Abstract
BACKGROUND
Trimethylamine N-oxide (TMAO), a gut microbiota metabolite from dietary phosphatidylcholine, has mechanistic links to atherosclerotic coronary artery disease (CAD) pathogenesis and is associated with adverse outcomes.
OBJECTIVES
We examined the relationship between plasma TMAO levels and the complexity and burden of CAD and degree of subclinical myonecrosis.
METHODS
We studied 353 consecutive stable patients with evidence of atherosclerotic CAD detected on elective coronary angiography between 2012 and 2014 who had high-sensitivity cardiac troponin T (hs-cTnT) measured. SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) scores and lesion characteristics were used to quantify atherosclerotic burden. Fasting plasma TMAO was measured by mass spectrometry.
RESULTS
In this prospective cohort study, median TMAO level was 5.5 μM (interquartile range [IQR]: 3.4 to 9.8 μM); median SYNTAX score was 11.0 (IQR: 4.0 to 18.5); and 289 (81.9%), 40 (11.3%) and 24 (6.8%) patients had low (0 to 22), intermediate (23 to 32), and high (≥33) SYNTAX scores, respectively. Plasma TMAO levels correlated (all p < 0.0001) with SYNTAX score (r = 0.61), SYNTAX score II (r = 0.62), and hs-cTnT (r = 0.29). Adjusting for traditional risk factors, body mass index, medications, lesion characteristic, renal function, and high-sensitivity C-reactive protein, elevated TMAO levels remained independently associated with a higher SYNTAX score (odds ratio [OR]: 4.82; p < 0.0001), SYNTAX score II (OR: 1.88; p = 0.0002) but not with subclinical myonecrosis (OR: 1.14; p = 0.3147). Elevated TMAO level was an independent predictor of the presence of diffuse lesions, even following adjustments for traditional risk factors and hs-cTnT (OR: 2.05; 95% confidence interval: 1.45 to 2.9; p = 0.0001).
CONCLUSIONS
Fasting plasma TMAO levels are an independent predictor for high atherosclerotic burden in patients with CAD.
Keywords: diffuse, focal, myonecrosis, risk factors, SYNTAX score, troponin T
INTRODUCTION
The incidence of atherosclerotic coronary artery disease (CAD), a common cardiovascular disease (CVD), has been increasing and remains a leading cause of death around the world (1,2). Despite the considerable attention to traditional risk factors (including age, sex, hypertension, dyslipidemia, smoking, and diabetes) and use of modern pharmacotherapies, including high-potency statin therapy, at least a 50% residual risk remains (2–4). Therefore, there is interest in identifying novel cardiovascular risk factors to improve both our understanding of the processes contributing to CVD pathogenesis that have not been explained by traditional or known risk factors as well as prevention and treatment of CVD (5).
There is growing appreciation that gut microbes participate in the overall metabolism of their host and contribute to and are associated with cardiometabolic disease phenotypes in both animal models of disease and in humans (6–9). In particular, a role for gut microbes as participants in the development of atherosclerosis and CVD has recently become appreciated (10–12). Specifically, a gut microbial metabolite, trimethylamine N-oxide (TMAO), has been shown to be atherogenic (12,13), and recent studies employing microbial transplantation into recipients confirmed a direct causal role for gut microbes in transmitting atherosclerosis susceptibility and overall TMAO production (10). TMAO arises from gut microbiota metabolism following ingestion of diets rich in phosphatidylcholine (PC or lecithin), the major dietary source of choline, and carnitine, an abundant nutrient in red meat (11,12,14,15). Moreover, elevated TMAO levels have been shown to predict future risk of major adverse cardiac events (MACE), increased prevalence of CVD, and show a relationship with the number of diseased coronary vessels (11,12,14). However, the relationship between plasma TMAO levels and detailed characterization and quantification of atherosclerosis burden has not been investigated.
The SYNTAX (SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery) trial produced the SYNTAX score, an angiographic scoring system to determine the complexity and burden of atherosclerotic CAD (16,17). The anatomical SYNTAX score has been shown to predict MACE and long-term prognosis risks among stable CAD patients who underwent coronary revascularization (18,19). However, due to some limitations of this score, including lack of clinical variables and a purely anatomical focus, the SYNTAX score II was recently developed with presumed improved prognostic value. It consists of a combination of 2 anatomical (SYNTAX score and unprotected left main CAD) and 6 clinical variables (age, creatinine clearance, left ventricular ejection fraction, sex, chronic obstructive pulmonary disease, and peripheral artery disease [PAD]). The SYNTAX score II has shown better long-term (4-year) mortality prediction between coronary artery bypass graft (CABG) surgery and percutaneous coronary intervention (PCI) than the original SYNTAX score (20). Indeed, patients with diffuse lesions present a higher risk for an adverse outcome after coronary revascularization due to a higher incidence of restenosis, poor run-off in the target coronary artery bypass graft, and increased risk of adverse cardiovascular outcomes (21–23).
