Abstract
Background
Patients with heart failure and preserved ejection fraction (HFpEF) display elevation in left heart pressures, but it is unclear how this affects pulmonary gas transfer or its determinants at rest and during exercise.
Objectives
Compare measures of gas exchange at rest and during exercise in subjects with HFpEF to age and gender-matched controls.
Methods
HFpEF patients (n=20) and controls (n=26) completed a recumbent cycle ergometry exercise test with simultaneous measurement of ventilation and gas exchange. Diffusion of the lungs for carbon monoxide (DLCO) and subcomponents, pulmonary capillary blood volume (VC) and alveolar-capillary membrane conductance (DM), were measured at rest, matched low-intensity (20W), and peak exercise. Stroke volume was measured by transthoracic echocardiography to calculate cardiac output.
Results
Compared to controls, HFpEF subjects displayed impaired diastolic function and reduced exercise capacity. HFpEF subjects demonstrated 24% lower DLCO at rest (11.0±2.3 vs 14.4±3.3 mL/mmHg/min, p<0.01), related to reductions in both DM (18.1±4.9 vs 23.1±9.1 mL/mmHg/min, p=0.04), and VC (45.9±15.2. vs 58.9±16.2 mL, p=0.01). DLCO was lower in HFpEF compared to controls in all stages of exercise, yet its determinants showed variable responses. With low-level exercise, HFpEF subjects demonstrated greater relative increases in VC, coupled with heightened ventilatory drive and more severe symptoms of dyspnea compared to controls. At 20W exercise, DM was markedly reduced in HFpEF compared to controls. From 20W to peak exercise, there was no further increase in VC in HFpEF subjects, which in tandem with reduced DM, led to 30% reduction in DLCO at peak exercise (17.3±4.2 vs 24.7±7.1 mL/mmHg/min, p<0.01).
Conclusion
Patients with HFpEF display altered pulmonary function and gas exchange at rest and especially during exercise which contributes to exercise intolerance. Novel therapies that improve gas diffusion may be effective to improve exercise tolerance in patients with HFpEF.
Keywords: Lung Diffusion, Exercise, HFpEF
INTRODUCTION
Elevation in pulmonary venous pressures with exercise is pathognomonic of heart failure with preserved ejection fraction (HFpEF)(1). Many studies have examined the hemodynamic mechanisms underlying filling pressure elevation in HFpEF(2–4), but very little is known about how the latter might alter pulmonary gas exchange and ventilatory mechanics to produce dyspnea. It is important to understand how the hemodynamic abnormalities developing during exercise in HFpEF affect the alveolar-pulmonary capillary interface in order to design novel therapeutics.
Acute elevation in pulmonary venous pressure can cause interstitial or alveolar edema, while sustained increases can cause pulmonary vascular remodeling(5). Increases in venous pressure during exercise in HFpEF may alter forces governing fluid distribution between the vascular, capillary wall, and alveolar spaces in the lung, potentially resulting in interstitial edema, impaired gas conductance, stiffer lungs, a more tachypneic pattern of breathing, greater ventilatory drive and ventilatory inefficiency, all of which may increase the work and cost of breathing and heighten symptoms of dyspnea during exercise(6).
The aim of this study was to comprehensively examine the pulmonary response to exercise in HFpEF, assessing measures of gas exchange, ventilatory drive and efficiency, and the diffusion capacity of the lungs for carbon monoxide (DLCO) and its subcomponents (pulmonary capillary blood volume [VC] and alveolar-capillary membrane conductance [DM]) in patients with HFpEF compared to healthy control participants at rest and during exercise. We hypothesized that patients with HFpEF would demonstrate reduced lung diffusion at rest and with exercise related to distinct patterns of change in capillary blood volume and membrane conductance.
METHODS
HFpEF subjects (n=20) with EF>50% and unequivocal signs and symptoms of heart failure (Framingham criteria) were studied prospectively in an outpatient, compensated state. Exclusion criteria included significant valvular or pericardial disease; infiltrative or hypertrophic cardiomyopathy, cor pulmonale, obstructive or restrictive pulmonary disease, unstable coronary disease, atrial fibrillation, pregnancy, primary renal or hepatic disease, and inability to exercise or to suspend cardiovascular medicines. Healthy controls without cardiovascular disease or diabetes (n=26) were recruited by advertisement.
Some clinical characteristics, cardiovascular function, and exercise capacity data from subjects in this study have previously been published(7); however, none of the data on pulmonary diffusion capacity, its subcomponents, or the relationships presented in this manuscript have been reported. All participants gave written informed consent after being provided a description of study requirements. The protocol was approved by the Mayo Clinic Institutional Review Board; all procedures conformed to the Declaration of Helsinki. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Exercise Testing Protocol
Subjects were instructed to avoid strenuous physical activity for 24 hours prior to arrival and were studied in the upright position in an ambulatory, compensated, fasting state in a quiet, temperature-controlled room (21°C). Additionally, all cardiovascular medicines were withheld for 24 hours prior to study. Ventilatory, gas exchange, heart rate and oxygen saturation data were measured continuously during exercise.
