Abstract
While electrospun nanofibers have demonstrated the potential for novel tissue engineering scaffolds, very little is known about the molecular mechanism of how cells sense and adapt to nanofibers. Here, we revealed the role of focal adhesion kinase (FAK), one of the key molecular sensors in the focal adhesion complex, in regulating mesenchymal stem cell (MSC) shaping on nanofibers. We produced uniaxially aligned and randomly distributed nanofibers from poly(L-lactic acid) to have the same diameters (about 130 nm) and evaluated MSC behavior on these nanofibers comparing with that on flat PLLA control. C3H10T1/2 murine MSCs exhibited upregulations in FAK expression and phosphorylation (pY397) on nanofibrous cultures as assessed by immunoblotting, and this trend was even greater on aligned nanofibers. MSCs showed significantly elongated and well-spread morphologies on aligned and random nanofibers, respectively. In the presence of FAK silencing via small hairpin RNA (shRNA), cell elongation length in the aligned nanofiber direction (cell major axis length) was significantly decreased, while cells still showed preferred orientation along the aligned nanofibers. On random nanofibers, MSCs with FAK-shRNA showed impaired cell spreading resulting in smaller cell area and higher circularity. Our study provides new data on how MSCs shape their morphologies on aligned and random nanofibrous cultures potentially via FAK-mediated mechanism.
Keywords: Aligned and random nanofibers, Mesenchymal stem cells, Cell alignment and spreading, Focal adhesion kinase, shRNA
1. Introduction
Successful tissue engineering scaffolds should meet several requirements on cytocompatibility, mechanical strength, porous architecture, degradation property, etc. To facilitate intracellular response and intercellular interaction in the scaffolds, scaffolds may better mimic in vivo extracellular matrix (ECM) environments. Nanofibers may be ideal for this goal considering their advantages of biomimicking ECM nanofilamentary architecture, high surface area-to-volume ratio, controllable mechanical property and porous structure, and a 3D environment for regulating cell-scaffold and cell-cell interaction [1].
Electrospinning has attracted a great deal of attention due to its capability to produce fibers of nanoscale diameter rapidly and cost-effectively from many raw materials. Our group’s effort on electrospinning has helped to open up broad applications of nanofibers from advanced supercomposites with nanofiber-reinforced interfaces to biomedical applications [2-4]. Many other studies have also shown that ECM-like nanofibers with tailored nanofiber architecture (diameter, aligned or random orientation, pore size, porosity), controlled degradability, and immobilized soluble signal can provide desired cell stimulatory cues to promote cell adhesion, orientation, proliferation, differentiation, and tissue formation [5,6]. Exploiting these advantages, electrospun nanofibers have been tested for engineering bone tissue [7,8], wound dressing [9,10], artificial vessel formation [11], neural tissue regeneration [12,13], and vehicles for drug delivery [14,15] and biomolecular transport [16].
Despite the successes of the nanofiber-based tissue engineering, very little is known about molecular mechanisms that govern cell-nanofiber interaction. We recently tested potential mechanism of cell-nanofiber interaction based on the hypothesis that RhoA kinase (ROCK), one of the key cytoskeletal tension signaling molecules, plays a role in controlling cell alignment on nanofibers [17]. The other key cellular mechanical structure, e.g., focal adhesion, may also play a vital role in cell-nanofiber interaction. In this study, we revealed the role of focal adhesion kinase (FAK), one of the key mechanistic sensors constituting the focal adhesion complex, in MSC morphology formation on nanofibers. FAK expression and phosphorylation in MSCs on aligned and random nanofibers were assessed relative to flat control. Then, the effects of FAK silencing on MSC alignment and spreading on test substrates were examined and compared with vector control. Our data on the FAK regulation in MSC-nanofiber interaction may provide an improved understanding on how MSCs sense and adapt to nanofibrous cultures.
