Abstract
Background
Patients with chronic heart failure (HF) secondary to left ventricular systolic dysfunction (LVSD) are frequently deficient in vitamin D. Low vitamin D levels are associated with a worse prognosis.
Objectives
The VINDICATE (VitamIN D treatIng patients with Chronic heArT failurE) study was undertaken to establish safety and efficacy of high-dose 25 (OH) vitamin D3 (cholecalciferol) supplementation in patients with chronic HF due to LVSD.
Methods
We enrolled 229 patients (179 men) with chronic HF due to LVSD and vitamin D deficiency (cholecalciferol <50 nmol/l [<20 ng/ml]). Participants were allocated to 1 year of vitamin D3 supplementation (4,000 IU [100 μg] daily) or matching non−calcium-based placebo. The primary endpoint was change in 6-minute walk distance between baseline and 12 months. Secondary endpoints included change in LV ejection fraction at 1 year, and safety measures of renal function and serum calcium concentration assessed every 3 months.
Results
One year of high-dose vitamin D3 supplementation did not improve 6-min walk distance at 1 year, but was associated with a significant improvement in cardiac function (LV ejection fraction +6.07% [95% confidence interval (CI): 3.20 to 8.95; p < 0.0001]); and a reversal of LV remodeling (LV end diastolic diameter -2.49 mm [95% CI: -4.09 to -0.90; p = 0.002] and LV end systolic diameter -2.09 mm [95% CI: -4.11 to -0.06 p = 0.043]).
Conclusions
One year of 100 μg daily vitamin D3 supplementation does not improve 6-min walk distance but has beneficial effects on LV structure and function in patients on contemporary optimal medical therapy. Further studies are necessary to determine whether these translate to improvements in outcomes. (VitamIN D Treating patIents With Chronic heArT failurE [VINDICATE]; NCT01619891)
Key Words: heart failure, left ventricular function, remodeling, vitamin D
Abbreviations and Acronyms: 1,25 (OH)2 vitamin D3, calcitriol; 25(OH) vitamin D2, ergocalciferol; 25 (OH) vitamin D3, cholecalciferol; AF, atrial fibrillation; CI, confidence interval; CMR, cardiac magnetic resonance; HF, heart failure; LV, left ventricular; PTH, parathyroid hormone; SR, sinus rhythm
Chronic heart failure (HF) secondary to left ventricular (LV) systolic dysfunction (LVSD) is a common condition affecting 5 million individuals in the United States (1) and a similar number in Western Europe (2). While the prognosis of chronic HF has improved substantially during the last 2 decades (3), mortality remains high with 50% of patients dying within 5 years of diagnosis 4, 5.
Patients suffering from cardiovascular disease are frequently deficient in the steroid hormone vitamin D, and vitamin D deficiency has been shown to be associated with the development of chronic HF in a number of studies 6, 7, 8, 9, 10. Approximately 90% of chronic HF patients have hypovitaminosis D (11), even in sunny climates (12). The agent has a range of pleiotropic effects that in the setting of chronic HF may impact on disease severity 13, 14, but despite this, clinical trials examining vitamin D supplementation in chronic HF patients have to date been inconclusive 15, 16.
The aim of the VINDICATE (VitamIN D treatIng patients with Chronic heArT failurE) study was to describe the safety and efficacy of long-term, high-dose vitamin D3 supplementation on submaximal exercise capacity and cardiac function in patients with chronic HF due to LVSD.
Methods
Study population
VINDICATE was a randomized placebo-controlled double-blind trial of vitamin D supplementation in vitamin D−deficient chronic HF patients on optimal medical therapy. Patients were eligible if they had stable (>3 months) New York Heart Association functional class II or III symptoms, a left ventricular ejection fraction (LVEF) ≤45% on maximally tolerated medical therapy (>3 months) and a 25(OH) vitamin D level of <50 nmol/l (<20 ng/ml).
Patients were ineligible if they were taking or had taken calcium or other vitamin supplements in the last 3 months; if their chronic HF was due to untreated valvular heart disease, anemia or thyrotoxicosis; if they had existing indications for vitamin D supplementation (e.g., previous osteoporotic fracture or symptoms of osteomalacia); if they had a history of primary hyperparathyroidism, sarcoidosis, tuberculosis or lymphoma, a cholecalciferol concentration at the time of screening >50 nmol/l (20 ng/ml); or if there was significant renal dysfunction (estimated glomerular filtration rate <30 ml/min).
Allocation and intervention
Patients enrolled into VINDICATE were allocated in blocks of 20 using minimization balancing for etiology of chronic HF (ischemic/non-ischemic), diabetes mellitus, sex, chronic obstructive pulmonary disease (requiring use of regular bronchodilators), and ethnic origin (Caucasian/non-Caucasian). Each participant was asked to take 2 tablets per day providing either a total of 100 μg cholecalciferol (4,000 IU daily) or placebo (Cultech, Port Talbot, Wales, United Kingdom).
The supplement and dose were chosen based upon guidelines for studies of vitamin D supplementation (17). These guidelines suggest that studies should: 1) aim to replace physiological requirements, supplementing between 75 and 250 μg/day; 2) last at least 9 months; 3) supplement with vitamin D3 (not D2); 4) assay supplements for potency; 5) include a regular serum measurement of 25(OH)D3 levels; and 6) aim to achieve serum levels in patients on active therapy between 100 and 160 nmol/l (40 ng/ml to 64 ng/ml). Also, on the basis of recent data demonstrating the adverse effect of hyperparathyroidism in chronic HF (18), we chose a dose likely to suppress parathyroid hormone (PTH) release. Our proof of concept study, using the same inclusion and exclusion criteria and protocol as VINDICATE, had previously demonstrated the efficacy of 4,000 IU daily to achieve positive remodeling with significant reductions in left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), and left ventricular end-diastolic dimension (LVEDD). The consort diagram and results from this study are presented in online supplementary datasets (Online Figure 1, Online Tables 1 and 2). A simple linear model-based trend test from this study demonstrated a significant decrease in PTH over the year (p = 0.0095) in those allocated vitamin D, with no such trend in patients allocated to the placebo arm (p = 0.977) (Online Figure 2) (19).
