Abstract
Background
L-threo-3,4-dihydroxyphenylserine (L-DOPS), a norepinephrine (NE) prodrug, is investigational for orthostatic hypotension, which occurs commonly in Parkinson’s disease. Adjunctive anti-parkinsonian drugs might interact with L-DOPS. We tested whether L-aromatic aminoacid decarboxylase inhibition by carbidopa (CAR) attenuates L-DOPS conversion to NE and blocks the pressor effect of L-DOPS, whereas catechol-O-methyltransferase inhibition by entacapone (ENT) interferes with L-DOPS metabolism and augments the pressor effect.
Methods
Twelve patients with autonomic failure took 400 mg of L-DOPS with 200 mg of placebo (PLA), CAR, or ENT on different days. Plasma L-DOPS, NE, and deaminated NE metabolites (dihydroxyphenylglycol [DHPG], dihydroxymandelic acid [DHMA]) were measured.
Results
L-DOPS+PLA and L-DOPS+ENT increased systolic pressure similarly (by 27 ± 8 and 24 ± 9 mm Hg at 3 hours). L-DOPS+CAR did not increase pressure. The peak increase in plasma NE (0.57 ± 0.11 nmol/L) averaged less than 1/15 000th that in L-DOPS and less than 1/35th that in DHPG+DHMA. CAR prevented and ENT augmented responses of plasma DHPG and DHMA to L-DOPS.
Conclusions
After L-DOPS administration plasma, NE levels do not increase sufficiently to increase blood pressure. Pressor responses to L-DOPS seem to reflect NE produced extraneuronally that escapes extensive enzymatic deamination and O-methylation and evokes vasoconstriction before reaching the systemic circulation.
Keywords: Dihydroxyphenylserine, norepinephrine, dihydroxyphenylglycol, dihydroxymandelic acid, orthostatic hypotension, autonomic failure, Parkinson’s disease, DHPG, DOPS
L-threo-3,4-dihydroxyphenylserine (L-DOPS) is a norepinephrine (NE) prodrug.1,2 Just as L-3,4-dihydroxyphenylalanine (L-DOPA, levodopa) is converted to dopamine via L-aromatic-aminoacid decarboxylase (LAAAD), L-DOPS is converted to NE. After oral ingestion of L-DOPS, circulating L-DOPS is taken up into cells via the neutral amino acid transporter (NAAT, Figure 1) and enzymatically decarboxylated by LAAAD, which is abundant in parenchymal cells of organs such as the liver, kidneys, and gut.
Figure 1.
Steps in the metabolism of L-threo-3,4-dihydroxyphenylserine (L-DOPS). After cellular uptake via the neutral amino acid transporter (NAAT), L-DOPS may be converted by DOPS aldolase to glycine and protocatechualdehyde, by catechol-O-methyltransferase (COMT) to form O-methyl-DOPS (O-Me-DOPS), or by L-aromatic-aminoacid-decarboxylase (LAAAAD) to form norepinephrine (NE). NE is metabolized by monoamine oxidase (MAO) to dihydroxyphenylglycolaldehyde (DOPEGAL). DOPEGAL is converted to dihydroxyphenylglycol (DHPG) by aldose/aldehyde reductase (AR) or to dihydroxymandelic acid (DHMA) by aldehyde dehydrogenase (ALDH). Because DHPG and DHMA are both catechols, they are subject to O-methylation catalyzed by COMT, forming methoxyhydroxyphenylglycol (MHPG) or vanillylmandelic acid (VMA). Carbidopa (CAR) inhibits LAAAD, and entacapone (ENT) inhibits COMT.