2. Materials and methods
2.1. Aligned and random nanofiber fabrication and characterization
Unidirectionally aligned and randomly distributed nanofibers were fabricated from poly(L-lactic acid) (PLLA, Mw = 152,000, Sigma-Aldrich) by electrospinning. Polymer solution was prepared by dissolving PLLA in dichloromethane (DCM)/n,n-dimethylformamide (DMF) (70/30) at 4% w/w. The solution was loaded into a 1 ml syringe with a 30-gauge needle and dispensed at 1 ml/h with a syringe pump. A voltage of 18 kV was applied using a DC power supply to generate the polymer jet. The electrospinning distance was fixed at 15 cm. For producing aligned nanofibers, a rotating collection disk was used (rotating at 1000 rpm). For random nanofibers, a flat stationary plate was used for collection. Both aligned and random nanofibers were collected on spin-cast PLLA films formed on 18 mm dia. round glass coverslips. Spin-casting of PLLA films was conducted at 4000 rpm for 25 sec using a 1% w/w PLLA solution in chloroform. Spin-cast PLLA films were also used as flat controls in cell culture assays. For scanning electron microscopy (SEM), nanofibers were coated with platinum/palladium using the sputter coater (Ted Pella) and observed by Quanta 200F SEM (FEI). Nanofiber diameter was measured from SEM images with ImageJ software.
2.2. Cell culture
C3H10T1/2 murine MSCs (ATCC, CCL-226) were cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Cells were seeded on test substrata (flat control, aligned and random nanofibers) using the growth media at 1×104 cells/cm2 and kept at 37°C and 5% CO2. Surfaces were not precoated with ECM proteins. Prior to cell culture, surfaces were treated with UV light for 2 h for sterilization.
2.3. Immunoblotting of FAK and phosphorylated FAK
Western blotting was used to assess FAK expression and phosphorylation. Methods followed our published protocols [17,18]. Cells were cultured for 24 h on test surfaces, washed with phosphate buffered saline (PBS), and harvested with 0.25% trypsin/ethylenediaminetetraacetic acid. The cells were centrifuged at 2000 rpm for 5 min, and then lysed with protein lysis buffer. Equal amounts of lysates underwent sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the proteins were transferred onto the nitrocellulose membrane. The membrane was blocked with 5% non-fat dry milk for 1 h, and then incubated at 4°C overnight with FAK antibody (Cell Signaling, 3285S) and phosphorylated FAK (pY397) antibody (BD Biosciences, 611806). The membrane was washed and then incubated with horseradish peroxidase-conjugated secondary antibody (Jackson Lab). Immuno-positive bands were detected by enhanced chemiluminescence. The band strength was quantified by ImageJ and normalized with the loading control, GAPDH. Tests were repeated three times.
2.4. Silencing FAK via shRNA
MSCs with stable FAK knockdown were produced by small hairpin RNA (shRNA). Detailed methodologies were described in our previous reports [19,20]. Briefly, FAK-shRNA plasmids (Santa Cruz, sc-35353-SH) were transfected into cells with transfection reagent. After exposing to the selection media having 2 μg/ml puromycin, cells with puromycin resistance were chosen for further passaging to establish cells with stable FAK knockdown. To produce vector control, the same process was repeated but with control shRNA plasmid (Santa Cruz, sc-108060).
2.5. Cell morphology measurements
Cell morphologies on test substrates without or with FAK-shRNA were quantified from SEM images using ImageJ. After 24 h of culturing, cells were fixed with 2.5% glutaraldehyde solution in PBS. Fixed cells were dehydrated by serially treating with ethanol/distilled water mixtures at 50, 80, 90, 95, 98, and 100% volumes of ethanol each continued for 10 min. After drying, cells were coated with platinum/palladium and imaged with SEM. Protocols for quantifying cell morphologies (major and minor axis lengths, area, circularity) and orientation angle followed those described in our previous work [17].
2.6. Statistics
Statistical analyses were completed by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc tests. The statistical significance is presented in the figures.
