Abstract
Natriuretic peptides are cardiac-derived hormones with a range of protective functions, including natriuresis, diuresis, vasodilation, lusitropy, lipolysis, weight loss, and improved insulin sensitivity. The actions are mediated through membrane bound guanylyl cyclases that lead to production of the intracellular second-messenger cGMP. A growing body of evidence demonstrates that genetic and acquired deficiencies of the natriuretic peptide system can promote hypertension, cardiac hypertrophy, obesity, diabetes mellitus, the metabolic syndrome, and heart failure. Clinically, natriuretic peptides are robust diagnostic and prognostic markers and augmenting natriuretic peptides is a target for therapeutic strategies in cardio-metabolic disease. This review will summarize current understanding and highlight novel aspects of natriuretic peptide biology.
Keywords: natriuretic peptides, guanylyl cyclase, cardio-metabolic, genetics, neprilysin
Natriuretic peptides and their receptors
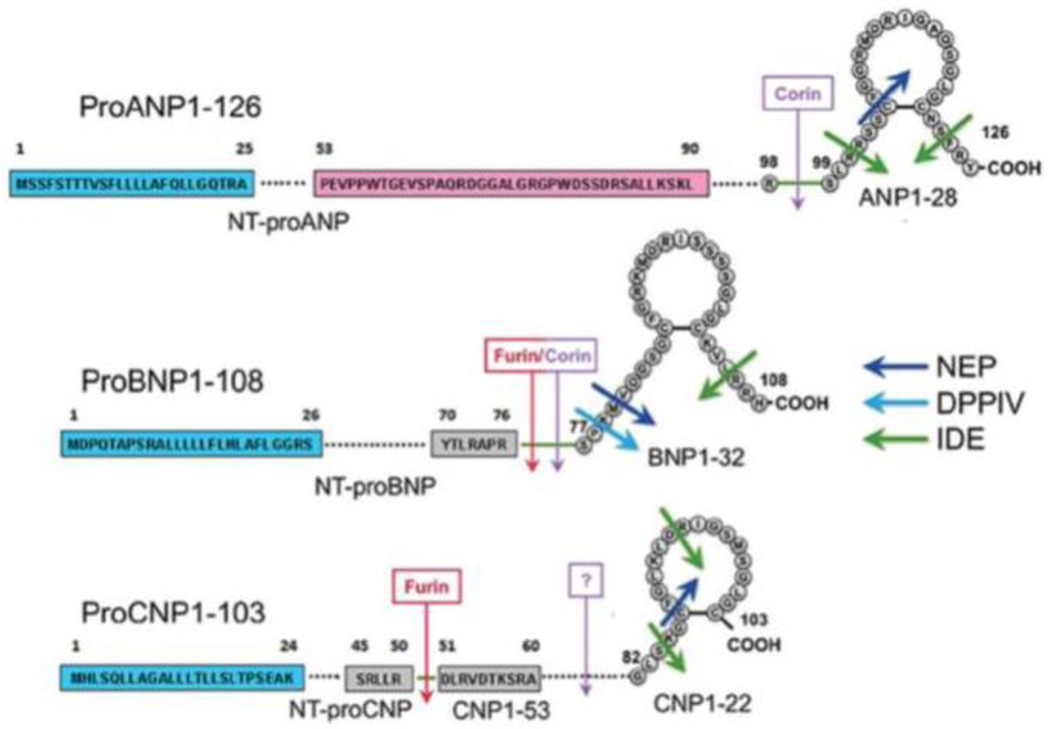
The natriuretic peptides are a family of cardiac-derived hormones that have pleiotropic cardiometabolic protective effects.1 Three natriuretic peptides, atrial (ANP), B-type (BNP), and C-type (CNP) have been described, with ANP being the first, identified by de Bold and colleagues and sequenced by Matsuo and Kangawa.2–7 In humans, these peptides are encoded by the NPPA (natriuretic peptide precursor A) and NPPB genes located in tandem on chromosome 1, and NPPC on chromosome 2.8–10 Mechanical stretch of cardiomyocytes and/or stimulation by endothelin, angiotensin II, the sympathetic nervous system, vasopressin, hypoxia, cold, or exercise induces the transcription factor GATA to bind the natriuretic peptide promoters.3, 11 The natriuretic peptide precursor genes are transcribed and translated into preprohormones that undergo post-translational processing and cleavage into biologically active carboxy-terminal and inactive amino-terminal fragments by the serine proteases corin and/or furin (Figure 1).12 ANP is predominantly synthesized, stored in preformed granules, and released from atrial cardiomyocytes; BNP is produced in atrial and ventricular cardiomyocytes; and CNP is largely derived from vascular endothelial cells and neurons.13–15 The bioactive carboxy-terminal natriuretic peptides have relatively short half-lives in the circulation, while the inactive amino-terminal fragments are more stable with longer half-lives.16
Figure 1.
The post-translational processing of natriuretic peptides. From Volpe M, Rubattu S, Burnett J, Jr. Natriuretic peptides in cardiovascular diseases: Current use and perspectives. Eur Heart J. 2014;35:419–425. (Permission pending)
Caption: Natriuretic peptide protein sequences and post-translational processing cleavage and degradation sites. NEP = neprilysin, DPPIV = dipeptidyl peptidase IV, IDE = insulin degrading enzyme.
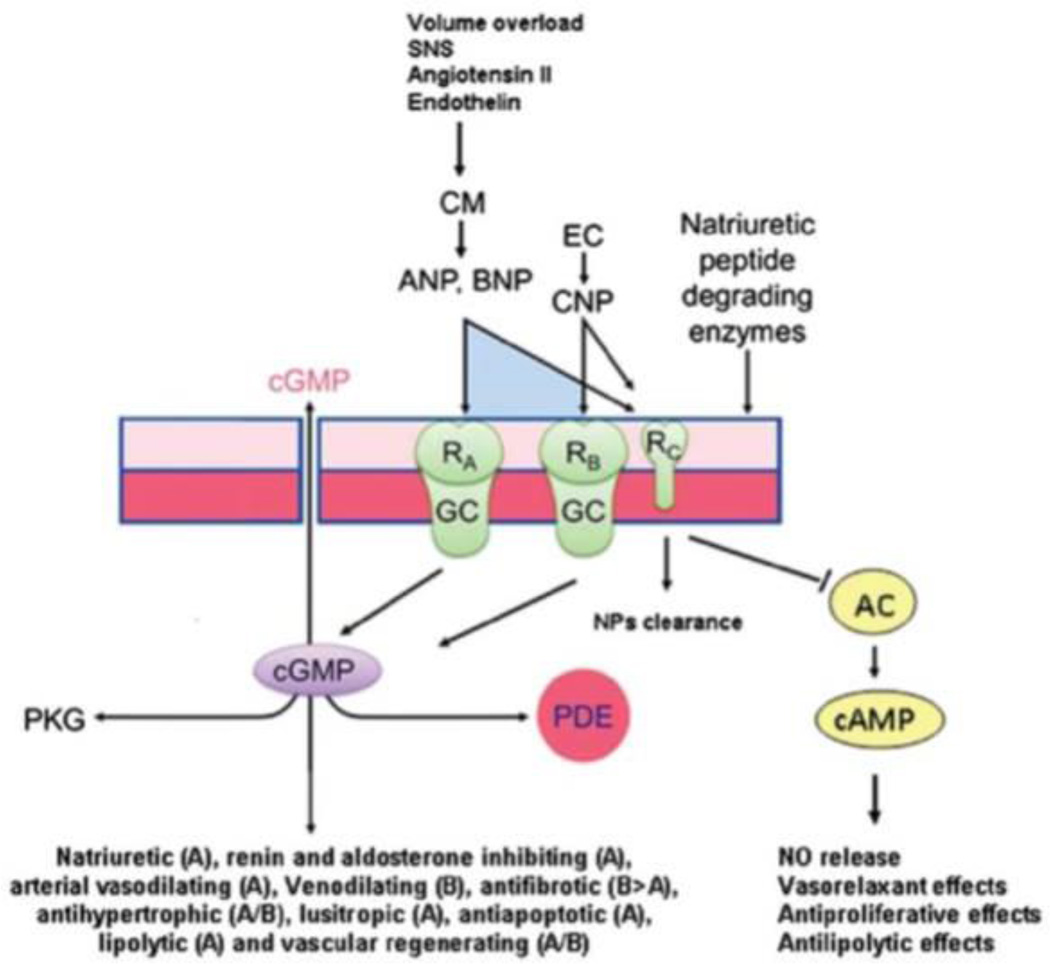
The natriuretic peptides exert their actions by binding guanylyl cyclase receptors A (GC-A for ANP and BNP) and B (GC-B for CNP), which are transmembrane proteins that catalyze the conversion of intracellular guanosine triphosphate into cyclic guanosine monophosphate (cGMP), which then increases intracellular protein kinase G (Figure 2).17 Natriuretic peptide receptor C (NPR-C) functions predominantly as a clearance receptor for all three natriuretic peptides, but also exerts effects on inhibitory G-proteins and adenylyl cyclase with activation of phospholipase C.18 Receptors for the natriuretic peptides are not only present on cardiomyocytes and fibroblasts, but also the kidneys, vascular and gastrointestinal smooth muscle, adrenals, brain, pancreas, adipocytes, chondrocytes, platelets, and the liver, suggesting that natriuretic peptides have biologic actions beyond natriuresis. In addition to clearance through NPR-C, natriuretic peptides are inactivated by neutral endopeptidases located within renal tubular cells and the vasculature, as well as insulin degrading enzyme and dipeptidyl peptidase-IV, and may also be passively excreted in the urine (Figure 1).12
Figure 2.
