Abstract
Background/aims
The novel prostaglandin E (EP) 3 and prostaglandin F (FP) receptor agonist ONO-9054 is effective in lowering intraocular pressure (IOP) in patients with ocular hypertension and open-angle glaucoma when administered once daily. This study compares the effects of morning (AM) versus evening (PM) dosing of ONO-9054 on tolerability and IOP lowering.
Methods
This was a single-centre, randomised, double-masked, two-sequence, placebo-controlled crossover study in 12 subjects with bilateral primary open-angle glaucoma or ocular hypertension. Two 14-day crossover regimens were separated by a 2-week washout: ONO-9054 (1 drop to each eye) in the morning (07:00) and vehicle in the evening (19:00) and vice versa. IOP was measured multiple times during select days. Ocular examinations also evaluated safety and tolerability.
Results
Mild ocular hyperaemia, reported by six subjects with PM dosing, was the most frequent adverse event. Mild to moderate dryness was also slightly more frequent after PM dosing. Maximum IOP reduction from baseline occurred on day 2 with decreases from baseline of −7.4 mm Hg (−30.8%) for AM dosing and −9.1 mm Hg, (−38.0%) for PM dosing; after 14 days, mean reduction in IOP was −6.8 mm Hg (−28.6%) for AM dosing and −7.5 mm Hg (−31.0%) for PM dosing.
Conclusions
PM dosing of ONO-0954 was associated with a slightly increased frequency of mild hyperaemia and mild to moderate dryness. Both dosing schedules provided sustained reduction in IOP.
Trial registration number
Keywords: Glaucoma, Intraocular pressure, Pharmacology
Introduction
Glaucoma is an insidious progressive optic neuropathy that often causes irreversible ganglion cell damage leading to permanent vision loss. The objective of glaucoma management is to preserve visual function by providing significant and sustained decrease in intraocular pressure (IOP) by means of pharmaceuticals, office-based laser procedures, minimally invasive glaucoma surgery and conventional surgical procedures.1–3
Pharmaceutical therapies for ocular hypertension (OHT) and glaucoma include several classes of drugs. Prostaglandin analogues (PGAs) reduce IOP by targeting the prostaglandin F (FP) receptor to increase outflow of aqueous humour, primarily through the uveoscleral pathway.4 In the USA, latanoprost, bimatoprost and travoprost are the most commonly prescribed PGAs used to target the FP receptor.5 Although current PGAs are considered the ‘gold standard’ for pharmaceutical reduction of IOP, new classes of PGA molecules with enhanced tolerability and additional therapeutic benefits are being evaluated. One area of investigation is prostaglandin E (EP) receptor agonists. The EP3 receptor is found in the trabecular meshwork and ciliary muscle,6 and has been demonstrated to augment reduction in IOP following the application of FP agonists in monkeys.7
Prodrug ONO-9054 is an isopropyl ester derivative of the biologically active free acid ONO-AG-367 and is a highly selective and potent agonist of both prostaglandin EP3 and FP receptors in vitro.8 Due to its dual receptor activity, the drug has potential to produce a more potent reduction of IOP than drugs that target the FP receptor.8
Although variable, IOP is often more elevated in the early morning hours.9–11 Thus, therapeutic efficacy of topical glaucoma medications should be effective at controlling IOP during this time. The objective of this crossover study was to assess the tolerability and the effect of morning (AM) versus evening (PM) dosing on IOP lowering of ophthalmic solution ONO-9054 in patients diagnosed with primary open-angle glaucoma (OAG) or OHT.
Materials and methods
Subjects
Twelve subjects with a confirmed diagnosis of bilateral OHT or chronic OAG aged 18–80 years were enrolled. Inclusion criteria included an IOP ≥22 mm Hg at 08:00 and ≥21 mm Hg at 10:00 in at least one eye, with ≤35 mm Hg at all measurements in both eyes on the 2 days preceding dosing (day −2 and day −1; 08:00 and 10:00). A best corrected visual acuity (BCVA) of at least 20/100, measured by Logarithm of Minimum Angle of Resolution (LogMAR=0.70 or better) was required at screening and on day 1. Other inclusion criteria included central corneal thickness of 500–600 μm at screening in both eyes, ocular cup-to-disc ratio ≤0.8 in both eyes and absence of visual field loss within the previous 6 months. All subjects gave written, informed consent and agreed to washout of all ocular drugs prior to the study.
