Abstract
Purpose
Whole exome sequencing (WES) is increasingly used as a diagnostic tool in medicine, but prior reports focus on predominantly pediatric cohorts with neurologic or developmental disorders. We describe the diagnostic yield and characteristics of whole exome sequencing in adults.
Methods
We performed a retrospective analysis of consecutive WES reports for adults from a diagnostic laboratory. Phenotype composition was determined using Human Phenotype Ontology terms.
Results
Molecular diagnoses were reported for 17.5% (85/486) of adults, lower than a primarily pediatric population (25.2%; p=0.0003); the diagnostic rate was higher (23.9%) in those 18–30 years of age compared to patients over 30 years (10.4%; p=0.0001). Dual Mendelian diagnoses contributed to 7% of diagnoses, revealing blended phenotypes. Diagnoses were more frequent among individuals with abnormalities of the nervous system, skeletal system, head/neck, and growth. Diagnostic rate was independent of family history information, and de novo mutations contributed to 61.4% of autosomal dominant diagnoses.
Conclusion
Early WES experience in adults demonstrates molecular diagnoses in a substantial proportion of patients, informing clinical management, recurrence risk and recommendations for relatives. A positive family history was not predictive, consistent with molecular diagnoses often revealed by de novo events, informing the Mendelian basis of genetic disease in adults.
Keywords: whole exome sequencing, adult patients
INTRODUCTION
Since its earliest described use seven years ago, sequencing and analysis of individual genomes has become a powerful tool for studying human genomic variation1–4 and identifying the cause of disease traits such as clinical neuropathy and inherited forms of hypertension.5,6 Genomic studies have demonstrated both the clinical and research utility of whole exome sequencing (WES) for detecting known and novel mutations in disease-causing genes across a variety of inheritance patterns.7–9 Clinically, genome-scale sequencing often follows exhaustive clinical evaluations unable to conclude an etiologic diagnosis. Genomic studies have proven especially useful for conditions with locus heterogeneity (long molecular differentials) or unexpected phenotypic variation.10 WES also allows for identification of pathologic variants in newly identified disease genes and future re-analysis of existing genomic data.11–13
The majority of diagnostic WES referrals are pediatric, with adult probands constituting just 16.2%,14 36.1%,15 and 12.2%11 of reported clinical cohorts. Several case reports and small studies have described the clinical utility of WES in adults, particularly in cases where an atypical phenotype or polygenic burden may confound a primary diagnosis,16–20 but no large-scale analyses of the use of WES by physicians caring for adult patients has been reported. To further explore the nature of genetic disease in adults, we describe indications for testing and diagnostic yield of clinical WES in adult patients referred by their physician to a single academic diagnostic laboratory.
MATERIALS AND METHODS
Patient Population
We performed a retrospective review of diagnostic WES in the Whole Genome Laboratory (WGL) at Baylor College of Medicine (BCM) during a three-year period (October 2011-November 2014), including tests from a large number of United States and international medical institutions. Cancer exomes, offered under a separate test code, were not included in this analysis. In all cases, WES was performed on the proband sample only (referred to here as proband-WES); although parental WES was not performed, available parental samples were obtained for variant confirmation by Sanger analysis. Of 4476 individuals having diagnostic proband-WES, 505 were 18 years or older at the time of referral. After excluding healthy adults and affected related individuals, 486 adults met criteria for study inclusion. The Institutional Review Board at BCM approved de-identified reporting of demographic and molecular data from this laboratory.
Whole Exome Sequencing and Variant Analysis
Library construction, exome capture using VCRome version 2.1,21 HiSeq next-generation sequencing, and data processing were performed as previously described.12 The diagnostic WES evaluation included a cSNP array performed for quality control, and mitochondrial genome sequencing after PCR amplification. The diagnostic reports described in the present manuscript were finalized prior to the laboratory’s implementation of an algorithm for copy number variant (CNV) identification using data from exome sequencing. Thus, the diagnostic rate reported here does not include additional diagnoses that might result from identification of exonic deletions or duplications using exome data. A molecular diagnosis required pathogenic or likely pathogenic variants in Mendelian disease genes consistent with the observed phenotype and expected inheritance.11,12 Variant classification was performed as previously described and consistent with guidelines set forth by the American College of Medical Genetics and Genomics (ACMG).22 Variants described in Supplemental Table 1 have been submitted to ClinVar as accession numbers SCV000245445-SCV000245563.
Data Analyses
Basic demographic information, ordering physician specialty, diagnostic indication, and family history were obtained from submitted clinical information. Primary and secondary findings were based on the clinical reports and addenda issued as of July 1, 2015. Patients who declined to receive reports of medically actionable secondary findings were excluded from secondary finding analyses.
Based on available clinical information, Human Phenotype Ontology (HPO) terms23 were designated for each subject, which allowed assignment of each case to one or more HPO phenotypic abnormality classes. Diagnostic rates and relative frequency of each phenotype class were determined computationally by analysis of HPO terms.
