Abstract
Background
The neonatal resuscitation program (NRP) recommends close monitoring of oxygenation during the resuscitation of newborns using a pulse oximeter. However, there are no guidelines for monitoring carbon dioxide (CO2) to assess ventilation. Considering that cerebral blood flow (CBF) correlates directly with PaCO2, continuous capnography monitoring of end-tidal CO2 (ETCO2) may limit fluctuations in PaCO2 and, therefore, CBF during resuscitation of asphyxiated infants.
Objective
To evaluate if continuous monitoring of ETCO2 with capnography during resuscitation of asphyxiated term lambs with meconium aspiration will prevent fluctuations in PaCO2 and carotid arterial blood flow (CABF).
Methods
Fifty-four asphyxiated term lambs with meconium aspiration syndrome were mechanically ventilated from birth to 60 min of age. Ventilatory parameters were adjusted based on clinical observation (chest excursion) and frequent arterial blood gas analysis in 24 lambs (control group) and 30 lambs (capnography group) received additional continuous ETCO2 monitoring. Left CABF was monitored. We aimed to maintain PaCO2 between 35–50 mmHg and ETCO2 between 30–45 mmHg.
Results
There was a significant correlation between ETCO2 and PaCO2 (R=0.7, p<0.001), between PaCO2 and carotid flow (R=0.52, p<0.001), and between ETCO2 and carotid flow (R=0.5, p<0.001). PaCO2 and CABF during the first 60 minutes of age showed significantly higher fluctuation in the control group compared to the capnography group.
Conclusion
Continuous monitoring of ETCO2 using capnography with mechanical ventilation during and after resuscitation in asphyxiated term lambs with meconium aspiration limits fluctuations in PaCO2 and CABF, and may potentially limit brain injury.
Keywords: capnography, carbon dioxide, carotid blood flow
Introduction
Ventilation of the lungs is the single most important and effective step in resuscitation [1]. Approximately 5 to 10% of newborn infants require some degree of active resuscitation at birth [2] and approximately 1–10% of infants born in hospital are reported to require assisted ventilation [1,3] with few of these infants being intubated in the delivery room [4]. Clinically, adequacy of ventilation is assessed by chest rise, auscultation and improving heart rate. The assessment of ventilation based on chest rise is unreliable [5,6] and in instances when chest rise was used to determine adequacy of ventilation, infants were hypocapnic at the time of admission [7]. Inaccurate clinical assessment of ventilation places newborns at risk for hypocapnia and/or hypercapnia. End-tidal capnography (end-tidal CO2, ETCO2) provides a graphical assessment of the partial pressure of carbon dioxide during expiration and is being increasingly used in the neonatal intensive care unit (NICU) [8].
Extremes of PaCO2 may prove detrimental in an asphyxiated infant by causing fluctuations in CBF [9]. Hypocapnia is associated with a decrease in brain tissue oxygen saturation (PbtO2) during hyperventilation [10]. Cumulative exposure to hypocapnia (PaCO2 <35 mmHg) has been associated with a higher rate of death or disability in infants with hypoxic ischemic encephalopathy (HIE) [11]. Therefore, in addition to maintaining oxygenation during resuscitation and in the post-resuscitation phase, it is crucial to avoid wide fluctuations in PaCO2 in infants with perinatal asphyxia [12].
Frequent evaluation of PaCO2 by blood gas analysis is not practical in the delivery room [13]. Studies have shown positive correlation between ETCO2 and PaCO2 in the neonatal intensive care unit (NICU) [8,14]. The reliability of ETCO2 during the period of transition in the delivery room is not known. Monitoring ETCO2 with waveform capnography during PPV in the delivery room may help avoid extreme fluctuations in PaCO2 levels. We hypothesize that ETCO2 correlates well with PaCO2 during transition and continuous monitoring of ETCO2 with capnography during resuscitation of asphyxiated term lambs with meconium aspiration will prevent fluctuations in PaCO2 and CBF.
Methods
The Institutional Animal Care and Use Committee (IACUC) at the State University of New York at Buffalo approved this study. Near term gestation fetal lambs from time-dated pregnant ewes (Newlife Pastures, Attica NY) were sedated with intravenous ketamine and diazepam, intubated and ventilated with 21–30% oxygen and ~2% isoflurane. The fetal lambs were exteriorized. Jugular and carotid lines were placed on the right side for access, to draw pre-ductal arterial blood gases (ABG) and to monitor systemic blood pressure. Ultrasound probes (Transonic systems, Ithaca, NY, USA) were placed around the left carotid artery and left pulmonary artery to measure blood flow. Systemic and pulmonary pressures were measured by transducing the right carotid and left pulmonary arteries. A catheter was placed in the left atrium to measure pressures.
As previously described [15], approximately 1 g/kg of human meconium was added to warm amniotic fluid from the ewe's uterus (4 ml/kg lamb weight) to a total volume of 5 ml/kg estimated weight and homogenized to form a viscous “thick” meconium solution. The solution was aspirated using an 18G needle with syringe. After draining excess lung liquid, a funnel with meconium in amniotic fluid was connected to the endotracheal tube. The meconium solution layered over the fetal lung liquid in the endotracheal tube and maintained at a height that hydrostatic pressure did not force meconium into the lungs. The umbilical cord was occluded for 5 min (cord occlusion #1) using an inflatable vascular occluding device resulting in approximately 10–15 gasps. Umbilical cord occlusion was released for 5 min to allow the lamb to recover and then occlusion was repeated for 5 more minutes (cord occlusion #2). Deep, gasping respirations aspirated meconium into the lung. At the end of this period, the endotracheal tube that was used to deliver meconium was removed. The umbilical cord was cut (delivery time) and lamb was placed under a radiant warmer. Following this period, the lambs were delivered and resuscitated as per NRP guidelines. Preductal saturations were recorded using Masimo Radical 7 pulse oximeter (Irvine, CA, USA). To monitor ETCO2 in the study group, a side stream capnography (SurgiVet 9004, Smith Medical, MA, USA) was used. According to the manufacturer, SurgiVet 9004 has an accuracy of ±2 mmHg with a display range of 0 to 100 mmHg. The capnograph continuously displayed the ETCO2 in mmHg with a waveform and respiratory rate.
