Abstract
The photoelectrochemical (PEC) water splitting performance of BiVO4 is partially hindered by insufficient photoresponse in the spectral region with energy below the band gap. Here, we demonstrate that the PEC water splitting efficiency of BiVO4 electrodes can be effectively enhanced by decorating Pd nanoparticles (NPs) and nanorods (NRs). The results indicate that the Pd NPs and NRs with different surface plasmon resonance (SPR) features delivered an enhanced PEC water splitting performance in the visible and near-infrared (NIR) regions, respectively. Considering that there is barely no absorption overlap between Pd nanostructures and BiVO4 and the finite-difference time domain (FDTD) simulation indicating there are substantial energetic hot electrons in the vicinity of Pd nanostructures, the enhanced PEC performance of Pd NP-decorated BiVO4 and Pd NR-decorated BiVO4 could both benefit from the hot electron injection mechanism instead of the plasmon resonance energy transfer process. Moreover, a combination of Pd NPs and NRs decorated on the BiVO4 electrodes leads to a broad-band enhancement across visible-NIR region.
Keywords: Plasmonic Pd, BiVO4, Photoelectrochemical water splitting, Surface plasmon resonance
Background
Solar hydrogen generation through photoelectrochemical (PEC) water splitting offers an efficient and sustainable solution to the global energy problem [1–5]. Recently, BiVO4 has emerged as a promising material for PEC water splitting due to its photoactivity in visible light region [6]. However, BiVO4 photoanodes suffer from rapid charge carrier recombination, slow water oxidation kinetics, and insufficient photoresponse in the spectral region with energy below the band gap of 2.4 eV [6], which limit its water splitting efficiency. Therefore, strategies such as doping [7–12], nanostructuring [13–18], and loading of oxygen evolution catalysts (OECs) [7, 9, 19, 20] have been adopted to improve the water splitting efficiency of BiVO4.
As reported, several dopants, such as W, Mo, and P, are reported to improve the PEC performance of BiVO4 [7–12]. Doped BiVO4 exhibits the improved carrier density, enhanced conductivity, or even increased hole diffusion length, thus resulting in enhanced PEC properties. Furthermore, the short diffusion length of photoexcited charge carriers is the main reason for the dominant electron-hole recombination in the bulk of BiVO4. To address this issue, the diffusion length for charge carriers can be shortened by nanostructuring, thereby reducing bulk recombination [13–18]. To increase the water oxidation kinetics, research efforts have been placed on the loading of the OECs on BiVO4 [7, 9, 19, 20]. Among these OECs, the Co-Pi and FeOOH catalysts can lead to a negative-shift of onset potentials of water oxidation and effectively enhance the magnitude of photocurrent [7, 9, 19]. However, through these strategies, the enhanced photoactivity of BiVO4 has only been achieved in the spectral region with energy above the band gap. To improve the PEC water splitting efficiency of BiVO4 in the spectral region with energy below the band gap or even in the near-infrared (NIR) region, whose energy accounts for 56.3 % of that of the solar spectrum [21], still remains a big challenge.
Recently, a new approach involving metal nanostructures in enhancing the photoactivity of TiO2 in the spectral region with energy below the band gap via plasmonic effect has received much attention [22–24]. Surface plasmon resonance (SPR) is an intrinsic property of metal nanostructures, in which the oscillation frequency is highly sensitive to their shape and size of the metal nanostructures as well as the dielectric constant of the surrounding environment [25–30]. The plasmonic metal nanostructures localize the optical energy by SPR and enhance the photoactivity of semiconductors through either near-field electromagnetic enhancement or hot electron injection [22–24]. For example, Hsu et al. reported that the performance of Au nanostructure-decorated TiO2 nanowires for PEC water splitting was enhanced across entire UV-visible region [23]. Although an enhancement in the PEC water splitting efficiency was also observed on BiVO4 electrodes with plasmonic metal nanostructures, such as Au and Ag [31–33], there are no reports that the photoactivity of BiVO4 can be improved in the spectral region with energy below the band gap or even in the NIR region by exploiting plasmonic metal nanostructures.
This work reports that the enhancement of PEC water splitting efficiency can be effectively extended into the visible-NIR region by the combination of Pd nanoparticles (NPs) and nanorods (NRs) with BiVO4 electrodes. The mechanisms of activity enhancement both in the visible and NIR regions have been discussed.