3. Results
3.1. Cell response is assessed on aligned and random nanofibers at the same nanofiber diameter and compared with flat control all under the same surface chemistry
SEM images of spin-cast PLLA flat control film and PLLA aligned and random nanofibers formed on the PLLA flat film are shown in Fig. 1. By tuning electrospinning conditions, we could successfully produce continuous nanofibers with desired orientation and diameter. To test the effects of nanofiber orientation (aligned, random), the other parameters were controlled to be the same. The diameters of aligned and random nanofibers were 128.4±34.7 nm and 126.7±32.6 nm, respectively. For aligned nanofibers, fiber orientation became disordered when the collection time was longer than 30 min, which may be due to the residual charge on collected fibers (as we published [21]). The collection time was adjusted not to exceed this time. Fabricating aligned and random nanofibers on spin-cast PLLA films improved the adhesion of electrospun fibers to the basal substrate. More importantly, this allowed to test the effects of nanofiber architectures in comparison with flat control all under the same surface chemistry of PLLA.
Fig. 1.
Aligned and random nanofibers were produced from PLLA to have the same diameter along with flat PLLA control. SEM images of spin-cast PLLA flat control and uniaxially aligned and randomly distributed PLLA nanofibers formed on spin-cast PLLA film.
3.2. FAK expression and phosphorylation in MSCs are increased on aligned and random nanofibers
We assessed FAK expression and phosphorylation at the auto-phosphorylation site (pY397) by immunoblotting (Fig. 2). The data after normalization showed that total FAK expression was increased for MSCs cultured on aligned nanofibers relative to flat control (*: p < 0.05). Cells on random nanofibers also showed increased FAK but did not reach statistical significance. FAK phosphorylation (p-FAK) at pY397 was significantly increased for MSCs on both aligned and random nanofibers compared with flat control (**: p < 0.01). The increase in p-FAK was greater on aligned nanofibers relative to random nanofibers (#: p < 0.05).
Fig. 2.
FAK expression and phosphorylation in MSCs were increased on aligned and random nanofibrous cultures. Immunoblotting of total FAK and FAK phosphorylation (p-FAK) at pY397 for C3H10T1/2 MSCs cultured on test substrates. The immuno-reactive band strengths were quantified and normalized to GAPDH. Results were compared with the flat control set at 1. Means and standard deviations (n = 3) are shown. *: p < 0.05 and **: p < 0.01 compared with flat control. #: p < 0.05 between aligned and random nanofibers.
3.3. MSCs display elongated and well-spread morphologies on aligned and random nanofibers, respectively
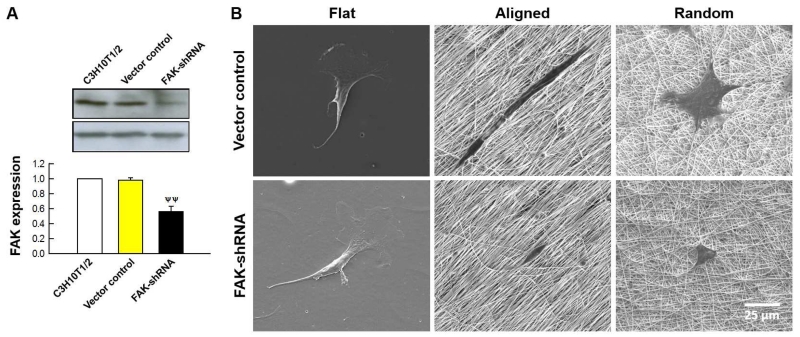
Fig 3B upper panels show examples of SEM images of MSCs seeded for 24 h on test surfaces. On aligned nanofibers, MSC vector control exhibited contact guidance, preferred cell orientation and elongation along the aligned nanofiber direction. Cells on random nanofibers also showed nanofiber-guided elongations but in random directions following local nanofiber orientations. There was no preferred cell orientation on the flat control, in which both filopodia extension and lamellipodia spreading were seen.
Fig. 3.
MSCs displayed elongated and well-spread morphologies on aligned and random nanofibers, respectively, which trend was suppressed by FAK-shRNA. (A) FAK-silenced MSCs were produced by shRNA (immunoblotting, ψψ: p < 0.01 with vector control). Reprinted from our previous publication [19] with permission from Elsevier. (B) SEM images of vector control and MSCs with FAK-shRNA cultured on test substrates.
