SUMMARY
Transplantation of human pluripotent stem cell (hPSC)-derived neurons is a promising avenue for treating disorders including Parkinson’s disease (PD). Precise control over engrafted cell activity is highly desired, as cells do not always integrate properly into host circuitry and can cause suboptimal graft function or undesired outcomes. Here, we show tunable rescue of motor function in a mouse model of PD, following transplantation of human midbrain dopaminergic (mDA) neurons differentiated from hPSCs engineered to express DREADDs (designer receptors exclusively activated by designer drug). Administering clozapine-N-oxide (CNO) enabled precise DREADD-dependent stimulation or inhibition of engrafted neurons, revealing D1 receptor-dependent regulation of host neuronal circuitry by engrafted cells. Transplanted cells rescued motor defects, which could be reversed or enhanced by CNO-based control of graft function, and activating engrafted cells drives behavioral changes in transplanted mice. These results highlight the ability to exogenously and noninvasively control and refine therapeutic outcomes following cell transplantation.
Graphical abstract
INTRODUCTION
Human pluripotent stem cells (hPSCs), including embryonic (hESCs) and induced pluripotent stem cells (hiPSCs) (Takahashi et al., 2007; Thomson et al., 1998), are a potential source for regenerative medicine. Following transplantation into model animals, hPSC-derived neuronal progenitors survive and mature, and contribute to behavioral recovery in animals with Parkinson’s disease (PD) (Grealish et al., 2014; Kirkeby et al., 2012; Kriks et al., 2011), Huntington’s disease (Ma et al., 2012), spinal cord injury (Lu et al., 2012), and epilepsy (Cunningham et al., 2014). These results highlight the potential of hPSCs in treating debilitating diseases like neurodegenerative disorders.
For stem cell therapy to succeed in neurological disorders, the engrafted cells must replace the lost neuronal types, connect to the precise targets, and receive proper innervations (Rossi and Cattaneo, 2002). However, transplanted neurons do not always integrate into the correct circuits and faithfully compensate the function of diseased neurons, which may result in incomplete/excessive therapeutic outcomes and, sometimes, even severe side effects. In PD, a degenerative disorder resulting from loss of dopamine (DA) neurons in the midbrain and insufficient DA release in the striatum, transplantation of human fetal ventral midbrain tissue into the striatum can restore DA release and ameliorate symptoms in some PD patients (Barker et al., 2013). However, many patients show incomplete functional recovery (Lindvall and Hagell, 2000), whereas some (15%–56%) exhibit uncontrolled movements, named graft-induced dyskinesia (GID) (Freed et al., 2001; Hagell et al., 2002; Olanow et al., 2003). These somewhat discouraging outcomes have led to a period of relative quiescence in the clinic trials of cell therapy in PD patients (Breger and Lane, 2013). Such outcomes are partly due to unregulated release of DA from engrafted cells (Freed et al., 2001; Maries et al., 2006). A similar scenario likely applies to cell therapy in other neurological and psychiatric diseases that require precise neural transmission. Therefore, the ability to regulate the graft activity will substantially raise the prospect of cell therapy.
One solution is to engineer a functional “switch” in the transplanted cells so that the neurotransmitter release from the engrafted neurons can be controlled at will. Chemogenetic tools, including DREADD (designer receptor exclusively activated by designer drug), enable regulation of cellular function by a designer drug (Sternson and Roth, 2014). DREADD is a family of engineered G protein-coupled receptors (GPCRs) that can precisely control three major GPCR signaling pathways (Gq, Gi, and Gs). These receptors can be activated by pharmacologically inert compounds such as clozapine-N-oxide (CNO), but not by their native ligand acetylcholine. CNO can be taken orally and can easily penetrate the blood-brain barrier, enabling remote control of the DREADD-expressing neurons in the brain (Alexander et al., 2009; Armbruster et al., 2007). Indeed, following transplantation of DREADD (hM3Dq)-expressing mouse induced dopaminergic (iDA) neurons (directly converted from fibroblasts), treatment of the grafted PD animals by CNO for three consecutive days resulted in motor function of PD animals closely comparable to those received by primary embryonic DA neurons, as assayed by apomorphine-induced rotation in the absence of CNO (Dell’Anno et al., 2014). This result suggests that the function of the graft may be augmented by chronic stimulation of the DREADD-expressing cells (Dell’Anno et al., 2014). However, it remains unknown whether the motor function of PD animals can be directly regulated through modulating the graft function in a drug-dependent and reversible manner. In particular, it is critical to determine if the graft-repaired circuit, and hence the therapeutic outcomes, can be tuned up or down remotely by regulating the function of engrafted neurons in real time.
We approached this question by engineering DREADD (both excitatory hM3Dq and inhibitory hM4Di receptor)-expressing hPSC lines by CRISPR (clustered regularly interspaced short palindromic repeats), differentiating them to midbrain dopaminergic (mDA) neurons, and transplanting the neurons into PD mice. We found that the activity of human mDA neurons is precisely regulated in vitro and in vivo, including the transmission between grafted mDA neurons and host striatal neurons. Importantly, peripheral application of CNO controls the motor behaviors of the transplanted PD mice in a drug-dependent and reversible manner.
RESULTS
Function of DREADD-Expressing Human mDA Neurons Is Regulated by CNO
To achieve stable and consistent expression of DREADDs in hPSC-derived mDA neurons, we knocked in hM4Di (the inhibitory DREADD)- or hM3Dq (the excitatory DREADD)-expressing cassette as well as the EGFP expression cassette (control) into the AAVS1 locus of H9 hESCs using CRISPR (Figures 1A and 1B). The integration of the transgene in the resulting clonal hESCs was confirmed by genomic PCR (Figures S1A and S1B, available online). Homozygous clones were selected (Figure S1B). These three hESC lines containing two copies of DREADD gene (or EGFP gene) were differentiated into mDA neurons. At day 18 of differentiation, coexpression of EN1/OTX2 and LMX1A/FOXA2 was found in >80% of total cells in all three lines (Figures S1C–S1E), suggesting efficient specification of ventral midbrain progenitors. By day 42, ~60% of the differentiated cells express tyrosine hydroxylase (TH) and >90% of TH-positive cells also expressed FOXA2 or EN1 (Figures 1C, 1D, S1F, and S1G), suggestive of an mDA neuronal identity. In the inhibitory hM4Di- or excitatory hM3Dq-expressing cells, mCherry was detected on the membrane and processes (Figure 1C). Thus, transgenes are robustly expressed in hESCs and differentiated mDA neurons, and the transgene expression does not interfere with mDA neuron differentiation.