Ventilation and Blood Gas Analyses
After delivery, the lambs were ventilated with the Servo 300A (Siemens, Monet Medical, Utah) using a pressure control mode of ventilation. Ventilation was initiated with a peak inspiratory pressure (PIP) of 30 cm H2O and a PEEP of 5 cm H2O. Arterial blood gases were drawn at birth, 1 minute and 5 minutes after birth followed by every 5 minutes for the first 30 minutes and every 15 minutes for the next 30 minutes. The lambs were ventilated in the first hour in both groups with a preductal oxygen saturation (SpO2) range of 85 – 99 %. The intervention group (capnography) was not randomized and was chosen based on the availability of capnography equipment.
In the control group (24 lambs), PaCO2 was targeted between 35–50 mmHg. PIP was adjusted by 2 cm H2O based on chest excursion and subsequently the rate (by 5 breaths/min) in an attempt to maintain PaCO2 in the target range. In the capnography group (30 lambs), in addition to maintaining PaCO2, ETCO2 was targeted between 30–45 mmHg as our preliminary data showed a variation of 5–10 mmHg compared to PaCO2. Continuous monitoring of ETCO2 with capnography with frequent ventilator adjustments based on ETCO2 values in addition to PaCO2 were performed in asphyxiated term lambs with meconium aspiration to limit fluctuations in PaCO2 and CBF.
Data Collection and Calculations
Data were continuously recorded using AcqKnowledge Acquisition & Analysis Software (BIOPAC systems, CA, USA). The variations in ETCO2, PaCO2 and left CABF were compared from 5 to 60 minutes of age. Data were compared after 5 minutes of age to allow pulmonary adaptation and transition to extra-uterine life [16]. Pulmonary vascular resistance (PVR) was calculated by dividing the difference in mean pulmonary arterial pressure and left atrial pressure by left pulmonary blood flow per minute corrected for body weight.
Statistics
Sample size and power