Exercise testing was conducted on a recumbent electronically braked cycle ergometer (Corival, Lode Medical Technology, Netherlands). The exercise protocol consisted of pedaling at a constant cadence of 65 rpm with an initial resistance of 0 Watts which was subsequently increased every 3 minutes by 20 Watts. Symptoms of fatigue were quantified by the rating of perceived exertion (RPE) on the Borg 6–20 scale. Subjects were verbally encouraged to continue the exercise protocol to maximal exertion, identified by RPE≥17. Symptoms of dyspnea were quantified by the Borg dyspnea score (0–10). Brachial blood pressure (BP) was obtained by auscultation by a single investigator during rest and at the end of each stage of exercise.
Ventilation and Expired Gas Analysis
Breath-by-breath oxygen consumption (VO2), carbon dioxide production (VCO2), minute ventilation (VE), tidal volume (VT), respiratory rate (fb), inspiratory time (TI), and total respiratory cycle time (TTOT) were measured continuously via metabolic measurement system through a mouth piece and pneumotachograph while wearing a nose clip (CPX/D, Medical Graphic, St. Paul, MN). Manual volume calibration was performed with a 3 liter syringe and gas calibration was performed with manufacturer-recommended gases of known concentration. All calibration procedures were conducted immediately prior to each testing protocol.
Aerobic capacity was assessed by the peak VO2 attained during exercise. Objective exercise effort was assessed by the peak respiratory exchange ratio (VCO2/VO2). Ventilatory efficiency was assessed by the slope of VE to VCO2, and ventilatory drive was assessed by the ratio of VT to TI (8). All analyses of ventilation and gas exchange data were conducted offline in a blinded fashion.
Pulmonary Diffusing Capacity and Subcomponents
The disappearance of carbon monoxide in concert with nitric oxide was measured for the assessment of alveolar-capillary membrane diffusion (DM) and pulmonary capillary blood volume (VC) as described in detail previously(9–11). Briefly, measurement of the diffusing capacity of the lungs for carbon monoxide (DLCO) and nitric oxide (DLNO) was conducted using the rebreathing technique with gases sampled on a mass spectrometer (Perkin-Elmer, 1100) and NO analyzer (Sievers Instruments, Boulder, CO) using a custom analysis software package(9,11). A 5-liter rebreathe bag was filled with 0.3% carbon monoxide (C18O), 40 parts per million (ppm) NO (diluted immediately before each measurement in the rebreathe bag from an 800-ppm gas mixture), 35% oxygen (O2), balance Nitrogen (N2). The isotope C18O was used in place of the more common C16O as the test gas because the molecular mass of C16O is nearly identical to that of N2 making these gases indistinguishable by the mass spectrometer(9,11).
The volume of gas used to fill the rebreathe bag was determined by the tidal volume of the subject. Consistent bag volumes were ensured using a timed switching circuit that, given a consistent flow rate from the tank, resulted in the desired volume. For each maneuver, subjects were switched into the rebreathe bag at the end of a normal expiration (end-expiratory lung volume, EELV) and instructed to nearly empty the bag with each breath for 10 consecutive breaths. The breathing frequency during the rebreathe maneuver was controlled with a metronome at a rate of 32 breaths per minute unless the intrinsic frequency was greater than 32 breaths per minute, at which time the metronome was switched off. The rebreathe bag was then emptied with a suction device and refilled immediately prior the next maneuver. All analyses of pulmonary diffusion and its subcomponents were conducted offline in a blinded fashion.
Measurement of Cardiac Output
Transthoracic echocardiography was used to measure stroke volume from the left ventricular outflow dimension and pulse wave Doppler as previously described(7). Stroke volume was multiplied by heart rate to calculate cardiac output (Q). Echo-Doppler measurements were interpreted offline in a blinded fashion and represent the mean of ≥3 consecutive beats collected by a trained cardiac sonographer.
Statistical Analysis
Continuous variables are reported as mean±SD. Between-group differences were compared by χ2 for categorical variables, ANOVA for Normally-distributed continuous variables and Wilcoxon Rank Sum or Kruskal-Walllis for non-Normally distributed continuous variables. Normality was evaluated for each variable by the Shapiro-Wilk W Test. Bonferroni correction was applied for multiple comparisons. Bivariate (Pearson coefficient) linear regression was performed to test associations between diffusing capacity of the lungs and its subcomponents and peak exercise capacity, ventilatory drive, and symptomology during exercise. Because HFpEF patients reach lower exercise workload on average than non-HF subjects, comparisons were made at both matched, low-level workload (20W) and at peak exercise. All statistical analyses were performed using SPSS 20.0 (SPSS, Inc. Chicago, IL) with graphical representation using GraphPad Prism 6 (GraphPad Software, Inc. La Jolla, CA).
RESULTS
Subject Characteristics
Subjects with HFpEF had higher body mass than controls but age, sex, comorbidity burden and renal function were similar in the two groups (Table 1). HFpEF subjects reported NYHA class II–III symptoms and displayed elevated BNP levels along with higher left atrial volume and greater E/e′ ratios, all consistent with elevated LV filling pressures. LV mass was higher in HFpEF whereas EF, heart rate and blood pressure were similar in HFpEF and controls. None of the subjects were treated with amiodarone or had sleep disordered breathing.
Table 1.
