Abstract
The auditory system relies on extraordinarily precise timing cues for the accurate perception of speech, music, and object identification. Epidemiological research has documented the age-related progressive decline in hearing sensitivity that is known to be a major health concern for the elderly. While smaller investigations indicate that auditory temporal processing also declines with age, such measures have not been included in larger studies. Temporal gap detection thresholds (TGDTs; an index of auditory temporal resolution) measured in 1071 listeners (18 to 98 years of age) were shown to decline at a minimum rate of 1.05 ms (15 percent) per decade. Age was a significant predictor of TGDT when controlling for audibility (partial correlation) and when restricting analyses to persons with normal hearing sensitivity (n = 434). The TDGTs were significantly better for males (3.5 ms; 51 percent) than females when averaged across the life span. These results highlight the need for indices of temporal processing in diagnostics, as treatment targets, and as factors in models of aging.
Keywords: aging, age-related hearing loss, presbycusis, auditory temporal processing, gap detection
1. Introduction
The auditory system is distinguished from other sensory systems by its remarkable speed, temporal precision, and the preservation of precise temporal coding at multiple levels within the central nervous system (CNS). Like other sensory systems and the CNS in general, the speed and precision of processing undergoes a progressive decline with advancing age (Eckert, 2011,Thompson, et al., 2014). Given linkage between auditory temporal processing and speech perception (Snell, et al., 2002,Tyler, et al., 1982), pitch perception (de Boer, 1976), and voice identification and separation (Rosen, 1992,Snyder and Alain, 2005), it is likely that declines in temporal processing contribute to the debilitating consequences of age-related hearing loss (presbycusis) including social isolation, general decline in health, and increased risk of dementia (Lin, et al., 2013). Reduced audibility (characterized clinically by elevated pure tone thresholds) and reduced temporal processing (typically measured only in the laboratory) are two principal hallmarks of age-related hearing loss. Both are known to compromise speech intelligibility in the presence of interfering sounds, which in turn is the number one complaint of persons with hearing loss. Due to their comorbidity, however, the relative contributions of audibility and temporal processing are often difficult to disassociate in older listeners.
Major epidemiological investigations and large laboratory data sets have documented the frequency-specific decline in auditory sensitivity with age (Allen and Eddins, 2010,Brant and Fozard, 1990,Cruickshanks, et al., 1998,Gates, et al., 1990,Hoffman, et al., 2010). The general pattern of results is a gradual loss of sensitivity at very high frequencies in early adulthood and with every passing decade, greater hearing loss that encroaches lower and lower frequency regions. This loss, however, is gender specific, with greater high-frequency loss in males, leading to a sloping audiogram, and greater low-frequency loss in females, leading to a flatter audiometric pattern in women. These changes in sensitivity with age accompany a cascade of corresponding changes in the region of hearing loss including altered loudness perception, loss of tuning or frequency selectivity, and overall reduction in speech intelligibility when background interference is present (Moore, 2007). Analysis of the pure-tone threshold data across multiple investigations indicates that auditory sensitivity declines at a rate of about 8 dB per decade in the 2000 to 4000 Hz frequency region between the ages of ~50 to 90 years (Allen and Eddins, 2010,Brant and Fozard, 1990,Cruickshanks, et al., 1998,Gates, et al., 1990,Hoffman, et al., 2010). On this basis, expected changes in intelligibility of conversational speech by decade can be estimated merely on the basis of reduced audibility alone using computational methods such as the speech intelligibly index (SII; ANSI, 2012). Estimates of the average decline in temporal resolution with age, analogous to declines in audibility with age, have not been reported but are needed to better capture the nature of presbycusis.
The association of temporal processing deficits and age are ubiquitous, as demonstrated by Humes and colleagues in their systematic review of the evidence (Humes, et al., 2012). They reported that the single most common measure of temporal processing associated with aging is temporal gap detection. The temporal gap detection task measures the smallest detectable silent interval separating preceding and trailing stimulus markers (usually noise or tones) following the method introduced by the elegant study of Plomp (Plomp, 1964). Since that time, the method has been used in laboratory and clinical investigations in a wide range of contexts using behavioral, electrophysiological, and neurophysiological methods. Typical behavioral estimates of temporal gap detection thresholds (TGDTs) for broadband noise in young, normal-hearing adults are between 2 and 3 ms as measured in humans and many animal species (Green, 1971). As the noise bandwidth is reduced, TGDTs tend to increase (are longer) due to a combination of reduced across-channel integration of temporal information and progressive increase in the inherent fluctuations of noise (for a review, see Eddins, 2004, Eddins and Green, 1995).
Studies of auditory temporal processing using a variety of measures, including temporal gap detection, reveal reduced performance with increasing age, leading to the logical question of whether reduced temporal processing in presbycusis is a result of the reduced audibility (i.e., hearing loss) associated with typical aging, changes in peripheral and/or central auditory processing associated with typical aging, or, in the worst case, both reduced audibility and age-related changes in peripheral and/or central auditory processing? Of the 13 TGDT investigations reviewed by Humes et al., several measured audibility and TGDTs in the same persons and used statistical methods such as partial correlation to estimate the relative contribution of age or audibility. Other studies cited in that review measured TDGTs in younger persons with normal audibility and older persons with near-normal audibility (so-called “golden ears”) so that across-group comparisons were minimally impacted by audibility differences. Twelve of those 13 studies were considered to have reported TGDTs that were un-confounded by hearing loss. Of those 12, nine reported a significant effect of age on TGDT, and more recently, others also have found an age effect (John et al., 2012,Palmer and Musiek, 2014), though not all have (Schoof and Rosen, 2014,Shen, 2014). Thus, most but not all evidence from the literature indicates that that advancing age, apart from audibility, leads to reduced temporal resolution as indexed by TGDTs.
