micro-CT demonstrating site of section; immunostaining demonstrating presence of BCSPs in Nfatc1-Cre/caACVR1fl/wt mice; B) FACS analysis characteristics of murine cartilage, bone, and ectopic bone in Nfatc1-Cre/caACVR1fl/wt mice; C) FACS analysis demonstrating the identification of proposed BCSPs in murine cartilage, bone, and ectopic bone in Nfatc1-Cre/caACVR1fl/wt mice. Asterisk represents population analyzed in successive panels; D) Quantification of BCSPs in murine cartilage (0.73 +/− 0.18%), bone (1.93 +/− 2.02%), and ectopic bone (8.48 +/− 4.49%) in Nfatc1-Cre/caACVR1fl/wt mice as a percent of viable cells. N=2 mice per group.
Pericytes are not enriched at the site of heterotopic ossification but are present and surround the site of ectopic bone formation
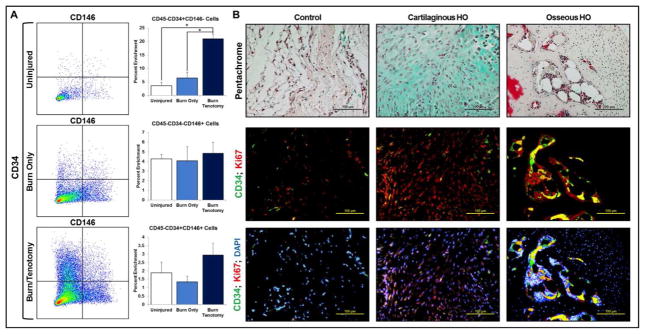
Recognizing that HO likely represents a heterogeneous mixture of cells, we next evaluated the presence of perivascular stem cells comprising two separate populations: pericytes (CD45-/CD34−/CD146+) 29–31 and adventitial cells (CD45-/CD34+/CD146−)30–33 in our models. Pericytes are known to have potential to undergo chondrogenic differentiation in vitro, have been implicated in ectopic calcification, and undergo in vitro osteogenic differentiation with overexpression of specific growth factors. Flow cytometry showed absence of enrichment for CD45-/CD34−/CD146+ pericytes (Fig 4A), while there was a significant enrichment of CD45-/CD34+/CD146− adventitial cells (Fig 4A). Immunostaining was then performed to assess the location of the enriched CD34+ cells showing that these were present throughout the cartilage and the endosteum of mature HO bone (Fig 4B). The presence of CD34+ cells was verified in the ectopic bone of mutant mice as well (Sup Fig 1).
Figure 4. Adventitial cells, but not pericytes, are enriched in HO.
(A) Presence of adventitial CD45-/CD34+/CD146- cells (Uninjured: 3.66 +/− 1.22%, Burn/Uninjured: 6.53 +/− 2.07%, Burn/Tenotomy: 21.03 +/− 1.72%), pericyte CD45-/CD34−/CD146+ cells (Uninjured: 4.28% +/− 0.43%, Burn/Uninjured: 4.07 +/− 1.48%, Burn/Tenotomy: 4.84 +/− 1.11%), and CD45-/CD34+/CD146+ endothelial cells (Uninjured: 1.89 +/− 0.63%, Burn/Uninjured: 1.35 +/− 0.33%, Burn/Tenotomy: 2.94 +/− 0.70%) three weeks after injury (N=3/group); (B) Sites of uninjured tissue (control), developing cartilage, and osteoid with respective immunofluorescent staining for CD34 and Ki67.
A systemic source contributes to BCSPs rarely
To determine whether BCSPs comprising the trauma-induced HO lesion are derived from a systemic or local source, we performed parabiosis between GFP-labeled and unlabeled wild type mice (Sup Fig 2A). Mice were joined together for 14 days to allow a shared circulation, followed by injury (burn/tenotomy) of the unlabeled wild-type mouse. Examination of the site three weeks after injury demonstrated rare presence of labeled BCSP cells (GFP+/AlphaV+/CD105+) located within the cartilage anlagen of the unlabeled mouse (Sup Fig 2B). These findings indicated that rarely BCSPs are derived from a systemic source outside of the local tissue environment.
