Abstract
Objectives
To determine, in newborn infants referred with elevated capillary thyroid-stimulating hormone (TSH), a threshold below which a frankly subnormal venous free thyroxine (fT4) level of <10 pmol/L is unlikely, so that treatment with levo-thyroxine (L-T4) might be deferred until venous thyroid function tests (TFTs) become available.
Subjects and methods
All infants referred in Scotland since 1979 with capillary TSH elevation were studied, with particular focus on infants screened using the AutoDELFIA assay between 2002 and 2013.
Results
Of the 321 infants referred with capillary TSH elevation using AutoDELFIA, 35 were excluded (fT4/TSH unavailable (12), venous sample either preceding or >10 days after capillary sampling (13, 10)), leaving 286 eligible for analysis (208 definite/probable hypothyroidism, 61 transient TSH elevation, 17 of uncertain thyroid status). Capillary TSH and venous T4 were strongly correlated (Spearman's rank correlation coefficient −0.707355). The optimal capillary TSH threshold for predicting a venous fT4 of <10 pmol/L was found to be >40 mU/L (90.3% sensitivity and 65.9% specificity compared with 90.25% and 59.1% for >35 mU/L and 88.3% and 68.2% for >45 mU/L). 93 infants (32.5%) had capillary TSH ≤40 mU/L at referral of whom 15 (9.7%) had venous fT4 <10 pmol/L, comprising seven with true congenital hypothyroidism, five with transient TSH elevation and three with uncertain status, two of whom died.
Conclusion
For infants in whom capillary TSH is ≤40 mU/L, it is reasonable to defer L-T4 treatment until venous TFT results are known provided that the latter become available quickly.
Keywords: Endocrinology, Screening, Neonatology
What is already known on this topic.
Most newborn infants referred with capillary thyroid-stimulating hormone (TSH) elevation on newborn screening have congenital hypothyroidism requiring treatment with levo-thyroxine (L-T4).
Some infants with capillary TSH elevation are found not to have congenital hypothyroidism so that treatment can either not be started or be discontinued.
What this study adds.
Infants in whom capillary TSH is ≤40 mU/L have a low probability (10%) of having a subnormal venous free thyroxine level of <10 pmol/L.
It is reasonable to defer L-T4 treatment in such infants until the results of venous thyroid function tests become available.
Introduction
Congenital hypothyroidism, the most common paediatric endocrine disorder, is very important clinically since severe cases will lead to irreversible mental handicap without prompt treatment.1 The reported incidence varies according to whether or not newborn screening is practised and if so by the cut-off used by the referring laboratory in terms of capillary thyroid-stimulating hormone (TSH) and thyroxine (T4). For example in Canada, where a capillary TSH threshold of 15 mU/L is used, the incidence of congenital hypothyroidism is 1 in 2600 births.2 Although capillary TSH screening for primary congenital hypothyroidism, which began in Scotland in 1979,3 has revolutionised the neurological outcome, some early treated infants still demonstrate a range of problems including subtle impairment of cognition, attention and memory deficit and vestibuloauditory problems,4–6 especially when postnatal care has been inadequate.6 Prompt treatment with levo-thyroxine (L-T4) of severe cases is therefore highly desirable.
When a newborn infant is referred with capillary TSH elevation, the physician needs to distinguish between congenital hypothyroidism requiring immediate treatment and milder thyroid dysfunction which may resolve without treatment. Following clinical assessment, a good venous blood sample for measurement of free thyroxine (fT4) and TSH is mandatory, since the result reflects the presence and severity of congenital hypothyroidism.7 However, at initial evaluation, only the capillary TSH value is available, and it may not be possible in all centres and in all countries to have the results of venous blood tests on the same day.