Herein, we aimed to examine the relationships between plasma TMAO levels and coronary artery atherosclerotic lesion complexity and burden quantified by SYNTAX scores and lesion characteristics.
METHODS
This is a single-center prospective cohort study approved by the Cleveland Clinic Institutional Review Board and all subjects provided written informed consent.
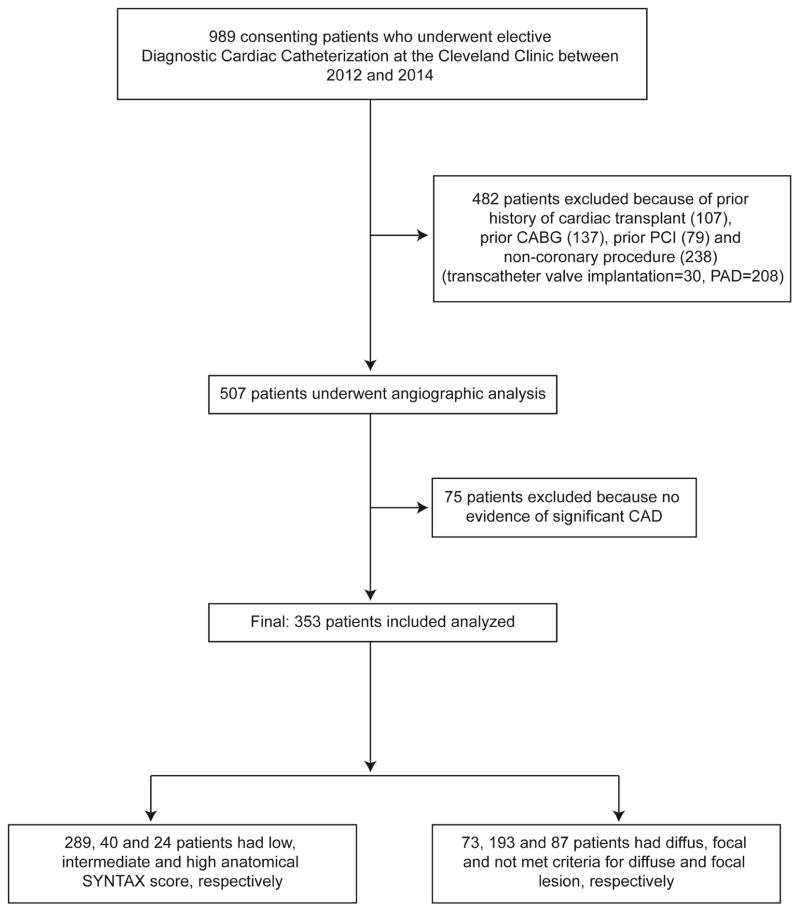
From the overall 989 patients who underwent elective diagnostic cardiac catheterization at the Cleveland Clinic between 2012 and 2014 without evidence of acute coronary syndrome (cardiac troponin T level: <0.03 μg/l), we specifically excluded patients with prior history of cardiac transplantation, PCI, CABG, and noncoronary artery procedure (structural heart procedure or PAD), and patients who had no evidence of significant CAD (total exclusion: 636 patients) (Figure 1). Therefore, 353 consecutive patients with evidence of significant CAD, defined by diameter stenosis ≥50% in vessels ≥1.5 mm, were included in this study.
FIGURE 1. CONSORT Diagram.
The flow diagram showing the process used to define the study population is depicted. CABG = coronary artery bypass surgery; PAD = peripheral artery disease; PCI = percutaneous coronary intervention; SYNTAX = SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery.
ANGIOGRAPHIC ANALYSIS
Images of coronary angiograms were obtained with Syngo Dynamics cardiovascular imaging software (Siemens Medical Solutions USA, Inc., Malvern, Pennsylvania). The SYNTAX score was calculated for each patient using a computer program consisting of sequential and interactive self-guided questions according to the SYNTAX score calculator version 2.11, and divided into tertiles according to the SYNTAX trial-defined low (0 to 22), intermediate (23 to 32), and high (≥33) SYNTAX score. The SYNTAX score II was calculated for each patient using a nomogram and stratified according to tertiles of SYNTAX score II for PCI as previously described (20,24). A diffuse lesion was defined as de novo CAD of >75% of the length of any segment(s) proximal to the lesion, at the site of the lesion, or distal to the lesion that had a vessel diameter of <2.0 mm and nondiffuse if the arteries contained no such lesion (25). A focal lesion was defined as de novo CAD of more than 50% reduction in luminal diameter and length of the lesion <20.0 mm. We indicated diffuse lesions if at least 1 diffuse lesion characteristic was present, focal lesions if pure focal lesions were present, and nondiffuse and nonfocal lesions if they did not meet the criteria for those lesions. All angiograms were reviewed by an interventional cardiologist who was blinded to TMAO level and clinical variable data.
