SUMMARY
The term “neurocardiology” refers to physiologic and pathophysiological interplays of the nervous and cardiovascular systems. This selective review provides an update about cardiovascular therapeutic implications of neurocardiology, with emphasis on disorders involving primary or secondary abnormalities of catecholamine systems. Concepts of scientific integrative medicine help understand these disorders. Scientific integrative medicine is not a treatment method or discipline but a way of thinking that applies systems concepts to acute and chronic disorders of regulation. Some of these concepts include stability by negative feedback regulation, multiple effectors, effector sharing, instability by positive feedback loops, allostasis, and allostatic load. Scientific integrative medicine builds on systems biology but is also distinct in several ways. A large variety of drugs and non-drug treatments are now available or under study for neurocardiologic disorders in which catecholamine systems are hyperfunctional or hypofunctional. The future of therapeutics in neurocardiology is not so much in new curative drugs as in applying scientific integrative medical ideas that take into account concurrent chronic degenerative disorders and interactions of multiple drug and non-drug treatments with each other and with those disorders.
Keywords: Autonomic nervous system, dysautonomia, neurocardiology, norepinephrine, sympathetic nervous system
Introduction: What is Neurocardiology?
“Neurocardiology” refers to physiological and pathophysiological interplays of the nervous and cardiovascular systems. Neurocardiology is therefore essentially interdisciplinary.
Neurocardiology is evolving as a discipline in clinical medicine. From initial emphases on behavior patterns in coronary artery disease [1] and the role of stress in hypertension [2], the field has by now expanded greatly to include many primary or secondary hyperfunctional and hypofunctional abnormalities of catecholamine systems (Table 1) that relate importantly to cardiovascular therapeutics.
Table 1.
Neurocardiologic disorders that feature abnormal catecholaminergic function
| Disorders in which physiologic changes in catecholaminergic system function worsen an independent pathologic state |
|---|
| Cardiovascular |
| Myocardial ischemia and infarction |
| Arrhythmias |
| Angina pectoris |
| Coronary spasm |
| Heart failure |
| Generalized and cardiac sympathoneural activation |
| Myocardial norepinephrine depletion |
| Prognosis |
| Arrhythmias and sudden death |
| Catecholaminergic polymorphic ventricular tachycardia (CPVT) |
| Psychiatric |
| Depression |
| Panic/anxiety |
| Renal disease |
| End stage renal disease |
| Chronic renal failure |
| Endocrine/metabolic |
| Hypothyroidism |
| Obesity, diabetes, and “Metabolic Syndrome” |
| Disorders where abnormal catecholaminergic function is etiologic |
| Hypofunctional states without central neurodegeneration |
| Acute, primary |
| Neurocardiogenic syncope |
| Spinal cord transection |
| Acute pandysautonomia |
| Sympathectomy |
| Acute, secondary |
| Drug-related (e.g., alcohol, tricyclic antidepressant, chemotherapy, opiate, barbiturates, benzodiazepines, sympatholytics, general anesthesia) |
| Seizures |
| Guillain–Barre syndrome |
| Alcohol |
| Chronic, primary |
| Pure autonomic failure |
| Horner’s syndrome |
| Familial dysautonomia |
| Carotid sinus syncope |
| Adie’s syndrome |
| Dopamine-β-hydroxylase deficiency |
| Sympathectomy |
| Chronic, secondary |
| Autonomic failure with peripheral neuropathy |
| Amyloid polyneuropathy |
| Hereditary amyloid polyneuropathy |
| Diabetic neuropathy |
| Diabetic autonomic neuropathy |
| Painful diabetic neuropathy |
| Quadriplegia |
| Chagas disease |
| Tabes dorsalis |
| Hyperfunctional states without central neurodegeneration |
| Acute, primary |
| Panic/anxiety |
| Acute, Secondary |
| Drug-related (e.g., nicotine, caffeine, cocaine, amphetamine, opiate withdrawal, tyramine “cheese effect”) |
| Stroke-related myocardial necrosis |
| Seizures |
| Guillain–Barre syndrome |
| Post-endarterectomy |
| Tetanus |
| Chronic, primary |
| Hypertension |
| Neurogenic hypertension |
| Baroreflex failure |
| Pheochromocytoma |
| Sporadic |
| Familial |
| Multiple endocrine neoplasia type II von Hippel–Lindau disease |
| Neurofibromatosis type I |
| Carotid body tumor |
| Hypofunctional states with central neurodegeneration |
| Multiple system atrophy (MSA) |
| MSA with sympathetic neurocirculatory failure (Shy–Drager Syndrome) |
| MSA with isolated parasympathetic failure |
| Parkinson’s disease with autonomic failure |
| Neurogenetic diseases |
| Genetic diseases with specific catecholaminergic phenotypes: synthesis |
| Tyrosine hydroxylase deficiency |
| Dihydropteridine reductase deficiency |
| L-DOPA-responsive dystonia |
| L-Aromatic-amino-acid decarboxylase deficiency |
| Menkes disease |
| Genetic diseases with specific catecholaminergic phenotypes: metabolism |
| Monoamine oxidase deficiency |
| Pseudopheochromocytoma |
| Mysterious or controversial entities |
| Chronic orthstatic intolerance |
| Postural Tachycardia syndrome (POTS) |
| Hyperdynamic circulation syndrome |
| Hyperadrenergic orthostatic intolerance |
| Mitral valve prolapse-dysautonomia syndrome |
| Chronic fatigue syndrome |
| Neurocardiogenic syncope |
| Neurasthenia |
| Chronic regional pain syndrome |
| Reflex sympathetic dystrophy |
| Fibromyalgia |
| Posttraumatic stress disorder |
| Human essential hypertension |
| “Type A” coronary-prone behavior pattern |
This review is not and cannot be comprehensive. The main purpose is not to describe in detail diagnostic and therapeutic strategies for each of the numerous common and rare neurocardiologic disorders but to convey a conceptual framework. The reader will find that large subject areas such as metabolic syndrome, cardiorenal failure, and dialysis-related cardiovascular morbidity, all of which include neurocardiologic facets, receive barely any mention. Instead, several disorders have been chosen to exemplify particular principles of scientific integrative medicine as applied to neurocardiology.
Catecholamine Systems and Neurocardiologic Disorders
In humans, the endogenous catecholamines are norepinephrine (NE), epinephrine (EPI), and dopamine (DA). NE is the main neurotransmitter mediating cardiovascular regulation by the sympathetic nervous system and is a major neurotransmitter in the central nervous system. EPI is the powerful chemical effector of the adrenomedullary hormonal system. DA is a key central neurotransmitter, is released with NE during sympathetically mediated exocytosis [3], and is an auto-paracrine effector [4].