Statistical Analyses
P-values for male:female ratios were determined using a two-tailed binomial test. Testing was also performed for association between phenotypic class and diagnostic rate. Because individual subjects can have multiple phenotypes and some phenotypic groups are sparsely represented, we adopted a permutation testing approach to determine the null distribution of test-statistics and corresponding p-values. We performed 10,000 Monte Carlo draws for which the overall diagnostic rate (defined as the number of diagnosed and undiagnosed cases) was held constant and subjects were randomly permuted with respect to diagnostic status; the ensemble of phenotypes for each individual case was retained. Analyses comprised the omnibus test considering all 22 phenotype classes at once against solved/unsolved status and for each individual class when assessed against the collapse of all other classes (2×2 table). A chi-square test statistic was determined for each two-way table, and p-values were determined by counting the number of realizations in which the value was equal or greater than the corresponding value for the study data and dividing by the number of permutations performed.
Role of the Funding Source
The National Human Genome Research Institute, National Cancer Institute, and National Institute of Neurological Disorders and Stroke had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
RESULTS
Demographics
Adults, defined as individuals 18 years of age and older, comprised 11.3% (505/4476) of all diagnostic WES cases in our clinical laboratory between October 2011 and November 2014. After eliminating related individuals or those referred without a clinical indication, 486 were included in the present analysis; 272 of whom were included in our previously published reports.11,12 WES for adults was ordered by 229 independent physicians; the majority of cases were sent by geneticists (61.2%), followed by neurologists (22.0%) and those with both neurology and genetics training (6.1%; Table 1).
Table 1.
Specialties of physicians referring adult patients for Whole Exome Sequencing (WES)
| Specialty | Number of referrals | Percentage of referrals |
|---|---|---|
| Genetics | 309 | 61.2% |
| Neurology | 111 | 22.0% |
| Neurogenetics | 31 | 6.1% |
| Endocrinology | 12 | 2.4% |
| Rheumatology | 7 | 1.4% |
| Primary care | 6 | 1.1% |
| Hematology | 4 | <1% |
| Gastroenterology | 4 | <1% |
| Cardiology | 3 | <1% |
| Obstetrics/gynecology | 3 | <1% |
| Oncology | 3 | <1% |
| Ophthalmology | 2 | <1% |
| Immunology | 2 | <1% |
| Toxicology | 1 | <1% |
| Urgent care | 1 | <1% |
| Urology | 1 | <1% |
| Unknown | 5 | <1% |
| Total | 505 |
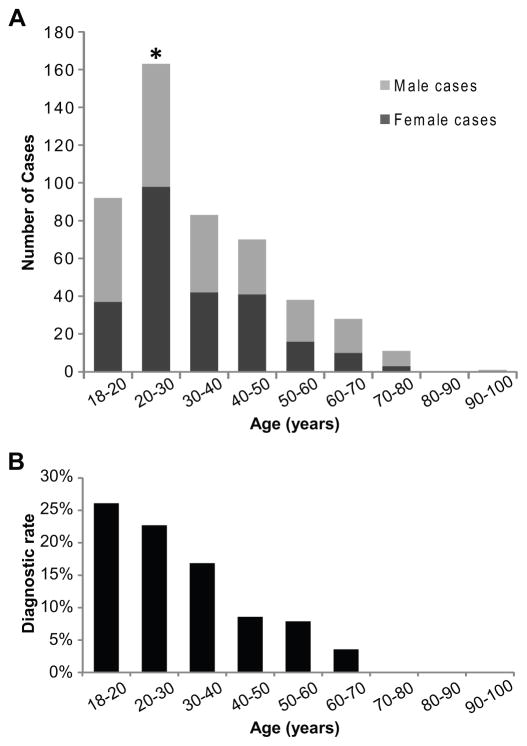
Most adult patients were 18–30 years of age (52.5%, 255/486), and only 2.5% (12/486) were older than 70 years of age (Figure 1A). Males (n=239) and females (n=247) were equally represented overall (p=0.75, binomial test) and within all age ranges except the predominantly female 20–30-year group (p=0.01, binomial test; Figure 1A). Ethnic or racial background was provided by the referring physician in 414 cases, of which mixed European Caucasian-descent was indicated in 71.7%, African American in 3.6%, Hispanic in 12.6% of individuals and mixed ethnic descent in 6.0%. Parental consanguinity was reported in 22 (4.5%) probands. Although WES was not performed on parental samples for the reported cases, both parental samples were available for Sanger analysis of selected variants in 52% of cases.
Figure 1. Age and sex of individuals undergoing WES.
(A) Total number of female (dark grey) and male (light grey) cases by age group. * indicates p<0.05 for significance of differences between male and female proportions within each age group. (B) Molecular diagnostic rate as percentage of total cases in each age group.
