Abstract
Hearing gradually declines with age in both animals and humans and this condition is known as age-related hearing loss. Here, we investigated the effects of deficiency of Sirt1, a member of the mammalian sirtuin family, on age-related cochlear pathology and associated hearing loss in C57BL/6 mice, a mouse model of early-onset age-related hearing loss. Sirt1 deficiency reduced age-related oxidative damage of cochlear hair cells and spiral ganglion neurons, and delayed the early onset of age-related hearing loss. In cultured mouse inner ear cell lines, Sirt1 knockdown increased cell viability under oxidative stress conditions, induced nuclear translocation of Foxo3a, and increased acetylation status of Foxo3a. This resulted in increased activity of the antioxidant enzyme catalase. In young wild-type mice, both Sirt1 and Foxo3a proteins resided in the cytoplasm of the supporting cells within the organ of Corti of the cochlea. Therefore, our findings suggest that SIRT1 promotes early-onset age-related hearing loss through suppressing FOXO3a-mediated oxidative stress resistance in the cochlea of C57BL/6 mice.
Keywords: Hearing loss, aging, inner ear, oxidative stress, sirtuin
1. Introduction
Sirtuins are a family of NAD+-dependent protein deacetylases that are known to extend lifespan in lower organisms (Finkel, et al., 2009). While earlier studies have shown that overexpression of sirtuins increases lifespan in lower organisms (Rogina and Helfand, 2004; Tissenbaum and Guarente, 2001; Viswanathan, et al., 2005), later studies revealed that overexpression of sirtuin genes do not increase lifespan when compared to a genetically standardized control strain in worms and flies (Burnett, et al., 2011). Caloric restriction (CR) extends lifespan and delays the onset of age-related diseases, including age-related hearing loss (AHL) in mammals (Someya, et al., 2010; Weindruch and Sohal, 1997). Earlier studies showed that CR increases lifespan by activating sirtuins in yeasts, worms, or flies (Lin, et al., 2000; Rogina and Helfand, 2004; Wang and Tissenbaum, 2006). These studies have led to the development of sirtuin activators as a potential strategy to delay aging and age-related diseases in humans (Baur, et al., 2006). However, subsequent studies revealed that sirtuins are not required for lifespan extension by CR in these organisms (Kaeberlein, 2010; Kenyon, 2010). Furthermore, overexpression of Sirt1, a member of the mammalian sirtuin family, increases apoptotic cell death in hearts and decreases cardiac function in mice (Alcendor, et al., 2007), while Sirt1 inhibition protects rat cortical neurons against oxidative stress (Li, et al., 2008). Hence, the roles of sirtuins in extending healthspan and lifespan have proved controversial.
Hearing gradually declines with age in mammals and this condition is known as age-related hearing loss (AHL) (Gates and Mills, 2005; Yamasoba, et al., 2013). Hearing loss is the third most prevalent chronic condition in older adults and affects 40% of people older than 65 years and 80% of people older than 85 years (Gates and Mills, 2005; Yamasoba, et al., 2013). Hearing loss also affects speech understanding (Frisina and Frisina, 1997), contributes to isolation and depression, and has been linked to dementia. AHL arises from age-dependent loss of sensory hair cells, spiral ganglion neurons, and/or stria vascularis atrophy in the cochlea of the inner ear. Hair cells are the sensory receptors that transduce sound stimuli into electrical responses (Hudspeth, 1997). The inner hair cells are the actual sensory receptors that relay their electrical response postsynaptically to the central auditory system through the auditory nerves or spiral ganglion neurons, whereas outer hair cells receive mostly efferent input. Stria vascularis is heavily vascularized and holds numerous capillary loops and small blood vessels that are essential for transporting oxygen, nutrients, and hormones into the cochlea. Hence, these cells are essential for maintaining auditory function, and extensive loss or degeneration of the hair cells or spiral ganglion neurons and/or atrophy of the stria vascularis results in hearing loss.
We have shown previously that Sirt3, a mitochondrial sirtuin, is required for the CR-mediated reduction of oxidative damage in the cochlear hair cells and spiral ganglion neurons and prevention of AHL in C57BL/6 (B6) mice, a mouse model of early-onset age-related hearing loss and one of the most widely used mouse models for the studies of aging (Someya, et al., 2010). In the current study, we examined the effects of Sirt1 deficiency on age-related cochlear pathology and associated hearing loss in B6 mice. Our results show that Sirt1 deficiency reduces age-related oxidative damage of cochlear hair cells and spiral ganglion neurons, and delays the early onset of AHL by enhancing Foxo3a-mediated oxidative stress resistance in the cochlea of B6 mice.
2. Materials and Methods
2.1. Animals
Male and female Sirt1+/− mice were a gift from Dr. Frederick W. Alt (Harvard University Medical School, Boston, MA) and have been described previously (Cheng, et al., 2003). Details on the methods used to house and feed mice have been described previously (Pugh, et al., 1999). Experiments were performed in accordance with protocols approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee. Only male wild-type (WT) and Sirt1+/− littermates were used in the current study.
2.2. Genotyping and DNA sequencing
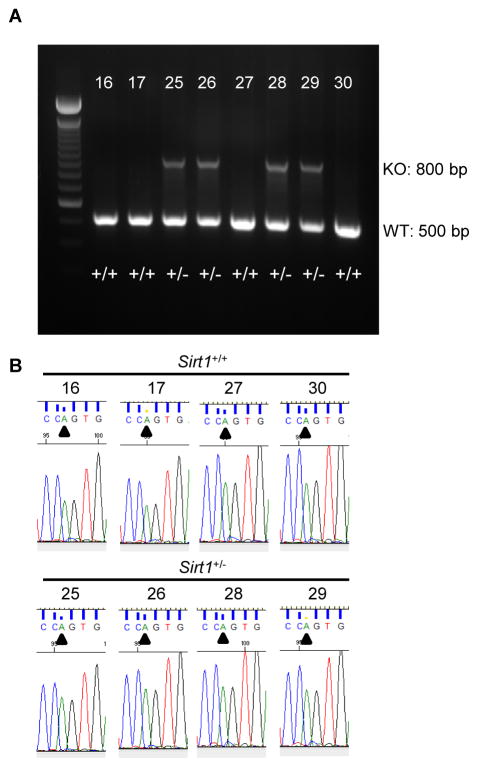
Sirt1 genotyping: Sirt1+/− males were mated with Sirt1+/− females, and the offspring from these mating were genotyped from DNA obtained by a tail clip at weaning. The following primers were used for genotyping: SIRT1SKO-F 5’-CTTGCACTTCAAGGGACCAA-3’; SIRT1SKO-R1 5’-GTATACCCACCACATCTGAG-3’; SIRT1SKO-R2 5’-CTACCACTCCTGGCTACCAA-3’. The PCR cycling parameters were as follows: 94°C for 3 min; 35 cycles of 94°C for 30 s, 56°C for 60 s, 72°C for 60 s; 72°C for 10 min. PCR products were separated on 1.5% agarose gel and the expected band size for WT and knockout allele were 500 and 800 bps, respectively (Fig. 1A).
Fig. 1.
Genotyping of Sirt1+/+ and Sirt1+/− mice. (A) PCR products were separated on 1.5% agarose gel and the expected band sizes for WT and knockout (KO) alleles were 500 and 800 bps, respectively. (B) The Cdh23 gene in WT and Sirt1+/− mice (n=4) was sequenced. All the Sirt1+/+ mice had the Cdh23753A/753A genotype, and all the Sirt1+/− mice had the Cdh23753A/753A genotype. Arrows indicate the Cdh23753A allele.
Cdh23 genotyping: Male and female Sirt1+/− mice have been backcrossed for 4 generations onto the C57BL/6J mouse strain that is homozygous for the recessive AHL-susceptibility allele Cdh23753A. To confirm that both Sirt1+/+ and Sirt1+/− mice have the same genotype for Cdh23, we amplified the DNA region containing the 753rd nucleotide in the Cdh23 gene by PCR reaction and sequenced the Cdh23 gene in the DNA obtained from tails of young Sirt1+/+ and Sirt1+/− mice (N=4 each group). The following primers were used for the PCR reaction: Cdh23-F 5’-GATCAAGACAAGACCAGACCTCTGTC-3’; Cdh23-R 5’-GAGCTACCAGGAACAGCTTGGGCCTG-3’. The size of amplified PCR product was 360 bps. We confirmed that all the Sirt1+/+ mice have the Cdh23753A/753A genotype, and all the Sirt1+/− mice have the Cdh23753A/753A genotype (Fig. 1B).
2.3. ABR hearing test
At 3 and 12 months of age, auditory brainstem responses (ABRs) were measured with a tone burst stimulus at 8, 16, and 32 kHz using an ABR recording system (Intelligent Hearing System, Miami, FL) as previously described (Someya, et al., 2010). Mice were anesthetized with a mixture of xylazine hydrochloride (10 mg/kg, i.m.) (Phoenix Urology of St. Joseph, St. Joseph, MO) and ketamine hydrochloride (40 mg/kg, i.m.) (Phoenix Urology of St. Joseph). We used 9–12 mice per group for ABR hearing assessment. Following the ABR hearing measurements, tissues from the same mice were used to conduct histopathological and biochemical analyses.