The crucial component of the venous blood sample is the T4 level, usually measured as fT4. Work of Mutlu et al8 in 296 newborns showed that on day 10 the 2.5th centile, 50th centile and 97.5th centiles for fT4 were 15.2, 22.5 and 32 pmol/L, respectively. It was on this basis that the European Society for Paediatric Endocrinology consensus group on congenital hypothyroidism defined the severity of hypothyroidism in terms of fT4 ranges, with <5 pmol/L as severe, 5–10 pmol/L as moderate and 10–15 pmol/L as mild hypothyroidism.7
When capillary TSH is grossly elevated, for example, >100 mU/L, with or without clinical features of hypothyroidism, the clinician will wish to start L-T4 treatment immediately since moderate or severe hypothyroidism is likely. The decision is less clear in an asymptomatic newborn with modest capillary TSH elevation, for example, 15–40 mU/L, where venous fT4 is unlikely to be frankly low, and more likely to be either mildly subnormal (10 to <15 pmol/L) or within the newborn reference range of 15–32 pmol/L. When the results of venous thyroid function tests (TFTs) will be available on the same day as venepuncture, the clinician can be guided by the fT4 levels. Although this same-day service will be routinely provided nowadays by laboratories in developed countries, the availability of venous results may still be problematic outside normal working hours and at weekends. Moreover, a same-day service may not be feasible in many countries with limited resources. In such situations, the clinician faces the dilemma as to whether to treat immediately or whether to await the venous results before committing the infant to several years of treatment with L-T4.
To date the relationship between capillary TSH levels measured in the course of newborn screening and serum thyroid hormone levels has not been explored. In this study, data from all infants referred by the Scottish Newborn Screening Programme with capillary TSH elevation has been reviewed with particular focus on infants evaluated by the AutoDELFIA assay since 2002. The aim is to determine a capillary TSH threshold below which decompensated primary hypothyroidism is unlikely so that immediate treatment with L-T4 is not indicated and can be deferred until venous TFT results become available.
Patients and methods
Since 1990, a computerised database has been kept of all infants referred by the newborn screening laboratory in Scotland since August 1979.3 9 This database records capillary TSH values (one, two or three samples depending on whether or not the laboratory have requested repeat tests) and venous total (t) T4 in nmol/L (prior to 1996) and venous fT4 in pmol/L thereafter measured on the first assessment sample prior to starting LT-4 replacement.
Patients were classified as having definite congenital hypothyroidism, probable congenital hypothyroidism, transient TSH elevation and status uncertain, as previously described.3 9 The definitions for these four categories take into account the fact that thyroid imaging has not been consistently carried out in all Scottish centres since screening began, although combined radioisotope and ultrasound scanning has become the norm in the West of Scotland since 1996.10
Analysis of capillary TSH and venous fT4 and tT4 data was carried out in relation to the four time periods during the study in which the assay and hence the normal, recall range and immediate referral cut-offs (mU/L) differed. These were:
1979–1982—Corning TSH radioimmunoassay (RIA): <25, 25–49, >50
1982–1989—in-house TSH RIA: <15, 15–39, >40
1989–2002—ImmunoDiagnosticsSystems TSH immunoradiometric assay (IRMA): <10, 10–39, >40
2002–present—Perkin-Elmer TSH dissociation-enhanced lanthanide fluorescence immunoassay (DELFIA): <8, 8–24, >25
The entire cohort of patients from the 34-year period between August 1979 and December 2013 in whom both capillary and venous TSH data were available was analysed, followed by more detailed analysis of patients screened by the AutoDELFIA method from 2002 onwards. Total thyroxine values were converted to fT4 using an approximate in-house conversion division factor of 7.25.11 Where there were two samples from the same day, the second sample value was taken. Data were excluded when venous testing took place more than 10 days after the initial capillary sample test. In cases where the date of venous sampling was missing, it was calculated as age at notification plus 1 day, based on Scottish data showing that median age at notification and start of treatment were 12 versus 13.5 days in 344 patients from 1979 to 1993 and 10 versus 11 days in 250 patients from 1994 to 2003, indicating an approximate 1 day difference.9
Statistical analysis
The Shapiro–Wilk W test was initially used to test data for normality and indicated a non-normal distribution; therefore, non-parametric tests were used for data analysis, values being expressed as median with either range or IQR. Correlation between venous fT4 and capillary TSH were analysed using Spearman’s rank correlation test and expressed as a coefficient of correlation with CIs. Receiver-operating characteristic (ROC) curve analysis was performed to explore the relationship between selected capillary TSH cut-offs and pretreatment venous fT4 levels, estimating the sensitivity and specificity at different capillary TSH cut-offs in predicting venous frank hypothyroidism, defined as fT4 <10 pmol/L. The optimal capillary TSH threshold was identified as that which had the highest specificity in association with a sensitivity approaching 90%. Venous fT4 data according to selected capillary TSH ranges were also expressed as ‘box and whisker’ plots using StatsDirect software (Altrincham, Cheshire, UK).
