Abstract
Complete duplication of large metazoan chromosomes requires thousands of potential initiation sites, only a small fraction of which are selected in each cell. Assembly of the replication machinery is highly conserved and tightly regulated during cell cycle, but the sites of initiation are highly flexible their temporal order of firing is regulated at the level of large-scale multi-replicon domains. Additionally, the number of replication forks needs to be quickly adjusted in response to stress to ensure complete replication and prevent genome instability. Here we argue that large genomes are divided into domains for exactly this reason. Domain structure abrogates the need for precise initiation sites and creates a scaffold for the evolution of other chromosome functions.
Introduction
Duplication of eukaryotic genomes is regulated at distinct levels ranging from the few kilobases at which initiation occurs to large-scale chromosome architecture and its three-dimensional organization within the nucleus (Figure 1). Eukaryotic cells have very large genomes (from 14 megabases in yeast to 3 gigabases in human); replicating this amount of DNA requires initiation at multiple replication origins. Classical experiments combined with the recent in vitro reconstitution of DNA replication from purified proteins (Yeeles et al., 2015) have unraveled a well-conserved mechanism ensuring that no molecule replicates more than once (Deegan and Diffley, 2016). However, ensuring that all DNA is completely replicated prior to cell division (the completion problem) is less well understood. The model of specific cis-elements of origin activity would require their distribution evenly throughout the genome, with no room for origin failure, and would restrain genome rearrangements during evolution (Gilbert, 2001). Instead, eukaryotes, even yeasts, have a great deal of flexibility in their initiation sites and assemble many more origins than necessary, recruiting backup or “dormant” origins when the primary origins fail (McIntosh and Blow, 2012). Cells ration limiting replication fork resources by firing sets of origins in a temporal sequence and waiting until a sufficient number of forks fuse before firing additional origins. Under conditions that block or slow down fork progression, it is critical to rapidly reduce the numbers of dangerously reactive stalled forks. We argue that haphazard firing of origins throughout the genome would impede this response, so instead cells initiate replication in spatially clustered groups of sites. Mammalian cells partition their genome into ~5,000 units of coordinated initiation referred as replication domains (RDs), each of which harbors ~6 of the ~30,000 origins that will be fired in each cell cycle. When faced with replication stress, cells redistribute resources quickly to complete replication within active RDs and inhibit initiation within RDs that have not yet initiated (Gilbert, 2007). RDs manifest structurally as topologically associating domains (TADs) and this structural organization may have been co-opted for other chromosome functions. For example, RD/TADs are replicated in a distinctive temporal order that is developmentally regulated and coordinated with changes in transcriptional activity and genome organization (Hiratani et al., 2010; Rivera-Mulia et al., 2015) while enhancer-promoter interactions are confined within these units to facilitate gene regulation. Here we develop a model in which large genomes are replicated in a domainal fashion to “divide and conquer” replication of large genomes, and that the establishment of stable structural units allows for both flexibility in origin usage and domain-level regulation of transcendent chromosome functions.
Figure 1.
The distinct scales of DNA replication regulation in eukaryotes. A) Replication origin regulation. The origin recognition complex (ORC) binds at all potential replication origins that become licensed by the CDT1-dependent recruitment of MCM-helicase complex (a double hexamer of the subunits MCM2-7) to form the pre-RC. All potential origins are licensed but only ~10% are activated. Additional factors are recruited, followed by DDK phosphorylation of distinct residues of the MCM complex that trigger the helicase activity, splitting the MCM hexamers and starting the bidirectional unwinding of the DNA strands. For simplicity, the depiction does not include all components of the pre-RC and pre-IC. For a detailed description see (Yeeles et al., 2015). B) DNA replication regulation at the replication domain scale. Synchronized firing of clusters of origins activated either early or late during S-phase partition the chromosomes into replication domains. C) DNA replication at the nuclear scale and its regulation during the cell cycle. RDs are segregated within the nucleus in such way that early replicating segments of the chromosomes occupy the nuclear interior while the late RDs are preferentially located close to the nuclear and nucleolar (N) periphery. Origin licensing occurs only from M to G1, then CDT1 is rapidly degraded and sequestrated by geminin blocking origin re-licensing although additional inhibitors might repress pre-RC activation during the G1-S transition (Sasaki et al., 2011). Then an induction of DDK/S-CDK in S-phase activate the pre-IC to initiate replication. The timing decision point (TDP) occurs early during G1 and precedes origins selection (ODP).
A bird’s eye view of DNA replication in eukaryotes
Regulation of DNA replication in eukaryotes has been the topic of several outstanding recent reviews (Boulos et al., 2015; Deegan and Diffley, 2016; Fragkos et al., 2015; Hyrien, 2015; Urban et al., 2015). Here we briefly highlight the mechanisms that ensure one complete round of DNA replication.
Once and only once
Preventing re-replication is achieved in two mutually exclusive steps: origins are first “licensed” by the assembly of pre-replication complex (pre-RC) under conditions that strictly prevent initiation and later, after a gap period, a subset of the pre-RCs are activated under conditions that strictly prevent pre-RC formation (Figure 1). This “two cycle engine” ensures that each molecule is duplicated once and only once per cell cycle and occurs through a highly conserved step-wise assembly process (Deegan and Diffley, 2016; Fragkos et al., 2015). Replication origin licensing commences with binding of the origin recognition complex (ORC, consisting of six subunits ORC1-6). Next, cell division cycle 6 (CDC6) and cdc10-dependent transcript 1 (CDT1) assist the loading of the helicase complex constituted by a double hexamer of minichromosomal maintenance proteins (MDM2-7). At this time in early G1 phase, the MCM helicase, now called a pre-RC, is inactive and wrapped around double-stranded DNA in an unusually stable complex (Deegan and Diffley, 2016; Kuipers et al., 2011). Evidence suggests that initially all pre-RCs or licensed origins have an equal potential to be activated later during S-phase while poorly understood mechanisms set differential potentials to these sites at a pre-restriction point stage of G1 phase termed the origin decision point (Sasaki et al., 2006). Later in G1 phase, after CDK and other species-specific mechanisms are in place to prevent pre-RC formation, the pre-initiation complex (pre-IC) forms through recruitment of factors such as MCM10, RECQ14, Treslin, CDC45, GINS, TOPBP1 and the DNA polymerase ε (Fragkos et al., 2015). Assembly of the pre-IC to initiate helicase activity requires phosphorylation of the MCM complex by Dbf4-dependent kinase (DDK) and recruitment of GINS and CDC45 by S phase-specific kinases cyclin-dependent kinase (S-CDK). Excess MCM that are not initiated are removed by the passing forks, by still poorly understood mechanisms (Fragkos et al., 2015). Thus, elaborate mechanisms are in place to ensure that no molecule replicates more than once. Importantly, none of these mechanisms invokes the requirement for initiation of replication to take place at specific sites (Gilbert, 2001), so the fact that some sites are utilized more frequently than others is likely for reasons that transcend cell cycle regulation of replication.