2.4. Cochlear histology
Following the ABR hearing measurements, the animals were sacrificed by cervical dislocation and the temporal bone was excised from the head and divided into cochlear and vestibular parts (Someya, et al., 2010). The cochlea was then excised, immersed in a fixative containing 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) in PBS solution for 1 day, and decalcified in 10% EDTA for 1 week. The paraffin-embedded specimens were sliced into 4-μm sections, mounted on silane-coated slides, stained with H&E, and observed under a light microscope (Leica). The Rosenthal's canal was divided into three regions: apical, middle and basal and the three regions were used for evaluation of cochlear histology. We used 4–5 mice per group for histopathological assessment. In each mouse, we evaluated every third modiolar section obtained from one cochlea for a total of ten sections. Tissues from the same animals were used for neuron counting, hair cell counting, and stria vascularis thickness measurement.
2.5. Cochlear cell counting
Spiral ganglion neuron counting: Spiral ganglion neurons (SG) were counted in the apical, middle, and basal regions of the cochlear sections using a 40X objective as previously described (Someya, et al., 2009). Ten sections of the apical, middle, and basal turns were evaluated in one cochlea per mouse. We used 4–5 mice per group for SGN counting. Hair cell counting: Outer (OH) hair cells and inner hair (IH) cells were counted in the apical, middle, and basal regions of the cochlear sections using a 40X objective (Someya, et al., 2009). Hair cells were identified by the presence of a nucleus. The OH cell survival percentage was calculated as the number of intact OH cells present among the 3 OH cells that should be observed in each turn of one cochlea in tissue sections of mice with normal hearing. The IH cell survival percentage was calculated as the number of intact IH cells present of the one IH cell that should be observed in each turn of one cochlea in tissue sections of mice with normal hearing. Ten sections of the apical, middle, and basal turns were evaluated in one cochlea per mouse. We used 4–5 mice per group for OHC and IHC counting.
2.6. Stria vascularis thickness measurements
Stria vascularis thickness was measured in 40X images of HE-stained mouse cochlear tissues. In the ImageJ program, the measurement was made by using a cursor to draw a line from the margin of the stria to the junction of the basal cells with the spiral ligament half-way between the attachment of Reissner’s membrane and the spiral prominence (Keithley, et al., 2005). Measurements were made at the basal, middle and apical regions of the cochlea for each mouse, and averages of each region were calculated for each mouse. Six to nine sections of the apical and middle turns were evaluated in one cochlea per mouse. We used 4 mice per group for stria vascularis thickness measurements.
2.7. Immunohistochemistry
For confocal-based immunohistochemistry, cochlear sections on slides were rehydrated and subjected to antigen retrieval (0.01 M NaCitrate pH 6.0 for 30 minutes at 60°C) and then incubated in diluted primary antibodies (Sirt1, Foxo3a and CoxIV) over night at 4°C. The following day, the slides were washed extensively and appropriate fluorescently labeled secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were applied for 2 hours at 37°C. The slides were then washed thoroughly and treated with DAPI (4’,6-diamidino-2-phenylindole, InVitrogen, Eugene, Oregon) to visualize DNA, and/or COXIV to visualize mitochondria. Cover slips were mounted with 60% glycerol in TBS containing p-phenylenediamine (to inhibit fluorescence quench). Preparations were viewed and digital images gathered with a Leica SP5 laser scanning confocal microscope. Figures were assembled using CorelDRAW 12 software. For light microscopy-based immunohistochemistry, cochlear sections were treated with anti-8-oxoguanine antibody (mouse monoclonal, 1:400 dilution, Abcam) to visualize 8-oxoguanine-positive cells or catalase antibody (mouse monoclonal, 1:50 dilution, Sigma-Aldrich) to visualize catalase-positive cells using the M.O.M kit (Vector laboratories, Burlingame, CA). Catalase-positive cells and 8-oxoguanine-positive cells were counted in the apical and middle regions using a 40X objective and the Cell Counter plugin of the ImageJ software. The 8-oxoguanine-positive cell percentage was calculated as the number of 8-oxoguanine-positive cells out of the total number of OH, IH, or SG cells. The catalase-positive cell percentage was calculated as the number of catalase-positive cells out of the total number of OH, IH, or SG cells. Six to nine sections of the apical and middle turns were evaluated in one cochlea per mouse. We used 4 mice per group for 8-oxoguanine-positive or catalase-positive cell counting.
2.8. Isolation of cytosol, nuclei, and mitochondria
Dissected inner ears or cultured cells were homogenized using a tissue grinder (Wheaton dounce tissue grinder, Fisher Scientific, Pittsburgh, PA) containing 1 ml of Tris buffer (10 mM Tris, 1 mM EDTA, 320 mM sucrose, pH 7.4) on ice and then centrifuged at 720 G (3000 rpm) for 5 min at 4°C to get a nuclear fraction (pellet). The supernatant was centrifuged at 12000 G for 10 min at 4°C to get a mitochondrial fraction (pellet) and a cytosolic fraction (supernatant). The pellets (nuclear fraction and mitochondrial fraction) were re-suspended vigorously with 200 μl or 100 μl of 1% NP-40 buffer (50 mM Tris, 250 mM NaCl, 1% NP40, pH 7.4) and after 30 min of incubation on ice, were centrifuged at 12000 G for 10 min at 4°C. Lamin B1, VDAC, or GAPDH was used as a nuclear, mitochondrial, or cytosolic marker respectively.
2.9. Western blotting
Fifty micrograms of total protein was fractionated by 10% of SDS-PAGE and transferred to nitrocellulose membranes (Bio-rad, Hercules, CA). Membranes were incubated with the primary antibody followed by the horseradish peroxidase-linked secondary antibody. A chemiluminescent detection reagent (ECL Prime, GE Healthcare Life Sciences, Logan, UT) or the Odyssey infrared scanner (LiCor Biosciences, Lincoln, NE) was used to visualize proteins. The band intensity was quantified using the ImageJ program and the levels of each protein were normalized by loading controls.
2.10. Cell line
HEI-OC1, a cochlear cell line, was a gift from Dr. Federico Kalinec (Department of Head and Neck Surgery, UCLA) and was maintained in high-glucose DMEM (Life technologies, Grand Island, NY) containing heat-inactivated 10% fetal bovine serum (HyClone FBS, GE Healthcare Life Sciences, Logan, UT) as previously described (Kalinec, et al., 2003). The HEI-OC1 cell line was derived from long-term cultures of cochleas of Immortomouse that was derived from CBA/Ca X C57BL/10 (Charles River, http://www.criver.com/products-services/basic-research/find-a-model/immortomouse).
2.11. Gene knockdown
To generate siRNA-mediated knockdown cells, HEI-OC1 cells (3X105) were plated on a 6 well plate one day before transfection. siRNA (Origene, Rockville, MD) targeted to mouse Sirt1 and scrambled siRNA were transfected with lipofectamine RNAi max (Life Technologies, Grand Island, NY) according to manufacturer’s instructions. After five days of incubation, the expression of Sirt1 protein was examined by western blotting.
2.12. In vitro oxidative stress test
The Sirt1 knockdown or control cells were replated on a 96 well plate (3X104/well) and treated with hydrogen peroxide at 0 to 2.8 mM for 2 hours. For cell viability measurements, after 22 hours, the media was replaced with DMEM containing 50 μg/mL neutral red (Sigma-Aldrich, St. Louis, MO) as previously described (Someya, et al., 2009). After 2 hours, 200 μl of a neutral red destaining solution composed of 50% ethanol, 49% deionized water, and 1% glacial acetic acid (Sigma-Aldrich, St. Louis, MO) was added to each well. The 96-well plate was placed on a plate shaker for 1 hour and the OD of the neutral red extract in each well was measured at 540 nm in a microplate spectrophotometer (BioTek, Winooski, VT). Each condition was run in duplicate.
2.13. Catalase activity assay
Catalase activity was measured using the catalase assay kit (Sigma-Aldrich, St. Louis, MO) according to the manufacturer’s instructions. In brief, 25 μl of samples (5~10 μg protein/μl) was mixed with 50 μl of 1X assay buffer and 25 μl of 200 mM H2O2 solution and incubated for 2 min at room temperature. The reaction was stopped by adding a stop solution (15 mM sodium azide in water). Then, 10 μl out of the 100 μl reaction mixture was mixed with 990 μl of the color reagent (150 mM potassium phosphate buffer, pH 7.0, containing 0.25 mM 4-aminoantipyrine and 2 mM 3,5-dichloro-2-hydroxybenzensulfonic acid) in a new tube by inversion. After 15 min of incubation for color development, the absorbance was measured at 520 nm in a spectrometer. Activity (μmoles/min/mg protein or U/mg protein) was calculated using the equation “ .”
2.14. Acetylation Levels of Foxo3a
Sirt1-knockdown cells and control cells were fractionated by Tris buffer, 1% NP-40 buffer containing deacetylase inhibitors, 10 mM nicotinamide, and 500 nM trichostatin. Nuclear lysates from Sirt1-knockdown cells and control cells were immunoprecipitated using the Pierce Classic IP kit (Thermo Scientific, Waltham, MA). One milligram of nuclear lysates were pre-cleared by the control agarose resin, combined with 3 μg of anti-acetyl lysine antibody (mouse monoclonal, Abcam, Boston, MA, Cat.#ab22550), and incubated on the shaker at 4°C for 16 hours. The antibody/lysate sample was added to Protein A/G agarose resin in a spin column and incubated on the shaker at 4°C for 1 hour. The column was centrifuged at 12000 G for 10 min and washed by IP lysis/wash buffer and 1X conditioning buffer. Fifty microliters of 2X sample buffer was then added to the column. The column was incubated at 100°C for 5 min and centrifuged at 12000 G for 10 min. To measure acetylation levels of Foxo3a, western blotting was performed using the immunoprecipitated samples using anti-Foxo3a antibody (rabbit polyclonal, used at 1:1000 dilution, Cell Signaling, Beverly, MA).