LABORATORY TESTING
After informed consent was obtained from all patients, fasting blood samples were collected using ethylenediaminetetraacetic acid (EDTA) tubes at the time of cardiac catheterization, which were then immediately processed and frozen at −80° C until analysis. TMAO levels in plasma were determined using stable isotope dilution high-performance liquid chromatography with online tandem mass spectrometry on an API 5000 triple quadrupole mass spectrometer (AB SCIEX, Framingham, Massachusetts), as previously described (12,26). The assay described shows inter- and intraday reproducibility (all coefficient of variations <7%) and accuracy (>98.5% across low, mid and high values). Routine laboratory tests and high-sensitivity C-reactive protein (hsCRP) were measured using the Architect ci8200 platform (Abbott Laboratories, Abbott Park, Illinois,), high-sensitivity cardiac troponin T (hs-cTnT) was measured by a high-sensitivity (fifth generation) assay on a Roche Cobas e411 platform (Roche Diagnostics, Indianapolis, Indiana). Estimated glomerular filtration rate (eGFR) was estimated using the Modification of Diet in Renal Disease equation.
STATISTICAL ANALYSIS
Continuous data are presented as mean ± SD or median (interquartile range [IQR]) and compared with Student t test or nonparametric test when appropriate. Categorical variables are presented as number (%) and compared between groups with chi-square tests. Spearman correlation analysis was used to examine the associations between TMAO and all clinical and laboratory variables. Comparisons among 3 or more groups were evaluated by 1-way analysis of variance or the Kruskal-Wallis test according to whether or not the distribution was normal. Ordinal logistic regression analysis adjusting for traditional Framingham risk factors (including age, sex, hypertension, diabetes mellitus, smoking, low-density lipoprotein [LDL] cholesterol, high-density lipoprotein [HDL] cholesterol, and triglycerides) and lesion characteristic, plus hsCRP, eGFR, body mass index (BMI), and medications (angiotensin-converting enzyme inhibitor [ACEI]/angiotensin receptor blocker [ARB], statins, and aspirin) were employed to examine the association of TMAO and higher SYNTAX score, SYNTAX score II, and hs-cTnT tertiles. Univariate and multivariate logistic regression analysis was used to determine independent predictors of diffuse or focal lesion; the variables were entered into the model including the above-mentioned traditional risk factors and hs-cTnT (log-transformed). Category-free net reclassification indexes (NRI) and area under the receiver-operating characteristic curves (AUC) were calculated to evaluate the incremental predictive ability of TMAO for predicting intermediate or high SYNTAX score (>22) and SYNTAX score II (>21) and adjusted for the same covariates from the regression model with traditional risk factors, lesion characteristic, hsCRP, eGFR, BMI, and medications (27). All analyses were performed using JMP Pro version 10 (SAS Institute, Cary, North Carolina) and R (3.1.2, Vienna, Austria). A p value < 0.05 is considered statistically significant.
RESULTS
PATIENT CHARACTERISTICS
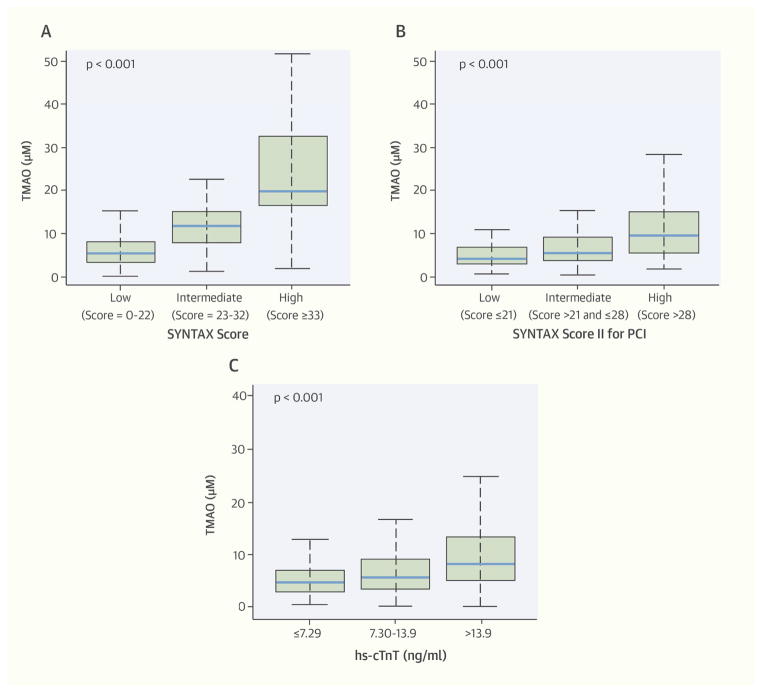
Baseline characteristics of the 353 patients included in our study are shown in Table 1. The mean age was 65 years old, 79% were men, 80% had hypertension, and 30% had diabetes. The median TMAO was 5.48 μM (IQR: 3.41 to 9.76 μM), median SYNTAX score was 11.0 (IQR: 4.0 to 18.5), and 289 (82%), 40 (11.3%), and 24 (6.8%) had low, intermediate, and high SYNTAX scores, respectively. The baseline characteristics according to SYNTAX score tertiles show that a patient with a higher SYNTAX score was more likely to be older and have hypertension and diabetes. In contrast, a history of smoking, BMI, and sex were similar across SYNTAX score tertiles (Table 1). TMAO levels were significantly higher with increasing SYNTAX score, SYNTAX score II, and hs-cTnT tertiles (Central Illustration).