Parkinson’s disease features loss of not only nigrostriatal dopaminergic but also locus ceruleus and sympathetic noradrenergic neurons,3,4 and sympathetic noradrenergic denervation manifests as orthostatic hypotension.5 Since 1989, L-DOPS has been marketed in Japan for treatment of Parkinson’s disease and of orthostatic hypotension. For the latter indication, L-DOPS is currently being developed for marketing in the United States. About 40% of patients with Parkinson’s disease have orthostatic hypotension,6 which is associated with and probably results from loss of noradrenergic nerves and failure of baroreflexive modulation of sympathetic noradrenergic outflows.7
Drugs used adjunctively in the treatment of Parkinson’s disease might affect the metabolism and effects of L-DOPS. The present study focused on carbidopa (CAR) and entacapone (ENT), both of which augment the therapeutic effect of levodopa by inhibiting metabolism of levodopa outside the brain.
NE produced from L-DOPS would be expected to undergo oxidative deamination catalyzed by monoamine oxidase (Figure 1) to form a catecholaldehyde, dihydroxyphenylglycolaldehyde (DOPEGAL). DOPEGAL is then converted to dihydroxyphenylglycol (DHPG) via aldehyde/aldose reductase8 or to dihydroxymandelic acid (DHMA) via aldehyde dehydrogenase.9 The sum of DHPG+DHMA therefore provides a measure of deaminated metabolites of NE.
Peripheral LAAAD inhibitors are administered with levodopa in Parkinson’s disease to decrease decarboxylation of levodopa outside the brain. This prevents nausea and vomiting due to dopamine formation outside the brain and increases levodopa availability for dopamine formation from levodopa inside the brain. If L-DOPS were used to treat orthostatic hypotension in Parkinson’s disease, peripheral LAAAD inhibition might diminish the efficiency of conversion of L-DOPS to NE in peripheral tissues. CAR is well known to inhibit LAAAD activity outside the brain and, by attenuating generation of NE from L-DOPS, CAR would be expected diminish increments in plasma levels of NE and of DHPG+ DHMA resulting from L-DOPS treatment.
Peripheral COMT inhibitors are used in Parkinson’s disease to enhance efficacy of levodopa by preventing its O-methylation before levodopa enters brain. Such drugs would be expected to do the same for L-DOPS, enhancing its conversion to NE outside the brain as well as diminishing O-methylation of NE and of deaminated metabolites of NE. This could be of benefit in treating orthostatic hypotension. Because L-DOPS, NE, DHPG, and DHMA are all catechols, they are subject to O-methylation catalyzed by catechol-O-methyltransferase. ENT inhibits catechol-O-methyltransferase outside the brain. Therefore, this drug would be expected to augment responses of plasma levels of L-DOPS as well as of NE and DHPG+DHMA after administration of L-DOPS.
In this study, we examined potential effects of CAR or ENT on the metabolic fate and indirect pressor action of L-DOPS. We expected that L-DOPS would increase plasma levels of NE and of its deaminated metabolites and thereby exert a pressor effect, that CAR would attenuate these neurochemical and physiological effects by inhibiting conversion of L-DOPS to NE via LAAAD, and that ENT would augment them, by inhibiting enzymatic O-methylation of L-DOPS, NE, and deaminated metabolites of NE.
METHODS
Subjects
We studied 12 patients with autonomic failure (mean age 66 ± 3 years, 8 men) in the US National Institutes of Health (NIH) Clinical Center. Seven patients had pure autonomic failure (PAF), 3 Parkinson’s disease, and 2 multiple system atrophy (MSA). All subjects were studied after having given informed consent to a research protocol approved by the Neuro Institutional Review Board of intramural NIH. The study was aborted in 1 of the MSA patients after a serious adverse event unrelated to the experimental procedures or test drugs, and only CAR and ENT data were obtained in that patient.
All the patients had previously been found to have neurogenic orthostatic hypotension in a different clinical research protocol. Orthostatic hypotension was defined by a decrease in systolic blood pressure of at least 20 mm Hg and in diastolic pressure at least 10 mm Hg between supine rest for at least 15 minutes and upright posture for 5 minutes (unless symptomatic or rapid hypotension necessitated return to the supine position before 5 minutes upright). Neurogenic orthostatic hypotension was defined by orthostatic hypotension coupled with abnormal beat-to-beat blood pressure associated with performance of the Valsalva maneuver.10
Drug Sources
Avance GmbH (Basel, Switzerland) provided the L-DOPS used in this study, under a cooperative research and development agreement. Avance GmbH did not design the study, collect or analyze the data, or write any part of this report. Avance GmbH did provide financial support for neurochemical assays.