In vivo cell transplantation of BCSPs
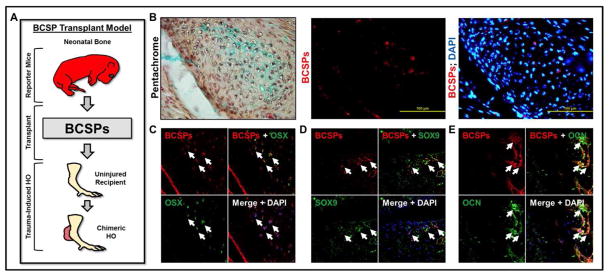
Next, we performed BCSP transplantation into the tendon transection site to demonstrate that these cells have the potential for in vivo chondrogenic and osteogenic differentiation. First, BCSPs (AlphaV+/CD105+/Tie2-/CD45-/Thy1-/6C3-) were isolated from neonatal mice and directly transplanted into the tenotomy site within six hours after injury (Fig 5A). Transplanted BCSPs could be identified within the cartilage of forming HO lesions three weeks after injury (Fig 5B). Furthermore, these BCSPs expressed Osterix (Fig 5C) and Sox9 (Fig 5D). Finally, BCSPs in areas of mature bone expressed Osteocalcin (Fig 5E).
Figure 5. Transplantation of BCSPs from neonatal mice into new tenotomy site.
(A) Schematic showing transplantation of BCSPs from neonatal mice into tenotomy site; (B) BCSPs from neonatal mice were injected into tenotomy sites and observed in Alcian-blue staining cartilage; (C) BCSPs from neonatal mice injected into tenotomy sites express Osterix; (D) BCSPs from neonatal mice injected into tenotomy sites express Sox9; (E) BCSPs from neonatal mice injected into tenotomy sites express Osteocalcin.
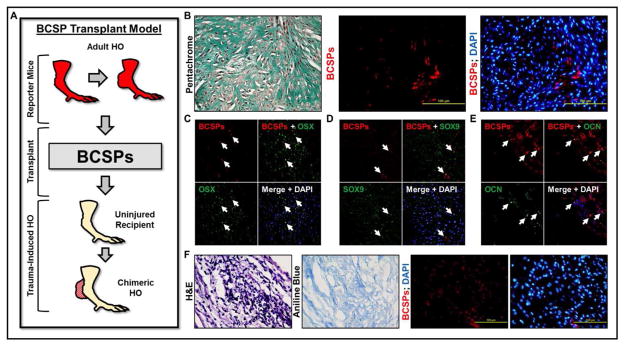
Next, BCSPs were isolated from the developing HO of mice three week after injury and transplanted into the tenotomy site of mice six hours after injury (Fig 6A). Again, transplanted BCSPs were identified in the lesion (Fig 6B). These BCSPs expressed Osterix (Fig 6C) and Sox9 (Fig 6D) in the developing cartilage and Osteocalcin (Fig 6E) in sites of mature bone. Finally, when BCSPs were isolated from developing HO and injected into uninjured tendon, these cells formed osteoid (Fig 6F).
Fig 6. Transplantation of BCSPs from the developing HO into new tenotomy site.
(A) Schematic showing transplantation of BCSPs from developing HO into tenotomy site (B) BCSPs from the HO site injected into separate tenotomy sites were identified in the lesion; (C) BCSPs from the HO site injected into separate tenotomy sites express Osterix; (D) BCSPs from the HO site injected into separate tenotomy sites express Sox9; (E) BCSPs from the HO site injected into separate tenotomy sites express Osteocalcin; (F) BCSPs from the HO site injected into uninjured tendon form osteoid.
DISCUSSION
Heterotopic ossification (HO) is the formation of extra-skeletal bone which occurs in patients with substantial trauma or BMP type I receptor mutations. Previously, HO has been shown to undergo multiple stages of development including proliferation of mesenchymal cells followed by cartilage formation and finally osteoid deposition7. Due to the similarity between endochondral ossification as observed in HO and normal bone development, we chose to focus on a known osteoprogenitor population which contributes to normal bone development.