Outcome variables
The pre-specified primary endpoint in VINDICATE was the difference in change in 6-min walk test distance (6MWT) (baseline to 12 months) between the 2 groups. Key pre-specified secondary endpoints included cardiac structure and function, and safety endpoints of serum calcium concentration, renal function, and vitamin D levels. Hypervitaminosis D was defined as 25(OH)D3 >200 nmol/l (80 ng/ml), and hypercalcemia as >2.6 nmol/l (10.4 mg/dl).
Study procedures
At baseline each patient performed a 6MWT according to standard criteria (20). Each patient also underwent echocardiography and blood sampling for serum calcium, serum creatinine, vitamin D, and PTH levels. Patients were also invited to undergo cardiac magnetic resonance (CMR) imaging to measure LV volumes. Subsequent visits took place at 3, 6, 9, and 12 months and blood draws were repeated at each visit for safety data.
Serum biochemistry
Serum 25(OH)D2 and 25(OH)D3 were analyzed by tandem mass spectrometry. Samples were prepared using a protein precipitation reagent containing deuterated cholecalciferol. The supernatant was analyzed on an API5000 LC-MS/MS (AB SCIEX, Warrington, United Kingdom) in atmospheric-pressure chemical ionization mode. The inter-assay coefficient of variability was <10% at all concentrations ranging from 12 nmol/l to 159 nmol/l (4.8 ng/ml to 63.7 ng/ml). Ergocalciferol and cholecalciferol concentrations were summed and reported as total 25(OH)D. We defined deficiency and insufficiency of vitamin D concentrations as <50 nmol/l (20 ng/ml) and <75 nmol/l (30 ng/ml), respectively 21, 22. We also measured serum calcium, creatinine, and PTH (Siemens Advia and Centaur, Siemens Healthcare Diagnostics, Camberley, United Kingdom). To confirm effective conversion of the supplement, we also measured 1,25(OH)D3 by radioimmunoassay (IDS, Boldon, United Kingdom) at baseline and 12 months.
Echocardiography
Echocardiography was performed on all patients at baseline and LV function was assessed according to European Society of Cardiology criteria using Simpson’s biplane measure to determine LVEF (23). Echocardiography was repeated at 12 months. Echocardiograms at both time points were analyzed offline at the end of the study by 2 senior echocardiographers blinded to patient treatment.
CMR imaging
CMR studies were performed on dedicated 1.5-T or 3-T CMR systems (Philips Healthcare, Best, the Netherlands). The same system was used for baseline and follow-up studies (at 12 months) of individual patients. A multislice multiphase data set covering the entire left ventricle in 10 to 12 short axis slices was acquired using a validated 2-dimensional balanced steady-state free precession pulse sequence (TR 2.8 ms, TE 1.4 ms, flip angle 55°, spatial resolution 2.0 mm × 2.0 mm × 10 mm, no interslice gap, 30 phases/cardiac cycle, 1 slice per breath-hold). Offline analysis by an experienced CMR observer using QMASS V7.0 software (Medis, Leiden, the Netherlands) blinded to study allocation derived end-diastolic and end-systolic LV volumes and LVEF.
Sample size
VINDICATE was powered to provide information on the patient-oriented outcome of 6MWT. A trial of iron supplementation in a similar patient group had demonstrated that improvements of 30 m to 40 m could be expected with this type of intervention (24). We assumed, based upon our preliminary data from a pilot study (19), that there would be a change between the 2 groups at 12 months of 30 m. The SD of change in 6MWT was estimated from these data; the upper limit of the 80% confidence interval (CI) (estimated using bootstrapping) was used in these calculations to allow for the small sample size in the proof of concept. This determined that 210 patients were required to have 90% power to show a difference in change in 6MWT of 28 m or more with 5% significance (SD = 62). We aimed to recruit 230 patients (115 per group) to allow for ∼10% dropout.
Statistical analysis
Differences in baseline variables between allocations were tested using Student t tests (continuous data) or the chi-square test (categorical data). The analysis of primacy for the main efficacy endpoints was based on analysis of covariance linear models relating differences in the final walk distance and imaging variables by treatment allocation, adjusting for baselines values and reported with 95% CIs (25). All significance tests were 2-sided and called significant at the 5% level. All analyses were conducted in Stata (version 14, StataCorp., College Station, Texas).
Ethical and safety considerations
A single unblinded observer with no involvement in the patients’ care or study follow-up (J.H.B.) reviewed each vitamin D result at each time point for safety. An agreed operating procedure for any subject who developed a serum vitamin D concentration >200 nmol/l (80 ng/ml) involved reducing the dose of treatment from 2 to 1 tablets per day to maintain patient blinding.
Results
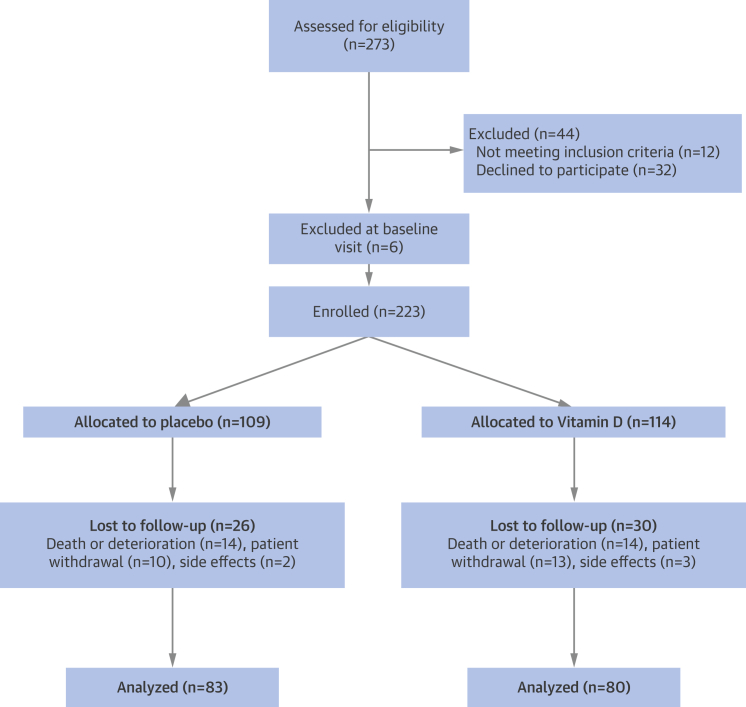
We enrolled 229 patients into VINDICATE. Six patients were found to be ineligible at the baseline visit, leaving 223 patients randomized to treatment. Figure 1 describes patient recruitment and loss to follow-up. A total of 163 patients completed the study. Baseline characteristics divided by treatment allocation are shown in Table 1. There were no important clinical differences at baseline between patients completing the study and those who dropped out. The 2 groups of completing participants were balanced for baseline clinical variables (Table 1).