Methods
Preparation of BiVO4 Electrodes
The BiVO4 electrodes were prepared as previously reported procedure [19]. Solutions for electrodeposition were prepared by dissolving 10 mM Bi(NO3)3 (98 %, Alfa Aesar) in a solution of 35 mM VOSO4 (97 %, Sigma Aldrich) at pH < 0.5 with HNO3 (65 %, Acros Organics). Then 2 M CH3COONa (≥99.0 %, Alfa Aesar) was added, raising the pH to ∼5.1, which was then adjusted to pH 4.7 with a few drops of HNO3. Acetate serves to stabilize other insoluble Bi (III) ions at pH 4.7. This mildly acidic pH condition must be used because at pH lower than 2 where Bi(III) is soluble, no film can be formed while at pH higher than 5, V (IV) precipitates from solution. A three-electrode cell was used for electrodeposition, with an fluorine-doped tin oxide (FTO, 8 Ω/□, Hartford Glass Co.) coated glass substrate as working electrode, a Ag/AgCl (4 M KCl) as reference electrode and a platinum foil as counter electrode. A potentiostat (Sloartron SI 1287) was used for electrodeposition. Deposition of amorphous Bi–V–O films was carried out potentiostatically at 1.9 V vs Ag/AgCl (4 M KCl) for 5 min at 70 °C (ca. 2 mA cm−2). The as-deposited films were converted to crystalline BiVO4 and amorphous V2O5 by annealing at 500 °C for 1 h in air, and pure BiVO4 was achieved by dissolving the V2O5 in 1 M KOH under stirring for 20 min.
Synthesis of Pd NPs and NRs
A simple but very efficient method that uses CO as reducing agent to synthesize Pd NPs and NRs has been developed and will be reported elsewhere. In brief, before the synthesis, 80 mM/L NaCl (≥99.0 %, Sigma Aldrich) and 40 mM/L PdCl2 (≥99.9 %, Sigma Aldrich) was dissolved in 50 mL CH3OH (≥99.9 %, Sigma Aldrich) to form Na2PdCl4 for preparation. Then, a flask was added 210-mg polyvinylpyrrolidone (K 30, average Mw 40,000, Sigma Aldrich) and 100 mL of 3.876 mM Na2PdCl4. To produce Pd NRs or NPs, 0.2- or 4.0-mL ultra-purity water (Millipore Milli-Q purification system, resistivity >18 MΩ cm) was added respectively. High-purity N2 with a flow rate of 200 mL/min was used for deaeration of the solution for 1 h under intense stirring. Then, high-purity CO with a flow rate of 200 mL/min was introduced to the flask under gentle stirring for 10 min. After that, a common balloon linked to the flask was blown up with CO to keep the reducing atmosphere for 4 h. During the whole synthesis process, the reaction system was keep at 30 °C.
Preparation of Pd Nanostructure-Decorated BiVO4 Electrodes
Pd nanostructure-decorated BiVO4 electrodes were prepared using an electrophoretic deposition process. A field of 15 V cm−1 was applied to deposit Pd nanostructures using a FTO counter electrode held at positive potentials relative to the working electrode (BiVO4 electrode). For the preparation process of Pd NP-decorated BiVO4 (NP-BiVO4) and Pd NR-decorated BiVO4 (NR-BiVO4) electrodes, the depositing time are 3 and 1 min, respectively. To prepare Pd NP- and NR-decorated BiVO4 (NP-NR-BiVO4) electrodes, Pd NRs were firstly deposited onto the surface of BiVO4 for 30 s and then Pd NPs were deposited for 90 s. After this deposition process, Pd nanostructure-decorated BiVO4 electrodes were rinsed by distilled water and then dried under atmospheric environment for 2 h.
Characterization
X-ray diffraction (XRD) was carried out using a Bruker AXS D8 Advance powder X-ray diffractometer with a Cu Kα (λ = 1.5418 Å) radiation source to confirm the purity and crystallinity of the prepared BiVO4 electrodes. UV-vis spectra were obtained using a Cary 5000 UV-vis-NIR spectrophotometer in diffuse reflectance mode. Scanning electron microscopy (SEM) images were collected with a field-emission SEM (Hitachi Situation-4800). Elemental compositions were determined by Energy Dispersive X-ray Spectroscopy (EDX) using the EDX detector on the Hitachi Situation-4800. Transmission electron microscopy (TEM) images were obtained with a JEOL 2100F at an accelerating voltage of 200 kV.
Photoelectrochemical Measurements
All photoelectrochemical measurements were carried out in a 0.1-M potassium phosphate buffer (KPi) at pH = 7, using a three-electrode setup, with a Ag/AgCl (4 M KCl) as reference electrode and a platinum foil as counter electrode. Linear scanning voltammograms (LSVs) were obtained with a scan rate of 10 mV s−1. White-light photocurrent measurements were conducted under simulated AM 1.5G solar illumination with a Xe lamp coupled with a Newport Sol3A Class AAA solar simulator (94023A-SR3). The light intensity of the solar light simulator was calibrated to 100 mW cm−2 by the standard reference of a Newport 91150 silicon solar cell. Visible (400–800 nm, 98.7 mW cm−2) and NIR (800–2000 nm, 363 mW cm−2) light measurements were conducted with this Xe lamp couple with spectral filters. Monochromatic photocurrent measurements were conducted with this Xe lamp coupled with a set of monochromatic filters. The whole working electrode was illuminated through the back side of FTO glass. The potential vs Ag/AgCl (4 M KCl) can be converted against the reversible hydrogen electrode (RHE) using the following equation:
Results and Discussion
Figure 1a shows XRD pattern of the BiVO4 film, which can be matched to monoclinic BiVO4 (JCPDS No. 14–0688). In addition, UV-vis absorption spectrum of the BiVO4 film was collected and its bandgap was estimated to be 2.5 eV, which agrees well with values for the bandgap of BiVO4 reported in the literature [19] (Fig. 1b).