In this analysis, we found that both trauma-induced and genetic HO demonstrate enrichment of BCSPs defined as AlphaV+/CD105+/Tie2-/CD45-/CD90-/BP1-; these cells, when isolated from neonatal mice, have previously been shown to give rise to bone, stroma, and cartilage when implanted in a kidney capsule26,27. BCSPs have also been recently shown to undergo expansion prior to fracture healing in post-natal mice indicating that their presence is not limited to embryonic bone development34. Similar to HO which develops in a variety of tissue types, implantation of these cells in fat and striated muscle also results in endochondral bone formation27. This population is enriched in both animal models of HO, consistent with its enrichment in human HO and in human traumatic wounds. That HO lesions form a marrow cavity is consistent with the previously demonstrated ability of BCSPs to form bone with stromal cavity27. Furthermore, our results demonstrate that these cells, when harvested from either neonatal mice or from developing HO, undergo osteogenic and chondrogenic differentiation on the basis of Osteocalcin, Osterix, and Sox9 expression. Overall, the shared presence of BCSPs in normally developing bone, healing fracture callus, and now in de novo ectopic bone suggests that these cells play a conserved role in post-natal bone formation.
Previous studies have identified other cell types including the Tie2-cre lineage and the GLAST-expressing lineage in models of exogenously administered bone morphogenetic protein (BMP). Interestingly, Tie2-cre lineage cells have not been seen within the mature ectopic bone3,4. Conversely, GLAST-expressing cells are not present in normal developing bone24. Another study has demonstrated that CD73+/CD105+/CD90- human skeletal muscle MSCs are clonally multi-potent and can give rise to all lineages present within HO35. Our BCSP panel includes CD105-positivity and CD90-negativity, but does not account for CD73-positivity. Unfortunately, due to the absence of lineage-tracing (Cre-expressing) mice specific to BCSP cells, it is not possible to determine whether BCSPs are entirely responsible for HO. However, our cell transplantation studies using BCSPs isolated from neonatal mice suggest that these cells are able to form bone in the wounded tendon environment after burn/tenotomy. These findings are strengthened by the identification and isolation of BCSPs from the injured tendon transection site which undergo chondrogenic and osteogenic differentiation upon transplantation into a second tendon transection site.
Though the identification of a single “HO precursor” cell is enticing, it is likely that HO is instead a heterogeneous composition of cells. Therefore, studying the cells which give rise to HO also requires an understanding of the cells which support its development. Initially we focused on the CD146+ pericyte cell population due to previous studies indicating its role in ectopic calcification, and capacity for in vitro chondrogenic and osteogenic differentiation36–38. These cells are present in the developing HO as well suggesting a role in HO development.
Our study is primarily limited by the fact that heterotopic bone is likely a heterogeneous composition of cells. Although BCSPs are certainly enriched at the site of HO, they do not represent a majority of cells in either the setting of trauma-induced or genetic HO. It is not possible to determine whether transitioning through the BCSP surface-marker phenotype is prerequisite for cells to contribute to endochondral ossification. Future studies may include knockouts of specific cell surface markers in order to determine whether loss of specific surface markers leads to absence of HO. Other cell types such as GLAST-expressing cells or Tie2-cre cells may also contribute to the lesion and whether these cells must transition through the BCSP phenotype is not clear. Therefore, it is not surprising that identification of a single progenitor cell for HO has been elusive, as it may not exist.
Overall, our findings demonstrate that a bone-cartilage-stromal progenitor cell surface phenotype which is present during normal bone development and uniquely gives rise to endochondral ossification, is also enriched during HO formation. Though a subset of cells responsible for HO, their presence during extra-skeletal bone formation suggests that their presence is not limited to normal skeletal development or skeletal healing.
METHODS
Ethics Statement
All animal experiments described were approved by the University Committee on Use and Care of Animals at the University of Michigan-Ann Arbor (Protocols: #05909). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal procedures were carried out in accordance with the guidelines provided in the Guide for the Use and Care of Laboratory Animals: Eighth Edition from the Institute for Laboratory Animal Research (ILAR, 2011). Institutional review board (IRB) approval was obtained through the University of Michigan (HUM051190).
Human Tissues
IRB approval was obtained from University of Michigan. Heterotopic ossification, native bone, and areas of surrounding soft tissue were excised using normal surgical techniques without excision of excess tissue. Each tissue specimen was freshly collected and immediately placed in sterile saline for further analysis.
Animals
C57/Bl6 male mice aged 6–8 weeks (Charles River, Wilmington, MA) were used for all experiments describing burn or burn/tenotomy. For our genetic model mice carrying the floxed constitutively active allele of ACVR1 (ACVR1 carrying Q207D mutation, ca-ACVR1fl/WT) and Nfatc1-Cre transgenic mice were used for breeding as previously described39–43. Resulting pups carrying both transgenes (Nfatc1-cre/ca-ACVR1fl/WT) were used as experimental mice. Littermates missing one or both transgenes were used as controls.