The standard deviation of the fluctuation in PaCO2 during the first 60 minutes in lambs with meconium aspiration is 11 mm Hg. To reduce this fluctuation (maximum PaCO2 – minimum PaCO2 in the first 60 minutes) by 10 mm Hg using capnography, we needed 20 lambs in each group with a type I error probability of 0.05 and power of 0.8. With the current sample size of 30 lambs in the capnography group and 24 control lambs, a probability (power) of 0.903 was achieved.
Statistical Methods
Pearson and Spearman correlations along with Bland-Altman plot were used in assessing ETCO2 and PaCO2 values. Normally distributed variables are presented as mean +/− standard deviation and skewed variables as median (range) values. The proportion of PaCO2 values outside the target range between the groups was compared by chi-square test. The fluctuation (difference between minimum and maximum values during 5 – 60 minutes of age represented as bar graphs) in PaCO2 and left CABF were analyzed by unpaired t-test. A p-value of <0.05 was considered significant.
Software
Data were analyzed using SPSS 22 software (IBM, Armonk, NY).
Results
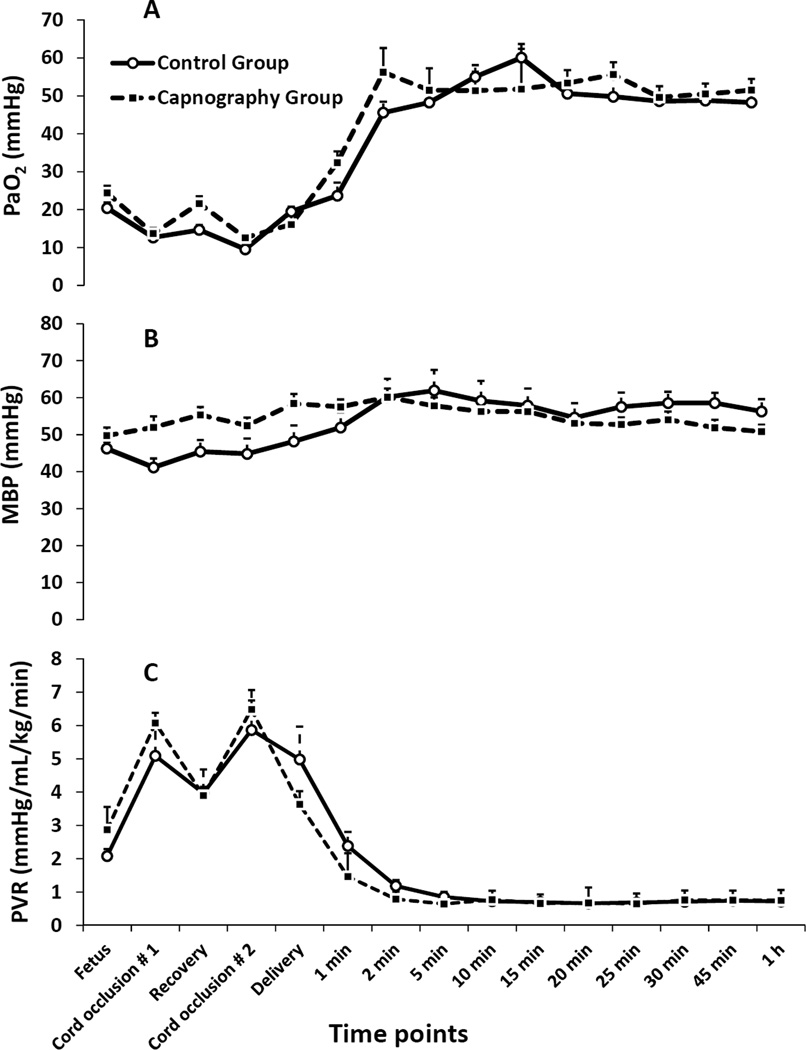
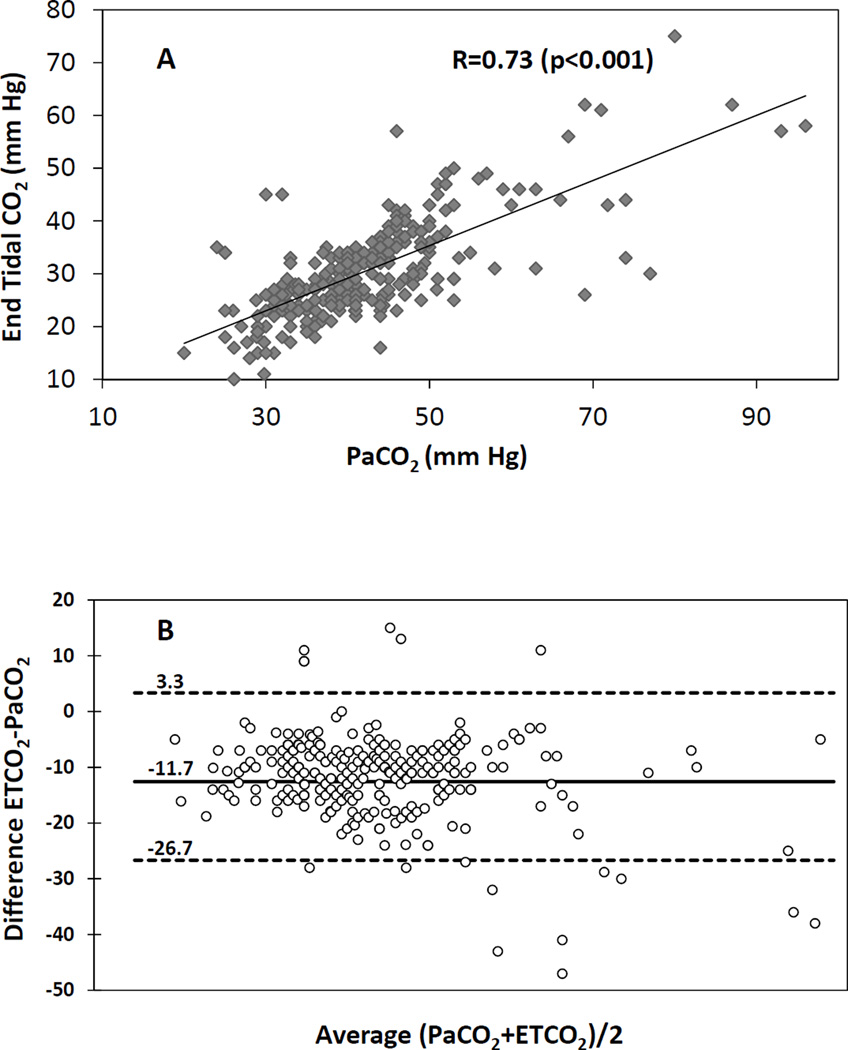
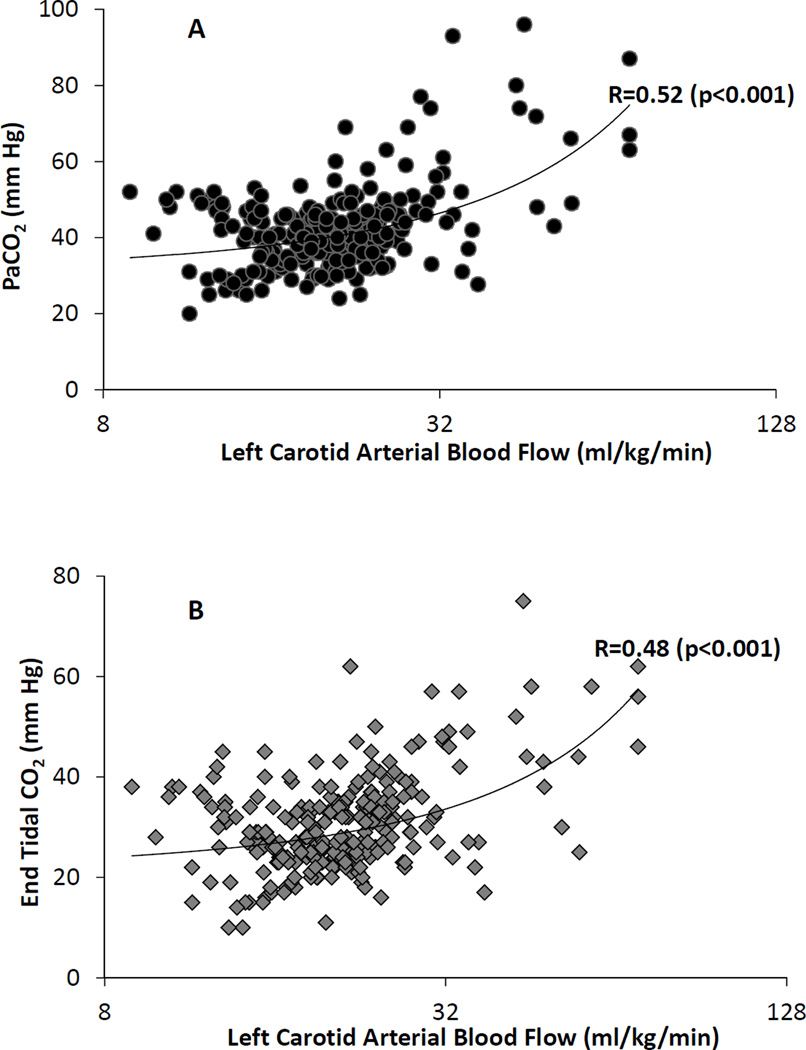
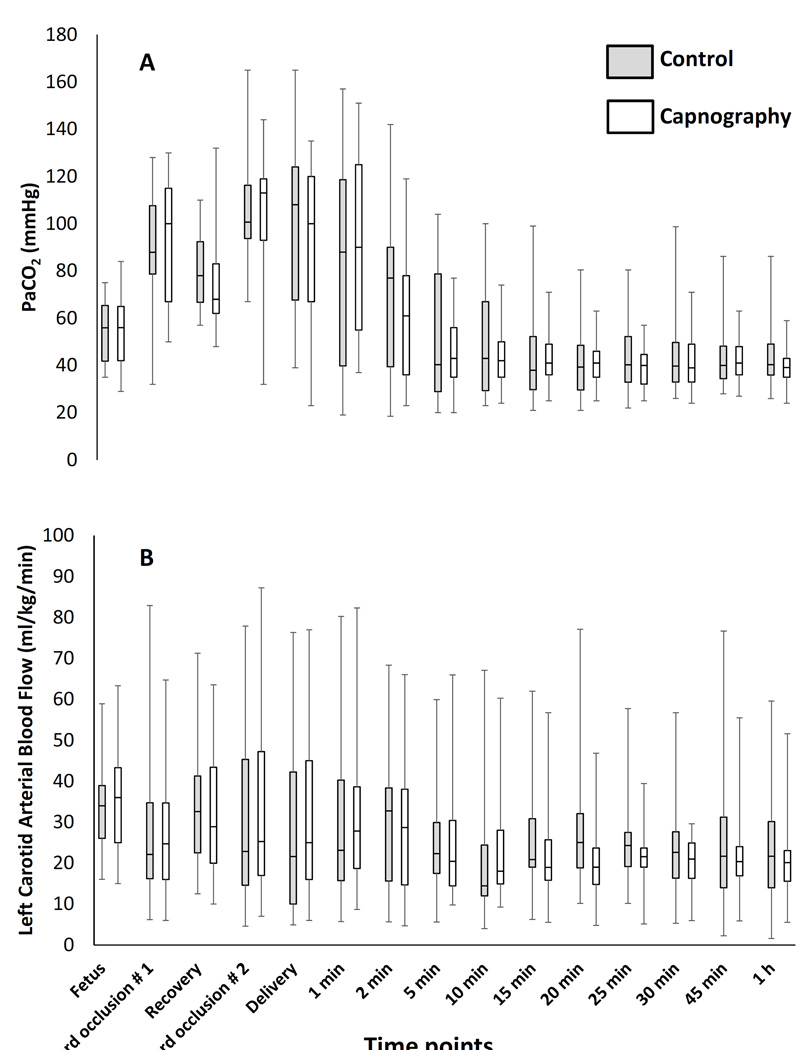
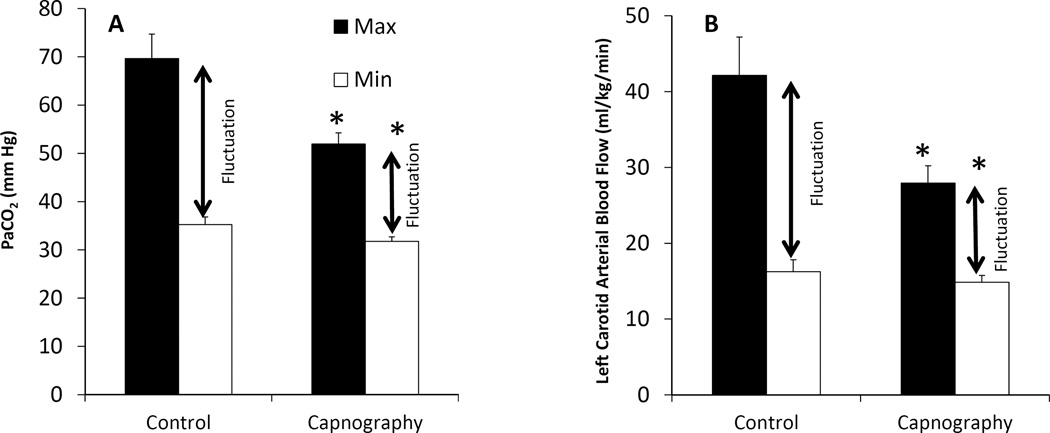
Umbilical cord occlusion and meconium aspiration resulted in severe hypercarbia and acidosis at birth (Table 1). The preductal oxygenation index (OI = mean airway pressure in cm H2O × FIO2 × 100 ÷ preductal PaO2) following meconium aspiration was 18.6±10 and 20.1±9 in the capnography and control groups respectively. The partial pressure of oxygen in arterial blood (PaO2), systemic mean blood pressure (MBP), PVR between the groups were not different during the first postnatal hour (Figure 1). There were 274 simultaneous blood and end-tidal CO2 measures obtained from the 30 capnography group lambs. The ETCO2 levels obtained during the first 2 minutes after birth were low compared to PaCO2 values and were excluded from correlation analysis. The correlation between ETCO2 