2.15. Antibodies
For confocal-based immunohistochemistry, primary antibodies used were as follows: Sirt1 (rabbit polyclonal, 1:50 dilution, Millipore, Bedford, MA), Foxo3a (rabbit polyclonal, 1:200 dilution, Sigma-Aldrich, St. Louis, MO), COX IV (mouse monoclonal, 1:100 dilution, Abcam, Boston MA). For western blotting, primary antibodies used were as follows: Sirt1 (mouse monoclonal, 1:1000 dilution, Sigma-Aldrich), Foxo3a (rabbit polyclonal, 1:1000 dilution, Cell Signaling, Beverly, MA), Catalase (mouse monoclonal, 1:2000 dilution, Sigma-Aldrich), SOD2 (mouse monoclonal, 1:1000 dilution, Abcam), Lamin B1 (rabbit polyclonal, 1:2000 dilution, Abcam), VDAC (rabbit polyclonal, 1:1000 dilution, Cell Signaling), GAPDH (rabbit polyclonal, 1:5000 dilution, Abcam). Secondary antibodies used were as follows: Mouse (1:5000 dilution, GE Healthcare Life Sciences, Piscataway, NJ) and rabbit (1:5000 dilution, GE Healthcare Life Sciences) secondary antibodies.
2.16. Statistical analysis
All statistical analyses were carried out by student T-test for 2 groups or by one-way ANOVA with post-Tukey multiple comparison tests for more than 3 groups using the Prism 4.0 statistical analysis program (GraphPad). All tests were 2-sided with statistical significance set at P < 0.05.
3. Results
3.1. Sirt1 deficiency delays the early onset of age-related hearing loss in B6 mice
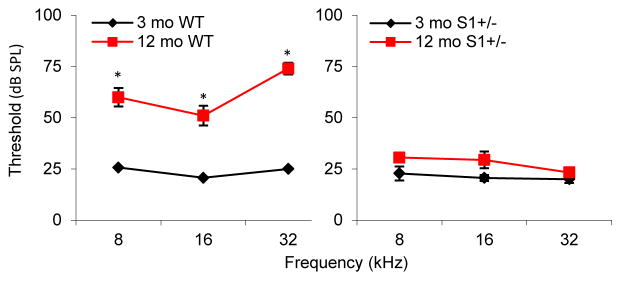
First, to investigate whether Sirt1 plays a role in maintaining auditory function or in age-related hearing loss, we conducted ABR (auditory brainstem response) hearing tests in young and middle-aged wild-type (WT) and Sirt1+/− mice that have been backcrossed onto the C57BL/6J strain for four generations. Because Sirt1−/− mice exhibited developmental defects and infrequently survived postnatally (Cheng, et al., 2003), only WT and Sirt1+/− mice were used in this study. To avoid obesity and other age-related diseases, the animals were not fed ad libitum, but were fed a control diet providing 85~90% of the average ad libitum food intake of these mice. We first confirmed that aging resulted in increased ABR hearing thresholds at the high (32 kHz), middle (16 kHz), and low (8 kHz) frequencies in middle-aged WT mice (Fig. 2, left), indicating age-related hearing loss. Surprisingly, aging did not result in increased ABR hearing thresholds in middle-aged Sirt1+/− mice at the same frequencies tested (Fig. 2, right), and there were no differences in hearing levels between young and middle-aged Sirt1+/− mice at 32, 16, and 8 kHz. Sirt1+/− mice were viable and fertile, and there were no differences in body weight between WT and Sirt1+/− mice at 3 or 12 months of age (data not shown).
Fig. 2.
Sirt1 deficiency delays the early onset of age-related hearing loss. ABR hearing thresholds were measured at 32, 16, and 8 kHz from WT (left) and Sirt1+/− (right) mice at 3 and 12 months of age. *Significantly different from 3-month-old WT mice (P < 0.05). Data are means ± SEM. S1=Sirt1.
3.2. Sirt1 deficiency protects cochlear hair cells and neurons
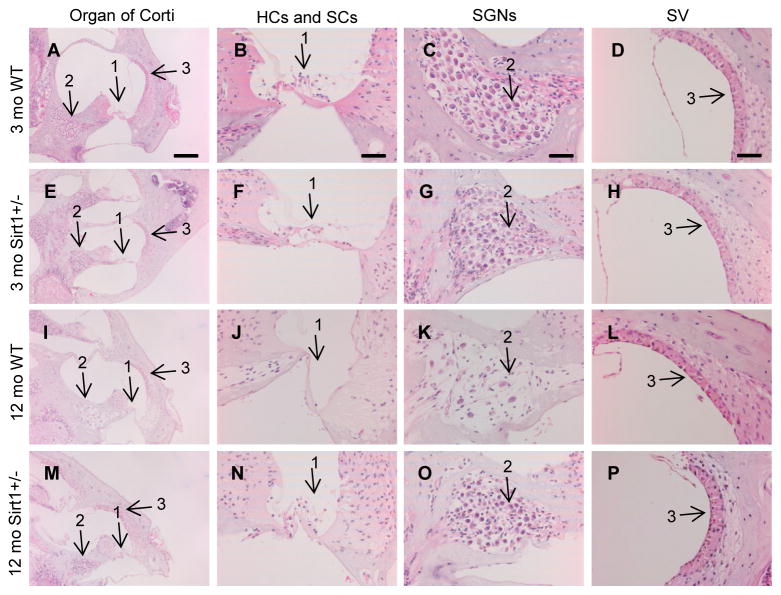
AHL arises from age-related loss of sensory hair cells, spiral ganglion neurons, and/or atrophy of the stria vascularis within the cochlea (Gates and Mills, 2005; Spongr et al., 1997; Yamasoba, et al., 2013). To validate the ABR hearing test results, we performed histological analysis on cochlear tissue sections from young and middle-aged WT and Sirt1+/− mice. In agreement with the hearing test results, basal regions of the cochleas from young WT and Sirt1+/− mice displayed no or only minor loss of sensory inner hair cells, outer hair cells (Fig. 3A, B, E, and F), or spiral ganglion neurons (Fig. 3C and G), and no or only minor degeneration of strial vascularis cells (Fig. 3D and H). In middle-aged mice, basal regions of the cochleas from WT mice displayed severe loss of outer hair and inner hair cells (Fig. 3I and J) and spiral ganglion neurons (Fig. 3K), while basal regions of the cochleas from age-matched Sirt1+/− mice displayed only minor loss or degeneration of these hair cells, spiral ganglion neurons (Fig. 3M–O), and increased stria vascularis thickness (Fig. 3P) when compared to WT mice (Fig. 3L).
Fig. 3.
Sirt1 deficiency protects cochlear cells. Organ of Corti in the basal cochlear regions from 3-month-old WT (A) and Sirt1+/− (E), and 12-month-old WT (I) and Sirt1+/− (M) mice. Hair and supporting cells in the organ of Corti in the basal cochlear regions from 3-month-old WT (B) and Sirt1+/− (F), and 12-month-old WT (J) and Sirt1+/− (N) mice. Spiral ganglion neurons in the basal cochlear regions from 3-month-old WT (C) and Sirt1+/− (G), and 12-month-old WT (K) and Sirt1+/− (O) mice. Strial vascularis in the basal cochlear regions from 3-month-old WT (D) and Sirt1+/− (H), and 12-month-old WT (L) and Sirt1+/− (P) mice. Arrows 1 indicate hair and supporting cells. Arrows 2 indicate spiral ganglion neuron regions. Arrows 3 indicate strial vascularis regions. HCs = hair cells, SCs = supporting cells, SGNs = spiral ganglion neurons, SV = strial vascularis. Scale bar = 100 μm (A) or 20 μm (B–D).
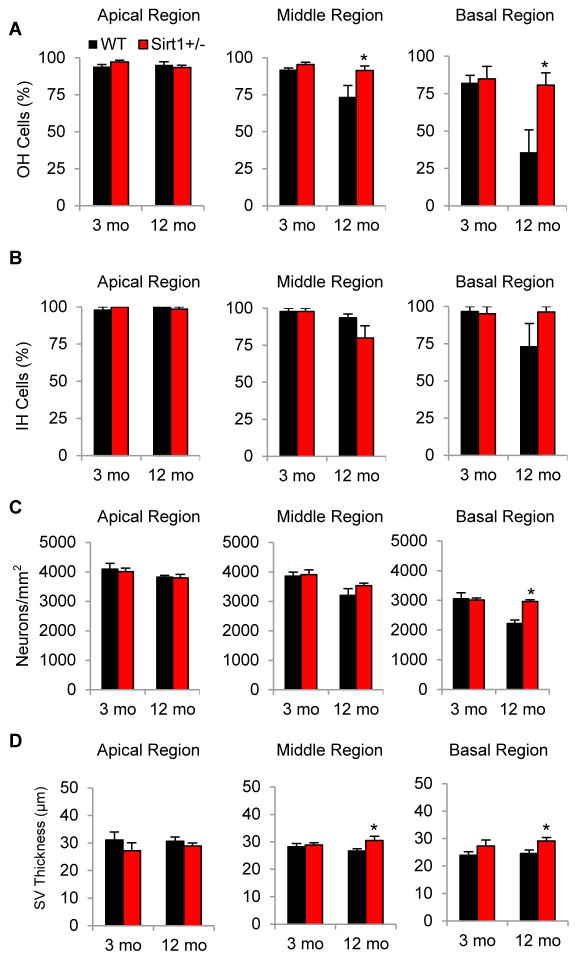
To confirm the histological observation results, we counted the numbers of cochlear hair cells and spiral ganglion neurons, and measured the thickness of stria vascularis of WT and Sirt1+/− mice at 3 and 12 months of age. Sirt1 deficiency increased the survival of outer hair cells (Fig. 4A) and spiral ganglion neurons (Fig. 4C), and increased stria vascularis thickness (Fig. 4D) in the basal regions of the cochlea. However, no significant changes between WT and Sirt1+/− mice were observed in the survival of inner hair cells in the basal region at 12 months of age (Fig. 4B). At 3 months of age, no significant changes between WT and Sirt1+/− mice were observed in the survival of outer hair cells, inner hair cells, or spiral ganglion neurons, or stria vascularis thickness in the basal cochlear regions (Fig. 4A–D). In the apical or middle cochlear regions, no significant changes between WT and Sirt1+/− mice were observed in the survival of inner hair cells or spiral ganglion neurons at 3 or 12 months of age (Fig. 4B and C). Collectively, these results demonstrate that Sirt1 deficiency protects cochlear outer hair cells and spiral ganglion neurons, and delays the early onset of AHL in B6 mice.