Trauma Induced Heterotopic Ossification
Mice in burn-only and burn/tenotomy groups received a 30% total body surface area (TBSA) partial-thickness burn injury to their dorsum. Briefly mice were anesthetized with 3–5% inhaled isoflurane and the left dorsum hair was clipped. Preoperative analgesia was provided with subcutaneous buprenorphine. The shaved area was then exposed to a metal block heated to 60°C in a hot-water bath for 18 seconds. Mice in the burn/tenotomy group then received a sharp dissection of the left Achilles tendon with sterile scissors immediately distal to the fuse-point of the fibula and tibia. The tenotomy site was closed with a single 5-0 vicryl stitch placed through the skin only. After injury all mice were allowed to recover and return to normal activity. Pain management was achieved with subcutaneous injections of buprenorphine every 12 hours for 3 days.
Parabiosis Model
Wild-type and GFP+ FVB mice were surgically joined together at the lateral midline44. Mice were monitored continuously for the first three days following joining, followed by twice daily evaluation for the next 11 days. Following a period of 14 days after joining, the unlabeled wild type mouse received the burn/tenotomy injury44.
Histologic Processing
In our trauma model, animals were euthanized for histology 3 weeks post-injury. In our genetic model, animals were survived to 3, 7, and 13 days after birth before sacrifice for histology.
Briefly skin was removed from the tenotomy site and the area of heterotopic bone formation was fixed in 10% buffered formalin solution for 24 hours at 4°C. Decalcification of the sample was completed with 19% ethylenediaminetetracetic acid (EDTA) solution for 28 days at 4C. Decalcified tissues were dehydrated through xylenes and graded ethanol for paraffin processing and embedding. Five-micron transverse sections were cut through from the area starting at the base of the talus distally to the lower gastrocnemius proximally. Sections were mounted on Superfrost plus slides (Fisher Scientific), and incubated for 4 hours at 56°C.
Microscopic Analysis
Fluorescent images were acquired using a Leica Upright SP5X Confocal Microscope with 2-Photon on an upright DMI 6000 stand with Supercontinuum “white-light laser” for selectable excitation wavelengths and a resonant scanner. At the University of Michigan Microscopy and Imaging Analysis Laboratory. Each site was imaged in all channels and images were overlaid before examination in Adobe Photoshop.
Tissue Digestion
Human samples were collected as described above and divided into four groups for digestion: native bone, heterotopic ossification, injured soft tissue, uninjured soft tissue.
Mice from the trauma model of HO group were survived for 3 weeks after injury. At time of sacrifice, tissue was collected from the hindlimbs bilaterally. In mice receiving burn tenotomy, the area of injury from the insertion of the tendon into the calcaneus to the distal gastrocnemius including encompassing areas of HO formation and surrounding soft tissue were harvested. In uninjured mice and those receiving burn only an analogous portion of the hindlimb soft tissue was collected.
Mice from the genetic model of HO group were survived to 21 days after birth. At time of sacrifice, native bone was collected from the diaphysis of the femur and tibia, cartilage was collected from the femoral head and the knee, and ectopic bone in mutant mice was collected from the ankle. Bone marrow was harvested from femurs of both wild type and Nfatc1-Cre/caACVR1fl/wt mutant mice for separate analysis without need for further digestion.
To obtain BCSP and pericyte populations for cell transplantation assays, post-natal tdT+ mice aged P4 were euthanized and mechanically dissociated.
All collected tissues were mechanically dissociated using either a sterile rongeur for human bone or sterile scissors for all murine samples. Tissues were digested for 120 minutes in 0.75% Collagenase 2 (Sigma-Aldrich) in Hanks Balanced Salt Solution (HBSS) at 37C under sustained agitation. Samples were filtered using successive 100- and 70-micron sterile strainers and digestion was quenched using equal parts 10% DMEM. Samples were centrifuges at 1,000 rpm for 5 minutes before removing the supernatant and washing in HBSS. This process was repeated in triplicate.
Flow Cytometry
Staining for BCSPs was performed using a previously described AlphaV+/CD105+/Tie2-/CD45-/CD90-/BP1- panel. Antibodies used included: AlphaV-PE (12-0512-83, eBioscience), Tie2-AlexaFluor 488 (334208, BioLegend), CD45-APC (17-0451-83, eBioscience), CD105-eFluor450 (48-1057-42, eBioscience), CD90-PE-Cyanine7 (25-0902-82, eBioscience), and BP1-Biotin (13-5891-85, eBioscience) conjugated with Streptavidin-PerCP-Cyanine5.5 (45-4317-82, eBioscience).