Ethical aspects
The Glasgow West Research Ethics Committee has previously approved data extraction from our database for audit and re-evaluation, provided that the data are anonymised. Since 2002, parents have given informed consent for data storage and for later anonymised data analysis and presentation. This project was registered with the Clinical Governance Support Unit of NHS Greater Glasgow and Clyde as a Quality Improvement Project.
Results
General information
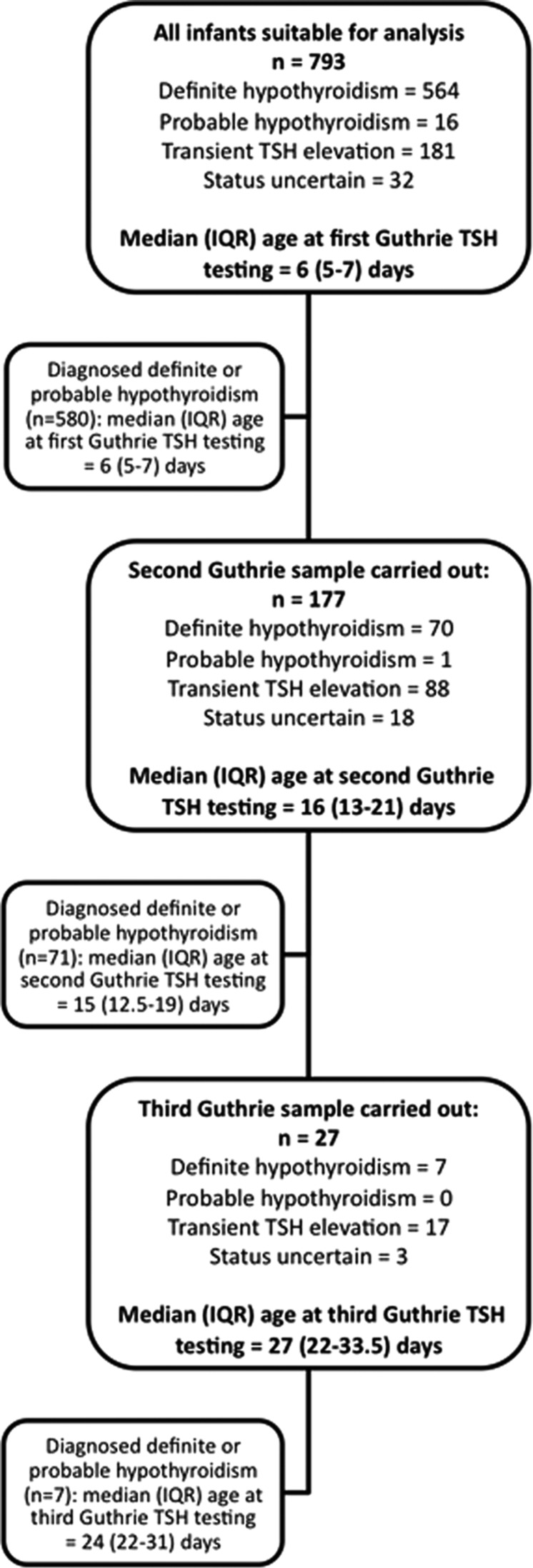
Between August 1979 and December 2013, 793 infants were referred by the screening laboratory and their outcome is shown in figure 1. Definite hypothyroidism was confirmed in 564 infants with probable hypothyroidism in 16, transient TSH elevation in 181 and status still uncertain in 32. Of the 564 infants with true congenital hypothyroidism 177 (31.3%) underwent a second capillary sample, while 7 (1.2%) underwent a third capillary sample. Recall rates were higher for the 181 infants with transient TSH elevation, 88 (48.6%) of whom required a second sample and 17 (9.4%) a third sample. Age at venepuncture was missing in 23 of the 793 infants (9 with definite and one with probable congenital hypothyroidism, one with status uncertain and 12 with transient TSH elevation) so that this was derived from age at notification as described above.
Figure 1.
Consort diagram showing outcome in 793 infants referred with capillary thyroid-stimulating hormone (TSH) elevation by the Scottish Newborn Screening laboratory between August 1979 and December 2013. Note that the infants who had two capillary screening samples performed (middle large box) are also included in the upper box since all infants had one sample taken. Those infants who had three capillary samples performed (lower large box) are included in both preceding boxes.