and PaCO2 following the first two minutes after birth improved, R=0.64 (rho-0.6, p=0.01). From 5 – 60 minutes after birth, the ETCO2 correlated well with PaCO2 with a correlation coefficient, R=0.73 (rho-0.7, p<0.001) (Figure 2A). The agreement between ETCO2 and PaCO2 is illustrated as a Bland-Altman plot [17]. The ETCO2 was lower than PaCO2 with a mean bias of −11.7±7.7 mm Hg with a 95% confidence interval of − 26.7, 3.3 (Figure 2B). Left CABF (ml/kg/min) had a modest correlation with PaCO2, R=0.52 (rho-0.31, p<0.001) (Figure 3A) and with ETCO2, R=0.48 (rho-0.48, p<0.0 (Figure 3B). There was a mean 19% increase in CABF for each KPa increase in PaCO2 (or 2.5% increase with 1 mm Hg increase in PaCO2). The respiratory rate (both spontaneous and ventilator setting of mandatory rate) during the entire study period did not correlate well with ETCO2 (0.13) or the PaCO2 (0.30). The percentage of PaCO2 outside the target range in the control group (61%) was significantly greater than the capnography group (43%) (P=0.002). The variance of PaCO2 and left CABF are depicted as box plots (Figure 4A, 4B) and the fluctuations as bar graphs (Figure 5A, 5B). The fluctuation of PaCO2 (30.5±18.9 vs. 20.0±11.4 mmHg, p=0.002) and the fluctuation between the left CABF (25.8±5.8 vs. 13.1±2.1ml/kg/min, p=0.008) were significantly higher in the control group compared to the capnography group.
Table 1.
Baseline characteristics and blood gas values prior to onset of resuscitation. Severe asphyxia induced by intermittent umbilical cord occlusion resulted in severe acidosis. The values represented in the table are at birth (mean±standard deviation) and were not different.
| Parameters | Capnography (N=30) |
Control (N=24) |
|---|---|---|
| Birth Weight (grams) | 3460±820 | 3220±780 |
| Gestational age (days) | 141±0.5 | 141±0.8 |
| Oxygenation index | 18.6±10 | 20.1±9 |
| SpO2 (%) | 49±29 | 49±25 |
| pH | 7.02±0.10 | 6.9±0.13 |
| PaCO2 (mmHg) | 102±29 | 107±25 |
| Base excess (mEq/L) | −8.7±2.1 | −10.4±3.8 |