The completion problem
In contrast to the elaborate mechanisms preventing re-replication, eukaryotic cells appear to ensure that all DNA is replicated by simply assembling many more pre-RCs than they need, thus enabling the potential to initiate bi-directional forks at any sites that may lag behind. Although mammalian cells replicate their genome by firing 20,000–50,000 origins, many types of evidence indicate that <10 % of the licensed origins are used in a given cell. Up to 250,000 initiation sites have been identified in various cell population based origin mapping methods but the heterogeneity of sites visualized on individual DNA fibers suggests that these ensemble methods are capturing only a fraction of the total spectrum of sites (Gilbert, 2012). Even in yeast only one in five of the potential origins are activated in S-phase (Kaykov and Nurse, 2015). Direct determination of the number of potential origins per cell requires measuring the number of MCM complexes assembled per cell, which has not been reliably accomplished. However, knockdown of MCMs has little effect on DNA replication rates in the absence of replication stress (Ibarra et al., 2008; Woodward et al., 2006) and live cell photobleaching indicates that nearly all MCMs are irreversibly loaded onto chromatin at the very onset of S phase (Kuipers et al., 2011). These excess MCMs become critical however, whenever replication fork progression is perturbed (Blow et al., 2011; Ibarra et al., 2008), due to DNA lesions, limiting resources or collisions between RNA and DNA polymerase (Lin and Pasero, 2012). Altogether the evidence is compelling that cells ensure completion of DNA synthesis by simply assembling a vast excess of pre-RCs, with no apparent requirement for assembly at particular sites.
Replication timing and Replication Domains
In eukaryotes, the numbers of replication initiation and fork proteins are insufficient to replicate the entire genome simultaneously (Collart et al., 2013; Mantiero et al., 2011). In fact, over-expression of these limiting factors results in too many origins firing synchronously during S phase, depletion of nucleotide pools and reduction in cell viability (Köhler et al., 2016; Mantiero et al., 2011; Toledo et al., 2013). As a result, initiation in all eukaryotes is extended over the course of S phase. In mammals, this occurs by synchronously firing clusters of origins creating large-scale (400–800 kb) RDs (Figure 1B). RDs are a functional readout of topologically-associated domains (TADs), large-scale structural units of chromosomes (Pope et al., 2014). What has been intriguing is the defined and evolutionarily conserved temporal order in which the genome is replicated in all eukaryotes; there is no a priori reason for such a temporal order simply to accomplish duplication of the genome (Ryba et al., 2010; Solovei et al., 2016; Yue et al., 2014). Moreover, this program changes dynamically during cell fate commitment, while conserving these same units (Hiratani et al., 2010; Rivera-Mulia et al., 2015). RT is intimately linked to 3D organization of the genome, with early replicating domains preferentially positioned towards the nuclear interior and late replicating domains located at the nuclear periphery and close to the nucleolus (Figure 1C). This spatial segregation of early and late replicating domains is also reflected by chromatin conformation mapping methods (Hi-C) (Rivera-Mulia and Gilbert, 2016; Ryba et al., 2010). Hence, RT is a universal principle of eukaryotic replication regulation, but there is no clear reason why a specific temporal order should emerge simply to duplicate the genome in pace with limiting resources.
Origin firing is subordinate to regulated replication timing of RDs
Intuitively, one would think that DNA replication should be regulated at the sites where it initiates. However, several lines of evidence indicate that the choreography of replication in mammalian cells occurs by first selecting RDs for initiation, followed by the stochastic selection of initiation sites within each domain.
RT establishment precedes origin selection
The first indication that RT is not dictated by deterministic “early or late” origins was the finding in yeast that initiation sites themselves are not programmed to fire early or late during S-phase. Ectopic insertion of normally early firing replication origins at late replicating genomic regions results in late activation of those origins (Ferguson and Fangman, 1992), in some cases attributed to the local chromatin context (Ebrahimi et al., 2010; Pohl et al., 2012; Yompakdee and Huberman, 2004). Later it was shown that RT is established prior to origin selection in mammalian cells (Dimitrova and Gilbert, 1999a). RT is established in a very early window during G1 (Figure 1C), referred as the timing decision point (TDP), coincident with the organization of chromatin into RDs/TADs and segregation of early and late RDs into distinct nuclear compartments that remain stable throughout the remainder of interphase (Dileep et al., 2015a). Specification of the origins that will fire in S-phase also occurs at a discrete time point (origin decision point; ODP) that is later in G1 than the TDP (J. R. Wu and Gilbert, 1997; 1996) and conditions can be identified in which RT is maintained but origin specification is not (Dimitrova et al., 2002). Hence, the regulation of replication is regulated by first establishing the temporal order of firing of the large-scale RDs, with the selection of origin sites being subordinate, at least in some conditions, unnecessary.
Origins fire stochastically within RDs
Identification of initiation sites has revealed a great deal of heterogeneity in origin selection (Figure 2A); rarely is the same origin used by two distinct cells in the same population, supporting a stochastic model of origin firing (Bechhoefer and Rhind, 2012; Borowiec and Schildkraut, 2011; Cayrou et al., 2011; Kaykov and Nurse, 2015). It is important to understand that stochastic regulation does not imply a random mechanism. Rather, all sites in the genome have a finite probability of harboring and activating an Mcm complex, often called “origin efficiency”, that is dictated by a complex combination of factors.
Figure 2.
Heterogeneity in replication origin selection and regulation of replication activation under stress. A) Multiple potential replication origins are licensed within each RD. Distinct cells within the same population use different origins under normal conditions. B) Regulation of origin activation under replication stress. When replication forks are stalled, checkpoint responses are induced to promote the activation of dormant origins within the RD while inhibiting the firing of origins in RDs that are activated later in S-phase (Gilbert, 2007).
Numerous methods to map the location of replication initiation sites have been discussed in detail elsewhere (Gilbert, 2010; Hyrien, 2015; Urban et al., 2015). Briefly, the most common methods are: a) the microscopic analysis of labeled replication fork polarity on individual DNA fibers (Herrick and Bensimon, 1999); b) trapping of DNA bubble structures formed at the initiation sites based on their retarded mobility in gel electrophoresis (Gilbert, 2010); c) identification of RNA-primed or BrdU labeled short nascent strands (SNS) (Gilbert, 2012; Urban et al., 2015); d) mapping the DNA template strand for Okazaki fragments (Petryk et al., 2016) and; e) chromatin immunoprecipitation (ChIP) against components of the pre-RC, which maps potential, but not activated origins (Dellino et al., 2013; MacAlpine et al., 2010). At present, these methods overlap only up to 35%, suggesting that each technique captures only a subset of initiation sites (Gilbert, 2012; Hyrien, 2015). Moreover, origins detected by ensemble methods query millions of cells and detect the approximate relative frequency of initiation (origin efficiency) at sites best detected by each method, while single molecule methods are low throughput but can potentially detect all origins. When compared directly, is clear that a small subset of ensemble-mapped origins are used on any given DNA fiber and that no two DNA molecules are replicated from the same cohort of origins (Cayrou et al., 2011; Czajkowsky et al., 2008; Kaykov and Nurse, 2015; Kaykov et al., 2016; Patel et al., 2006), supporting a stochastic mechanism for origin firing. Nonetheless, DNA fiber studies reveal that origins are fired coordinately in spatial clusters consistent with RDs (REFS for origin clusters on fibers).