Median (IQR) age at first capillary TSH testing was 6 days (5–7 days) for all infants and 6 days (5–7 days) after excluding infants with transient TSH elevation and status uncertain. A second capillary test was required in 177 infants, median (IQR) age at testing 16 days (13–21 days). For infants subsequently diagnosed with definite or probable hypothyroidism, median age at second testing was 15 days (12.5–19 days). A third capillary test was required in 27 infants, median (IQR) age 27 days (22–33.5 days). Of these, seven were diagnosed with definite hypothyroidism, median (IQR) age at third testing 24 days (22–31 days).
Analysis of cohort from 2002 onwards using the AutoDELFIA assay
Between January 2002 and December 2013, a total of 321 infants were referred by the Scottish Newborn Screening laboratory using the same AutoDELFIA assay. Thirty-five were excluded from analysis due to the age at venous sample >10 days after the capillary test (10 infants), venous TSH or fT4/tT4 unavailable (12 infants) and venous sample taken prior to first capillary sample (13 infants). This left 286 infants who met our criteria for analysis: 208 with definite and probable hypothyroidism, 61 with transient TSH elevation and 17 of uncertain thyroid status. Age at venepuncture was missing and hence derived from notification in one infant with definite congenital hypothyroidism and two with transient TSH elevation.
Correlation between capillary TSH and venous fT4 testing
There was a strong correlation between capillary TSH at screening and confirmatory venous fT4 testing following referral with a highly significant Spearman’s rank correlation coefficient of −0.707355 (p<0.0001, 95% CI after using Fisher’s Z transformed −0.760912 to −0.644225).
Sensitivity and specificity of different capillary TSH cut-off levels in predicting low venous fT4
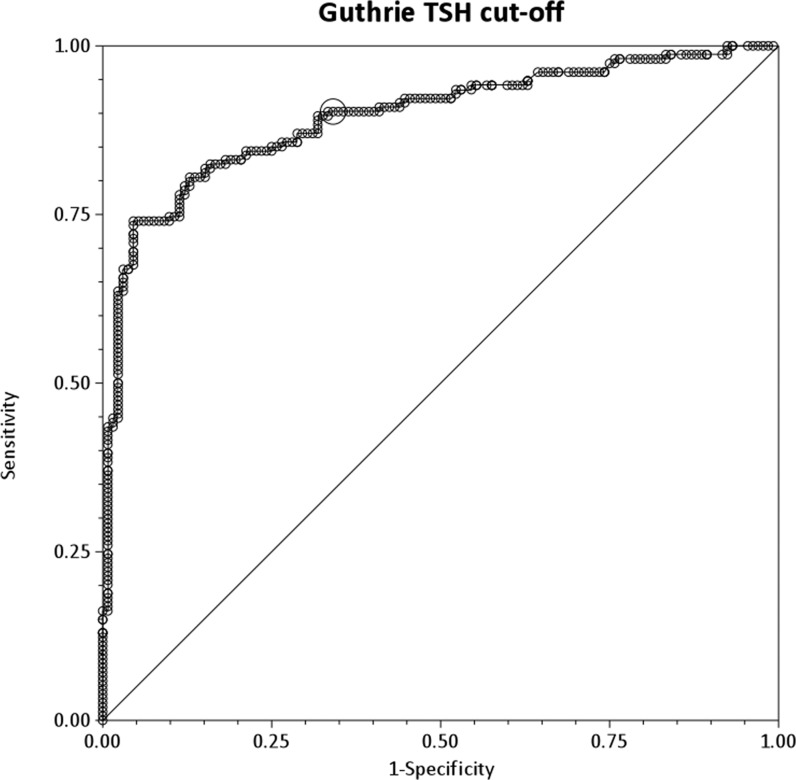
Table 1 and figure 2 show that a cut-off value of >40 mU/L for capillary TSH gives a 90.3% sensitivity (95% CI 84.4 to 94.4)and a 65.9% specificity (95% CI 57.2 to 73.9) for predicting a venous fT4 value of <10 pmol/L, which would indicate the need for treatment with L-T4. Therefore, a capillary TSH cut-off of >40 mU/L would detect 90.3% of those infants with frankly low venous fT4, while 65.9% of infants with fT4 >10 pmol/L would have capillary TSH values of ≤40 mU/L. Moreover, the area under the ROC curve is 0.89 (95% CI 0.86 to 0.93), indicating that referral capillary TSH is a strong predictor of subsequent fT4 values. A capillary TSH threshold of >35 mU/L has almost identical sensitivity but lower specificity (59.1%), while sensitivity is lower (88.3%) for a cut-off of >45 mU/L and specificity is lower (53.8%) for a cut-off of >30 mU/L compared with the >40 mU/L cut-off.