Staining for pericytes was performed as performed using the following antibodies: Anti-CD146-PE (12-1469-41, eBioecience), Anti-Mouse CD34-FITC (11-0341-81, eBioscience), and Anti-Mouse CD45-APC (17-0451-82, eBioscience).
Samples were incubated for 30 minutes at 4°C with an additional 15 minutes for the addition of Streptavidin- PerCP-Cyanine5.5 for the BCSPs. Following incubation samples were washed three times and filtered through a 45 micron mesh filter before being run on a FACSAria II (BD Biosciences) Cell Sorter at the University of Michigan Flow Cytometry Core. Viability was assessed by propridium iodide staining at time of sorting and analysis. Greater than 10,000 events were collected for each population. All analyses were carried out in FlowJo.
Cells from burn/tenotomy soft tissue or uninjured P4 mice were concurrently sorted to generate purified BCSPs and BCSPs-depleted subpopulations.
In vivo cell transplantation
Unlabeled wild type C57Bl/6 mice were the recipients of labeled BCSP populations flow sorted from either uninjured mice (age P4) or from the burn/tenotomy site three weeks after injury. All donor BCSP populations were generated from labeled, tdT+ mice. Recipient mice underwent burn/tenotomy as described above within 12 hours of transplantation. Subsequently, donor-derived BCSPs suspended in Matrigel (50,000 cells/10uL) were injected directly into the tendon transection site. Recipient mice were then euthanized within 3 and 5 weeks after injury to evaluate the distribution of BCSPs within the developing HO lesion. Separately, mice were injected with BCSPs derived from the tenotomy site into uninjured tendon to assess their ability to independently form osteoid.
Immunofluorescence
Tissue was processed for histology as described above. After rehydration antigen retrieval was cared out in pH6.0 buffered sodium citrate (10mM) with 0.05% Tween at 90°C for 30 minutes. Samples were then blocked for 1 hour at room temperature (Blocking: 1% BSA, 2% normal donkey serum, 0.1% Cold Water fish skin gelatin, 0.1% TritonX-100, 0.05% Tween-20, and 0.3 M glycine in PBS). After blocking samples were incubated overnight at 4°C in 1:50 dilutions of the following primary antibodies: Endoglin Antibody (RM0030-6J9; Santa Cruz), Rabbit Anti-Integrin alpha V/CD51 (bs-2250R, Bioss), Anti-Mouse/Rat CD90.1-Biotin (13-0900-81, eBioscience), BP1-Biotin (13-5891-85, eBioscience), Tie2-Biotin (13-5987-81, eBioscience), and CD45-Biotin (13-0451-81, eBioscience), Sox9 (sc-17341, Santa Cruz), Osteocalcin (sc-18319, Santa Cruz), Osterix (bs-1110R, BIOSS). Samples were then washed in PBS and the following secondary antibodies were applied: Donkey anti-Rat AlexaFluor 594 (Life Technologies), Donkey anti-Rabbit AlexaFluor 488 9Life Technologies), and Streptavidin APC (17-4317-82, eBioscience). Tissues were incubated in secondary for 1 hour at room temperature and washed thoroughly. Samples were then incubabted for 15 minutes at room temperature in a 1:10000 dilution of Hoechst 33342 (Life Technologies) before being rinsed in PBS. Samples were mounted with Prolong Gold Antifade Reagent (Life Technologies) and cover slips were applied.
Statistical Analysis
Means and SDs for flow cytometry values were calculated from numerical data, as presented in the text, figures, and figure legends. In figures, bar graphs represent means, whereas error bars represent one SD. Statistical analysis was performed using an appropriate analysis of variance when more than two groups were compared, followed by a post hoc Student’s t test to directly compare two groups. P values and sample size are included in the figure legends.