TABLE 1.
Baseline Characteristics
| SYNTAX Score | |||||
|---|---|---|---|---|---|
|
| |||||
| Variables | Total (N = 353) | Low (0–22) (n = 289) | Intermediate (23–32) (n = 40) | High (≥33) (n = 24) | p Value |
|
| |||||
| Age, yrs | 65.0 ± 11.0 | 64.0 ± 11.0 | 67.0 ± 9.0 | 68.0 ± 10.0 | 0.02 |
|
| |||||
| Male | 79.0 | 78.0 | 88.0 | 75.0 | 0.35 |
|
| |||||
| BMI, kg/m2 | 29.4 (26.4–33.4) | 29.0 (29.3–30.6) | 30.2 (28.9–32.9) | 32.7 (29.2–34.7) | 0.22 |
|
| |||||
| Systolic BP, mm Hg | 128.8 ± 19.4 | 128.6 ± 18.8 | 125.1 ± 21.9 | 137.4 ± 21.9 | 0.06 |
|
| |||||
| Diabetes mellitus | 30.3 | 26.0 | 47.5 | 54.2 | 0.001 |
|
| |||||
| Hypertension | 80.5 | 77.5 | 92.5 | 95.8 | 0.012 |
|
| |||||
| Current smoker | 60.2 | 59.9 | 60.0 | 65.2 | 0.40 |
|
| |||||
| Unprotected left main CAD | 5.7 | 1.7 | 22.5 | 25 | <0.001 |
|
| |||||
| LDL cholesterol, mg/dl | 81 (63–104) | 82.0 (64.0–105.0) | 70.0 (51.0–85.0) | 78.0 (62.0–103.0) | 0.03 |
|
| |||||
| HDL cholesterol, mg/dl | 45.0 (36–55) | 45.0 (37.0–55.0) | 43.0 (30.0–59.0) | 42.0 (35.0–49.0) | 0.26 |
|
| |||||
| Triglyceride, mg/dl | 123.0 (84–159) | 119.0 (84.0–154.0) | 146 (92.0–215.0) | 142.0 (88.0–178.0) | 0.09 |
|
| |||||
| eGFR, ml/min/1.73 m2 | 95.33 (72.5–116.1) | 97.44 (75.4–115.6) | 85.68 (54.4–123.1) | 79.78 (59.9–114.3) | 0.004 |
|
| |||||
| Medication: | |||||
| Aspirin | 74.0 | 64.0 | 75.0 | 75.0 | 0.98 |
| ACEI or ARB | 36.0 | 44.0 | 68.0 | 42.0 | <0.001 |
| Statin | 66.0 | 70.0 | 70.0 | 50.0 | 0.203 |
| Beta-blocker | 58.0 | 68.0 | 62.0 | 58.0 | 0.79 |
|
| |||||
| TMAO, μM | 5.48 (3.41–9.76) | 4.84 (3.1–7.5) | 12.02 (7.66–15.34) | 19.69 (16.1–26.28) | <0.001 |
|
| |||||
| hs-cTnT, ng/l | 10.41 (7.03–18.04) | 9.73 (6.86–15.83) | 16.82 (9.74–27.65) | 12.39 (8.49–28.78) | 0.003 |
|
| |||||
| Diffuse lesion characteristics | 20.7 | 17.0 | 32.5 | 45.8 | <0.001 |
Values are mean ± SD, %, or median (interquartile range).
ACEI = angiotensin-converting enzyme inhibitors; ARB = angiotensin receptor blocker; BMI = body mass index; BP = blood pressure; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; hs-cTnT = high-sensitivity cardiac troponin T; LDL = low-density lipoprotein; SYNTAX = SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery; TMAO = trimethylamine N-oxide.