Fig. 4.
Sirt1 deficiency reduces age-related loss of hair cells and spiral ganglion neurons in the cochlea. (A) OH (outer hair) cell survival (%) of apical, middle, and basal cochlear regions of WT and Sirt1+/− mice was measured at 3 and 12 months of age (n = 5). (B) IH (inner hair) cell survival (%) of apical, middle, and basal cochlear regions of WT and Sirt1+/− mice was measured at 3 and 12 months of age (n =5). (C) SGN (spiral ganglion neuron) survival (SGN density) of apical, middle, and basal cochlear regions of WT and Sirt1+/− mice was measured at 3 and 12 months of age (n = 5). (D) SV (strial vascularis) thickness of apical, middle, and basal cochlear regions of WT and Sirt1+/− mice was measured at 3 and 12 months of age (n = 5). *Significantly different from 12-month-old WT mice (P < 0.05).
3.3. Sirt1 deficiency protects cochlear hair cells and neurons from oxidative stress
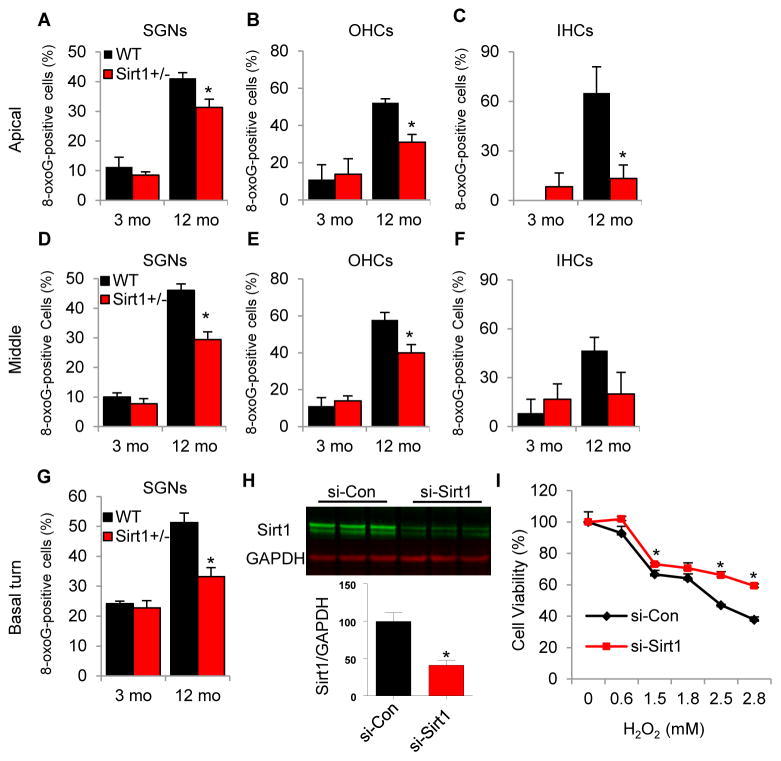
It is well documented that oxidative damage contributes to age-related loss of sensory hair cells, spiral ganglion neurons, and/or atrophy of the stria vascularis within the cochlea in mammals (Yamasoba, et al., 2013). Hence, we hypothesized that Sirt1 deficiency may protect cochlear sensory hair cells and neurons through the reduction of oxidative damage in the cochlea. To test this hypothesis, 8-oxoguanine, a common oxidative nuclear DNA damage marker, was immunostained with anti-8-oxoguanine antibody and observed under light microscopy. We found that middle-aged Sirt1+/− mice displayed significantly fewer 8-oxoguanine-positive cells in the outer hair cells and spiral ganglion neurons in the middle cochlear regions when compared to age-matched WT mice (Fig. 5D and E). In the apical cochlear regions, middle-aged Sirt1+/− mice also displayed significantly fewer 8-oxoguanine-positive cells in the inner hair cells, outer hair cells, and spiral ganglion neurons when compared to age-matched WT mice (Fig. 5A–C). In the basal cochlear regions, middle-aged Sirt1+/− mice displayed significantly fewer 8-oxoguanine-positive cells in the spiral ganglion neurons when compared to age-matched WT mice (Fig. 5G). At 3 months of age, no significant changes were observed in the numbers of 8-oxoguanine-positive cells in the outer hair cells, inner hair cells, or spiral ganglion neurons between WT and Sirt1+/− mice (Fig. 5A–G).
Fig. 5.
Sirt1 deficiency reduces oxidative nuclear DNA damage in the cochlea, while Sirt1 knockdown increases cell viability in cultured mouse inner ear cells under oxidative stress conditions. (A–G) The numbers of 8-oxoguanine-positive cells were counted in the outer hair cells (B, E) inner hair cells (C, F), and spiral ganglion neurons (A, D, G) in the apical (A–C), middle (D–F), and basal (G) cochlear regions from WT and Sirt1+/− mice at 3 and 12 months of age. *Significantly different from age-matched WT mice (P < 0.05). Data are means ± SEM. (H) Western blotting analysis of Sirt1 protein levels in Sirt1 knockdown mouse inner ear cells (si-Sirt1) and control cells (si-Con): Quantification of Sirt1 proteins (lower panel) from (upper panel). (I) Sirt1 knockdown increased the viability of cultured mouse inner ear cells treated with hydrogen peroxide (H2O2) (n = 3) (0–2.8 mM). *Significantly different from control cells (P < 0.05).
To further confirm the roles of Sirt1 in oxidative stress resistance of cochlear cells, we conducted in vitro oxidative stress tests using hydrogen peroxide (H2O2), followed by cell viability tests in cultured mouse inner ear cell lines (HEI-OC1) that were transfected with siRNA targeted to mouse Sirt1. We first confirmed that treatment with siRNA reduced Sirt1 protein expression levels by 67% compared to controls (treatment with scrambled siRNA) in the HEI-OC1 cells (Fig. 5H). Hydrogen peroxide reduced cell viability in a dose-dependent manner in the control cells; however, Sirt1 knockdown cells displayed increased cell viability at multiple concentrations of H2O2 compared to control cells (Fig. 5I). Collectively, these results demonstrate that Sirt1 deficiency protects cochlear hair cells and neurons from oxidative stress.
3.4. Sirt1 resides in the cytosol of the supporting cells within the organ of Corti of the cochlea
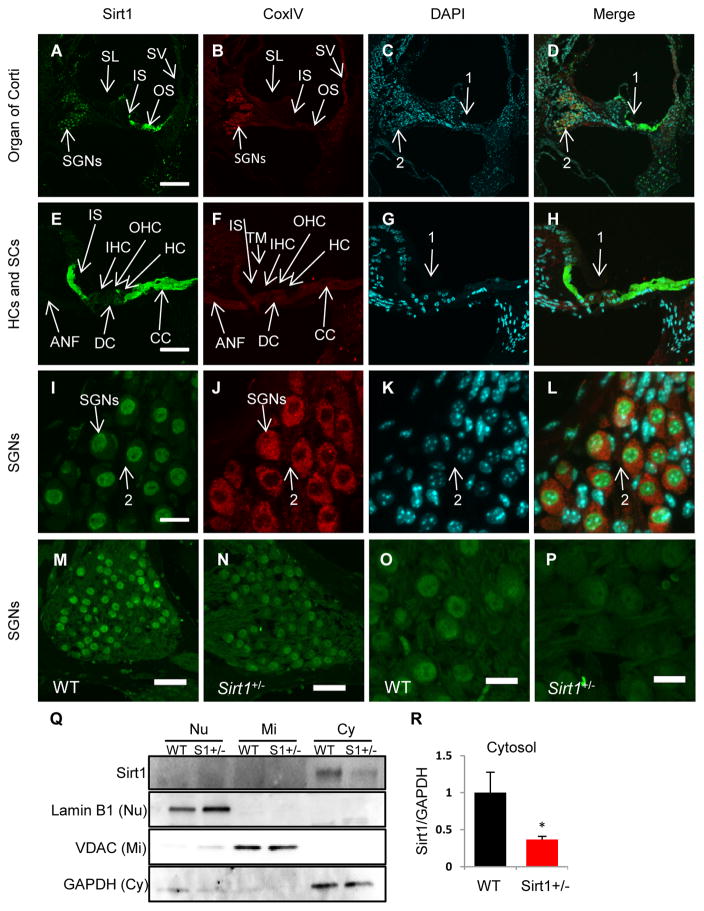
To investigate the localization of Sirt1 in the cochlea of young WT mice, Sirt1 was immunostained with anti-Sirt1 antibody and observed by confocal microscopy. Fig. 6A–D show an area of the organ of Corti in the cochlea at low magnification: Sirt1 immunostaining was detected as a very strong signal in the cytosol of some of the supporting cells of the organ of Corti, including the cells of Claudius (Fig. 6E–H) that provide mechanical support to the outer hair cells, and inner sulcus cells that enclose the sensory inner hair cells (Merchant and Nadol, 2010). There was also a signal for Sirt1 immunostaining in the nuclei of the neuronal cells of the spiral ganglion (Fig. 6I–L). When comparing signal intensities for the Sirt1 immunolocalization between WT and Sirt1+/− cochlear tissues, the spiral ganglion neurons from the Sirt1+/− mice clearly showed a lower level of Sirt1 than the WT (Fig. 6M–P). All conditions for labeling and imaging were identical and the images were gathered on the same day using confocal settings first optimized for the WT and then used on the Sirt1+/− cochlear tissues. To confirm these immunostaining results, we performed western blotting to measure Sirt1 protein levels in the cytosol, nuclei, and mitochondria in the inner ear tissues from 3–4 months old WT and Sirt1+/− mice. We found that Sirt1 protein was expressed in the cytosol in the cochleas of WT mice (Fig. 6Q), while Sirt1+/− mice displayed a 63% decrease in Sirt1 protein levels in the cytosol of the cochlea compared to WT mice (Fig. 6R). Together, these results demonstrate that Sirt1 is expressed in the cochlea and resides in the cytosol of the supporting cells within the organ of Corti, including Claudius and inner sulcus cells.