Catecholamines not only can precipitate acute events such as stress cardiopathy [5] and emotion-related sudden death [6] but also contribute to chronic conditions such as metabolic syndrome [7] and hypertension [8,9]. Progressive degenerative loss of catecholamine neurons in the brain and periphery underlies the movement disorder and dysautonomia that characterize Parkinson disease (PD) [10,11].
Concepts of Scientific Integrative Medicine
Scientific integrative medicine is not a treatment method or discipline but a way of thinking that applies systems concepts to understand clinical disorders. Multiple simultaneous degenerations combined with effects of multiple drugs and remedies and myriad interactions between the degenerations and the treatments constitute the bulk of modern medical practice. The scientific integrative medicine approach provides a framework for understanding highly complex and dynamic challenges to our integrity as organisms and in turn for developing novel treatments based on this complexity and dynamism [12].
Negative Feedback Regulation
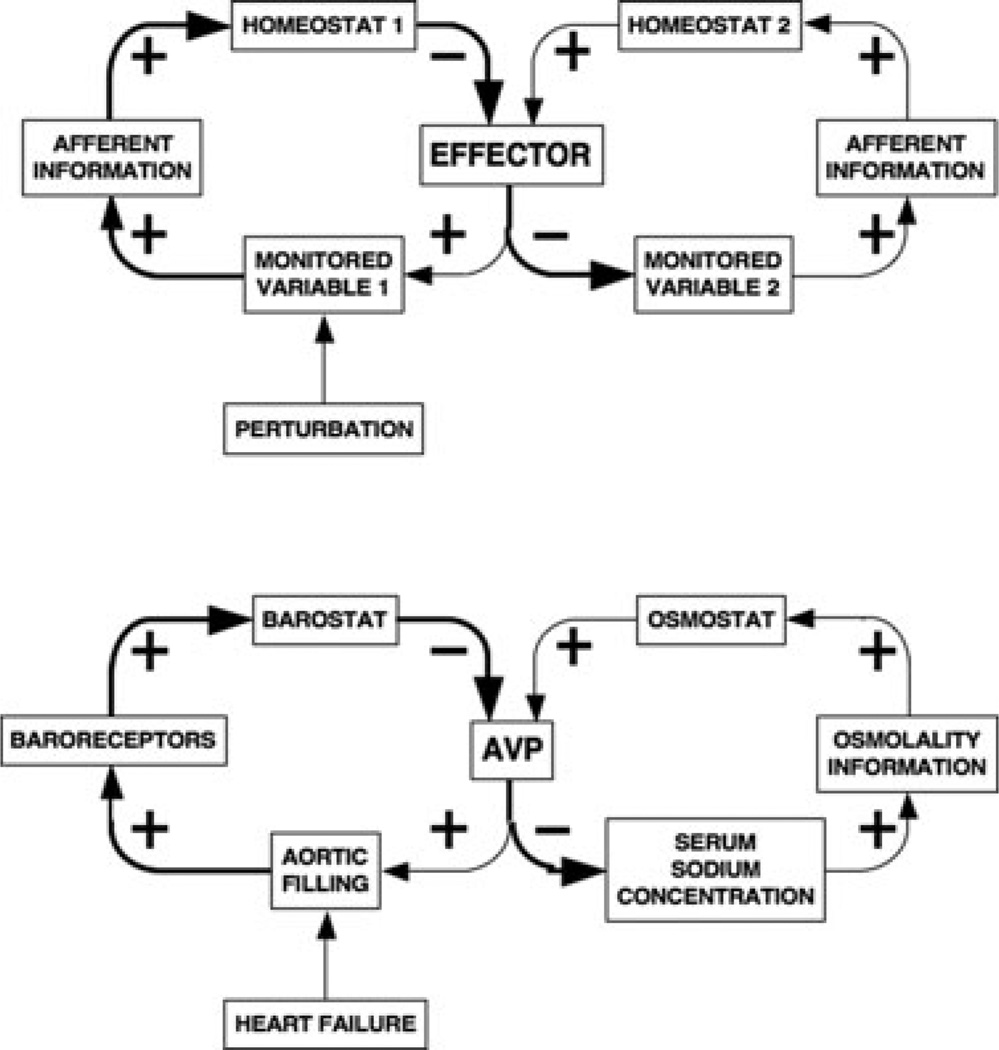
Physiological homeostatic systems entail negative feedback regulation of numerous monitored variables, including core temperature, blood pressure, serum osmolality, glucose levels, and metabolic rate. Conceptually, each system depends on a comparator—a homeostat—to compare afferent information about the monitored variables with set points or other criteria for responding (Figure 1).
Figure 1.
A physiological homeostatic system. As the level of the monitored variable changes, afferent information is compared with a set point or other algorithm for responding, and the sensed discrepancy leads to altered activities of effectors. Note the odd number of (−) signs, indicating a negative feedback loop. In response to a continuous perturbation, the level of the monitored variable reaches an apparent steady state.
Disruption of a negative feedback loop, by preventing afferent information from reaching the brain, inability to process the information and regulate effector functions correctly, or dysfunction or loss of effectors, increases fluctuations in levels of monitored variables [13]. Positive feedback loops are inherently unstable, and conversion from a negative to a positive neurocirculatory feedback loop presages rapid decompensation. For instance, one can understand transitions from heat stress to heat shock and from compensated to decompensated heart failure in terms of positive feedback loops.
Multiple Effectors
Multiple effectors regulate levels of most monitored variables of the body (Figure 2). Having available multiple effectors extends the range of control, allows at least some regulation of the monitored variable if a particular effector fails (compensatory activation), and enables elaboration of specific, adaptive effector patterns. Thus, the sympathetic noradrenergic system, adrenomedullary hormonal system, and hypothalamic–pituitary–thyroid axis are effectors for regulation of core temperature, and hypophysectomy, thyroidectomy, and hypothyroidism all activate the sympathetic noradrenergic system compensatorily [14,15].
Figure 2.
Compensatory activation. One advantage of multiple effectors is compensatory activation of alternative effectors if one effector fails, enabling control of the monitored variable. For instance, thyroidectomy augments sympathetic nervous system (SNS) responses to cold exposure.
Compensatory activation of sympathetic nerves in the heart can for long periods be a major source of homeostasis in the face of intrinsic cardiovascular degeneration. Eventually, however, the same activation can induce neurocirculatory positive feedback loops, resulting in cardiovascular instability, rapid worsening of clinical status, and death. Associations of poor prognosis with a high rate of appearance of NE in the coronary sinus (cardiac NE spillover), a large arterial-cardiac venous increment in plasma NE, or decreased uptake and increased washout of cardiac 123I-metaiodobenzylguanidine-derived radioactivity occur in diverse disorders including congestive heart failure [16,17], ventricular arrhythmias [18], dilated cardiomyopathy [19], diabetes mellitus [20], metabolic syndrome [21], and chronic renal failure [22] and fit with this notion.