CORRELATIONS WITH PLASMA TMAO LEVELS
Plasma TMAO levels were strongly correlated with both SYNTAX score and SYNTAX score II (Spearman’s correlation: r = 0.61 and 0.62, respectively; both p <0.0001). Plasma TMAO was also correlated with hs-cTnT, a measure of subclinical myonecrosis since all hs-cTnT levels were below the diagnostic cutoff for myocardial infarction (r = 0.29; p < 0.0001). Following ordinal logistic regression analysis adjusting for traditional risk factors (including age, sex, hypertension, diabetes mellitus, smoking, LDL cholesterol, HDL cholesterol, and triglyceride) and lesion characteristic, elevated TMAO levels remained independently associated with a higher tertile of SYNTAX score (adjusted odds ratio [OR]: 4.68; 95% confidence interval [CI]: 2.35 to 9.29; p < 0.0001), SYNTAX score II (adjusted OR: 2.02; 95% CI: 1.72 to 3.01; p = 0.0001), and hs-cTnT (adjusted OR: 1.34; 95% CI: 1.02 to 1.78; p = 0.037) (Table 2). However, when further adjusted for hsCRP, BMI, eGFR, and medications, the association between TMAO and hs-cTnT was no longer significant, whereas SYNTAX score and SYNTAX score II remained significant (Table 2).
TABLE 2.
Association between TMAO and SYNTAX Scores and hs-cTnT
| Variable | SYNTAX Score | SYNTAX Score II | hs-cTnT* | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Unadjusted | 6.57 (3.37–12.8) | <0.0001 | 2.28 (1.72–3.01) | <0.0001 | 1.56 (1.2–2.02) | 0.0008 |
| Adjusted Model† | 4.82 (2.43–9.57) | <0.0001 | 1.86 (1.36–2.60) | 0.0002 | 1.14 (0.88–1.47) | 0.3147 |
Subclinical myonecrosis = hs-cTnT >0.01 ng/ml.
Included age, sex, triglyceride, HDL cholesterol, LDL cholesterol, diabetes mellitus, hypertension, smoking, diffuse lesion, focal lesion, log high sensitivity C-reactive protein, BMI, log eGFR, statins, ACEI or ARB, aspirin, and beta-blocker.
CI = confidence interval; OR = odds ratio; other abbreviations as in Table 1.
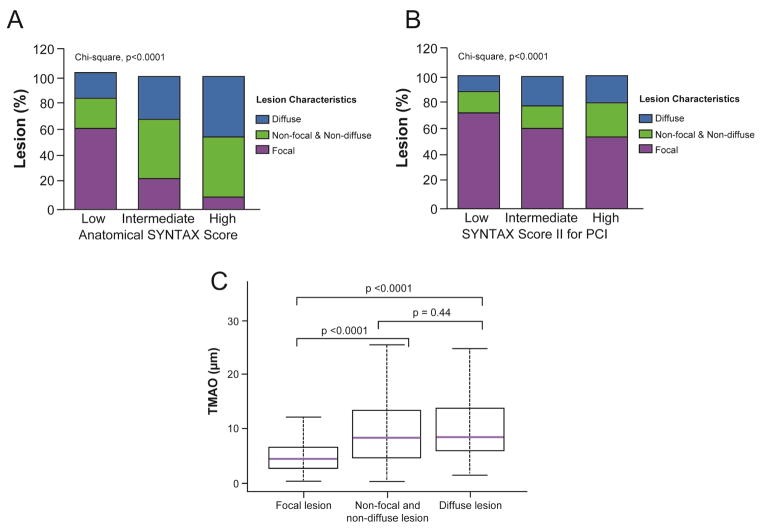
Plasma TMAO levels were significantly higher in patients with diffuse lesions than in patients with focal lesions (8.4 μM [IQR: 5.7 to 14.0] vs. 4.4 μM [IQR: 5.2 to 13.5]; p < 0.0001) (Figure 2C). Furthermore, plasma TMAO was significantly higher in patients with nonfocal and nondiffuse lesions than in patients with focal lesions (8.3 μM [IQR: 4.5 to 13.4] vs. 4.4 μM [IQR: 5.2 to 13.5]; p < 0.0001) (Figure 2C). Interestingly, the frequency of diffuse lesions was significantly increased with increasing SYNTAX score and SYNTAX score II tertiles (17% in low SYNTAX score vs. 45.8% in high SYNTAX score and 11.4% in low SYNTAX score II vs. 20.7% in high SYNTAX score II; p < 0.0001 for all) (Figures 2A and 2B). In contrast, the frequency of focal lesions was significantly lower with increasing SYNTAX score and SYNTAX score II tertiles (p < 0.0001) (Figures 2A and 2B). Following multivariate adjustments, elevated TMAO level remained associated with increased likelihood of having diffuse lesions (adjusted OR: 2.05; 95% CI: 1.45 to 2.90; p = 0.0001) and decreased likelihood of having focal lesions (adjusted OR: 0.46; 95% CI: 0.31 to 0.68; p = 0.0001).