Fig. 1.
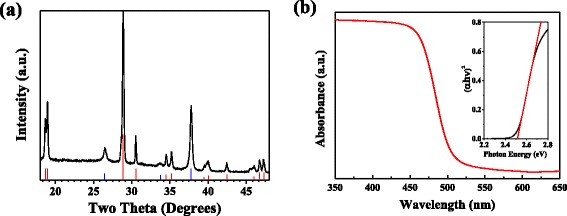
a XRD pattern of the BiVO4 film. The vertical lines indicate JCPDS diffraction peaks of FTO (blue) and monoclinic BiVO4 (red) and b UV-vis absorption spectrum of the BiVO4 film. Inset shows a Tauc plot of BiVO4 for direct bandgap
Figure 2 shows SEM image and EDX maps of as-prepared NP-NR-BiVO4 composite film and TEM images of Pd nanostructures. SEM image demonstrates that the BiVO4 film is composed of grains with no obvious feature. Although no Pd NPs and NRs can be observed in the SEM image due to their small size (Fig. 2f, g), EDX maps clearly show that Pd element presents a uniform distribution on the surface of BiVO4 film, with the Pd/Bi weight percentage being 2.17 %, indicating that Pd NPs and NRs are successfully decorated on the surface of BiVO4 film. EDX analyses for NP-BiVO4 and NR-BiVO4 electrodes show that the Pd/Bi weight percentages are 2.15 and 1.9 %, respectively.
Fig. 2.
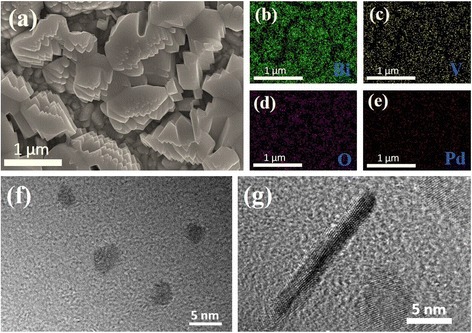
a Typical SEM image and b–e EDX maps of NP-NR-BiVO4 film for four elements. TEM images of Pd f NPs and g NRs
Figure 3a shows linear scanning voltammograms (LSVs) of NP-BiVO4 and bare BiVO4 electrodes recorded in 1-M KPi solution in the dark and under AM 1.5G illumination (100 mW cm−2). The dark scans collected in the potential range between −0.4 and 0.8 V reveal a small background current of ∼10−7 A/cm2. Under AM 1.5G illumination, NP-BiVO4 and bare BiVO4 electrodes show a steady increase in photocurrent with applied potential. Importantly, NP-BiVO4 electrodes exhibited substantially larger photocurrent density than bare BiVO4 electrodes. The photocurrent density is ca. 0.336 mA cm−2 at 0.6 V on NP-BiVO4 electrode, which is about 3.3 times higher than that on bare BiVO4 electrode. Furthermore, chronoamperometric curves were collected during a period of 1 h at 0.3 V under AM 1.5G illumination. As shown in Fig. 3b, during such a relatively long period, the enhancement is still noticeable, suggesting that the photoactivity of BiVO4 electrodes can be enhanced under simulated solar light illumination by decoration of Pd NPs on the surface.
Fig. 3.
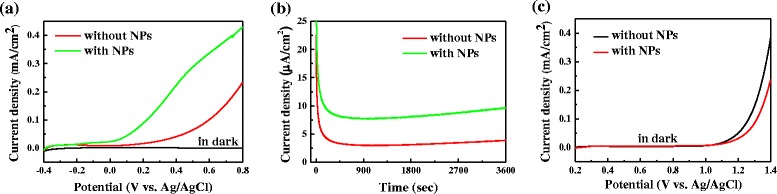
PEC performance of bare BiVO4 and NP-BiVO4 electrodes: a LSVs in the dark and under AM 1.5G illumination, b I–t curves under AM 1.5G illumination at 0.3 V, and c LSV in the potential range from 0.2 to 1.4 V under dark condition
Figure 3c shows LSVs of bare BiVO4 and NP-BiVO4 electrodes collected in the potential range from 0.2 to 1.4 V under dark condition. There is a decrease in current on NP-BiVO4 electrode in the potential ranges between 1.1 and 1.4 V, which could rule out the catalytic effect of Pd NPs on water splitting. Such a current decrease could be ascribed to the fact that the coverage of Pd NPs on the surface of BiVO4 reduces the interfacial surface area between BiVO4 and the electrolyte, and thereby hindering the water oxidation.