Excluded from the study were subjects with history of severe ocular trauma in either eye, intraocular or ocular laser surgery within the previous 3 months, refractive surgery within the previous 6 months and any condition preventing reliable screening or ocular assessment. Prohibited medications included recent ocular, inhaled, intranasal or systemic steroids; β-adrenergic blockers; adrenergic agonists; ocular allergy medications; carbonic anhydrase inhibitors or cholinergic agonists.
Study design
This was a phase I, randomised, double-masked, placebo-controlled, two-sequence crossover study (clintrials.gov: NCT01670266) with a total dosing time of 4 weeks. All procedures were performed at West Coast Clinical Trials (Costa Mesa, California 92626, USA) between 5 October 2012 and 9 May 2013. The study protocol was approved by the Aspire Institutional Review Board (Santee, California, USA) and the study conducted in accordance with the ethical principles of Good Clinical Practice and the Declaration of Helsinki. Subjects, clinical site personnel (with the exception of the medication coordinator), study investigators and the Sponsor were masked to the treatment.
Following a washout of IOP-lowering drugs for up to 28 days during screening, 12 subjects underwent two 14-day treatment periods (days 1–15 and 29–43) separated by a 14-day washout (see online supplementary figure S1). Subjects were examined on days −1–2, 14–15, 28–30 and 42–43; follow-up occurred on day 49. During the first treatment period, six subjects were randomised to receive one drop (approximately 30 μL) of ONO-9054 (30 μg/mL) to both eyes in the morning (07:00±30 min) and placebo (vehicle) solution in the evening (07:00±30 min; Sequence 1). The remaining six subjects received active drug in the evening and placebo in the morning (Sequence 2). After washout, each subject group crossed over to the alternate dosing schedule.
Randomisation occurred at the study unit: a randomisation table was prepared by an unmasked designee who assigned each subject to either treatment. The medication coordinator dispensed appropriate study medication. ONO-9054 and placebo were administered as clear colourless liquid ophthalmic solutions.
Safety and tolerability
Adverse events were recorded from first administration to follow-up and coded using the Medical Dictionary for Drug Regulatory Affairs (MedDRA) dictionary (V.15.1). Clinical laboratory evaluations, vital signs and 12-lead electrocardiographs were assessed.
Ocular examinations including symptomatology, BCVA, pupillometry and slit lamp examinations were conducted at various time points on days −2, −1, 1, 2, 7, 14, 15, 21, 27, 28, 29, 42, 43 and 49. Assessments of pachymetry, indirect ophthalmology, cup-to-disc ratio, fundus imaging, impression cytology and visual field were conducted on days −2, −1, 15, 21, 28, 43 and 49.
The ONO-9054 30 μg/mL dose was selected for study because it is well tolerated and produces hyperaemia at levels adequate to enable the analysis of modest exacerbation or improvement of hyperaemia with AM or PM dosing.9 Ocular hyperaemia was evaluated by comparison with standardised photographs of conjunctival hyperaemia in subjects on glaucoma drug therapy (Ora Calibra Redness Scale V.6.b, under license from Ora, Andover, Massachusetts, USA). The hyperaemia scale was ≥0 or 0.5=none; 1 or 1.5=mild; 2 or 2.5=moderate; 3=severe.
Subjects rated dosing sequence tolerability (photophobia, itching, tearing, dryness, discharge) as 0=absent, 1=mild, 2=moderate, 3=severe with stinging or burning or 4=severe with blurred or dim vision.
Pharmacodynamics
Goldmann applanation tonometry conducted with a masked observer and recorder was used to assess duration of IOP lowering. IOP was measured at 08:00, 10:00, 12:00, 16:00, 20:00 and 22:00 on days 1, 2, 7, 14 and 15 and compared with corresponding baseline measurements on day −1. IOPs measured at the same time points on days 29, 30, 35, 42 and 43 were compared with corresponding baseline measurements on day 28.
Pharmacokinetics
On days 1, 14, 29 and 42, blood samples were taken before dosing (AM or PM) and 5, 10, 20, 30 and 45 min and 1, 1.5, 2, and 3 h after dosing. Plasma ONO-9054 and ONO-AG-367 concentrations were determined using validated liquid chromatography/tandem mass spectrometry methods at Quintiles BioSciences. Plasma concentration versus time data were analysed using Phoenix WinNonlin V.6.2.1, using a standard non-compartmental model. The following parameters were derived: maximum observed concentration (Cmax), time to Cmax (Tmax) and elimination half-life (T½).