3.4. FAK-shRNA suppresses MSC elongation on aligned nanofibers and spreading on random nanofibers
To further test FAK involvement in MSC-nanofiber interaction, MSCs with silenced FAK were established via shRNA. As in Fig. 3A, MSCs with FAK-shRNA showed significantly reduced FAK expression relative to parent cells and vector control. In contrast to small interference RNA (siRNA) that produces only transient silencing, shRNA provides sustainable knockdown and less off-target effect via endogenous machinery [22]. We confirmed sustainable interference after subcultures (not shown here).
SEM images of MSCs with FAK-shRNA are shown in Fig 3B lower panels. Most noticeably, MSCs with FAK-shRNA had less elongated morphology on aligned nanofibers relative to its counterpart (vector control). Also, MSCs with FAK-shRNA showed less cell spreading on random nanofibers. These changes in cell morphologies were quantified in Fig. 4A. For vector control, cell elongation on aligned nanofibers resulted in significantly larger cell major axis length, smaller minor axis length, smaller area, and lower circularity in comparisons with both flat control (**: p < 0.01) and random nanofibers (##: p < 0.01). FAK-shRNA induced significant changes in cell morphologies (marked as ψψ: p < 0.01 compared with vector control). Major axis lengths were significantly reduced on both aligned and random nanofibers in the presence of FAK-shRNA. Decreases in minor axis length and cell area by FAK-shRNA were significant on random nanofibers. These changes resulted in a significant increase in circularity on random nanofibers for FAK-shRNA.
Fig. 4.
In the presence of FAK-shRNA, cell major axis length on aligned nanofibers was significantly decreased (while contact-guided cell orientation was still seen) and cell spreading on random nanofibers was impaired. (A) Quantified cell morphologies for vector control and MSCs with FAK-shRNA cultured on test substrates. Methods for quantifying cell morphologies followed our published protocols [17]. Briefly, major axis length was obtained as the longest cell length and minor axis length as the length perpendicular to the major axis length. Circularity, defined as 4πA/p2 where A is a cell area and p is a perimeter, becomes close to 1 for a more circular cell. Means and standard error of measurements are shown (n = 105-251 total cell measurements in each case). Among vector controls, comparison with flat control is shown as **: p < 0.01 and comparison between aligned and random nanofibers as ##: p < 0.01. Comparisons between vector control and FAK-shRNA on each test substrates are shown with ψψ: p < 0.01. (B) Histograms of cell orientation angles. Contact-guided cell orientation was clearly seen for the vector control on aligned nanofibers. Even with FAK-shRNA, cells showed similar orientation angle histogram on aligned nanofibers as that of the vector control. On flat control and random nanofibers, random cell orientation was observed for both vector control and FAK-shRNA.
Histograms of cell orientation angles quantified relative to the nanofiber direction are shown in Fig. 4B. For flat control and random nanofibers arbitrary direction was set as 0°, while for aligned nanofibers 0° was set along the longitudinal direction of the aligned nanofibers. Then, the angle with the cell major axis was obtained. Contact-guided cell orientation by aligned nanofibers is clearly seen for vector control. Notably, even with FAK-shRNA cells still exhibited nanofiber-guided orientation producing very similar orientation angle distribution as that of the vector control (although the cell major axis length on aligned nanofibers was significantly reduced by FAK-shRNA as in Fig. 4A). On flat control and random nanofibers, both vector control and FAK-shRNA showed random cell orientation angles.
4. Discussion
Since nanofibers can be fabricated to mimic the fibrous components of the native ECM, they may provide biomimetic cues essential for effectively constructing cell morphology and organization. Understanding underlying molecular mechanisms that govern cell adaptation to biomimicry parameters of nanofibers (aligned vs. random, diameter, porous structure, etc.) may help design new and improved nanofiber scaffolds. Based on this rationale, this study aimed to reveal the role of focal adhesion signaling, FAK, in MSC shaping on aligned and random nanofibers.