The present data were collected in the context of the standard intake protocol from a long-running programmatic study of age-related hearing loss and comorbid medical disorders funded by the National Institute on Aging of the National Institutes of Health. The measures considered here include pure-tone thresholds that index audibility and monaural (better ear) TGDTs as a proxy measure of temporal processing. Data are reported for a large subject cohort (n = 1071; 462 males) ranging in age from 18 to 98 years. Such a cohort provides the statistical power to identify robust relationships between temporal processing, audibility, age, and gender and the cross-sectional data provide a prediction of the rate of decline in TGDT with age.
1. Methods
2.1. Subjects
Participants included 1071 adults (462 males) 18.0 to 97.9 years of age. Inclusion criteria included negative history of head injury, ear disease, ear surgery, or conductive hearing loss. Audiometric data are reported in the Results section. Participants provided written consent, as approved by university Institutional Review Board and were paid an hourly rate.
2.2. Stimuli
Stimuli were low-pass filtered (either at 1 kHz or 4 kHz) Gaussian noise bursts presented at 70-dB SPL in the presence of a continuous wide-band noise (low-pass filtered at 10 kHz) presented at 50-dB SPL. Each stimulus consisted of a pre-gap noise burst 40-ms in duration and a post-gap noise burst 110-ms in duration. Individual bursts were shaped with a 1-ms cosine-squared rise-fall window. In the signal interval, a silent gap was introduced. The pre-gap burst, silent period (signal interval only), and post-gap burst were concatenated and the full stimulus was gated with a 10-ms cosine squared rise-fall window. Stimulus generation and presentation via insert earphones (Etymotic ER-3A) was handled by TDT hardware (Tucker-Davis Technologies) at a sampling rate of either 40000 Hz (System 2 hardware) or 24414 Hz (System 3 hardware).
2.3. Procedure
The temporal gap detection task was part of a larger, 3-hour test battery and typically occurred in the second half of that session. Audiometric and temporal gap detection measurements were conducted in a double-walled sound-attenuating chamber. The TGDTs were measured via two-interval, two-alternative forced-choice procedure with feedback via an adaptive, 2-down-1-up tracking rule estimating 70.7% correct detection (Levitt, 1971). The initial gap duration was 50 ms. Initial step size was 10 ms, which was reduced to 4 ms after 2 reversals. The maximum possible gap duration was 50 ms and the minimal possible gap duration was 2 ms. Thresholds were based on the average of two 40-trial blocks, in which the first two reversals were discarded. Stimulus presentation and response collection was controlled through custom software (System 2) or TDT SykofizX 2.0 software (System 3).
2.4. Statistical analyses
In the primary analyses, participants were separated into three broad age groups: younger (>18 and <=40 years; 74 males and 69 females), middle-aged (>40 and <=65; 103 males and 197 females), and older (>65; 285 males and 343 females). As these participants were part of a larger study on age-related hearing loss, there is a bias in sample size towards the middle-aged and older groups. Across groups, the mean age was 62.9 years and the median age was 68.0 years. The overall ratio of males to females was roughly 3:4.
Secondary analyses included a subset of listeners who had pure-tone thresholds less than or equal to 25 dB HL at all octave frequencies from 250 to 4000 Hz, allowing for a comparison of temporal gap detection thresholds as a function of age in groups of persons having clinically normal hearing thresholds (ANSI, 2010). In addition to controlling for substantial changes in pure-tone threshold with age, this reduced dataset had the unplanned advantage of creating more similar sample sizes within the three age-groups described above. This can be explained by the effect of age on hearing sensitivity: for younger listeners, only 2 participants were excluded due to elevated pure-tone thresholds, whereas progressively greater proportions of participants were excluded from the middle-aged and older groups. Because exclusions were inversely proportional to the original sample sizes, the end result was more similar sample sizes in the derived subset. In all, a total of 637 listeners did not meet the pure tone threshold inclusion criteria, leaving the younger group with 141 participants (72 male and 69 female), the middle-aged group with 172 listeners (49 male, and 123 female), and the older group with 121 listeners (27 male and 94 female). The mean age of the full subset was 49.7 years, and the median age was 56.4 years. The ratio of males to females was roughly 1:2.