The natriuretic peptide system responds to hemodynamic and metabolic stimuli through activation of guanylyl cyclase receptors resulting in cardiometabolic protect effects. From Volpe M, Rubattu S, Burnett J, Jr. Natriuretic peptides in cardiovascular diseases: Current use and perspectives. Eur Heart J. 2014;35:419–425. (Permission pending)
Caption: Cardiomyocytes and endothelial cells are stimulated to release natriuretic peptides, which bind receptors with guanylyl cyclase activity. This activation leads to increased intracellular cyclic GMP with beneficial downstream effects mediated through protein kinase G and phosphodiesterase. CNP also inhibits adenylate cyclase to reduce cAMP levels through the natriuretic peptide receptor-C.
Biologic effects of natriuretic peptides: experimental evidence
Genetic models
Experimental evidence supports the broad range of cardiovascular and metabolic actions of the natriuretic peptides. Transgenic overexpression or knock-out mouse models for each of the natriuretic peptides and their receptors provides consistent evidence of these hormones protective cardio-metabolic effects (Table 1).11 Overexpression of NPPA, NPPB, and GC-A leads to blood pressure lowering and protection against salt-sensitive hypertension.19–22 Knockout of NPR-C yields a similar phenotype of lower blood pressure.23 Mice overexpressing the BNP gene (NPPB) are also resistant to obesity and demonstrate lower glucose and insulin concentrations compared with wild-type mice, a finding attributed to increased skeletal muscle mitochondrial content and fatty acid oxidation.24–26 In contrast, NPPA, NPPB, and GC-A knockout mice exhibit hypertension, salt-sensitivity, cardiac hypertrophy, cardiac fibrosis, and susceptibility to heart failure, as well as obesity.27–37 Alterations in the corin protein (corresponding to known human genetic variants) that lead to reduced cleavage of natriuretic peptide prohormone into the active peptide also result in salt-sensitive hypertension and cardiac hypertrophy.38, 39
Table 1.
Summary of the phenotypes associated with genetic manipulation of the natriuretic peptide system in animals. From Gardner DG, Chen S, Glenn DJ, Grigsby CL. Molecular biology of the natriuretic peptide system: Implications for physiology and hypertension. Hypertension. 2007;49:419–426. (Permission pending)
| Gene Disruption | Phenotype/Physiology |
|---|---|
| ANP overexpression | Hypotension, decrease in hypoxic hypertension, normal salt excretion, increased H2O intake and excretion |
| ANP knockout (Nppa−/−) | Hypertension, BP-independent right and left ventricular hypertrophy, impaired Na and Cl excretion |
| BNP overexpression | Hypotension, skeletal overgrowth, resistance to immune-mediated renal injury |
| BNP knockout (Nppb−/−) | Load dependent ventricular tibrortic lesions, no hypertrophy, no hypertension |
| CNP knockout (Nppc−/−) | Dwarfism, early death |
| CNP overexpression (chondrocyte targeted) | Rescue of dwarfism phenotype |
| NPR-A (GC-A) overexpression | Hypotension, protection against salt-sensitive hypertension |
| NPR-A (GC-A) Knockout (Npr1−/−) | Salt-resistant hypertension, BP-independent ventricular hypertrophy, increase in sudden death, enhanced NHE-1 activity, increased susceptibility to heart failure |
| NPR-A targeted knockout | |
| Cardiomyocyte | Hypertrophy, increase in hypertrophy markers, hypotension |
| Smooth muscle | Loss of ANP response, volume dependent hypertension |
| Vascular endothelium | Arterial hypertension and cardiac hypertrophy, increased plasma volume |
| NPR-B (GC-B) knockout (Npr2−/−) | Dwarfism, neuronal disorders, female Infertility |
| NPR-B (GC-B) dominant-negative overexpression (rat) |
BP-independent cardiac hypertrophy, increased congestive heart failure, elevated heart rate |
| NPR-C knockout (Npr3−/−) | Hypotension, bone overgrowth, reduced blood volume |
Non-genetic experiments
In vitro experiments and in vivo data highlight the role of the natriuretic peptides in cardiovascular and metabolic physiology. Animals exposed to infusion of ANP or BNP have lower blood pressure, not only through increased natriuresis and diuresis, but also through arterial and veno-dilation, increased vascular permeability (shifting volume from the intracellular to extracellular space), and direct suppression of the renin-angiotensin-aldosterone and sympathetic nervous systems.3, 40, 41 CNP administration induces marked venodilation.3 The natriuretic and diuretic effects are due to 1) enhanced glomerular filtration through simultaneous dilation of afferent arterioles and constriction of efferent arterioles and 2) direct effects on renal tubular cells through antagonism of angiotensin II and vasopressin.42–44 The vasodilatory effects of ANP and BNP are also mediated centrally in the brainstem through decrease of sympathetic outflow.41, 45, 46
ANP inhibits growth of cardiac fibroblasts and can induce cardiomyocyte apoptosis.47–49 Similar to ANP, CNP is a potent inhibitor of cardiac fibroblasts and exerts anti-fibrotic effects,50 which may be in part mediated by PKG dependent phosphorylation of Smad3 resulting in less nuclear translocation when stimulated by transforming growth factor-β.51 Through p38 MAPK, natriuretic peptides also exhibit anti-mitogenic properties with some indication of anti-neoplastic potential through reduction of inflammation and cell adhesion processes as well.52, 53
The p38 MAPK pathway may also modulate the effect of natriuretic peptides on the induction of brown adipose tissue from white adipocytes.54 Further supporting a role for the natriuretic peptides in the control of energy homeostasis, exposure of cultured adipocytes to physiologic doses of ANP and/or BNP promote cGMP dependent activation of hormone sensitive-lipase leading to lipolysis.55,56
Biologic effects of natriuretic peptides: clinical evidence
Genetic variants
The biologic importance of the natriuretic peptide system is supported by the finding that the NPPA gene is highly conserved across species.57 Nevertheless, genetic variants in the natriuretic peptides, their receptors, and activating proteases have been identified in humans and their associations with cardio-metabolic phenotypes described.57 The results of these genetic variation studies in humans parallel the evidence from animal models regarding the role of the natriuretic peptide system.
A number of variants in the promoter, coding, intronic, and 3’ untranslated region of the NPPA gene have been characterized (Table 2).57 Candidate gene studies in Japanese and Italian individuals have associated a C-664G variant with lower circulating ANP, hypertension, and left ventricular hypertrophy.58,59, 60 There are mixed data regarding another missense variant, rs5063, which results in a valine to methionine substitution and has been linked to lower blood pressure among Chinese individuals and participants in the Women’s Genome Health study, although this was not observed among Japanese individuals. 58, 61,62, 63 In other populations, the rs5063 variant was associated with an increased risk of hypertension or stroke.64, 65,66 Interestingly, the rs5065 (2238 T>C) variant in exon 3 has been associated with a decreased risk of hypertension,67 but higher risk of myocardial infarction and stroke, that may be mediated through altered NPR-C activation and resultant endothelial dysfunction.68–72 Nonetheless, many of the aforementioned candidate genes have not been reliably reproduced in large-scale population genetic studies nor in meta-analyses of GWAS studies.