Clinical Characteristics and Resting Cardiovascular Function
| Control (n=26) | HFpEF (n=20) | p-value | |
|---|---|---|---|
| Clinical Characteristics | |||
| Age (years) | 65±9 | 67±11 | 0.4 |
| Sex (% Female) | 69 | 75 | 0.7 |
| Body Mass Index (kg/m2) | 29.1±5.5 | 34.5±6.8 | 0.004 |
| NYHA class I/II/III | 26/0/0 | 0/9/11 | <0.0001 |
| Hypertension (%) | 62 | 85 | 0.11 |
| Smoking Hx (%) | 0 | 10 | 0.18 |
| eGFR (ml/min) | 80±18 | 82±39 | 0.8 |
| Plasma BNP (pg/ml) | 37 (16, 61) | 175 (58, 200) | 0.0003 |
| Hemoglobin (gm/dl) | 14.0±1.8 | 13.1±1.3 | 0.08 |
| Loop Diuretic (%) | 0 | 60 | 0.0005 |
| Resting Hemodynamics and Echocardiography | |||
| Heart Rate (bpm) | 70±11 | 68±13 | 0.7 |
| Systolic BP (mmHg) | 133±15 | 129±20 | 0.4 |
| LV Mass Index (mg/m2) | 82±24 | 87±27 | 0.5 |
| Ejection Fraction (%) | 58±5 | 60±6 | 0.2 |
| LA volume index (ml/m2) | 31±7 | 45±14 | 0.0001 |
| E/E′ ratio | 11±4 | 20±8 | 0.0004 |
| Cardiac Index (L/min*m2) | 2.3±0.6 | 2.3±0.6 | 0.6 |
Data are Mean±SD. Final column reflects two-tailed unpaired t-test or Fisher’s exact test for sex. Abbreviations: GFR, glomerular filtration rate; BNP, B-type natriuretic peptide; BP, blood pressure; LA, left atrium; E, early mitral inflow velocity; E`, early mitral valve tissue inflow velocity.
Exercise Performance
Exercise time, peak workload, VO2 at ventilatory threshold, peak VO2 and percent predicted peak VO2 were all markedly impaired in HFpEF compared to controls (Table 2). Borg effort and dyspnea scores in HFpEF were higher at matched submaximal workload (20W), indicating greater perceived difficulty with low level exercise at matched workload (Figure 1).
Table 2.
Exercise Performance
| Control (n=26) | HFpEF (n=20) | p-value | |
|---|---|---|---|
| Exercise Time (seconds) | 840±239 | 495±210 | <0.001 |
| Peak Workload (watts) | 93±27 | 55±23 | <0.001 |
| Peak VO2 (mL/kg/min) | 18.6±3.3 | 12.7±3.2 | <0.001 |
| % Predicted Peak VO2 (%) | 94±22 | 57±19 | <0.001 |
| Peak Respiratory Exchange Ratio | 1.09±0.07 | 1.05±0.09 | 0.11 |
| VO2 at VAT (mL/kg/min) | 14.2±2.5 | 10.4±2.3 | <0.001 |
| VE/VCO2 slope | 33.5±3.0 | 36.0.±5.0 | 0.05 |
| 20W Borg Effort (6–20) | 8.6±1.4 | 11.3±2.4 | <0.001 |
| 20W Borg Dyspnea (0–10) | 0.8±0.7 | 2.8±1.5 | <0.001 |
| Peak Borg Effort (6–20) | 16.2±1.8 | 15.7±2.2 | 0.45 |
| Peak Borg Dyspnea (0–10) | 5.2±1.9 | 5.5±2.0 | 0.61 |
Data are Mean±SD. Final column reflects two-tailed unpaired t-test. Abbreviations: VO2, oxygen consumption; VAT, ventilatory anaerobic threshold; VE, minute ventilation; VCO2, carbon dioxide production.
Figure 1.
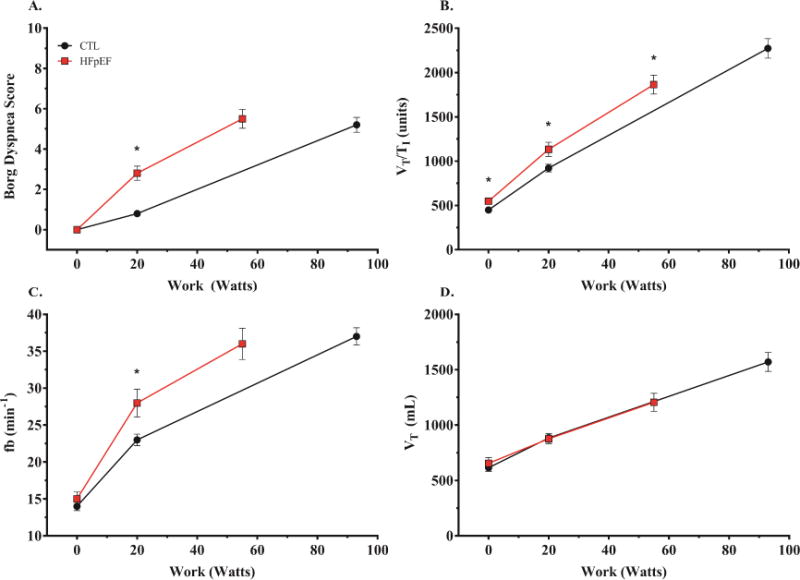
Borg dyspnea score (A), ventilatory drive (B=VT/TI), breathing frequency (C=fb), and tidal volume (D=VT), as a function of workload during exercise. *p <0.05 versus control,
At peak, Borg scores were similar between HFpEF and controls, consistent with maximal subjective effort in all groups, but Borg scores relative to work performed were higher in HFpEF. There was no difference in peak respiratory exchange ratio between HFpEF and control subjects.