Figure 1.
Consort Diagram Demonstrating Patient Enrollment and Disposition for VINDICATE
VINDICATE = VitamIN D treatIng patients with Chronic heArT failurE.
Table 1.
Patient Demographics (VINDICATE) at Randomization: Intention-to-Treat Population
| Total (n = 163) | Placebo (n = 83) | Vitamin D (n = 80) | |
|---|---|---|---|
| Male | 129 (79.1) | 62 (74.7) | 67 (83.8) |
| Age, yrs | 68.7 ± 13.10 | 69.0 ± 13.78 | 68.5 ± 12.45 |
| Caucasian | 146 (90.0) | 74 (89.0) | 72 (90.0) |
| Etiology | |||
| Ischemic heart disease | 94 (57.7) | 50 (60.2) | 44 (55.0) |
| Nonischemic cardiomyopathy | 61 (37.4) | 29 (34.9) | 32 (40.0) |
| Valvular heart disease | 8 (4.9) | 4 (4.8) | 4 (5.0) |
| Diabetes mellitus | 37 (22.7) | 20 (24.1) | 17 (21.3) |
| BMI, kg/m2 | 30.0 ± 11.41 | 30.3 ± 14.36 | 29.8 ± 7.26 |
| NYHA functional class | |||
| II | 145 (89.0) | 71 (85.5) | 74 (92.5) |
| III | 18 (11.0) | 12 (14.5) | 6 (7.5) |
| Beta blockers | 155 (95.1) | 79 (95.2) | 76 (95.0) |
| ACEi/ARB | 150 (92.0) | 76 (91.6) | 74 (92.5) |
| Furosemide dose, mg/day | 61.4 ± 46.38 | 64.4 ± 52.07 | 58.6 ± 41.00 |
| Digoxin | 29 (18.0) | 15 (18.3) | 14 (17.7) |
| Spironolactone | 83 (51.2) | 41 (50.0) | 42 (52.5) |
| Device (ICD or CRT) | 48 (29.5) | 27 (32.5) | 21 (26.3) |
| Atrial fibrillation, | 68 (45.0) | 33 (42.9) | 35 (47.3) |
| Baseline heart rate, beats/min | 70.5 ± 13.10 | 72.7 ± 14.72 | 68.2 ± 10.86 |
| Systolic BP, mm Hg | 120.3 ± 20.81 | 122.9 ± 22.44 | 117.6 ± 18.74 |
| Diastolic BP, mm Hg | 71.2 ± 13.21 | 72.8 ± 14.96 | 70.0 ± 10.99 |
| 6-min walk test, m | 292.9 (120.35) | 283.7 (116.84) | 302.2 (123.81) |
| LVEF % | 26.1 ± 10.68 | 26.5 ± 10.62 | 25.6 ± 10.80 |
| LVEDD, mm | 57.8 ± 7.58 | 58.0 ± 6.49 | 57.6 ± 8.62 |
| LVESD, mm | 50.3 ± 8.50 | 50.7 ± 7.58 | 49.8 ± 9.42 |
| LVEDV, ml | 163.0 ± 66.60 | 164.1 ± 60.07 | 161.8 ± 73.58 |
| LVESV, ml | 115.4 ± 59.39 | 119.4 ± 53.30 | 111.0 ± 63.58 |
| 25(OH) Vitamin D, nmol/l | 37.3 ± 22.56 | 36.4 ± 20.24 | 38.2 ± 24.81 |
| Parathyroid hormone, pmol/l | 11.4 ± 8.09 | 11.7 ± 7.50 | 11.0 ± 8.75 |
| Creatinine, μmol/l | 96 ± 29.3 | 94.4 ± 29.42 | 96.6 ± 29.26 |
Values are n (%) or mean ± SD. Conversion factors: vitamin D nmol/l · 0.4 = ng/ml; creatinine mmol/l · 0.11 = mg/dl; calcium mmol/l · 4 = mg/dl; parathyroid hormone pmol/l · 9.4 = pg/ml.
ACEi = angiotensin-converting enzyme inhibitor; ARB = aldosterone receptor blocker; BMI = body mass index; BP = blood pressure; CRT = cardiac resynchronization therapy; ICD = implantable cardioverter defibrillator; LVEDD = left ventricular end-diastolic diameter; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic diameter; LVESV = left ventricular end-systolic volume; NYHA = New York Heart Association functional class; VINDICATE = VitamIN D treatIng patients with Chronic heArT failurE.