Anchorage-dependent cells adhere to ECM via focal adhesion complex. Various linker proteins including FAK, vinculin, paxillin, talin, etc. participate in the focal adhesion complex as physical connectors when ECM-bound integrins are linked to cytoskeletons. Such physical linker proteins can also behave as signaling moderators. Specifically, FAK, a tyrosine-phosphorylated kinase, has been proposed to play a vital role in cell adhesion and spreading and in the signal transduction generated by focal adhesion, thus modulating downstream cell functions such as gene expression, proliferation, survival, differentiation, and motility [23-25]. In our previous study utilizing randomly distributed nanopit topographies [18], we demonstrated that FAK may be involved in cell-nanotopography interaction. We showed that FAK expression and pY397 phosphorylation were increased for osteoblastic cells cultured on nanopit textures with specific pit depths (ca. 10-20 nm) compared with flat control.
Here, we tested the role of FAK in the nanofiber control of MSCs. MSCs displayed elongated and well-spread morphologies on aligned and random nanofibers, respectively, in contrast to flat control (Fig. 3B). This was observed at the same nanofiber diameter (about 130 nm) for both nanofibers and under the same surface chemistry (PLLA) for all three test surfaces. FAK expression and phosphorylation showed increasing trends for MSCs cultured on nanofibers (Fig. 2), suggesting that FAK may mediate MSC-nanofiber interaction. Similar but slightly different results were recently reported for skin fibroblasts [26]. When fibroblasts were cultured on polycaprolactone (PCL) nanofibers, cells showed increased FAK phosphorylation (pY397) on aligned nanofibers relative to random nanofibers, but the total FAK expression was not changed on two nanofibers. Both nanofibers had about 300 nm diameters, but results were not compared with flat control. Another study reported the effect of nanofiber diameter on FAK activation [27]. When osteoblastic MG63 cells were cultured on gelatin nanofibers with small (110 nm) and large (600 nm) diameters, both random nanofibers, cells seeded on small diameter nanofibers showed noticeable increases in FAK expression and activation relative to large diameter nanofibers. However, aligned nanofibers were not tested and results were not compared with flat control. In contrast to these reports demonstrating positive correlation between nanofibrous culture and FAK (including our current study), one study reported an opposite result. Submandibular salivary gland ductal epithelial cells seeded on poly-l-lactic-co-glycolic acid (PLGA) random nanofibers (250 nm diameter) showed diffused and decreased focal adhesion formation and phosphorylation relative to flat control as assessed by immunofluorescence and immunoblotting [28]. The difference might originate from the cell type, but with limited number of reports on this topic it is difficult to draw a conclusion due to other differences in biomaterial type, nanofiber architecture, and diameter.
It is also noteworthy that some other studies reported FAK upregulation with nanofibers but only under the aid of additional stimulatory cues. For example, cells on nanofibers could display increased FAK activation when nanofibers were further supplemented with carbon nanotubes [29], coated with polydopamine [30], or when bone morphogenetic protein (BMP) soluble signal was added [31]. These results, in combination with ours, suggest that nanofibers may be used to trigger focal adhesion signaling via potential intrinsic effects from nanofiber architecture and also with added chemical or soluble signals.
Our study is as far as we know the first to assess FAK expression and phosphorylation in MSCs using aligned and random nanofibers at the same diameter with appropriate flat control under the same surface chemistry. Our immunoblotting data quantitatively evidenced FAK activity upregulation via nanofibrous cultures. We further demonstrated the role of FAK in MSC-nanofiber interaction via FAK-shRNA. On aligned nanofibers, FAK-shRNA led to a significant reduction in cell major axis length relative to the vector control. On random nanofibers, FAK-shRNA induced significant decreases in both major and minor axis lengths resulting in smaller cell area and higher circularity. The observations that MSCs with interfered FAK displayed significantly impaired responses in nanofiber-guided cell elongation and spreading suggest that FAK may play a vital role in MSC sensing and adaptation to nanofibrous cultures. Relevant studies referenced above [26-31] measured FAK expressions on nanofibers, but did not test the role of FAK via interference methods.