Ventilation, Gas Exchange, and Lung Diffusion at Rest and during Exercise
There were no differences in VO2 or measures of ventilation and breathing pattern at rest (Table 3). In contrast, DLCO was significantly lower in the HFpEF patients compared to control participants. Compared to controls, subjects with HFpEF displayed lower DM, reflecting impaired membrane gas transfer, along with lower VC, reflecting pulmonary capillary oligemia despite evidence of higher left heart filling pressures, as noted above (Table 3, Figure 2).
Table 3.
Cardiopulmonary function, gas exchange, and lung diffusion at rest and during exercise
| Control (n=26) | HFpEF (n=20) | p-value | |
|---|---|---|---|
| Rest | |||
| VO2 (mL/kg/min) | 3.60±0.51 | 3.29±0.82 | 0.13 |
| Q (L/min) | 4.4±1.2 | 4.9±1.2 | 0.15 |
| SV (mL/beat) | 63.9±17.6 | 74.1±20.9 | 0.09 |
| HR (beat/min) | 70±11 | 68±13 | 0.7 |
| VE (L/min) | 8.5±1.9 | 9.5±2.4 | 0.14 |
| fb (breaths/min) | 14±3 | 15±4 | 0.5 |
| VT (mL) | 615±166 | 655±218 | 0.5 |
| VE/VCO2 (ratio) | 38.6±5.0 | 40.1±6.4 | 0.4 |
| VT/TI (ratio) | 448±120 | 547±122 | 0.01 |
| DLCO (mL/mmHg/min) | 14.4±3.3 | 11.0±2.3 | <0.001 |
| DM (mL/mmHg/min) | 23.1±9.1 | 18.1±4.9 | 0.04 |
| VC (mL) | 58.9±16.2 | 45.9±15.2 | 0.01 |
| Matched Workload (20 W) | |||
| VO2 (mL/kg/min) | 9.6±1.2 | 8.9±1.41 | 0.06 |
| Q (L/min) | 8.2±2.3 | 7.2±1.4 | 0.12 |
| SV (mL/beat) | 86.2±21.7 | 77.8±19.1 | 0.09 |
| HR (beat/min) | 85±17 | 94±15 | 0.04 |
| VE (L/min) | 20.1±4.4 | 24.0±5.3 | 0.01 |
| fb (breaths/min) | 23±4 | 28±8 | 0.02 |
| VT (mL) | 883±197 | 876±188 | 0.9 |
| VE/VCO2 (ratio) | 34.3±4.2 | 36.0±5.0 | 0.2 |
| VT/TI (ratio) | 922±225 | 1133±340 | 0.02 |
| DLCO (mL/mmHg/min) | 17.4±3.6 | 14.5±4.0 | 0.01 |
| DM (mL/mmHg/min) | 26.9±6.6 | 21.5±6.1 | 0.008 |
| VC (mL) | 67.6±23.3 | 64.9±24.9 | 0.7 |
| Peak Exercise | |||
| VO2 (mL/kg/min) | 18.6±3.3 | 12.7±3.2 | <0.001 |
| Q (L/min) | 13.2±3.7 | 9.2±2.5 | 0.004 |
| SV (mL/beat) | 91.6±22.1 | 80.0±25.1 | 0.11 |
| HR (beat/min) | 144±20 | 119±22 | <0.001 |
| VE (L/min) | 56.7±14.3 | 42.9±12.4 | 0.002 |
| fb (breaths/min) | 37±6 | 36±9 | 0.9 |
| VT (mL) | 1570±433 | 1206±344 | 0.005 |
| VE/VCO2 (ratio) | 34.5±3.6 | 37.1±4.7 | 0.05 |
| VT/TI (ratio) | 2273±560 | 1864±452 | 0.01 |
| DLCO (mL/mmHg/min) | 24.7±7.1 | 17.3±4.2 | <0.001 |
| DM (mL/mmHg/min) | 39.6±12.9 | 27.2±6.3 | 0.001 |
| VC (mL) | 86.1±24.3 | 66.4±24.9 | 0.01 |
Data are Mean±SD. Final column reflects two-tailed unpaired t-test. Abbreviations: VO2, oxygen consumption; VE, minute ventilation; fb, breathing frequency; VT, tidal volume; VCO2, carbon dioxide production; DLCO, diffusing capacity of the lungs for carbon monoxide; DM, alveolar-capillary membrane conductance; VC, pulmonary capillary blood volume.
Figure 2.