Effector Sharing
Different homeostatic systems can share effectors (Figure 3). Sharing of the adrenomedullary hormonal system by the barostat and glucostat can explain hyperglycemia in any of several emergencies such as hemorrhagic shock [23], stroke [24], sepsis [25], and myocardial infarction [26]. Not surprisingly, treatment of hyperglycemia by insulin infusion in these situations does not improve outcome. Analogously, sharing of the vasopressin system by the barostat and osmostat can explain hyponatremia in heart failure [27,28]. From the principle of effector sharing one may predict that the most efficient means to reverse hyperglycemia and hyponatremia in these conditions is to treat the underlying cause.
Figure 3.
Effector sharing. Sharing of an effector by multiple homeostats can explain unpredicted consequences and syndromic features of disease processes. For instance, in heart failure, decreased aortic filling increases levels of vasopressin (AVP), which, as the anti-diuretic hormone, promotes retention of free water, explaining hyponatremia attending heart failure.
Stress, Allostasis, and Allostatic Load
The “homeostat” theory defines stress as a condition in which expectations, whether genetically programmed, established by prior learning, or deduced from circumstances, do not match current or anticipated perceptions of the internal or external environment, and the discrepancy elicits patterned, compensatory responses [12]. One can conceptualize stress in terms of an error signal that reflects the difference between afferent information about conditions as sensed and a set point for responding that is determined by a regulator [29], as shown in Figure 4.
Figure 4.
Homeostatic definitions of stress and allostatic load. In stress the organism senses a discrepancy between afferent information about a monitored variable and a set point and other instructions for responding, altering activities of effectors to decrease the discrepancy. Allostatic load reflects wear and tear, which, if sustained and substantial enough, decreases effector efficiency, further activating the effector and accelerating wear and tear. Allostatic load can therefore eventuate in a destabilizing and pathologic positive feedback loop.
Steady state levels of monitored variable can be modified by changing the set point or other instructions for responding. Allostasis refers to this “other sameness.” The flexibility comes at the cost of wear and tear—allostatic load (Figure 4). Allostatic load can lead eventually to a positive feedback loop and rapid system failure.
By way of analogy, suppose you went on sabbatical for a year and that when you left you forgot to close a large window in your home. The temperature would be controlled at the programmed settings, but the air conditioner would be on more in the summer and the furnace would be on more in the winter. With these appliances being on more of the time there would be more wear and tear on them, and they would eventually become less efficient. This means they would be on more of the time, and this would accelerate the wear and tear—a positive feedback loop. In fact, when you returned, you might find that the entire heating and cooling system had failed. According to the notion of allostatic load, it is by way of prolonged activation of effectors to maintain allostasis that chronic stress can contribute to the development of chronic degenerative diseases.
Applications to Neurocardiologic Disorders
Heart Attack and Sudden Death
Patients with acute myocardial infarction have a high risk of lethal arrhythmias, reflecting activation of catecholamine systems both as compensatory responses to decreased cardiac pumping efficiency and as autonomic concomitants of distress. High circulating EPI levels and augmented NE release from cardiac sympathetic nerves increase ventricular automaticity. EPI decreases serum potassium levels [30], adding to arrhythmogenicity.
Takotsubo Cardiopathy
Takotsubo cardiomyopathy refers to a relatively recently described form of acute, reversible cardiomyopathy in which apical akinesia gives the heart the shape of a takotsubo, a Japanese fishing pot for trapping octopus [31]. Takotsubo cardiomyopathy occurs with relatively high incidence in elderly women soon after exposure to severe emotional distress [5]. Symptoms mimic acute myocardial infarction; however, coronary angiography fails to demonstrate coronary occlusion. The condition can trigger sudden cardiac failure or death, yet in survivors cardiac function typically normalizes within a few weeks. Some patients seem susceptible to repeated episodes. This is an area of current research.
Takotsubo cardiomyopathy features remarkably high plasma catecholamine levels [5] and depressed cardiac contractile function. Proposed pathogenetic mechanisms include coronary microvascular spasm (resulting at least partly from coronary sympathetic nervous activation) and cardiotoxicity from neuronal NE and hormonal EPI [31], which may interact to precipitate multiple positive feedback loops.
Heart Failure
Heart failure entails markedly increased cardiac sympathetic nerve traffic [32] and therefore increased delivery of NE to myocardial cells. High local catecholamine levels promote ventricular hypertrophy and predispose to arrhythmias. Both increased wall stiffness and arrhythmias worsen the heart’s pumping efficiency, in turn reflexively evoking further increases in sympathetic nerve traffic to the heart—a positive feedback loop.
Catecholamine increases the work of the heart. In a patient with coronary stenoses, the rate of oxygen utilization may exceed that of oxygen delivery via coronary perfusion. Lack of oxygen delivery to the sympathetic nerves themselves in the heart tends to make their storage vesicles “leaky,” augmenting NE release even for the same rate of sympathetic nerve traffic [33].
Increased sympathetic nervous system outflows augment cardiac filling because of decreased venous capacitance, direct and indirect sodium-retaining effects, and increased total peripheral resistance. Cardiac overfilling leads to accumulation of fluid in the lungs, producing hypoxemia, acidemia, and distress, all of which stimulate catecholamine systems, worsening cardiac overfilling. As noted above for takotsubo cardiopathy, acute severely increased catecholamine levels can decrease rather than increase cardiac pumping efficiency, precipitating a rapidly life-threatening positive feedback loop manifesting in fulminant pulmonary edema.
Not surprisingly, in congestive heart failure the plasma NE level constitutes an independent prognostic factor and correlates with functional status [34,35].
Hyponatremia occurs commonly in heart failure. One way to conceptualize the basis for this association is from sharing of the vasopressin/anti-diuretic hormone (ADH) effector by two homeostats, the barostat and the osmostat. Decreased efficiency of cardiac ejection releases the vasopressin system from baroreflex restraint, and vasopressin levels in the bloodstream increase. Because of increased vasopressin levels, the kidneys retain “free water,” and serum osmolality and serum sodium concentrations fall. The most appropriate treatment for hyponatremia attending heart failure is not hypertonic saline (which could precipitate pulmonary edema) or water restriction but alleviation of the heart failure.
Hypertension
The principle of multiple effectors can help explain the perennial controversy about the role of the sympathetic noradrenergic and adrenomedullary hormonal systems in the development and maintenance of essential hypertension [9,36,37]. Multiple effectors determine blood pressure, and disruption of sympathetic outflow to study its pathophysiological role leads to compensatory activation of the other effectors.