Supplementary Material
References
- 1.Shore EM, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nature genetics. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 2.Kan L, Kessler JA. Evaluation of the cellular origins of heterotopic ossification. Orthopedics. 2014;37:329–340. doi: 10.3928/01477447-20140430-07. [DOI] [PubMed] [Google Scholar]
- 3.Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2012;27:1004–1017. doi: 10.1002/jbmr.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medici D, et al. Conversion of vascular endothelial cells into multipotent stem-like cells. Nature medicine. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis TA, et al. Heterotopic ossification in complex orthopaedic combat wounds: quantification and characterization of osteogenic precursor cell activity in traumatized muscle. The Journal of bone and joint surgery. 2011;93:1122–1131. doi: 10.2106/JBJS.J.01417. [DOI] [PubMed] [Google Scholar]
- 6.Nelson ER, Wong VW, Krebsbach PH, Wang SC, Levi B. Heterotopic ossification following burn injury: the role of stem cells. Journal of burn care & research: official publication of the American Burn Association. 2012;33:463–470. doi: 10.1097/BCR.0b013e31825af547. [DOI] [PubMed] [Google Scholar]
- 7.Peterson JR, et al. Treatment of heterotopic ossification through remote ATP hydrolysis. Science translational medicine. 2014;6:255ra132. doi: 10.1126/scitranslmed.3008810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banovac K. The effect of etidronate on late development of heterotopic ossification after spinal cord injury. J Spinal Cord Med. 2000;23:40–44. doi: 10.1080/10790268.2000.11753507. [DOI] [PubMed] [Google Scholar]
- 9.Banovac K, Williams JM, Patrick LD, Haniff YM. Prevention of heterotopic ossification after spinal cord injury with indomethacin. Spinal cord. 2001;39:370–374. doi: 10.1038/sj.sc.3101166. [DOI] [PubMed] [Google Scholar]
- 10.Cassar-Pullicino VN, et al. Sonographic diagnosis of heterotopic bone formation in spinal injury patients. Paraplegia. 1993;31:40–50. doi: 10.1038/sc.1993.7. [DOI] [PubMed] [Google Scholar]
- 11.Dewan AK, et al. Assessing mechanical integrity of spinal fusion by in situ endochondral osteoinduction in the murine model. J Orthop Surg Res. 2010;5:58. doi: 10.1186/1749-799X-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garland DE. Clinical observations on fractures and heterotopic ossification in the spinal cord and traumatic brain injured populations. Clinical orthopaedics and related research. 1988:86–101. [PubMed] [Google Scholar]
- 13.van Kuijk AA, Geurts AC, van Kuppevelt HJ. Neurogenic heterotopic ossification in spinal cord injury. Spinal cord. 2002;40:313–326. doi: 10.1038/sj.sc.3101309. [DOI] [PubMed] [Google Scholar]
- 14.Ellerin BE, et al. Current therapy in the management of heterotopic ossification of the elbow: a review with case studies. Am J Phys Med Rehabil. 1999;78:259–271. doi: 10.1097/00002060-199905000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Holguin PH, Rico AA, Garcia JP, Del Rio JL. Elbow anchylosis due to postburn heterotopic ossification. The Journal of burn care & rehabilitation. 1996;17:150–154. doi: 10.1097/00004630-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Kung TA, Jebson PJ, Cederna PS. An individualized approach to severe elbow burn contractures. Plastic and reconstructive surgery. 2012;129:663e–673e. doi: 10.1097/PRS.0b013e3182450c0c. [DOI] [PubMed] [Google Scholar]
- 17.Ploumis A, Belbasis L, Ntzani E, Tsekeris P, Xenakis T. Radiotherapy for prevention of heterotopic ossification of the elbow: a systematic review of the literature. J Shoulder Elbow Surg. 2013;22:1580–1588. doi: 10.1016/j.jse.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 18.Ring D, Jupiter JB. Excision of heterotopic bone around the elbow. Tech Hand Up Extrem Surg. 2004;8:25–33. doi: 10.1097/00130911-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Tsionos I, Leclercq C, Rochet JM. Heterotopic ossification of the elbow in patients with burns. Results after early excision. The Journal of bone and joint surgery. 2004;86:396–403. doi: 10.1302/0301-620x.86b3.14480. [DOI] [PubMed] [Google Scholar]
- 20.Yang SC, et al. Early surgical management for heterotopic ossification about the elbow presenting as limited range of motion associated with ulnar neuropathy. Chang Gung Med J. 2002;25:245–252. [PubMed] [Google Scholar]
- 21.Kaplan FS, et al. Dysregulation of the BMP-4 signaling pathway in fibrodysplasia ossificans progressiva. Ann N Y Acad Sci. 2006;1068:54–65. doi: 10.1196/annals.1346.008. [DOI] [PubMed] [Google Scholar]