Divide and conquer
Why would origin specification be subordinate to domain organization? Studies of the responses of cells to replication stress provide a logical paradigm (Ge and Blow, 2010; Gilbert, 2007). When mammalian cells experience replication stress, they respond with a Chk1-dependent pathway that simultaneously activates dormant origins within their activated domain, while inhibiting initiation of all domains that have not yet initiated. Single stranded DNA created by stalled forks recruits ATR, activating Chk1 kinase, which subsequently triggers the firing of nearby dormant origins. However, low levels of Chk1 throughout the nucleus inhibit replication initiation at uninitiated RDs (Toledo et al., 2013), resulting in a redistribution of limiting resources to the sites of replication stress. Although the mechanisms are still unclear, evidence suggest that it may operate through differential modulation of S-CDK activity in activated vs. unactivated RDs (Thomson et al., 2010). Here, we suggest that by compartmentalizing large genomes into defined replication units, cells reduce the complexity of replicon distribution. When problems are encountered, they can more easily complete what they have started within each RD in order to rapidly reduce the number of forks per RD to only those two emanating from the RD, while RDs that have not yet initiated are kept silent until the source of the replication stress is resolved (Figure 2B). This provides a biological rationale for regulating replication at the level of large domains rather than individual origins.
Many factors affect the stochastic probability of replication initiation within replication domains
It is clear that different genomic sites have different probabilities of initiation, but what determines that probability? Here we review some of the many features that have been found to correlate with or be necessary for initiation in certain contexts. What is clear is that no feature has a one to one correspondence with origin efficiency. More likely, origin efficiency results from a combination of events that affect pre-RC assembly and activation.
DNA sequence
Initial characterization of budding yeast origins identified ARS elements as consensus sequences capable of initiating replication in plasmids or minichromosomes, but more recent studies reveal that sequences associated with replication initiation sites are highly flexible (Bogenschutz et al., 2014; Gros et al., 2014). Hence, even in budding yeasts, replication origins are specified by more than just DNA sequences. At the other end of the spectrum, during the early stages of Drosophila and Xenopus development, initiation exhibits no detectable dependency upon DNA sequence (Gilbert et al., 1995; Harland and Laskey, 1980; Hyrien and Méchali, 1993; Sasaki et al., 1999). Moreover, analysis of allele-specific replication initiation in mammal cells revealed variations in the initiation efficiency of clustered origins associated with replication asynchrony (Bartholdy et al., 2015; Mukhopadhyay et al., 2014). Finally, one intriguing study detected a single nucleotide polymorphism (SNP) upstream of the FMR1 gene in individuals with fragile X syndrome that was associated with delayed RT and loss of origin activity at the FMR1 locus (Gerhardt et al., 2014). Hence, although DNA sequences might have potential roles in regulating origin efficiencies, replication initiation does not require specific sequences.
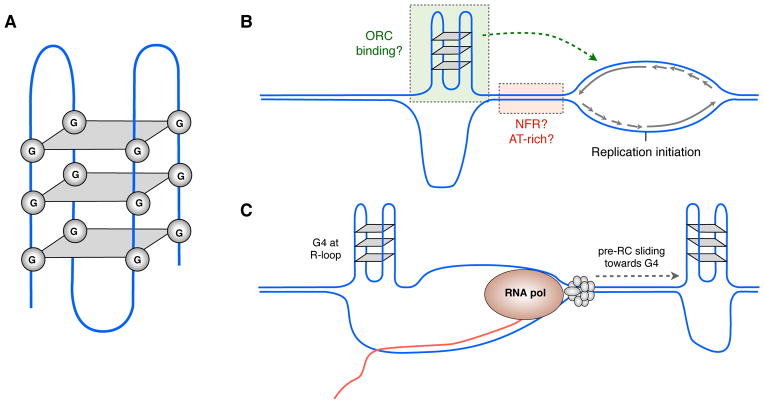
Genome-wide mapping of SNS by deep sequencing has provided some new clues about origin distribution and efficiency (Hyrien, 2015). Highly efficient origins (origins that are frequently used) are enriched in regions with high GC content (Cayrou et al., 2011), more specifically at repeated elements (origin G-rich repeated elements, OGRE) that tend to form G-quadruplexes (G4) (Besnard et al., 2012; Cayrou et al., 2011; Picard et al., 2014). G4 are four-stranded nucleic acid structures stabilized by bonds between guanines that have generated great attention (Figure 3A–B). Although the enrichment of origins at G4 might be overestimated (Foulk et al., 2015), some evidence supports a role for G4 structures in origin efficiency: ORC preferentially binds to G4 motifs in ssDNA (Hoshina et al., 2013), G4 visualization revealed nuclear foci during S-phase (Biffi et al., 2013) and specific helicases unwind G4 (Bochman et al., 2010; Hiom, 2010; Y. Wu and Brosh, 2010). Moreover, a series of small deletions in two well characterized chicken origins confirmed that G4 motifs are necessary for high origin efficiency (Valton et al., 2014). However, G4 motifs are clearly neither necessary nor sufficient for initiation as a very large amount of potential G4 DNA structures (> 370,000) have been predicted in the human genome (Huppert and Balasubramanian, 2007) and up to 700,000 G4 structures can be formed in vitro (Chambers et al., 2015) while most replication origins detected by bubble trap do not contain G4 (Mesner et al., 2013). Altogether, G4 may affect origin efficiencies in certain chromatin contexts (Figure 3C).
Figure 3.
G-quadruplexes (G4) and replication origin specification. A) G4 structure. Rings of guanine quartets are stacked on top each other and stabilized by hydrogen bonds. B) G4 might cooperate with additional cis elements (such as NFRs or AT-rich regions) to specify the more efficient location of replication initiation (modified from (Valton et al., 2014). C) Gene expression can influence origin selection by generating R-loops that enhance G4 formation or “pushing” the pre-RC towards G4 motifs.
Origin activity and chromatin landscape
Multiple chromatin modifications have been linked to origin selection, and have been reviewed in detail (Fragkos et al., 2015). In general, transcription activating modifications are associated with higher origin density and efficiency, while inhibitory modifications are observed in regions depleted of origins. However, transcriptionally silent regions of the genome replicate very rapidly (Koren and McCarroll, 2014), indicating the presence of a large number of inefficient origins that escape detection by SNS methods. In Drosophila, activating chromatin marks correlate with ORC binding and firing of early origins (Eaton et al., 2011). Initiation sites are often enriched near DNaseI hypersensitive sites (DHSs) and nucleosome free regions (NFR) (Besnard et al., 2012; Cayrou et al., 2011; Mukhopadhyay et al., 2014). Consistently, ORC binding to potential origins and pre-RC activation is affected by local chromatin structure, with active origins located preferentially at NFRs (MacAlpine et al., 2010). In concordance, ORC binding sites are enriched at open chromatin (Liu et al., 2012; Lubelsky et al., 2014). Moreover, the correlation between replication initiation and chromatin is high enough that DHSs can predict RT (Gindin et al., 2014). However, as with G4, only a fraction of DHSs overlap with the mapped origins and vice versa. In summary, multiple correlations between chromatin features and replication origins have been established but no general rules have emerged.
Transcription and its regulatory factors affect replication origin selection
A substantial percentage of replication origins are found near promoters (Dellino et al., 2013; Fragkos et al., 2015; Valenzuela et al., 2011) and there is evidence that origin efficiencies can be influenced by nearby transcription factors (Knott et al., 2012; Lombraña et al., 2015; Puzzi et al., 2015). Some of these effects may be due to the formation of hybrid RNA-DNA molecules during transcription, referred as R-loops, which have been recently associated with diverse cellular processes (Sollier and Cimprich, 2015). R-loops can persist long enough to expose ssDNA with the potential to form G4 (Lombraña et al., 2015). Alternatively, it has been proposed that transcriptional activity might displace pre-RCs affecting origin selection (Dimitrova and Gilbert, 1999b; Gilbert, 2001; Sasaki et al., 2006). Consistently, RNA polymerases can displace MCM complex and since it remains functional after sliding along dsDNA, replication initiation can take place at alternative sites (Gros et al., 2015). Hence, one possibility is that transcription could “push” loaded MCM helicases along DNA until reaching a bulky G4 structure, explaining some of the correlation of origins and G4 (Figure 3D). However, initiation also takes place in transcriptionally silent regions, so transcription is unlikely to be necessary for origin specification.