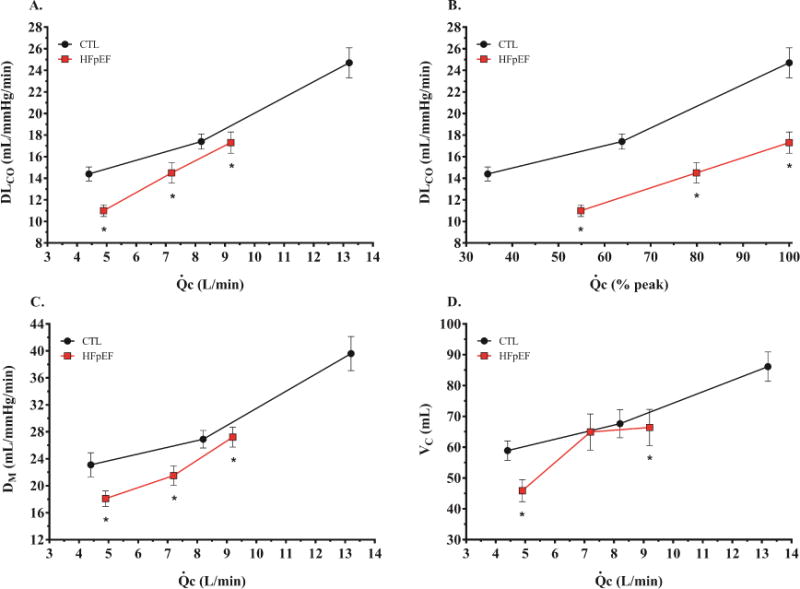
Diffusing capacity of the lungs for carbon monoxide (A=DLCO), DLCO/Q ratio (B), alveolar-capillary membrane conductance (C=DM), and pulmonary capillary blood volume (D=VC) as a function of cardiac output during exercise. *p <0.05 versus control.
During matched submaximal workload (20W), VO2 tended to be lower in the HFpEF (p=0.06, Table 3). The DLCO was lower in the HFpEF group compared to controls at 20W, mediated exclusively by reduced DM (Table 3). Notably, VC increased dramatically from baseline in the HFpEF group to 20W exercise, to a level that was not different from controls (Figure 2). Despite lower absolute values in HFpEF, relative changes in DLCO and DM from rest to 20W exercise were similar in HFpEF and controls. In contrast, the relative change in VC was 2-fold greater in HFpEF than controls during low-level exercise (Figure 3A). This greater increase in VC at 20W was coupled with higher VE and VT/TI (index of ventilatory drive) in the HFpEF group compared to the control group (Table 3). The increase in VE in HFpEF was due to higher fb, with no difference in VT, indicating a more tachypneic breathing pattern (Figure 1).
Figure 3.
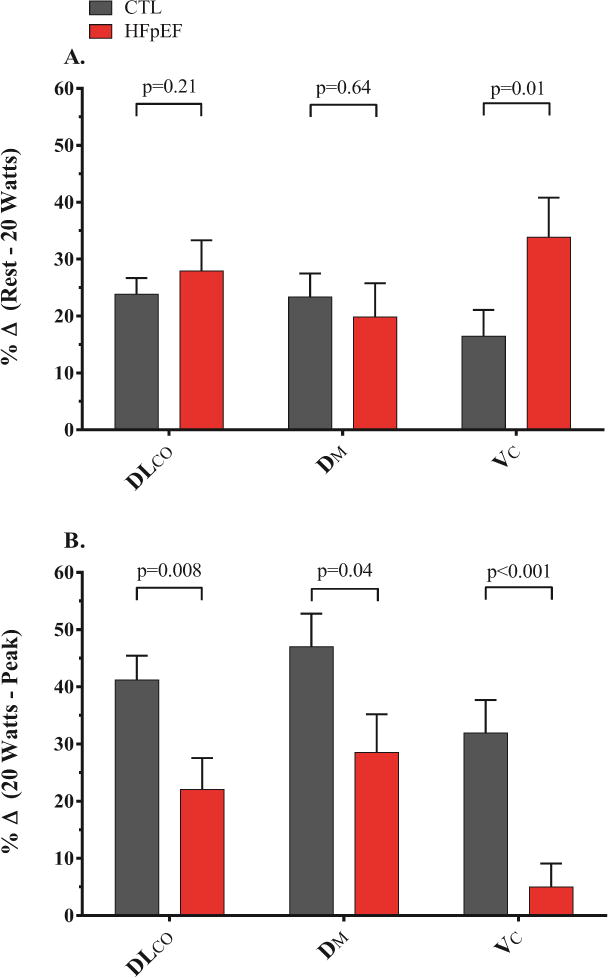
(A) Percent change from rest to matched submaximal absolute workload matched submaximal absolute workload (20 Watts) and (B) Percent change from matched submaximal absolute workload (20 Watts) to peak exercise for the diffusing capacity of the lungs for carbon monoxide (DLCO), alveolar-capillary membrane conductance (DM), and pulmonary capillary blood volume (VC) during exercise.
At peak exercise, VE was lower in the HFpEF group compared to the control group, mediated by reduced VT (Table 3). However, relative to the work performed, VT responses were similar in the two groups, while fb was persistently elevated in HFpEF relative to exercise workload (Figure 1). The DLCO during peak exercise in HFpEF was again lower compared to the controls, as a function of both a reduction in DM and VC (Table 3, Figure 2). From 20W exercise up to peak, there was proportionately greater increase in DLCO, DM and VC in controls as compared to HFpEF (Figure 3B). Notably, there was no further increase in VC from 20W to peak exercise in HFpEF subjects, indicating that pulmonary capillary blood volume reserve had become saturated during low-level exercise.