Figure 1. Establishment, mDA Neuron Differentiation, and Functional Assessment of the DREADD-Expressing hESC Lines.
(A) Schematic diagram showing the strategy for functional regulation of human mDA neurons transplanted into PD mice.
(B) Schematic depiction of the strategy for knockin of EGFP-, hM3Dq-, or hM4Di-expressing cassette into AAVS1 locus. Exons are shown as orange boxes. The vertical arrows indicate targeting site by sgRNA T2 in AAVS1 locus. Donor plasmids are as follows: SA-Pur, splice acceptor sequence followed by a T2A self-cleaving peptide sequence and the puromycin resistance gene, and CAG, synthetic CAGGS promoter containing the actin enhancer and the cytomegalovirus early promoter. mCherry is fused to the C terminus of receptors.
(C) Immunostaining of day 42 cultures derived from hM4Di- or hM3Dq-expressing hESCs shows markers for mDA neurons. Ho, Hoechst. Scale bar, 25 µm.
(D) Quantification of cellular differentiation presented in (C).
(E and F) Whole-cell patch clamping of differentiated human mDA neurons shows representative changes in membrane potential on 10-week-old cultures (E) and spontaneous action potentials (sAP) on 12-week-old cultures (F) upon treatment with and wash of CNO (40 µM).
(G and H) The statistical analysis of changes in membrane potential (G; n = 4–9 for each group) and frequency of spontaneous action potentials (H) upon treatment with and wash of CNO (40 µM) (n = 6–9 for each group). Data are represented as mean ± SEM. Paired t test. **p < 0.01, ***p < 0.001.
See also Figure S1.
By patch-clamping analysis on 70-day-old cultures, we found that expression of hM4Di or hM3Dq receptors had no effect on the resting membrane potential of human mDA neurons in the absence of CNO, compared to EGFP-expressing mDA neurons (Figures 1E and 1G). CNO treatment induced a hyperpolarization of the resting membrane potential in neurons expressing the inhibitory hM4Di (n = 9, p < 0.001) (Figures 1E and 1G). In contrast, in the excitatory hM3Dq-expressing mDA neurons, CNO treatment induced depolarization of the resting membrane potential (n = 6, p < 0.01) and elicited action potentials (Figures 1E and 1G). However, CNO treatment had no effect on the resting membrane potential of EGFP-expressing human mDA neurons (n = 4, p > 0.05) (Figures 1E and 1G).
Most mDA neurons in older cultures (12 weeks) also exhibit spontaneous action potentials. Expression of hM4Di or hM3Dq receptors had no effect on the frequency of spontaneous action potentials of human mDA neurons in the absence of CNO, compared to EGFP-expressing mDA neurons (Figures 1F and 1H). CNO treatment abrogated spontaneous action potentials in the inhibitory hM4Di-expressing mDA neurons (n = 9, p < 0.001), which recovered after washing away the drug (Figures 1F and 1H). In contrast, CNO treatment increased the frequency of the spontaneous action potentials in the excitatory hM3Dq-expressing neurons (n = 6, p < 0.01) and sustained even after washing away the drug (Figures 1F and 1H). CNO treatment had no effect on the frequency of spontaneous action potentials in control EGFP-expressing human mDA neurons (n = 6, p > 0.05) (Figures 1F and 1H). The dopaminergic neuron identity was confirmed by immunostaining of biocytin-labeled cells. Of the total 73 neurons we successfully recorded, 45 neurons were TH positive (Figure S1H). Together, these results show that the activity of DREADD-expressing human mDA neurons can be regulated by CNO in vitro.
DREADD-Expressing mDA Neurons Innervate Host Striatum Extensively
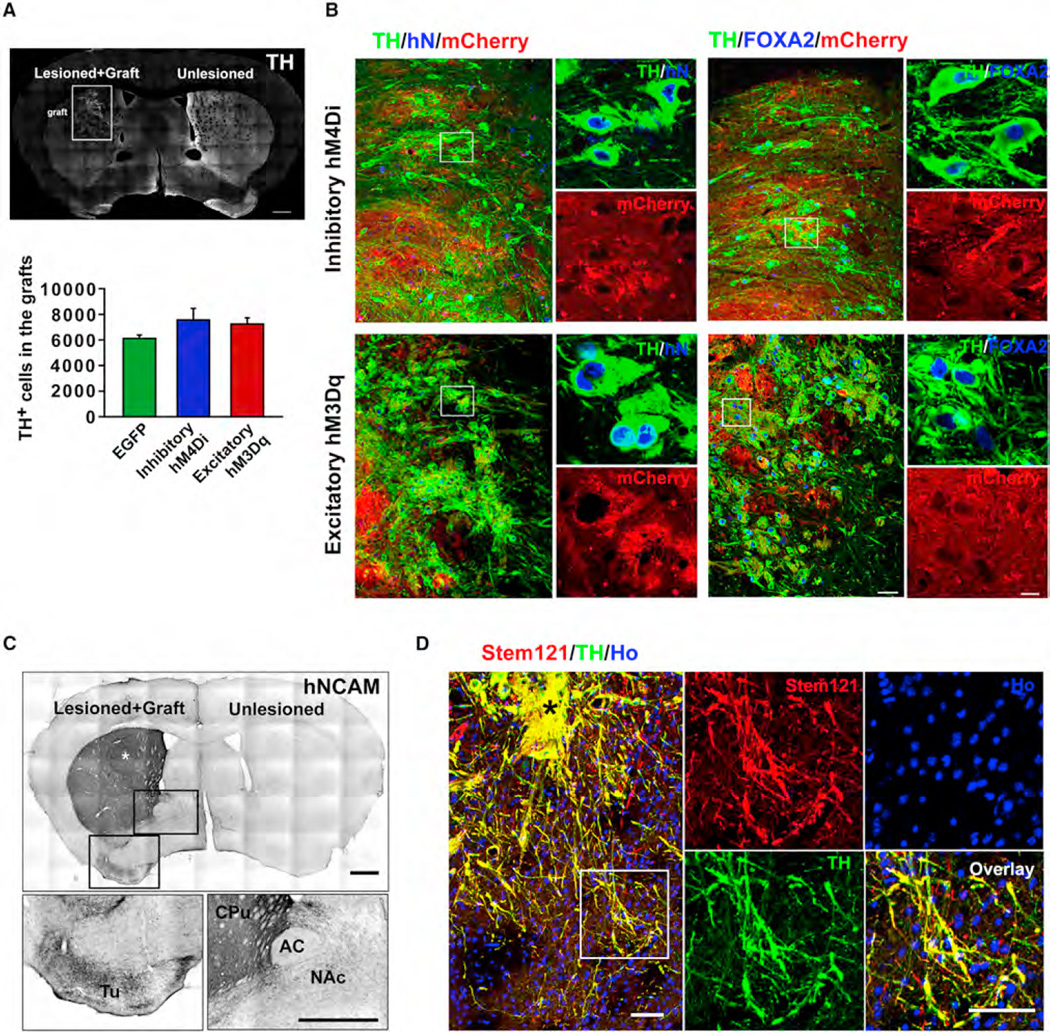