A very strong correlation between early replication and transcriptional activity has been observed in all eukaryotes (Hiratani et al., 2010; Rivera-Mulia et al., 2015; Schübeler et al., 2002; Woodfine et al., 2004). Evidence from replication in yeast revealed trans-acting factors that influence origin selection. Early replication segments in budding yeast contain binding sites to forkhead proteins (Fkh1 and Fkh2) that stimulate origin activation by recruiting Cdc45 to the pre-RC. Additionally, clusters of early replicating origins are established by their interaction with Fkh1 and Fkh2 (Aparicio, 2013; Knott et al., 2012; Ostrow et al., 2014). Moreover, analysis of ARS elements in budding yeast identified a late consensus sequence (LCS) enriched at telomeres that can delay replication initiation (Yompakdee and Huberman, 2004). Late replication is regulated by the binding of the LCS by to Rap1 interacting factor (Rif1) that recruits protein phosphatase 1 (PP1) to counteract DDK phosphorylation of MCMs, thereby delaying origin activation at late replicating domains (Hayano et al., 2012; Peace et al., 2014; Renard-Guillet et al., 2014). Rif1 is highly conserved in eukaryotes (Sreesankar et al., 2012) and disruption of Rif1 in mammals also increases levels of MCM phosphorylation by DDK (Yamazaki et al., 2012). Moreover, Rif1 is enriched at late RDs and might be involved in the spatial organization of the chromatin (Cornacchia et al., 2012; Foti et al., 2016). To date, Rif1 is one of only two trans-acting factors known to regulate replication initiation in metazoans. The other is DNA polymerase theta, which also regulates replication at the level of RDs by unknown mechanism (Fernandez-Vidal et al., 2014). Altogether, evidence suggests that origin selection is highly flexible and affected by multiple factors. Hence, a persistent question is whether initiation sites matter at all or if once RDs and their chromatin landscape are stablished, DNA replication can be initiated at any location within the RDs.
Developmental regulation of DNA replication
Unlike origin specification, for which changes during development are mostly quantitative rather than qualitative (Bartholdy et al., 2015), RT is coordinately regulated during development across entire RDs. Although we have yet to understand the biological significance of either a defined RT program or its developmental regulation, the units of regulation (RDs) remain the same in all cell types, suggesting their universal importance to cell function. RT changes during differentiation occur across half of the genome and analysis of multiple cell types allowed the identification of distinct types of domains: constitutive early replicating domains (CE), constitutive late replicating domains (CE) and developmentally regulated domains (Figure 4A). A surprise from these studies is that genome-wide correlations of chromatin properties to RT are driven by the constitutive half of the genome, including transcriptional activity (Rivera-Mulia et al., 2015), ORC binding sites (Lubelsky et al., 2014), origin efficiency (Besnard et al., 2012; Comoglio et al., 2015), gene density and GC content (Hiratani et al., 2010; Rivera-Mulia et al., 2015), DHSs (Pope et al., 2014), MNase sensitivity (Takebayashi et al., 2012) and Rif1 (Dileep et al., 2015a). By contrast, these same properties do not correlate with RT of developmentally regulated domains (Rivera-Mulia and Gilbert, 2016). This includes the distribution of replication origins. When origins are mapped using SNS methods, they are enriched in CE domains, while developmental domains have the same low level of origins as CL domains but, even when very early replicating (Besnard et al., 2012; Cayrou et al., 2011; 2015). Hence, studies of developmental regulation of RT have challenged many longstanding assumptions. They also further support the notion that the distribution of origins is independent of RT regulation (Figure 4B).
Figure 4.
Dynamic changes in replication regulation during development. A) RT changes during development defining distinct classes of RDs: constitutive domains (replicating always either early or late) and developmental regulated domains that switch between early and late replication. B) Developmentally regulated domains are distinct from constitutive RDs.
Co-opting genome integrity for cell fate specification
Once such a domain structure would be established, it could be co-opted for other functions. For example, why is RT regulated during development? Wouldn’t a single RT program suffice to faithfully duplicate all genomes? Most housekeeping genes are in the CE domains, so an active chromatin landscape may have evolved signals for early RT. However, early RT also increases gene dosage, which may be important for specific genes in certain cell types (Voichek et al., 2016). Moreover, it would make sense to spatially consolidate chromatin with similar functions, or to sequester chromatin with higher densities of parasitic transposons. Thus, nuclear bodies such as nucleoli compartmentalize chromosome functions and domains in close proximity tend to replicate at similar times. Consistently, spatio-temporal regulation of RT is highly conserved from ciliates to humans (Figure 1D of (Solovei et al., 2016)). The structure of RDs also provides a convenient scaffold on which to restrict enhancer-promoter interactions (Ren and Yue, 2015), which could then feed forward with transcription factors themselves reinforcing the structure necessary for regulating RT (Dekker and Mirny, 2016). Replicating these domains in a specific temporal order could then serve to assemble different types of chromatin at different times, compartmentalize constitutive vs. developmental outputs, silence genes and regulate mutation rates (Sima and Gilbert, 2014) in different segments of the genome. Testing this model will require a thorough understanding of what regulates both structure and function at this organizational scale. A prediction would be that disruption of 3D organization would lead to rapid genome instability under replication stress. However, to date such manipulations (e.g. CTCF depletion) have had little to no effect on 3D genome compartmentalization or RT (Dileep et al., 2015b; Zuin et al., 2014).