Researchers have debated for many years whether baroreceptor “debuffering” increases “resting” blood pressure—that is, whether debuffering produces a form of neurogenic hypertension. Increased sympathetic outflow to the kidneys seems to be a major determinant of the renal function curve that relates natriuresis to renal perfusion pressure [38,39]. Release of renal sympathetic outflow from baroreceptor restraint could lead to a long-term increase in blood pressure by resetting the renal function curve and compensatorily activating the renin–angiotensin–aldosterone system.
Two recent developments involve devices rather than drugs to treat chronic hypertension. One is based on afferent baroreflex activation and the other on renal sympathetic denervation. Chronic carotid sinus electrical stimulation produces sustained decreases in blood pressure in hypertensive dogs, even during adrenergic blockade [40]. Clinical trials of a carotid sinus stimulator for resistant hypertension are currently under way. A recent acute study of hypertensive patients reported a rapid depressor response to carotid stimulation that was associated with sympathoinhibition [41]. Although muscle sympathetic nerve activity decreased sharply beginning soon after the start of the stimulation, sympathetic activity subsequently increased toward baseline. A catheter-based system for radiofrequency ablation of renal sympathetic innervation has been introduced, also for resistant hypertension [42]. This approach is derived from substantial preclinical literature that renal sympathetic nerves contribute to sodium retention and blood pressure especially during stress. Renal adrenergic stimulation augments activity of the renin– angiotensin–aldosterone system, and in the central nervous system angiotensin modulates baroreflex regulation of renal sympathetic neural outflow [43]. Given the multiplicity of effectors regulating blood pressure one may predict substantial interindividual variability and complex determinants of efficacy of both these devices.
Baroreflex Failure
In humans, baroreceptor “debuffering” by surgical denervation or local anesthesia of the carotid sinus area increases blood pressure acutely and concurrently increases values for indices of sympathetic nervous system activity. Irradiation-induced arterial baroreceptor denervation, while predisposing to episodes of paroxysmal hypertension and very high plasma NE levels, does not necessarily produce sustained hypertension [13]. On the other hand, as noted above carotid sinus electrical stimulation produces chronic decreases in blood pressure in hypertensive dogs.
Patients with orthostatic hypotension (OH) in the setting of chronic autonomic failure typically have supine hypertension, which can be quite severe. In general, drugs that raise blood pressure during orthostasis worsen supine hypertension, posing a major therapeutic challenge. OH in such patients is associated with baroreflex-cardiovagal and baroreflex-sympathoneural failure. Conversely, because of inability to modulate release of NE reflexively from sympathetic nerves, baroreflex failure likely is a key determinant of OH. In addition, patients with pure autonomic failure or Parkinson disease with orthostatic hypotension have evidence for loss of cardiac and extra-cardiac noradrenergic innervation, which likely interacts with baroreflex failure to produce OH. Dysfunctional baroreflexes might also contribute to deleterious sympathetic stimulation in heart failure [44].
Beginning in the early 1980s, attempts were made to develop a “prosthetic baroreceptor” that could prevent or ameliorate OH by controlled i.v. infusion of NE [45]. More recent case reports have noted sometimes spectacular improvement in orthostatic tolerance by patient-controlled i.v. infusion of pressors without continuous pressure monitoring [46]. Development of a pharmacologic or electronic [47] system to modulate or replace baroreflexes would be a major step forward for treating combined OH with supine hypertension and possibly heart failure [48].
Primary Neurocardiologic Disorders
Pheochromocytoma
The clearest evidence for a role of high circulating levels of catecholamines in clinical hypertension comes from patients with pheochromocytoma, rare tumors of catecholamine-synthesizing cells. Despite their rarity, pheochromocytomas are important in medicine, for two reasons. First, the tumors typically are benign, and surgical removal of a pheochromocytoma can cure the hypertension. Second, in response to seemingly minor perturbations such as anesthesia induction, pheochromocytomas can release their catecholamine contents into the bloodstream, resulting in paroxysms of high blood pressure or acute heart failure that can be lethal.
Because of the commonness and nonspecificity of symptoms and signs of pheochromocytoma, such as hypertension that is difficult to control, headache, anxiety, pallor, sweating, fast pulse rate, and palpitations, and the potentially catastrophic consequences of missing the diagnosis, clinicians frequently attempt to exclude pheochromocytoma, despite its rarity. In such patients, the most sensitive screening test is plasma free metanephrines [49].
Pheochromocytoma provides an experiment of nature for learning about long-term consequences of high catecholamine levels. These include not only myocardial hypertrophy but also dilated cardiomyopathy. Removal of the tumor can be lifesaving and reverse even dilated cardiomyopathy [50].
Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT)
Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) is a primary inherited arrhythmia syndrome that often manifests as episodes of syncope in the first or second decade of life and predisposes to unexplained sudden cardiac death in young adulthood. Mutations of genes encoding voltage gated ion channels (channelopathies) underlie a predisposition to ventricular arrhythmias in response to endogenous catecholamines. In particular, mutations of the gene for ryanodine receptor RYR2 account for about 70% of the CPVT cases and cause the autosomal dominant form of the disease [51,52]. It is thought that the mutation leads to disruption of intracellular Ca2+ homeostasis, with cAMP-induced aberrant Ca2+ release from the sarcoplasmic reticulum into the cytoplasm. β-Adrenoceptor activation, which increases cAMP generation, may therefore precipitate a pathogenic positive feedback loop in which ventricular arrhythmia rapidly increases cardiac sympathetic and adrenomedullary hormonal system outflows, in turn exacerbating aberrant Ca2+ release.
Because symptoms and signs are likely to be brought on by emotional or physical stress, clinical evaluation by echocardiography or electrocardiography in the resting state can yield false negative results. Other disorders predisposing to sudden cardiac death in young adulthood include congenital long QT syndrome and Brugada syndrome.
β-Adrenoceptor blockers are a mainstay of treatment for CPVT. An automated defibrillator may have to be implanted. Treatment for CPVT also includes left sympathectomy. Such treatment leaves open the theoretical possibilities of denervation supersensitivity of cardiac adrenoceptors and compensatory activation of the adrenomedullary hormonal system; however, plasma levels of catecholamines have not been assessed in CPVT with or without therapeutic cardiac denervation.
Primary Chronic Autonomic Failure
Failure of the sympathetic noradrenergic system causes a fall in blood pressure when the patient stands up—orthostatic hypotension (OH). Sympathetic nervous system failure also produces postprandial hypotension, exercise-related hypotension, a tendency to relatively slow pulse rate, exercise intolerance, and fatigue.
Most physicians lump together all forms of autonomic failure. In contrast, the current presentation distinguishes orthostatic or postprandial symptoms and hemodynamic abnormalities as manifestations specifically of failure of sympathetic noradrenergic function.