Levodopa and drugs that inhibit LAAAD or catechol-O-methyltransferase were withdrawn throughout the period of study. For parkinsonism and anxiety related to withdrawal of levodopa, alternative drugs such as serotonin reuptake blockers, antianxiety agents, or dopamine receptor agonists were used if needed at constant doses during the study.
Study Design
Subjects were tested in 3 treatment phases. In each phase, a single oral dose of 400 mg (four 100-mg capsules) of L-DOPS was given at about 10:00 AM, with CAR (200 mg), ENT (200 mg), or placebo (PLA, 200 mg), in 2 identical capsules containing 100 mg of material. Each subject served as his or her own control. Subjects received the test drugs on 3 different days (usually Monday, Thursday, and the following Monday). The sequence of drugs given with L-DOPS was randomized. The Pharmaceutical Development Service of the NIH Clinical Center dispensed L-DOPS, CAR, ENT, and PLA according to a code to which the investigators were blinded until all the hemodynamic and neurochemical data for the subject had been recorded.
Experimental Procedures
On each day, while the patient was supine with his or her head on a pillow, an intravenous (IV) catheter was inserted in an arm vein for drawing blood samples. Electrocardiographic and finger and brachial cuffs were applied for noninvasive measurements of beat-to-beat blood pressure and heart rate. At least 15 minutes after IV catheter insertion, baseline hemodynamic data were collected and a blood sample (about 5 mL) drawn through the IV catheter was transferred to a heparinized glass tube and placed immediately on ice. The patient was then brought to a full upright position using a motorized tilt table, with hemodynamic monitoring continuing. At about 5 minutes (less if the blood pressure was declining rapidly), a blood sample was drawn. The patient was then returned to the supine position. At about 10 AM, the patient then took L-DOPS with the experimental medication orally with water. Hemodynamics were measured and blood sampled again at 1, 2, 3, 6, and 24 hours after administration of L-DOPS with CAR, ENT, or PLA. A blood sample was also obtained through an indwelling IV catheter with the patient supine at 48 hours, without formal hemodynamic measurements. Between evaluation points on the day of drug administration, the IV catheter was kept in place, and the patient was allowed to ambulate and to take a hospital diet; however, the patient lay supine with head on pillow for at least 15 minutes before experimental measurements. To minimize effects of meal ingestion, subjects were allowed to eat 1 or 2 granola bars and drink water between sampling points. Subjects were allowed to drink water ad libitum.
Neurochemical Assays
Plasma levels of catechols were assayed by alumina extraction followed by liquid chromatography with electrochemical detection.11,12 The assay method allowed measurement of plasma levels of DHMA, which had a chromatographic retention time between that of L-DOPS and DHPG.13 Intra-assay coefficients of variation for L-DOPS ranged from 1% to 4%, with a lower limit of detection of about 1 nmol/L.
Data Analysis and Statistics
Differences in neurochemical data between ENT or CAR treatment and PLA treatment were compared by t tests for dependent means. Trends over time were assessed by repeated-measures analyses of variance using Kaleidagraph 4.01 (Synergy Software, Reading, Pa). In the event of empty cells, all data for the subject were cleared. Relationships between hemodynamic and neurochemical measures were assessed by linear regression. Mean values were expressed ± standard error of the mean (SEM). Although it was expected that L-DOPS would increase plasma NE and blood pressure levels, 2-tailed tests were used. A P value less than .05 defined statistical significance.
RESULTS
L-DOPS
In all subjects and for all 3 treatment combinations, plasma L-DOPS levels increased progressively over time after administration of L-DOPS. Peak L-DOPS levels (8898 ± 1961 nmol/L for L-DOPS+PLA) were attained at 3 hours (Figure 2). After 6 hours, plasma L-DOPS decreased in an approximately mono-exponential manner with a half time of about 4 hours. There were no significant differences among the L-DOPS+PLA, L-DOPS+CAR, and L-DOPS+ENT treatments in peak levels or rates of decline of L-DOPS.