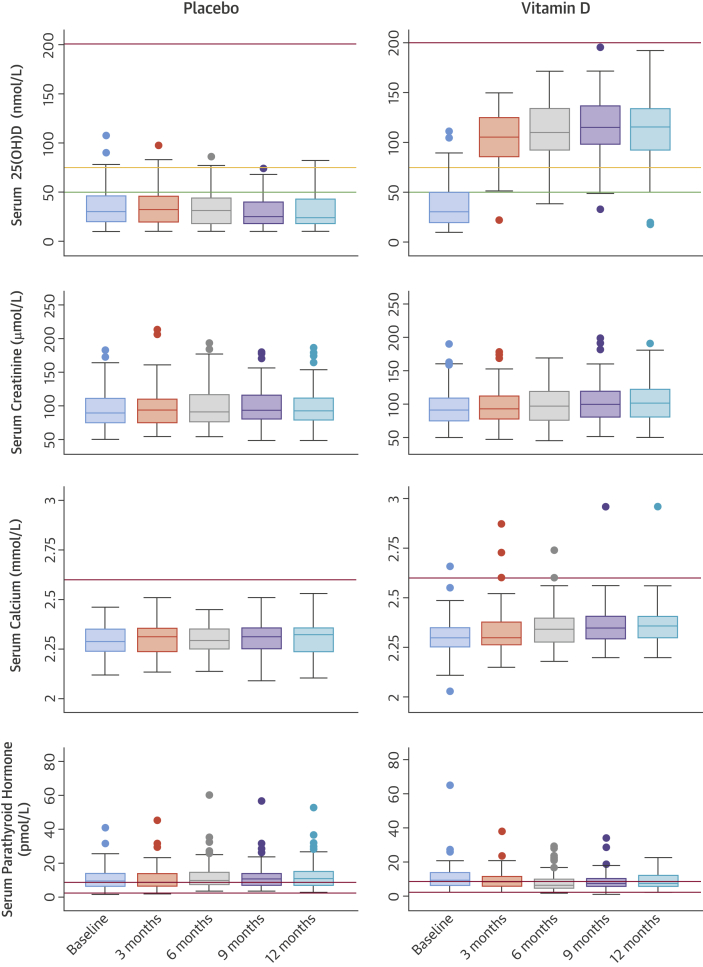
The vitamin D3 supplement was well-tolerated and achieved sustained normal serum 25(OH)D concentrations by 3 months post-randomization, indicating excellent adherence to treatment (Figure 2). Patients in the placebo arm had lower median concentrations of 25(OH)D at 12-months post-randomization, (24.5 nmol/l; range: 10.0 to 81.8 nmol/l [9.8 ng/ml; range: 4 to 32.7 ng/ml]) than patients in the active supplement arm (115 nmol/l; range: 17.8 to 193 nmol/l [46 ng/ml; range: 7.1 to 77.2 ng/ml]; p < 0.0001) confirming the effectiveness of the vitamin D supplementation in normalizing vitamin D levels. The supplement also effectively normalized 1,25 (OH)2 vitamin D3 (calcitriol) levels to 121 pmol/l (range: 40 to 331 pmol/l [46.5 pg/ml; range: 15.4 to 127.3 pg/ml]) at 12 months and also suppressed PTH levels, leading to lower PTH levels in subjects allocated vitamin D (8.70 pmol/l; range: 1.28 to 22.2 pmol/l [82 ng/ml, range: 12 to 209 ng/ml]) than those allocated placebo (10.80 pmol/l; range: 2.80 to 53.10 pmol/l [102 ng/ml, range: 26 to 499 ng/ml]); analysis of covariance difference in mean change was -3.63 pmol/l, 95% CI: -5.24 to -2.03 pmol/l (-34 ng/ml, 95% CI: -49 to -19 ng/ml); p < 0.0001.
Figure 2.
Median and Interquartile Ranges for Vitamin D, Creatinine, Calcium, and Parathyroid Concentrations at 3 Monthly Time Points in VINDICATE by Treatment Allocation
Vitamin D concentrations are described in relation to deficiency (green line), sufficiency (orange line), and the accepted upper limit for hypervitaminosis D (red line). Serum calcium levels are described in relation to upper limit of normal range (red line), and serum parathyroid hormone concentrations in relation to the normal range (between red lines). Conversion factors: vitamin D nmol/l · 0.4 = ng/ml; creatinine mmol/l · 0.11 = mg/dl; calcium mmol/l · 4 = mg/dl; parathyroid hormone pmol/l · 9.4 = pg/ml. VINDICATE = VitamIN D treatIng patients with Chronic heArT failurE.
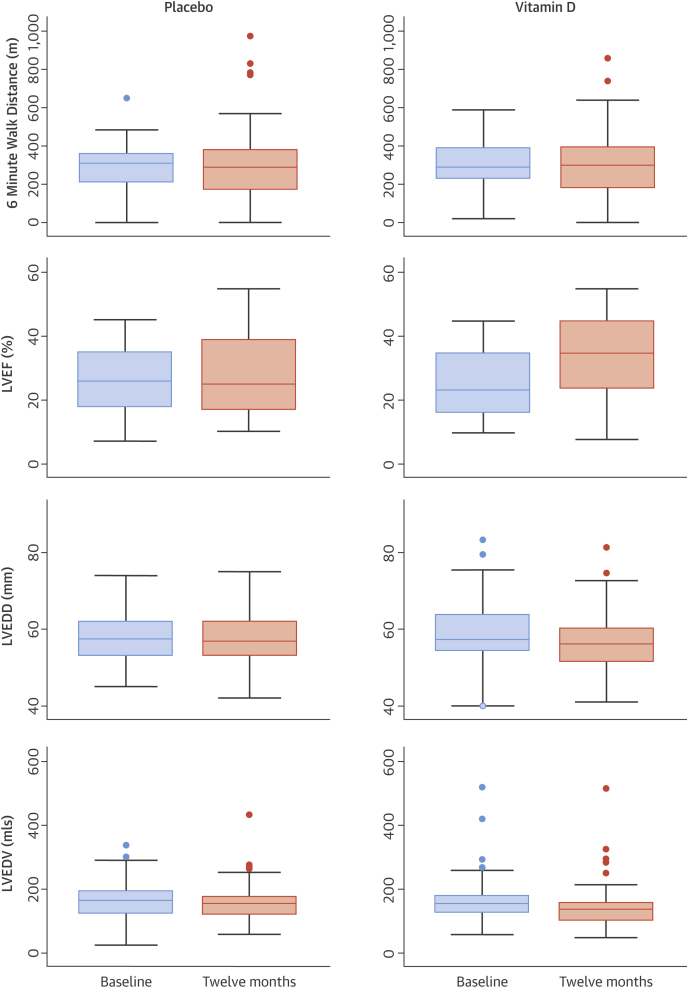
No patient was observed to suffer hypervitaminosis D according to our pre-specified safety concentration of 200 nmol/l (80 ng/ml) 25(OH)D and no subject required a down-titration of dose. One patient with borderline hypercalcemia at baseline (2.66 mmol/l [10.64 mg/dl]) had persistent hypercalcemia throughout the study, and 1 other patient with hypercalcemia at 3 months (2.73 mmol/l [10.9 mg/dl]) had a normal calcium level by 6 months and throughout the remainder of the study (Figure 2). There was no concerning change in renal function (Figure 2) and there were no study drug-related admissions or adverse events. Twelve months of 4,000 IU of cholecalciferol did not improve or preserve 6MWT distance in chronic HF patients (Figure 3).