Figure 1.
(A) Preductal arterial oxygen tension in mmHg (PaO2), (B) mean systemic blood pressure in mmHg (MBP) and (C) pulmonary vascular resistance (PVR) in mmHg/ml/kg/min are represented by graphs. Data from fetal baseline values, cord occlusion for asphyxiation, resuscitation and the first 60 minutes of postnatal period are shown in the graph. Data were analyzed from 5 to 60 minutes of life. PaO2, MBP and PVR were similar between groups.
Figure 2.
(A) Scatter plot of end-tidal carbon dioxide (ETCO2) and arterial carbon dioxide (PaCO2) values from 5–60 minutes of age showed good correlation (r-0.73, rho-0.7, p<0.001). (B) The agreement between ETCO2 and PaCO2 is illustrated as Bland Altman plot. The ETCO2 was lower than PaCO2 with a mean bias of −11.7+/− 7.7 mm Hg with confidence interval of −26.7, 3.3 mm Hg.
Figure 3.
(A) Scatter plot of left carotid arterial blood flow and PaCO2 showed a moderate correlation (R-0.52, rho-0.31, p<0.001). (B) Scatter plot of left carotid arterial blood flow and ETCO2 showed a moderate correlation (r-0.48, rho-0.48, p<0.001). The left carotid arterial blood flow is plotted on a logarithmic scale.
Figure 4.
(A) The variance of PaCO2 (mmHg) and (B) left carotid arterial blood flow are depicted as box plot between the groups.
Figure 5.
(A) The fluctuations in PaCO2 (mmHg) is depicted as a bar diagram in the capnography group and control group. The amplitude of fluctuations (difference between the mean maximum and minimum values) were significantly different (p=0.002). (B) The fluctuations in left carotid arterial blood flow (ml/kg/min) are depicted as bar diagrams between the groups. The fluctuations (difference between the mean maximum and minimum values) were significantly different (p=0.008).
The median (range) PaCO2 from 5 – 60 minutes of age was 40 (20–77) mmHg in the capnography group and 40 (20–104) mmHg in the control group. Median (range) left CABF was 20 (3–59) ml/kg/min in the capnography group and 23 (5–90) ml/kg/min in the control group.