Molecular Diagnoses
A molecular (genetic) diagnosis was reported in 85/486 (17.5%) adults (Table S1) including 6 individuals who received two molecular diagnoses each – about 7% of all molecularly diagnosed cases. The molecular diagnostic rate in adults is significantly lower than observed in a primarily pediatric population (25.2%, two-tailed p=0.0003, Fisher exact test).11 When stratified by age group, diagnostic rates were higher (23.9%, 61/255) in cases between 18 and 30 years of age, but declined to 10.4% (24/231, two-tailed p=0.0001, Fisher exact test) in cases greater than 30 years of age (Figure 1B). The diagnostic rate was similar when WES was ordered by geneticists (56/296, 18.9%; 95% CI 14.87–23.77%) or neurologists and neurogeneticists (26/139, 18.7%; 95% CI 12.22–25.18%). All other specialties combined had a low diagnostic rate but represent a small number of cases, (3/51, 5.9%; 95% CI 0–12.37%).
Although a majority of the molecular diagnoses were in nuclear genes, mitochondrial genome sequencing included in the WES test yielded three diagnoses (one individual with two overlapping large mitochondrial deletions and one missense mutation each in MT-ATP6 and MT-ND6, Table S1). SNP array data identified one 1.55-Mb pathologic deletion CNV on Xq23 including PLS3 in a male (case 70), one 4.5-Mb deletion on 15q11.2-q13 providing a diagnosis of Angelman syndrome (case 68), and one 1.38-Mb deletion on 17q12 including HNF1B (case 33). The remaining diagnoses were based on a total of 111 distinct variants reported from WES data, of which 60 were novel at the time of reporting (Table S1). Single nucleotide variants (SNVs) comprised 75.7% (84/111) of the variants, similar to 76.7% (543/708, two-tailed p=0.81, Fisher exact test) in a primarily pediatric series,11 resulting in 57 missense, 16 nonsense, 10 splice site, and one initiation codon mutation. Short insertion or deletion variants (indels) comprised 23.4% (26/111) of diagnostic variants compared to 22.2% (157/708, two-tailed p=0.81, Fisher exact test) in a primarily pediatric series.11 Of note, the diversity of underlying Mendelian disorders is demonstrated by only 4 genes (DYRK1A, FLG, BRAF, and NSD1) having variants reported in two or more unrelated individuals accounting for a total of nine (10.6%) molecularly diagnosed cases.
The reported mode of inheritance of molecular diagnoses was most frequently autosomal dominant (48.4%), followed by autosomal recessive (40.7%), X-linked (7.7%), and mitochondrial (3.3%; Table 2). This is similar to a primarily pediatric population11, where 53.1% (280/527, two-tailed p=0.43, Fisher exact test) of diagnoses were autosomal dominant, 34.3% (181/527, p=0.28) autosomal recessive, 12.3% (65/527, p=0.29) X-linked, and 0.2% mitochondrial inheritance. A majority of both autosomal dominant and recessive syndromes included neurologic, muscular/musculoskeletal, or multiple affected organ systems features (Table S1). Considering all dominant molecular diagnoses, 75.0% (33/44) were either de novo events and/or novel (not previously described) variants in disease genes.
Table 2.
Modes of inheritance observed across 91 molecular diagnoses in 85 cases
| Mode of Inheritance | Number of Diagnoses | Percent of Diagnoses (%) |
|---|---|---|
| Autosomal dominant | 44 | 48.4% |
| De novo | 27 | |
| Inherited, parental mosaicism | 2 | |
| Inherited, no mosaicism | 4 | |
| Inheritance unknown | 11 | |
| Autosomal recessive | 37 | 40.7% |
| Compound heterozygous SNVs | 26 | |
| Homozygous | 11 | |
| Homozygous, uniparental disomy | 1 | |
| X-linked | 7 | 7.7% |
| De novo | 2 | |
| Mitochondrial | 3 | 3.3% |
| Dual diagnoses | 12 (6 cases) | 11.0% |
| Autosomal dominant + Autosomal dominant | 6 (3 cases) | |
| Autosomal recessive + Autosomal recessive | 2 (1 case) | |
| Autosomal dominant + Autosomal recessive | 4 (2 cases) |
The 37 autosomal recessive molecular diagnoses included only 11 homozygous disease alleles (29.7%, 11/37; Table 2). Uniparental disomy of chromosome 9 led to a homozygous SIGMAR1 mutation and juvenile amyotrophic lateral sclerosis in case 25. Parental consanguinity, reported in 4.5% (22/486) of all adult cases, was overrepresented among those with diagnoses based on homozygous variants (5/10, 50%) and autosomal recessive diagnoses (5/37, 13.5%, two-tailed p=0.02, Fisher exact test). Only recessive diagnoses were reported in cases with consanguineous parents. Three of seven X-linked diagnoses were in female patients; two of these were de novo diagnoses of Cornelia de Lange syndrome, caused by mutations in SMC1A or HDAC8 (case 34 and 41).
Phenotypes
Each individual’s phenotype as provided by the referring physician was completely represented by an average of 10 HPO terms. HPO terms representing neurologic and developmental disorders were frequent: the terms ‘motor delay’, ‘intellectual disability’, ‘seizures’, and ‘delayed speech and language development’ each occurred in over 100 of the 486 cases (Table 3). To evaluate the overall phenotypic composition of this patient population, cases were assigned to one or more of 22 HPO phenotype classes based on their HPO terms. Neoplasm-related HPO terms were always assigned to the neoplasm class. Abnormalities of the nervous system, musculature, and skeletal system were the most frequent phenotype classes (Figure 2A).