Statistical considerations
Sample size was based on practical considerations. The Safety Set comprised all subjects enrolled in the study who received at least one drop of study medication of any strength (including placebo) instilled in either eye and was used for analysis of pharmacodynamic end points. The Pharmacokinetic Analysis Population included all enrolled subjects who received ONO-9054 and had at least one postdose pharmacokinetic sample.
Results
Disposition and demographic characteristics
Twelve subjects were enrolled and randomised; all completed the study as planned. Each subject received a cumulative total of 28 doses of ONO-9054 30 μg/mL and 28 doses of placebo in each eye. The study population was predominantly white (10/12) and male (8/12) with a mean age of 68.3 years (range: 50–79 years (table 1)). Half of subjects had been treated previously with either a topical carbonic anhydrase inhibitor (6/12) or PGA (6/12).
Table 1.
Subject demographics
| Parameter | Sequence 1 (QAM/QPM) (n=6) | Sequence 2 (QPM/QAM) (n=6) | All (n=12) |
|---|---|---|---|
| Age, gender, race and ethnicity | |||
| Age (in years) | |||
| Mean | 71.5 (±9.0) | 65.0 (±8.0) | 68.3 (±8.8) |
| Median | 76 | 68 | 69.5 |
| Min, max | 59, 79 | 50, 71 | 50, 79 |
| Age, n (%) | |||
| 50–59 | 1 (16.7) | 1 (16.7) | 2 (16.7) |
| 60–69 | 1 (16.7) | 3 (50.0) | 4 (33.3) |
| >69 | 4 (66.7) | 2 (33.3) | 6 (50.0) |
| Gender, n (%) | |||
| Male | 4 (66.7) | 4 (66.7) | 8 (66.7) |
| Female | 2 (33.3) | 2 (33.3) | 4 (33.3) |
| Race, n (%) | |||
| White | 4 (66.6) | 6 (100.0) | 10 (83.3) |
| Asian | 2 (33.3) | 0 (0.0) | 2 (16.7) |
| Ethnicity, n (%) | |||
| Not Hispanic | 6 (100.0) | 6 (100.0) | 12 (100.0) |
| Diagnosis | |||
| Ocular conditions n (%) | |||
| Ocular HTN | 1 (16.7) | 5 (83.3) | 6 (50.0) |
| OAG | 5 (83.3) | 1 (16.7) | 6 (50.0) |
| Previous IOP-lowering drugs, n (%) | |||
| TCAI | 4 (66.7) | 2 (33.3) | 6 (50.0) |
| PGA | 4 (66.7) | 2 (33.3) | 6 (50.0) |
| β-Blocker | 2 (33.3) | 1 (16.7) | 3 (25.0) |
| Other | 3 (50.0) | 2 (33.3) | 5 (41.7) |
| None | 0 (0.0) | 1 (16.7) | 1 (8.3) |
HTN, hypertension; IOP, intraocular pressure; OAG, open-angle glaucoma; PGA, prostaglandin analogue; QAM, once daily morning; QPM, once daily evening; TCAI, topical carbonic anhydrase inhibitors.
Safety and tolerability
There were no episodes of photophobia, tearing or discharge. Itching was mild and transient, occurring in two subjects during PM dosing and one subject during AM dosing; there was no apparent difference in frequency between AM and PM dosing. Dryness was mild-moderate occurring in three subjects during PM dosing and one subject during AM dosing.
Hyperaemia was absent to normal in all subjects at baseline. Peak hyperaemia scores during each 14-day sequence were Grade 2 (moderate) for two subjects (16.7%) in the pooled AM dose group, Grade 3 (severe) for one subject (8.3%) in the PM dose group (table 2). Peak scores were recorded 15 h post dose in the AM group and 13 h post dose in the PM group. Mean scores decreased with continued dosing in both groups: after 14 days of dosing, hyperaemia was rated as none to mild (Grade ≤1) for 10/12 (83.3%) subjects in the AM dose group and 9/12 (75.0%) in the PM dose group.
Table 2.