To explore the possible reason for the enhanced PEC water splitting on the NP-BiVO4 electrodes, chronoamperometric curves were collected for bare BiVO4 and NP-BiVO4 electrodes under a set of monochromatic light illumination in the visible region. Figure 4a shows the photocurrent response of PEC water splitting on BiVO4 with and without the decoration of Pd NPs under 500 nm (60 mW cm−2) illumination, where the photocurrent on NP-BiVO4 is ca. 4 times of that on pristine BiVO4 electrode. The enhancement factor of photocurrent on the NP-BiVO4 electrode as a function of excitation wavelength was imposed on the absorption spectrum of synthesized Pd NPs stock solution, as shown in Fig. 4b. It can be seen that the enhancement factor strongly depends on the excitation wavelength and that it exhibits a similar trend with Pd NPs absorption spectral feature. Since the Pd absorption spectrum is a consequence of the SPR of Pd NPs, the result suggests that the Pd SPR could be responsible for the observed photocurrent enhancement [24]. Additionally, the photoresponses of bare BiVO4 and NP-BiVO4 electrodes under NIR illumination are obtained and shown in Fig. 4c. The photocurrent collected from NP-BiVO4 electrode slightly decreases in NIR region in comparison with BiVO4 electrode. This result is reasonable since the SPR feature of Pd NPs is located at 573 nm, which is far away from NIR region, and the SPR excitation of Pd NPs cannot occur under NIR illumination.
Fig. 4.
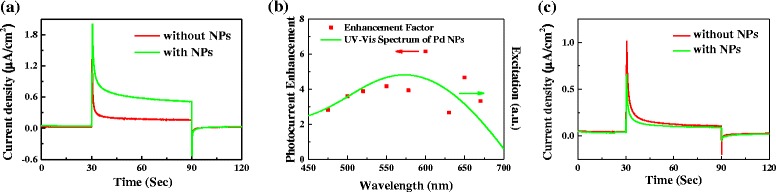
I–t curves at 0.4 V for bare BiVO4 and NP-BiVO4 electrodes under a monochromatic light (500 nm) and c NIR light illumination. b Symbols: photocurrent enhancement for NP-BiVO4 electrode as a function of excitation wavelength. Solid curve: absorption spectrum of the Pd NPs stock solution
To extend the photoresponse to NIR region for the PEC water splitting, Pd NRs were decorated on the surface of BiVO4 because the SPR featured peak is located at ca. 900 nm (see Fig. 5a). Figure 5b–d displays the PEC properties of NR-BiVO4 and bare BiVO4 electrodes. As shown in Fig. 5b, the photocurrent density of NR-BiVO4 electrode under AM 1.5G illumination decreased dramatically compared to bare BiVO4 electrode. The photocurrent density on the NR-BiVO4 and BiVO4 electrodes at 0.6 V are 42 and 146 μA cm−2, respectively. To reveal the plasmonic effect of Pd NRs on the photoresponse of BiVO4 electrodes, photocurrent response for the NR-BiVO4 and bare BiVO4 electrodes was conducted under visible light and NIR light illumination, respectively. As shown in Fig. 5c, the photocurrent for the NR-BiVO4 electrode obtained under visible light illumination greatly decreased relative to bare BiVO4 electrode. Intriguingly, NR-BiVO4 electrode exhibited much enhanced photoactivity under NIR light illumination, as shown in Fig. 5d, indicating that the SPR excitation of Pd NRs is responsible for the enhanced photoactivity of NR-BiVO4 electrodes under NIR light illumination. To the best of our knowledge, this is the first report that the photoactivity of BiVO4 for water splitting can be enhanced in the NIR region by loading plasmonic metal nanostructures [31–33]. As for the photocurrent decrease under visible light and AM 1.5G illumination, it could be attributed to the fact that the presence of Pd NRs not only block the light absorption of BiVO4 but reduce the interfacial surface area between BiVO4 and the electrolyte, thus hindering the transfer of photoexcited holes into the interface between BiVO4 and the electrolyte for water oxidation.
Fig. 5.