Conclusions
Bechhoefer and Rhind have previously explained how a distinct temporal order of replication can arise from stochastic origin firing in small genomes (Bechhoefer and Rhind, 2012). However, this model is insufficient to explain the existence of domain level regulation, which is most obviously seen in large genomes but remnants of domain-organization exist even in yeast (McCune et al., 2008; Mizuguchi et al., 2014; Rhind and Gilbert, 2013). Genome partitioning is also observed in bacteria, however the mechanisms that regulate the 3D organization in prokaryotes are dictated mostly by the supercoiling properties of DNA (Le et al., 2013). Here, we argue that domain organization in metazoans facilitates genome integrity when replicating large genomes through a divide and conquer mechanism (Figure 2B). In this view, a timing program is used to ration resources, while executing this program in units (RDs) serves to consolidate damage in the face of replication stress. Once domain structure is established, origin specification and distribution are much less important because each domain can be replicated in a timely fashion from one or a few clustered origins regardless of their specific locations. Moreover, when faced with replication stresses, domain structure consolidates replicons to rapidly reduce the number of dangerously reactive replication forks. This domain structure, established to maintain genome integrity, could then be co-opted for other chromosomal functions. We submit that this replication-centric view of genome organization provides a useful framework for understanding the complexity of genome structure and function relationships in the context of large genomes.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants GM083337 and GM085354 (D.M.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aparicio OM. Location, location, location: it’s all in the timing for replication origins. Genes & Development. 2013;27:117–128. doi: 10.1101/gad.209999.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholdy B, Mukhopadhyay R, Lajugie J, Aladjem MI, Bouhassira EE. Allele-specific analysis of DNA replication origins in mammalian cells. Nat Comms. 2015;6:7051–4. doi: 10.1038/ncomms8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechhoefer J, Rhind N. Replication timing and its emergence from stochastic processes. Trends in Genetics. 2012;28:374–381. doi: 10.1016/j.tig.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard E, Babled A, Lapasset L, Milhavet O, Parrinello H, Dantec C, Marin J-M, Lemaitre JM. Unraveling cell type-specific and reprogrammable human replication origin signatures associated with G-quadruplex consensus motifs. Nat Struct Mol Biol. 2012;19:837–844. doi: 10.1038/nsmb.2339. [DOI] [PubMed] [Google Scholar]
- Biffi G, Tannahill D, McCafferty J, Balasubramanian S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nature Chemistry. 2013;5:182–186. doi: 10.1038/nchem.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ, Ge XQ, Jackson DA. How dormant origins promote complete genome replication. Trends Biochem Sci. 2011;36:405–414. doi: 10.1016/j.tibs.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochman ML, Sabouri N, Zakian VA. Unwinding the functions of the Pif1 family helicases. DNA Repair (Amst ) 2010;9:237–249. doi: 10.1016/j.dnarep.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz NL, Rodriguez J, Tsukiyama T. Initiation of DNA Replication from Non-Canonical Sites on an Origin-Depleted Chromosome. 2014;9:e114545. doi: 10.1371/journal.pone.0114545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec JA, Schildkraut CL. Open sesame: activating dormant replication origins in the mouse immunoglobulin heavy chain (Igh) locus. Curr Opin Cell Biol. 2011;23:284–292. doi: 10.1016/j.ceb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulos RE, Drillon G, Argoul F, Arneodo A, Audit B. Structural organization of human replication timing domains. FEBS Lett. 2015;589:2944–2957. doi: 10.1016/j.febslet.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Cayrou C, Ballester B, Peiffer I, Fenouil R, Coulombe P, Andrau J-C, van Helden J, Méchali M. The chromatin environment shapes DNA replication origin organization and defines origin classes. Genome Res. 2015;25:1873–1885. doi: 10.1101/gr.192799.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrou C, Coulombe P, Vigneron A, Stanojcic S, Ganier O, Peiffer I, Rivals E, Puy A, Laurent-Chabalier S, Desprat R, Méchali M. Genome-scale analysis of metazoan replication origins reveals their organization in specific but flexible sites defined by conserved features. Genome Res. 2011;21:1438–1449. doi: 10.1101/gr.121830.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers VS, Marsico G, Boutell JM, Di Antonio M, Smith GP, Balasubramanian S. High-throughput sequencing of DNA G-quadruplex structures in the human genome. Nat Biotechnol. 2015;33:877–881. doi: 10.1038/nbt.3295. [DOI] [PubMed] [Google Scholar]
- Collart C, Allen GE, Bradshaw CR, Smith JC, Zegerman P. Titration of Four Replication Factors Is Essential for the Xenopus laevis Midblastula Transition. Science. 2013;341:893–896. doi: 10.1126/science.1241530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoglio F, Schlumpf T, Schmid V, Rohs R, Beisel C, Paro R. High-resolution profiling of Drosophila replication start sites reveals a DNA shape and chromatin signature of metazoan origins. Cell Reports. 2015;11:821–834. doi: 10.1016/j.celrep.2015.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornacchia D, Dileep V, Quivy J-P, Foti R, Tili F, Santarella-Mellwig R, Anthony C, Almouzni G, Gilbert DM, Buonomo SBC. Mouse Rif1 is a key regulator of the replication-timing programme in mammalian cells. EMBO J. 2012 doi: 10.1038/emboj.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowsky DM, Liu J, Hamlin JL, Shao Z. DNA combing reveals intrinsic temporal disorder in the replication of yeast chromosome VI. Journal of Molecular Biology. 2008;375:12–19. doi: 10.1016/j.jmb.2007.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deegan TD, Diffley JFX. MCM: one ring to rule them all. Current Opinion in Structural Biology. 2016;37:145–151. doi: 10.1016/j.sbi.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Dekker J, Mirny L. The 3D Genome as Moderator of Chromosomal Communication. Cell. 2016;164:1110–1121. doi: 10.1016/j.cell.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellino GI, Cittaro D, Piccioni R, Luzi L, Banfi S, Segalla S, Cesaroni M, Mendoza-Maldonado R, Giacca M, Pelicci PG. Genome-wide mapping of human DNA-replication origins: levels of transcription at ORC1 sites regulate origin selection and replication timing. Genome Res. 2013;23:1–11. doi: 10.1101/gr.142331.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dileep V, Ay F, Sima J, Vera DL, Noble WS, Gilbert DM. Topologically associating domains and their long-range contacts are established during early G1 coincident with the establishment of the replication-timing program. Genome Res. 2015a;25:1104–1113. doi: 10.1101/gr.183699.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dileep V, Rivera-Mulia JC, Sima J, Gilbert DM. Large-Scale Chromatin Structure-Function Relationships during the Cell Cycle and Development: Insights from Replication Timing. Cold Spring Harb Symp Quant Biol. 2015b doi: 10.1101/sqb.2015.80.027284. [DOI] [PubMed] [Google Scholar]
- Dimitrova DS, Gilbert DM. The Spatial Position and Replication Timing of Chromosomal Domains Are Both Established in Early G1 Phase. Mol Cell. 1999a;4:983–993. doi: 10.1016/S1097-2765(00)80227-0. [DOI] [PubMed] [Google Scholar]