Figure 2.
Plasma mean (± SEM) concentrations of (top) L-threo-3,4-dihydroxyphenylserine (L-DOPS) and (bottom) norepinephrine (NE) as functions of time after oral administration of L-DOPS (400 mg) with 200 mg of placebo (PLA, open circles), 200 mg of carbidopa (CAR, black circles), or 200 mg of entacapone (ENT, black squares).
Norepinephrine
Plasma NE increased as a function of time after L-DOPS administration (for L-DOPS+PLA, F = 13.5, P < .0001; for L-DOPS+ENT, F = 8.1, P < .0001); however, for L-DOPS+CAR plasma NE did not change significantly (Figure 2, bottom). For all 3 treatment combinations, the increments in plasma NE levels were only very small fractions of the L-DOPS levels. For instance, at 3 hours after L-DOPS+PLA, plasma NE increased by 0.47 ± 0.16 nmol/L from baseline, less than 1/15000th the corresponding L-DOPS level.
Trends in plasma NE levels differed by little among the 3 treatment groups (Figure 2). At 6 hours after L-DOPS administration, the mean increment in plasma NE was larger after L-DOPS+ENT treatment than after L-DOPS+CAR (P = .03), but plasma NE increments did not differ between the L-DOPS+ENT and L-DOPS+PLA treatments, and peak NE did not differ among the 3 treatments.
Blood Pressure
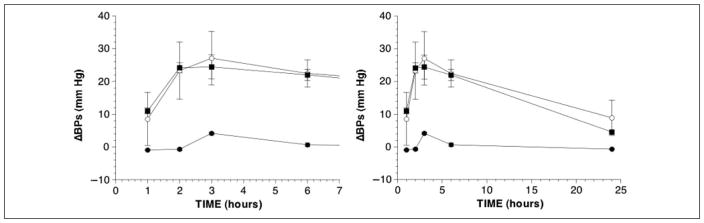
Systolic blood pressure increased after L-DOPS+PLA (F = 2.9, P = .02) and L-DOPS+ENT (F = 3.7, P = .007). At 6 hours, the increments from baseline averaged 25 ± 5 mm Hg for L-DOPS+PLA and 22 ± 5 mm Hg for L-DOPS+ENT. In remarkable contrast, systolic pressure failed to increase after L-DOPS+CAR (Figure 3). Neither ENT nor CAR affected diastolic pressure or heart rate responses to L-DOPS (Table I).
Figure 3.
Mean (± SEM) changes in systolic blood pressure from baseline as functions of time after oral administration of L-threo-3,4-dihydroxyphenylserine (L-DOPS) (400 mg) with 200 mg of placebo (PLA, open circles), 200 mg of carbidopa (CAR, black circles), or 200 mg of entacapone (ENT, black squares).
TABLE I.
Mean (± SEM) Values for Diastolic Blood Pressure and Heart Rate After Oral Administration of 400 mg of L-DOPS, With 200 mg of Placebo (PLA), 200 mg of Carbidopa (CAR), or 200 mg of Entacapone (ENT) in 11 Subjects
| Variable | Baseline | 1 h | 2 h | 3 h | 6 h | 24 h |
|---|---|---|---|---|---|---|
| Diastolic blood pressure, mm Hg | ||||||
| PLA | 83 ± 4 | 87 ± 5 | 90 ± 6 | 90 ± 8 | 91 ± 6 | 86 ± 3 |
| CAR | 84 ± 4 | 81 ± 5 | 82 ± 6 | 85 ± 5 | 84 ± 6 | 84 ± 4 |
| ENT | 79 ± 5 | 87 ± 6 | 93 ± 5 | 91 ± 7 | 91 ± 5 | 82 ± 4 |
| Heart rate, beats/min | ||||||
| PLA | 66 ± 3 | 65 ± 4 | 67 ± 3 | 69 ± 5 | 67 ± 4 | 66 ± 3 |
| CAR | 66 ± 3 | 69 ± 4 | 66 ± 3 | 65 ± 3 | 61 ± 3 | 66 ± 3 |
| ENT | 68 ± 3 | 65 ± 2 | 66 ± 4 | 64 ± 4 | 68 ± 3 | 68 ± 4 |
Deaminated NE Metabolites
In contrast with small increases in plasma NE levels, there were robust increases in plasma DHPG levels after both L-DOPS+PLA (F = 3.74, P = .003) and L-DOPS+ENT (F = 12.1, P < .0001; Figure 4, top). Plasma DHPG did not increase after L-DOPS+CAR (Figure 4). Peak increments in plasma DHPG were larger with L-DOPS+ENT than L-DOPS+PLA (P = .001).