To determine if the human neuron function is regulated in vivo, we transplanted the transgenic human mDA neuronal progenitors into a mouse model of PD. This model, created by unilateral 6-hydroxydopamine (6-OHDA)-induced lesion in the nigra, is characterized by the specific loss of DA neurons in the midbrain and dopaminergic denervation in the striatum and consequent motor impairments. Its motor behaviors directly correlate to the function of DA neurons (Iancu et al., 2005; Schober, 2004). Among 105 mice, 11 died after surgery, and 69 of the remaining 94 mice (73.4%) exhibited a stable PD model, as evidenced by >6 ipsilateral amphetamine-induced rotation/min and greater than 90% (92.6% ± 2.9%) reduction in TH immunoreactivity in caudate-putamen (CPu) and loss of endogenous DA neurons in the substantia nigra compacta (Figures S3A–S3C). Six months following transplantation, grafts were present in all the transplanted animals (Figure 2A). Stereological measurement indicated similar numbers and proportions of TH+ cells in the grafts, i.e., 7,555 ± 913 (61.8% ± 1.1%), 7,245 ± 517 (59.4% ± 2.7%), and 6,110 ± 254 (61.1% ± 1.0%) cells in the grafts with hM4Di-, hM3Dq-, and EGFP-expressing cells, respectively (Figure 2A). Other cells in the grafts included GABA (<1%), 5-HT (3.7% ± 0.9%)-positive neurons, and GFAP-positive glial cells (3.2% ± 0.1%) (averaged from all of three groups) (Figure S2). In all the transplanted groups, TH+ neurons were present throughout the graft (Figures 2B, S3D, and S3E). These engrafted TH+ neurons coexpressed the human cell marker human nuclei (hN) and FOXA2, a marker expressed by mDA neurons (Figures 2B and S3D). In addition, mCherry was detected on the membrane and cell bodies of these TH+ neurons in the hM4Di and hM3Dq cell grafts (Figure 2B). These results indicate that the transgenic cells robustly differentiate to mDA neurons and retain transgene expression in vivo.
Figure 2. Maturation of and Innervation by Human DREADD-Expressing mDA Neurons in PD Mice.
(A) TH expression and graft morphology at 6 months after striatal transplantation of human mDA neuron progenitors expressing hM4Di. Scale bar, 500 µm. Boxed area indicates graft location. Stereological analysis shows the total number of TH-positive cells in the graft expressing EGFP, hM4Di, or hM3Dq at 6months after transplantation. Data are presented as mean ± SEM; n = 8–10 for each group.
(B) Immunostaining images show that the grafted TH-positive cells coexpress human nuclei (hN), FOXA2, and mCherry. Boxed regions are magnified on the right. Scale bar, 50 µm for large images, 10 µm for magnified images.
(C) Immunostaining of hNCAM shows fibers from hM3Dq-expressing mDA neuron-enriched human graft 6 months after intrastriatal transplantation. Boxed areas are amplified below. White asterisk indicates the graft site. Scale bar, 500 µm. CPu, caudate-putamen; Tu, olfactory tubercle; NAc, nucleus accumbens; AC, anterior commissure.
(D) Immunostaining shows outgrowth of fibers from hM3Dq graft and presence of fibers in the host tissue. Black asterisk indicates graft. Boxed area is amplified on the right. Scale bar, 25 µM.
See also Figures S2–S4.
To assess graft-derived innervations, we used human neural cell adhesion molecule (hNCAM) antibody, which allows selective and highly sensitive visualization of the engrafted human neurons and their projections (Grealish et al., 2014). As shown in Figure 2C, in hM3Dq group, extensive hNCAM+ fibers were present throughout the transplanted caudate-putamen (CPu), the target region of substantia nigra dopaminergic neurons. Dense hNCAM+ fibers were also observed in the CPu away from the graft core (Figure S3F). In addition, hNCAM+ fibers were also present in nucleus accumbens (NAc) and olfactory tubercle (Tu), the target region of ventral tegmental area (VTA) mDA neurons, though at a lesser density (Figure 2C). Costaining of human cytoplasmic marker (Stem121), TH, or mCherry revealed that graft-derived human fibers expressed TH and penetrated into the host tissue (Figures 2D and S4A). A similar pattern and degree of reinnervations of host striatum by grafted human TH fibers were also evidenced in EGFP group (Figures S3H and S4B) and hM4Di group (Figures S3G and S4C). These results demonstrate that human mDA graft innervates the host striatum extensively.