At any cardiac output, absolute DLCO and DM values were consistently lower in HFpEF subjects than controls, while VC was lower in HFpEF only at baseline and peak exercise (Figure 2). Peak VO2 was directly correlated with exercise increases in DLCO (r=0.68, p<0.0001) and DM (r=0.69, p<0.0001). In contrast, peak VO2 was only modestly associated with changes in VC (r=0.33, p=0.02).
DISCUSSION
This prospective study is the first to evaluate pulmonary gas diffusion both at rest and during exercise in patients with HFpEF, with separation of individual determinants of gas transfer including membrane conductance (DM) and pulmonary capillary blood volume (VC). We demonstrate that patients with HFpEF have important limitations to gas transfer as evidenced by global reduction in the diffusing capacity of the lung (DLCO) both at rest and during exercise.
Examination of the individual determinants of lung diffusion reveals a complex and dynamic interplay: with the onset of low-level exercise, there is greater increase in VC in subjects with HFpEF compared to controls, presumably related to the greater increase in pulmonary venous pressures. Despite the greater rise in VC with low-level exercise in HFpEF, DLCO remains lower than controls because of persistently decreased DM. From low level to peak exercise, there is no further increase in VC in HFpEF subjects, in contrast to steady increases in VC with increasing cardiac output observed in controls. This indicates a limitation in pulmonary vascular recruitment with maximal exercise, which coupled with impaired DM, greatly limits gas transfer during exercise in subjects with HFpEF. Diffusion abnormalities in HFpEF were associated with lower aerobic capacity and were coupled to greater symptoms of dyspnea, more profound tachypnea, and increased ventilatory drive relative to exercise workload. These data provide new insight into the mechanisms by which hemodynamic abnormalities developing during exercise in patients with HFpEF alter pulmonary function and gas exchange to contribute to symptoms of exercise intolerance. Novel therapies that improve gas diffusion through either hemodynamic or non-hemodynamic mechanisms may be effective to improve exercise tolerance in patients with HFpEF.
Determinants of Pulmonary Diffusing Capacity
Diffusing capacity of the lungs for carbon monoxide (DLCO) describes the conductance of gas from alveolus across the alveolar-capillary membrane to bind hemoglobin in erythrocytes(9). The reciprocal of this conductance (1/DLCO) thus represents the total resistance to gas transfer in the lungs and is determined by the sum of resistances imposed by the alveolar-capillary membrane (1/DM) as well as the pulmonary capillary blood (1/θ*VC).(9,12) Previous studies in patients with HF and reduced EF (HFrEF) have shown that DLCO and DM are reduced compared to controls when measured at rest, and that the extent of this impairment is correlated with greater burden of pulmonary vascular disease, worse ventilatory inefficiency, greater HF severity, lower exercise capacity, and increased mortality(13–16). The current results show that these limitations in resting gas diffusion are also present in patients with HFpEF, and that the extent of limitation is associated with impaired exercise capacity, hyperpnea and symptoms of dyspnea.
Pulmonary Diffusion Reserve in Heart Failure
In the normal alveolar-capillary interface, DLCO increases linearly with cardiac output during exercise. This is typically attributed to increased DM and VC due to capillary and alveolar distention and recruitment resulting in increased surface area available for gas exchange, along with more homogenous distribution of red cells within and among the pulmonary capillaries (17). Previous studies in HFrEF patients have evaluated changes in global gas diffusion (DLCO) during exercise. Smith and colleagues found that the increase in DLCO relative to Qc was impaired in HFrEF during low-level exercise (30W)(18). Olson et al. later confirmed and extended upon this observation showing reduced DLCO both at low-level and peak exercise in HFrEF(19). The current results in HFpEF reveal a similar picture, with impaired recruitment of pulmonary gas diffusion reserve during exercise compared to controls.
The primary novel finding in the current study is the evaluation of the individual determinants of lung diffusion during exercise, which has not been studied to date in any HF population. Agostoni and colleagues examined DLCO, DM and VC at baseline and then 2 minutes following exercise in patients with HFrEF and controls(15). The authors observed that while VC increased after exercise in HFrEF, DM decreased, consistent with the development of interstitial pulmonary edema. Similar reductions in DM have been observed following acute saline infusion in patients with HFrEF(13,14).
In contrast, the current study did not observe a reduction in DM during exercise, though absolute values of DM were lower in HFpEF compared to control for any given workload. The reason for the discrepant results may relate to the timing of DM assessment, which was during exercise in the current study as opposed to after exercise had been completed in the study of Agostoni(15). Indeed, following cessation of exercise, many of the factors that dictate recruitment of DM (e.g. distention of alveolar septae from increased ventilation, opening of capillaries with increased cardiac output) are no longer present. The slope of increase in DM relative to cardiac output was similar in cases and controls (Figure 2); arguing against the development of interstitial pulmonary edema during exercise in HFpEF, despite greater increase in VC with submaximal exercise (Figures 2–3). It may be that chronic remodeling of the alveolar-capillary membrane protects against the development of interstitial edema during transient increases in pulmonary venous pressure during exercise in HFpEF, but at the cost of impediment to gas transfer at the membrane (i.e. lower DM)(20,21). Cardiac output reserve was depressed in HFpEF subjects, and it cannot be determined from these data whether DM and VC responses to exercise might have differed if Qc were closer to normal.