Using cardiac sympathetic neuroimaging one can now distinguish pathophysiological subtypes of autonomic failure. PAF and PD+OH are invariably associated with severe loss of sympathetic nerves in the left ventricular myocardium. In contrast, most patients with multiple system atrophy (MSA) have intact sympathetic innervation. These findings have led to a clinical pathophysiological classification of neurogenic OH [53] and to somewhat different treatment regimens.
Pure Autonomic Failure
PAF features persistent, consistent OH in the absence of signs of central nervous system disease and other known causes of OH. In PAF, OH results from generalized loss of sympathetic noradrenergic nerves coupled with baroreflex failure. Because of the loss of noradrenergic nerves, drugs that release NE, such as tyramine, yohimbine, amphetamine, and ephedrine, produce relatively small increases in blood pressure compared to drugs that directly stimulate adrenoceptors, such as midodrine and phenylephrine. Patients with PAF can have large, beneficial increases in blood pressure in response to adrenoceptor agonists, consistent with “denervation supersensitivity” [54].
Multiple System Atrophy
MSA is a progressive neurodegenerative disease that involves dysregulation of multiple components of the autonomic nervous system and lesions of multiple central neurotransmitter systems [55]. Most MSA patients have OH. In MSA, OH usually reflects loss of ability to modulate sympathetic nerve traffic to intact nerve terminals. This is a major difference from PAF or PD+OH. MSA patients typically have deficient orthostatic increments in plasma NE levels, a feature shared with PAF and PD+OH.
Parkinson Disease with Orthostatic Hypotension
Autonomic failure and OH occur fairly commonly in PD and can come on early in the disease course [56,57]. Studies during the past decade have found consistently that all patients with PD+OH have cardiac sympathetic denervation. PD+OH patients also have evidence of extra-cardiac sympathetic noradrenergic denervation [58,59]. The combination of cardiac and extra-cardiac sympathetic denervation with baroreflex failure probably explains OH attending PD.
Because most patients with PD who do not have OH nevertheless have at least some loss of cardiac sympathetic nerves [60], PD appears to be not only a movement disorder but also a form of dysautonomia. Cardiac sympathetic denervation can precede the movement disorder by several years [61], providing a potential biomarker to detect the pathogenetic process in at-risk individuals and to track effects of putative neuroprotective agents.
Cardiac denervation in PD+OH might produce symptoms such as fatigue and dyspnea on exertion that mimic mild heart failure.
Controversial Neurocardiologic Disorders
Many different diagnostic appellations have been suggested for psychoneurocardiologic syndromes. The length of the list probably indicates not so much the variety of conditions as ignorance about pathophysiological mechanisms.
Postural Tachycardia Syndrome
Patients with the postural tachycardia syndrome (postural orthostatic tachycardia syndrome, POTS) have an excessive increase in pulse rate during standing, usually without OH. The finding of OH does not exclude a diagnosis of POTS, however, as delayed OH [62] can occur. Patients with POTS have several nonspecific symptoms, such as chronic fatigue, heat intolerance, exercise intolerance, headache, chest pain, palpitations, disturbed sleep, decreased ability to concentrate (“brain fog”), and a tendency to panic or anxiety in threatening situations.
Researchers have thought that the tachycardia in POTS reflects increased cardiac sympathetic nerve traffic in compensation for decreased venous return to the heart or inadequately increased total peripheral resistance when the patient stands up. Either abnormality could disinhibit cardiac sympathetic outflow from baroreflexive restraint.
There are many potential causes for an excessive orthostatic decrease in venous return to the heart. The possibilities of blood volume depletion or excessive dependent pooling of blood have drawn particular attention. Consistent with excessive blood pooling in the legs, pelvis, or lower abdomen during orthostasis, inflation of a military antishock trousers suit reduces substantially the increase in heart rate in response to orthostasis in patients with POTS.
In “partial dysautonomia” or “neuropathic POTS” it is thought that there is a patchy loss of sympathetic innervation, such as in the legs or abdominal organs [63]. When the patient stands up, the blood would pool because of failure of the arterioles or veins to constrict, and the sympathetic nervous system supply to the heart would be stimulated reflexively. POTS may also be a manifestation of dysfunction of the renin–angiotensin–aldosterone system, a major system regulating sodium balance and extracellular fluid volume [64]. Rarely, POTS can result from failure to inactivate NE by reuptake via the cell membrane NE transporter [65].
In “hyperadrenergic orthostatic intolerance,” the problem is thought to be a primary abnormality in the functioning or regulation of the sympathetic nervous system itself. In a related syndrome, the “hyperdynamic circulation syndrome,” the patients have a fast pulse rate all the time, variable high blood pressure, increased heart rate responses to the drug isoproterenol, and increased plasma NE and EPI levels at rest and during provocative maneuvers [66,67]. β-Adrenoceptor blockers or benzodiazepines improve the syndrome. It is unclear whether patients with this syndrome have an increased frequency of later development of established hypertension.
In “inappropriate sinus tachycardia,” the heart rate is increased substantially even during supine rest. Radiofrequency ablation of the sinus node is considered for patients with inappropriate sinus tachycardia who are resistant to treatment with medications.
Neurocardiogenic Syncope
Neurocardiogenic syncope (vasovagal syncope, reflex syncope, neurally mediated hypotension, the common faint) is the most common cause of sudden loss of consciousness in the general population. Patients with frequent neurocardiogenic syncope can feel unwell between episodes, with an inability to tolerate prolonged standing, chronic fatigue, headache, and heat intolerance, as in POTS.
Neurocardiogenic syncope typically involves a particular evoked neuroendocrine pattern of central neural origin. EPI levels are high at the time of the acute episode [68,69], while activity of the sympathetic noradrenergic system is not as increased—“sympathoadrenal imbalance” [70,71]. Bradycardia can be prominent, indicating stimulation of parasympathetic cardiovagal outflow; however, heart transplant recipients can faint [72,73], and cholinergic blockade fails to prevent neurocardiogenic syncope, indicating that the neurally mediated hypotension does not depend solely on increased vagal tone. Studies have disagreed remarkably as to whether skeletal muscle sympathetic outflow falls abruptly [74–77] or is maintained [78]. Although EPI-induced relaxation of blood vessels in skeletal muscle could decrease vascular resistance in skeletal muscle and in the body as a whole, non-selective β-adrenoceptor blockade is not universally effective in preventing tilt-induced neurocardiogenic syncope [79]. Neurocardiogenic syncope is attended by other neuroendocrine changes, including elevated levels of β-endorphin [80], atrial natriuretic peptide, corticotropin, and vasopressin [81,82].
It therefore appears that there are multiple determinants of the hypotension that characterizes fainting [83]. Experimental attempts to elucidate contributions of these determinants by blocking effectors may yield false negative results because of compensatory activation of alternative effectors.