Maximum hyperaemia score according to dosing sequences
| Ocular hyperaemia | Sequence 01:00 (n=6) | Sequence 02:00 (n=6) | Sequence 13:00 (n=6) | Sequence 14:00 (n=6) | Pooled AM (n=12) | Pooled PM (n=12) | |
|---|---|---|---|---|---|---|---|
| Study day/period | Grade | n/N (%) | n/N (%) | n/N (%) | n/N (%) | n/N (%) | n/N (%) |
| Post first dose through follow-up | 0 | 0/6 (0.0) | 1/6 (16.7) | 0/6 (0.0) | 0/6 (0.0) | 1/12 (8.3) | 0/12 (0.0) |
| 0.5 | 0/6 (0.0) | 4/6 (66.7) | 1/6 (16.7) | 0/6 (0.0) | 4/12 (33.3) | 1/12 (8.3) | |
| 1 | 5/6 (83.3) | 0/6 (0.0) | 5/6 (83.3) | 4/6 (66.7) | 5/12 (41.7) | 9/12 (75.0) | |
| 1.5 | 0/6 (0.0) | 0/6 (0.0) | 0/6 (0.0) | 0/6 (0.0) | 0/12 (0.0) | 0/12 (0.0) | |
| 2 | 1/6 (16.7) | 1/6 (16.7) | 0/6 (0.0) | 1/6 (16.7) | 2/12 (16.7) | 1/12 (8.3) | |
| 2.5 | 0/6 (0.0) | 0/6 (0.0) | 0/6 (0.0) | 0/6 (0.0) | 0/12 (0.0) | 0/12 (0.0) | |
| 3 | 0/6 (0.0) | 0/6 (0.0) | 0/6 (0.0) | 1/6 (16.7) | 0/12 (0.0) | 1/12 (8.3) | |
Sequence 1, (AM→PM); sequence 2, (PM→AM); AM, once daily morning; PM, once daily evening.
Score interpretation: ≥0 or 0.5, no hyperaemia; 1 or 1.5, mild hyperaemia; 2 or 2.5, moderate hyperaemia; 3, severe hyperaemia; when two eyes in the same subject had different results, the worse (more severe grade) score was used.
Overall, seven subjects reported 13 adverse events: six subjects reported 11 adverse events in the PM group and two subjects reported two adverse events in the AM group (table 3). The most frequent adverse events reported were hyperaemia and dry eye. Hyperaemia was only recorded as an adverse event if there were any additional clinical changes, or if the condition was reported by the subject. Six subjects (50.0%) in the PM group reported ocular hyperaemia, which occurred during the first 14 days of dosing. All adverse events were judged mild. There were no ocular adverse events among subjects in the AM dosing sequence; six subjects experienced eye disorders in the PM dosing sequence.
Table 3.
Adverse events
| System organ class preferred term |
Pooled AM (n=12) n (%) |
Pooled PM (n=12) n (%) |
All (n=12) n/% |
|---|---|---|---|
| Number of AEs/number of subjects (% of subjects with ≥1 AE) | 2/2 (16.7) | 11/6 (50.0) | 13/7 (58.3) |
| Eye disorders | 0 (0.0) | 6 (50.0) | 6 (50.0) |
| Dry eye | 0 (0.0) | 3 (25.0) | 3 (25.0) |
| Eye pruritus | 0 (0.0) | 1 (8.3) | 1 (8.3) |
| Ocular hyperaemia | 0 (0.0) | 6 (50.0) | 6 (50.0) |
| Gastrointestinal disorders | 1 (8.3) | 0 (0.0) | 1 (8.3) |
| Dyspepsia | 1 (8.3) | 0 (0.0) | 1 (8.3) |
| Skin and subcutaneous tissue disorders | 1 (8.3) | 1 (8.3) | 2 (16.7) |
| Dermatitis contact | 0 (0.0) | 1 (8.3) | 1 (8.3) |
| Urticaria | 1 (8.3) | 0 (0.0) | 1 (8.3) |
AE, adverse event.
No clinically significant progressive changes were noted in any other ocular assessments (slit-lamp examination, BCVA, pupillometry, pachymetry, indirect ophthalmology, cup-to-disc ratio, fundus imaging, impression cytology and visual field) or in clinical laboratory evaluations, vital signs or 12-lead electrocardiographs.