Figure 4.
Plasma mean (± SEM) concentrations of (top) dihydroxyphenylglycol (DHPG) and (bottom) dihydroxymandelic acid (DHMA) as functions of time after oral administration of L-threo-3,4-dihydroxyphenylserine (L-DOPS) (400 mg) with 200 mg of placebo (PLA, open circles), 200 mg of carbidopa (CAR, black circles), or 200 mg of entacapone (ENT, black squares).
Plasma DHMA also clearly increased after L-DOPS administration (for L-DOPS+PLA, F = 4.4, P = .0002; Figure 4, bottom). Levels of plasma DHMA continued to increase between 3 and 6 hours after L-DOPS, whereas levels of DHPG decreased in L-DOPS+ENT or did not change significantly in L-DOPS+PLA treated groups.
The sum of DHPG+DHMA, representing deaminated metabolites of NE, increased remarkably after L-DOPS administration (for L-DOPS+PLA, F = 4.6, P = .0007). Administration of L-DOPS+CAR virtually abolished DHPG+DHMA responses to L-DOPS, whereas ENT augmented them.
Across the 6 testing times from baseline through 24 hours after L-DOPS administration, mean systolic pressure varied with mean plasma NE (for L-DOPS+ PLA, r = 0.91, P = .01), and pressor responses varied with changes in plasma NE from baseline (r = 0.90, P = .04). Analogously, systolic pressure varied with plasma DHPG (r = 0.93, P = .006) and pressor responses with DHPG responses (r = 0.94, P = .02). As expected for a precursor–product relationship, mean changes in plasma DHPG from baseline correlated with mean changes in plasma NE from baseline (r = 0.98, P = .0008). The line of best fit for the relationship between the mean increment in systolic pressure and the mean increment in plasma NE was shifted downward for the L-DOPS+CAR treatment compared with the L-DOPS+PLA and L-DOPS+ENT treatments (Figure 5, left). The line of best fit for the relationship between the mean increment in systolic pressure and the mean increment in plasma DHPG was shifted to the right for the L-DOPS+ENT treatment compared with L-DOPS+PLA (Figure 5, right).
Figure 5.
Mean (± SEM) changes in systolic blood pressure from baseline as functions of (left) changes in plasma norepinephrine (NE) from baseline and (right) changes in plasma dihydroxyphenylglycol (DHPG) from baseline after oral administration of L-threo-3,4-dihydroxyphenylserine (L-DOPS) (400 mg) with 200 mg of placebo (PLA, open circles), 200 mg of carbidopa (CAR, black circles), or 200 mg of entacapone (ENT, black squares). In the left panel, lines of best fit for ENT have long dashes, for PLA short dashes, and for CAR are solid.
DISCUSSION
Although the results of this study generally confirm that in humans oral L-DOPS acts as an NE prodrug that raises blood pressure,1 increments in plasma NE levels after L-DOPS administration were remarkably small—too small to explain the pressor effect of the drug. As discussed below, the findings indicate that extensive metabolism of NE produced mainly extraneuronally from L-DOPS severely limits plasma NE responses to L-DOPS, leading us to propose that NE produced from L-DOPS acts on cardiovascular adrenoceptors to raise blood pressure before NE reaches the systemic circulation.