2. Results
3.1. Audiometric measures
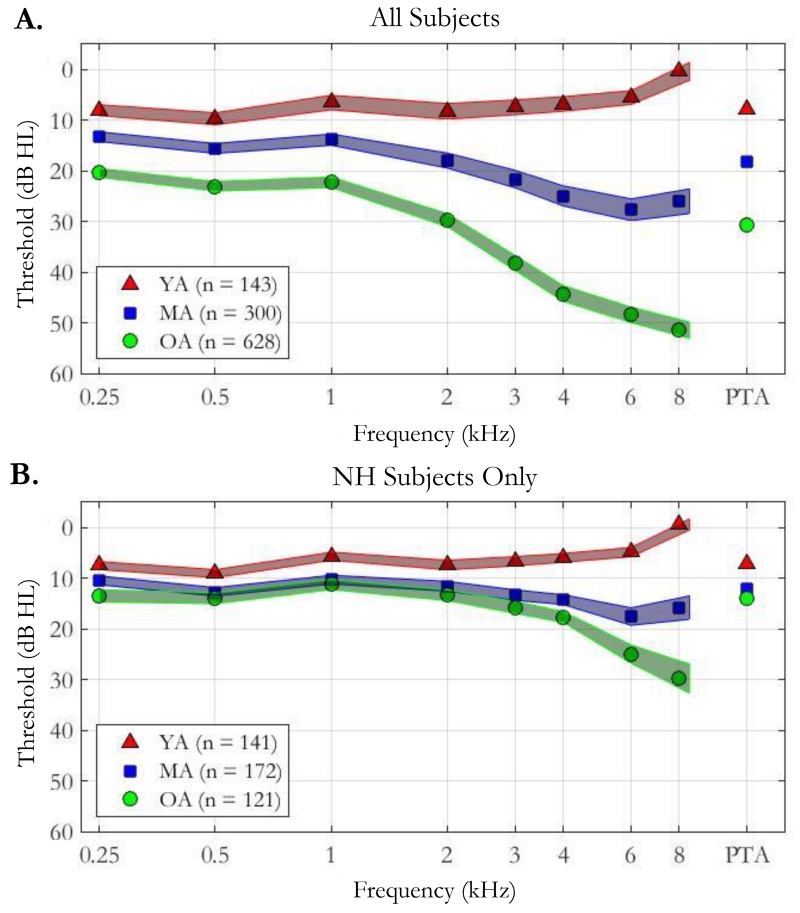
The upper panel of Figure 1A shows the pure-tone audiograms in the better ear (corresponding to the ear to which TGDT stimuli were presented) for all younger (YA; red triangles), middle-aged (MA; blue squares), and older (OA; green circles) adults. Colored regions depict the 95% confidence intervals for each mean pure-tone threshold. Thresholds for the middle- and older-aged groups reveal stereotypic signs of presbycusis including gradually increasing thresholds from low to high frequencies. Three-frequency pure tone averages (PTAs) were computed as the mean threshold at 500, 1000, and 2000 Hz and are reported on the right side of each panel in Figure 1 as separate symbols. From these data, it is clear that there was a significant threshold difference among groups, as confirmed by a comparison of means for PTAs (F2,1068 = 268, p < 0.001). A post-hoc Bonferroni test indicated significant differences between each possible pair of age groups (p < 0.001).
Figure 1.
Pure tone thresholds (dB HL) at octave frequencies for all subjects (top panel) and the normal-hearing (NH) subset (bottom panel) separated by age group: younger adults (YA; red triangles) between the ages of 18 and 40, middle-aged adults (MA; blue squares) between the ages of 40 and 65, and older adults (OA; green circles) greater than 65 years. Pure tone average (PTA), measured as the average pure tone threshold at 500, 1000, and 2000 Hz, is shown for each age group on the righthand side of each panel. Shaded regions depict 95% confidence regions. The NH group was determined by omitting listeners from the full dataset who had poorer than 25 dB HL at any octave frequencies up to 4000 Hz.
As described in the Methods, a subset of listeners with normal audiometric thresholds was extracted from the full dataset for further analysis. Although the normal-hearing subset was meant to limit threshold differences between age groups, it is evident from Figure 1B that a small but consistent difference in pure-tone thresholds remained across age group. A comparison of means PTAs in the normal-hearing subset was significant (F2,431 = 108, p < 0.001), and post-hoc Bonferroni tests show all three age groups to be significantly different from each other (p < 0.001). Such differences are common in studies that include older listeners with “normal hearing.” Nevertheless, each group had clinically normal hearing at frequencies below 4 kHz.
3.2. Temporal gap detection - Effect of age
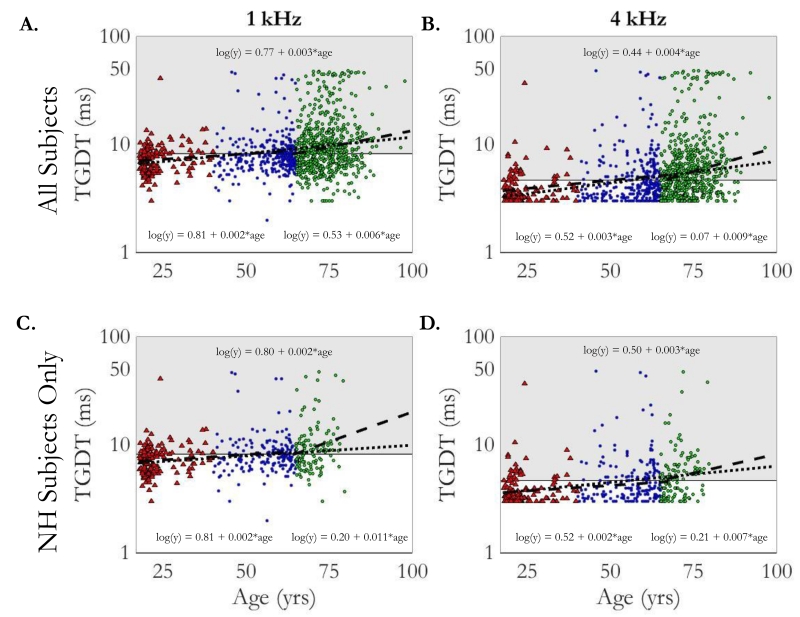
The individual TGDTs (in ms; logarithmic-scaled ordinate) are plotted in Figure 2 as a function of age (in yrs; linear-scaled abscissa). Panels A and B present data for all subjects in the 1 kHz and 4 kHz low-pass-filtered noise conditions, respectively, and panels C and D present the thresholds for only the normal-hearing subset (see Method section for details). Different colors and markers highlight the age groups: younger adults (YA; red triangles), middle-aged adults (MA; blue squares), and older adults (OA; green circles). The grey region in each plot represents thresholds falling outside the 95% confidence interval of the YA group. Figure 2 leads to four immediate observations: 1) TGDTs are longer (poorer) with increasing age; 2) TGDTs are more variable with increasing age; 3) TGDTs are longer for all subjects in the 1 kHz condition (μ = 10.4 ms, σ = 8.1 ms) than the 4 kHz condition (μ = 6.8 ms, σ = 7.9 ms); and 4) the results for the normal-hearing subset (panels C and D) are similar in pattern to the full set (panels A and B).
Figure 2.