Figure 3.
Median and Interquartile Ranges for 6-Minute Walk Test Distance, and LVEF, LVEDD, and LVEDV Measured by Echocardiography at Baseline and Final Visit in VINDICATE by Treatment Allocation
LVEDD = left ventricular end-diastolic dimension; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; VINDICATE = VitamIN D treatIng patients with Chronic heArT failurE.
At 12 months, patients in the vitamin D arm had a greater improvement in echocardiographic measures of LV function compared with patients randomized to placebo. Changes in the treatment versus placebo arms were as follows: for LVEF +7.65% (95% CI: 5.21% to 10.09%) and +1.36% (95% CI: -0.38% to 3.11%), respectively (p < 0.0001); for LVEDD -2.45 mm (95% CI: -3.70 to -1.21 mm) and 0.08 mm (95% CI: -1.25 to 1.10 mm), respectively (p = 0.002); and for LVESD were -2.72 mm (95% CI: -4.52 to -0.92 mm) and -0.99 mm (95% CI: -2.31 to 0.33 mm), respectively (p = 0.043). Changes in LV volumes in the treatment versus the placebo arms were: LVEDV -16.47 ml (95% CI: -25.71 to -7.22 ml) and -3.83 ml (95% CI: -13.36 to 5.70 ml), respectively (p = 0.04); and LVESV -18.77 ml (95% CI: -25.96 to 9.59 ml) and -8.49 ml (95% CI: -17.98 to 1.01 ml), respectively (p = 0.041) (Table 2, Figure 3). There was a dose-response relationship between the increase in vitamin D levels and the increase in LVEF (coefficient 0.04; p = 0.023) and decrease in LVEDV (coefficient -0.02; p = 0.035).
Table 2.
Change in Primary and Secondary Outcome Variables in VINDICATE at 12 Months Post-Randomization: Intention-to-Treat Population
| Endpoint | Randomized Treatment | Mean Change After 12 Months | ANCOVA Difference in Mean Change |
p Value |
|---|---|---|---|---|
| Primary outcome | ||||
| 6-min walk distance, m | Placebo | 10.10 (-20.77 to 40.96) | -24.11 (-65.81 to 17.60) | 0.255 |
| Vitamin D | -12.56 (-40.80 to 15.68) | |||
| Secondary outcomes | ||||
| LVEF, % | Placebo | 1.36 (-0.38 to 3.11) | 6.07 (3.20 to 8.94) | <0.001 |
| Vitamin D | 7.65 (5.21 to 10.09) | |||
| LVEDD, mm | Placebo | -0.08 (-1.25 to 1.10) | -2.49 (-4.09 to -0.90) | 0.002 |
| Vitamin D | -2.45 (-3.70 to -1.21) | |||
| LVESD, mm | Placebo | -0.99 (-2.31 to 0.33) | -2.09 (-4.11 to -0.06) | 0.043 |
| Vitamin D | -2.72 (-4.52 to -0.92) | |||
| LVEDV, ml | Placebo | -3.83 (-13.36 to 5.70) | -13.11 (-25.63 to -0.60) | 0.040 |
| Vitamin D | -16.47 (-25.71 to -7.22) | |||
| LVESV, ml | Placebo | -8.49 (-17.98 to 1.01) | -12.65 (-24.76 to -0.54) | 0.041 |
| Vitamin D | -18.77 (-25.96 to -9.59) | |||
Values are mean change (95% confidence intervals); 95% significance shown in bold.
ANCOVA = analysis of covariance; other abbreviations as in Table 1.
Enrollment into VINDICATE did not mandate CMR imaging, and one-third of patients in VINDICATE had cardiac devices incompatible with CMR imaging. Only 69 patients volunteered to undergo baseline CMR scanning. The CMR data are further limited as a result of withdrawal or death during follow-up (n = 8), device implantation between baseline and follow-up (n = 2 implantable cardioverter defibrillators), patient refusal to undergo a second scan (n = 17), and technical problems with the second scan, such that we only had 34 patients with serial CMR images. Baseline characteristics of these patients are shown in Online Table 3. Patients agreeing to serial CMR scans were younger (61.5 years [range: 36.7 to 84.8 years] vs. 71.3 years [range: 28.1 to 92.3 years]; p < 0.0001) had better renal function (creatinine: 86 μmol/l [range: 43 to 114 μmol/l] vs. 102 μmol/l [range: 48 to 245 μmol/l]; p = 0.007) and were non-significantly less deficient in 25-(OH) vitamin D at baseline (43.9 nmol/l [range: 10.0 to 90.4 nmol/l] vs. 35.94 nmol/l [range: 10.0 to 111.0 nmol/l] or 17.6 ng/ml [range: 4.0 to 36.2 ng/ml] vs. 14.4 ng/ml [range: 4.0 to 44.4 ng/ml]; p = 0.07), but were otherwise similar to patients who declined CMR scanning including the change in vitamin D from baseline to completion (p = 0.64). The data from serial CMR scans showed improvements in cardiac function with vitamin D, but were not statistically significant possibly due to insufficient statistical power: for LVEF 4.12% (95% CI: -0.11% to 8.35%) versus 1.19% (95% CI: -3.20% to 5.59%; p = 0.317), for LVEDV -26.12 ml (95% CI: -63.27 to 11.04 ml) versus –0.10 ml (95% CI: -12.88 to 13.07 ml; p = 0.168) and for LV -29.61 ml (95% CI: -72.40 to 13.18 ml) versus –1.36 ml (95% CI: -19.19 to 16.48 ml; p = 0.206). There was however, a dose response relationship in our CMR data, with a relationship between increases in vitamin D and reductions in LVEDV (coefficient -0.19; p = 0.050) and LVESV (coefficient -0.20; p = 0.083).