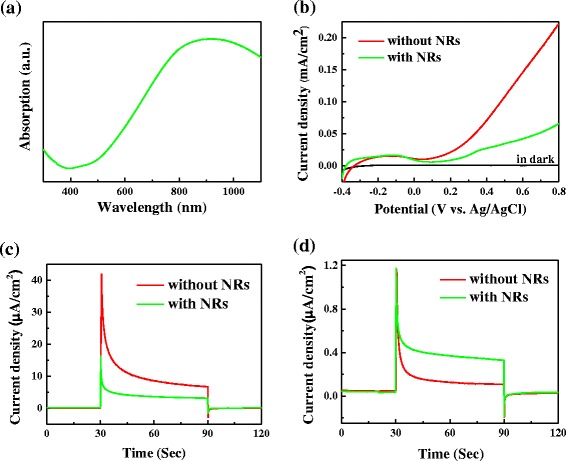
a UV-vis absorption spectrum of Pd NR stock solution. PEC performance of bare BiVO4 and NR-BiVO4 electrodes: b LSVs in the dark and under AM 1.5G illumination; I–t curves at 0.4 V with c visible light and d NIR light illumination
Inspired by the facts that the NP-BiVO4 and NR-BiVO4 electrodes exhibit respectively enhanced PEC water splitting efficiency in the visible and NIR region, a combination of Pd NPs and Pd NRs with BiVO4 electrodes was fabricated by decorating a mixture of Pd NPs and NRs onto the surface of BiVO4 electrode. Figure 6 shows the PEC properties of NP-NR-BiVO4 and bare BiVO4 electrodes. As shown in Fig. 6a, NP-NR-BiVO4 electrode exhibited an enhanced photocurrent density in the potential range from 0.0 to 0.8 V. Importantly, chronoamperometric curves collected under visible and NIR light illumination as shown in Fig. 6b, c demonstrate that the NP-NR-BiVO4 electrode exhibited an enhanced PEC water splitting efficiency in both visible and NIR regions. Although the enhancement factors are not high enough, there must be an optimal loading for Pd NPs and NRs on the BiVO4 to achieve much better PEC performance. Nevertheless, this study is beyond the scope of the current work.
Fig. 6.
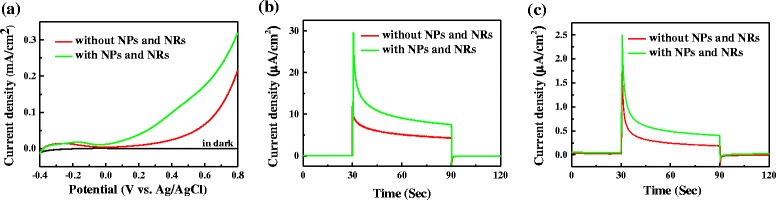
PEC performance of bare BiVO4 and NP-NR-BiVO4 electrodes: a LSVs in the dark and under AM 1.5G illumination; I–t curves collected at 0.4 V with b visible light and c NIR light illumination
There are three energy transfer mechanisms by which SPR can enhance the concentration of charge carriers in a nearby semiconductor and therefore increase the photocurrent in a PEC cell. These mechanisms include resonant photon scattering, plasmon resonance energy transfer (PRET), and hot electron injection. First, the mechanism of resonant photon scattering can be ruled out for Pd nanostructure-decorated-BiVO4 electrodes containing Pd NPs with the diameter around 3.5 nm and Pd NRs with length and width of 11.8 and 1.3 nm, respectively, because it normally occurs in plasmonic metal nanostructures larger than 50 nm in size [34–36]. Second, the effect of PRET is normally observed at wavelengths where the plasmon resonance and semiconductor absorption overlap [37]. Whereas, the SPR feature of Pd NPs and NRs is located at 573 and 900 nm, respectively, indicating that there is barely no absorption overlap between Pd nanostructures and BiVO4 with an absorption band edge around 520 nm. Thus, the effect of PRET can also be ruled out for Pd nanostructure-decorated-BiVO4 electrodes. Third, the hot electron injection from plasmonic metal nanostructures into the conduction band of semiconductors is another possible process following the SPR excitation [38, 39]. Considering that the hot electrons accompany with an enhanced localized electric-field intensity in the vicinity of plasmonic metal nanostructures, the finite-difference time domain (FDTD) simulation was performed to calculate the spatial distribution of local electric-field intensity at the interface between Pd nanostructures and BiVO4 as a function of the wavelength of incident photons. Figure 7a, b shows typical FDTD simulation for Pd NP-BiVO4 and Pd NR-BiVO4 at excitation wavelengths of 500 and 900 nm, respectively. One can clearly observe that the electric-field intensity in the vicinity of Pd NP and Pd NR was greatly enhanced, indicating that there are substantial energetic hot electrons. Thus, in the present work, the hot electron injection mechanism could be the major contributor to the enhanced PEC performance of the Pd nanostructures decorated-BiVO4 electrodes in both visible and NIR regions. As schematically presented in Fig. 7c, under visible or NIR light illumination, Pd NP or NR acts as a sensitizer, absorbing resonant photons, generating the energetic hot electrons from the SPR excitation. Then, the hot electrons pass over the Schottky barrier at the Pd/BiVO4 interface and inject into the conduction band of BiVO4. Schottky barrier at the interface also helps the transferred hot electrons accumulate in the conduction band of BiVO4, preventing them from traveling back to the Pd nanostructures [32, 40].
Fig. 7.