FIGURE 2. TMAO, Atherosclerotic Burden, and Lesion Characteristics.
Diffuse, nonfocal and nondiffuse, and focal lesion frequency was compared according to SYNTAX score (A) and SYNTAX score II (B) for PCI tertiles, fasting trimethylamine N-oxide (TMAO) levels were compared according to lesion characteristics (C).
PREDICTION OF HIGH ATHEROSCLEROTIC BURDEN
High atherosclerosis burden was identified by the presence of intermediate or high SYNTAX score and SYNTAX score II. We tested whether TMAO levels could help predict enhanced coronary atherosclerotic burden, and whether addition of TMAO to models comprised of traditional risk factors, lesion characteristic, hsCRP, eGFR, BMI, and medications helped to predict enhanced CAD atherosclerotic burden. Addition of TMAO to the fully-adjusted model showed that elevated TMAO levels significantly improved NRI and trended toward improvement in AUC for predicting high atherosclerosis burden, albeit not at a statistically significant level (SYNTAX score: NRI = 0.87; p < 0.001; AUC from 0.83 to 0.88; p = 0.07; and SYNTAX score II: NRI = 0.58; p < 0.001; AUC from 0.92 to 0.93; p = 0.07).
DISCUSSION
The main finding of our study is the strongly significant association between fasting plasma TMAO levels in an independent and contemporary cohort of stable patients with CAD and quantitative indexes of coronary atherosclerosis burden (quantified by the SYNTAX score and SYNTAX score II). An additional major finding is the observed correlation between TMAO levels and evidence of subclinical myonecrosis (quantified by hs-cTnT) in patients with stable CAD undergoing elective coronary angiography (28), even though renal insufficiency can be a confounder. Furthermore, elevated TMAO levels were found to serve as an independent predictor of higher SYNTAX score, higher SYNTAX score II, and elevated hs-cTnT, even following adjustments for traditional risk factors and lesion characteristic on coronary artery angiogram. Higher plasma TMAO level was also shown to serve as an independent predictor of the presence of diffuse coronary lesion characteristics, which often represent high severity and atherosclerosis burden of CAD. Moreover, the addition of TMAO resulted in a significant increase in the NRI for prediction of high atherosclerosis burden over traditional risk factors, lesion characteristic, hsCRP, eGFR, BMI, and medications. Taken together, our findings further demonstrate that TMAO levels are associated with greater coronary atherosclerotic burden.
Gut microbes play an important role in global host metabolism, including the production of vitamins and other essential nutrients, and regulation of many aspects of host immunity (6–8). Changes in gut microbial composition has been linked to diseases such as obesity, insulin resistance, chronic kidney disease, inflammatory bowel disease, and CVD (12,29–34). TMAO has previously been shown to directly promote pro-atherosclerotic effects in vivo using animal model studies. The present study extends these observations by showing striking and independent associations between TMAO and enhanced coronary artery atherosclerotic burden. They are thus consistent with the gut microbial TMAO pathway being mechanistically linked with CAD development and the pathogenesis of CVD (12–14,35).
Previous studies showed that the SYNTAX score is a useful tool to risk stratify outcomes in stable patients with complex CAD (with or without unprotected left main CAD) who underwent revascularization by PCI or CABG, and they independently predicted MACE and all-cause mortality, which increased in the higher SYNTAX score tertiles (18,19). A high SYNTAX score is also a marker of systemic atherosclerotic burden, which likely correlates with poor prognosis (16,17).