Changes in Pulmonary Capillary Blood Volume during Exercise in HFpEF
Reduction in exercise capacity in many patients with HFpEF is determined largely by inadequate cardiac output reserve, which, when coupled with stress-induced elevations in pulmonary venous pressures, markedly limits exercise capacity(1,4,22). In this light, we can envision a setting where the decrements in cardiac reserve coupled with elevated filling pressures would exacerbate hemodynamic pooling in the pulmonary circulation. This theoretical construct is supported by the present study which demonstrates a greater increase in VC from rest to low intensity work (20 Watts) in HFpEF patients. This rapid rise during the onset of exercise corresponds to the time where the largest increase in left heart filling pressures is observed in patients with HFpEF(1,23). Indeed, 80% of the total increase in pulmonary venous pressure that will occur during exercise in HFpEF is observed in the first 1.5 minutes of exercise at 20W(1). We speculate that increases in venous return in this early stage cannot be accommodated by the left ventricle in HFpEF because of diastolic reserve limitation(4), such that during onset of exercise, right ventricular output transiently exceeds left ventricular output, and blood thus pools in the pulmonary circulation. This is consistent with the greater relative increase in VC in the HFpEF patients observed at 20W. With higher levels of exercise, a new steady state may be reached in HFpEF patients, where left and right-sided cardiac outputs are matched, even as they both remain lower than what is observed in controls, as in the current study and others(22). Alternatively, it may simply be that the ability to recruit greater pulmonary capillary volume becomes rapidly saturated in HFpEF during low-level exercise due to vascular remodeling.
Limitations
Body mass index was greater in HFpEF subjects compared to controls, though this seems unlikely to influence gas diffusion in the lungs because lung size does not vary with body composition. Invasive left ventricular filling pressures were not assessed as part of this study but are well known to increase dramatically during exercise in people with HFpEF, and measures reflective of resting LV filling pressures (BNP, E/e′, left atrial volume) were all increased in HFpEF compared to controls. Future study is required to clarify how hemodynamic derangements relate to observed changes in gas diffusion in HFpEF. Controls in the current study displayed somewhat depressed exercise capacity and mild ventilatory inefficiency. However, all were recruited a priori based upon the absence of known cardiovascular disease, and the mildly abnormal exercise findings in this group would only bias any observed group differences toward the null. Right ventricular function is known to be abnormal in HFpEF but was not assessed in the current study(24).
Conclusions
The results of this study confirm our hypothesis that patients with HFpEF demonstrate significant reductions in pulmonary gas diffusion capacity during exercise when compared to healthy control subjects. The impairment of DLCO reserve is related to abnormalities in DM and VC that vary as a function of exercise intensity, with persistent depression in DM throughout exercise, presumably related to alveolar-capillary remodeling, with greater increase in VC early during exercise followed by failure to increase VC further reflecting deficits in vascular recruitment reserve. These data provide important new insights into the pulmonary effects of hemodynamic abnormalities developing during exercise in HFpEF, and demonstrate that patients with HFpEF have impaired lung diffusion both at rest and during graded exercise that is related to abnormalities in lung conductance and capillary blood volume. Further study is warranted to determine how these abnormalities in lung diffusion might be targeted therapeutically to improve exercise capacity and tolerance in people with HFpEF.
Perspectives
Competency in Medical Knowledge 1
Patients with HFpEF display abnormal pulmonary gas transfer at rest that is related to abnormalities in lung membrane conductance and pulmonary capillary oligemia, despite evidence of elevated filling pressures.
Competency in Medical Knowledge 2
With low-level exercise where filling pressures increase dramatically, there is greater increase in pulmonary capillary blood volume in HFpEF that is coupled to increased ventilatory drive and greater symptoms of dyspnea.
Competency in Medical Knowledge 3
Up to maximum exercise, there is persistently impaired gas transfer related to reduced membrane conductance and inability to further recruit the pulmonary capillary vasculature in HFpEF.
Translational Outlook 1
Novel therapies that target pulmonary gas transfer, through either hemodynamic or non-hemodynamic mechanisms, may help improve exercise tolerance in patients with HFpEF.
Acknowledgments
We would like to thank the participants who contributed to the successful conduct of this research. TPO was supported by AHA-12GRNT11630027, BDJ by HL71478, and BAB was supported by grants from the Mayo Clinic CTSA, NIH (UL RR024150), and the Marie Ingalls Career Development Award in Cardiovascular Research.