Table 1.
Sensitivity and specificity of different capillary TSH cut-off levels in predicting a venous fT4 of <10 pmol/L in 286 Scottish infants referred with capillary TSH elevation
| Capillary TSH cut-off (mU/L whole blood) | Sensitivity | Specificity |
|---|---|---|
| >10 | 0.987013 | 0.106061 |
| >20 | 0.961039 | 0.356061 |
| >30 | 0.922078 | 0.537879 |
| >35 | 0.902597 | 0.590909 |
| >40 | 0.902597 | 0.659091 |
| >45 | 0.883117 | 0.681818 |
| >50 | 0.857143 | 0.719697 |
| >60 | 0.831169 | 0.795455 |
| >70 | 0.818182 | 0.848485 |
| >80 | 0.792208 | 0.878788 |
| >90 | 0.75974 | 0.886364 |
| >100 | 0.746753 | 0.901515 |
| >110 | 0.74026 | 0.939394 |
| >120 | 0.694805 | 0.954545 |
Sensitivity refers to the proportion of infants with fT4 of <10 pmol/L whose capillary TSH is above a given cut-off value, while specificity refers to the proportion of infants with fT4 ≥10 pmol/L whose capillary TSH is below the cut-off value. fT4, free thyroxine; TSH, thyroid-stimulating hormone.
Figure 2.
Receiver-operating characteristic curve analysis of capillary thyroid-stimulating hormone (TSH) levels predicting a venous free T4 value of ≤10 pmol/L in 286 newborn infants with TSH elevation on newborn screening.
Of the 286 infants analysed, 93 (32.5%) had capillary TSH values of ≤40 mU/L at referral comprising 32 (11.1%) with TSH 25–40 mU/L at first testing and immediate referral; 57 (19.9%) with TSH 8 to ≤25 mU/L on first sample and 8–40 mU/L at second sample prior to referral and 4 with TSH 8–40 mU/L on third sample.
Comparison between 1979–2001 and 2002–2013 data
Data from 1979 to 2001 showed higher sensitivity (96.4% vs 90.3%) but much lower specificity (22.6% vs 65.9%) for detecting venous fT4 <10 pmol/L with a capillary TSH cut-off of >40 mU/L (area under the ROC curve 0.83494).
Venous fT4 levels according to capillary TSH cut-off in infants screened between 2002 and 2013 using the AutoDELFIA method
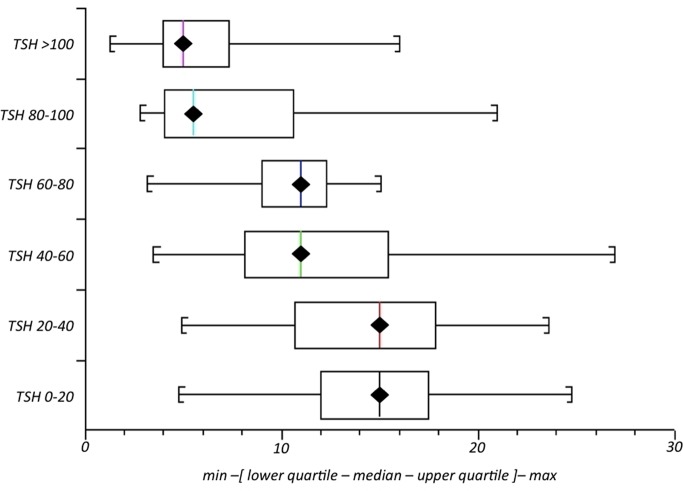
Figure 3 shows a box and whisker plot of fT4 values grouped according to capillary TSH ranges. Median venous fT4 was 15 pmol/L for capillary TSH values of between 0 and 20 mU/L (n=53). Median fT4 was also 15 pmol/L for capillary TSH values of 20–40 mU/L (n=48); 11 for values of 40–60 mU/L (n=28); 11 for values of 60–80 mU/L (n=19) and 5 pmol/L when capillary TSH was >80 mU/L (n=138).