Discussion
Perinatal asphyxia is a major cause of HIE and is often associated with meconium aspiration syndrome [18,19]. The initial delivery room management of an unstable newborn infant is important to ensure transition to extra uterine life and optimize lifelong neurodevelopmental outcome [20]. Birth asphyxia is associated with fluctuations in CBF in the early post-resuscitation period [21]. Monitoring PCO2 is not only important to avoid fluctuations in CBF and but also to maintain moderately elevated PaCO2 during the first minutes of resuscitation to restore CBF [22,23]. In addition elevated PCO2 may be beneficial and is associated with higher 5 minute apgar scores after moderate acidemia [24]. However, a persistent or excessive increase in brain perfusion during the postnatal period may be associated with reperfusion injury in HIE [25]. Wide variations in arterial PCO2 values are common immediately following asphyxia and resuscitation [26], and may contribute to fluctuations in CBF. A recent retrospective study has shown severe abnormal neurodevelopmental outcomes to be associated with high PaCO2 variability over 72 hours in whole body cooled HIE neonates [12]. Our study aimed at avoiding fluctuations in PaCO2 and studying its impact on CABF in a term asphyxiated lamb model in the immediate transition phase.
Current NRP guidelines focus on maintaining normoxemia using a pulse oximeter in the delivery room [1]. However, hypercapnia even in the presence of normoxemia results in significant increase in CBF in newborn piglets [27]. Severe hypercarbia is commonly seen immediately following asphyxia (Table 1). Resuscitation with PPV can result in either over ventilation with hypocapnia or under ventilation with hypercapnia. In this study, we have demonstrated that capnography, when combined with blood gas monitoring is a simple intervention that is superior to frequent blood gas sampling alone at the bedside in reducing fluctuation in PaCO2.
Pryds et al have evaluated the effect of blood pressure and PaCO2 on CBF in newborn infants with varying severity of HIE and compared them to normal control infants. Following very severe asphyxial injury leading to an isoelectric EEG, vasoparalysis results in a pressure-passive cerebral circulation that is non-responsive to PaCO2 [28]. However, CBF in neonates with severe asphyxial injury leading to burst-suppression on EEG was pressure-passive but was responsive to changes in PaCO2 (~ 2% mean change in flow for every mmHg change in PaCO2 compared to 3.4% in control neonates without asphyxia) [28]. Carotid blood flow increased by 2.5% for every mmHg increase in PaCO2 in the current study consistent with published literature. Hence in infants with moderate to severe asphyxia avoiding hypocapnia and hypercapnia during and immediately after resuscitation and maintaining stable systemic blood pressure may be important in minimizing reperfusion injury.
We found poor correlation between ETCO2 and PaCO2 (R – 0.11) during the first 2 minutes of resuscitation. This is probably due to poor lung aeration secondary to alveolar fluid, poor access to alveolar gas, low pulmonary blood flow and severe lung disease. Hooper et al have similarly demonstrated that increasing ETCO2 levels in newly born lambs, and preterm rabbits are reflective of increasing tidal volume and improved lung aeration after birth [29]. Similarly, Schmolzer et al have shown the relationship between ETCO2 and establishing tidal volume during the first couple of minutes in human neonates [16]. These findings suggest that ETCO2 values may not correlate well with PaCO2 in the first few minutes of resuscitation. In the presence of an increasing or stable heart rate, we recommend that adjustments to ventilation (such as decreasing PIP) should not be made based on ETCO2 values during the first couple of minutes of resuscitation, prior to establishment of lung aeration in asphyxiated neonates. An increasing ETCO2 in the first couple of minutes may be a sign of better lung aeration and increasing pulmonary blood flow.
A good correlation between ETCO2 and PaCO2 has been demonstrated in NICU patients, including extremely low birth weight patients [14]. In the current study, we used a model of perinatal asphyxia with combined metabolic and respiratory acidosis, significant parenchymal lung disease and airway obstruction. In spite of the presence of parenchymal and airway disease, we found a good correlation between PaCO2 and ETCO2. The presence of increased physiologic dead space and the meconium aspiration in lambs could have contributed to the increased difference between PaCO2 and ETCO2 (mean of −11.7) in our study as compared to previous studies (Figure 2B).
Term infants have much greater cerebrovascular sensitivity to PaCO2 than preterm infants [30]. Wyatt et al calculated that the percentage change in cerebral blood volume increased per kilopascal change in PaCO2 from 4% at 26 weeks to 25% at 40 weeks [30]. We studied the effect of fluctuations of PaCO2 on the left CABF in a controlled setting while maintaining normoxia with similar systemic and PVR between the groups. Fluctuations in PaCO2 outside the target range (35–50 mmHg) could be considered clinically relevant, as hypocarbia and hypercarbia has been reported when clinical methods are used for ventilation. By reducing fluctuations in PaCO2, we were able to maintain the left CABF in a narrow range in the capnography group (Figure 5).
Strengths and Limitations
This study was conducted in a model of perinatal asphyxia and lung disease (meconium aspiration) with severe respiratory acidosis, a condition where variations in cerebral and pulmonary blood flows can influence outcome. An asphyxiated model with cardiac arrest would have provided more information on the effect of fluctuation of CO2 on CBF. The use of hand bagging or T-piece resuscitator instead of ventilator for resuscitation may have yielded different results that are more pertinent to delivery room resuscitation. A wide target (35–50 mm Hg) for ETCO2 was a limitation and could have contributed to fluctuation in capnography group. Given the variability in ETCO2 values, choosing a narrow target range (35–40 mm Hg) could have further reduced fluctuation in the capnography group. A major limitation of the current study is that it was unblinded, and not randomized where the lambs were assigned to the group based on availability of capnography equipment in the laboratory animal-operating suite. Less frequent blood gas analysis (as seen in many clinical settings) may have yielded potentially increased fluctuation in cerebral blood flow in the control group leading to different results. Finally, we did not have access to volumetric data (tidal volume) in our capnograph instrument and access to this information may have helped us wean PIP and rate on the ventilator.