Non-Drug and Drug Treatments for Catecholaminergic Neurocardiologic Disorders
Clinical neurocardiologic disorders are often treatable. In this section, treatments are divided into non-drug, drugs for hyper-catecholaminergic states, and drugs for hypo-catecholaminergic states.
Non-Drug Treatments
Elevation of the Head of the Bed
In patients with OH, elevation of the head of the bed at night improves the ability to tolerate standing up in the morning. Because such patients typically have supine hypertension [84], mechanisms of this benefit probably include decreased pressure natriuresis.
Salt Intake
High salt intake tends to increase extracellular fluid volume. Clinicians usually recommend a high salt diet for patients with chronic orthostatic intolerance or OH. Normally when a person takes in a high salt diet, the kidneys increase the amount of salt in the urine, and this limits the increase in blood volume. Drugs that promote retention of sodium by the kidneys, such as fludrocortisone, are usually required for high salt intake to increase body fluid volume and blood pressure effectively.
Meals
Eating a large meal leads to shunting of blood toward the gut. Patients with chronic orthostatic intolerance or with OH therefore usually are advised to take frequent small meals.
Compression Hose
Compression hose or other compression garments tend to decrease the amount of orthostatic pooling of blood in veins. This can decrease leakage of fluid from the veins into the tissues and decrease pedal swelling. In patients with veins that fill up or leak excessively during standing, compression garments can improve toleration of prolonged standing. In patients with OH, the problem may be less with the veins than with the arteries and arterioles. Wearing compression garments therefore may be disappointing in the management of OH.
Temperature
Patients with dysautonomias often have an inability to tolerate extremes of external temperature. When exposed to the heat, patients with failure of the sympathetic nervous system may not sweat adequately to maintain the core temperature by evaporative heat loss. Patients with chronic orthostatic intolerance, such as from postural tachycardia syndrome (POTS), can have heat intolerance because of loss of blood volume by sweating or shunting of blood away from the brain. When exposed to cold, patients with sympathetic nervous system failure may not constrict blood vessels adequately in the skin, so that the body temperature falls (hypothermia).
Exercise
As a person exercises, arterioles tend to relax, due at least partly to the accumulation of byproducts of metabolism. The sympathetic nervous system normally counters this tendency. Activation of sympathetic nerves to the heart during exercise increases the force and rate of the heartbeat, and the cardiac output increases. Patients with sympathetic neurocirculatory failure are thought to have a decreased ability to increase the cardiac output during exercise, producing a decrease in blood pressure, shortness of breath, or early exhaustion.
Patients with inadequate sympathetic responses feel worse after than during exercise, probably because of cessation of muscle pumping. It is important for such patients to stay hydrated and avoid activities like eating a large meal or driving in a hot car after exercise.
Pacemakers and Sinus Node Ablation
Insertion of a cardiac pacemaker can prevent fainting in patients with neurocardiogenic syncope or POTS. This is an area of active research and some controversy, because a low pulse rate at the time of fainting might not cause and might even be the result of low blood flow to the brain. In patients with POTS and chronic fatigue, a pacemaker may not alleviate fatigue.
Patients with “inappropriate” sinus tachycardia may undergo therapeutic sinus node ablation. This is helpful if the tachycardia does not reflect compensation via cardiac sympathetic stimulation for another problem such as hypovolemia; eliminating the compensation would make the patient worse.
Neurosurgery
Some patients with chronic orthostatic intolerance have brainstem anatomic change called Chiari malformation, in which the cerebellar tonsils lie below the foramen magnum. Neurosurgery can correct the malformation, but the orthostatic intolerance does not necessarily disappear. This is a controversial topic; patients should seek a second opinion before submitting to this procedure.
Bilateral thoracic sympathectomies or sympathotomies are done for refractory palmar hyperhidrosis [85–87]. Iontophoresis, botulinum toxin injection, and glycopyrrolate cream are alternatives. Because sweating is mediated mainly by sympathetic cholinergic fibers, autonomic neurosurgery is usually effective; however, a variety of expected and unexpected consequences can result, including ectopic (e.g., plantar) hyperhidrosis, gustatory sweating, Horner syndrome, and decreased heart rate responses to exercise. The latter seems to be related to partial cardiac denervation [88]. Anecdotally, fatigue, altered mood, blunted emotion, and decreased ability to concentrate can develop after bilateral thoracic sympathectomies.
Drug Treatments
In this section, drug treatments of neurocardiologic disorders are classified in terms of therapy for hypercatecholaminergic and hypocatecholaminergic states.
Hypercatecholaminergic
Clonidine
Clonidine is an α2 adrenoceptor agonist that acts in the central nervous system to decrease sympathetic nervous system outflows and in the periphery at presynaptic receptors to decrease NE release from sympathetic nerve terminals [89]. Therefore, even though clonidine stimulates a type of α-adrenoceptor, clonidine normally decreases blood pressure.
Clonidine usually causes drowsiness and often causes a dry mouth. Sedation from clonidine has limited its clinical use.
Clonidine normally decreases plasma NE levels. In patients with pheochromocytoma plasma NE can be increased because of release of NE into the bloodstream independently of the sympathetic nervous system. In such patients failure of clonidine to reduce plasma NE constitutes a positive diagnostic test result [90,91]. Conversely, the combination of a high plasma NE level and a large fall in blood pressure in response to clonidine may identify patients with “hypernoradrenergic hypertension” [92]. In such patients, clonidine can be very effective in lowering the blood pressure. Clonidine is also effective in treating withdrawal from addictive drugs and in treating panic disorder.
Adrenoceptor Blockers
α-, β-, and combined α- and β-adrenoceptor blockers are used commonly to treat hypercatecholaminergic states such as POTS. Although β-adrenoceptor blockers reliably decrease heart rate, they do not necessarily alleviate other symptoms of POTS such as fatigue, “brain fog,” and exercise intolerance. Nonselective β-adrenoceptors have been disappointing in preventing neurocardiogenic syncope and can even prolong asystole in the cardioinhibitory form of the condition [79].
In pheochromocytoma, treatment of hypertension with β-blockers alone is contraindicated, because this leaves unopposed α-adrenoceptor occupation by circulating NE. β-Adrenoceptor blockers are effective for hyperdynamic circulation syndrome [67].
β-Adrenoceptor blockers can precipitate pulmonary edema in patients with acute heart failure. On the other hand, β-blocker treatment of cardiomyopathy can reduce long-term morbidity and morbidity, possibly by attenuating cardiac hypertrophy and decreasing predisposition to arrhythmias from sympathetic stimulation.