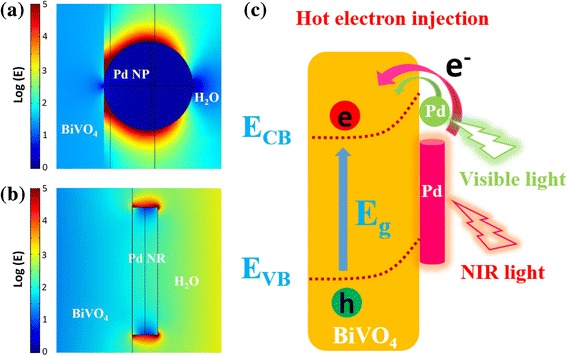
Spatial distribution of electric-field for a NP-BiVO4 and b NR-BiVO4 with excitation wavelengths of 500 and 900 nm, respectively. The incident light is along a specific direction (z axis). c Schematic illustration of the plasmon-induced charge carrier transfer under visible or NIR light illumination at Pd/BiVO4 interface
Conclusions
The photoactivity of Pd NPs and NRs decorated BiVO4 electrodes for PEC water splitting can be effectively enhanced across the visible-NIR region. The enhanced photoactivity in both visible and NIR regions was ascribed to the hot electron injection upon SPR excitation of Pd NPs and NRs, respectively. The present work aimed at enhancing the photoactivity of BiVO4 electrodes and at extending from the visible to the NIR region inspired us to design other plasmonic metal nanostructure-decorated semiconductor photoelectrodes for more effective utilization of the solar spectrum.
Abbreviations
EDX, energy dispersive X-ray spectroscopy; FDTD, finite-difference time domain; LSVs, linear scanning voltammograms; NIR, near-infrared; NP-BiVO4, Pd NPs decorated BiVO4; NP-NR-BiVO4, Pd NPs and NRs decorated BiVO4; NPs, nanoparticles; NR-BiVO4, Pd NRs decorated BiVO4; NRs, nanorods; OECs, oxygen evolution catalysts; PEC, photoelectrochemical; PRET, plasmon resonance energy transfer; SEM, scanning electron microscopy; SPR, surface plasmon resonance; TEM, transmission electron microscopy; XRD, X-ray diffraction.
Footnotes
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
WWY carried out the experiments and drafted the manuscript. YJX participated in the experimental design and the statistical analysis. LLZ and ZQZ helped to revise the manuscript. DDL and QXM participated in the coordination and discussion of the study. YSW performed the FDTD simulation. Hui Yang supervised the whole work. All authors have read and approved the final manuscript.
Acknowledgements
We would like to thank the National Basic Research Program of China (973 Program) (2012CB932800) and the National Natural Science Foundation of China (21533005).
Contributor Information
Zhiqing Zou, Email: zouzq@sari.ac.cn.
Hui Yang, Phone: +86 21 20325112, Email: yangh@sari.ac.cn.
References
- 1.Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238:37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- 2.Zou ZG, Ye JH, Sayama K, Arakawa H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature. 2001;414:625–627. doi: 10.1038/414625a. [DOI] [PubMed] [Google Scholar]
- 3.Chen XB, Shen SH, Guo LJ, Mao SS. Semiconductor-based photocatalytic hydrogen generation. Chem Rev. 2010;110:6503–6570. doi: 10.1021/cr1001645. [DOI] [PubMed] [Google Scholar]
- 4.Qiu YC, Leung S-F, Wei ZH, Lin QF, Zheng XL, Zhang YG, Fan ZY, Yang SH. Enhanced charge collection for splitting of water enabled by an engineered three-dimensional nanospike array. J Phys Chem C. 2014;118:22465–22472. doi: 10.1021/jp507800t. [DOI] [Google Scholar]
- 5.Qiu YC, Leung SF, Zhang QP, Hua B, Lin QF, Wei ZH, Tsui KH, Zhang YG, Yang SH, Fan ZY. Efficient photoelectrochemical water splitting with ultrathin films of hematite on three-dimensional nanophotonic structures. Nano Lett. 2014;14:2123–2129. doi: 10.1021/nl500359e. [DOI] [PubMed] [Google Scholar]
- 6.Park Y, McDonald KJ, Choi K-S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem Soc Rev. 2013;42:2321–2337. doi: 10.1039/C2CS35260E. [DOI] [PubMed] [Google Scholar]
- 7.Zhong DK, Choi S, Gamelin DR. Near-complete suppression of surface recombination in solar photoelectrolysis by “Co-Pi” catalyst-modified W:BiVO4. J Am Chem Soc. 2011;133:18370–18377. doi: 10.1021/ja207348x. [DOI] [PubMed] [Google Scholar]
- 8.Ye H, Park HS, Bard AJ. Screening of electrocatalysts for photoelectrochemical water oxidation on W-doped BiVO4 photocatalysts by scanning electrochemical microscopy. J Phys Chem C. 2011;115:12464–12470. doi: 10.1021/jp200852c. [DOI] [Google Scholar]
- 9.Pilli SK, Furtak TE, Brown LD, Deutsch TG, Turner JA, Herring AM. Cobalt-phosphate (Co-Pi) catalyst modified Mo-doped BiVO4 photoelectrodes for solar water oxidation. Energy Environ Sci. 2011;4:5028–5034. doi: 10.1039/c1ee02444b. [DOI] [Google Scholar]