Human mDA Grafts Modulate Glutamatergic Transmission onto Striatal Medium Spiny Neurons via D1 Receptors
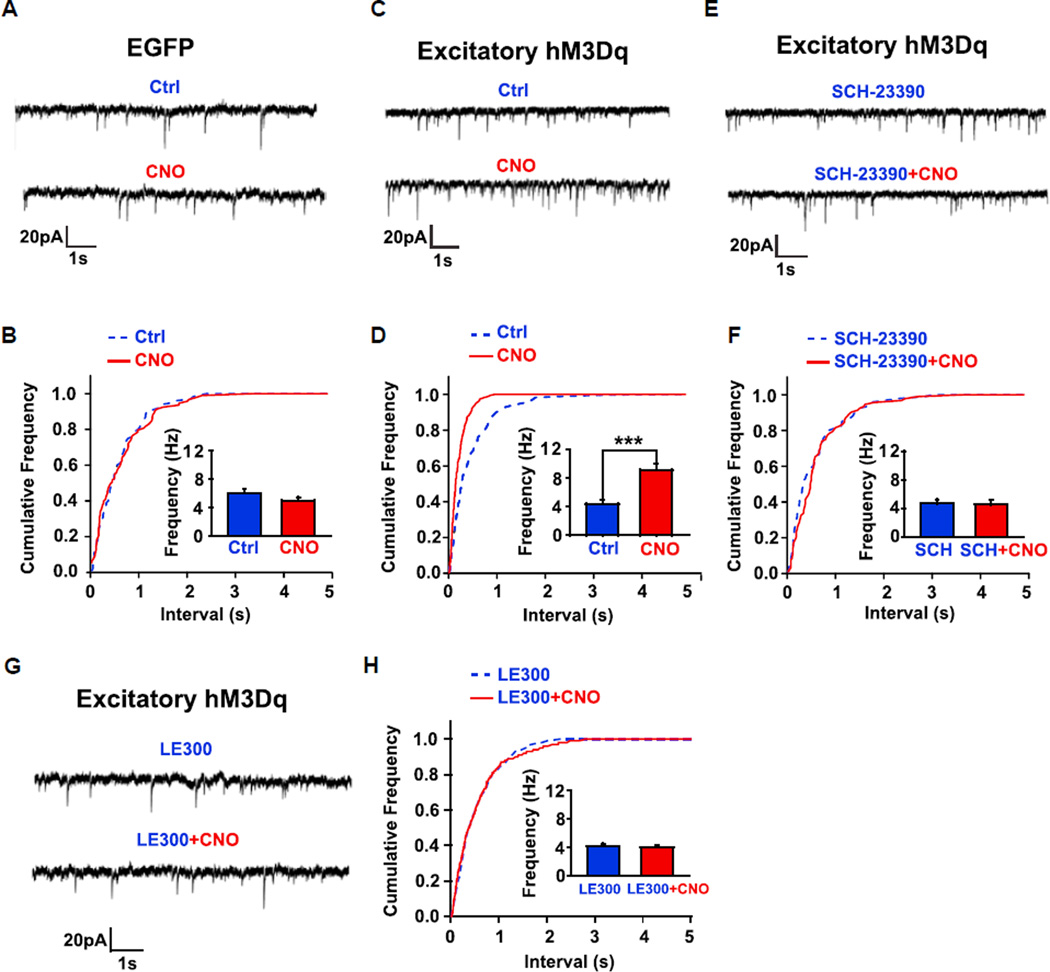
Dopaminergic neurons, by modulating the cortical and thalamic glutamatergic inputs into the medium spiny neurons (MSNs), regulate motor function (André et al., 2010; Tritsch and Sabatini, 2012). To determine if the engrafted mDA neurons functionally integrate into the host circuitry, we recorded spontaneous excitatory postsynaptic currents (sEPSCs) from MSNs near the graft in acute brain slices from the EGFP or excitatory hM3Dq transplant groups. As shown in Figure 3C, CNO treatment in slices from the hM3Dq group significantly increased the frequency of sEPSCs. Plotting of a cumulative distribution of sEPSC intervals and statistical analysis with a Kolmogorov-Smirnov (K-S) test showed that CNO induced a clear shift toward shorter intervals between sEPSCs (Figure 3D) and the shift was statistically significant (p < 0.01). The mean sEPSC frequency in the pooled eight cells increased (Figure 3D; n = 8, p < 0.001). In contrast, CNO treatment had no effect on the frequency of sEPSCs of host MSNs in slices from the EGFP-expressing human mDA transplanted group (Figures 3A and 3B). These results suggest that the CNO-induced increase in sEPSC frequency of host MSNs is the result of functional regulation by the engrafted DREADD-expressing cells. The frequency of sEPSCs is an index of presynaptic glutamate release. These results indicate that activation of transplanted DREADD-expressing human mDA neurons regulates host glutamatergic transmission onto MSNs.
Figure 3. sEPSCs in Host GABA Neurons in Response to Activation of Human mDA Neuron-Enriched Grafts.
(A, C, E, and G) Typical whole-cell patch-clamp recordings of sEPSCs in striatal GABA neurons of the brain slices that received EGFP (A) or hM3Dq (C, E, and G) cell transplantation with the absence or presence of CNO (40 µM) (A, C, E, and G), or presence of SCH-23390 (10 µM) (E) or LE300 (10 nM) (G). Holding potential is −70 mV.
(B, D, F, and H) Cumulative distributions of the interevent intervals of sEPSCs for (A), (C), (E), and (G), respectively. **p < 0.01 in (D), K-S test. The insets show the mean sEPSC frequency before and after CNO treatment in the absence (B, n = 6; D, n = 8), or presence of 10 mM SCH-23390 (F, n = 4) or 10 nM LE300 (H, n = 5). Data are presented as mean ± SEM. Paired t test. ***p < 0.001.