Table 2.
Summary of NPPA gene variants in humans and their associated clinical phenotype. From Rubattu S, Sciarretta S, Volpe M. Atrial natriuretic peptide gene variants and circulating levels: Implications in cardiovascular diseases. Clin Sci (Lond). 2014;127:1–13. (permission pending)
| NPFA variant | Hypertension | LVH | cv acute events | AF | MS | HF |
|---|---|---|---|---|---|---|
| −664C>G | G allele more frequent in young subjects with HT [25]: C allele more frequent in Japanese subjects with HT [27] |
G allele associated with increased LVH in HT [26] |
No association of either allele with stroke and AMI [46,47] |
No association of either allele with AF in high risk Italian patients [69] |
– | – |
| rs5063 (664G>A) | A allele associated with lower DBP [35]: A allele associated with BP progression [36,37]; no association in japanese patients [27] |
– | A allele associated with increased risk or stroke [59]; common allele associated with higher risk of acute events [53] |
A allele associated with increased risk of lone AF in Chinese patients [67]; no association with AF in North American subjects [68] |
– | – |
| Hpall | Variant allele associated with increased risk of HT [36,39]; common allele associated with increased risk of HT [40] |
– | Variant allele associated with increased risk of stroke [59] |
– | – | – |
| rs5065 (2238T>C) | C allele associated with a decreased risk of HT [41] |
C allele associated with an increased risk of stroke, AMI and MACE [45–48,50]; no association with stroke [59]; no association with CV events [52]; C allele associated with a greater response to diuretic in HT [52] |
No association of C allele with AF in both North American and Italian patients [68,69] |
Allele variant associated with ANP plasma levels in NYHA class III–IV [71] |
||
| rs5068 | Allele variant associated with a decreased risk of HT [29] |
Allele variant associated with decreased occurrence of LVH [44] |
– | – | Allele variant associated with a decreased risk of the MS [62,63] |
No association of allele variant with HF [72] |
LVH = left ventricular hypertrophy, CV = cardiovascular, AF = atrial fibrillation, MS = metabolic syndrome, HF = heart failure, HT = hypertension, AMI = acute myocardial infarction, DBP = diastolic blood pressure, BP = blood pressure, MACE = major adverse cardiovascular events.
The most statistically robust findings to date have derived from studies of white individuals, given the larger sample sizes. For instance, from a meta-analysis of data from the Framingham Heart Study, the Malmo Diet and Cancer Study, and the Finrisk study, the rs5068 A/G variant in the 3’ untranslated region of the NPPA gene is associated with higher circulating ANP levels at a genome-wide level of significance (among carriers of the minor allele, G, P = 8 × 10−70). The G allele has been associated with lower blood pressure, less hypertension, and less ventricular hypertrophy.73, 74 Additional studies demonstrate that the rs5068 A/G variant relates to a favorable metabolic profile as evidenced by lower body mass index, smaller waist circumference, higher HDL, lower C-reactive protein, as well as less susceptibility to heart failure.75, 76 Recently, Arora and colleagues elucidated the molecular mechanism by which the rs5068 variant influenced ANP production. The variant is in the non-coding 3’ UTR of the NPPA gene, a region that is targeted by micro-RNAs. ANP expression was modulated through negative regulation by a specific microRNA, miR-425, which binds to the site of rs5068. Thus, individuals with the AG allele combination are resistant to miR-425, and therefore have higher circulating ANP levels and less hypertension compared with AA homozygote individuals.77
Genetic variants in other natriuretic peptide and related genes have also been described. The rs198388 (presence of A allele) and 198389 (presence of the C allele) variants in the NPPB gene are associated with lower blood pressure, improved left ventricular diastolic function, reduced left ventricular remodeling, and lower risk of diabetes mellitus.73, 78–81 A functional deletion mutation in the 5’ flanking region of the natriuretic peptide receptor GC-A gene reduces transcription and is associated with hypertension and ventricular hypertrophy among Japanese individuals.82 In genome wide association studies, NPR-C variants are associated with hypertension in Caucasian and Asian individuals.80, 81 Less is known about variants in NPPC and GC-B.57 Missense variants in CORIN (555T>I and 568Q>P), which encodes a serine protease that cleaves natriuretic prohormones into the active carboxy- and inactive amino- terminal peptides, has been found to present in approximately 9% of African-Americans and is associated with a greater risk for hypertension and cardiac hypertrophy.83, 84
Physiologic studies
The beneficial cardiovascular effects of natriuretic peptides have also been demonstrated through infusions of ANP, BNP, and CNP. All three natriuretic peptides induce vasodilation, with ANP and BNP also lowering blood pressure.85, 86 Infusion of ANP, BNP, or CNP may also limit post-acute myocardial infarction adverse cardiac modeling.87–89 In the setting of heart failure, ANP and BNP infusions decrease pulmonary capillary wedge pressure and systemic vascular resistance, leading to increased stroke volume.90–94
Natriuretic peptides not only influence myocardial structure and function, but also exert positive influences on the vasculature. Cultured endothelial cells exposed to ANP or CNP demonstrated reduced expression of adhesion molecules (MCP-1 and P-selectin), which are needed for leukocyte infiltration into atherosclerotic plaques.95, 96 CNP also inhibits coronary vascular smooth muscle proliferation in models of atherosclerosis,97–99 reduces platelet leukocyte aggregation, and limits thrombus formation through reduction in PAI-1, perhaps through NPR-C.100–102
The beneficial metabolic effects of natriuretic peptides have also been demonstrated in humans. Infusion of ANP at physiologic levels induced lipid mobilization from subcutaneous adipose tissue with a concomitant increase in lipid oxidation by skeletal muscle.25, 26, 103 BNP has been demonstrated to lower glucose levels,104 while both ANP and BNP converted white to brown fat through mitochondrial uncoupling protein-1 and p38 MAPK.54 It has also been suggested that exercise induced lipolysis may be mediated through ANP.105
Epidemiologic associations
The cross-sectional associations between circulating natriuretic peptide levels and cardiovascular and metabolic disease have been examined in epidemiologic studies. An inverse relationship has been demonstrated between plasma natriuretic peptide levels and body mass index.106, 107 Similarly, low levels of NT-proBNP and NT-proANP have been found in individuals with the metabolic syndrome and/or left ventricular hypertrophy.108–111 The beneficial effects of natriuretic peptides on endothelial function has also been demonstrated in the Framingham Heart Study.112 Congruent with the animal studies, low natriuretic peptide levels have been associated with the development of diabetes mellitus.113, 114
Natriuretic peptides as biomarkers
While experimental, population genetic, and natriuretic peptide infusion studies demonstrate inverse associations between natriuretic peptide levels and cardio-metabolic disease, clinical studies of natriuretic peptides as prognostic biomarkers typically yield positive associations between circulating natriuretic peptide levels and adverse cardiovascular outcomes.16 This apparent paradox is attributable to the fact that natriuretic peptides are counter-regulatory hormones that are released in response to cardiac stress. In population studies, higher natriuretic peptide levels, even within what might be considered a “normal” range, are commonly seen in the setting of subclinical cardiovascular disease. Thus, the elevated natriuretic peptide levels observed in clinical biomarker studies reflect normal physiologic responses to elevated cardiac wall stress.
For example, among individuals without prevalent cardiovascular disease in the Framingham Offspring Study and in Copenhagen, higher natriuretic peptide levels were positively and significantly associated with cardiovascular mortality115 or major adverse cardiovascular events,116 respectively. Among persons with stable coronary artery disease or acute coronary syndromes, higher natriuretic peptides were significantly and positively associated with greater risk for recurrent cardiovascular events and/or death.117–121 Similarly, higher natriuretic peptide levels are associated with worse outcomes among individuals with heart failure.122–125 However, further evidence for the beneficial effects of natriuretic peptides, even in the setting of subclinical or over cardiovascular disease, comes from therapeutic trials in which augmentation of natriuretic peptides led to favorable cardiovascular effects.