Table 3.
Human Phenotype Ontology (HPO) terms occurring in over 10% of adult exome cases
| HPO term | Number of occurrences | Percent of cases |
|---|---|---|
| Motor delay | 123 | 25.3% |
| Intellectual disability | 121 | 24.9% |
| Seizures | 113 | 23.3% |
| Delayed speech and language development | 110 | 22.6% |
| Abnormality of movement | 78 | 16.0% |
| Spasticity | 78 | 16.0% |
| Hypertonia | 78 | 16.0% |
| Ataxia | 75 | 15.4% |
| Scoliosis | 68 | 14.0% |
| Muscular hypotonia | 64 | 13.2% |
| Abnormality of brain morphology | 63 | 13.0% |
| Abnormal face shape | 62 | 12.8% |
| Joint hypermobility | 60 | 12.3% |
| Short stature | 58 | 11.9% |
| Abnormality of the eye | 58 | 11.9% |
| Abnormality of the skin | 51 | 10.5% |
Figure 2. Phenotypic spectrum of individuals undergoing WES.
(A) Scaled representation of relative frequency of each phenotype class within this series. Individual cases may be counted in multiple classes. (B) Diagnostic rate for each phenotype class [grey bars, left y-axis] and percent of all cases for each phenotype class [blue line, right y-axis]. * indicates Monte Carlo p<0.05 for the association between diagnostic rate and phenotype class.
To better understand intellectual disability (ID) in adults, we analyzed the subset of cases with the HPO term ‘Neurodevelopmental abnormality’, or one of several daughter terms. Of 53 such individuals with molecular diagnoses, 66.1% (37/56) of their diagnoses were autosomal dominant (Table S1), although in five cases the diagnosis explained components of the phenotype but not the ID. De novo mutations were reported in 75.0% (24/32) of autosomal dominant ID diagnoses. One of the remaining eight cases exhibited evidence of parental mosaicism; parental samples were unavailable in six cases.
Diagnostic rate was dependent on phenotype by a Monte Carlo analysis of 10,000 randomizations of diagnosis status (p=0.0053). Diagnostic rates within each phenotype class varied, with abnormalities of the nervous system, skeletal system, musculature, head and neck, and growth trending toward higher diagnostic rates, suggesting the presence of these features can portend a favorable impact on establishing a diagnosis using WES (Figure 2B). Conversely, abnormalities of the abdomen, genitourinary, hematologic, immune, and respiratory systems, as well as oncologic findings, were associated with a reduced diagnostic rate. The highest diagnostic rate was seen in subjects with neurodevelopmental abnormalities (27.7%, 53/191).
A family history overlapping with part or all of the proband’s phenotype did not affect the molecular diagnosis rate, with a 15.5% (34/219) diagnostic rate among cases with a positive family history not significantly different from the 19.1% (51/267) diagnostic rate in cases with a negative family history (two-tailed p=0.34, Fisher exact test). In 6 cases reporting a positive family history, WES was also performed on an affected sibling (4 cases) or parent (2 cases) but no molecular diagnosis was reported.
Secondary Findings
Seven medically actionable findings meeting ACMG criteria for secondary findings24 were reported in six adult probands (1.2%, 6/482, 95% CI 0.25–2.23%) including one patient with both a pathogenic BRCA1 mutation, previously identified in her mother, and a deleterious DSC2 mutation associated with arrhythmogenic right ventricular cardiomyopathy.25 There was a significantly higher frequency of ACMG secondary findings in pediatric probands tested during the same interval (3.1%, 114/3648, two-tailed p=0.020, Fisher exact test). The WGL reported a medically actionable finding outside the ACMG guidelines11 in six adult patients (1.2%, 6/481) including: two mitochondrial gene mutations associated with increased risk of aminoglycoside-induced nonsyndromic hearing loss, two cardiomyopathy genes (ABCC9, AKNRD1), one cancer susceptibility gene (RAD51D), and one novel loss-of-function mutation in a cholesterol metabolism gene (APOB, for which ACMG recommends reporting only known pathogenic mutations). Concurrently, 1.3% (49/3648) of pediatric probands had medically actionable findings outside the current ACMG gene list (two-tailed p=1, Fisher exact test). Carrier status for autosomal recessive conditions was reported in 24 of 486 cases in disorders recommended for testing as part of reproductive planning by the ACMG26 cystic fibrosis, Tay-Sachs disease, Canavan disease, Gaucher disease, sickle cell anemia, and Niemann-Pick disease type A.