DA regulates glutamatergic inputs to the MSNs via D1 receptors (André et al., 2010). We found that pretreatment of slices with SCH23390, a specific D1 receptor antagonist, abrogated the CNO-induced increase in sEPSC frequency in host MSNs (Figures 3E and 3F; n = 4, p > 0.05), suggesting that the increased host glutamatergic transmission onto MSNs after activation of transplanted human mDA graft was mediated by D1 receptors. Similar results were obtained by using another D1 receptor antagonist, LE 300, which is highly specific for D1 receptor (Witt et al., 2000). Pretreatment of LE 300 abrogated CNO-induced increase in sEPSC frequency in host MSNs (Figures 3G and 3H; n = 5, p > 0.05), further confirming that the increased host glutamatergic transmission onto MSNs is mediated by D1 receptors.
Motor Behaviors of Transplanted Mice Are Controlled by CNO
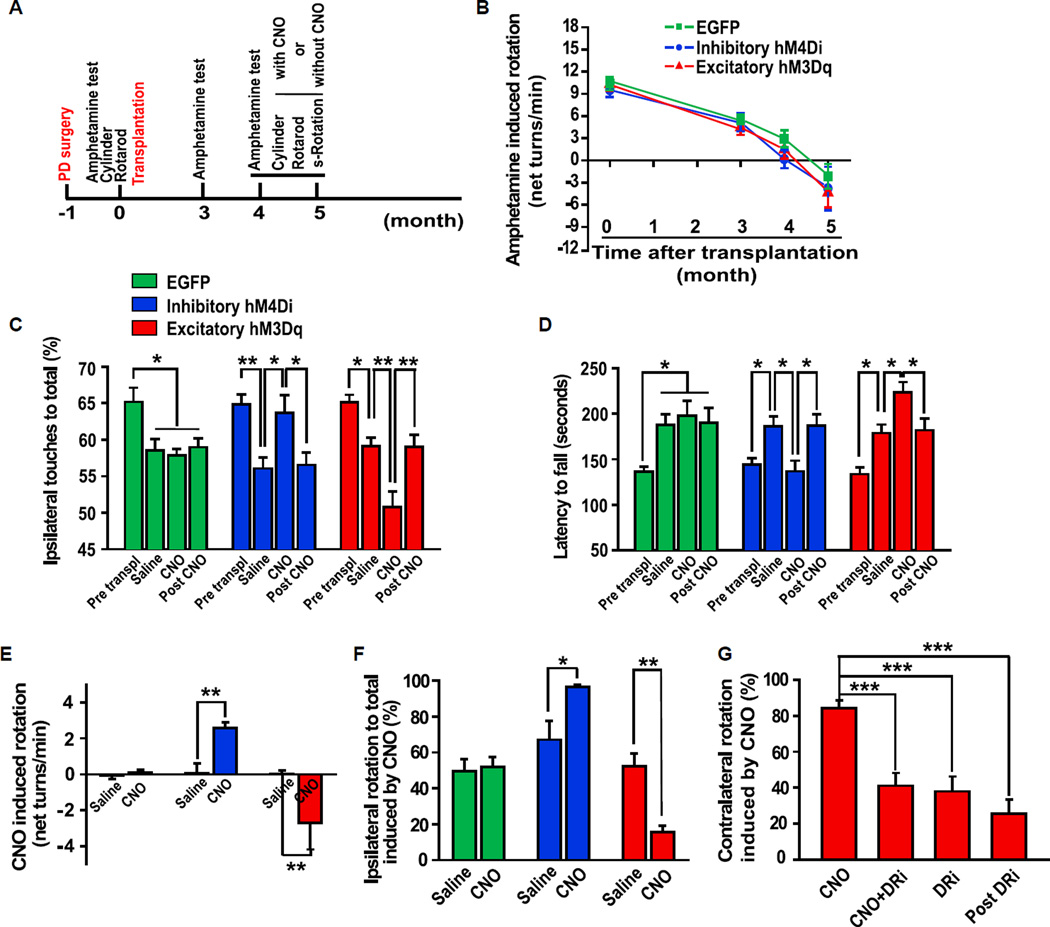
To determine if the behaviors of the transplanted animals are controlled by regulating the function of grafted cells, we assessed motor behaviors of the mice with or without CNO treatment (Figure 4A). Amphetamine-induced rotation test revealed that the PD mice grafted with EGFP-, hM4Di-, or hM3Dq-expressing human mDA-rich neurons all recovered 4 months post-transplant (n = 10 for each group, p < 0.001) (Figure 4B). Extended observation showed overcompensation in amphetamine-induced rotation test (Figure 4B). No statistic difference was observed between these three groups 4 or 5 months post-transplantation (n = 10 for each group, p > 0.05). These results suggest that human mDA neurons, expressing DREADDs or GFP, can rescue motor defects of the PD animals in a similar manner.
Figure 4. Control of Motor Behaviors of Transplanted PD Mice.
(A) Schematic depiction of the experimental process of animal model, transplantation, and behavioral analysis. s-Rotation, spontaneous rotation.
(B) Amphetamine-induced rotation shows the changes of rotation behavior from all of the three groups grafted with mDA neurons expressing EGFP, hM4Di, or hM3Dq over the 5 months posttransplantation. n = 10 for each group.
(C) Cylinder test shows the preferential ipsilateral touches before and after striatal transplantation of human mDA neurons as well as treatment with and 2 days after withdrawal of CNO. n = 10 for each group.
(D) Rotarod test shows the changes in latency to fall before and after striatal transplantation of human mDA neurons as well as treatment with and 2 days after withdrawal of CNO. n = 10 for each group.
(E and F) Spontaneous rotation test shows CNO-induced change in net ipsilateral rotations (E) and preferential ipsilateral rotations (F) in EGFP, hM4Di, and hM3Dq groups. n = 7–10 for each group.
(G) CNO-induced preferential contralateral rotation was inhibited by combinatorial pretreatment of DA receptor antagonists (DRi) SCH-23390 and Raclopride. n = 10.
Data are presented as mean ± SEM. One-way ANOVA followed by Tukey’s posthoc test in (C), (D), and (G). Paired t test in (E) and (F). *p < 0.05, **p < 0.01, ***p < 0.001.