Individual temporal gap detection thresholds (TGDTs) are shown as function of age for all subjects in the top row (panels A and B) and for the normal-hearing subset in the bottom row (panels C and D). The left column shows data for the 1 kHz low-pass-filtered noise (panels A and C) and the right column shows data for the 4 kHz low-pass-filtered noise (panels B and D). Colors and markers indicate age group (YA, MA, OA) consistent with those in Figure 1. Grey regions represent thresholds falling above the 95% confidence interval of the YA group thresholds. Change-point linear regression fits (dashed line) and equations (below the fits) are provided for each dataset. A single linear fit (dotted line) and equation (above the fit) is also provided for comparison..
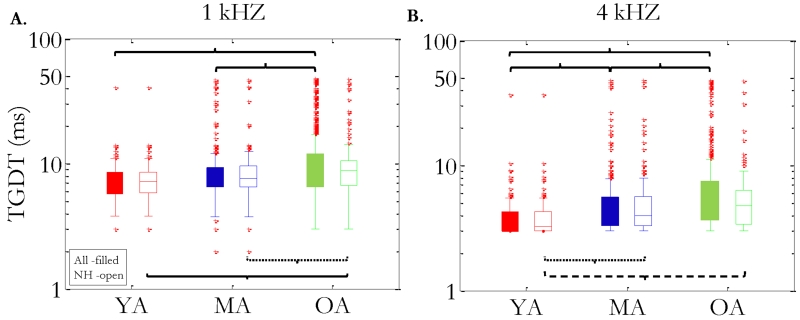
Boxplots of the full dataset (filled boxes) are presented in Figure 3A. To test differences in TGDTs between conditions and across age groups, data were submitted to a two-way (noise condition × age group), repeated-measures analysis of variance (ANOVA). As expected from visual inspection, there was a highly significant main effect of noise condition (F1,1068 = 357, p < .001) and age group (F2,1068 = 22, p < .001), and no interaction was present. Due to the limits of the TGDT measure, which constrained thresholds between 2 ms and 50 ms, non-parametric tests were also run. A Kruskal-Wallis H test showed that there was a statistically significant difference in TGDT threshold between the three age groups for both the 1 kHz (χ2[2]=44.9, p < .001) and 4 kHz (χ2[2]=112.1, p < .001) noise conditions. In the full dataset, nonparametric post-hoc analyses of median age-group differences indicate that the older group was significantly different from both younger and middle-aged groups in both noise conditions (p < 0.001), whereas the younger and middle-aged groups were only significantly different in the 4 kHz noise condition (p < 0.001).
Figure 3.
Boxplot summary of all the full dataset (filled boxes) and normal-hearing subset (open boxes) for the two low-pass-filtered noise conditions (1 kHz: left panel; 4 kHz: right panel). Data are subdivided into age groups (YA, MA, and OA), and box colors are consistent with previous figures. Red markers (+) denote thresholds that fell outside of two times the interquartile range from the median. Tests of significance are represented by brackets (p < .001: solid line; p < .005; dashed line; p < .05: dotted line).
The lack of a significant difference between younger and middle-aged groups indicate that a more rapid decline in temporal processing may occur at a later stage in life, such as the case with age-related sensitivity loss. To characterize an accelerating effect of age on TGDTs, a change-point regression model was evaluated for the log-transformed TGDTs and age from the full dataset. This analysis, which assumed there was an age at which threshold worsens at an accelerated pace, fits two linear regressions with a change-point at ever increasing ages until the difference in slopes of the fits pass a significance test at the 0.05 level. The resulting fits (dashed lines) and equations (below the fits) are provided in the respective panels of Figure 2. For the 1-kHz-bandwidth condition, the change point occurred at 64.4 years., For the 4-kHz-bandwidth condition, the change point occurred at 67.25 years. Comparison of the slopes of the two-segment fits before and after the change point reveal steeper slopes, by 50%, for the 4 kHz noise than the 1 kHz noise (0.003 vs 0.002 before the change point and 0.009 vs 0.006 after the change point). Initial intercept in the 1kHz condition was higher than the 4 kHz condition, which was another indication of poorer thresholds overall for the 1 kHz condition. These linear fits also can be described in terms of the age effect on gap detection thresholds by decade by taking the anti-log of ten times the slope – specifically, before the respective change-points there was an expected threshold increase of 1.05 ms (1 kHz condition) and 1.07 ms (4 kHz condition) for each increase of ten years, whereas after the change-points, thresholds worsened at a rate of 1.15 and 1.23 ms per decade, respectively. . As a percentage, these rates amount to an increase before the change-point of 15% and 29% per decade relative to expected threshold at 18 years old. Reflecting that secondary acceleration after the change-points, thresholds worsened by 14% and 25% per decade relative to the corresponding change-point age. Lastly, a single regression was performed on each dataset for comparison with the change-point regression model. The resulting fit (dotted line) and equation (above the fit) are also given in Fig 2. The function of the single fit closely resembles the fits prior to the change-point age in the above analysis, and they are considered the most conservative estimate of temporal processing declines with age.
3.3. Effect of audibility
It is well known that, in normal-hearing listeners, TGDTs are inversely proportional to the sensation level of the stimulus such that TGDTs increase as the sensation level (SL) decreases below about 30-dB SL (Buus and Florentine, 1985). It is possible that the relationship between age and TGDTs shown above may have been confounded by corresponding changes in pure-tone threshold with age. To examine this possibility, the pure-tone average (PTA; average pure tone threshold at 500, 1000, and 2000 Hz) for each subject was used as a surrogate for audibility (mean PTAs are plotted on the right-hand side of Figure 1). A partial correlation analysis was conducted between age and TGDTs, controlling for audibility, resulting in a highly significant, yet small correlation (see Table 1). Likewise, a partial correlation analysis was conducted between audibility and gap detection thresholds, controlling for age, resulting in a non-significant correlation (see Table 2). As a comparison to analyses above, a two-way, repeated-measures analysis of covariance (ANCOVA; noise condition × age group) was conducted with PTA as a covariate. Main effects persisted for noise condition (F1,1067 = 171, p < 0.001) and age (F2,1067 = 6.6, p = 0.001), while PTA was indeed found to be a significant covariate (F1,1067 = 10.1, p < 0.005). The highly significant correlations indicate that TDGTs clearly decline with age when controlling for audibility while the relatively low correlations are consistent with the scatter seen in Figures 2 and 3.