Natriuretic peptides as a therapeutic target
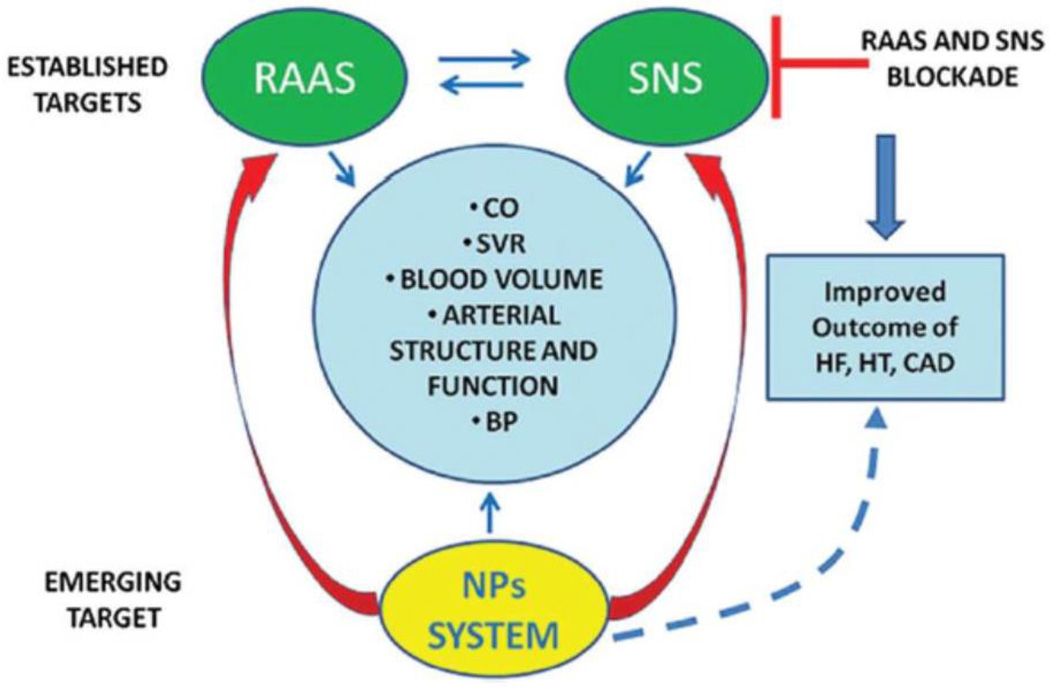
Most strategies for the prevention and treatment of cardiovascular disease have been directed at blocking the deleterious effects of the renin-angiotensin-aldosterone axis and sympathetic nervous system.12 Given that hypertension, obesity, and insulin-resistance are major risk factors for the development of cardiovascular disease and natriuretic peptides guard against the development and progression of these disorders, natriuretic peptides are attractive therapeutic targets (Figure 3).
Figure 3.
Natriuretic peptides as novel therapeutic targets in cardiometabolic disease. Adapted from Volpe M, Rubattu S, Burnett J, Jr. Natriuretic peptides in cardiovascular diseases: Current use and perspectives. Eur Heart J. 2014;35:419–425. (Permission pending)
Caption: Natriuretic peptides exert beneficial effects on cardiac and vascular function directly and through inhibition of the renin-angiotensin-aldosterone axis and sympathetic nervous systems, making them ideal and novel targets for cardiovascular protection.
Therapeutic approaches have included intravenous infusions of recombinant ANP or BNP, oral inhibitors of neutral endopeptidases, and synthetic or “designer” natriuretic peptide analogues.3 Intravenous infusions of ANP and BNP have been tested in clinical trials for hypertension126 and heart failure with favorable hemodynamic effects, but no clear benefit on long-term clinical outcomes.94, 127 Furthermore, oral formulations of ANP and BNP are not stable, currently limiting their therapeutic application in chronic disease.
An alternative strategy to direct supplementation of natriuretic peptides is to limit the breakdown of endogenous natriuretic peptides. Inhibitors of neutral endopeptidases are stable when given orally and the first to be tested in hypertension was candoxatril; however, this agent was not of substantial benefit because of simultaneous vasoconstriction due to increases in endothelin-1 and angiotensin II.128, 129 Subsequently, combined inhibition of neutral endopeptidases and angiotensin converting enzyme with omapatrilat was evaluated in hypertensive and heart failure patients in the OCTAVE, OVERTURE, and IMPRESS trials. Blood pressure was lower in omapatrilat treated patients compared to those treated with enalapril alone; however, omapatrilat was associated with a higher frequency of angioedema and symptomatic hypotension, without demonstration of superior efficacy, thereby preventing approval for clinical use.130–132 More recently, neutral endopeptidase inhibition has been combined with angiotensin receptor blockade to avoid the angioedema seen with ACE inhibition. The angiotensin receptor and neprilysin inhibitor (ARNI) LCZ696 is efficacious for lowering blood pressure among patients with essential hypertension, without increased angioedema compared with valsartan alone.133 In a phase II study of heart failure with preserved ejection fraction patients (PARAMOUNT), LCZ696 was superior to valsartan alone in reducing NT-proBNP levels over 12 weeks of follow up.134 Recently, LCZ696 was demonstrated to be superior to enalapril for reducing cardiovascular death and heart failure hospitalizations in patients with heart failure and reduced ejection fraction (PARADIGM-HF),135 lending further support for the therapeutic benefit of natriuretic peptides.
“Designer” or synthetic natriuretic peptides that are more stable than native natriuretic peptides have been developed. For example, the ANP analog carperitide promotes vasodilation, natriuresis, and inhibition of the renin-angiotensin-aldosterone axis and is approved in Japan for treatment of acute decompensated heart failure.136 Another analog, M-ANP, which is more resistant to neutral endopeptidase degradation than native ANP, has been designed and has favorable anti-hypertensive effects.137 A novel chimeric molecule, CD-NP, has been engineered by combining the 15 amino acid carboxy-terminus of dendrapsis natriuretic peptide with CNP, resulting in a protein that is able to activate both GC-A and GC-B. This chimeric peptide demonstrates potent natriuresis and diuresis, as well as anti-fibrotic and anti-proliferative properties.138
Summary
Natriuretic peptides are cardiac-derived hormones and the principal counter-regulatory system guarding against salt-retention, volume expansion, cardiac stress, and remodeling. They also modulate energy metabolism, lipolysis, weight loss, and insulin sensitivity. Low natriuretic peptide activity can be associated with increased risk for hypertension, obesity, and diabetes mellitus, conditions that are increasing in prevalence and are major risk factors for cardiovascular disease. Consequently, natriuretic peptides are attractive targets for therapeutic approaches to cardio-metabolic disease.
Acknowledgments
Funding: National Heart, Lung, and Blood Institute grants K12 HL109019 and R01-HL-102780; the National Center for Advancing Translational Sciences of the National Institutes of Health award numbers UL1TR000445.