DISCUSSION
Clinical WES in adult patients had a diagnostic rate of 17.5% in our series, which is significantly lower than 25.2% previously reported in a primarily pediatric population (two-tailed p=0.0003, Fisher exact test).11 Within this cohort of adult patients referred for WES, a positive family history was not predictive of molecular diagnosis, and molecular diagnoses often resulted from de novo events, informing the structure of Mendelian diseases in adults. The association of family history with diagnosis rate may be impacted by the underlying clinical phenotypes in these adult patients referred for WES. For example, cancer diagnoses were a rare indication for WES in this case population. Inheritance patterns of the diagnoses were similar to the primarily pediatric population;11 we observed an unexpected high prevalence of de novo mutations, which may be driven by the high frequency of neurodevelopmental abnormalities in this series. Like that observed in a pediatric population, three of seven X-linked diagnoses were X-linked dominant traits in females.11 The small number but higher rate of mitochondrial diagnoses (3/86) compared to a contemporary primarily pediatric population11 (1/504) may represent lack of available mitochondrial analyses when these adults were initially evaluated as children. Small insertion or deletion variants, which are less robustly detected by WES analysis algorithms,27 comprise nearly 25% of disease-causing variants in both pediatric and adult series,11 underscoring the need for improved detection and genotyping of these variants. We anticipate that implementation of the most updated ACMG guidelines28 for variant interpretation may allow improved reporting of likely pathogenic variants and evidence-based assessment of loss of function variants in WES reporting. The clinical utility of molecular diagnosis through WES in this adult population is demonstrated through medical management recommendations, anticipatory guidance, and provision of recurrence risk for patients and families, as recently described by the ACMG.10
The diagnostic indications in this adult case series were predominantly neurologic, for example 39.3% with neurodevelopmental delay, perhaps reflecting the specialty of ordering physicians. However, this phenotypic spectrum differed markedly from that within adult genetics clinics.11,14,15,29 For example, the 9.7% of adult WES cases with oncologic phenotypes is less than the 35% in adult genetics clinic,29 likely reflecting both the well-defined nature of many familial cancer syndromes and the availability of comprehensive next-generation hereditary cancer gene panels. Neurologic (14%) and cardiovascular (13%) phenotypes represent the next most common indications in adult genetics clinic, while 81% and 27% of adult WES cases, respectively, have such phenotypes. These differences illustrate the selective use of WES in the adult genetics clinic, in addition to its increasing use in adult neurology and neurogenetics centers. As the number of disease-gene and disease-variant associations continues to grow, combined with reporting of clinical sequencing studies from different patient populations (see for example https://cser-consortium.org), we will gain improved knowledge as to which adult patients are most appropriate for WES as a clinical test across the medical spectrum.
Diagnoses were more common among individuals with specific phenotypes including abnormalities of the head and neck, skeletal system, musculature, nervous system, or growth. Exploration of the relationship of pairwise combinations of phenotypes to the likelihood of molecular diagnosis did not find significant differences for specific pairs of anomalies in this cohort although larger studies may be needed to investigate this issue with sufficient power.
The “Clan Genomics” hypothesis for human disease traits posits that variants arising in the proband (i.e. de novo mutations), or a recent antecedent in the family or clan, are important to disease trait manifestation.30 Such de novo or recent events can cause dominant and X-linked diagnoses, or autosomal recessive diagnoses in the setting of consanguinity which allows for rapid attainment of homozygosity for newly occurring variants within the family or clan. Although no de novo recessive variants were present in our cohort, homozygous variants accounted for 29.7% of autosomal recessive diagnoses, and 7/11 homozygous variants were novel. Parental consanguinity was reported in 50% of homozygous mutations not explained by uniparental disomy. The high proportion of homozygous novel variants in autosomal recessive diagnoses taken together with parental consanguinity support the potential role of recently emerging variants in recessive disease.
We further observed pathogenic de novo events in 28.6% (2/7) of X-linked and 61.4% (27/44) of autosomal dominant diagnoses, all reported in adults less than 30 years, including two de novo diagnoses (PRICKLE2, CREBBP) in one individual with intellectual disability and seizures (Table S1). Importantly, when parental samples were available, de novo events were detected in 81.8% (27/33) of dominant diagnoses, which is similar to the 86.7% (208/240) reported in a primarily pediatric population.11 The diagnostic rate for autosomal dominant disease is impacted by parental sample availability, particularly for missense mutations not previously documented to be disease-associated. We report 24 missense variants among 44 total autosomal dominant diagnoses (55%), 13 of which were novel. Determination of pathogenicity for these novel missense variants required parental samples, as 85% (11/13) were de novo, one inherited from an affected mother, and one from an unaffected mosaic father. These findings suggest that the lower diagnostic rate in individuals over 30 years of age may be explained, at least in part, by limited parental sample availability. Overall, these data demonstrate that interpretation of novel missense variants as pathogenic frequently relies on de novo status in autosomal dominant disorders, and support a role for trio-WES for detection of de novo variants. That de novo mutations underlie a high proportion of genetic disease in adults is unexpected and illustrates that physicians who rely on a positive family history to refer for genetic testing may miss such diagnoses.