Table 1.
Pearson r Correlation Coefficients Among Age and Two Noise Condition TGDTs (Above the Diagonal) and Partial Correlations Controlling for Audibility (Below the Diagonal).
| Age | 1kHz-TGDT | 4kHz-TGDT | |
|---|---|---|---|
| Age | - | .21*** | .21*** |
| 1kHz-TGDT | .13*** | - | .80*** |
| 4kHz-TGDT | .11*** | .79*** | - |
*, **, *** indicates significance at the 0.01, 0.05, and 0.001 level, respectively.
Table 2.
Pearson r Correlation Coefficients Among Audibility and Two Noise Condition TGDTs (Above the Diagonal) and Partial Correlations Controlling for Age (Below the Diagonal).
| PTA | 1kHz-TGDT | 4kHz-TGDT | |
|---|---|---|---|
| PTA | - | .17*** | .20*** |
| 1kHz-TGDT | .05 | - | .80*** |
| 4kHz-TGDT | .09** | .79*** | - |
*, **, *** indicates significance at the 0.05, 0.01, and 0.001 level, respectively.
As another means to parse the potential impact of age versus pure tone threshold on TGDTs, the full dataset was reduced to include only listeners with clinically normal pure-tone thresholds (Figure 3, open boxes). Data were submitted to a two-way (noise condition × age group), repeated-measures (ANCOVA) with PTA as a covariate. Results indicate significant main effects of noise condition (F1,430 = 343, p < 0.001) and age group (F2,430 = 4.1, p < 0.02) while PTA was not a significant covariate (F1,430 = 0.76, p = 0.382). Whereas no interaction was previously found between age group and noise condition, the reduced dataset did show a significant interaction (F2,430 = 3.9, p < 0.05). The interaction between noise condition and age group in the normal-hearing subset is likely driven by a raised threshold at an earlier age in the 4-kHz-condition relative to the 1-kHz-condition. The brackets below the boxplots in Figure 3 highlight statistically significant differences, and show that the middle-aged adults perform more similarly to younger adults in the 1-kHz-condition, but their thresholds were significantly longer (poorer) than younger adults in the 4-kHz-condition. The larger age-related difference in the 4-kHz condition may reflect an age-related reduction in spectral integration of temporal information (i.e., the ability to combine information from multiple auditory filters; Eddins and Green, 1995), however, we know of no empirical evidence that supports or refutes this conjecture. . For comparison with the full dataset, the normal-hearing subset was submitted to linear regression models before and after the previously determined change-point years. The model equations are shown in Fig 2. Due to the bias in the normal-hearing subset to preserve more of the younger listeners, linear fits before the change-point age were nearly identical with the full dataset. . Following the change-point, however, there was some distinct differences between datasets. Thresholds worsened at a rate of 1.29 ms and 1.17 ms per decade in the 1 kHz and 4 kHz conditions, respectively, which indicated a greater rate of decline for the narrower bandwidth condition and a marginally smaller rate of decline for the wider bandwidth condition than previously found in the full dataset. Similar to the full set, however, the rate of increase in TGDTs was roughly 16% per decade for the 1 kHz condition and 24% per decade for the 4 kHz condition, so any absolute differences in slopes should be viewed with caution.
3.4. Effect of gender
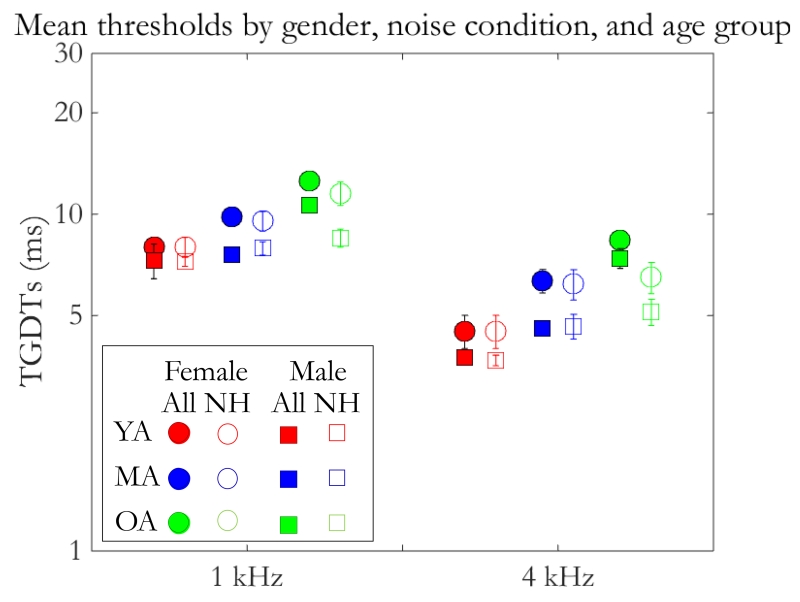
Because of the nature of large sample sizes, it is sometimes possible to investigate contributing factors to an effect that would otherwise be unlikely to be identified in smaller sample sizes. Due to the considerable representation of each gender among the age groups in the full dataset, it was of interest to test whether gender was also a factor in age-related changes in TGDTs. As can be seen in Figure 4, TGDTs were, on average, longer in each age group for female than for male listeners (mean of 10.3 ms versus 6.8 ms, respectively). Data were submitted to a three-way (noise condition × age group × gender) ANCOVA with PTA as a covariate. Thresholds for the two noise conditions were significantly different from each other (F1,1064=153, p < 0.001), as were age group (F2,1064 = 5.8, p < 0.005) and gender (F1,1064 = 8.8, p < 0.005). There were no significant interactions among factors. For the normal-hearing subset, there were main effects of noise condition (F1,427 = 39.3, p < 0.001) and gender (F1,427 = 7.3, p < .01), but not age group; there were also no significant interactions.