Pharmacodynamics
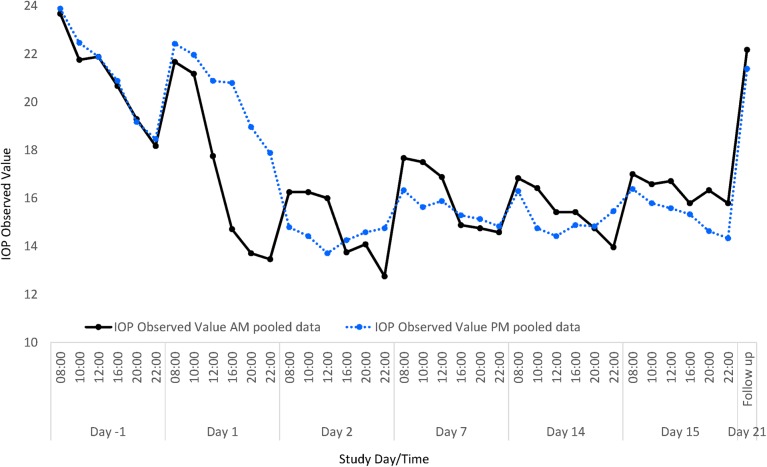
Visual inspection of time plots showed that the initial rate of decline in IOP was greater with AM dosing in both treatment sequences (figure 1). Greater diurnal fluctuation was observed during the first week of AM dosing than PM dosing in both sequences than in following weeks; a cumulative effect produced a dampening of IOP fluctuation during the fourth week of treatment, independent of AM or PM dosing. Observed values of IOP were similar for the pooled AM and pooled PM dose groups at baseline and decreased from 08:00 to the final measurement of the day at 22:00.
Figure 1.
Observed intraocular pressure (IOP) values following morning (AM) and evening (PM) dosing of ONO-9054. IOP decreased from baseline in both cohorts, and this was maintained throughout the dosing period. Data from both dosing sequences were pooled to produce data for AM (♦) and PM (▴) dosing.
Maximum reduction in IOP for pooled data was observed at 08:00 on the second day of dosing in each sequence (day 2 or 30): −7.4 mm Hg (−30.8% from baseline) for AM dosing (1 h post dose) and −9.1 mm Hg (−38.0%) for PM dosing (12 h post dose). After 14 days of dosing, reductions in IOP were similar to those observed on day 2. On day 14, in pooled results from both AM sequences, mean IOP was 16.4 mm Hg and reduction relative to baseline at 08:00 was −6.8 mm Hg (−28.6%). Twenty-four hours post dose on day 15 at 08:00, reduction relative to baseline was −6.7 mm Hg (−27.5%). In pooled results from both PM sequences after 14 days of dosing on day 15, mean IOP was 16.4 mm Hg and reduction relative to baseline at 08:00 (12 h after dose) was −7.5 mm Hg (−31.0%). At 24 h post dose at 20:00, mean observed IOP was 14.6 mm Hg and reduction relative to baseline was −4.5 mm Hg (−22.5%). After 14 days of dosing, the actual values for IOP were 16.8 and 16.4 mm Hg at 08:00 h on day 14 in the AM dosing group and day 15 in the PM dosing group and 14.0 and 14.3 mm Hg for AM and PM dosing, respectively, at 22:00 h.
Although pooled data (table 4) indicated that a greater number of subjects responded to treatment with IOP lowering to levels ≤18 mm Hg during the 14-day AM treatment intervals (10/12 subjects, 83.3%) versus PM treatment intervals (8/12 subjects, 66.7%), greater numbers of subjects in the pooled 14-day PM treatment intervals than AM treatment intervals met IOP lowering targets of ≤17 mm Hg (8/12 subjects, 66.7%, vs 5/12, 41.7%) and ≤16 mm Hg (7/12 subjects, 58.3%, vs 3/12, 25%).
Table 4.