Discussion
VINDICATE aimed to examine the effect of high-dose vitamin D3 supplementation in patients with chronic HF secondary to LVSD who underwent optimal medical therapy. The results demonstrate that 4,000 IU vitamin D3 given for 12 months is safe, well tolerated, and not associated with concerning adverse biochemical effects (Central Illustration).
Central Illustration.
Vitamin D in Chronic Heart Failure: Impact on Left Ventricular Structure and Function
Vitamin D improves left ventricular ejection fractions and reduces left ventricular dimensions and volumes.
There was no effect of vitamin D supplementation on the primary endpoint of 6MWT distance but there were statistically significant and prognostically and clinically relevant improvements in the secondary outcomes of LVEF and LV dimensions and volumes, thus suggesting that vitamin D is leading to beneficial reverse remodeling.
New therapies for serious chronic conditions including chronic HF are often expensive, increasingly technical, and frequently fail to meet the rigorous demands of large phase 3 clinical trials. Vitamin D might be an inexpensive and safe additional option for chronic HF patients and may have beneficial effects on multiple features of the syndrome (13).
Patients with chronic HF are frequently deficient in vitamin D, and low vitamin D levels increase the risk of incident chronic HF (26), and are associated with more severe disease and worse outcomes in patients with chronic HF 6, 7, 8, 9, 12. Supplementation to treat or prevent osteoporotic fractures might be associated with a lower incidence of chronic HF (10).
However, despite the publication of studies exploring various doses and forms of vitamin D supplementation in patients with chronic HF, there remains considerable uncertainty regarding the benefits of this therapeutic approach. In the first study by Schleithoff et al. (15), 93 subjects received 50 μg vitamin D3 + calcium (Ca2+) per day for 9 months or placebo + Ca2+. There was a trend to improvement of LV function measured by echocardiography and a smaller increase in pro-inflammatory cytokines during follow-up in those randomized to vitamin D. Both groups were given Ca2+ and both groups had some improvement in LV function with no differences between them. Witham et al. (16) examined vitamin D2 supplementation in 105 elderly patients. Subjects were randomized to 2 doses of 100,000 IU of vitamin D2 or placebo at baseline and 10 weeks and assessed at 20 weeks. There was no effect on walk distance or immune function, and a slight deterioration in quality of life. The population in that study was heterogeneous; patients with and without LVSD were included, mean N-terminal B-type natriuretic peptide levels and daily furosemide doses were lower than those seen in a usual HF population, medical therapy was not optimized, the duration of treatment was short, patients who were randomized to vitamin D remained deficient (43.4 nmol/l [17.4 ng/ml]), and PTH was not suppressed (27). Although Boxer et al. 28, 29 did not demonstrate improvements in cardiac function or objective measures of muscle strength and exercise capacity in 64 chronic HF patients (of whom 34 underwent echocardiography) randomized to weekly doses of 50,000 IU of vitamin D3 for 6 months, there was an improvement in serum aldosterone and quality of life in those allocated the supplement. In an open-label study, Schroten et al. (30) demonstrated a reduction in plasma renin concentration after 6 weeks of 2,000 IU vitamin D3 daily in 101 patients with chronic HF. Finally, Dalbeni et al. (31) noted an increase in LVEF of almost 7% after only 25 weeks in 13 patients randomized to 600,000 IU vitamin D3 at baseline and 2 further doses of 100,000 IU at 10 weeks and 20 weeks, whereas 10 patients randomized to placebo had a reduction in LVEF of more than 4%. The authors did not comment on cardiac dimensions and there was an increase in natriuretic peptide levels in both groups. In contrast to these studies, VINDICATE is a double-blind, placebo-controlled study of an oral non−calcium-based daily supplement of 4,000 IU of vitamin D3 administered for 12 months in patients with chronic HF due to LVSD on otherwise optimal medical therapy. The supplement led to consistent biochemical evidence of replenishment and an effective suppression of PTH levels.
The primary endpoint of VINDICATE was change in 6MWT distance. The study was based upon pilot data and powered to detect a 28-m difference between the 2 groups at 12 months (19). The variability in the walk distance measure at baseline was much greater than predicted from our pilot study, such that our sample size only had 7% post hoc power to detect a difference between the groups. VINDICATE was therefore underpowered to detect a clinically relevant change in walk distance. Six-minute walk distance is an increasingly frequently used patient-oriented outcome measure, but has greater variability than objective surrogate endpoints (20). The findings from VINDICATE have implications for future studies using 6-min walk distance as an outcome measure.
However, our secondary endpoints of cardiac function and structure measured by echocardiography were highly statistically and clinically significant, with improvements in LVEF, and LV dimensions and volumes. Similar changes were seen in a subgroup of patients agreeing to serial CMR imaging, although they did not reach conventional levels of statistical significance due to lack of power.
A pathophysiological hallmark of chronic HF secondary to LVSD is a progressive increase in LV cavity dimensions and impaired contractility, a process known as LV remodeling (32). Current accepted therapies for chronic HF which afford HF patients improvements in survival such as angiotensin converting enzyme inhibitors (33), beta-adrenoceptor antagonists 34, 35, and cardiac resynchronization therapy (36) have also been shown to have a favorable effect on LV remodeling by delaying progression of, or reversing LV dilatation. The degree of favorable remodeling induced by these treatments is related to long-term outcomes (37). It is therefore plausible that the improvements in cardiac function demonstrated in VINDICATE have the potential to improve outcomes.
How does vitamin D contribute to beneficial remodeling?
Vitamin D deficiency could contribute to adverse remodeling through 2 major pathways. Vitamin D deficiency could lead to cardiomyocyte dysfunction by interfering with Ca2+ transport (38) at a cellular concentration. HF is a condition of intracellular calcium overload that adversely affects both contraction and relaxation. Furthermore, vitamin D deficiency might contribute to cardiomyocyte hypertrophy, interstitial inflammation, and fibrosis (39). Hence, vitamin D deficiency could contribute to a more rapid progression to HF following myocardial damage due to more aggressive adverse remodeling (40).