Even with FAK-shRNA, MSCs still showed preferred cell orientation along the aligned nanofiber direction (cell orientation angle histograms, Fig. 4B). This might be partly correlated with the FAK silencing via shRNA which produced about 50% silencing (Fig. 3A). A future study may adopt a complete elimination using the recently developed CRISPR-Cas9 knock-out [32]. However, since FAK may be critically required for cell survival and growth [23-25], CRISPR-Cas9 knock-out might not fully guarantee MSC growth on nanofibers. While MSCs with FAK-shRNA still displayed nanofiber-guided orientation, FAK silencing effect could be detected with significantly decreased major axis length (Fig. 4A) as described above.
Beyond cell shaping, focal adhesion signaling governed by nanofibers may affect downstream behaviors including migration, growth, differentiation, etc. On polystyrene (PS) nanofibers, C2C12 myoblasts showed increased migration speed when having a spindle cell shape on aligned nanofibers, which was attributed to increased focal adhesion formation assessed by paxillin immunofluorescence (FAK was not tested) [33]. In our recent study (on plain glass slides) [20], FAK played a regulatory role in MSC migration under flow, e.g., fluid shear-induced MSC migration was significantly suppressed by FAK-shRNA possibly due to decreased focal adhesion turnover required for migration. In the study on the nanofiber diameter [27], osteogenic differentiation by MG63 cells had positive correlation with nanofiber-induced FAK activation. Our previous study also showed that FAK may be critically required for MSC commitment such as adipogenesis [19]. Combined, the question of how FAK activation in MSCs modulated by nanofibrous cultures affects their migration, proliferation, and lineage commitment and differentiation will be the research topics of interest.
In conclusion, to reveal the FAK regulation in MSC alignment and spreading on nanofibers, we produced aligned and random PLLA nanofibers with the same average diameters and assessed cell behavior in comparison with flat PLLA control. MSCs exhibited upregulated FAK expression and pY397 phosphorylation with nanofibrous cultures. With FAK-shRNA, cell major axis length along the aligned nanofiber direction was significantly decreased, while MSCs still showed preferred orientation along the aligned nanofibers. FAK-shRNA also impaired MSC spreading on random nanofibers, resulting in lower cell area and higher circularity. Our results may provide an improved insight into how MSCs shape their morphologies on nanofibrous cultures, demonstrating a potential importance of the FAK-mediated mechanism in MSC-nanofiber interaction.
Supplementary Material
Highlights.
Use aligned and random nanofibers at the same fiber diameter with proper flat control
FAK expression and phosphorylation are increased by aligned and random nanofibers
Elongated and well-spread morphologies on aligned and random nanofibers, respectively
FAK-shRNA suppresses MSC elongation and spreading on nanofibrous cultures
Acknowledgments
Funding supports from NSF (DMR-1310534, CMMI-1463636), NIH (1R01HL125736-01; MPI: Kamenskiy/MacTaggart), and ONR (N000141410663) (all to YD); NSF CAREER Award 1351570, Osteology Foundation Grant 12-006, NE DHHS Stem Cell Project 2015-06, Nebraska Research Initiative, and Nebraska Tobacco Biomedical Seed Grant (all to JYL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure. None.