Analysis of intraocular pressure target following 14 days morning (AM) or evening (PM) dosing
| Pressure target (mm Hg) | Pooled AM 25 h post AM dose n/N (%) |
Pooled PM 25 h post PM dose n/N (%) |
|---|---|---|
| >18 | 2/12 (16.7) | 4/12 (33.3) |
| ≤18 | 10/12 (83.3) | 8/12 (66.7) |
| ≤17 | 5/12 (41.7) | 8/12 (66.7) |
| ≤16 | 3/12 (25.0) | 7/12 (58.3) |
| ≤15 | 3/12 (25.0) | 5/12 (41.7) |
| ≤14 | 1/12 (8.3) | 3/12 (25.0) |
| ≤13 | 0/12 (0) | 1/12 (8.3) |
Pharmacokinetics
Plasma concentrations of ONO-9054 were below the limit of quantification in all subjects. ONO-AG-367 was detectable (see online supplementary figures S2 and S3). Maximum plasma concentrations on day 1 were achieved after approximately 10 min for each dosing schedule (mean Tmax=0.17 h). Maximum plasma concentrations were slightly higher for AM than for PM dosing (mean Cmax 23.4 pg/mL vs 20.4 pg/mL) and T½ was slightly shorter (mean T½ 0.84 h vs 0.96 h).
Discussion
ONO-9054 was well tolerated in both dosing groups, although there were some indications that ONO-9054 might have been better tolerated in the AM dosing sequence. Most adverse events occurred in the PM dosing group, although they were all considered mild. Notably, there were no ocular adverse events in the AM dosing group; ocular adverse events were only reported in the PM dosing group. Dry eye has been reported for several pharmacological treatments of OAG,4 and visual function is known to be significantly better in the mornings than in the evenings due to regeneration of the tear film while the eyes are closed.12 13 As such, the dryness reported here is likely to originate from the active compound potentiating dryness accumulating throughout the day. There is a possibility that the frequency of adverse events reported in this study may have been augmented because of the twice-daily dosing schedule used to implement the crossover design. Subjects received vehicle twice daily—once as placebo and once carrying ONO-9054. Ocular irritation may have been exacerbated as this administration was double what subjects would usually experience on a once-daily schedule.
The most common local side effect of FP receptor agonists is mild to moderate ocular hyperaemia: rates of 5–20% with latanoprost, 35–50% with travoprost and 15–55% with bimatoprost have been reported.14 Given the small population of the current study, comparisons with previously published rates would be unreliable; two (17%) subjects in both the AM and PM groups experienced hyperaemia and the incidence of ocular hyperaemia was similar in both dosing schedules. There were no meaningful pharmacokinetic differences between AM and PM dosing.
IOP displays diurnal variation. Given that IOP is often highest when measured on awakening, there is a possibility that the most serious IOP-related damage occurs early in the morning, before patients have the opportunity to instil medication into their eyes. Administration of ONO-9054 led to reductions in IOP over the 14-day dosing period irrespective of whether it was administered AM or PM. ONO-9054 produced substantial IOP lowering as early as day 2 of the study. Similar peak and trough IOP values were observed with either AM or PM dosing.
After 14 days, ONO-9054 achieved IOP reductions comparable with those of bimatoprost, latanoprost and travoprost reported after 1 month.4 For exploratory purposes, this study which was designed to provide a gross comparison of AM and PM dosing included only 12 subjects. A crossover study design has benefits compared with a parallel design in reducing variability by using within-subject comparisons. Typically, such a design would be expected to have a greater power than the equivalent parallel design; however, no sample size assumptions were made and the effects of AM and PM administration of ONO-9054 were not compared statistically. Data for maximum reductions in IOP from baseline and subjects with IOP ≤16 mm Hg indicate that PM dosing may have had a slightly greater effect on IOP lowering than AM dosing, although a thorough examination of efficacy would require further study in more subjects. Many patients do not take glaucoma treatments in the prescribed manner despite the availability of effective topical therapies. Non-adherence in patients with glaucoma has been reported to range from 64% to 80% depending on the definition used.15 Therefore, effective treatments for OHT and glaucoma will need to combine acceptable tolerability, pharmacodynamic activity and ease of administration. It is acknowledged that the inclusion and exclusion criteria are not representative of all patients with OAG. However, this was considered to be an appropriate population to study for this exploratory early phase study.
In conclusion, both AM and PM once-daily dosing of ONO-9054 provided a sustained reduction in IOP with minimal hyperaemia and few adverse tolerability effects in this population studied.
Supplementary Material
Acknowledgments
The authors would like to thank study staff and participants. Dr Justin Cook and Dr Ellen Robertshaw of Niche Science and Technology provided medical writing and submission support; Ono Pharma UK paid for this service.
Footnotes
Contributors: All authors contributed to the conception and design of the study and acquisition, analysis and interpretation of the data. All authors critically revised the content of this manuscript, and have approved this final version for publication. All authors are accountable for all aspects of the work in relation to the accuracy and integrity of the scientific content.