ABBREVIATIONS
- HFpEF
Heart failure with preserved ejection fraction
- DLCO
Diffusion of the lungs for carbon monoxide
- DM
Alveolar-capillary membrane conductance
- VC
Pulmonary capillary blood volume
- EF
Ejection fraction
- RPE
Rating of perceived exertion
- BP
Blood pressure
- VO2
Volume of oxygen consumed
- VCO2
Volume of carbon dioxide produced
- VE
Minute ventilation
- VT
Tidal volume
- fb
Breathing frequency
- TI
Inspiratory time
- TTOT
Total respiratory cycle time
- Q
Cardiac output
- BNP
Brain natriuretic peptide
- E
Early mitral inflow velocity
- è
Medial mitral annular velocity
- LV
Left ventricle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Relationship with industry to disclose: none
References
- 1.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure-abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 3.Westermann D, Kasner M, Steendijk P, et al. Role of left ventricular stiffness in heart failure iwth normal ejection fraction. Circulation. 2008;117:2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 4.Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–969. doi: 10.1136/hrt.2010.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation. 2012;126:975–990. doi: 10.1161/CIRCULATIONAHA.111.085761. [DOI] [PubMed] [Google Scholar]
- 6.Olson TP, Snyder EM, Johnson BD. Exercise-disordered breathing in chronic heart failure. Exerc Sport Sci Rev. 2006;34:194–201. doi: 10.1249/01.jes.0000240022.30373.a2. [DOI] [PubMed] [Google Scholar]
- 7.Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lind FG, Hesser CM. Breathing pattern and lung volumes during exercise. Acta Physiol Scand. 1984;120:123–129. doi: 10.1111/j.1748-1716.1984.tb07381.x. [DOI] [PubMed] [Google Scholar]
- 9.Ceridon ML, Beck KC, Olson TP, Bilezikian JA, Johnson BD. Calculating alveolar capillary conductance and pulmonary capillary blood volume: comparing the multiple- and single-inspired oxygen tension methods. J Appl Physiol. 2010;109:643–653. doi: 10.1152/japplphysiol.01411.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyder EM, Olson TP, Johnson BD, Frantz RP. Influence of sildenafil on lung diffusion during exposure to acute hypoxia at rest and during exercise in health humans. Eur J Appl Physiol. 2008;103:421–430. doi: 10.1007/S00421-008-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamhane RM, Johnson RLJ, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest. 2001;120:1850–1856. doi: 10.1378/chest.120.6.1850. [DOI] [PubMed] [Google Scholar]
- 12.Forster RE, Roughton FL, Cander L, Briscoe WA, Kreuzer F. Apparent pulmonary diffusing capacity for CO at varying alveolar O2 tensions. J Appl Physiol. 1957;11:277–289. doi: 10.1152/jappl.1957.11.2.277. [DOI] [PubMed] [Google Scholar]
- 13.Guazzi M, Agostoni P, Bussotti M, Guazzi MD. Impeded alveolar-capillary gas transfer with saline infusion in heart failure. Hypertension. 1999;34:1202–1207. doi: 10.1161/01.hyp.34.6.1202. [DOI] [PubMed] [Google Scholar]
- 14.Puri S, Dutka DP, Baker BL, Hughes JMB, Cleland JGF. Acute saline infusion reduces alveolar-capillary membrane conductance and increases airflow obstruction in patients with left ventricular dysfunction. Circulation. 1999;99:1190–1196. doi: 10.1161/01.cir.99.9.1190. [DOI] [PubMed] [Google Scholar]
- 15.Agostoni P, Cattadori G, Bianchi M, Wasserman K. Exercise-induced pulmonary edema in heart failure. Circulation. 2003;108:2666–2667. doi: 10.1161/01.CIR.0000097115.61309.59. [DOI] [PubMed] [Google Scholar]
- 16.Guazzi M, Pontone G, Brambilla R, Agostoni P, Reina G. Alveolar-capillary membrane gas conductance: a novel prognostic indicator in chronic heart failure. Eur Heart J. 2002;23:467–476. doi: 10.1053/euhj.2001.2803. [DOI] [PubMed] [Google Scholar]
- 17.Hsia CCW, McGrayer DG, Ramanathan M. Reference values of pulmonary diffusion cpacity during exercise by a rebreathing technique. Am J Rep Crit Care Med. 1995;152:658–665. doi: 10.1164/ajrccm.152.2.7633723. [DOI] [PubMed] [Google Scholar]
- 18.Smith AA, Cowburn PJ, Parker ME, et al. Impaired pulmonary diffusion during exercise in patients with chronic heart failure. Circulation. 1999;100:1406–1410. doi: 10.1161/01.cir.100.13.1406. [DOI] [PubMed] [Google Scholar]
- 19.Olson LJ, Snyder EM, Beck KC, Johnson BD. Reduced rate of alveolar-capillary recruitment and fall of pulmonary diffusing capacity during exercise in patients with heart failure. J Card Fail. 2006;12:299–306. doi: 10.1016/j.cardfail.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Townsley MI, Fu Z, Mathieu-Costello O, West JB. Pulmonary microvascular permeability: Responses to high vascular pressure after induction of pacing-induced heart failure in dogs. Circ Res. 1995;77:317–325. doi: 10.1161/01.res.77.2.317. [DOI] [PubMed] [Google Scholar]
- 21.Lee YS. Electron microscopic studies on the alveolar-capillary barrier in the patients of chronic pulmonary edema. Jpn Circ J. 1979;43:945–954. doi: 10.1253/jcj.43.945. [DOI] [PubMed] [Google Scholar]
- 22.Abudiab MM, Redfield MM, Melenovsky V, et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785. doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 24.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]