References
- [1].Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv. Drug. Deliv. Rev. 2007;59:1413–1433. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- [2].Dzenis Y. Material science. Spinning continuous fibers for nanotechnology. Science. 2004;304:1917–1919. doi: 10.1126/science.1099074. [DOI] [PubMed] [Google Scholar]
- [3].Chew SY, Wen Y, Dzenis Y, Leong KW. The role of electrospinning in the emerging field of nanomedicine. Curr. Pharm. Des. 2006;12:4751–4770. doi: 10.2174/138161206779026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dzenis Y. Materials science. Structural nanocomposites. Science. 2008;319:419–420. doi: 10.1126/science.1151434. [DOI] [PubMed] [Google Scholar]
- [5].Teo W, He W, Ramakrishna S. Electrospun scaffold tailored for tissue-specific extracellular matrix. Biotechnol. J. 2006;1:918–929. doi: 10.1002/biot.200600044. [DOI] [PubMed] [Google Scholar]
- [6].Lim SH, Mao H. Electrospun scaffolds for stem cell engineering. Adv. Drug. Deliv. Rev. 2009;61:1084–1096. doi: 10.1016/j.addr.2009.07.011. [DOI] [PubMed] [Google Scholar]
- [7].Schofer MD, Roessler PP, Schaefer J, Theisen C, Schlimme S, Heverhagen JT, Voelker M, Dersch R, Agarwal S, Fuchs-Winkelmann S, Paletta JR. Electrospun PLLA nanofiber scaffolds and their use in combination with BMP-2 for reconstruction of bone defects. PLoS One. 2011;6:e25462. doi: 10.1371/journal.pone.0025462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Seyedjafari E, Soleimani M, Ghaemi N, Shabani I. Nanohydroxyapatite-coated electrospun poly(l-lactide) nanofibers enhance osteogenic differentiation of stem cells and induce ectopic bone formation. Biomacromolecules. 2010;11:3118–3125. doi: 10.1021/bm1009238. [DOI] [PubMed] [Google Scholar]
- [9].Kumbar SG, Nukavarapu SP, James R, Nair LS, Laurencin CT. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials. 2008;29:4100–4107. doi: 10.1016/j.biomaterials.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhu X, Cui W, Li X, Jin Y. Electrospun fibrous mats with high porosity as potential scaffolds for skin tissue engineering. Biomacromolecules. 2008;9:1795–1801. doi: 10.1021/bm800476u. [DOI] [PubMed] [Google Scholar]
- [11].Hajiali H, Shahgasempour S, Naimi-Jamal MR, Peirovi H. Electrospun PGA/gelatin nanofibrous scaffolds and their potential application in vascular tissue engineering. Int. J. Nanomedicine. 2011;6:2133–2141. doi: 10.2147/IJN.S24312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- [13].Xie J, MacEwan MR, Schwartz AG, Xia Y. Electrospun nanofibers for neural tissue engineering. Nanoscale. 2010;2:35–44. doi: 10.1039/b9nr00243j. [DOI] [PubMed] [Google Scholar]
- [14].Sill TJ, von Recum HA. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- [15].Yoon H, Kim G. A three-dimensional polycaprolactone scaffold combined with a drug delivery system consisting of electrospun nanofibers. J. Pharm. Sci. 2011;100:424–430. doi: 10.1002/jps.22310. [DOI] [PubMed] [Google Scholar]
- [16].Ji W, Sun Y, Yang F, van den Beucken JJ, Fan M, Chen Z, Jansen JA. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011;28:1259–1272. doi: 10.1007/s11095-010-0320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Andalib MN, Lee JS, Ha L, Dzenis Y, Lim JY. The role of RhoA kinase (ROCK) in cell alignment on nanofibers. Acta Biomater. 2013;9:7737–7745. doi: 10.1016/j.actbio.2013.04.013. [DOI] [PubMed] [Google Scholar]
- [18].Lim JY, Dreiss AD, Zhou Z, Hansen JC, Siedlecki CA, Hengstebeck RW, Cheng J, Winograd N, Donahue HJ. The regulation of integrin-mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography. Biomaterials. 2007;28:1787–1797. doi: 10.1016/j.biomaterials.2006.12.020. [DOI] [PubMed] [Google Scholar]