- Dimitrova DS, Gilbert DM. DNA replication and nuclear organization: prospects for a soluble in vitro system. Crit Rev Eukaryot Gene Expr. 1999b;9:353–361. doi: 10.1615/critreveukargeneexpr.v9.i3-4.200. [DOI] [PubMed] [Google Scholar]
- Dimitrova DS, Prokhorova TA, Blow JJ, Todorov IT, Gilbert DM. Mammalian nuclei become licensed for DNA replication during late telophase. J Cell Sci. 2002;115:51–59. doi: 10.1242/jcs.115.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton ML, Prinz JA, MacAlpine HK, Tretyakov G, Kharchenko PV, MacAlpine DM. Chromatin signatures of the Drosophila replication program. Genome Res. 2011;21:164–174. doi: 10.1101/gr.116038.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi H, Robertson ED, Taddei A, Gasser SM, Donaldson AD, Hiraga S-I. Early initiation of a replication origin tethered at the nuclear periphery. J Cell Sci. 2010;123:1015–1019. doi: 10.1242/jcs.060392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BM, Fangman WL. A position effect on the time of replication origin activation in yeast. Cell. 1992;68:333–339. doi: 10.1016/0092-8674(92)90474-Q. [DOI] [PubMed] [Google Scholar]
- Fernandez-Vidal A, Guitton-Sert L, Cadoret JC, Drac M, Schwob E, Baldacci G, Cazaux C, Hoffmann JS. A role for DNA polymerase θ in the timing of DNA replication. Nat Comms. 2014;5:4285. doi: 10.1038/ncomms5285. [DOI] [PubMed] [Google Scholar]
- Foti R, Gnan S, Cornacchia D, Dileep V, Bulut-Karslioglu A, Diehl S, Buness A, Klein FA, Huber W, Johnstone E, Loos R, Bertone P, Gilbert DM, Manke T, Jenuwein T, Buonomo SCB. Nuclear Architecture Organized by Rif1 Underpins the Replication-Timing Program. Mol Cell. 2016;61:260–273. doi: 10.1016/j.molcel.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulk MS, Urban JM, Casella C, Gerbi SA. Characterizing and controlling intrinsic biases of lambda exonuclease in nascent strand sequencing reveals phasing between nucleosomes and G-quadruplex motifs around a subset of human replication origins. Genome Res. 2015;25:725–735. doi: 10.1101/gr.183848.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkos M, Ganier O, Coulombe P, Méchali M. DNA replication origin activation in space and time. Nat Rev Mol Cell Biol. 2015;16:360–374. doi: 10.1038/nrm4002. [DOI] [PubMed] [Google Scholar]
- Ge XQ, Blow JJ. Chk1 inhibits replication factory activation but allows dormant origin firing in existing factories. The Journal of Cell Biology. 2010;191:1285–1297. doi: 10.1083/jcb.201007074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt J, Tomishima MJ, Zaninovic N, Colak D, Yan Z, Zhan Q, Rosenwaks Z, Jaffrey SR, Schildkraut CL. The DNA replication program is altered at the FMR1 locus in fragile X embryonic stem cells. Mol Cell. 2014;53:19–31. doi: 10.1016/j.molcel.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DM. Replication origins run (ultra) deep. Nat Struct Mol Biol. 2012;19:740–742. doi: 10.1038/nsmb.2352. [DOI] [PubMed] [Google Scholar]
- Gilbert DM. Evaluating genome-scale approaches to eukaryotic DNA replication. Nat Rev Genet. 2010;11:673–684. doi: 10.1038/nrg2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DM. Replication origin plasticity, Taylor-made: inhibition vs recruitment of origins under conditions of replication stress. Chromosoma. 2007;116:341–347. doi: 10.1007/s00412-007-0105-9. [DOI] [PubMed] [Google Scholar]
- Gilbert DM. Making Sense of Eukaryotic DNA Replication Origins. Science. 2001;294:96–100. doi: 10.1126/science.1061724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DM, Miyazawa H, DePamphilis ML. Site-specific initiation of DNA replication in Xenopus egg extract requires nuclear structure. Mol Cell Biol. 1995;15:2942–2954. doi: 10.1128/mcb.15.6.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindin Y, Valenzuela MS, Aladjem MI, Meltzer PS, Bilke S. A chromatin structure-based model accurately predicts DNA replication timing in human cells. Molecular Systems Biology. 2014;10:722–722. doi: 10.1002/msb.134859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros J, Devbhandari S, Remus D. Origin plasticity during budding yeast DNA replication in vitro. EMBO J. 2014;33:621–636. doi: 10.1002/embj.201387278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros J, Kumar C, Lynch G, Yadav T, Whitehouse I, Remus D. Post-licensing Specification of Eukaryotic Replication Origins by Facilitated Mcm2-7 Sliding along DNA. Mol Cell. 2015;60:797–807. doi: 10.1016/j.molcel.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland RM, Laskey RA. Regulated replication of DNA microinjected into eggs of Xenopus laevis. Cell. 1980;21:761–771. doi: 10.1016/0092-8674(80)90439-0. [DOI] [PubMed] [Google Scholar]
- Hayano M, Kanoh Y, Matsumoto S, Renard-Guillet C, Shirahige K, Masai H. Rif1 is a global regulator of timing of replication origin firing in fission yeast. Genes & Development. 2012;26:137–150. doi: 10.1101/gad.178491.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick J, Bensimon A. Single molecule analysis of DNA replication. Biochimie. 1999;81:859–871. doi: 10.1016/s0300-9084(99)00210-2. [DOI] [PubMed] [Google Scholar]
- Hiom K. FANCJ: solving problems in DNA replication. DNA Repair (Amst ) 2010;9:250–256. doi: 10.1016/j.dnarep.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Hiratani I, Ryba T, Itoh M, Rathjen J, Kulik M, Papp B, Fussner E, Bazett-Jones DP, Plath K, Dalton S, Rathjen PD, Gilbert DM. Genome-wide dynamics of replication timing revealed by in vitro models of mouse embryogenesis. Genome Res. 2010;20:155–169. doi: 10.1101/gr.099796.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshina S, Yura K, Teranishi H, Kiyasu N, Tominaga A, Kadoma H, Nakatsuka A, Kunichika T, Obuse C, Waga S. Human origin recognition complex binds preferentially to G-quadruplex-preferable RNA and single-stranded DNA. Journal of Biological Chemistry. 2013;288:30161–30171. doi: 10.1074/jbc.M113.492504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert JL, Balasubramanian S. G-quadruplexes in promoters throughout the human genome. 2007;35:406–413. doi: 10.1093/nar/gkl1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyrien O. Peaks cloaked in the mist: the landscape of mammalian replication origins. The Journal of Cell Biology. 2015;208:147–160. doi: 10.1083/jcb.201407004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyrien O, Méchali M. Chromosomal replication initiates and terminates at random sequences but at regular intervals in the ribosomal DNA of Xenopus early embryos. EMBO J. 1993;12:4511–4520. doi: 10.1002/j.1460-2075.1993.tb06140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra A, Schwob E, Méndez J. Excess MCM proteins protect human cells from replicative stress by licensing backup origins of replication. Proceedings of the National Academy of Sciences. 2008;105:8956–8961. doi: 10.1073/pnas.0803978105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaykov A, Nurse P. The spatial and temporal organization of origin firing during the S-phase of fission yeast. Genome Res. 2015;25:391–401. doi: 10.1101/gr.180372.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaykov A, Taillefumier T, Bensimon A, Nurse P. Molecular Combing of Single DNA Molecules on the 10 Megabase Scale. Sci Rep. 2016;6:19636. doi: 10.1038/srep19636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott SRV, Peace JM, Ostrow AZ, Gan Y, Rex AE, Viggiani CJ, Tavaré S, Aparicio OM. Forkhead transcription factors establish origin timing and long-range clustering in S.cerevisiae. Cell. 2012;148:99–111. doi: 10.1016/j.cell.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren A, McCarroll SA. Random replication of the inactive X chromosome. Genome Res. 2014;24:64–69. doi: 10.1101/gr.161828.