The majority of individuals with de novo diagnoses (26/29) had disorders that included neurodevelopmental delay with onset in childhood, representing 13.6% (26/191) of all cases involving neurodevelopmental delay, and providing a molecular explanation for the developmental phenotype in 24 of these. These findings are similar to prior reports that between 16%31 and 55%32 of pediatric ID cases, and 16% of a variety of pediatric developmental disorders,33 have de novo autosomal dominant molecular diagnoses by trio-WES. This suggests greater phenotypic overlap with pediatric genetics cases among these individuals with de novo mutations than is present in the adult WES cases as a whole. Additionally, over half of these diagnoses were made in genes with disease associations discovered in the last decade. Therefore, the ascertainment of frequent de novo mutations in our adult cohort is potentially attributable to both limited genetic diagnostic testing capabilities and limited disease gene knowledge when these individuals initially presented as children.
While phenotypic similarity between young adults presenting for WES and pediatric cases potentially contributes to increased diagnoses in younger adults (Figure 1), other factors contribute to a lower overall diagnostic rate in adults. Interestingly, recurrent molecular diagnoses (diagnostic variants found in the same gene in multiple subjects) were observed in only 10.6% (9/85) of molecularly diagnosed adult cases, compared to 56.0% of cases in a primarily pediatric population.11 These findings may reflect the smaller number of adult cases, but may also indicate a greater diversity of underlying genetic disorders in adults.
Diagnosis of adults with genetic disease provides a unique challenge. Effects of environmental exposures or signs of more common/complex non-Mendelian medical disease may obscure adult phenotypes. Lack of parental samples may limit recognition of de novo variants, and adult phenotypes may be milder, more heterogeneous, and with variable onset, possibly due to rare hypomorphic alleles. Other limitations of WES, such as difficulties in detecting CNV and repeat expansion, are magnified in adult patients for whom chromosomal microarray or repeat expansion analysis may not have been performed prior to WES. The greater clinical availability of molecular diagnostic tools such as WES has allowed diagnoses that were not possible ten or even five years ago, but this same success underscores the need for a more complete interrogation of individual genomic variation, particularly rare variant alleles and copy number variation, as these both contribute to adult disease.34
Despite the present limitations and clear areas for further discovery (gene function identification), WES is a clinically useful diagnostic tool with the ability to deconvolute complicated adult phenotypic presentations and diagnose exceedingly rare conditions. This advantage is evident in several of the cases described in Table S1, such as the rare diagnosis of glutamate formiminotransferase deficiency (OMIM #229100), Sotos syndrome in an individual with an atypical phenotype not ascertained in childhood (OMIM #117550), blended phenotypes due to coexistence of multiple genetic conditions,11,12 and molecular diagnoses such as myotonia congenita (OMIM #255700) and Kufor-Rakeb syndrome (OMIM #606693) that inform clinical management of symptoms (Table S1).35,36
Identification of secondary, potentially actionable mutations provides opportunities to inform medical surveillance and disease prevention.37 The ACMG recommends actively searching for such actionable findings in all cases undergoing clinical WES,24 and this practice will likely increase quality of life and be cost-effective.38 We identified findings in 1.2% of adults, similar to that previously reported in adult controls (92/6503, 1.4%, p=1.00)39 but significantly lower than the 3.1% frequency in pediatric cases in our clinical laboratory (p=0.020).11 Differences may reflect the elimination of secondary findings in adults who have already manifested features (or had a positive family history) related to these later-onset conditions than children.40
We report experiences with WES as a diagnostic tool in an adult population referred to an academic diagnostic laboratory, a group for which phenotype heterogeneity, aging and environmental exposures increase the diagnostic challenges. The adult patients referred for WES were phenotypically similar to pediatric cohorts, with a predominance of developmental or neurologic disorders.11 Molecular diagnoses were more common in young adults. The observation of two molecular genetic diagnoses in about 7% of those with diagnoses is similar to findings from our previous studies of 6.5%12 and 4.6%11. Molecular diagnoses and medically actionable secondary findings provided anticipatory information, management and surveillance guidance, and in some cases, disease-specific treatment options that may result in a significant improvement in quality of life. The presence of a positive family history did not predict molecular diagnosis, and the high contribution of de novo events potentially informs the Mendelian basis of genetic disease in adults. Moreover, the paucity of recurrent molecular diagnoses suggests much remains to be learned about the underlying genes and genetic architecture of adult genetic disease.
Supplementary Material
Acknowledgments
JEP was supported by the Medical Genetics Research Fellowship Program NIH/NIGMS NIH T32 GM07526. WW was supported by the Career Development Award K23NS078056 from the National Institute of Neurological Disorders and Stroke (NINDS). This work was funded in part by grants U01 HG006485 (SEP) from the National Human Genome Research Institute (NHGRI) and National Cancer Institute (NCI), U54 HG006542 (JRL) from the NHGRI and National Heart, Lung, and Blood Institute (NHLBI), U54-HG003273 (RAG) from the NHGRI, and R01 NS058529 (JRL) from the NINDS.