Funding: This study was funded by Ono Pharma Japan.
Competing interests: AF, TO and CLW are employees of ONO Pharmaceuticals Co. CR-R, DTR and AW conducted this work as paid employees of ONO Pharma USA. MSB, CQ and IA acted as consultants to Ono and were remunerated for their contributions.
Ethics approval: Aspire Institutional Review Board (Santee, California, USA).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ishida N, Odani-Kawabata N, Shimazaki A, et al. Prostanoids in the therapy of glaucoma. Cardiovasc Drug Rev 2006;24:1–10. 10.1111/j.1527-3466.2006.00001.x [DOI] [PubMed] [Google Scholar]
- 2.National Institute for Health and Care Excellence (NICE). Glaucoma: Diagnosis and management of chronic open angle glaucoma and ocular hypertension. https://www.nice.org.uk/guidance/cg85 (accessed 31 Mar 2015). [PubMed]
- 3.American Optometric Association (AOA). Optometric clinical practice guideline: Care of the patient with open angle glaucoma. 2011. http://www.aoa.org/documents/optometrists/CPG-9.pdf (accessed 31 Mar 2015).
- 4.Lin L, Zhao YJ, Chew PT, et al. Comparative efficacy and tolerability of topical prostaglandin analogues for primary open-angle glaucoma and ocular hypertension. Ann Pharmacother 2014;48:1585–93. 10.1177/1060028014548569 [DOI] [PubMed] [Google Scholar]
- 5.Lee A, McCluskey P. Chemical utility and differential effects of prostaglandin analogs in the management of raised intraocular pressure and ocular hypertension. Clinical Ophthalmology 2010;4:741–64 10.1167/iovs.14-16181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlötzer-Schrehardt U, Zenkel M, Nüsing RM. Expression and localization of FP and EP prostanoid receptor subtypes in human ocular tissues. Invest Ophthalmol Vis Sci 2002;43:1475–87. [PubMed] [Google Scholar]
- 7.Gabelt BT, Hennes EA, Bendel MA, et al. Prostaglandin subtype-selective and non-selective IOP-lowering comparison in monkeys. Ocul Pharmacol Ther 2009;25:1–8. 10.1089/jop.2008.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamane S, Karakawa T, Nakayama S, et al. IOP-lowering effect of ONO-9054 – a novel dual agonist of prostanoid EP3 and FP receptors – in monkeys. Invest Ophthalmol Vis Sci 2015;56:2547–52. [DOI] [PubMed] [Google Scholar]
- 9.Drance SM. Diurnal variation of intraocular pressure in treated glaucoma. Arch Ophthalmol 1963;70:302–11. 10.1001/archopht.1963.00960050304004 [DOI] [PubMed] [Google Scholar]
- 10.Sacca SC, Rolando M, Marletta A, et al. Fluctuations of intraocular pressure during the day in open angle glaucoma, normal tension glaucoma and normal subjects. Ophthalmologica 1998;212:115–19. 10.1159/000027290 [DOI] [PubMed] [Google Scholar]
- 11.Zeimer RC, Wilensky JT, Gieser DK. Presence and rapid decline of early morning intraocular pressure peaks in glaucoma. Ophthalmology 1990;97:547–50. 10.1016/S0161-6420(90)32543-5 [DOI] [PubMed] [Google Scholar]
- 12.Abelson MB, Ousler GW, Maffei C. Dry eye in 2008. Curr Opin Ophthalmol 2009;20:282–6. 10.1097/ICU.0b013e32832b7578 [DOI] [PubMed] [Google Scholar]
- 13.Walker PM, Lane KJ, Ousler GW III, et al. Diurnal variation of visual function and the signs and symptoms of dry eye. Cornea 2010;29:607–12. 10.1097/ICO.0b013e3181c11e45 [DOI] [PubMed] [Google Scholar]
- 14.Parrish RK, Palmberg P, Sheu WP, et al. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol 2003;135:688–703. 10.1016/S0002-9394(03)00098-9 [DOI] [PubMed] [Google Scholar]
- 15.Dreer LE, Girkin C, Mansberger SL. Determinants of medication adherence to topical glaucoma therapy. J Glaucoma 2012;21:234–40. 10.1097/IJG.0b013e31821dac86 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.