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C, Koalick D, Fabricius A, Parplys AC, Borgmann K, Pospiech H, Grosse F. Cdc45 is limiting for replication initiation in humans. Cell Cycle. 2016;15:974–985. doi: 10.1080/15384101.2016.1152424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers MA, Stasevich TJ, Sasaki T, Wilson KA, Hazelwood KL, McNally JG, Davidson MW, Gilbert DM. Highly stable loading of Mcm proteins onto chromatin in living cells requires replication to unload. The Journal of Cell Biology. 2011;192:29–41. doi: 10.1083/jcb.201007111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TBK, Imakaev MV, Mirny LA, Laub MT. High-Resolution Mapping of the Spatial Organization of a Bacterial Chromosome. Science. 2013;342:731–734. doi: 10.1126/science.1242059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YL, Pasero P. Interference Between DNA Replication and Transcription as a Cause of Genomic Instability. CG. 2012;13:65–73. doi: 10.2174/138920212799034767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, McConnell K, Dixon M, Calvi BR. Analysis of model replication origins in Drosophila reveals new aspects of the chromatin landscape and its relationship to origin activity and the prereplicative complex. Mol Biol Cell. 2012;23:200–212. doi: 10.1091/mbc.E11-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombraña R, Almeida R, Álvarez A, Gómez M. R-loops and initiation of DNA replication in human cells: a missing link? Front Genet. 2015;6 doi: 10.3389/fgene.2015.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubelsky Y, Prinz JA, DeNapoli L, Li Y, Belsky JA, Belsky JA, MacAlpine DM. DNA replication and transcription programs respond to the same chromatin cues. Genome Res. 2014;24:1102–1114. doi: 10.1101/gr.160010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlpine HK, Gordân R, Powell SK, Hartemink AJ, MacAlpine DM. Drosophila ORC localizes to open chromatin and marks sites of cohesin complex loading. Genome Res. 2010;20:201–211. doi: 10.1101/gr.097873.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantiero D, Mackenzie A, Donaldson A, Zegerman P. Limiting replication initiation factors execute the temporal programme of origin firing in budding yeast. EMBO J. 2011;30:4805–4814. doi: 10.1038/emboj.2011.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune HJ, Danielson LS, Alvino GM, Collingwood D, Delrow JJ, Fangman WL, Brewer BJ, Raghuraman MK. The Temporal Program of Chromosome Replication: Genomewide Replication in clb5Δ Saccharomyces cerevisiae. Genetics. 2008;180:1833–1847. doi: 10.1534/genetics.108.094359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh D, Blow JJ. Dormant origins, the licensing checkpoint, and the response to replicative stresses. Cold Spring Harb Perspect Biol. 2012;4:a012955–a012955. doi: 10.1101/cshperspect.a012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesner LD, Valsakumar V, Cieslik M, Pickin R, Hamlin JL, Bekiranov S. Bubble-seq analysis of the human genome reveals distinct chromatin-mediated mechanisms for regulating early- and late-firing origins. Genome Res. 2013;23:1774–1788. doi: 10.1101/gr.155218.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi T, Fudenberg G, Mehta S, Belton J-M, Taneja N, Folco HD, FitzGerald P, Dekker J, Mirny L, Barrowman J, Grewal SIS. Cohesin-dependent globules and heterochromatin shape 3D genome architecture in S. pombe Nature. 2014 doi: 10.1038/nature13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay R, Lajugie J, Fourel N, Selzer A, Schizas M, Bartholdy B, Mar J, Lin CM, Martin MM, Ryan M, Aladjem MI, Bouhassira EE. Allele-specific genome-wide profiling in human primary erythroblasts reveal replication program organization. PLoS Genet. 2014;10:e1004319. doi: 10.1371/journal.pgen.1004319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow AZ, Nellimoottil T, Knott SRV, Fox CA, Tavaré S, Aparicio OM. Fkh1 and Fkh2 bind multiple chromosomal elements in the S. cerevisiae genome with distinct specificities and cell cycle dynamics. PLoS ONE. 2014;9:e87647. doi: 10.1371/journal.pone.0087647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PK, Arcangioli B, Baker SP, Bensimon A, Rhind N. DNA replication origins fire stochastically in fission yeast. Mol Biol Cell. 2006;17:308–316. doi: 10.1091/mbc.E05-07-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peace JM, Ter-Zakarian A, Aparicio OM. Rif1 Regulates Initiation Timing of Late Replication Origins throughout the S. cerevisiae. Genome. 2014;9:e98501. doi: 10.1371/journal.pone.0098501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryk N, Kahli M, d’Aubenton-Carafa Y, Jaszczyszyn Y, Shen Y, Silvain M, Thermes C, Chen CL, Hyrien O. Replication landscape of the human genome. Nat Comms. 2016;7:10208. doi: 10.1038/ncomms10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Cadoret J-C, Audit B, Arneodo A, Alberti A, Battail C, Duret L, Prioleau M-N. The spatiotemporal program of DNA replication is associated with specific combinations of chromatin marks in human cells. PLoS Genet. 2014;10:e1004282. doi: 10.1371/journal.pgen.1004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl TJ, Brewer BJ, Raghuraman MK. Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication in Saccharomyces cerevisiae. PLoS Genet. 2012;8:e1002677. doi: 10.1371/journal.pgen.1002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope BD, Ryba T, Dileep V, Yue F, Wu W, Denas O, Vera DL, Wang Y, Hansen RS, Canfield TK, Thurman RE, Cheng Y, Gülsoy G, Dennis JH, Snyder MP, Stamatoyannopoulos JA, Taylor J, Hardison RC, Kahveci T, Ren B, Gilbert DM. Topologically-associating domains are stable units of replication-timing regulation. Nature. 2014;515:402–405. doi: 10.1038/nature13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzi L, Marchetti L, Peverali FA, Biamonti G, Giacca M. DNA-protein interaction dynamics at the Lamin B2 replication origin. Cell Cycle. 2015;14:64–73. doi: 10.4161/15384101.2014.973337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Yue F. Transcriptional Enhancers: Bridging the Genome and Phenome. Cold Spring Harb Symp Quant Biol. 2015:027219. doi: 10.1101/sqb.2015.80.027219. [DOI] [PubMed] [Google Scholar]
- Renard-Guillet C, Kanoh Y, Shirahige K, Masai H. Temporal and spatial regulation of eukaryotic DNA replication: from regulated initiation to genome-scale timing program. Semin Cell Dev Biol. 2014;30:110–120. doi: 10.1016/j.semcdb.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Rhind N, Gilbert DM. DNA replication timing. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Mulia JC, Buckley Q, Sasaki T, Zimmerman J, Didier RA, Nazor K, Loring JF, Lian Z, Weissman S, Robins AJ, Schulz TC, Menendez L, Kulik MJ, Dalton S, Gabr H, Kahveci T, Gilbert DM. Dynamic changes in replication timing and gene expression during lineage specification of human pluripotent stem cells. Genome Res. 2015;25:1091–1103. doi: 10.1101/gr.187989.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Mulia JC, Gilbert DM. Replication timing and transcriptional control: beyond cause and effect-part III. Curr Opin Cell Biol. 2016;40:168–178. doi: 10.1016/j.ceb.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryba T, Hiratani I, Lu J, Itoh M, Kulik M, Zhang J, Schulz TC, Robins AJ, Dalton S, Gilbert DM. Evolutionarily conserved replication timing profiles predict long-range chromatin interactions and distinguish closely related cell types. Genome Res. 2010;20:761–770. doi: 10.1101/gr.099655.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Li A, Gillespie PJ, Blow JJ, Gilbert DM. Evidence for a mammalian late-G1 phase inhibitor of replication licensing distinct from geminin or Cdk activity. Nucleus. 2011;2:455–464. doi: 10.4161/nucl.2.5.17859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Ramanathan S, Okuno Y, Kumagai C, Shaikh SS, Gilbert DM. The Chinese hamster dihydrofolate reductase replication origin decision point follows activation of transcription and suppresses initiation of replication within transcription units. Mol Cell Biol. 2006;26:1051–1062. doi: 10.1128/MCB.26.3.1051-1062.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Sawado T, Yamaguchi M, Shinomiya T. Specification of regions of DNA replication initiation during embryogenesis in the 65-kilobase DNApolalpha-dE2F locus of Drosophila melanogaster. Mol Cell Biol. 1999;19:547–555. doi: 10.1128/MCB.19.1.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schübeler D, Scalzo D, Kooperberg C, van Steensel B, Delrow J, Groudine M. Genome-wide DNA replication profile for Drosophila melanogaster: a link between transcription and replication timing. Nat Genet. 2002;32:438–442. doi: 10.1038/ng1005. [DOI] [PubMed] [Google Scholar]