Figure 3.
Median (range) venous free T4 according to range of capillary thyroid-stimulating hormone (TSH) in 286 infants with raised TSH on newborn screening.
Analysis of patients screened between 2002 and 2013 with venous fT4 <10 pmol/L in whom capillary TSH was ≤40 mU/L
Of the 154 patients with frankly subnormal fT4 of <10 pmol/L, 15 (9.7%) had capillary TSH values at the time of referral of <40 mU/L and their details are given in table 2. All but three had been referred after a repeat capillary TSH had been requested, and two infants (patients 8 and 15) were referred after the second repeat. Seven infants were found to have true hypothyroidism, five had transient TSH elevation and status was uncertain in three patients, of whom two died. Although venous TSH was higher than capillary TSH (consistent with assay differences and also with a rise having occurred between capillary and venous sampling), the degree of elevation was modest in relation to the venous fT4 being <10 pmol/L. Thus, venous TSH was <30 mU/L in four patients (patients 1, 7, 8, 15) and only 30–60 mU/L in a further four patients (patients 2, 3, 5, 9). Moreover, fT4 was severely reduced at <5 pmol/L in three of the patients (patients 1, 10, 12).
Table 2.
Data on 15 infants referred with elevated capillary TSH in whom capillary TSH was <40 mU/L and venous fT4 was <10 pmol/L
| Patient no. | BW (kg) | GA (weeks) | Sick? (Y/N) | cTSH (mU/L) (age in days) | vTSH (mIU/L) (age in days) | vfT4 (pmol/L) | Comment | First cTSH (mU/L) (age in days) | Other cTSH (mU/L) (age in days) |
|---|---|---|---|---|---|---|---|---|---|
| True congenital hypothyroidism | |||||||||
| 1 | 2.95 | 39 | N | 9.5* (12) | 22.7 (18) | 4.8 | Probable dyshormonogenesis on radio-isotope scan | 23.2 (5) | |
| 2 | 4.22 | 42 | N | 21.8* (17) | 49.9 (20) | 8.0 | Confirmed CH (rising TSH on diagnostic challenge) but no imaging performed | 9.42 (5) | |
| 3 | 3.54 | 39 | N | 25.7 (6) | 55.0 (15) | 9.0 | Dyshormonogenesis (no mutation found) | ||
| 4 | 2.8 | 40 | N | 26.4* (18) | N/A (22) | 7.1 | Dyshormonogenesis; IUGR | 15.3 (6) | |
| 5 | 3.08 | 38 | N | 14.3* (15) | 52.45 (24) | 6.6 | Confirmed CH but cause unknown (no uptake on RIS but on L-T4 for > 4 weeks prior to imaging) | 13.7 (6) | |
| 6 | 3.52 | 40 | N | 34.9* (14) | >100 (16) | 6.0 | Dyshormonogenesis (confirmed TPO defect) | 10.16 (5) | |
| 7 | 1.66 | 28 | Y | 32.3 (4) | 27.0 (9) | 7.5 | Respiratory distress syndrome with small IVH in perinatal period). Confirmed CH (TSH 15.9 mU/L after >1 year on L-T4 therapy) | ||
| Status uncertain | |||||||||
| 8 | 3.5 | 39 | Y | 15.0† (25) | 11.2 (29) | 6.6 | Interrupted aortic arch and Di George (died aged 3 months) | 10 (6) | 20 (16) |
| 9 | 1.04 | 29 | Y | 21.0* (15) | 41.8 (17) | 7.2 | Down syndrome with AVSD and chronic lung disease (died aged 6 months) | 7.4 (6) | 75 (23) NB after vTFTs |
| 10 | 1.49 | 31 | N | 21.0* (10) | 150 (15) | <5.0 | PDA (resolved). No imaging performed. Still on L-T4 | 5.77 (5) | 48.4 (17) NB after vTFTs |
| Transient TSH elevation | |||||||||
| 11 | 3.83 | 40 | N | 27.0* (27) | N/A (36) | 9.7 | No imaging; treatment never started | 16 (4) | |
| 12 | 2.9 | 37 | Y | 15.0* (18) | 110 (24) | 4.8 | ECMO for Group B Streptococcus sepsis | 14 (14) | |
| 13 | 3.65 | 37 | N | 33.0* (18) | 75.0 (22) | 9.8 | PAX8 mutation and thyrotropin receptor polymorphism | 9 (6) | |
| 14 | 3.01 | 37 | Y | 9.8 (10) | 73.5 (17) | 5.1 | Renal and lung dysplasia, imperforate anus | 182.03 (39) NB after vTFTs | |
| 15 | 1.01 | 27 | Y | 11.2† (28) | 17.9 (36) | 9.4 | Preterm/VLBW | 1.49 (5) at 28 weeks GA | 11.8 (25) |
*Repeat sample.†Second repeat sample.