See also Movies S1, S2, S3, and S4.
We next assessed the CNO effect on the behavior of the transplanted mice using the cylinder test, rotarod test, and spontaneous rotation test. These tests have been shown to correlate well with the extent of animal recovery from lesion (Heuer et al., 2012; Iancu et al., 2005). More importantly, these tests avoid amphetamine-induced DA release via reverse transport, which is largely independent of neuronal activity (Steinbeck et al., 2015). Cylinder test, a measure of forelimb akinesia, showed that all groups presented preferential ipsilateral touches after 6-OHDA lesion, a result from disability of the contralateral forelimb. The ipsilateral touching preferences were significantly reduced (closer to 50%) after transplantation of human mDA-rich neurons in all three groups (n = 10, p < 0.05, in EGFP group; n = 10, p < 0.01, in hM4Di group; n = 10, p < 0.05, in hM3Dq group). Strikingly, CNO treatment of animals with the inhibitory hM4Di transplant reintroduced the preferential ipsilateral touches (n = 10, p < 0.05) (Figure 4C, blue bars). In contrast, in the excitatory hM3Dq transplanted group, CNO treatment further decreased percentage of ipsilateral touches (n = 10, p < 0.01) (Figure 4C, red bars). CNO treatment had no effect on the percentage of ipsilateral touches in mice receiving EGFP-expressing graft (n = 10, p > 0.05) (Figure 4C, green bars). These results suggest that the recovery of forelimb akinesia is dependent on the activity of the transplanted cells, and the degree of recovery can be adjusted by regulating the function of grafted cells. The CNO effect in both groups of animals returned to basal levels (saline treatment) 48 hr after CNO withdrawal (n = 10, p < 0.05, in hM4Di group; n = 10, p < 0.01, in hM3Dq group) (Figure 4C), demonstrating the reversible functional modulation of the DREADD-expressing grafts.
In rotarod test, the latency to fall was significantly increased in all of the three transplantation groups when compared with its cognate pretransplantation control (n = 10 for each group, p < 0.05). CNO treatment decreased the latency to fall in the inhibitory hM4Di animals (n = 10, p < 0.05) but increased the latency in the excitatory hM3Dq transplantation group (n = 10, p < 0.05). In contrast, it had no effect in the EGFP group (n = 10, p > 0.05) (Figure 4D). These effects returned to basal levels 48 hr after CNO withdrawal (n = 10, p < 0.05, in hM4Di group; n = 10, p < 0.05, in hM3Dq group.) (Figure 4D). These results demonstrate the causal role of human mDA grafts in the recovery of the motor function in the PD mice and suggest a significant added benefit of activation of human mDA graft.
Spontaneous rotation test is another means to assess motor balance without a need of amphetamine-elicited artificial DA release (Kim et al., 2002). As shown in Figure 4E, the EGFP mDA transplanted animals did not show obvious changes with or without CNO treatment. However, CNO-treated animals exhibited more ipsilateral than contralateral rotations in the inhibitory hM4Di group when compared with those without CNO (n = 7, p < 0.01). Ipsilateral rotation ratio was also calculated as a more accurate readout to reflect the rotation bias. As shown in Figure 4F, the ipsilateral rotation ratio increased from 67.13% ± 10.35% (without CNO) to 96.45% ± 1.10% when treated with CNO (n = 7, p < 0.05), suggesting a loss of function of the inhibitory hM4Di-expressing grafted cells, which is elicited by CNO (see also Movies S1 and S2). In contrast, CNO treatment of the excitatory hM3Dq group induced significant contralateral net rotation (n = 10, p < 0.01) (Figure 4E; Movies S3 and S4), which translates to the ipsilateral rotation ratio of 15.55% ± 3.63% in the presence of CNO from 52.21% ± 7.13% without CNO (n = 10, p < 0.01) (Figure 4F). This result suggests a gain of function of the excitatory hM3Dq cells activated by CNO.
To validate that the CNO effect on mouse motor behaviors is mediated by DA released from grafted human mDA neurons, we applied DA receptor antagonists SCH-23390 (for D1 receptor) and Raclopride (for D2 receptor) before CNO treatment of the excitatory hM3Dq transplanted animals. As shown in Figure 4G, pretreatment with DA receptor antagonists abolished CNO-induced contralateral rotation, suggesting a requirement of DA receptor activation in this process.
DISCUSSION
By building a functional switch (DREADD) into hPSCs, we have shown that the function of the transgenic hPSC-derived neurons is enhanced or inhibited by the designer drug CNO via regulation of membrane potentials and firing frequency. Importantly, these DREADD-expressing human mDA neurons, transplanted into the striatum of PD mice, can be regulated “remotely” via peripheral CNO application. These results not only reveal how the transplanted mDA neurons contribute to behavioral improvement but also suggest the possibility of the strategy for achieving refined therapeutic outcomes or even artificial control of corresponding behaviors.
How neural transplantation contributes to animal behavioral improvement was not well defined until the availability of tools that precisely regulate the activity of grafted cells. By using optogenetics, Studer and colleagues showed that amplitudes of evoked excitatory postsynaptic potentials (EPSPs) in MSNs, induced by electrical stimulation of the corpus callosum, are decreased by light-evoked inhibition of grafted halorhodopsin-expressing DA neurons (Steinbeck et al., 2015). These results suggest that the grafted DA neurons regulate host MSNs via a postsynaptic mechanism. Using the DREADD system, we showed that activation of grafted human mDA neurons enhanced the sEPSC frequency on host MSNs. The frequency of sEPSCs is an index of presynaptic glutamate release. These results suggest a presynaptic regulation of host MSNs by grafted human mDA neurons. Intrastriatal grafted hPSC-derived DA neurons release DA in vivo (Kirkeby et al., 2012; Steinbeck et al., 2015). We have confirmed that the CNO-induced increase in sEPSC frequency is mediated by human cell-derived DA as two D1 receptor antagonists completely abrogate the CNO effect. These results demonstrate that engrafted human mDA neuron-repaired/regulated neuronal circuitry is mediated by DA/DA D1 receptors. Thus, by manipulating graft function through the DREADD system, our study provides insight into the mechanisms of cell therapy in PD. A similar strategy could be applied to investigation of circuitry mechanisms underlying graft function and behavioral outcomes by remotely activating or inhibiting transplanted cells in other neurological/psychiatric conditions.