From the approximately monoexponential decline in plasma L-DOPS (Figure 2), the half-time for removal of L-DOPS from the plasma is about 4 hours, in line with previous findings.11 The corresponding half-time for NE is about 1.5 minutes.14 Based on these differences, the expected steady-state concentration of L-DOPS would be about 240/1.5 or 160 times the steady-state increment in the concentration of NE. Instead, with L-DOPS+PLA treatment, the actual values were 8898 and 0.47 nmol/L at 3 hours (L-DOPS:NE ratio 18,931) and 7555 and 0.57 nmol/L at 6 hours (ratio 13,254). The observed ratios were therefore 83 to 118 times those expected from the difference in plasma clearances alone. From these calculations, we infer that virtually all of the NE formed from cytoplasmic L-DOPS is stored or metabolized, and only about 1% enters the systemic circulation unchanged.
The data about plasma DHPG and DHMA levels and effects of ENT on those levels indicate that monoamine oxidase and catechol-O-methyltransferase metabolize most of the NE formed from L-DOPS. In contrast with plasma NE, plasma DHPG and DHMA levels increased markedly after L-DOPS administration. By far the main neuronal metabolite of NE is DHPG.15 Therefore, the finding of large DHMA responses to L-DOPS suggests metabolism of NE after extraneuronal uptake of L-DOPS via the neutral amino acid transporter and subsequent intracellular conversion of L-DOPS to NE. Because sympathetic nerves do not express catechol-O-methyltransferase, the inhibition of catechol-O-methyltransferase by ENT-augmented plasma DHPG responses provides additional support for the notion of mainly extraneuronal production and metabolism of NE derived from L-DOPS.
The continuing rise of DHMA levels while plasma levels of DHPG fell or did not change was consistent with conversion of DHPG to DHMA, justifying use of their summed levels as an index on NE deamination. The peak increment in plasma DHMA+DHPG with L-DOPS+PLA treatment averaged 23.1 nmol/L, 35 times the mean peak increment in plasma NE (0.66 nmol/L). Assuming similar plasma clearances of NE, DHMA, and DHPG, these findings suggest that after NE is formed from L-DOPS, about 97% undergoes oxidative deamination. With the L-DOPS+ENT treatment, the peak increments in plasma DHPG+DHMA and NE averaged 41.3 and 0.77 nmol/L, corresponding to a 54-fold difference. Comparison of the L-DOPS+ENT results with the L-DOPS+PLA results therefore suggests that substantial proportions of the DHPG and DHMA derived from L-DOPS via NE undergo enzymatic O-methylation. Thus, monoamine oxidase with or without catechol-O-methyltransferase figures very prominently in the fate of NE made from L-DOPS. The similarity in plasma NE responses after L-DOPS+ENT and after L-DOPS+PLA suggests that relatively little of circulating NE formed from L-DOPS is O-methylated. Taken together, intracellular metabolism can explain why after L-DOPS administration, increments in plasma NE levels are very small.
Patients with chronic autonomic failure have severe attenuation of baroreflexes. When NE is infused intravenously into such patients, they have exaggerated pressor responses.16 Denervation super-sensitivity of α-adrenoceptors in patients with Parkinson’s disease or pure autonomic failure would be expected to augment further the pressor responses for given circulating NE levels.17 Even so, at 3 hours after L-DOPS+PLA treatment, the observation of a systolic pressure response averaging 27 mm Hg when the mean increment in plasma NE was only 0.47 nmol/L leads us to propose that the pressor response to L-DOPS depends on NE exiting the cells in which it is produced and acting on α-adrenoceptors on nearby cardiovascular smooth muscles to produce a pressor effect before the NE reaches the systemic circulation. Moreover, NE delivered from mesenteric organs to the liver via the portal vein would undergo extensive hepatic metabolism before the NE could reach the systemic circulation.