Potential role of monitoring ETCO2 during neonatal resuscitation in the DR
We speculate that the use of capnography while providing PPV through an endotracheal tube will limit fluctuations in PaCO2 and that maintaining normoxia may benefit asphyxiated, unstable term infants by avoiding fluctuations of CBF. This may potentially lead to better outcomes in infants at risk for hypoxic injury. In addition, capnography may also be able to measure the effectiveness of chest compressions and identify return of spontaneous circulation in patients with cardiac arrest [31]. It is also possible that in conditions with a marked increase in physiologic dead space, a low ETCO2 (with normal PaCO2) may lead to hypoventilation. Caution must be exercised while interpreting ETCO2 values during the first few minutes of resuscitation prior to establishment of lung aeration. Periodic blood gas analysis and trending with ETCO2 is recommended during prolonged resuscitation.
Conclusion
Based on our results, continuous end-tidal CO2 during and immediately after resuscitation is feasible and may limit fluctuations in PaCO2 and cerebral blood flow in an asphyxiated term infant with tracheal intubation. Further clinical and translational studies evaluating capnography with volumetric data during resuscitation and the post-resuscitation phases with neurodevelopmental follow-up are needed before advocating its routine use in clinical practice.
Acknowledgments
Funded by: American Academy of Pediatrics, Neonatal Resuscitation Program (SL) and 1R01HD072929-0 (SL)
References
- 1.Kattwinkel J, Perlman JM, Aziz K, Colby C, Fairchild K, Gallagher J, Hazinski MF, Halamek LP, Kumar P, Little G, McGowan JE, Nightengale B, Ramirez MM, Ringer S, Simon WM, Weiner GM, Wyckoff M, Zaichkin J. Part 15: Neonatal resuscitation: 2010 american heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S909–S919. doi: 10.1161/CIRCULATIONAHA.110.971119. [DOI] [PubMed] [Google Scholar]
- 2.Saugstad OD. Practical aspects of resuscitating asphyxiated newborn infants. Eur J Pediatr. 1998;157(Suppl 1):S11–S15. doi: 10.1007/pl00014284. [DOI] [PubMed] [Google Scholar]
- 3.Palme-Kilander C. Methods of resuscitation in low-apgar-score newborn infants--a national survey. Acta Paediatr. 1992;81:739–744. doi: 10.1111/j.1651-2227.1992.tb12094.x. [DOI] [PubMed] [Google Scholar]
- 4.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, Saugstad OD, Simeoni U, Speer CP, Halliday HL European Association of Perinatal M. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants - 2010 update. Neonatology. 2010;97:402–417. doi: 10.1159/000297773. [DOI] [PubMed] [Google Scholar]
- 5.Brugada M, Schilleman K, Witlox RS, Walther FJ, Vento M, Te Pas AB. Variability in the assessment of 'adequate' chest excursion during simulated neonatal resuscitation. Neonatology. 2011;100:99–104. doi: 10.1159/000322009. [DOI] [PubMed] [Google Scholar]
- 6.Poulton DA, Schmolzer GM, Morley CJ, Davis PG. Assessment of chest rise during mask ventilation of preterm infants in the delivery room. Resuscitation. 2011;82:175–179. doi: 10.1016/j.resuscitation.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Tracy M, Downe L, Holberton J. How safe is intermittent positive pressure ventilation in preterm babies ventilated from delivery to newborn intensive care unit? Arch Dis Child Fetal Neonatal Ed. 2004;89:F84–F87. doi: 10.1136/fn.89.1.F84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu CH, Chou HC, Hsieh WS, Chen WK, Huang PY, Tsao PN. Good estimation of arterial carbon dioxide by end-tidal carbon dioxide monitoring in the neonatal intensive care unit. Pediatr Pulmonol. 2003;35:292–295. doi: 10.1002/ppul.10260. [DOI] [PubMed] [Google Scholar]
- 9.Stiris T, Odden JP, Hansen TW, Hall C, Bratlid D. The effect of arterial pco2-variations on ocular and cerebral blood flow in the newborn piglet. Pediatr Res. 1989;25:205–208. doi: 10.1203/00006450-198902000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Schneider GH, Sarrafzadeh AS, Kiening KL, Bardt TF, Unterberg AW, Lanksch WR. Influence of hyperventilation on brain tissue-po2, pco2, and ph in patients with intracranial hypertension. Acta Neurochir Suppl. 1998;71:62–65. doi: 10.1007/978-3-7091-6475-4_20. [DOI] [PubMed] [Google Scholar]
- 11.Pappas A, Shankaran S, Laptook AR, Langer JC, Bara R, Ehrenkranz RA, Goldberg RN, Das A, Higgins RD, Tyson JE, Walsh MC Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. Hypocarbia and adverse outcome in neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2011;158:752–758. e751. doi: 10.1016/j.jpeds.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen G, Al Shafouri N, Narvey M, Vallance JK, Srinivasan G. High blood carbon dioxide variability and adverse outcomes in neonatal hypoxic ischemic encephalopathy. J Matern Fetal Neonatal Med. 2015:1–4. doi: 10.3109/14767058.2015.1015983. [DOI] [PubMed] [Google Scholar]