Footnotes
CONFLICT OF INTEREST
Baylor College of Medicine (BCM) and Miraca Holdings Inc. have formed a joint venture with shared ownership and governance of the Baylor Miraca Genetics Laboratories (BMGL), which performs clinical exome sequencing. JAR, ZN, FX, REP, MW, ALB, CME, YY, RAG, JRL, and SEP are employees of BCM and derive support through a professional services agreement with the BMGL. SEP and JRL serve on the Scientific Advisory Board of the BMGL. RAG serves as interim Chief Scientific Officer of the BMGL. ALB serves as Chief Medical Officer of the BMGL.
MB is the founder of Codified Genomics Inc., and derives personal fees from Illumina Inc. SD is the CEO and co-founder of PanGenomics Clinical Genetics Center in India. JAR reports personal fees from Signature Genomic Laboratories, PerkinElmer, Inc., in the past 36 months. RAG reports consulting fees from GE-Clarient. JRL has stock ownership in 23 and Me, is a paid consultant for Regeneron Pharmaceuticals, has stock options in Lasergen, Inc and is a co-inventor on multiple United States and European patents related to molecular diagnostics for inherited neuropathies, eye diseases and bacterial genomic fingerprinting. Other authors have no disclosures relevant to the manuscript.
Other authors have no potential conflicts to disclose.
References
- 1.Wheeler DA, Srinivasan M, Egholm M, et al. The complete genome of an individual by massively parallel DNA sequencing. Nature. 2008;452(7189):872–876. doi: 10.1038/nature06884. [DOI] [PubMed] [Google Scholar]
- 2.Levy S, Sutton G, Ng PC, et al. The diploid genome sequence of an individual human. PLoS Biol. 2007;5(10):e254. doi: 10.1371/journal.pbio.0050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert TJ, Molla MN, Muzny DM, et al. Direct selection of human genomic loci by microarray hybridization. Nat Methods. 2007;4(11):903–905. doi: 10.1038/nmeth1111. [DOI] [PubMed] [Google Scholar]
- 4.Bainbridge MN, Wang M, Burgess DL, et al. Whole exome capture in solution with 3 Gbp of data. Genome Biol. 2010;11(6):R62. doi: 10.1186/gb-2010-11-6-r62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lupski JR, Reid JG, Gonzaga-Jauregui C, et al. Whole-genome sequencing in a patient with Charcot-Marie-Tooth neuropathy. N Engl J Med. 2010;362(13):1181–1191. doi: 10.1056/NEJMoa0908094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyden LM, Choi M, Choate KA, et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482(7383):98–102. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worthey EA, Mayer AN, Syverson GD, et al. Making a definitive diagnosis: successful clinical application of whole exome sequencing in a child with intractable inflammatory bowel disease. Genet Med. 2011;13(3):255–262. doi: 10.1097/GIM.0b013e3182088158. [DOI] [PubMed] [Google Scholar]
- 8.Iglesias A, Anyane-Yeboa K, Wynn J, et al. The usefulness of whole-exome sequencing in routine clinical practice. Genet Med. 2014;16(12):922–931. doi: 10.1038/gim.2014.58. [DOI] [PubMed] [Google Scholar]
- 9.Wangler MF, Gonzaga-Jauregui C, Gambin T, et al. Heterozygous de novo and inherited mutations in the smooth muscle actin (ACTG2) gene underlie megacystis-microcolon-intestinal hypoperistalsis syndrome. PLoS Genet. 2014;10(3):e1004258. doi: 10.1371/journal.pgen.1004258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ACMG Board of Directors. Clinical utility of genetic and genomic services: a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2015 doi: 10.1038/gim.2015.41. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Muzny DM, Xia F, et al. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312(18):1870–1879. doi: 10.1001/jama.2014.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Muzny DM, Reid JG, et al. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N Engl J Med. 2013;369(16):1502–1511. doi: 10.1056/NEJMoa1306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bainbridge MN, Hu H, Muzny DM, et al. De novo truncating mutations in ASXL3 are associated with a novel clinical phenotype with similarities to Bohring-Opitz syndrome. Genome Med. 2013;5(2):11. doi: 10.1186/gm415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farwell KD, Shahmirzadi L, El-Khechen D, et al. Enhanced utility of family-centered diagnostic exome sequencing with inheritance model-based analysis: results from 500 unselected families with undiagnosed genetic conditions. Genet Med. 2014 doi: 10.1038/gim.2014.154. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Deignan JL, Dorrani N, et al. Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA. 2014;312(18):1880–1887. doi: 10.1001/jama.2014.14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Szybowska M. A novel mutation c. 4003 G>C in the CREBBP gene in an adult female with Rubinstein-Taybi syndrome presenting with subtle dysmorphic features. Am J Med Genet A. 2010;152A(11):2939–2941. doi: 10.1002/ajmg.a.33693. [DOI] [PubMed] [Google Scholar]