References
- 1.Wang TJ. The natriuretic peptides and fat metabolism. N Engl J Med. 2012;367:377–378. doi: 10.1056/NEJMcibr1204796. [DOI] [PubMed] [Google Scholar]
- 2.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 3.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo H, Kangawa K. Human and rat atrial natriuretic polypeptides (hANP & rANP) purification, structure and biological activity. Clin Exp Hypertens A. 1984;6:1717–1722. doi: 10.3109/10641968409046065. [DOI] [PubMed] [Google Scholar]
- 5.Oikawa S, Imai M, Ueno A, Tanaka S, Noguchi T, Nakazato H, et al. Cloning and sequence analysis of cDNA encoding a precursor for human atrial natriuretic polypeptide. Nature. 1984;309:724–726. doi: 10.1038/309724a0. [DOI] [PubMed] [Google Scholar]
- 6.Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 7.Sudoh T, Minamino N, Kangawa K, Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990;168:863–870. doi: 10.1016/0006-291x(90)92401-k. [DOI] [PubMed] [Google Scholar]
- 8.Nemer M, Chamberland M, Sirois D, Argentin S, Drouin J, Dixon RA, et al. Gene structure of human cardiac hormone precursor, pronatriodilatin. Nature. 1984;312:654–656. doi: 10.1038/312654a0. [DOI] [PubMed] [Google Scholar]
- 9.Arden KC, Viars CS, Weiss S, Argentin S, Nemer M. Localization of the human B-type natriuretic peptide precursor (NPPB) gene to chromosome 1p36. Genomics. 1995;26:385–389. doi: 10.1016/0888-7543(95)80225-b. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa Y, Itoh H, Yoshitake Y, Inoue M, Yoshimasa T, Serikawa T, et al. Molecular cloning and chromosomal assignment of the mouse C-type natriuretic peptide (CNP) gene (Nppc): comparison with the human CNP gene (NPPC) Genomics. 1994;24:383–387. doi: 10.1006/geno.1994.1633. [DOI] [PubMed] [Google Scholar]
- 11.Gardner DG, Chen S, Glenn DJ, Grigsby CL. Molecular biology of the natriuretic peptide system: implications for physiology and hypertension. Hypertension. 2007;49:419–426. doi: 10.1161/01.HYP.0000258532.07418.fa. [DOI] [PubMed] [Google Scholar]
- 12.Volpe M, Rubattu S, Burnett J., Jr Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J. 2014;35:419–425. doi: 10.1093/eurheartj/eht466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimura M, Yasue H, Okumura K, Ogawa H, Jougasaki M, Mukoyama M, et al. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation. 1993;87:464–469. doi: 10.1161/01.cir.87.2.464. [DOI] [PubMed] [Google Scholar]
- 14.Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/01.cir.90.1.195. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa O, Ogawa Y, Itoh H, Suga S, Komatsu Y, Kishimoto I, et al. Rapid transcriptional activation early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J Clin Invest. 1995;96:1280–1287. doi: 10.1172/JCI118162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn M. Molecular physiology of natriuretic peptide signalling. Basic Res Cardiol. 2004;99:76–82. doi: 10.1007/s00395-004-0460-0. [DOI] [PubMed] [Google Scholar]
- 18.Rubattu S, Sciarretta S, Morriello A, Calvieri C, Battistoni A, Volpe M. NPR-C: a component of the natriuretic peptide family with implications in human diseases. J Mol Med (Berl) 2010;88:889–897. doi: 10.1007/s00109-010-0641-2. [DOI] [PubMed] [Google Scholar]
- 19.Steinhelper ME, Cochrane KL, Field LJ. Hypotension in transgenic mice expressing atrial natriuretic factor fusion genes. Hypertension. 1990;16:301–307. doi: 10.1161/01.hyp.16.3.301. [DOI] [PubMed] [Google Scholar]
- 20.Veress AT, Chong CK, Field LJ, Sonnenberg H. Blood pressure and fluid-electrolyte balance in ANF-transgenic mice on high- and low-salt diets. Am J Physiol. 1995;269:R186–R192. doi: 10.1152/ajpregu.1995.269.1.R186. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa Y, Itoh H, Tamura N, Suga S, Yoshimasa T, Uehira M, et al. Molecular cloning of the complementary DNA and gene that encode mouse brain natriuretic peptide and generation of transgenic mice that overexpress the brain natriuretic peptide gene. J Clin Invest. 1994;93:1911–1921. doi: 10.1172/JCI117182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliver PM, John SW, Purdy KE, Kim R, Maeda N, Goy MF, et al. Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc Natl Acad Sci U S A. 1998;95:2547–2551. doi: 10.1073/pnas.95.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsukawa N, Grzesik WJ, Takahashi N, Pandey KN, Pang S, Yamauchi M, et al. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci U S A. 1999;96:7403–7408. doi: 10.1073/pnas.96.13.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58:2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest. 2012;122:4675–4679. doi: 10.1172/JCI64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birkenfeld AL, Boschmann M, Moro C, Adams F, Heusser K, Franke G, et al. Lipid mobilization with physiological atrial natriuretic peptide concentrations in humans. J Clin Endocrinol Metab. 2005;90:3622–3628. doi: 10.1210/jc.2004-1953. [DOI] [PubMed] [Google Scholar]
- 27.John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, et al. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 28.John SW, Veress AT, Honrath U, Chong CK, Peng L, Smithies O, et al. Blood pressure and fluid-electrolyte balance in mice with reduced or absent ANP. Am J Physiol. 1996;271:R109–R114. doi: 10.1152/ajpregu.1996.271.1.R109. [DOI] [PubMed] [Google Scholar]
- 29.Melo LG, Veress AT, Chong CK, Pang SC, Flynn TG, Sonnenberg H. Salt-sensitive hypertension in ANP knockout mice: potential role of abnormal plasma renin activity. Am J Physiol. 1998;274:R255–R261. doi: 10.1152/ajpregu.1998.274.1.R255. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Oparil S, Feng JA, Li P, Perry G, Chen LB, et al. Effects of pressure overload on extracellular matrix expression in the heart of the atrial natriuretic peptide-null mouse. Hypertension. 2003;42:88–95. doi: 10.1161/01.HYP.0000074905.22908.A6. [DOI] [PubMed] [Google Scholar]
- 31.Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, et al. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995;378:65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 32.Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci U S A. 2000;97:4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, et al. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci U S A. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez MJ, Garbers DL, Kuhn M. The guanylyl cyclase-deficient mouse defines differential pathways of natriuretic peptide signaling. J Biol Chem. 1997;272:23064–23068. doi: 10.1074/jbc.272.37.23064. [DOI] [PubMed] [Google Scholar]
- 35.Knowles JW, Esposito G, Mao L, Hagaman JR, Fox JE, Smithies O, et al. Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient mice. J Clin Invest. 2001;107:975–984. doi: 10.1172/JCI11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kishimoto I, Rossi K, Garbers DL. A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase-A) inhibits cardiac ventricular myocyte hypertrophy. Proc Natl Acad Sci U S A. 2001;98:2703–2706. doi: 10.1073/pnas.051625598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, et al. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest. 2003;111:1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W, Liao X, Fukuda K, Knappe S, Wu F, Dries DL, et al. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;103:502–508. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang W, Cui Y, Shen J, Jiang J, Chen S, Peng J, et al. Salt-sensitive hypertension and cardiac hypertrophy in transgenic mice expressing a corin variant identified in blacks. Hypertension. 2012;60:1352–1358. doi: 10.1161/HYPERTENSIONAHA.112.201244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charles CJ, Espiner EA, Richards AM. Cardiovascular actions of ANF: contributions of renal, neurohumoral, and hemodynamic factors in sheep. Am J Physiol. 1993;264:R533–R538. doi: 10.1152/ajpregu.1993.264.3.R533. [DOI] [PubMed] [Google Scholar]