- 10.Ye H, Lee J, Jang JS, Bard AJ. Rapid screening of BiVO4-based photocatalysts by scanning electrochemical microscopy (SECM) and studies of their photoelectrochemical properties. J Phys Chem C. 2010;114:13322–13328. doi: 10.1021/jp104343b. [DOI] [Google Scholar]
- 11.Luo WJ, Yang ZS, Li ZS, Zhang JY, Liu JG, Zhao ZY, Wang ZQ, Yan SC, Yu T, Zou ZG. Solar hydrogen generation from seawater with a modified BiVO4 photoanode. Energy Environ Sci. 2011;4:4046–4051. doi: 10.1039/c1ee01812d. [DOI] [Google Scholar]
- 12.Jo WJ, Jang J, Kong K, Kang HJ, Kim JY, Jun H, Parmar KPS, Lee JS. Phosphate doping into monoclinic BiVO4 for enhanced photoelectrochemical water oxidation activity. Angew Chem Int Ed. 2012;51:3147–3151. doi: 10.1002/anie.201108276. [DOI] [PubMed] [Google Scholar]
- 13.McDonald KJ, Choi KS. A new electrochemical synthesis route for a BiOI electrode and its conversion to a highly efficient porous BiVO4 photoanode for solar water oxidation. Energy Environ Sci. 2012;5:8553–8557. doi: 10.1039/c2ee22608a. [DOI] [Google Scholar]
- 14.Su J, Guo L, Yoriya S, Grimes CA. Aqueous growth of pyramidal-shaped BiVO4 nanowire arrays and structural characterization: application to photoelectrochemical water splitting. Cryst Growth Des. 2010;10:856–861. doi: 10.1021/cg9012125. [DOI] [Google Scholar]
- 15.He HC, Berglund SP, Rettie AJE, Chemelewski WD, Xiao P, Zhang YH, Mullins CB. Synthesis of BiVO4 nanoflake array films for photoelectrochemical water oxidation. J Mater Chem A. 2014;2:9371–9379. doi: 10.1039/c4ta00895b. [DOI] [Google Scholar]
- 16.Yoon H, Mali MG, Choi JY, Kim MW, Choi SK, Park H, Al-Deyab SS, Swihart MT, Yarin AL, Yoon SS. Nanotextured pillars of electrosprayed bismuth vanadate for efficient photoelectrochemical water splitting. Langmuir. 2015;31:3727–3737. doi: 10.1021/acs.langmuir.5b00486. [DOI] [PubMed] [Google Scholar]
- 17.Rao PM, Cai LL, Liu C, Cho IS, Lee CH, Weisse JM, Yang PD, Zheng XL. Simultaneously efficient light absorption and charge separation in WO3/BiVO4 core/shell nanowire photoanode for photoelectrochemical water oxidation. Nano Lett. 2014;14:1099–1105. doi: 10.1021/nl500022z. [DOI] [PubMed] [Google Scholar]
- 18.Su JZ, Guo LJ, Bao NZ, Grimes CA. Nanostructured WO3/BiVO4 heterojunction films for efficient photoelectrochemical water splitting. Nano Lett. 2011;11:1928–1933. doi: 10.1021/nl2000743. [DOI] [PubMed] [Google Scholar]
- 19.Seabold JA, Choi K-S. Efficient and stable photo-oxidation of water by a bismuth vanadate photoanode coupled with an iron oxyhydroxide oxygen evolution catalyst. J Am Chem Soc. 2012;134:2186–2192. doi: 10.1021/ja209001d. [DOI] [PubMed] [Google Scholar]
- 20.Wang DE, Li RG, Zhu J, Shi JY, Han JF, Zong X, Li C. Photocatalytic water oxidation on BiVO4 with the electrocatalyst as an oxidation cocatalyst: essential relations between electrocatalyst and photocatalyst. J Phys Chem C. 2012;116:5082–5089. doi: 10.1021/jp210584b. [DOI] [Google Scholar]
- 21.Cho S, Lee MJ, Kim MS, Lee S, Kim YK, Lee DH, Lee CW, Cho KH, Chung JH. Infrared plus visible light and heat from natural sunlight participate in the expression of MMPs and type I procollagen as well as infiltration of inflammatory cell in human skin in vivo. J Dermatol Sci. 2008;50:123–133. doi: 10.1016/j.jdermsci.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Zhang ZH, Zhang LB, Hedhili MN, Zhang HN, Wang P. Plasmonic gold nanocrystals coupled with photonic crystal seamlessly on TiO2 nanotube photoelectrodes for efficient visible light photoelectrochemical water splitting. Nano Lett. 2013;13:14–20. doi: 10.1021/nl3029202. [DOI] [PubMed] [Google Scholar]
- 23.Pu YC, Wang G, Chang KD, Ling Y, Lin YK, Fitzmorris BC, Liu CM, Lu X, Tong Y, Zhang JZ, Hsu YJ, Li Y. Au nanostructure-decorated TiO2 nanowires exhibiting photoactivity across entire UV-visible region for photoelectrochemical water splitting. Nano Lett. 2013;13:3817–3823. doi: 10.1021/nl4018385. [DOI] [PubMed] [Google Scholar]