Fig. 6.
Sirt1 is prominently localized in the cytosol of the supporting cells of the organ of Corti within the cochlea. (A–L) Sirt1 staining (green) (A,E,I), COX IV staining (mitochondrial marker) (red) (B, F, J), DAPI staining (nuclear marker) (C, G, K), and merged staining (D, H, L) were detected in the organ of Corti regions (A–D), hair cells (HCs) and their supporting cells (SCs) (EH), or spiral ganglion neurons (SGNs) (I–L) from 3-month-old WT mice. Arrows indicate hair and supporting cells in the organ of Corti region. Arrows 2 indicate spiral ganglion neurons. Figures were taken from the basal turn (A–H). Scale bar = 200 μm (A), 50 μm (E) or 15 μm (I). SV=stria vascularis, SL=spiral lympus, SGN=spiral ganglion neurons, IS=inner sulcus cells, OS=outer sulcus region, IHC=inner hair cells, OHC=outer hair cells, DC=Deiters’ cell, HC=Hensen’s cell, CC=Claudius cell, ANF=auditory nerve fibers, TM=tectorial membrane. (M–P) Sirt1 staining in the spiral ganglion neuron regions of WT (M, O) and Sirt1+/− (N, P) mice. Scale bar = 40 μm (M–N) or 15 μm (O–P). (Q, R) Western blotting analysis of Sirt1 protein levels in the cochlea from 3-month-old WT mice: Quantification of Sirt1 proteins in the cytosol (R) from (Q). Lamin B1, VDAC, and GAPDH were used as a nuclear, mitochondrial, and cytosolic markers respectively. S1=Sirt1, Nu=nuclear, Mi=mitochondria, Cy=cytosol.
3.5. Foxo3a resides in the cytosol of the supporting cells within the organ of Corti of the cochlea
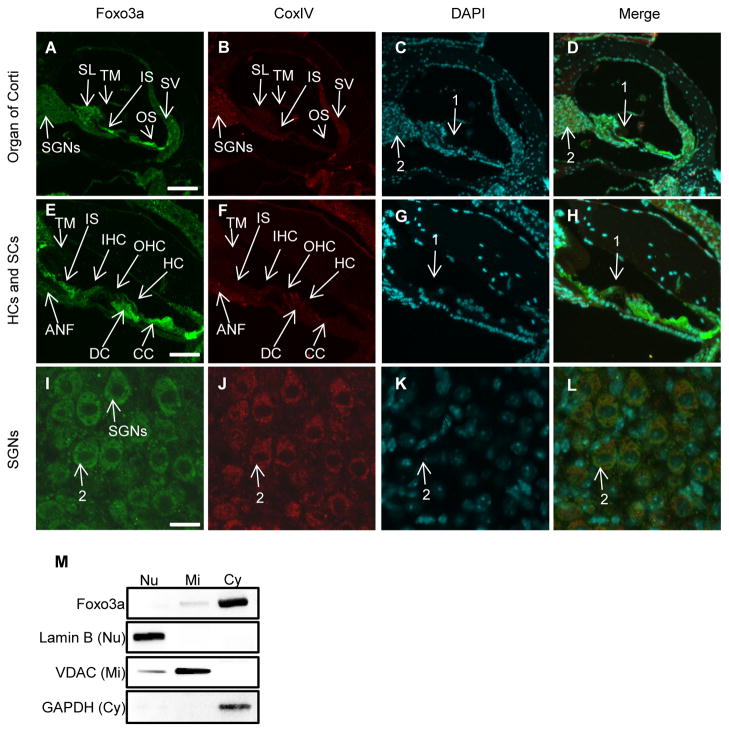
The forkhead transcription factor FOXO3a regulates oxidative stress resistance by directly binding to the promoters of the antioxidant genes encoding catalase or manganese superoxide dismutase (SOD2) in a variety of species (Calnan and Brunet, 2008; Huang and Tindall, 2007). Earlier studies have shown that Sirt1 directly inhibits Foxo3a activity by deacetylating Foxo3a in mammalian cells (Motta, et al., 2004). Hence, we hypothesized that Sirt1 deficiency may result in the activation of Foxo3a, which in turn activates catalase or SOD2, thereby reducing oxidative stress in the cochlear hair cells and their supporting cells. To test this hypothesis, we first investigated the localization of Foxo3a proteins in the cochlea of young WT mice using confocal microscopy. Fig. 7A–D show an area of the organ of Corti in the cochlea at low magnification: Foxo3a immunostaining was detected as a very strong signal in the cytosol of some of the supporting cells of the organ of Corti, including Claudius cells, Deiters’ cells that hold the basal end of the outer hair cells, and inner sulcus cells (Fig. 7E–H) (Merchant and Nadol, 2010). Inner and outer hair cells were also labeled, but to a lesser extent than the supporting cells within the organ of Corti. The large neurons of the spiral ganglion and cells of the stria vascularis also exhibited staining (Fig. 7A–D and I–L). In all cases, cytosolic staining was apparent. If nuclear staining was present, it was minimal compared to the cytoplasmic staining within the same cell. To confirm these immunostaining results, we performed western blotting to measure Foxo3a protein levels in the cytosol, nuclei, and mitochondria in the inner ear tissues from 3–4 months old WT mice. We found that Foxo3a proteins were expressed in the cytosol in the cochlear cells of WT mice (Fig. 7M). Together, these observations demonstrate that Foxo3a is expressed in the cochlea, and both Foxo3a and Sirt1 reside in the cytosol of the supporting cells within the organ of Corti, including Claudius and inner sulcus cells.
Fig. 7.
Foxo3a is prominently localized in the cytosol of the supporting cells of the organ of Corti within the cochlea. (A–L) Foxo3a staining (green) (A, E, I), CoxIV staining (mitochondrial marker) (red) (B, F, J), DAPI staining (nuclear marker) (C, G, K), and merged staining (D, H, L) were detected in the organ of Corti regions (A–D), hair cells (HCs) and their supporting cells (SCs) (E–H), or spiral ganglion neurons (SGNs) (I–L) from 3-month-old WT mice. Arrows 1 indicate hair and supporting cells in the organ of Corti region. Arrows 2 indicate spiral ganglion neurons. Figures were taken from the apical turn (A–H). Scale bar = 200 μm (A), 50 μm (E) or 15 μm (I). SV=stria vascularis, SL=spiral lympus, SGN=spiral ganglion neurons, IS=inner sulcus cells, OS=outer sulcus region, IHC=inner hair cells, OHC=outer hair cells, DC=Deiters’ cell, HC=Hensen’s cell, CC=Claudius cell, ANF=auditory nerve fibers, TM=tectorial membrane. (M) Western blotting analysis of Foxo3a protein levels in the cochlea from 3-month-old WT mice. Lamin B, VDAC, and GAPDH were used as nuclear, mitochondrial, and cytosolic markers respectively. Nu=nuclear, Mi=mitochondria, Cy=cytosol.