Imidazoline Receptor Agonists
Clonidine is an imidazoline. Research comparing clonidine with α-methyl-norepinephrine, which like clonidine is an α2 adrenoceptor agonist, led to the discovery of a class of imidazoline receptors, stimulation of which decreases sympathetic noradrenergic outflows. An endogenous clonidine-displacing substance—agmatine [93]—and the synthetic drugs moxonidine (Physiotens) and rilmenidine exemplify imidazoline I1 receptor agonists. Moxonidine decreases blood pressure and sympathetic outflow [94]. A trial of moxonidine in patients with symptomatic heart failure and reduced ejection fraction was stopped due to excessive early mortality in the moxonidine treated group [95]. This adverse outcome might have reflected recruitment of cardiac sympathetic outflow to maintain cardiac performance in the setting of intrinsic myocardial dysfunction, so that blocking the compensatory activation was deleterious. Moxonidine is available in several countries but has not been approved by the US FDA.
α-Methyl-Para-Tyrosine
α-Methyl-para-tyrosine (metyrosine, Demser) competitively antagonizes tyrosine hydroxylase, the rate-limiting enzymatic step in catecholamine biosynthesis. Plasma levels of DOPA, NE, dihydroxyphenylacetic acid, and dihydroxyphenylglycol decrease [96]. α-Methyl-para-tyrosine is used to decrease catecholamine biosynthesis in patients with pheochromocytoma prior to surgery [97]. Because of inhibition of catecholamine synthesis, the drug produces depressed mood and parkinsonism as long-term side effects [98].
Carbidopa
Carbidopa, which is combined with levodopa in Sinemet, inhibits decarboxylation of levodopa to DA outside the brain. Although carbidopa effectively inhibits L-aromatic-amino-acid decarboxylase, attained plasma L-DOPA concentrations are so high (about 10,000 nmol/L) that plasma levels of the DA metabolite dihydroxyphenylacetic acid typically increase by more than 20-fold (from about 7 to about 180 nmol/L), implying that patients taking levodopa/ carbidopa actually have substantially increased production and metabolism of DA outside the brain.
Ganglion Blockade
Ganglion blockers such as trimethaphan (TRI) and pentolinium (PEN) interfere with ganglionic transmission by blocking neuronal acetylcholine receptors. These drugs produce symptoms and signs of both parasympathetic and sympathetic failure, including dry mouth, constant pulse rate, decreased sweating, fixed pupils, and OH. Plasma NE levels fall [99] to a greater extent than do plasma dihydroxyphenylglycol levels [100], consistent with ongoing NE turnover due to net leakage from vesicles into the neuronal cytoplasm.
Ganglion blockers are not used to treat hypercatecholaminergic states, because the drugs always produce OH. They are used for research purposes, to evaluate the contribution of sympathetic innervation to blood pressure. Patients with MSA have large declines in blood pressure during ganglion blockade, revealing a post-ganglionic sympathoneural contribution to supine hypertension in this disease [101], whereas patients with PAF have small declines, due to sympathetic post-ganglionic denervation.
Reserpine and Tetrabenazine
Reserpine is a classical drug derived from the root of the Rauwolfia serpentina (Indian snakeroot) plant. A highly lipophilic drug, reserpine enters monoaminergic neurons and chromaffin cells and irreversibly blocks the type-1 and type-2 vesicular monoamine transporters. By blocking vesicular recycling, reserpine administration rapidly increases net leakage of NE from vesicles into the cytosol and therefore depletes sympathoneural NE stores [102].
After reserpine administration, increased oxidative deamination of cytosolic NE catalyzed by monoamine oxidase results in increased formation of dihydroxyphenylglycol (DHPG), and so plasma DHPG levels at first increase [103]. Subsequently, as vesicular NE stores become depleted, plasma DHPG decreases to low levels. Although reserpine is an effective antihypertensive agent, it is rarely used clinically because of side effects such as depression, orthostatic hypotension, diarrhea, and hypothermia.
Tetrabenazine also inhibits vesicular recycling of monoamines. Because it depletes DA stores it is used to treat hyperkinetic movement disorders such as chorea, tics associated with Tourette’s syndrome, and tardive dyskinesias [104]. The drug might prove useful for hyperadrenergic states and offers a better safety profile than reserpine.
Hypocatecholaminergic
Fludrocortisone
Fludrocortisone (Florinef), a synthetic mineralocorticoid that closely resembles the body’s main salt-retaining steroid, aldosterone, is used widely to treat chronic orthostatic intolerance and OH. For fludrocortisone to work requires that the patient be on a high-salt diet. The patient gains “fluid weight,” and blood pressure increases. Because of the tendency of fludrocortisone to waste potassium, fludrocortisone can cause a fall in the serum potassium level, and so patients taking fludrocortisone should have periodic checks of their serum potassium level.
Fludrocortisone given for OH can cause or worsen high blood pressure when the patient is lying down. The clinician must then balance the long-term increased risk of stroke, heart failure, or renal failure against the immediate risk of fainting or falling from OH.
Midodrine
Midodrine (Proamatine) is a vasoconstrictor that works by stimulating α-adrenoceptors in blood vessel walls. Midodrine is used frequently for neurogenic OH [105]. By stimulating α-adrenoceptors directly, midodrine acts like synthetic NE. In patients with OH associated with sympathetic noradrenergic denervation, denervation supersensitivity can render midodrine very effective in raising blood pressure.
In using midodrine to treat elderly men with OH, the clinician should be aware that stimulation of α-adrenoceptors can worsen symptoms and signs of prostate hypertrophy, such as urinary retention, urgency, and decreased urinary stream. α1-Adrenoceptor blockers are effective in treating benign prostatic hypertrophy, and such drugs interfere with midodrine.
Somatostatin
Somatostatin (Octreotide) has been reported to be helpful for orthostatic and postprandial hypotension [106]. The drug is expensive and must be injected.
Vasopressin
Vasopressin (or Desmopressin, which is 1-desamino-8-D-arginine vasopressin) sprayed into the nostrils can exert a systemic pressor effect.
Amphetamines
Amphetamines such as methylphenidate (Ritalin) and dextroamphetamine (Dexedrine) can be effective to treat symptoms of chronic fatigue syndrome such as fatigue and decreased ability to concentrate [107]. Methylphenidate has been reported to increase plasma EPI but not NE levels [99], whereas amphetamine increases plasma NE levels [79]. The potential for addiction or abuse limits clinical use of these drugs. It is reasonable to propose that such drugs might be effective for chronic orthostatic intolerance or OH; however, no controlled studies have been done for this sort of application.
Pyridostigmine
Pyridostigmine (Mestinon) is an inhibitor of acetylcholinesterase. The drug therefore increases delivery of endogenous acetylcholine to muscarinic and nicotinic receptors. The latter effect augments ganglionic neurotransmission, and via increased postganglionic sympathetic noradrenergic outflows, blood pressure increases. A potential therapeutic advantage of pyridostigmine to treat OH is that the drug may increase blood pressure more during orthostasis, when sympathetic outflow is increased, than during supine rest [108].