- Sima J, Gilbert DM. Complex correlations: replication timing and mutational landscapes during cancer and genome evolution. Curr Opin Genet Dev. 2014;25:93–100. doi: 10.1016/j.gde.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollier J, Cimprich KA. Breaking bad: R-loops and genome integrity. Trends Cell Biol. 2015;25:514–522. doi: 10.1016/j.tcb.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovei I, Thanisch K, Feodorova Y. How to rule the nucleus: divide et impera. Curr Opin Cell Biol. 2016;40:47–59. doi: 10.1016/j.ceb.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Sreesankar E, Senthilkumar R, Bharathi V, Mishra RK, Mishra K. Functional diversification of yeast telomere associated protein, Rif1, in higher eukaryotes. BMC Genomics. 2012;13:1. doi: 10.1186/1471-2164-13-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi S-I, Dileep V, Ryba T, Dennis JH, Gilbert DM. Chromatin-interaction compartment switch at developmentally regulated chromosomal domains reveals an unusual principle of chromatin folding. Proc Natl Acad Sci USA. 2012;109 doi: 10.1073/pnas.1207185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Gillespie PJ, Blow JJ. Replication factory activation can be decoupled from the replication timing program by modulating Cdk levels. The Journal of Cell Biology. 2010;188:209–221. doi: 10.1083/jcb.200911037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo LI, Altmeyer M, Rask MB, Lukas C, Larsen DH, Povlsen Lou Klitgaard, Bekker-Jensen S, Mailand N, Bartek J, Lukas J. ATR Prohibits Replication Catastrophe by Preventing Global Exhaustion of RPA. Cell. 2013;155:1088–1103. doi: 10.1016/j.cell.2013.10.043. [DOI] [PubMed] [Google Scholar]
- Urban JM, Foulk MS, Casella C, Gerbi SA. The hunt for origins of DNA replication in multicellular eukaryotes. F1000Prime Rep. 2015;7:30. doi: 10.12703/P7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela MS, Chen Y, Davis S, Yang F, Walker RL, Bilke S, Lueders J, Martin MM, Aladjem MI, Massion PP, Meltzer PS. Preferential localization of human origins of DNA replication at the 5′-ends of expressed genes and at evolutionarily conserved DNA sequences. 2011;6:e17308. doi: 10.1371/journal.pone.0017308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valton A-L, Hassan-Zadeh V, Lema I, Boggetto N, Alberti P, Saintomé C, Riou J-F, Prioleau M-N. G4 motifs affect origin positioning and efficiency in two vertebrate replicators. EMBO J. 2014;33:732–746. doi: 10.1002/embj.201387506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voichek Y, Bar-Ziv R, Barkai N. Expression homeostasis during DNA replication. Science. 2016;351:1087–1090. doi: 10.1126/science.aad1162. [DOI] [PubMed] [Google Scholar]
- Woodfine K, Fiegler H, Beare DM, Collins JE, McCann OT, Young BD, Debernardi S, Mott R, Dunham I, Carter NP. Replication timing of the human genome. Hum Mol Genet. 2004;13:191–202. doi: 10.1093/hmg/ddh016. [DOI] [PubMed] [Google Scholar]
- Woodward AM, Göhler T, Luciani MG, Oehlmann M, Ge X, Gartner A, Jackson DA, Blow JJ. Excess Mcm2-7 license dormant origins of replication that can be used under conditions of replicative stress. The Journal of Cell Biology. 2006;173:673–683. doi: 10.1083/jcb.200602108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JR, Gilbert DM. The replication origin decision point is a mitogen-independent, 2-aminopurine-sensitive, G1-phase event that precedes restriction point control. Mol Cell Biol. 1997;17:4312–4321. doi: 10.1128/mcb.17.8.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JR, Gilbert DM. A distinct G1 step required to specify the Chinese hamster DHFR replication origin. Science. 1996;271:1270–1272. doi: 10.1126/science.271.5253.1270. [DOI] [PubMed] [Google Scholar]
- Wu Y, Brosh RM., Jr G-quadruplex nucleic acids and human disease. FEBS Journal. 2010;277:3470–3488. doi: 10.1111/j.1742-4658.2010.07760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Ishii A, Kanoh Y, Oda M, Nishito Y, Masai H. Rif1 regulates the replication timing domains on the human genome. EMBO J. 2012 doi: 10.1038/emboj.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeeles JTP, Deegan TD, Janska A, Early A, Diffley JFX. Regulated eukaryotic DNA replication origin firing with purified proteins. Nature. 2015;519:431–435. doi: 10.1038/nature14285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yompakdee C, Huberman JA. Enforcement of late replication origin firing by clusters of short G-rich DNA sequences. J Biol Chem. 2004;279:42337–42344. doi: 10.1074/jbc.M407552200. [DOI] [PubMed] [Google Scholar]
- Yue F, Cheng Y, Breschi A, Vierstra J, Wu W, Ryba T, Sandstrom R, Ma Z, Davis C, Pope BD, Shen Y, Pervouchine DD, Djebali S, Thurman RE, Kaul R, Rynes E, Kirilusha A, Marinov GK, Williams BA, Trout D, Amrhein H, Fisher-Aylor K, Antoshechkin I, Desalvo G, See LH, Fastuca M, Drenkow J, Zaleski C, Dobin A, Prieto P, Lagarde J, Bussotti G, Tanzer A, Denas O, Li K, Bender M, Zhang M, Byron R, Groudine M, McCleary D, Pham L, Ye Z, Kuan S, Edsall L, Wu YC, Rasmussen MD, Bansal MS, Kellis M, Keller CA, Morrissey CS, Mishra T, Jain D, Dogan N, Harris RS, Cayting P, Kawli T, Boyle AP, Euskirchen G, Kundaje A, Lin S, Lin Y, Jansen C, Malladi VS, Cline MS, Erickson DT, Kirkup VM, Learned K, Sloan CA, Rosenbloom KR, de Sousa BL, Beal K, Pignatelli M, Flicek P, Lian J, Kahveci T, Lee D, Kent WJ, Santos MR, Herrero J, Notredame C, Johnson A, Vong S, Lee K, Bates D, Neri F, Diegel M, Canfield T, Sabo PJ, Wilken MS, Reh TA, Giste E, Shafer A, Kutyavin T, Haugen E, Dunn D, Reynolds AP, Neph S, Humbert R, Hansen RS, De Bruijn M, Selleri L, Rudensky A, Josefowicz S, Samstein R, Eichler EE, Orkin SH, Levasseur D, Papayannopoulou T, Chang KH, Skoultchi A, Gosh S, Disteche C, Treuting P, Wang Y, Weiss MJ, Blobel GA, Cao X, Zhong S, Wang T, Good PJ, Lowdon RF, Adams LB, Zhou XQ, Pazin MJ, Feingold EA, Wold B, Taylor J, Mortazavi A, Weissman SM, Stamatoyannopoulos JA, Snyder MP, Guigo R, Gingeras TR, Gilbert DM, Hardison RC, Beer MA, Ren B Consortium TME. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515:355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuin J, Dixon JR, van der Reijden MIJA, Ye Z, Kolovos P, Brouwer RWW, van de Corput MPC, van de Werken HJG, Knoch TA, van IJcken WFJ, Grosveld FG, Ren B, Wendt KS. Cohesin and CTCF differentially affect chromatin architecture and gene expression in human cells. Proc Natl Acad Sci USA. 2014;111:996–1001. doi: 10.1073/pnas.1317788111. [DOI] [PMC free article] [PubMed] [Google Scholar]