The present study included autonomic failure patients in different diagnostic groups that might have different neurochemical and hemodynamic responses to L-DOPS. In particular, patients with generalized sympathetic denervation could have augmented pressor responses for given NE responses because of denervation supersensitivity; and patients on an inhibitor of monoamine oxidase could have augmented NE and pressor responses due to metabolism by monoamine oxidase of NE produced from L-DOPS.
CAR at a dose of 200 mg virtually abolished systolic pressure and plasma DHPG and DHMA responses to L-DOPS, indicating that CAR effectively inhibited LAAAD. Much lower doses are normally used for adjunctive treatment of Parkinson’s disease. Therefore, the present results do not exclude L-DOPS raising blood pressure in patients on levodopa–carbidopa combinations. In chronic autonomic failure, blood pressure often increases during the day. Because the present study did not include a control treatment with PLA alone, the present results also cannot exclude time-related increases in blood pressure independent of L-DOPS treatment. Given the small, variable, and transient pressor responses to L-DOPS+CAR treatment, effects of time of day are likely to be minor.
L-DOPS is a catechol and is known to be a substrate for catechol-O-methyltransferase; however, neither CAR nor ENT significantly augmented peak L-DOPS levels or prolonged the half-time of decline in plasma L-DOPS. These findings are consistent with catechol-O-methyltransferase and other enzymes acting as an intracellular sink for L-DOPS taken up from the circulation.
Oxidative deamination of NE produced from cytoplasmic L-DOPS results in formation of the catecholaldehyde, DOPEGAL, and hydrogen peroxide, and the action of aldehyde dehydrogenase on DOPEGAL to form DHMA also generates hydrogen peroxide. The present findings of large increases in plasma DHPG and DHMA levels after L-DOPS administration therefore imply that L-DOPS leads to substantial generation of hydrogen peroxide, which is well known to be an oxidative stressor. DOPEGAL itself also may be cytotoxic.18 Because oxidative deamination with attendant hydrogen peroxide and catecholaldehyde production seems to be a major route of metabolism of NE produced from L-DOPS, future studies should consider possible toxicity from oxidative stress during long-term L-DOPS treatment.
In conclusion, L-DOPS administration elicits increases in blood pressure and correlated increases in plasma levels of NE and its metabolites, consistent with L-DOPS acting as an NE prodrug. Only a very small proportion of L-DOPS leads to NE in the systemic circulation, because NE generated extraneuronally from L-DOPS undergoes substantial intracellular metabolism. Although plasma DHPG normally reflects neuronal metabolism of NE,19 after L-DOPS administration increments in plasma DHPG (and DHMA) probably reflect extraneuronal production and metabolism of NE derived from L-DOPS. Accordingly, the pressor response to L-DOPS may depend on effects of NE that take place before NE enters the systemic circulation. A combination of L-DOPS with an inhibitor of monoamine oxidase might enhance therapeutic effects of L-DOPS on blood pressure and mitigate theoretically possible toxic effects of L-DOPS.
Footnotes
Financial disclosure: The research reported here was supported by the Division of Intramural Research of the National Institute of Neurological Disorders and Stroke, US National Institutes of Health, Bethesda, Maryland. Avance GmbH (Basel, Switzerland) provided financial support for neurochemical assays.