- 41.Schultz HD, Gardner DG, Deschepper CF, Coleridge HM, Coleridge JC. Vagal C-fiber blockade abolishes sympathetic inhibition by atrial natriuretic factor. Am J Physiol. 1988;255:R6–R13. doi: 10.1152/ajpregu.1988.255.1.R6. [DOI] [PubMed] [Google Scholar]
- 42.Marin-Grez M, Fleming JT, Steinhausen M. Atrial natriuretic peptide causes pre-glomerular vasodilatation and post-glomerular vasoconstriction in rat kidney. Nature. 1986;324:473–476. doi: 10.1038/324473a0. [DOI] [PubMed] [Google Scholar]
- 43.Harris PJ, Thomas D, Morgan TO. Atrial natriuretic peptide inhibits angiotensin-stimulated proximal tubular sodium and water reabsorption. Nature. 1987;326:697–698. doi: 10.1038/326697a0. [DOI] [PubMed] [Google Scholar]
- 44.Dillingham MA, Anderson RJ. Inhibition of vasopressin action by atrial natriuretic factor. Science. 1986;231:1572–1573. doi: 10.1126/science.3006248. [DOI] [PubMed] [Google Scholar]
- 45.Yang R, Jin H, Wyss JM, Chen YF, Oparil S. Salt supplementation does not alter the pressor effect of blocking atrial natriuretic peptide in nucleus tractus solitarii. Hypertension. 1992;20:242–246. doi: 10.1161/01.hyp.20.2.242. [DOI] [PubMed] [Google Scholar]
- 46.Steele MK, Gardner DG, Xie PL, Schultz HD. Interactions between ANP and ANG II in regulating blood pressure and sympathetic outflow. Am J Physiol. 1991;260:R1145–R1151. doi: 10.1152/ajpregu.1991.260.6.R1145. [DOI] [PubMed] [Google Scholar]
- 47.Itoh H, Pratt RE, Dzau VJ. Atrial natriuretic polypeptide inhibits hypertrophy of vascular smooth muscle cells. J Clin Invest. 1990;86:1690–1697. doi: 10.1172/JCI114893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao L, Gardner DG. Natriuretic peptides inhibit DNA synthesis in cardiac fibroblasts. Hypertension. 1995;25:227–234. doi: 10.1161/01.hyp.25.2.227. [DOI] [PubMed] [Google Scholar]
- 49.Wu CF, Bishopric NH, Pratt RE. Atrial natriuretic peptide induces apoptosis in neonatal rat cardiac myocytes. J Biol Chem. 1997;272:14860–14866. doi: 10.1074/jbc.272.23.14860. [DOI] [PubMed] [Google Scholar]
- 50.Furuya M, Aisaka K, Miyazaki T, Honbou N, Kawashima K, Ohno T, et al. C-type natriuretic peptide inhibits intimal thickening after vascular injury. Biochem Biophys Res Commun. 1993;193:248–253. doi: 10.1006/bbrc.1993.1616. [DOI] [PubMed] [Google Scholar]
- 51.Li P, Wang D, Lucas J, Oparil S, Xing D, Cao X, et al. Atrial natriuretic peptide inhibits transforming growth factor beta-induced Smad signaling and myofibroblast transformation in mouse cardiac fibroblasts. Circ Res. 2008;102:185–192. doi: 10.1161/CIRCRESAHA.107.157677. [DOI] [PubMed] [Google Scholar]
- 52.Vesely DL. Family of peptides synthesized in the human body have anticancer effects. Anticancer Res. 2014;34:1459–1466. [PubMed] [Google Scholar]
- 53.Nojiri T, Hosoda H, Tokudome T, Miura K, Ishikane S, Otani K, et al. Atrial natriuretic peptide prevents cancer metastasis through vascular endothelial cells. Proc Natl Acad Sci U S A. 2015;112:4086–4091. doi: 10.1073/pnas.1417273112. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moro C, Lafontan M. Natriuretic peptides and cGMP signaling control of energy homeostasis. Am J Physiol Heart Circ Physiol. 2013;304:H358–H368. doi: 10.1152/ajpheart.00704.2012. [DOI] [PubMed] [Google Scholar]
- 56.Sengenes C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345–1351. [PubMed] [Google Scholar]
- 57.Rubattu S, Sciarretta S, Volpe M. Atrial natriuretic peptide gene variants and circulating levels: implications in cardiovascular diseases. Clin Sci (Lond) 2014;127:1–13. doi: 10.1042/CS20130427. [DOI] [PubMed] [Google Scholar]
- 58.Kato N, Sugiyama T, Morita H, Nabika T, Kurihara H, Yamori Y, et al. Genetic analysis of the atrial natriuretic peptide gene in essential hypertension. Clin Sci (Lond) 2000;98:251–258. [PubMed] [Google Scholar]
- 59.Rubattu S, Evangelista A, Barbato D, Barba G, Stanzione R, Iacone R, et al. Atrial natriuretic peptide (ANP) gene promoter variant and increased susceptibility to early development of hypertension in humans. J Hum Hypertens. 2007;21:822–824. doi: 10.1038/sj.jhh.1002228. [DOI] [PubMed] [Google Scholar]
- 60.Rubattu S, Bigatti G, Evangelista A, Lanzani C, Stanzione R, Zagato L, et al. Association of atrial natriuretic peptide and type a natriuretic peptide receptor gene polymorphisms with left ventricular mass in human essential hypertension. J Am Coll Cardiol. 2006;48:499–505. doi: 10.1016/j.jacc.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 61.Zhang S, Mao G, Zhang Y, Tang G, Wen Y, Hong X, et al. Association between human atrial natriuretic peptide Val7Met polymorphism and baseline blood pressure, plasma trough irbesartan concentrations, and the antihypertensive efficacy of irbesartan in rural Chinese patients with essential hypertension. Clin Ther. 2005;27:1774–1784. doi: 10.1016/j.clinthera.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 62.Conen D, Glynn RJ, Buring JE, Ridker PM, Zee RY. Natriuretic peptide precursor a gene polymorphisms and risk of blood pressure progression and incident hypertension. Hypertension. 2007;50:1114–1119. doi: 10.1161/HYPERTENSIONAHA.107.097634. [DOI] [PubMed] [Google Scholar]
- 63.Conen D, Cheng S, Steiner LL, Buring JE, Ridker PM, Zee RY. Association of 77 polymorphisms in 52 candidate genes with blood pressure progression and incident hypertension: the Women’s Genome Health Study. J Hypertens. 2009;27:476–483. doi: 10.1097/hjh.0b013e32832104c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rutledge DR, Sun Y, Ross EA. Polymorphisms within the atrial natriuretic peptide gene in essential hypertension. J Hypertens. 1995;13:953–955. doi: 10.1097/00004872-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Beige J, Ringel J, Hohenbleicher H, Rubattu S, Kreutz R, Sharma AM. HpaII-polymorphism of the atrial-natriuretic-peptide gene and essential hypertension in whites. Am J Hypertens. 1997;10:1316–1318. doi: 10.1016/s0895-7061(97)00305-1. [DOI] [PubMed] [Google Scholar]
- 66.Rubattu S, Ridker P, Stampfer MJ, Volpe M, Hennekens CH, Lindpaintner K. The gene encoding atrial natriuretic peptide and the risk of human stroke. Circulation. 1999;100:1722–1726. doi: 10.1161/01.cir.100.16.1722. [DOI] [PubMed] [Google Scholar]
- 67.Niu W. The Relationship between Natriuretic Peptide Precursor a Gene T2238C Polymorphism and Hypertension: A Meta-Analysis. Int J Hypertens. 2011;2011:653698. doi: 10.4061/2011/653698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gruchala M, Ciecwierz D, Wasag B, Targonski R, Dubaniewicz W, Nowak A, et al. Association of the ScaI atrial natriuretic peptide gene polymorphism with nonfatal myocardial infarction and extent of coronary artery disease. Am Heart J. 2003;145:125–131. doi: 10.1067/mhj.2003.52. [DOI] [PubMed] [Google Scholar]
- 69.Sciarretta S, Marchitti S, Bianchi F, Moyes A, Barbato E, Di Castro S, et al. C2238 atrial natriuretic peptide molecular variant is associated with endothelial damage and dysfunction through natriuretic peptide receptor C signaling. Circ Res. 2013;112:1355–1364. doi: 10.1161/CIRCRESAHA.113.301325. [DOI] [PubMed] [Google Scholar]
- 70.Rubattu S, Stanzione R, Di Angelantonio E, Zanda B, Evangelista A, Tarasi D, et al. Atrial natriuretic peptide gene polymorphisms and risk of ischemic stroke in humans. Stroke. 2004;35:814–818. doi: 10.1161/01.STR.0000119381.52589.AB. [DOI] [PubMed] [Google Scholar]