Figure 4.
Average (mean) thresholds are plotted with respect to gender (Female: circles; Male: squares), noise condition (abscissa), and age group (YA: red; MA: blue; OA: green). Unfilled markers aside each filled marker represent mean thresholds in the normal-hearing subset from the neighboring condition in the full dataset, respectively. Error bars show ±1 standard error of the mean (SEM).
4. Discussion
By virtue of the different low-pass filter cutoff frequencies, the two noise conditions evaluated here resulted in a difference in gap detection thresholds on average from 10.4 to 6.8 ms for the 1 kHz and 4 kHz conditions, respectively. This difference is consistent with the results of several previous investigations, including those in which the upper cutoff frequency of a low-pass noise was a parameter (Fitzgibbons, 1983) and in studies in which the width of a bandpass noise was increased (Eddins, et al., 1992,Glasberg and Moore, 1992,Shailer and Moore, 1983,Shailer and Moore, 1985). These data indicate an inverse relationship between noise bandwidth and TGDTs that may be explained in terms of integration of synchronous envelope cues introduced by the temporal gap in broadband noise. As the noise bandwidth is increased, two factors contribute to improved gap detection thresholds. First, broader bandwidth permits integration of temporal information across increasing numbers of peripheral auditory filters. Second, as the auditory filter bandwidth increases (as with increasing center frequency), the inherent fluctuations in the output of the filter are less salient, allowing the envelope fluctuation introduced by the temporal gap to increase in salience (Eddins, et al., 1992,Grose, 1991). The results of the present study are in agreement with these previous studies, as listeners tended to perform better in the wider, 4-kHz cutoff noise condition.
Changes in temporal resolution have long been thought to be associated with changes to audibility (Fitzgibbons and Wightman, 1982,Florentine and Buus, 1984). However, it is unclear the degree to which audibility is directly responsible for poorer temporal resolution, or whether alternative factors are underlying the effect. In the present, large-scale study, partial correlation and covariate analyses found audibility to impact gap detection thresholds due to its confounding status with age. Nevertheless, correlation analyses indicated that audibility was not a good predictor of gap detection thresholds when controlling for age. This is aligned with previous reports that hearing loss does not have a dominant impact on indices of temporal resolution once age is taken into account. Previous studies of the effect of age on temporal resolution have reported mixed results. In some early cases, TGDTs were reported to be higher and more variable with age (Mazelova, et al., 2003,Moore, et al., 1992,Schneider, et al., 1994,Snell, 1997), although, like the present data, the vast majority of thresholds for older subjects fell within the same range as for the younger adults. The review by Humes and colleagues (Humes, et al., 2012) observed that 9 of 12 gap detection studies – in which age was not confounded by hearing loss – showed significant effects of age on thresholds. A consistent quantitative measure of the effect of age on temporal resolution, however, is difficult to ascertain from the literature, as the majority of studies report TGDTs for a variety of stimuli from younger and older normal-hearing listeners ranging in size from 10 to 40 participants per group. Nevertheless, these studies nearly all conclude to some degree that age is directly related to poorer temporal resolution as measured by gap detection thresholds.
The current results also reveal rather dramatic differences in TGDTs by gender, with females having significantly and substantially poorer TGDTs than males for each of the three age groups considered. This is the first report of such large differences (3.5 ms when averaged across age group) between genders in a TGDT task. Thus, while gender differences in pure-tone thresholds increased with age (poorer for male than female), the gender differences in TGDT were present across all age ranges (and were poorer for female than male). Greater changes with age in pure-tone threshold for male than female subjects further support the consensus that the observed age-related changes in auditory temporal processing are not the results of audibility limitations. Studies using other temporal processing measures also have indicated better temporal processing for male than female listeners (e.g., temporal order thresholds; Szymaszek, et al., 2006,Wittmann and Szelag, 2003). Both neurobiological (Geffen, et al., 2000,Rammsayer and Lustnauer, 1989) and cognitive factors (Wittmann and Szelag, 2003) have been considered as potential factors underlying better temporal order thresholds in men, and similar reasoning can be applied to the present results.
The data reported here represent the largest known set of monaural TGDTs combined with audiometric measures across such a wide range in ages. The subset with audiometric thresholds within the normal range (n = 434) is more than 10 times the size of the next comparable study of temporal resolution and aging (c.f., Snell, 1997) using noise stimuli, and nearly 2.5 times the size of the largest study in the literature that used tonal stimuli (c.f., Humes, et al., 2009). The present data indicate that TGDTs steadily worsen through early adulthood at roughly 1.05 ms per decade, and in the 6th decade of life, increase at rates as high as 1.23 ms per decade. Among the various, chronic conditions afflicting older adults, hearing loss is one of the most prevalent, after arthritis and hypertension (Cruickshanks, et al., 1998). Therefore, it is essential that we understand the major factors related to age-related hearing loss in order to properly diagnose and treat those who are affected by this debilitating condition. When controlling for audibility (either statistically or through data paring), age was a significant predictor of TGDTs. These results clearly indicate a deficit in temporal resolution independent of reduced hearing sensation associated with age-related hearing loss. Secondary analyses also confirm previous assertions that temporal processing varies across gender lines – an outcome that would have otherwise been difficult to ascertain with smaller sample sizes. These results indicate that, like reduced audibility, poor temporal resolution is a key diagnostic variable and potential treatment target associated with age-related hearing loss. This report provides data needed to adequately model age-related declines in audition.