References
- 1.Kaufmann H, Saadia D, Voustianiouk A, et al. Norepinephrine precursor therapy in neurogenic orthostatic hypotension. Circulation. 2003;108:724–728. doi: 10.1161/01.CIR.0000083721.49847.D7. [DOI] [PubMed] [Google Scholar]
- 2.Blaschko H, Burn JH, Langemann H. The formation of noradrenaline from dihydroxyphenylserine. Br J Pharmacol Chemother. 1950;5:431–437. doi: 10.1111/j.1476-5381.1950.tb00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- 4.Amino T, Orimo S, Takahashi A, Uchihara T, Mizusawa H. Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Pathol. 2005;15:29–34. doi: 10.1111/j.1750-3639.2005.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziegler MG, Lake CR, Kopin IJ. The sympathetic-nervous-system defect in primary orthostatic hypotension. N Engl J Med. 1977;296:293–297. doi: 10.1056/NEJM197702102960601. [DOI] [PubMed] [Google Scholar]
- 6.Allcock LM, Ullyart K, Kenny RA, Burn DJ. Frequency of orthostatic hypotension in a community based cohort of patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1470–1471. doi: 10.1136/jnnp.2003.029413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein DS, Eldadah BA, Holmes C, et al. Neurocirculatory abnormalities in Parkinson disease with orthostatic hypotension: independence from levodopa treatment. Hypertension. 2005;46:1–7. doi: 10.1161/01.HYP.0000188052.69549.e4. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura M, Eisenhofer G, Kopin IJ, et al. Aldose reductase: an aldehyde scavenging enzyme in the intraneuronal metabolism of norepinephrine in human sympathetic ganglia. Auton Neurosci. 2002;96:131–139. doi: 10.1016/s1566-0702(01)00385-x. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhofer G, Goldstein DS, Stull R, Ropchak TG, Keiser HR, Kopin IJ. Dihydroxyphenylglycol and dihydroxymandelic acid during intravenous infusions of noradrenaline. Clin Sci. 1987;73:123–125. doi: 10.1042/cs0730123. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein DS, Tack C. Non-invasive detection of sympathetic neurocirculatory failure. Clin Auton Res. 2000;10:285–291. doi: 10.1007/BF02281111. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein DS, Holmes C, Kaufmann H, Freeman R. Clinical pharmacokinetics of the norepinephrine precursor L-threo-DOPS in primary chronic autonomic failure. Clin Auton Res. 2004;14:363–368. doi: 10.1007/s10286-004-0221-z. [DOI] [PubMed] [Google Scholar]
- 12.Holmes C, Eisenhofer G, Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatog B Biomed Applic. 1994;653:131–138. doi: 10.1016/0378-4347(93)e0430-x. [DOI] [PubMed] [Google Scholar]
- 13.Kawamura M, Kopin IJ, Kador PF, Sato S, Tjurmina O, Eisenhofer G. Effects of aldehyde/aldose reductase inhibition on neuronal metabolism of norepinephrine. J Auton Nerv Syst. 1997;66:145–148. doi: 10.1016/s0165-1838(97)00086-6. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein D, Horwitz D, Keiser HR, Polinsky RJ, Kopin IJ. Plasma l-[3H]norepinephrine, d-[14C]norepinephrine, and d,l-[3H]isoproterenol kinetics in essential hypertension. J Clin Invest. 1983;72:1748–1758. doi: 10.1172/JCI111134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhofer G, Goldstein DS, Ropchak TG, Nguyen HQ, Keiser HR, Kopin IJ. Source and physiological significance of plasma 3,4-dihydroxyphenylglycol and 3-methoxy-4-hydroxyphenylglycol. J Auton Nerv Syst. 1988;24:1–14. doi: 10.1016/0165-1838(88)90130-0. [DOI] [PubMed] [Google Scholar]
- 16.Polinsky RJ, Kopin IJ, Ebert MH, Weise V. Pharmacologic distinction of different orthostatic hypotension syndromes. Neurology. 1981;31:1–7. doi: 10.1212/wnl.31.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Senard JM, Valet P, Durrieu G, et al. Adrenergic supersensitivity in parkinsonians with orthostatic hypotension. Eur J Clin Invest. 1990;20:613–619. doi: 10.1111/j.1365-2362.1990.tb01909.x. [DOI] [PubMed] [Google Scholar]
- 18.Burke WJ, Li SW, Chung HD, et al. Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: Role in neurodegenerative diseases. Neurotoxicology. 2004;25:101–115. doi: 10.1016/S0161-813X(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein DS, Eisenhofer G, Stull R, Folio CJ, Keiser HR, Kopin IJ. Plasma dihydroxyphenylglycol and the intraneuronal disposition of norepinephrine in humans. J Clin Invest. 1988;81:213–220. doi: 10.1172/JCI113298. [DOI] [PMC free article] [PubMed] [Google Scholar]