- [19].Lee JS, Ha L, Kwon IK, Lim JY. The role of focal adhesion kinase in BMP4 induction of mesenchymal stem cell adipogenesis. Biochem. Biophys. Res. Commun. 2013;435:696–701. doi: 10.1016/j.bbrc.2013.05.045. [DOI] [PubMed] [Google Scholar]
- [20].Riehl BD, Lee JS, Ha L, Lim JY. Fluid-flow-induced mesenchymal stem cell migration: role of focal adhesion kinase and RhoA kinase sensors. J. R. Soc. Interface. 2015;12:20141351. doi: 10.1098/rsif.2014.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu L, Dzenis YA. Analysis of the effects of the residual charge and gap size on electrospun nanofiber alignment in a gap method. Nanotechnology. 2008;19:355307. doi: 10.1088/0957-4484/19/35/355307. [DOI] [PubMed] [Google Scholar]
- [22].Rao DD, Vorhies JS, Senzer N, Nemunaitis J. siRNA vs. shRNA: similarities and differences. Adv. Drug. Deliv. Rev. 2009;61:746–759. doi: 10.1016/j.addr.2009.04.004. [DOI] [PubMed] [Google Scholar]
- [23].Tomakidi P, Schulz S, Proksch S, Weber W, Steinberg T. Focal adhesion kinase (FAK) perspectives in mechanobiology: implications for cell behaviour. Cell Tissue Res. 2014;357:515–526. doi: 10.1007/s00441-014-1945-2. [DOI] [PubMed] [Google Scholar]
- [24].Cox BD, Natarajan M, Stettner MR, Gladson CL. New concepts regarding focal adhesion kinase promotion of cell migration and proliferation. J. Cell. Biochem. 2006;99:35–52. doi: 10.1002/jcb.20956. [DOI] [PubMed] [Google Scholar]
- [25].Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- [26].Huang C, Fu X, Liu J, Qi Y, Li S, Wang H. The involvement of integrin β1 signaling in the migration and myofibroblastic differentiation of skin fibroblasts on anisotropic collagen-containing nanofibers. Biomaterials. 2012;33:1791–1800. doi: 10.1016/j.biomaterials.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sisson K, Zhang C, Farach-Carson MC, Chase DB, Rabolt JF. Fiber diameters control osteoblastic cell migration and differentiation in electrospun gelatin. J. Biomed. Mater. Res. A. 2010;94:1312–1320. doi: 10.1002/jbm.a.32756. [DOI] [PubMed] [Google Scholar]
- [28].Sequeira SJ, Soscia DA, Oztan B, Mosier AP, Jean-Gilles R, Gadre A, Cady NC, Yener B, Castracane J, Larsen M. The regulation of focal adhesion complex formation and salivary gland epithelial cell organization by nanofibrous PLGA scaffolds. Biomaterials. 2012;33:3175–3186. doi: 10.1016/j.biomaterials.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ostrovidov S, Shi X, Zhang L, Liang X, Kim SB, Fujie T, Ramalingam T,M, Chen M, Nakajima K, Al-Hazmi F, Bae H, Memic A, Khademhosseini A. Myotube formation on gelatin nanofibers - multi-walled carbon nanotubes hybrid scaffolds. Biomaterials. 2014;35:6268–6277. doi: 10.1016/j.biomaterials.2014.04.021. [DOI] [PubMed] [Google Scholar]
- [30].Lin CC, Fu SJ. Osteogenesis of human adipose-derived stem cells on poly(dopamine)-coated electrospun poly(lactic acid) fiber mats. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016;58:254–263. doi: 10.1016/j.msec.2015.08.009. [DOI] [PubMed] [Google Scholar]
- [31].Schofer MD, Veltum A, Theisen C, Chen F, Agarwal S, Fuchs-Winkelmann S, Paletta JR. Functionalisation of PLLA nanofiber scaffolds using a possible cooperative effect between collagen type I and BMP-2: impact on growth and osteogenic differentiation of human mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2011;22:1753–1762. doi: 10.1007/s10856-011-4341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sheets K, Wunsch S, Ng C, Nain AS. Shape-dependent cell migration and focal adhesion organization on suspended and aligned nanofiber scaffolds. Acta Biomater. 2013;9:7169–7177. doi: 10.1016/j.actbio.2013.03.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.