- 13.Kleinman ME, de Caen AR, Chameides L, Atkins DL, Berg RA, Berg MD, Bhanji F, Biarent D, Bingham R, Coovadia AH, Hazinski MF, Hickey RW, Nadkarni VM, Reis AG, Rodriguez-Nunez A, Tibballs J, Zaritsky AL, Zideman D, Pediatric B Advanced Life Support Chapter C. Part 10: Pediatric basic and advanced life support: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2010;122:S466–S515. doi: 10.1161/CIRCULATIONAHA.110.971093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rozycki HJ, Sysyn GD, Marshall MK, Malloy R, Wiswell TE. Mainstream end-tidal carbon dioxide monitoring in the neonatal intensive care unit. Pediatrics. 1998;101:648–653. doi: 10.1542/peds.101.4.648. [DOI] [PubMed] [Google Scholar]
- 15.Lakshminrusimha S, Mathew B, Nair J, Gugino SF, Koenigsknecht C, Rawat M, Nielsen L, Swartz DD. Tracheal suctioning improves gas exchange but not hemodynamics in asphyxiated lambs with meconium aspiration. Pediatr Res. 2015;77:347–355. doi: 10.1038/pr.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmolzer GM, Hooper SB, Wong C, Kamlin CO, Davis PG. Exhaled carbon dioxide in healthy term infants immediately after birth. J Pediatr. 2015 doi: 10.1016/j.jpeds.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 18.Hofer N, Jank K, Resch E, Urlesberger B, Reiterer F, Resch B. Meconium aspiration syndrome--a 21-years' experience from a tertiary care center and analysis of risk factors for predicting disease severity. Klin Padiatr. 2013;225:383–388. doi: 10.1055/s-0033-1361105. [DOI] [PubMed] [Google Scholar]
- 19.Espinheira MC, Grilo M, Rocha G, Guedes B, Guimaraes H. Meconium aspiration syndrome - the experience of a tertiary center. Rev Port Pneumol. 2011;17:71–76. [PubMed] [Google Scholar]
- 20.Saugstad OD. Delivery room management of term and preterm newly born infants. Neonatology. 2015;107:365–371. doi: 10.1159/000381159. [DOI] [PubMed] [Google Scholar]
- 21.Ilves P, Lintrop M, Metsvaht T, Vaher U, Talvik T. Cerebral blood-flow velocities in predicting outcome of asphyxiated newborn infants. Acta paediatrica. 2004;93:523–528. doi: 10.1080/08035250410024745. [DOI] [PubMed] [Google Scholar]
- 22.Saugstad OD International Liason Committee on R. New guidelines for newborn resuscitation--a critical evaluation. Acta Paediatr. 2011;100:1058–1062. doi: 10.1111/j.1651-2227.2011.02301.x. [DOI] [PubMed] [Google Scholar]
- 23.Solas AB, Kalous P, Saugstad OD. Reoxygenation with 100 or 21% oxygen after cerebral hypoxemia-ischemia-hypercapnia in newborn piglets. Biol Neonate. 2004;85:105–111. doi: 10.1159/000074966. [DOI] [PubMed] [Google Scholar]
- 24.Kro GA, Yli BM, Rasmussen S, Noren H, Amer-Wahlin I, Rosen KG, Stray-Pedersen B, Saugstad OD. Association between umbilical cord artery pco(2) and the apgar score; elevated levels of pco(2) may be beneficial for neonatal vitality after moderate acidemia. Acta Obstet Gynecol Scand. 2013;92:662–670. doi: 10.1111/aogs.12090. [DOI] [PubMed] [Google Scholar]
- 25.Wintermark P, Hansen A, Gregas MC, Soul J, Labrecque M, Robertson RL, Warfield SK. Brain perfusion in asphyxiated newborns treated with therapeutic hypothermia. AJNR American journal of neuroradiology. 2011;32:2023–2029. doi: 10.3174/ajnr.A2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engle WD, Laptook AR, Perlman JM. Acute changes in arterial carbon dioxide tension and acid-base status and early neurologic characteristics in term infants following perinatal asphyxia. Resuscitation. 1999;42:11–17. doi: 10.1016/s0300-9572(99)00081-7. [DOI] [PubMed] [Google Scholar]
- 27.Hansen NB, Brubakk AM, Bratlid D, Oh W, Stonestreet BS. The effects of variations in paco2 on brain blood flow and cardiac output in the newborn piglet. Pediatr Res. 1984;18:1132–1136. doi: 10.1203/00006450-198411000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Pryds O, Greisen G, Lou H, Friis-Hansen B. Vasoparalysis associated with brain damage in asphyxiated term infants. J Pediatr. 1990;117:119–125. doi: 10.1016/s0022-3476(05)72459-8. [DOI] [PubMed] [Google Scholar]
- 29.Hooper SB, Fouras A, Siew ML, Wallace MJ, Kitchen MJ, te Pas AB, Klingenberg C, Lewis RA, Davis PG, Morley CJ, Schmolzer GM. Expired co2 levels indicate degree of lung aeration at birth. PloS one. 2013;8:e70895. doi: 10.1371/journal.pone.0070895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wyatt JS, Edwards AD, Cope M, Delpy DT, McCormick DC, Potter A, Reynolds EO. Response of cerebral blood volume to changes in arterial carbon dioxide tension in preterm and term infants. Pediatr Res. 1991;29:553–557. doi: 10.1203/00006450-199106010-00007. [DOI] [PubMed] [Google Scholar]
- 31.Chalak LF, Barber CA, Hynan L, Garcia D, Christie L, Wyckoff MH. End-tidal co(2) detection of an audible heart rate during neonatal cardiopulmonary resuscitation after asystole in asphyxiated piglets. Pediatr Res. 2011;69:401–405. doi: 10.1203/PDR.0b013e3182125f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]