AVSD, atrioventricular septal defect; BW, birthweight; CH, congenital hypothyroidism; cTSH, capillary thyroid-stimulating hormone; ECMO, extra-corporeal membrane oxygenation; fT4, free thyroxine; GA, gestational age; IUGR, intrauterine growth restriction; IVH, intraventricular haemorrhage; L-T4, levo-thyroxine; N/A, not available; NB, nota bene; PDA, patent ductus arteriosus; TPO, thyroperoxidase; TSH, thyroid-stimulating hormone; vfT4, venous free thyroxine; VLBW, very low birth weight; vTFT, venous thyroid function test.
Discussion
This study shows that even when capillary TSH is only just above the Scottish threshold for referral (≥8 mU/L), there is a risk that venous thyroid hormones will be low. This was the case in 15 infants who were found to have frankly low venous fT4 (<10 pmol/L) on initial venous sampling despite only mild capillary TSH elevation, ranging between 9.5 and 34.9 mU/L. Thus, two infants with subnormal fT4 (patients 1 and 14 in table 2) had capillary TSH values of <10 mU/L, while three (patients 8, 12 and 15) had values between 10 and 20 mU/L and two (patients 9 and 10) had values between 20 and 25 mU/L. These findings are in keeping with work from Italy showing that capillary TSH cut-offs of 10 and 12 mU/L were superior to 20 mU/L in detecting thyroid dysgenesis.12 It follows that infants referred with capillary TSH elevation values just above the national or regional screening threshold should be investigated promptly, since true congenital hypothyroidism may be discovered in this situation.
In the present study, data from 1979 to 2002 (before the current assay came into use) show that while a threshold of >40 mU/L for capillary TSH was sensitive in predicting a low fT4, specificity was much lower. By contrast, data from 2002 to 2013 using the AutoDELFIA assay give information of superior predictive value, demonstrating that just over 90% of infants who are found to have venous fT4 <10 pmol/L will have capillary TSH values of ≥40 mU/L.
The infants with modest capillary TSH elevation (values ranging between 8 and 40 mU/L) tended to be those requiring repeat sampling rather than direct referral. Thus, over half of the infants referred with capillary TSH values of ≤40 mU/L had been referred after a second (or in four cases a third) capillary test.
Analysis of the current study suggests that if capillary TSH is >40 mU/L, it is advisable to institute L-T4 treatment without delay unless venous TFTs are available on the same day, since the specificity of 66% for predicting a venous fT4 of 10 pmol/L means that only 34% of such infants will have fT4 values of ≥10 pmol/L. By contrast, if capillary TSH is ≤40 mU/L and the results of venous TFTs are not going to be available on the same day, there is a case for deferring L-T4 treatment. This should only be considered in situations when the infant is clinically euthyroid and when venous blood results will be available within a few days. The interval between the capillary TSH test leading to referral (whether initial, first or second repeat) should also be taken into consideration.
An alternative approach is to ‘play safe’ and to always institute L-T4 treatment in any infant with initial capillary TSH ≥25 mU/L or repeat capillary TSH ≥8 mU/L in whom the venous blood results are not immediately available. However, this strategy deprives the clinician of being able to observe the venous TSH and fT4 trends when the latter are within the reference range, may adversely affect uptake on radioisotope scanning due to TSH suppression13 and may commit the infant and family to 2 or 3 years of potentially unnecessary treatment, followed by the need for re-evaluation.7
We conclude that in situations where it is not feasible to obtain the results of venous blood tests on the same day, those infants with capillary TSH values of ≤40 mU/L who appear clinically well with no symptoms or signs of hypothyroidism (eg, jaundice, poor feeding, cold extremities) and in whom blood results will be available shortly do not necessarily require immediate treatment. However, immediate treatment is advised when the infant is symptomatic irrespective of the capillary TSH level.