In our study, plasma TMAO levels were correlated with both the SYNTAX score (anatomical factors) as well as the SYNTAX score II (both anatomical and clinical factors). We also found elevated plasma TMAO to be an independent predictor for the presence of diffuse lesion characteristics, which represent markers of higher atherosclerosis burden and are associated with adverse outcomes in CAD (22,36). By contrast, elevated plasma TMAO levels showed an inverse and independent association with the presence of focal lesions, which indicate a lower atherosclerotic burden. Importantly, despite the present study cohort being comprised of stable subjects without evidence of acute coronary syndrome (cardiac troponin T level, <0.03 μg/l) at presentation, elevated plasma TMAO was associated with evidence of subclinical myonecrosis, as indicated by a higher level of hs-cTnT. It is thus conceivable that subclinical myonecrosis or high severity and atherosclerosis burden of CAD may occur in the setting of elevated plasma TMAO levels. Taken together, our current findings provided the additional support for a pro-atherogenic effect of elevated systemic levels of TMAO. The mechanism of the relation between plasma TMAO and atherosclerotic burden might be explained by our previous studies that showed higher plasma TMAO correlated with greater atherosclerotic plaque size in both arterial wall and aortic root in mice on diets supplemented with either TMAO or choline (12). TMAO may have direct biological activity that facilitates the development or propagation of atherosclerosis plaque and suppression reverse cholesterol transport in in vivo mouse models (12,13). Moreover, recent studies suggest that flavin monooxygenase 3 (FMO3), the major host enzyme responsible for forming TMAO from gut microbe-generated trimethylamine, is a master regulator of tissue cholesterol and sterol metabolism (37). Furthermore, results from recent studies in mice fed a high-fat diet also suggest that dietary TMAO may exacerbate impaired glucose tolerance, obstruct hepatic insulin signaling, and promote adipose tissue inflammation, which were related to the complexity and degree of atherosclerosis burden of CAD (38). It is also of interest that recent studies have further implicated FMO3 in atherosclerosis development in rodent models of diabetes (39).
STUDY LIMITATIONS
This was a single, tertiary referral center that recruited patients at the point of cardiac evaluation; therefore, we cannot exclude selection bias for patients undergoing diagnostic cardiac catheterization (especially with relatively preserved renal function). Because the patient population was a contemporary cohort (the last patient was enrolled in 2014), we do not have long-term outcome data. Despite all subjects being recruited at the time of coronary angiography while fasting for at least 10 h in anticipation of coronary angiography, we cannot exclude the potential for dietary intake of choline or TMAO (e.g., large consumption of some fish species) within 24 h prior to blood sampling. Also, the relatively low number of the patients in the intermediate and high SYNTAX score tertiles is a limitation in our study. Furthermore, improvement in AUC for a model is often very small in magnitude, yet the category-free NRI may tend to overstate the incremental value of a biomarker.
CONCLUSIONS
Fasting plasma TMAO level is an independent predictor of high atherosclerotic burden in patients with CAD. Higher TMAO levels can predict the presence of increased atherosclerotic burden and complexity, as evidenced by higher SYNTAX scores and diffuse lesion characteristics. Despite the associative nature in this cross-sectional study, these findings are consistent with numerous prior mechanistic demonstrations linking TMAO to the pathogenesis of atherosclerosis, and the multiple reported findings demonstrating associations between TMAO and cardiovascular risks. Further investigations into the mechanisms of elevated TMAO leading to atherosclerotic burden and myonecrosis are warranted.
FIGURE 3. CENTRAL ILLUSTRATION Relationship Between TMAO and Measures of CAD Burden and Subclinical Myonecrosis.
Trimethylamine N-oxide (TMAO) has links to coronary artery disease (CAD) pathogenesis and is associated with adverse outcomes. One way to measure CAD complexity and burden is via the SYNTAX score that came from the SYNTAX (SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery) trial; the more recent SYNTAX score II has been shown to have improved prognostic ability. In 353 stable patients with evidence of atherosclerotic CAD, concentrations of TMAO were significant higher with increasing SYNTAX score (A), SYNTAX score II (B), and subclinical myonecrosis (quantified by high-sensitivity cardiac troponin T [hs-cTnT]) tertiles (C).
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
The intestinal microbe-generated phosphatidylcholine metabolite TMAO is related to the pathogenesis of atherosclerotic coronary artery disease.
TRANSLATIONAL OUTLOOK
Further research is needed to determine whether dietary or pharmacological interventions that reduce plasma levels of TMAO can prevent or retard the progression of coronary atherosclerosis.
Acknowledgments
SOURCES OF FUNDING: This research was supported by grants from the National Institutes of Health and the Office of Dietary Supplements (R01HL103866, P20HL113452, R01DK106000). The GeneBank study has been supported by NIH grants P01HL076491, P01HL098055, R01HL103931, and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439). Dr. Wang was partially supported by an American Heart Association Scientist Development Grant 12SDG12050473. Dr. Hazen is also partially supported by a gift from the Leonard Krieger endowment. Mass spectrometry studies were performed on instruments housed in a facility supported in part by a Center of Innovations Award by AB SCIEX.