3.6. Sirt1 deficiency activates Foxo3a and increases catalase activity in cochlear hair cells
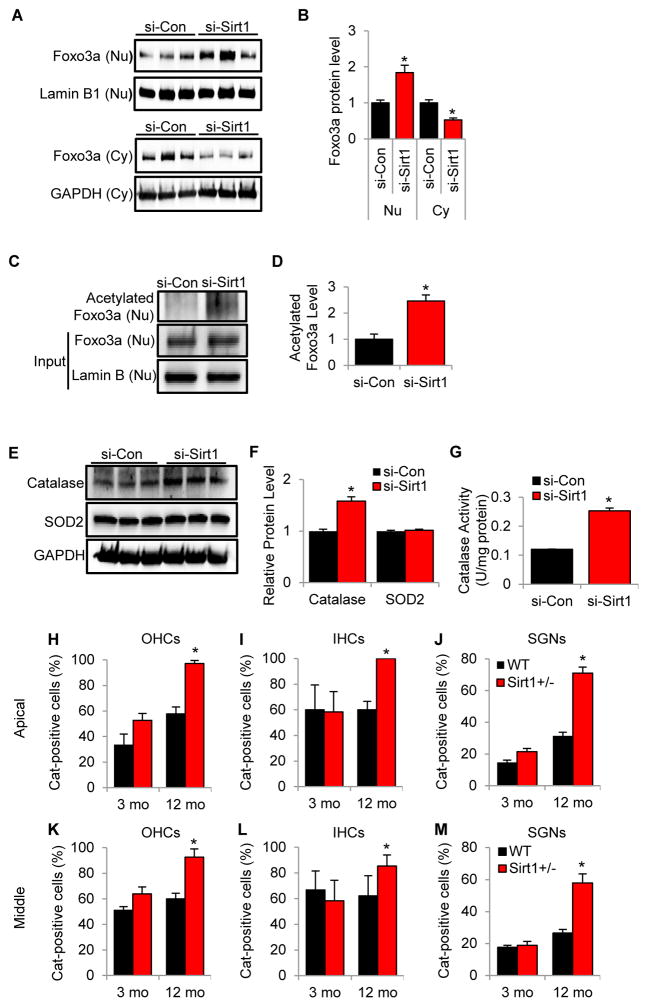
In response to external or internal stimuli or when activated through posttranslational modifications such as acetylation, Foxo3a proteins translocate to the nucleus and bind to its targeted genes (Huang and Tindall, 2007; Calnan and Brunet, 2008). Hence, we hypothesized that Sirt1 deficiency or knockdown may promote the translocation of Foxo3a to the nucleus where Foxo3a can directly bind and activate catalase or SOD2 within the cochlea. To test this hypothesis, we first performed western blotting to measure Foxo3a protein levels in the nuclei and cytosol in the HEI-OC1 cells that were transfected with siRNA targeted to mouse Sirt1. We found that Sirt1 knockdown significantly increased nuclear Foxo3a protein levels when compared to control cells (Fig. 8A and B). Next, to investigate whether Sirt1 knockdown increases the acetylation status of nuclear Foxo3a, we measured the acetylation levels of Foxo3a in the nuclei of siRNA-mediated Sirt1 knockdown mouse inner ear cells. We found that Sirt1 knockdown induced a 2.46 fold increase in acetylation (Fig. 8C and D).
Fig. 8.
Sirt1 knockdown or deficiency activates Foxo3a and increases catalase activity in the cochlear sensory cells. (A, B) Western blotting analysis of Foxo3a protein levels in Sirt1-knockdown HEI-OC1 cells (si-Sirt1). (B) Quantification of Foxo3a proteins in the nuclei and cytosol from (A). *Significantly different from control cells (si-Con) (P < 0.05). Data are means ± SEM. (C, D) Endogenous acetylated Foxo3a proteins in the nuclei from control (si-Con) and Sirt1-knockdown HEI-OC1 cells (si-Sirt1) were isolated by immunoprecipitation with anti-acetyl-lysine antibody followed by western blotting with anti-Foxo3a antibody (n = 3). (D) Quantification of the amounts of total acetylated Foxo3a from (C). *Significantly different from control cells (P < 0.05). Data are means ± SEM. (E, F) Western blotting analysis of catalase and SOD2 protein levels in control and Sirt1-knockdown HEI-OC1 cells. (F) Quantification of catalase and SOD2 proteins levels from (E). (G) Catalase activities were measured in control and Sirt1-knockdown HEI-OC1 cells (n = 3). (H–M) The numbers of catalase (Cat)-positive cells were counted in the outer hair cells (H, K), inner hair cells (I, L), and spiral ganglion neurons (J, M) in the apical (H–J) and middle (K–M) cochlear regions from WT and Sirt1+/− mice (n = 5) at 3 and 12 months of age. *Significantly different from age-matched WT mice (P < 0.05). Data are means ± SEM.
Earlier studies have shown that when activated, Foxo3a directly binds to the promoters of the genes encoding catalase and SOD2 in the nuclei (Calnan and Brunet, 2008; Huang and Tindall, 2007). To test the hypothesis that Sirt1 knockdown increases the activity of catalase or SOD2, we measured the protein levels of catalase and SOD2 in siRNA-mediated Sirt1 knockdown mouse inner ear cells. We found that Sirt1 knockdown increased the protein levels of catalase, but not SOD2 (Fig. 8E and F). We then measured the activity of catalase in siRNA-mediated Sirt1 knockdown mouse inner ear cells. We found that Sirt1 knockdown significantly increased catalase activity in mouse inner ear cells (Fig. 8G). To confirm these in vitro test results in mouse cochlear tissues, catalase was immunostaining was performed with anti-catalase antibody and observed by light microscopy. We found that middle-aged Sirt1+/− mice displayed significantly more catalase-positive cells in the inner hair cells, outer hair cells, and spiral ganglion neurons in the apical and middle cochlear regions when compared to age-matched WT mice (Fig. 8H-M). At 3 months of age, no significant changes were observed in the numbers of the catalase-positive cells in inner hair cells, outer hair cells, or spiral ganglion neurons between WT and Sirt1+/− mice (Fig. 8H–M). Collectively, these results demonstrate that Sirt1 deficiency promotes the translocation of Foxo3a to the nucleus where it activates catalase in the sensory hair cells in the organ of Corti of the cochlea.
4. Discussion
4.1. Sirt1 plays a role in early onset of age-related hearing loss in C57BL/6 mice
It has been proposed that sirtuins play an essential role in extending healthspan and lifespan in mammals (Finkel, et al., 2009). In agreement with this idea, there is a significant reduction of Sirt1 expression in mouse cochlea and auditory cortex during aging (Xiong, et al., 2014), while inhibition of Sirt1 leads to an increase in apoptosis in the mouse inner ear cell line (HEI-OC1) (Xiong, et al., 2015). Xiong et al have also shown that Sirt1 protein was strongly expressed in the nuclei of the inner hair cells and weakly expressed in the outer hair cells (Xiong et al, 2014). In the current study, however, Sirt1 was detected as a very strong signal in the cytosol of some of the supporting cells within the organ of Corti. We note that because the report by Xiong et al did not provide information on Sirt1 expression in the supporting cells of the organ of Corti or a low magnification image of the organ or Corti, it is not clear whether Sirt1 was detected in the supporting cells in their study. We speculate that the discrepancy between their study and our study may be due to different antibodies and/or different microscopy used: our study used confocal microscopy which can provide finer details that cannot be detected in a non-confocal, standard fluorescent microscope. In the current study, we also verified antibody specificity using cochlear tissues of Sirt1+/− mice, and verified our Sirt1 sub-cellular localization result by measuring Sirt1 protein levels in the nuclei, cytosol, and mitochondria of the cochlear tissues by Western blot.
We were also unable to verify the anti-aging effects of Sirt1 on age-related hearing loss in mice. Indeed, our study suggests that Sirt1 may have an opposing role in promoting at least one feature of mammalian aging, AHL. Importantly, the current studies were conducted in the same animal facility using the same dietary feeding protocol and mouse genetic background as those of our previous Sirt3 knockout mouse study (Someya, et al., 2010): Both Sirt1+/− and Sirt3−/− mice were backcrossed onto the C57BL/6 mouse strain for 4 generations, and hence, the Sirt1 and Sirt3 knockout mouse studies were conducted on the same genetic background. In both studies, animals were individually housed in the same mouse room and received the same diets. In the Sirt3 knockout mouse study, both 12-month-old WT and Sirt3−/− mice displayed age-related hearing loss. In contrast, in the current study, we found that 12-month-old Sirt1+/− mice maintained normal hearing at the middle and high frequencies, while age-matched WT mice displayed age-related hearing loss, indicating that Sirt1 plays a key role in early-onset AHL. Hence, these two independent studies provide strong evidence that under the same housing and diet conditions and when compared on the same genetic background, Sirt1, but not Sirt3, promotes early onset of AHL in B6 mice. We note that recent findings support the anti-aging effects of Sirt3 on hearing loss: activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss in mice (Brown, et al., 2014), while there is a decrease in Sirt3 expression associated with accumulation of ROS in the auditory cortex of an aging rat model (Zeng, et al., 2014).
The C57BL/6J mouse strain is homozygous for the recessive AHL-susceptibility allele Cdh23753A, that is known to promote early onset of AHL by 12–15 months of age (Noben-Trauth, et al., 2003). Hence, the mechanisms underlying early-onset of cochlear pathology and associated hearing loss in B6 mice might be different from the mechanisms underlying normal or late-onset age-related hearing loss. To confirm that Sirt1 promotes AHL as our hearing and histological analyses indicate, further studies are needed in Sirt1+/− mice in the background of a model of late-onset age-related hearing loss such as the CBA/CaJ mouse strain, which does not carry the Cdh23753A allele and does not display age-related hearing loss until 17–20 months of age (Zheng, et al., 1999). However, not only did we find that Sirt1 plays a key role in early-onset AHL in B6 mice, we also found Sirt1 is involved in regulation of antioxidant activity in cochlear cells and that Sirt1 knockdown in HEI-OC1 cells results in increased resistance to oxidative stress. Therefore, we speculate that Sirt1 may also promote late-onset AHL in CBA/CaJ mice.