The ability to control the function of grafted human cells opens a possibility for refining therapeutic outcome. Our present study, using transplantation of human mDA neurons in PD mice as a model system, suggests the potential applicability of such a strategy in patients. In the PD mice transplanted with the hM4Di-expressing human mDA-rich neural cells, CNO treatment can effectively dampen the overcompensation by the engrafted cells. In contrast, in those with the excitatoryhM3Dqcells, behavioral outcomes can be enhanced by CNO treatment. Both effects are reversible, highlighting the modulatory effects. In an analogy, in patients with suboptimal efficacy, activation of the graft function may improve the therapeutic outcome. In contrast, in patients with unwanted effects, dampening the graft activity may mitigate side effects. Thus, DREADD-mediated modulation would enable refinement of transplant therapy.
While such regulation of graft function may also be achieved by optogenetic tools, technical hurdles, including the requirement of an implant in the brain for delivering light, limited size of laser illumination (usually 1 mm in distance for blue light in tissue, which is not enough for a much larger human brain; Aravanis et al., 2007), and inability to illuminate the transplanted cells in distance (migrate away from the transplant site), limit its ready translation to clinical use (Sternson and Roth, 2014). In contrast, the functional regulation of DREADD-expressing human grafts can be achieved remotely simply by an oral medication, regardless of the site and volume of transplanted cells. Furthermore, like many other GPCR-based drug targets, DREADD-mediated signal transduction can be regulated by agonists in a dose-dependent manner (Alexander et al., 2009), enabling fine-tuning of the transplanted cell function. Hence, the DREADD system is translatable and has broader application for cell therapy in clinic. Of course, CNO may be metabolized to clozapine in human, though at a low level (~10%), which may cause side effects. Nevertheless, no apparent effect has been observed in humans receiving CNO (Jann et al., 1994), consistent with its pharmacological inertness (Armbruster et al., 2007). Our finding that graft function can be precisely regulated in drug-dependent and reversible manner suggests that CNO or other designer drugs may be taken when needed, but not necessarily continuously, further minimizing potential side effects of designer drugs.
Modulation of functional (behavioral) outputs of transplanted human cells in animals through a simple peripheral application of a designer drug not only allows interrogation of the casual role of human grafts but more importantly enables potential clinical translation by refining therapeutic outcomes. Of course, genetic engineering of the donor cells introduces an additional step that is subjected to regulatory examination before clinical application. To minimize potential safety concerns involving genetic modification, we have employed the site-specific genome editing method CRISPR (Jinek et al., 2012), instead of random viral infection (Steinbeck et al., 2015), to generate hPSCs with two copies of DREADD gene in the defined locus. This enables defined genome targeting as well as consistent and predictable expression of DREADDs in derived human cells. Therefore, engineering of human stem cells with functional switches (DREADD), as indicated by the proof-of-principle study here, will likely substantially expand the scope of stem cell therapy.
EXPERIMENTAL PROCEDURES
Generation of Inhibitory hM4Di-, Excitatory hM3Dq-, or EGFP-Expressing hESC Lines
hESCs (line WA09 [WiCell], passages 20–40) were cultured and electroporated as previously described (Chen et al., 2015). Briefly, Rho-kinase (ROCK) inhibitor (0.5 µM, Calbiochem, H-1152P)-pretreated hESCs were digested, dispersed into single cells, and electroporated with 15 µg Cas9 plasmid, 15 µg sgRNA T2 plasmid, and 30 µg donor plasmid in 500 µL of electroporation buffer (5 mM KCl, 5 mM MgCl2, 15 mM HEPES, 102.94 mM Na2HPO4, and 47.06 mM NaH2PO4 [pH 7.2]) using the Gene Pulser Xcell System (Bio-Rad) at 250 V, 500 µF in a 0.4 cm cuvette (Phenix Research Products). Cells were subsequently plated onto mouse embryonic fibroblast (MEF) feeder layer in six-well plates. Individual colonies were picked up after drug selection and identified by genomic PCR. Detailed information of donor plasmids is given in Supplemental Experimental Procedures.
Generation of Midbrain DA Neurons
Differentiation of DA neurons from transgene-expressing hESCs was carried out as described (Xi et al., 2012), with modification detailed in Supplemental Experimental Procedures.
Animal Surgery and Cell Transplantation
All animal experiments were conducted according to protocols approved by the animal care and use committee at University of Wisconsin-Madison. Details are given in Supplemental Experimental Procedures.
Whole-Cell Patch-Clamp and Brain Slice Recording
Whole-cell patch-clamp recordings were performed as described in Supplemental Experimental Procedures. The identity of recorded cells was revealed by injecting biocytin (1%, Sigma) followed by immunostaining.
Behavioral Tests
Amphetamine-induced rotation test, cylinder test, and rotarod test are carried out as described in Supplemental Experimental Procedures.
Statistical Analyses
SPSS software was used for statistical analysis. In all studies, data were analyzed by paired t test or one-way ANOVA followed by Tukey’s multiple comparison test. Statistical significance was determined at p < 0.05.
Supplementary Material
In Brief.
Chen et al. show that motor function in PD mice can be reversed or enhanced following transplantation of dopamine neurons differentiated from hPSCs engineered to express DREADDs. Administering CNO noninvasively and reversibly modulates neuronal graft function, highlighting the potential to exogenously control and refine therapeutic outcomes following cell transplantation.
Highlights.