However, adverse remodeling is also the result of persistent neurohormonal activation, particularly that of the renin angiotensin aldosterone system (RAAS) which strongly contributes to deteriorating cardiac function, cardiomyocyte loss, and interstitial fibrosis (41). Inhibition of the RAAS leads to attenuated or reverse LV remodeling in patients with HF (42). Vitamin D deficiency heightens RAAS activity 30, 43, whereas vitamin D supplementation seems to reduce renin synthesis (44) and plasma renin activity (43).
Study limitations
VINDICATE was performed at a single center. However, the study was based upon results from a randomized, placebo-controlled pilot study in 53 patients using the same dose for 12 months that also showed a favorable effect of vitamin D on cardiac structure and function (19). We did not examine the effect of vitamin D supplementation in patients with chronic HF and preserved ejection fraction, a group of patients who may warrant such investigation.
Conclusions
VINDICATE has demonstrated that high-dose vitamin D3 supplementation is safe, well-tolerated, and associated with a clinically relevant improvement in cardiac function in chronic HF patients already taking current optimal therapies.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: In patients with chronic HF, vitamin D deficiency is common, and high-dose vitamin D3 supplementation is safe, well tolerated, and associated with a favorable effect on cardiac function.
TRANSLATIONAL OUTLOOK: Further studies are needed to establish the mechanism by which correction of vitamin D3 deficiency improves cardiac function in patients with systolic HF and the generalizability of this response.
Acknowledgments
The authors acknowledge the consistent administrative support provided by Mrs. Andrea Marchant and Miss Lisa Trueman that made VINDICATE possible. They also acknowledge methodological and analytical advice offered by Dr. David A. Cairns of the Leeds Clinical Trials Research Unit, University of Leeds.
This research took place in the National Institute for Health Research Leeds Cardiovascular Clinical Research Facility at Leeds Teaching Hospitals NHS Trust.
Footnotes
VINDICATE was approved by the regional ethics committee [12/YH/0206], funded by the Medical Research Council-UK. Dr. Witte has received unrestricted research funding from Medtronik UK; and holds an NIHR Clinician Scientist Fellowship. Dr. Gierula holds an NIHR-HCS Doctoral Fellowship. Dr. M. T. Kearney is a British Heart Foundation Professor; and has received research funding from Medtronic UK. Dr. Paton is funded by a Leeds Charitable Foundation Fellowship. Dr. Cubbon holds an Intermediate Fellowship from the British Heart Foundation. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Appendix
For supplemental tables and figures, please see the online version of this article.
Appendix
References
- 1.Heidenreich P.A., Albert N.M., Allen L.A., et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosterd A., Hoes A.W. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cubbon R.M., Gale C.P., Kearney L.C., et al. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circ Heart Fail. 2011;4:396–403. doi: 10.1161/CIRCHEARTFAILURE.110.959882. [DOI] [PubMed] [Google Scholar]
- 4.Barker W.H., Mullooly J.P., Getchell W. Changing incidence and survival for heart failure in a well-defined older population, 1970–1974 and 1990–1994. Circulation. 2006;113:799–805. doi: 10.1161/CIRCULATIONAHA.104.492033. [DOI] [PubMed] [Google Scholar]
- 5.Jhund P.S., Macintyre K., Simpson C.R., et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation. 2009;119:515–523. doi: 10.1161/CIRCULATIONAHA.108.812172. [DOI] [PubMed] [Google Scholar]
- 6.Zittermann A., Schleithoff S.S., Frisch S., et al. Circulating calcitriol concentrations and total mortality. Clin Chem. 2009;55:1163–1170. doi: 10.1373/clinchem.2008.120006. [DOI] [PubMed] [Google Scholar]
- 7.Pilz S., März W., Wellnitz B., et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93:3927–3935. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- 8.Schierbeck L.L., Jensen T.S., Bang U., Jensen G., Kober L., Jensen J.E. Parathyroid hormone and vitamin D—markers for cardiovascular and all-cause mortality in heart failure. Eur J Heart Fail. 2011;13:626–632. doi: 10.1093/eurjhf/hfr016. [DOI] [PubMed] [Google Scholar]
- 9.Liu L., Chen M., Hankins S.R., et al. Serum 25-hydroxyvitamin D concentration and mortality from heart failure and cardiovascular disease, and premature mortality from all-cause in United States adults. Am J Cardiol. 2012;110:834–839. doi: 10.1016/j.amjcard.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Ford J.A., MacLennan G.S., Avenell A., et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746–755. doi: 10.3945/ajcn.113.082602. [DOI] [PubMed] [Google Scholar]
- 11.Kim D.H., Sabour S., Sagar U.N., Adams S., Whellan D.J. Prevalence of hypovitaminosis D in cardiovascular disease (from the National Health and Nutrition Examination Survey 2001-2004) Am J Cardiol. 2008;102:1540–1544. doi: 10.1016/j.amjcard.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 12.Ameri P., Ronco D., Casu M., et al. High prevalence of vitamin D deficiency and its association with left ventricular dilation: an echocardiography study in elderly patients with chronic heart failure. Nutr Metab Cardiovasc Dis. 2010;20:633–640. doi: 10.1016/j.numecd.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Witte K.K., Byrom R. Micronutrients for chronic heart failure: end of the road or path to enlightenment? J Am Coll Cardio HF. 2014;2:318–320. doi: 10.1016/j.jchf.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Gardner D.G., Chen S., Glenn D.J. Vitamin D and the heart. Am J Physiol Regul Integr Comp Physiol. 2013;305:R969–R977. doi: 10.1152/ajpregu.00322.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schleithoff S.S., Zittermann A., Tenderich G., et al. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, radomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 16.Witham M.D., Crighton L.J., Gillespie N.D., Struthers A.D., McMurdo M.E. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail. 2010;3:195–201. doi: 10.1161/CIRCHEARTFAILURE.109.907899. [DOI] [PubMed] [Google Scholar]