A recent study from the International Mouse Phenotyping Consortium (IMPC) showed that young mice homozygous for a Sirt1 knockout mutation exhibited significantly increased ABR thresholds compared with C57BL/6 control mice (https://www.mousephenotype.org/phenoview/?gid=363&qeid=MP:0004738&ctrl=2357729&pt=0.0001). A possible explanation for this contradicting result is that this was a preliminary screening test for auditory function using two young Sirt1+/− heterozygous mice (n=2) and two young Sirt1−/− homozygous mice (n=2) compared to C57BL/6 mice (n=353) as controls. It is also possible that the Sirt1−/− homozygous mice tested at MRC Harwell exhibited higher ABR thresholds due to developmental defects in the inner ear and/or central nervous system since Sirt1−/− homozygous mice infrequently survive postnatally, exhibit development defects, and are small (Cheng et al., 2003). We acknowledge that because there are only a few reports on the role of Sirt1 in age-related hearing loss in mice, further studies are needed to confirm the reports by the IMPC, Xiong et al, and our findings in middle-aged wild-type and Sirt1+/− heterozygous mice in the C57BL/6 background that are publicly available from IMPC or Jackson Laboratory.
Using transgenic mice with heart-specific overexpression of Sirt1 on the FVB mouse background, Alcendor et al have reported that high heart-specific overexpression of Sirt1 (12.5-fold) increased apoptosis and decreased cardiac function. However, the same report has shown that moderate heart-specific overexpression of Sirt1 (2.5~7.5-fold) protected the heart from oxidative stress induced by paraquat and increased catalase expression in mice (Alcendor et al., 2007). A possible explanation for the contradicting result is that because the cochlea is the receptor organ for hearing that is constantly exposed to noise and/or noise-induced oxidative stress throughout the lifespan and/or because B6 mice are more susceptible to age- and/or noise-related hair cell damage due to the Cdh23 mutation, Sirt1 may act as a pro-aging molecule in the cochlear cells of B6 mice. Again, further studies are needed to confirm the report by Alcendor et al as to how high or moderate inner ear-specific overexpression of Sirt1 influences the progression of age-related hearing loss in mice on the C57BL/6 background.
There are also a number of cell line and primary culture studies that support a protective effect of Sirt1 against oxidative stress. For example, Sirt1 inhibitors enhanced apoptosis and catalase expression, while Sirt1 overexpression downregulated catalase in kidney cell lines (HK-2 cell lines (Hasegawa et al., 2008). Under hydrogen peroxide treatment, Sirt1 overexpression also rescued H2O2-induced apoptosis through the upregulation of catalase and the nuclear accumulation of Foxo3a. In mouse myoblast cell lines, SIRT1 inhibitors increased antimycin A-induced ROS levels and apoptosis (Hori et al., 2013). In addition, the SIRT1 activator resveratrol and NAD+ suppressed apoptosis, while siRNA-mediated knockdown of Sirt1 abolished those effects of resveratrol. In primary astrocytes derived from C57BL/6 mice, upregulation of Sirt1 induced by glucose deprivation increased the expression levels of SOD2 and catalase, while inhibition of SIRT1 increased the acetylation of Foxo4, and decreased the expression levels of SOD2 and catalase (Cheng et al., 2014). Furthermore, in cultured endothelial progenitor cells (EPCs) obtained from human umbilical cord blood, SIRT1 protein levels were increased by hydrogen peroxide, while hydrogen peroxide treatment dose-dependently induced apoptosis in EPCs (Wang et al., 2015). SIRT1 overexpression also reduced H2O2-induced apoptosis and decreased the total FOXO3a protein expression. Collectively, although those four studies were performed exclusively in cell lines or primary cultures, their results strongly support a protective effect of SIRT1 against oxidative stress. In the current study using mouse inner ear cell lines (HEI-OC1), we showed that Sirt1 knockdown increased resistance to H2O2-induced cell death, catalase activity, and Foxo3a-mediated oxidative stress resistance, suggesting a role of Sirt1 in promoting oxidative stress. Our in vitro findings were verified and supported by in vivo tests, including ABR hearing tests, histopathological analyses of cochlear tissues, and immunostaining of oxidative DNA damage marker, Sirt1, catalase and Foxo3 proteins in the cochlear tissues of young and/or old WT and Sirt1+/− mice. A possible explanation for these contradicting results is that the role of Sirt1 in oxidative stress in the cochlear cells of B6 mice might be quite different from those in other cell types or other organs such as kidney cells, myocytes, astrocytes and/or EPCs of other mouse strains because because B6 mice are more susceptible to age- and/or noise-related hair cell damage due to the Cdh23 mutation. Nevertheless, given that there are only a few reports on the roles of Sirt1 in oxidative stress in mouse inner ear cells lines or primary cochlear cultures, further studies are needed to verify our in vitro test reports as well as the other in vitro study reports.
4.2. Effects of Cdh23 genotype on the progression of age-related hearing loss in knockout and transgenic mice
The Sirt1 KO mice used in the current study were derived from TC1 ES cells that were originated from the129/SvEvTacfBR strain (Deng, et al., 1996) and were maintained on the 129/Sv substrain background (Cheng, et al., 2003). There are a number of 129 substrains that exhibit extensive genetic variations due to admixtures with other strains during their derivations and subsequent genetic divergence (Simpson, et al., 1997). In agreement with this report, 129P1/ReJ, 129P3/J, and 129X1/SvJ mice carry the Cdh23753A allele, while 129S1/SvlmJ, 129S6/SvEv-Mostm1Ev/J, and 129T2/SvEmsJ carry the Cdh23753G allele (Johnson, et al., 2006). To address the possible modifying effects that different 129 substrain backgrounds might have on hearing assessments of Sirt1+/+ and Sirt1+/− mice on the B6 background that is homozygous for the recessive AHL-susceptibility allele Cdh23753A, we sequenced the Cdh23 gene in young Sirt1+/+ and Sirt1+/− mice and confirmed that both the Sirt1+/+ and Sirt1+/− mice used in this study have the same genotype for Cdh23 (Fig. 1B). We note that when assessing auditory function in knockout/transgenic mice, knowing which 129 substrain was used to generate the mutant as well as controlling for strain background effects are critical. For example, when a knockout mouse line is backcrossed onto the B6 mouse that has the Cdh23753A/753A genotype or CBA/CaJ mouse that has the Cdh23753G/753G genotype, the mutant mouse could have the Cdh23753G/753G genotype, while its wild-type counterpart could have the Cdh23753A/753A genotype, depending on the original background of the mouse line and/or the location of the mutated gene (e.g., if the gene is located close to the Cdh23 gene on Chromosome 10). Hence, to avoid any confounding results, it is critical to confirm that both the mutant and its wild-type control mice have the same Cdh23 genotype (e.g., Cdh23753A/753A, Cdh23753G/753A or Cdh23753G/753G) when assessing auditory function. Lastly, although we assume all of the phenotypic differences observed between WT and Sirt1+/− mice are due to the effects of Sirt1 genotypes, we acknowledge that there might still be other genetic variants from the 129 background strains that contribute to phenotypic differences in these animals.
4.2. Tissue homeostasis, supporting cells, and, SIRT1/FOXO3a-mediated regulation of oxidative stress in damaged cochlea
When sensory hair cells die, non-sensory supporting cells play a key role in the maintenance of epithelial barrier integrity and preserving ion homeostasis (Jagger et al., 2014; Gale and Jagger, 2010). In non-mammalian vertebrates such as birds, when sensory hair cells die, the dying hair cells are eliminated. New hair cells arise from the adjacent non-sensory supporting cells and the spaces they formerly occupied are rapidly filled by the supporting cells. In the cochlea of mammals, when hair cell dies, there is no regeneration of sensory hair cells (Taylor et al., 2012; Jagger et al., 2014). The hair cell lesion is filled by the adjacent supporting cells and the columnar epithelium is remodeled as a flat epithelium (Taylor et al., 2012; Jagger et al., 2014).
The loss of hair cells is thought to trigger repair responses within the adjacent supporting cells and those repair processes are dependent on gap junctional intercellular communication (Jagger et al., 2014). Gap junctions are sites of direct communication between adjacent cells and all cochlear supporting cells are directly connected to adjacent supporting cells with gap junctions that allow ions and small molecules to flow between the supporting cells (Forge et al., 2002; Jagger et al., 2014). In the current study, we have demonstrated that both Sirt1 and Foxo3a proteins reside in the supporting cells of the organ of Corti of the cochlea in B6 mice. Hence, we speculate that when outer hair cells become damaged or are lost in the cochlea of B6 mice, the dying hair cells may trigger repair responses within the adjacent supporting cells, which in turn activate Sirt1 through gap junctional communication. This may lead to Foxo3a inhibition in the supporting cells, which in turn results in increased oxidative stress within the dying outer hair cells, promoting the elimination of the dying cells from the epithelium. Hence, in B6 mice that are more susceptible to age- and/or noise-related hair cell damage due to the Cdh23 mutation, Sirt1 may play a role in the repair or remodeling process of the epithelium when hair cells become damaged or are lost.
A large body of evidence indicates that Insulin/IGF-1 and FOXO signaling influence lifespan extension in response to dietary restriction in a variety of species (Fontana et al., 2010; Kenyon, 2010). Genetic variants of the FOXO family member FOXO3a have also been shown to be associated with human longevity (Kuningas et al, 2007; Li et al, 2009; Flachsbart et al, 2009). It is also well-established that FOXO3a regulates oxidative stress resistance by directly binding to the promoters of the antioxidant genes encoding catalase or manganese superoxide dismutase (SOD2) in a variety of species (Calnan and Brunet, 2008; Huang and Tindall, 2007). In agreement with these reports, overexpression of Cat (catalase) results in reduced age-related pathology in the heart, reduces cochlear pathology, delays the onset of age-related hearing loss and increases lifespan in C57BL/6 mice (Schriner et al., 2005; Someya et al., 2009). Furthermore, catalase expression and activity are reduced in aged mouse brain (Mo et al., 1995). In contrast, catalase knockout mice do not show any developmental defects or gross abnormalities (Ho et al., 2004), and lenses from these Cat KO mice do not show increased susceptibility to oxidative stress, suggesting that Cat deficiency is compensated by other antioxidant enzymes. Therefore, although catalase deficiency may be compensated by another antioxidant enzyme, we speculate that catalase overexpression or FOXO3a-mediated catalase activation may play a role in oxidative stress resistance in the cochlea of rodents and humans. Lastly, although we have demonstrated that Sirt1 can activate Foxo3a by promoting the translocation of Foxo3a to the nucleus and by increasing the acetylation status of nuclear Foxo3a in mouse inner ear cell lines, we acknowledge that these cell cultures test results are not verified in the cochlea of middle-aged WT and Sirt1+/− mice. Therefore, further research is needed to verify our in vitro test results and proposed hypothesis in the cochlea of B6 as well as CBA/CaJ mice.