Footnotes
Contributors: Data for this paper were provided by SS and JJ, collated and analysed by TP and the paper was written by MDCD with the help of MGS, TP, JJ and SS.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Deladoey J, Ruel J, Giguère Y, et al. . Is the incidence of congenital hypothyroidism really increasing? A 20-year retrospective population-based study in Quebec. J Clin Endocrinol Metab 2011;96:2422–9. 10.1210/jc.2011-1073 [DOI] [PubMed] [Google Scholar]
- 2.Grosse SD, Van Vliet G. Prevention of intellectual disability through screening for congenital hypothyroidism: how much and at what level? Arch Dis Child 2011;96:374–9. 10.1136/adc.2010.190280 [DOI] [PubMed] [Google Scholar]
- 3.Ray M, Muir T, Kennedy R, et al. . An audit of congenital hypothyroidism in Scotland 1979–1993. Arch Dis Child 1997;76:411–15. 10.1136/adc.76.5.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rovet JF. Children with congenital hypothyroidism and their siblings: do they really differ? Pediatrics 2005;115:e52–7. 10.1542/peds.2004-1492 [DOI] [PubMed] [Google Scholar]
- 5.Oerbeck B, Sundet K, Kate BF, et al. . Congenital hypothyroidism: no adverse effects of high dose thyroxine treatment on adult memory, attention, and behaviour. Arch Dis Child 2005;90:132–7. 10.1136/adc.2003.043935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Léger J, Ecosse E, Roussey M, et al. , French Congenital Hypothyroidism Study Group. Subtle health impairment and socioeducational attainment in young adult patients with congenital hypothyroidism diagnosed by neonatal screening: a longitudinal population-based cohort study. J Clin Endocrinol Metab 2011;96:1771–82. 10.1210/jc.2010-2315 [DOI] [PubMed] [Google Scholar]
- 7.Léger J, Olivieri A, Donaldson M, et al. , ESPE-PES-SLEP- JSPE-APEG-ISPAE, and the Congenital Hypothyroidism Consensus Conference Group. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis and management of congenital hypothyroidism. J Clin Endocrinol Metab 2014;81:80–103. 10.1159/000358198 [DOI] [PubMed] [Google Scholar]
- 8.Mutlu M, Karaguzel G, Alıyazicioğlu Y, et al. . Reference intervals for thyrotropin and thyroid hormones and ultrasonographic thyroid volume during the neonatal period. J Matern Fetal Neonatal Med 2012;25:120–4. 10.3109/14767058.2011.561894 [DOI] [PubMed] [Google Scholar]
- 9.Jones JH, Mackenzie J, Croft GA, et al. . Improvement in screening performance and diagnosis of congenital hypothyroidism in Scotland 1979–2003. Arch Dis Child 2006;91:680–5. 10.1136/adc.2005.088427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry RJ, Maroo S, Maclellan AC, et al. . Combined ultrasound and isotope scanning is more informative in the diagnosis of congenital hypothyroidism than single scanning. Arch Dis Child 2006;91:972–6. 10.1136/adc.2006.096776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones JH, Gellén B, Paterson WF, et al. . Effect of high versus low initial doses of L-thyroxine for congenital hypothyroidism on thyroid function and somatic growth. Arch Dis Child 2008;93:940–4. 10.1136/adc.2007.120618 [DOI] [PubMed] [Google Scholar]
- 12.Corbetta C, Weber G, Cortinovis F, et al. . A 7-year experience with low blood TSH cutoff levels for neonatal screening reveals an unsuspected frequency of congenital hypothyroidism (CH). Clin Endocrinol (Oxf) 2009;71:739–45. 10.1111/j.1365-2265.2009.03568.x [DOI] [PubMed] [Google Scholar]
- 13.Lucas-Herald A, Jones J, Attaie M, et al. . Diagnostic and predictive value of ultrasound and isotope thyroid scanning, alone and in combination, in infants referred with thyroid-stimulating hormone elevation on newborn screening. J Pediatr 2014;164:846–54. 10.1016/j.jpeds.2013.11.057 [DOI] [PubMed] [Google Scholar]