- 22.Shore EM, Gannon FH, Kaplan FS. Fibrodysplasia ossificans progressiva why do some people have two skeletons? J Clin Rheumatol. 1997;3:84–89. doi: 10.1097/00124743-199704001-00019. [DOI] [PubMed] [Google Scholar]
- 23.Shore EM, Gannon FH, Kaplan FS. Fibrodysplasia ossificans progressiva: why do some people have two skeletons? Rev Rhum Engl Ed. 1997;64:92S–97S. [PubMed] [Google Scholar]
- 24.Kan L, Peng CY, McGuire TL, Kessler JA. Glast-expressing progenitor cells contribute to heterotopic ossification. Bone. 2013;53:194–203. doi: 10.1016/j.bone.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal S, et al. BMP signaling mediated by constitutively active Activin type 1 receptor (ACVR1) results in ectopic bone formation localized to distal extremity joints. Developmental biology. 2015 doi: 10.1016/j.ydbio.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan CK, et al. Clonal precursor of bone, cartilage, and hematopoietic niche stromal cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:12643–12648. doi: 10.1073/pnas.1310212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan CK, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160:285–298. doi: 10.1016/j.cell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marecic O, et al. Identification and characterization of an injury-induced skeletal progenitor. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:9920–9925. doi: 10.1073/pnas.1513066112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen J, et al. Pericyte Antigens in Perivascular Soft Tissue Tumors. International journal of surgical pathology. 2015;23:638–648. doi: 10.1177/1066896915591272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James AW, et al. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem cells translational medicine. 2012;1:510–519. doi: 10.5966/sctm.2012-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James AW, et al. An abundant perivascular source of stem cells for bone tissue engineering. Stem cells translational medicine. 2012;1:673–684. doi: 10.5966/sctm.2012-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corselli M, et al. Identification of perivascular mesenchymal stromal/stem cells by flow cytometry. Cytometry. Part A: the journal of the International Society for Analytical Cytology. 2013;83:714–720. doi: 10.1002/cyto.a.22313. [DOI] [PubMed] [Google Scholar]
- 33.Corselli M, et al. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem cells and development. 2012;21:1299–1308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tevlin R, et al. Skeletal Stem Cell Niche Aberrancies Underlie Impaired Fracture Healing in a Mouse Model of Type 2 Diabetes. Plastic and reconstructive surgery. 2015;136:73. [Google Scholar]
- 35.Downey J, et al. Prospective heterotopic ossification progenitors in adult human skeletal muscle. Bone. 2015;71:164–170. doi: 10.1016/j.bone.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Schor AM, Allen TD, Canfield AE, Sloan P, Schor SL. Pericytes derived from the retinal microvasculature undergo calcification in vitro. Journal of cell science. 1990;97(Pt 3):449–461. doi: 10.1242/jcs.97.3.449. [DOI] [PubMed] [Google Scholar]
- 37.Canfield AE, Sutton AB, Hoyland JA, Schor AM. Association of thrombospondin-1 with osteogenic differentiation of retinal pericytes in vitro. Journal of cell science. 1996;109(Pt 2):343–353. doi: 10.1242/jcs.109.2.343. [DOI] [PubMed] [Google Scholar]
- 38.Doherty MJ, et al. Vascular pericytes express osteogenic potential in vitro and in vivo. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 1998;13:828–838. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- 39.Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB., 3rd BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development. 2001;128:4449–4461. doi: 10.1242/dev.128.22.4449. [DOI] [PubMed] [Google Scholar]
- 40.Fukuda T, et al. Generation of a mouse with conditionally activated signaling through the BMP receptor, ALK2. Genesis. 2006;44:159–167. doi: 10.1002/dvg.20201. [DOI] [PubMed] [Google Scholar]
- 41.Kaartinen V, et al. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development. 2004;131:3481–3490. doi: 10.1242/dev.01214. [DOI] [PubMed] [Google Scholar]
- 42.Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Developmental biology. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 43.Thomas PS, Rajderkar S, Lane J, Mishina Y, Kaartinen V. AcvR1-mediated BMP signaling in second heart field is required for arterial pole development: implications for myocardial differentiation and regional identity. Developmental biology. 2014;390:191–207. doi: 10.1016/j.ydbio.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinha M, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.