- 71.Barbato E, Bartunek J, Mangiacapra F, Sciarretta S, Stanzione R, Delrue L, et al. Influence of rs5065 atrial natriuretic peptide gene variant on coronary artery disease. J Am Coll Cardiol. 2012;59:1763–1770. doi: 10.1016/j.jacc.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 72.Cannone V, Huntley BK, Olson TM, Heublein DM, Scott CG, Bailey KR, et al. Atrial natriuretic peptide genetic variant rs5065 and risk for cardiovascular disease in the general community: a 9-year follow-up study. Hypertension. 2013;62:860–865. doi: 10.1161/HYPERTENSIONAHA.113.01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–353. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jujic A, Leosdottir M, Ostling G, Gudmundsson P, Nilsson PM, Melander O, et al. A genetic variant of the atrial natriuretic peptide gene is associated with left ventricular hypertrophy in a non-diabetic population--the Malmo preventive project study. BMC Med Genet. 2013;14:64. doi: 10.1186/1471-2350-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cannone V, Boerrigter G, Cataliotti A, Costello-Boerrigter LC, Olson TM, McKie PM, et al. A genetic variant of the atrial natriuretic peptide gene is associated with cardiometabolic protection in the general community. J Am Coll Cardiol. 2011;58:629–636. doi: 10.1016/j.jacc.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cannone V, Cefalu AB, Noto D, Scott CG, Bailey KR, Cavera G, et al. The atrial natriuretic peptide genetic variant rs5068 is associated with a favorable cardiometabolic phenotype in a Mediterranean population. Diabetes Care. 2013;36:2850–2856. doi: 10.2337/dc12-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arora P, Wu C, Khan AM, Bloch DB, Davis-Dusenbery BN, Ghorbani A, et al. Atrial natriuretic peptide is negatively regulated by microRNA-425. J Clin Invest. 2013;123:3378–3382. doi: 10.1172/JCI67383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ellis KL, Newton-Cheh C, Wang TJ, Frampton CM, Doughty RN, Whalley GA, et al. Association of genetic variation in the natriuretic peptide system with cardiovascular outcomes. J Mol Cell Cardiol. 2011;50:695–701. doi: 10.1016/j.yjmcc.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 79.Meirhaeghe A, Sandhu MS, McCarthy MI, de Groote P, Cottel D, Arveiler D, et al. Association between the T-381C polymorphism of the brain natriuretic peptide gene and risk of type 2 diabetes in human populations. Hum Mol Genet. 2007;16:1343–1350. doi: 10.1093/hmg/ddm084. [DOI] [PubMed] [Google Scholar]
- 80.International Consortium for Blood Pressure Genome-Wide Association S. Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet. 2011;43:531–538. doi: 10.1038/ng.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakayama T, Soma M, Takahashi Y, Rehemudula D, Kanmatsuse K, Furuya K. Functional deletion mutation of the 5’-flanking region of type A human natriuretic peptide receptor gene and its association with essential hypertension and left ventricular hypertrophy in the Japanese. Circ Res. 2000;86:841–845. doi: 10.1161/01.res.86.8.841. [DOI] [PubMed] [Google Scholar]
- 83.Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–2410. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 84.Rame JE, Drazner MH, Post W, Peshock R, Lima J, Cooper RS, et al. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;49:857–864. doi: 10.1161/01.HYP.0000258566.95867.9e. [DOI] [PubMed] [Google Scholar]
- 85.Vesely DL, Douglass MA, Dietz JR, Gower WR, Jr, McCormick MT, Rodriguez-Paz G, et al. Three peptides from the atrial natriuretic factor prohormone amino terminus lower blood pressure and produce diuresis, natriuresis, and/or kaliuresis in humans. Circulation. 1994;90:1129–1140. doi: 10.1161/01.cir.90.3.1129. [DOI] [PubMed] [Google Scholar]
- 86.Nakamura M, Arakawa N, Yoshida H, Makita S, Hiramori K. Vasodilatory effects of C-type natriuretic peptide on forearm resistance vessels are distinct from those of atrial natriuretic peptide in chronic heart failure. Circulation. 1994;90:1210–1214. doi: 10.1161/01.cir.90.3.1210. [DOI] [PubMed] [Google Scholar]
- 87.Hayashi M, Tsutamoto T, Wada A, Maeda K, Mabuchi N, Tsutsui T, et al. Intravenous atrial natriuretic peptide prevents left ventricular remodeling in patients with first anterior acute myocardial infarction. J Am Coll Cardiol. 2001;37:1820–1826. doi: 10.1016/s0735-1097(01)01233-5. [DOI] [PubMed] [Google Scholar]
- 88.Chen HH, Martin FL, Gibbons RJ, Schirger JA, Wright RS, Schears RM, et al. Low-dose nesiritide in human anterior myocardial infarction suppresses aldosterone and preserves ventricular function and structure: a proof of concept study. Heart. 2009;95:1315–1319. doi: 10.1136/hrt.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soeki T, Kishimoto I, Okumura H, Tokudome T, Horio T, Mori K, et al. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J Am Coll Cardiol. 2005;45:608–616. doi: 10.1016/j.jacc.2004.10.067. [DOI] [PubMed] [Google Scholar]
- 90.Yoshimura M, Yasue H, Morita E, Sakaino N, Jougasaki M, Kurose M, et al. Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure. Circulation. 1991;84:1581–1588. doi: 10.1161/01.cir.84.4.1581. [DOI] [PubMed] [Google Scholar]
- 91.Semigran MJ, Aroney CN, Herrmann HC, Dec GW, Jr, Boucher CA, Fifer MA. Effects of atrial natriuretic peptide on myocardial contractile and diastolic function in patients with heart failure. J Am Coll Cardiol. 1992;20:98–106. doi: 10.1016/0735-1097(92)90144-c. [DOI] [PubMed] [Google Scholar]
- 92.Marcus LS, Hart D, Packer M, Yushak M, Medina N, Danziger RS, et al. Hemodynamic and renal excretory effects of human brain natriuretic peptide infusion in patients with congestive heart failure A double-blind, placebo-controlled, randomized crossover trial. Circulation. 1996;94:3184–3189. doi: 10.1161/01.cir.94.12.3184. [DOI] [PubMed] [Google Scholar]
- 93.Mills RM, LeJemtel TH, Horton DP, Liang C, Lang R, Silver MA, et al. Sustained hemodynamic effects of an infusion of nesiritide (human b-type natriuretic peptide) in heart failure: a randomized, double-blind, placebo-controlled clinical trial Natrecor Study Group. J Am Coll Cardiol. 1999;34:155–162. doi: 10.1016/s0735-1097(99)00184-9. [DOI] [PubMed] [Google Scholar]
- 94.Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, et al. Intravenous nesiritide a natriuretic peptide in the treatment of decompensated congestive heart failure Nesiritide Study Group. N Engl J Med. 2000;343:246–253. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 95.Scotland RS, Cohen M, Foster P, Lovell M, Mathur A, Ahluwalia A, et al. C-type natriuretic peptide inhibits leukocyte recruitment and platelet-leukocyte interactions via suppression of P-selectin expression. Proc Natl Acad Sci U S A. 2005;102:14452–14457. doi: 10.1073/pnas.0504961102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weber NC, Blumenthal SB, Hartung T, Vollmar AM, Kiemer AK. ANP inhibits TNF-alpha-induced endothelial MCP-1 expression--involvement of p38 MAPK and MKP-1. J Leukoc Biol. 2003;74:932–941. doi: 10.1189/jlb.0603254. [DOI] [PubMed] [Google Scholar]
- 97.Ikeda M, Kohno M, Yasunari K, Yokokawa K, Horio T, Ueda M, et al. Natriuretic peptide family as a novel antimigration factor of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997;17:731–736. doi: 10.1161/01.atv.17.4.731. [DOI] [PubMed] [Google Scholar]
- 98.Kohno M, Yokokawa K, Yasunari K, Kano H, Minami M, Ueda M, et al. Effect of natriuretic peptide family on the oxidized LDL-induced migration of human coronary artery smooth muscle cells. Circ Res. 1997;81:585–590. doi: 10.1161/01.res.81.4.585. [DOI] [PubMed] [Google Scholar]
- 99.Ueno H, Haruno A, Morisaki N, Furuya M, Kangawa K, Takeshita A, et al. Local expression of C-type natriuretic peptide markedly suppresses neointimal formation in rat injured arteries through an autocrine/paracrine loop. Circulation. 1997;96:2272–2279. doi: 10.1161/01.cir.96.7.2272. [DOI] [PubMed] [Google Scholar]
- 100.Bouchie JL, Hansen H, Feener EP. Natriuretic factors nitric oxide suppress plasminogen activator inhibitor-1 expression in vascular smooth muscle cells Role of cGMP in the regulation of the plasminogen system. Arterioscler Thromb Vasc Biol. 1998;18:1771–1779. doi: 10.1161/01.atv.18.11.1771. [DOI] [PubMed] [Google Scholar]
- 101.Yoshizumi M, Tsuji H, Nishimura H, Masuda H, Kunieda Y, Kawano H, et al. Natriuretic peptides regulate the expression of tissue factor and PAI-1 in endothelial cells. Thromb Haemost. 1999;82:1497–1503. [PubMed] [Google Scholar]