Highlights.
We evaluated auditory temporal acuity and hearing sensitivity in adults 18 to 98 yrs
Temporal gap detection thresholds (TGDTs) increased 15% per decade
Of the 1071 subjects, a subset (n = 434) had normal audiometric thresholds
TGDT increase was not predicted by audiometric thresholds in full group or subset
TGDTs across the life span were 51% poorer for female than male subjects
Acknowledgements
We thank Francis Mapes, Elizabeth Hicks, and Robert Nutt for their contributions to data collection, Professor Brent Small for contributions to statistical analyses, and Eric Hoover for editorial suggestions. This work was supported by NIH NIA award PO1 AG009524. E.J.O was supported by NIH NIDCD award F32 DC013724.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors have no actual or potential conflicts of interest.
References
- Allen PD, Eddins DA. Presbycusis phenotypes form a heterogeneous continuum when ordered by degree and configuration of hearing loss. Hearing Res. 2010;264(1-2):10–20. doi: 10.1016/j.heares.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANSI . Methods for Manual Pure-tone Threshold Audiometry. American National Standards Institute; New York: 2010. ANSI S321-2010. [Google Scholar]
- ANSI . Methods For Calculation of the Speech Intelligibility Index. Acoustical Society of America; New York: 2012. ANSI S35-1997 R2012. [Google Scholar]
- Brant LJ, Fozard JL. Age-changes in pure-tone hearing thresholds in a longitudinal study of normal human aging. The Journal of the Acoustical Society of America. 1990;88(2):813–20. doi: 10.1121/1.399731. [DOI] [PubMed] [Google Scholar]
- Buus S, Florentine M. Gap detection in normal and impaired listeners: The effect of level and frequency. In: Michelsen A, editor. Time Resolution in Auditory Systems. Springer Berlin Heidelberg; 1985. pp. 159–79. [Google Scholar]
- Cruickshanks KJ, Wiley TL, Tweed TS, Klein BEK, Klein R, Mares-Perlman JA, Nondahl DM. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin - The epidemiology of hearing loss study. Am J Epidemiol. 1998;148(9):879–86. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- de Boer E. On the “residue” and auditory pitch perception. In: Keidel WD, Neff WD, editors. Handbook of Sensory Physiology. Springer; Berlin: 1976. [Google Scholar]
- Eckert MA. Slowing down: age-related neurobiological predictors of processing speed. Front Neurosci. 2011;5 doi: 10.3389/fnins.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddins DA. Temporal resolution in listeners with hearing impairment. In: Kent R, editor. Encyclopedia of Communication Disorders. MIT Press; Cambridge: 2004. [Google Scholar]
- Eddins DA, Green DM. Temporal integration and temporal resolution. In: Moore BCJ, editor. Hearing. Academic Press; New York: 1995. [Google Scholar]
- Eddins DA, Hall JW, 3rd, Grose JH. The detection of temporal gaps as a function of frequency region and absolute noise bandwidth. The Journal of the Acoustical Society of America. 1992;91(2):1069–77. doi: 10.1121/1.402633. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ. Temporal gap detection in noise as a function of frequency, bandwidth, and level. The Journal of the Acoustical Society of America. 1983;74(1):67–72. doi: 10.1121/1.389619. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Wightman FL. Gap detection in normal and hearing-impaired listeners. The Journal of the Acoustical Society of America. 1982;72(3):761–5. doi: 10.1121/1.388256. [DOI] [PubMed] [Google Scholar]
- Florentine M, Buus S. Temporal gap detection in sensorineural and simulated hearing impairments. Journal of speech and hearing research. 1984;27(3):449–55. doi: 10.1044/jshr.2703.449. [DOI] [PubMed] [Google Scholar]
- Gates GA, Cooper JC, Kannel WB, Miller NJ. Hearing in the elderly - the Framingham cohort, 1983-1985. 1. Basic Audiometric Test-Results. Ear Hearing. 1990;11(4):247–56. [PubMed] [Google Scholar]
- Geffen G, Rosa V, Luciano M. Sex differences in the perception of tactile simultaneity. Cortex; a journal devoted to the study of the nervous system and behavior. 2000;36(3):323–35. doi: 10.1016/s0010-9452(08)70844-x. [DOI] [PubMed] [Google Scholar]
- Glasberg BR, Moore BC. Effects of envelope fluctuations on gap detection. Hearing Res. 1992;64(1):81–92. doi: 10.1016/0378-5955(92)90170-r. [DOI] [PubMed] [Google Scholar]
- Green DM. Temporal auditory acuity. Psychol Rev. 1971;78(6):540–&. doi: 10.1037/h0031798. [DOI] [PubMed] [Google Scholar]
- Grose JH. Gap detection in multiple narrow bands of noise as a function of spectral configuration. The Journal of the Acoustical Society of America. 1991;90(6):3061–8. doi: 10.1121/1.401780. [DOI] [PubMed] [Google Scholar]
- Hoffman HJ, Dobie RA, Ko CW, Themann CL, Murphy WJ. Americans hear as well or better today compared with 40 years ago: Hearing threshold Levels in the Unscreened Adult Population of the United States, 1959-1962 and 1999-2004. Ear Hearing. 2010;31(6):725–34. doi: 10.1097/AUD.0b013e3181e9770e. [DOI] [PubMed] [Google Scholar]