- 24.Ingram DB, Linic S. Water splitting on composite plasmonic-metal/semiconductor photoelectrodes: evidence for selective plasmon-induced formation of charge carriers near the semiconductor surface. J Am Chem Soc. 2011;133:5202–5205. doi: 10.1021/ja200086g. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z, Lin YY, Yin M, Zhang HF, Cheng CW, Lu LF, Xue XZ, Fan HJ, Chen XY, Li DD. Understanding the enhancement mechanisms of surface plasmon-mediated photoelectrochemical electrodes: a case study on Au nanoparticle decorated TiO2 nanotubes. Adv Mater Interfaces. 2015;2:1500169. [Google Scholar]
- 26.Jain PK, El-Sayed MA. Noble metal nanoparticle pairs: effect of medium for enhanced nanosensing. Nano Lett. 2008;8:4347–4352. doi: 10.1021/nl8021835. [DOI] [PubMed] [Google Scholar]
- 27.Malinsky MD, Kelly KL, Schatz GC, Van Duyne RP. Chain length dependence and sensing capabilities of the localized surface plasmon resonance of silver nanoparticles chemically modified with alkanethiol self-assembled monolayers. J Am Chem Soc. 2001;123:1471–1482. doi: 10.1021/ja003312a. [DOI] [Google Scholar]
- 28.Haes AJ, Van Duyne RP. A nanoscale optical biosensor: sensitivity and selectivity of an approach based on the localized surface plasmon resonance spectroscopy of triangular silver nanoparticles. J Am Chem Soc. 2002;124:10596–10604. doi: 10.1021/ja020393x. [DOI] [PubMed] [Google Scholar]
- 29.Xiong YJ, Wiley B, Chen JY, Li ZY, Yin YD, Xia YN. Corrosion-based synthesis of single-crystal Pd nanoboxes and nanocages and their surface plasmon properties. Angew Chem Int Ed Engl. 2005;44:7913–7917. doi: 10.1002/anie.200502722. [DOI] [PubMed] [Google Scholar]
- 30.Liu YT, Xu Z, Yin M, Fan HW, Cheng WJ, Lu LF, Song Y, Ma J, Zhu XF. Enhanced photoelectrocatalytic performance of alpha-Fe2O3 thin films by surface plasmon resonance of Au nanoparticles coupled with surface passivation by atom layer deposition of Al2O3. Nanoscale Res Lett. 2015;10:374. doi: 10.1186/s11671-015-1077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang LW, Lin CY, Valev VK, Reisner E, Steiner U, Baumberg JJ. Plasmonic enhancement in BiVO4 photonic crystals for efficient water splitting. Small. 2014;10:3970–3978. doi: 10.1002/smll.201400970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang LW, Herrmann LO, Baumberg JJ. Size dependent plasmonic effect on BiVO4 photoanodes for solar water splitting. Sci Rep. 2015;5:16660. doi: 10.1038/srep16660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdi FF, Dabirian A, Dam B, van de Krol R. Plasmonic enhancement of the optical absorption and catalytic efficiency of BiVO4 photoanodes decorated with Ag@SiO2 core-shell nanoparticles. Phys Chem Chem Phys. 2014;16:15272–15277. doi: 10.1039/c4cp01583e. [DOI] [PubMed] [Google Scholar]
- 34.Evanoff DD, Chumanov G. Synthesis and optical properties of silver nanoparticles and arrays. ChemPhysChem. 2005;6:1221–1231. doi: 10.1002/cphc.200500113. [DOI] [PubMed] [Google Scholar]
- 35.Burda C, Chen XB, Narayanan R, El-Sayed MA. Chemistry and properties of nanocrystals of different shapes. Chem Rev. 2005;105:1025–1102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 36.Kelly KL, Coronado E, Zhao LL, Schatz GC. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B. 2003;107:668–677. doi: 10.1021/jp026731y. [DOI] [Google Scholar]
- 37.Warren SC, Thimsen E. Plasmonic solar water splitting. Energy Environ Sci. 2012;5:5133–5146. doi: 10.1039/C1EE02875H. [DOI] [Google Scholar]
- 38.Linic S, Christopher P, Ingram DB. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat Mater. 2011;10:911–921. doi: 10.1038/nmat3151. [DOI] [PubMed] [Google Scholar]
- 39.Tian Y, Tatsuma T. Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles. J Am Chem Soc. 2005;127:7632–7637. doi: 10.1021/ja042192u. [DOI] [PubMed] [Google Scholar]
- 40.Qian K, Sweeny BC, Johnston-Peck AC, Niu WX, Graham JO, DuChene JS, Qiu JJ, Wang YC, Engelhard MH, Su D, Stach EA, Wei WD. Surface plasmon-driven water reduction: gold nanoparticle size matters. J Am Chem Soc. 2014;136:9842–9845. doi: 10.1021/ja504097v. [DOI] [PubMed] [Google Scholar]