Highlights.
Sirt1 deficiency delays the early onset of age-related hearing loss in C57BL/6 mice
Sirt1 deficiency protects cochlear hair cells and spiral ganglion neurons
Sirt1 deficiency increases oxidative stress resistance in mouse cochlea
Sirt1 and Foxo3a interact in the cytosol of the supporting cells of the organ of Corti.
Acknowledgments
This research was supported by National Institutes of Health (NIH) Grants RO1 AG021905 (T.A.P.), R01 DC012552 (S.S.), R01 DC014437 (S.S.), and R03 DC011840 (S.S.), and American Federation for Aging Research Grant 12388 (S.S.). We thank L. VanEkeris and S. Kinoshita for histological processing.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100(10):1512–21. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W, Sauve A, Verdin E, Jaffrey SR. Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 2014;20(6):1059–68. doi: 10.1016/j.cmet.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvari M, Piper MD, Hoddinott M, Sutphin GL, Leko V, McElwee JJ, Vazquez-Manrique RP, Orfila AM, Ackerman D, Au C, Vinti G, Riesen M, Howard K, Neri C, Bedalov A, Kaeberlein M, Soti C, Partridge L, Gems D. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477(7365):482–5. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27(16):2276–88. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100(19):10794–9. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Takeuchi H, Sonobe Y, Jin S, Wang Y, Horiuchi H, Parajuli B, Kawanokuchi J, Mizuno T, Suzumura A. Sirtuin 1 attenuates oxidative stress via upregulation of superoxide dismutase 2 and catalase in astrocytes. J Neuroimmunol. 2014;269(1–2):38–43. doi: 10.1016/j.jneuroim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84(6):911–21. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–91. doi: 10.1038/nature08197. nature08197 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachsbart F, Caliebe A, Kleindorp R, Blanché H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A. 2009;106(8):2700–5. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328(5976):321–6. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A, Becker D, Casalotti S, Edwards J, Marziano N, Nickel R. Connexins and gap junctions in the inner ear. Audiol Neurootol. 2002;7(3):141–5. doi: 10.1159/000058299. [DOI] [PubMed] [Google Scholar]
- Frisina DA, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hearing research. 1997;106(1–2):95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366(9491):1111–20. doi: 10.1016/S0140-6736(05)67423-5. S01406736(05)67423-5 [pii] [DOI] [PubMed] [Google Scholar]
- Gale JE, Jagger DJ. Cochlear supporting cells. In: Fuchs PA, editor. The oxford handbook of auditory science: the ear. Oxford: Oxford UP; 2010. pp. 307–327. [Google Scholar]
- Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, Washida N, Tokuyama H, Hayashi K, Itoh H. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem Biophys Res Commun. 2008;372(1):51–6. doi: 10.1016/j.bbrc.2008.04.176. [DOI] [PubMed] [Google Scholar]
- Ho YS, Xiong Y, Ma W, Spector A, Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem. 2004;279(31):32804–12. doi: 10.1074/jbc.M404800200. [DOI] [PubMed] [Google Scholar]
- Hori YS, Kuno A, Hosoda R, Horio Y. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS One. 2013;8(9):e73875. doi: 10.1371/journal.pone.0073875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120(Pt 15):2479–87. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. How hearing happens. Neuron. 1997;19(5):947–50. doi: 10.1016/s0896-6273(00)80385-2. [DOI] [PubMed] [Google Scholar]
- Jagger DJ, Nickel R, Forge A. Gap junctional coupling is essential for epithelial repair in the avian cochlea. J Neurosci. 2014;34(48):15851–60. doi: 10.1523/JNEUROSCI.1932-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Noben-Trauth K. Strain background effects and genetic modifiers of hearing in mice. Brain Res. 2006;1091(1):79–88. doi: 10.1016/j.brainres.2006.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M. Lessons on longevity from budding yeast. Nature. 2010;464(7288):513–9. doi: 10.1038/nature08981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec GM, Webster P, Lim DJ, Kalinec F. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol Neurootol. 2003;8(4):177–89. doi: 10.1159/000071059. 71059. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Wang X, Fischel-Ghodsian N, Johnson KR. Cu/Zn superoxide dismutase and age-related hearing loss. Hearing research. 2005;209(1–2):76–85. doi: 10.1016/j.heares.2005.06.009. S0378-5955(05)00212-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–12. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kuningas M, Mägi R, Westendorp RG, Slagboom PE, Remm M, van Heemst D. Haplotypes in the human Foxo1a and Foxo3a genes; impact on disease and mortality at old age. Eur J Hum Genet. 2007;15(3):294–301. doi: 10.1038/sj.ejhg.5201766. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang WJ, Cao H, Lu J, Wu C, Hu FY, Guo J, Zhao L, Yang F, Zhang YX, Li W, Zheng GY, Cui H, Chen X, Zhu Z, He H, Dong B, Mo X, Zeng Y, Tian XL. Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum Mol Genet. 2009;18(24):4897–904. doi: 10.1093/hmg/ddp459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8(1):38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289(5487):2126–8. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Merchant SN, Nadol JB. Schuknecht's Pathology of the Ear. 3. 2010. pmph usa. [Google Scholar]
- Mo JQ1, Hom DG, Andersen JK. Decreases in protective enzymes correlates with increased oxidative damage in the aging mouse brain. Mech Ageing Dev. 1995;81(2–3):73–82. doi: 10.1016/0047-6374(95)01586-o. [DOI] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116(4):551–63. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nature genetics. 2003;35(1):21–3. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh TD, Klopp RG, Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol Aging. 1999;20(2):157–65. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- Quan Y, Xia L, Shao J, Yin S, Cheng CY, Xia W, Gao WQ. Adjudin protects rodent cochlear hair cells against gentamicin ototoxicity via the SIRT3-ROS pathway. Sci Rep. 2015;5:8181. doi: 10.1038/srep08181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101(45):15998–6003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308(5730):1909–1911. doi: 10.1126/science.1106653. 1106653 [pii] [DOI] [PubMed] [Google Scholar]
- Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16(1):19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- Someya S, Xu J, Kondo K, Ding D, Salvi RJ, Yamasoba T, Rabinovitch PS, Weindruch R, Leeuwenburgh C, Tanokura M, Prolla TA. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci U S A. 2009;106(46):19432–7. doi: 10.1073/pnas.0908786106. 0908786106 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143(5):802–12. doi: 10.1016/j.cell.2010.10.002. S0092-8674(10)01138-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J Acoust Soc Am. 1997;101(6):3546–53. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282(9):6823–32. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- Taylor RR, Jagger DJ, Forge A. Defining the cellular environment in the organ of Corti following extensive hair cell loss: a basis for future sensory cell replacement in the Cochlea. PLoS One. 2012;7(1):e30577. doi: 10.1371/journal.pone.0030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410(6825):227–30. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell. 2005;9(5):605–15. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tissenbaum HA. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech Ageing Dev. 2006;127(1):48–56. doi: 10.1016/j.mad.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Cao Q, Wang F, Huang LY, Sang TT, Liu F, Chen SY. SIRT1 Protects Against Oxidative Stress-Induced Endothelial Progenitor Cells Apoptosis by Inhibiting FOXO3a via FOXO3a Ubiquitination and Degradation. J Cell Physiol. 2015;230(9):2098–107. doi: 10.1002/jcp.24938. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337(14):986–94. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasoba T, Lin FR, Someya S, Kashio A, Sakamoto T, Kondo K. Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hearing research. 2013;303:30–8. doi: 10.1016/j.heares.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Dai M, Ou Y, Pang J, Yang H, Huang Q, Chen S, Zhang Z, Xu Y, Cai Y, Liang M, Zhang X, Lai L, Zheng Y. SIRT1 expression in the cochlea and auditory cortex of a mouse model of age-related hearing loss. Exp Gerontol. 2014;51:8–14. doi: 10.1016/j.exger.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Xiong H, Pang J, Yang H, Dai M, Liu Y, Ou Y, Huang Q, Chen S, Zhang Z, Xu Y, Lai L, Zheng Y. Activation of miR-34a/SIRT1/p53 signaling contributes to cochlear hair cell apoptosis: implications for age-related hearing loss. Neurobiol Aging. 2015;36(4):1692–701. doi: 10.1016/j.neurobiolaging.2014.12.034. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hearing research. 1999;130(1–2):94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Yang Y, Hu Y, Sun Y, Du Z, Xie Z, Zhou T, Kong W. Age-related decrease in the mitochondrial sirtuin deacetylase Sirt3 expression associated with ROS accumulation in the auditory cortex of the mimetic aging rat model. PLoS One. 2014;9(2):e88019. doi: 10.1371/journal.pone.0088019. [DOI] [PMC free article] [PubMed] [Google Scholar]