- 102.Kairuz EM, Barber MN, Anderson CR, Kanagasundaram M, Drummond GR, Woods RL. C-type natriuretic peptide (CNP) suppresses plasminogen activator inhibitor-1 (PAI-1) in vivo. Cardiovasc Res. 2005;66:574–582. doi: 10.1016/j.cardiores.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 103.Moro C, Crampes F, Sengenes C, De Glisezinski I, Galitzky J, Thalamas C, et al. Atrial natriuretic peptide contributes to physiological control of lipid mobilization in humans. FASEB J. 2004;18:908–910. doi: 10.1096/fj.03-1086fje. [DOI] [PubMed] [Google Scholar]
- 104.Heinisch BB, Vila G, Resl M, Riedl M, Dieplinger B, Mueller T, et al. B-type natriuretic peptide (BNP) affects the initial response to intravenous glucose: a randomised placebo-controlled cross-over study in healthy men. Diabetologia. 2012;55:1400–1405. doi: 10.1007/s00125-011-2392-1. [DOI] [PubMed] [Google Scholar]
- 105.Moro C, Pillard F, de Glisezinski I, Klimcakova E, Crampes F, Thalamas C, et al. Exercise-induced lipid mobilization in subcutaneous adipose tissue is mainly related to natriuretic peptides in overweight men. Am J Physiol Endocrinol Metab. 2008;295:E505–E513. doi: 10.1152/ajpendo.90227.2008. [DOI] [PubMed] [Google Scholar]
- 106.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 107.Fox ER, Musani SK, Bidulescu A, Nagarajarao HS, Samdarshi TE, Gebreab SY, et al. Relation of obesity to circulating B-type natriuretic peptide concentrations in blacks: the Jackson Heart Study. Circulation. 2011;124:1021–1027. doi: 10.1161/CIRCULATIONAHA.110.991943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khan AM, Cheng S, Magnusson M, Larson MG, Newton-Cheh C, McCabe EL, et al. Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community-based studies. J Clin Endocrinol Metab. 2011;96:3242–3249. doi: 10.1210/jc.2011-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Olsen MH, Hansen TW, Christensen MK, Gustafsson F, Rasmussen S, Wachtell K, et al. N-terminal pro brain natriuretic peptide is inversely related to metabolic cardiovascular risk factors and the metabolic syndrome. Hypertension. 2005;46:660–666. doi: 10.1161/01.HYP.0000179575.13739.72. [DOI] [PubMed] [Google Scholar]
- 110.Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation. 2007;115:1345–1353. doi: 10.1161/CIRCULATIONAHA.106.655142. [DOI] [PubMed] [Google Scholar]
- 111.Rubattu S, Sciarretta S, Ciavarella GM, Venturelli V, De Paolis P, Tocci G, et al. Reduced levels of N-terminal-proatrial natriuretic peptide in hypertensive patients with metabolic syndrome and their relationship with left ventricular mass. J Hypertens. 2007;25:833–839. doi: 10.1097/HJH.0b013e32803cae3c. [DOI] [PubMed] [Google Scholar]
- 112.Kathiresan S, Gona P, Larson MG, Vita JA, Mitchell GF, Tofler GH, et al. Cross-sectional relations of multiple biomarkers from distinct biological pathways to brachial artery endothelial function. Circulation. 2006;113:938–945. doi: 10.1161/CIRCULATIONAHA.105.580233. [DOI] [PubMed] [Google Scholar]
- 113.Magnusson M, Jujic A, Hedblad B, Engstrom G, Persson M, Struck J, et al. Low plasma level of atrial natriuretic peptide predicts development of diabetes: the prospective Malmo Diet and Cancer study. J Clin Endocrinol Metab. 2012;97:638–645. doi: 10.1210/jc.2011-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lazo M, Young JH, Brancati FL, Coresh J, Whelton S, Ndumele CE, et al. NH2-terminal pro-brain natriuretic peptide and risk of diabetes. Diabetes. 2013;62:3189–3193. doi: 10.2337/db13-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 116.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293:1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 117.Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666–675. doi: 10.1056/NEJMoa042330. [DOI] [PubMed] [Google Scholar]
- 118.Sabatine MS, Morrow DA, de Lemos JA, Omland T, Sloan S, Jarolim P, et al. Evaluation of multiple biomarkers of cardiovascular stress for risk prediction and guiding medical therapy in patients with stable coronary disease. Circulation. 2012;125:233–240. doi: 10.1161/CIRCULATIONAHA.111.063842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schnabel R, Lubos E, Rupprecht HJ, Espinola-Klein C, Bickel C, Lackner KJ, et al. B-type natriuretic peptide and the risk of cardiovascular events and death in patients with stable angina: results from the AtheroGene study. J Am Coll Cardiol. 2006;47:552–558. doi: 10.1016/j.jacc.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 120.Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB, Whooley MA. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA. 2007;297:169–176. doi: 10.1001/jama.297.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 122.van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–1506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 123.Anand IS, Fisher LD, Chiang YT, Latini R, Masson S, Maggioni AP, et al. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107:1278–1283. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 124.Maisel A, Hollander JE, Guss D, McCullough P, Nowak R, Green G, et al. Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT) A multicenter study of B-type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath. J Am Coll Cardiol. 2004;44:1328–1333. doi: 10.1016/j.jacc.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 125.Gottlieb SS, Kukin ML, Ahern D, Packer M. Prognostic importance of atrial natriuretic peptide in patients with chronic heart failure. J Am Coll Cardiol. 1989;13:1534–1539. doi: 10.1016/0735-1097(89)90344-6. [DOI] [PubMed] [Google Scholar]
- 126.Volpe M, Mele AF, Indolfi C, De Luca N, Lembo G, Focaccio A, et al. Hemodynamic and hormonal effects of atrial natriuretic factor in patients with essential hypertension. J Am Coll Cardiol. 1987;10:787–793. doi: 10.1016/s0735-1097(87)80271-1. [DOI] [PubMed] [Google Scholar]
- 127.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 128.Ando S, Rahman MA, Butler GC, Senn BL, Floras JS. Comparison of candoxatril and atrial natriuretic factor in healthy men Effects on hemodynamics, sympathetic activity, heart rate variability, and endothelin. Hypertension. 1995;26:1160–1166. doi: 10.1161/01.hyp.26.6.1160. [DOI] [PubMed] [Google Scholar]
- 129.Bevan EG, Connell JM, Doyle J, Carmichael HA, Davies DL, Lorimer AR, et al. Candoxatril, a neutral endopeptidase inhibitor: efficacy and tolerability in essential hypertension. J Hypertens. 1992;10:607–613. [PubMed] [Google Scholar]
- 130.Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103–111. doi: 10.1016/j.amjhyper.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 131.Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) Circulation. 2002;106:920–926. doi: 10.1161/01.cir.0000029801.86489.50. [DOI] [PubMed] [Google Scholar]
- 132.Rouleau JL, Pfeffer MA, Stewart DJ, Isaac D, Sestier F, Kerut EK, et al. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet. 2000;356:615–620. doi: 10.1016/s0140-6736(00)02602-7. [DOI] [PubMed] [Google Scholar]
- 133.Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375:1255–1266. doi: 10.1016/S0140-6736(09)61966-8. [DOI] [PubMed] [Google Scholar]
- 134.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 135.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 136.Suwa M, Seino Y, Nomachi Y, Matsuki S, Funahashi K. Multicenter prospective investigation on efficacy and safety of carperitide for acute heart failure in the ‘real world’ of therapy. Circ J. 2005;69:283–290. doi: 10.1253/circj.69.283. [DOI] [PubMed] [Google Scholar]
- 137.McKie PM, Cataliotti A, Boerrigter G, Chen HH, Sangaralingham SJ, Martin FL, et al. A novel atrial natriuretic peptide based therapeutic in experimental angiotensin II mediated acute hypertension. Hypertension. 2010;56:1152–1159. doi: 10.1161/HYPERTENSIONAHA.110.159210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McKie PM, Sangaralingham SJ, Burnett JC., Jr CD-NP: an innovative designer natriuretic peptide activator of particulate guanylyl cyclase receptors for cardiorenal disease. Curr Heart Fail Rep. 2010;7:93–99. doi: 10.1007/s11897-010-0016-6. [DOI] [PubMed] [Google Scholar]