- 17.Vasquez A., Cannell J. Calcium and vitamin D in preventing fractures: data are not sufficient to show inefficacy. BMJ. 2005;331:108–109. doi: 10.1136/bmj.331.7508.108-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahokas R.A., Sun Y., Bhattacharya S.K., Gerling I.C., Weber K.T. Aldosteronism and a proinflammatory vascular phenotype. Role of Mg2+, Ca2+ and H2O2 in peripheral blood mononuclear cells. Circulation. 2005;111:51–57. doi: 10.1161/01.CIR.0000151516.84238.37. [DOI] [PubMed] [Google Scholar]
- 19.Lowry J., Gierula J., Byrom R., et al. Vitamin d supplementation improves the size and function of the left ventricle in patients with heart failure. Eur Heart J. 2015;36(Suppl 1):A665–A666. [Google Scholar]
- 20.Olsson L.G., Swedberg K., Clark A.L., Witte K.K., Cleland J.G. Six minute corridor walk test as an outcome measure for the assessment of treatment in randomized, blinded intervention trials of chronic heart failure: a systematic review. Eur Heart J. 2005;26:778–793. doi: 10.1093/eurheartj/ehi162. [DOI] [PubMed] [Google Scholar]
- 21.Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 22.Rosen C.J. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364:248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 23.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Anker S.D., Comin Colet J., Filippatos G., et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S., Paul J., Nantha-Aree M., et al. Empirical comparison of four baseline covariate adjustment methods in analysis of continuous outcomes in randomized controlled trials. Clin Epidemiol. 2014;6:227–235. doi: 10.2147/CLEP.S56554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zittermann A., Fischer J., Schleithoff S.S., et al. Patients with congestive heart failure and healthy controls differ in vitamin D-associated lifestyle factors. Int J Vitam Nutr Res. 2007;77:280–288. doi: 10.1024/0300-9831.77.4.280. [DOI] [PubMed] [Google Scholar]
- 27.Begg G.A., Cleland J.G., Witte K.K. Letter by Begg et al. regarding the article “The effects of vitamin D supplementation on physical function and quality of life in older heart failure patients: a randomized controlled trial.”. Circ Heart Fail. 2010;3:e24. doi: 10.1161/CIRCHEARTFAILURE.110.952549. [DOI] [PubMed] [Google Scholar]
- 28.Boxer R.S., Hoit B.D., Schmotzer B.J., Stefano G.T., Gomes A., Negrea L. The effect of vitamin d on aldosterone and health status in patients with heart failure. J Card Fail. 2014;20:334–342. doi: 10.1016/j.cardfail.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boxer R.S., Kenny A.M., Schmotzer B.J., Vest M., Fiutem J.J., Piña I.L. A randomized controlled trial of high dose vitamin D3 in patients with heart failure. J Am Coll Cardiol HF. 2013;1:84–90. doi: 10.1016/j.jchf.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroten N.F., Ruifrok W.P., Kleijn L., et al. Short-term vitamin D3 supplementation lowers plasma renin activity in patients with stable chronic heart failure: an open-label, blinded end point, randomized prospective trial (VitD-CHF trial) Am Heart J. 2013;166:357–364. doi: 10.1016/j.ahj.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Dalbeni A., Scaturro G., Degan M., Minuz P., Delva P. Effects of six months of vitamin D supplementation in patients with heart failure: a randomized double-blind controlled trial. Nutr Metab Cardiovasc Dis. 2014;24:861–868. doi: 10.1016/j.numecd.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Mann D.L., Bristow M.R. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 33.Abdulla J., Barlera S., Latini R., et al. A systematic review: effects of angiotensin converting enzyme inhibition on left ventricular volumes and ejection fraction in patients with a myocardial infarction and in patients with left ventricular dysfunction. Eur J Heart Fail. 2007;9:129–135. doi: 10.1016/j.ejheart.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Packer M., Antonopoulos G.V., Berlin J.A., Chittams J., Konstam M.A., Udelson J.E. Comparative effects of carvedilol and metoprolol on left ventricular ejection fraction in heart failure: results of a meta analysis. Am Heart J. 2001;141:899–907. doi: 10.1067/mhj.2001.115584. [DOI] [PubMed] [Google Scholar]
- 35.van de Ven L.L., van Veldhuisen D.J., Goulder M., Zilahi Z., Meyer W.R., Willenheimer R. The effect of treatment with bisoprolol-first versus enalapril-first on cardiac structure and function in heart failure. Int J Cardiol. 2010;144:59–63. doi: 10.1016/j.ijcard.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 36.Foley P.W., Chalil S., Khadjooi K., Irwin N., Smith R.E., Leyva F. Left ventricular reverse remodelling, long-term clinical outcome, and mode of death after cardiac resynchronization therapy. Eur J Heart Fail. 2011;13:43–51. doi: 10.1093/eurjhf/hfq182. [DOI] [PubMed] [Google Scholar]
- 37.Cohn J.N., Ferrari R., Sharpe N. Cardiac remodelling—concept and clinical implications: a consensus paper from an international forum on cardiac remodelling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 38.Weisshaar R.E., Simpson R.U. Involvement of vitamin D3 with cardiovascular function II direct and indirect effects. Am J Physiol. 1987;253:E675–E683. doi: 10.1152/ajpendo.1987.253.6.E675. [DOI] [PubMed] [Google Scholar]
- 39.Chen S., Law C.S., Grigsby C.L., et al. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124:1838–1847. doi: 10.1161/CIRCULATIONAHA.111.032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber K.T., Weglicki W.B., Simpson R.U. Macro- and micronutrient dyshomeostasis in the adverse structural remodelling of myocardium. Cardiovasc Res. 2009;81:500–508. doi: 10.1093/cvr/cvn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Artaza J.N., Mehrotra R., Norris K.C. Vitamin D and the cardiovascular system. Clin J Am Soc Nephrol. 2009;4:1515–1522. doi: 10.2215/CJN.02260409. [DOI] [PubMed] [Google Scholar]
- 42.Konstam M.A., Kramer D.G., Patel A.R., Maron M.S., Udelson J.E. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. J Am Coll Cardiol Img. 2011;4:98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Xiang W., Kong J., Chen S., et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrin Metab. 2005;288:E125–E132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 44.Li Y.C., Kong J., Wei M., Chen Z.F., Liu S.Q., Cao L.P. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.