- Humes LE, Busey TA, Craig JC, Kewley-Port D. The effects of age on sensory thresholds and temporal gap detection in hearing, vision, and touch. Atten Percept Psychophys. 2009;71(4):860–71. doi: 10.3758/APP.71.4.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Dubno JR, Gordon-Salant S, Lister JJ, Cacace AT, Cruickshanks KJ, Gates GA, Wilson RH, Wingfield A. Central presbycusis: A review and evaluation of the evidence. J Am Acad Audiol. 2012;23(8):635–66. doi: 10.3766/jaaa.23.8.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John AB, Hall JW, 3rd, Kreisman BM. Effects of advancing age and hearing loss on gaps-in-noise test performance. American journal of audiology. 2012;21(2):242–50. doi: 10.1044/1059-0889(2012/11-0023). doi:10.1044/1059-0889(2012/11-0023) [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. The Journal of the Acoustical Society of America. 1971;49(2):467–77. [PubMed] [Google Scholar]
- Lin FR, Yaffe K, Xia J, Xue QL, Harris TB, Purchase-Helzner E, Satterfield S, Ayonayon HN, Ferrucci L, Simonsick EM, Health ABCSG. Hearing loss and cognitive decline in older adults. JAMA internal medicine. 2013;173(4):293–9. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelova J, Popelar J, Syka J. Auditory function in presbycusis: peripheral vs. central changes. Experimental gerontology. 2003;38(1-2):87–94. doi: 10.1016/s0531-5565(02)00155-9. [DOI] [PubMed] [Google Scholar]
- Moore BCJ. Cochlear Hearing Loss: Physiological, Psychological and Technical Issues. 2nd ed. Wiley and Sons; Chichester: 2007. [Google Scholar]
- Moore BCJ, Peters RW, Glasberg BR. Detection of temporal gaps in sinusoids by elderly subjects with and without hearing-loss. The Journal of the Acoustical Society of America. 1992;92(4):1923–32. doi: 10.1121/1.405240. [DOI] [PubMed] [Google Scholar]
- Palmer SB, Musiek FE. Electrophysiological gap detection thresholds: effects of age and comparison with a behavioral measure. J Am Acad Audiol. 2014;25(10):999–1007. doi: 10.3766/jaaa.25.10.8. doi:10.3766/jaaa.25.10.8. [DOI] [PubMed] [Google Scholar]
- Plomp R. Rate of Decay of Auditory Sensation. The Journal of the Acoustical Society of America. 1964;36(2):277–&. [Google Scholar]
- Rammsayer T, Lustnauer S. Sex differences in time perception. Percept Motor Skill. 1989;68(1):195–8. doi: 10.2466/pms.1989.68.1.195. [DOI] [PubMed] [Google Scholar]
- Rosen S. Temporal information in speech: acoustic, auditory and linguistic aspects. Philos T Roy Soc B. 1992;336(1278):367–73. doi: 10.1098/rstb.1992.0070. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK, Kowalchuk D, Lamb M. Gap detection and the precedence effect in young and old adults. The Journal of the Acoustical Society of America. 1994;95(2):980–91. doi: 10.1121/1.408403. [DOI] [PubMed] [Google Scholar]
- Schoof T, Rosen S. The role of auditory and cognitive factors in understanding speech in noise by normal-hearing older listeners. Frontiers in aging neuroscience. 2014;6:307. doi: 10.3389/fnagi.2014.00307. doi:10.3389/fnagi.2014.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shailer MJ, Moore BC. Gap detection as a function of frequency, bandwidth, and level. The Journal of the Acoustical Society of America. 1983;74(2):467–73. doi: 10.1121/1.389812. [DOI] [PubMed] [Google Scholar]
- Shailer MJ, Moore BC. Detection of temporal gaps in bandlimited noise: effects of variations in bandwidth and signal-to-masker ratio. The Journal of the Acoustical Society of America. 1985;77(2):635–9. doi: 10.1121/1.391881. [DOI] [PubMed] [Google Scholar]
- Shen Y. Gap detection and temporal modulation transfer function as behavioral estimates of auditory temporal acuity using band-limited stimuli in young and older adults. Journal of speech, language, and hearing research: JSLHR. 2014;57(6):2280–92. doi: 10.1044/2014_JSLHR-H-13-0276. doi:10.1044/2014_JSLHR-H-13-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell KB. Age-related changes in temporal gap detection. The Journal of the Acoustical Society of America. 1997;101(4):2214–20. doi: 10.1121/1.418205. [DOI] [PubMed] [Google Scholar]
- Snell KB, Mapes FM, Hickman ED, Frisina DR. Word recognition in competing babble and the effects of age, temporal processing, and absolute sensitivity. The Journal of the Acoustical Society of America. 2002;112(2):720–7. doi: 10.1121/1.1487841. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Alain C. Age-related changes in neural activity associated with concurrent vowel segregation. Brain Res. 2005;24(3):492–9. doi: 10.1016/j.cogbrainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Szymaszek A, Szelag E, Sliwowska M. Auditory perception of temporal order in humans: The effect of age, gender, listener practice and stimulus presentation mode. Neurosci Lett. 2006;403(1-2):190–4. doi: 10.1016/j.neulet.2006.04.062. [DOI] [PubMed] [Google Scholar]
- Thompson JJ, Blair MR, Henrey AJ. Over the hill at 24: Persistent age-related cognitive motor decline in reaction times in an ecologically valid video game task begins in early adulthood. Plos One. 2014;9(4) doi: 10.1371/journal.pone.0094215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler RS, Summerfield Q, Wood EJ, Fernandes MA. Psychoacoustic and phonetic temporal processing in normal and hearing-impaired listeners. The Journal of the Acoustical Society of America. 1982;72(3):740–52. doi: 10.1121/1.388254. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Szelag E. Sex differences in perception of temporal order. Percept Motor Skill. 2003;96(1):105–12. doi: 10.2466/pms.2003.96.1.105. [DOI] [PubMed] [Google Scholar]