ABBREVIATIONS AND ACRONYMS
- CABG
coronary artery bypass graft
- CAD
coronary artery disease
- CVD
cardiovascular disease
- hs-cTnT
high-sensitivity cardiac troponin T
- PAD
peripheral arterial disease
- PCI
percutaneous coronary intervention
- SYNTAX score
SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery score
- TMAO
trimethylamine N-oxide
Footnotes
DISCLOSURE: High sensitivity cardiac troponin T testing reagents were provided by Roche Diagnostics. Dr. Wang, Dr. Levison and Dr. Hazen are named as co-inventor on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Wang and Dr. Levison report that they have the right to receive royalty payment for inventions or discoveries related to cardiovascular diagnostics from Cleveland Heart Lab. Dr. Hazen reports having been paid as a consultant for the following companies: Esperion, P&G. Dr. Hazen reports receiving research funds from Abbott, PG, Pfizer Inc., Roche Diagnostics and Takeda. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Cleveland Heart Lab., Siemens, Esperion, and Frantz Biomarkers, LLC. All other authors have no relationships to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 5.Wang TJ. New cardiovascular risk factors exist, but are they clinically useful? Eur Heart J. 2008;29:441–4. doi: 10.1093/eurheartj/ehm644. [DOI] [PubMed] [Google Scholar]
- 6.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 7.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–8. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 8.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 9.Nabel EG. Cardiovascular disease. N Engl J Med. 2003;349:60–72. doi: 10.1056/NEJMra035098. [DOI] [PubMed] [Google Scholar]
- 10.Gregory JC, Buffa JA, Org E, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647–60. doi: 10.1074/jbc.M114.618249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koeth RA, Levison BS, Culley MK, et al. gamma-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20:799–812. doi: 10.1016/j.cmet.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.al-Waiz M, Mikov M, Mitchell SC, Smith RL. The exogenous origin of trimethylamine in the mouse. Metabolism. 1992;41:135–6. doi: 10.1016/0026-0495(92)90140-6. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda N, Kogame N, Iijima R, Nakamura M, Sugi K. Carotid artery intima-media thickness and plaque score can predict the SYNTAX score. Eur Heart J. 2012;33:113–9. doi: 10.1093/eurheartj/ehr399. [DOI] [PubMed] [Google Scholar]
- 17.Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–27. [PubMed] [Google Scholar]
- 18.Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–38. doi: 10.1016/S0140-6736(13)60141-5. [DOI] [PubMed] [Google Scholar]
- 19.Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–72. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 20.Farooq V, van Klaveren D, Steyerberg EW, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet. 2013;381:639–50. doi: 10.1016/S0140-6736(13)60108-7. [DOI] [PubMed] [Google Scholar]
- 21.McLean RC, Nazarian SM, Gluckman TJ, et al. Relative importance of patient, procedural and anatomic risk factors for early vein graft thrombosis after coronary artery bypass graft surgery. J Cardiovasc Surg (Torino) 2011;52:877–85. [PMC free article] [PubMed] [Google Scholar]
- 22.Rodes-Cabau J, Gutierrez M, Courtis J, et al. Importance of diffuse atherosclerosis in the functional evaluation of coronary stenosis in the proximal-mid segment of a coronary artery by myocardial fractional flow reserve measurements. Am J Cardiol. 2011;108:483–90. doi: 10.1016/j.amjcard.2011.03.073. [DOI] [PubMed] [Google Scholar]
- 23.Ertan C, Ozeke O, Gul M, et al. Association of prediabetes with diffuse coronary narrowing and small-vessel disease. J Cardiol. 2014;63:29–34. doi: 10.1016/j.jjcc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Xu B, Genereux P, Yang Y, et al. Validation and comparison of the long-term prognostic capability of the SYNTAX score-II among 1,528 consecutive patients who underwent left main percutaneous coronary intervention. JACC Cardiovasc Interv. 2014;7:1128–37. doi: 10.1016/j.jcin.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Serruys PW, Onuma Y, Garg S, et al. Assessment of the SYNTAX score in the Syntax study. EuroIntervention. 2009;5:50–6. doi: 10.4244/eijv5i1a9. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. doi: 10.1016/j.ab.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 28.Omland T, de Lemos JA, Sabatine MS, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–47. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–9. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 30.Vaziri ND, Wong J, Pahl M, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–15. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 31.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumas ME, Kinross J, Nicholson JK. Metabolic phenotyping and systems biology approaches to understanding metabolic syndrome and fatty liver disease. Gastroenterology. 2014;146:46–62. doi: 10.1053/j.gastro.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Tang WH, Buffa JA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35:904–10. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujii K, Mintz GS, Kobayashi Y, et al. Vascular remodeling and plaque composition between focal and diffuse coronary lesions assessed by intravascular ultrasound. Am J Cardiol. 2004;94:1067–70. doi: 10.1016/j.amjcard.2004.06.071. [DOI] [PubMed] [Google Scholar]
- 37.Warrier M, Shih DM, Burrows AC, et al. The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell Rep. 2015 Jan 14; doi: 10.1016/j.celrep.2014.12.036. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118:476–81. doi: 10.1016/j.jbiosc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Miao J, Ling AV, Manthena PV, et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. doi: 10.1038/ncomms7498. [DOI] [PMC free article] [PubMed] [Google Scholar]