- 17.Prada CE, Gonzaga-Jauregui C, Tannenbaum R, et al. Clinical utility of whole-exome sequencing in rare diseases: Galactosialidosis. Eur J Med Genet. 2014;57(7):339–344. doi: 10.1016/j.ejmg.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collison FT, Xie YA, Gambin T, et al. Whole Exome Sequencing Identifies an Adult-onset Case of Methylmalonic Aciduria and Homocystinuria Type C (cblC) with Non-syndromic Bull’s Eye Maculopathy. Ophthalmic Genet. 2015 doi: 10.3109/13816810.2015.1010736:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Garay ML, McGuire AL, Pereira S, Caskey CT. Personalized genomic disease risk of volunteers. Proc Natl Acad Sci U S A. 2013;110(42):16957–16962. doi: 10.1073/pnas.1315934110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caskey CT, Gonzalez-Garay ML, Pereira S, McGuire AL. Adult genetic risk screening. Annu Rev Med. 2014;65:1–17. doi: 10.1146/annurev-med-111212-144716. [DOI] [PubMed] [Google Scholar]
- 21.Bainbridge MN, Wang M, Wu Y, et al. Targeted enrichment beyond the consensus coding DNA sequence exome reveals exons with higher variant densities. Genome Biol. 2011;12(7):R68. doi: 10.1186/gb-2011-12-7-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards CS, Bale S, Bellissimo DB, et al. ACMG recommendations for standards for interpretation and reporting of sequence variations: Revisions 2007. Genet Med. 2008;10(4):294–300. doi: 10.1097/GIM.0b013e31816b5cae. [DOI] [PubMed] [Google Scholar]
- 23.Kohler S, Doelken SC, Mungall CJ, et al. The Human Phenotype Ontology project: linking molecular biology and disease through phenotype data. Nucleic Acids Res. 2014;42(Database issue):D966–974. doi: 10.1093/nar/gkt1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green RC, Berg JS, Grody WW, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15(7):565–574. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beffagna G, De Bortoli M, Nava A, et al. Missense mutations in desmocollin-2 N-terminus, associated with arrhythmogenic right ventricular cardiomyopathy, affect intracellular localization of desmocollin-2 in vitro. BMC Med Genet. 2007;8:65. doi: 10.1186/1471-2350-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross SJ, Pletcher BA, Monaghan KG, Professional P, Guidelines C. Carrier screening in individuals of Ashkenazi Jewish descent. Genet Med. 2008;10(1):54–56. doi: 10.1097/GIM.0b013e31815f247c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DePristo MA, Banks E, Poplin R, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eble TN, Nagamani SC, Franco LM, Plon SE, Blazo M, Dhar SU. The practice of adult genetics: a 7-year experience from a single center. Am J Med Genet A. 2013;161A(1):89–93. doi: 10.1002/ajmg.a.35684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lupski JR, Belmont JW, Boerwinkle E, Gibbs RA. Clan genomics and the complex architecture of human disease. Cell. 2011;147(1):32–43. doi: 10.1016/j.cell.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Ligt J, Willemsen MH, van Bon BW, et al. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med. 2012;367(20):1921–1929. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- 32.Rauch A, Wieczorek D, Graf E, et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380(9854):1674–1682. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- 33.Wright CF, Fitzgerald TW, Jones WD, et al. Genetic diagnosis of developmental disorders in the DDD study: a scalable analysis of genome-wide research data. Lancet. 2015;385(9975):1305–1314. doi: 10.1016/S0140-6736(14)61705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiVincenzo C, Elzinga CD, Medeiros AC, et al. The allelic spectrum of Charcot-Marie-Tooth disease in over 17,000 individuals with neuropathy. Mol Genet Genomic Med. 2014;2(6):522–529. doi: 10.1002/mgg3.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Statland JM, Bundy BN, Wang Y, et al. Mexiletine for symptoms and signs of myotonia in nondystrophic myotonia: a randomized controlled trial. JAMA. 2012;308(13):1357–1365. doi: 10.1001/jama.2012.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams DR, Hadeed A, al-Din AS, Wreikat AL, Lees AJ. Kufor Rakeb disease: autosomal recessive, levodopa-responsive parkinsonism with pyramidal degeneration, supranuclear gaze palsy, and dementia. Mov Disord. 2005;20(10):1264–1271. doi: 10.1002/mds.20511. [DOI] [PubMed] [Google Scholar]
- 37.Green RC, Lupski JR, Biesecker LG. Reporting genomic sequencing results to ordering clinicians: incidental, but not exceptional. JAMA. 2013;310(4):365–366. doi: 10.1001/jama.2013.41703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennette CS, Gallego CJ, Burke W, Jarvik GP, Veenstra DL. The cost-effectiveness of returning incidental findings from next-generation genomic sequencing. Genet Med. 2014 doi: 10.1038/gim.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amendola LM, Dorschner MO, Robertson PD, et al. Actionable exomic incidental findings in 6503 participants: challenges of variant classification. Genome Res. 2015 doi: 10.1101/gr.183483.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jurgens J, Ling H, Hetrick K, et al. Assessment of incidental findings in 232 whole-exome sequences from the Baylor-Hopkins Center for Mendelian Genomics. Genet Med. 2015 doi: 10.1038/gim.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.