CRISPR-mediated DREADD knockin enables precise regulation of hPSC-derived neurons
Engrafted mDA neurons regulate host neuronal circuitry in striatum via D1 receptors
Graft activity can be noninvasively and reversibly controlled by DREADD ligands
Regulation of DREADD-expressing human graft activity dictates animal behavior
Acknowledgments
We thank Dr. Bryan L. Roth for the generous gift of the AAV-DIO-hM4DimCherry and AAV-DIO-hM3Dq-mCherry plasmids and Dr. A. Bhattacharyya for helpful comments on the manuscript. This study was supported in part by the NIH-NINDS (NS045926, NS076352, and NS086604), NIH-NIMH (MH099587 and MH100031), the National Natural Science Foundation of China (81370030), the Bleser Family Foundation, the Busta Foundation, and the NICHD (HD076892 and HD03352). Su-Chun Zhang is a cofounder of Brainxell, Inc.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, and four movies and can be found with this article online at http://dx.doi.org/10.1016/j.stem.2016.03.014.
AUTHOR CONTRIBUTIONS
Y.C. and S.-C.Z. conceived the study. M.X. and Y.C. did the cell line construction, differentiation, behavior tests, and histology experiments. Y.D. performed the electrophysiology experiments. A.H., J.C., H. L., and W. Z. helped with some of the experiments. Y.C., M.X., Y.D., and A.H. collected and analyzed data. Y.C., M.X., Y.D., and S.-C.Z. wrote the manuscript. S.-C.Z supervised the project.
REFERENCES
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André VM, Cepeda C, Cummings DM, Jocoy EL, Fisher YE, William Yang X, Levine MS. Dopamine modulation of excitatory currents in the striatum is dictated by the expression of D1 or D2 receptors and modified by endocannabinoids. Eur. J. Neurosci. 2010;31:14–28. doi: 10.1111/j.1460-9568.2009.07047.x. [DOI] [PubMed] [Google Scholar]
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 2007;4:S143–S156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker RA, Barrett J, Mason SL, Björklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 2013;12:84–91. doi: 10.1016/S1474-4422(12)70295-8. [DOI] [PubMed] [Google Scholar]
- Breger LS, Lane EL. L-DOPA and graft-induced dyskinesia: different treatment, same story? Exp. Biol. Med. (Maywood) 2013;238:725–732. doi: 10.1177/1535370213488478. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cao J, Xiong M, Petersen AJ, Dong Y, Tao Y, Huang CT, Du Z, Zhang SC. Engineering human stem cell lines with inducible gene knockout using CRISPR/Cas9. Cell Stem Cell. 2015;17:233–244. doi: 10.1016/j.stem.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M, Cho JH, Leung A, Savvidis G, Ahn S, Moon M, Lee PK, Han JJ, Azimi N, Kim KS, et al. hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell. 2014;15:559–573. doi: 10.1016/j.stem.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Anno MT, Caiazzo M, Leo D, Dvoretskova E, Medrihan L, Colasante G, Giannelli S, Theka I, Russo G, Mus L, et al. Remote control of induced dopaminergic neurons in parkinsonian rats. J. Clin. Invest. 2014;124:3215–3229. doi: 10.1172/JCI74664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Grealish S, Diguet E, Kirkeby A, Mattsson B, Heuer A, Bramoulle Y, Van Camp N, Perrier AL, Hantraye P, Björklund A, Parmar M. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell. 2014;15:653–665. doi: 10.1016/j.stem.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagell P, Piccini P, Björklund A, Brundin P, Rehncrona S, Widner H, Crabb L, Pavese N, Oertel WH, Quinn N, et al. Dyskinesias following neural transplantation in Parkinson’s disease. Nat. Neurosci. 2002;5:627–628. doi: 10.1038/nn863. [DOI] [PubMed] [Google Scholar]
- Heuer A, Smith GA, Lelos MJ, Lane EL, Dunnett SB. Unilateral nigrostriatal 6-hydroxydopamine lesions in mice I: motor impairments identify extent of dopamine depletion at three different lesion sites. Behav. Brain Res. 2012;228:30–43. doi: 10.1016/j.bbr.2011.11.027. [DOI] [PubMed] [Google Scholar]
- Iancu R, Mohapel P, Brundin P, Paul G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav. Brain Res. 2005;162:1–10. doi: 10.1016/j.bbr.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Jann MW, Lam YW, Chang WH. Rapid formation of clozapine in guinea-pigs and man following clozapine-N-oxide administration. Arch. Int. Pharmacodyn. Ther. 1994;328:243–250. [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Auerbach JM, Rodríguez-Gómez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sánchez-Pernaute R, Bankiewicz K, McKay R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Hagell P. Clinical observations after neural transplantation in Parkinson’s disease. Prog. Brain Res. 2000;127:299–320. doi: 10.1016/s0079-6123(00)27014-3. [DOI] [PubMed] [Google Scholar]
- Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Hu B, Liu Y, Vermilyea SC, Liu H, Gao L, Sun Y, Zhang X, Zhang SC. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell. 2012;10:455–464. doi: 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maries E, Kordower JH, Chu Y, Collier TJ, Sortwell CE, Olaru E, Shannon K, Steece-Collier K. Focal not widespread grafts induce novel dyskinetic behavior in parkinsonian rats. Neurobiol. Dis. 2006;21:165–180. doi: 10.1016/j.nbd.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann. Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- Rossi F, Cattaneo E. Opinion: neural stem cell therapy for neurological diseases: dreams and reality. Nat. Rev. Neurosci. 2002;3:401–409. doi: 10.1038/nrn809. [DOI] [PubMed] [Google Scholar]
- Schober A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D, Mosharov EV, Studer L. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat. Biotechnol. 2015;33:204–209. doi: 10.1038/nbt.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu. Rev. Neurosci. 2014;37:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt T, Hock FJ, Lehmann J. 7-Methyl-6,7,8,9,14,15-hexahydro-5H-benz[d]indolo[2,3-g]azecine: a new heterocyclic system and a new lead compound for dopamine receptor antagonists. J. Med. Chem. 2000;43:2079–2081. doi: 10.1021/jm9911478. [DOI] [PubMed] [Google Scholar]
- Xi J, Liu Y, Liu H, Chen H, Emborg ME, Zhang SC. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells. 2012;30:1655–1663. doi: 10.1002/stem.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.