Yohimbine
Yohimbine exerts effects opposite to those of clonidine. Yohimbine increases sympathetic neural outflows and blocks α2-adrenoceptors on sympathetic nerve terminals, thereby increasing plasma NE levels [109]. Yohimbine challenge testing can assess whether a patient with neurogenic OH has releasable NE stores [110], which can be a target for treatment. Yohimbine challenge testing can also reveal excessive NE release in patients with anxiety or panic disorder. In patients with neurocardiogenic syncope yohimbine can improve orthostatic tolerance [76]. Yohimbine administration evokes large increases in blood pressure and plasma NE levels in patients with MSA [111], which can be useful in differential diagnosis from PAF and PD+OH [112] and might improve orthostatic tolerance [113].
Tyramine
Indirectly acting sympathomimetic amines such as dextroamphetamine and tyramine release NE from sympathetic nerve endings and increase plasma NE levels. These drugs are substrates for both the cell membrane norepinephrine transporter (NET) and vesicular monoamine transporter (VMAT). Probably by intravesicular alkalinization they enhance NE leakage from storage vesicles into the axoplasm. They also interfere with the efficiency of the NET, resulting in transport of the axoplasmic NE into the extracellular fluid. In humans, infusion of tyramine or dextroamphetamine therefore increases plasma NE levels [114,115]. During tyramine infusion, plasma DHPG levels increase more than do plasma NE levels [116], probably because of buildup of NE in the axoplasm.
Foodstuffs such as hard cheeses and red wines contain large amounts of tyramine. Normally dietary tyramine is metabolized in the gastrointestinal tract and liver before the amine can enter the systemic circulation. In patients taking an MAO inhibitor, tyramine is able to reach the sympathetic nerve terminals, and after neuronal and vesicular uptakes of tyramine paroxysmal hypertension can result from release of vesicular NE—a phenomenon termed the “cheese effect” [117]. Because of the susceptibility to severe hypertension due to the cheese effect MAO inhibitors have not had wide usage as antidepressants, despite their clinical efficacy. Intentional combination of tyramine with an MAO inhibitor can be used to treat neurogenic OH [118].
Contamination of TYR infusates with DA can increase plasma DA levels artifactually [119,120]. During infusion of relatively uncontaminated TYR, individual values for changes in plasma DA concentrations are positively correlated with those in NE [3], consistent with a neuronal source of plasma DA.
Isoproterenol
Infusion of the β-adrenoceptor agonist isoproterenol (ISO) increases plasma NE levels [121,122]. The increases probably reflect a combination of agonist occupation of β2-adrenoceptors on sympathetic nerves [123] and reflexive stimulation of sympathetic outflows in response to systemic vasodilation. Patients with chronic autonomic failure associated with generalized sympathetic noradrenergic denervation have attenuated plasma NE responses to infused ISO [58].
COMT Inhibitors (Entacapone)
Entacapone (Comtan) is an inhibitor of catechol-O-methyltransferase (COMT). Because levodopa is a catechol, entacapone treatment inhibits metabolic breakdown of levodopa to 3-methoxytyrosine [124]. Treatment of PD with levodopa/carbidopa/entacapone (Stalevo) is becoming increasingly common. In humans, entacapone does not increase peak plasma DOPA concentrations but does increase the area under the curve for DOPA concentrations vs. time [125,126]. Addition of entacapone to levodopa/carbidopa is therefore thought to be potentially beneficial in patients with PD and the “on-off” phenomenon.
ENT does not appreciably affect plasma levels of catecholamines [127], likely reflecting the relatively minor effects of nonneuronal uptake and O-methylation on the fate of NE released from sympathetic nerves. On the other hand, ENT augments cardiac responses to infused EPI and ISO, and the drug combination can precipitate ventricular arrhythmias [128].
MAO Inhibitors
Monoamine oxidase (MAO) figures much more prominently in the metabolic fate of DA and NE than does COMT, because sympathetic nerves do not express COMT. Plasma levels of dihydroxyphenylacetic acid, the main deaminated metabolite of DA, and of dihydroxyphenylglycol, the main deaminated metabolite of NE, therefore exceed by far those of the corresponding O-methylated metabolites.
There are two isoforms of MAO, MAO-A and MAO-B. Sympathetic nerves express MAO-A, whereas most extraneuronal cells express both MAO-A and MAO-B. Clorgyline selectively inhibits MAO-A, and selegiline (Deprenyl, Eldepryl) and rasagiline selectively inhibit MAO-B. Because of the MAO-B selectivity, selegiline and rasagiline are not associated with the “cheese effect.” MAO-B inhibitors are used commonly in adjunctive treatment of PD. The drugs do not appear to worsen OH [129].
NET Inhibitors
Tricyclic antidepressants, cocaine, and amphetamines inhibit the cell membrane NE transporter (NET). Effects of these agents on plasma levels of NE and its metabolites are complex, because these drugs all enter the brain and can affect sympathetic neuronal outflows.
Desipramine, a classical tricyclic anti-depressant, inhibits skeletal muscle sympathetic nerve traffic and decreases NE spillover in the body as a whole, the forearm, and the kidneys [130]. In contrast, desipramine increases plasma EPI levels, indicating differential effects on sympathetic noradrenergic and adrenomedullary outflows [131]. NET inhibition also decreases plasma dihydroxyphenylglycol levels, an effect that has been attributed to decreased NE turnover [132]. Desipramine abolishes plasma dihydroxyphenylglycol responses and augments plasma NE responses to yohimbine [132].
In humans, intra-nasal cocaine increases blood pressure substantially. After taking into account baroreflexive sympathoinhibition, skeletal muscle sympathetic outflow is increased [133]. In cocaine addicts, i.v. cocaine increases plasma NE and EPI levels [134].
Duloxetine, atomoxetine, and reboxetine are non-tricyclic antidepressants that block the NET. In humans, duloxetine increases plasma NE levels and reduces the plasma DHPG:NE ratio [135]. Reboxetine does not affect plasma NE levels during supine rest [136], possibly because of counter-balancing effects of reuptake inhibition and decreased sympathetic neuronal outflow [137]. Reboxetine decreases plasma DHPG levels [138]. Amitriptyline also tends to increase plasma NE and decrease plasma DHPG levels, consistent with some NET inhibition [138].
L-DOPS
L-threo-3,4-dihydroxyphenylserine (L-DOPS), a NE pro-drug, is an investigational agent for neurogenic OH.
After L-DOPS administration, plasma NE levels increase [139] but by surprisingly little and insufficiently to increase blood pressure [140]. In contrast with small increases in plasma NE levels, there are robust increases in plasma DHPG and dihydroxymandelic acid (DHMA) levels. The pressor response to L-DOPS therefore seems mainly to reflect actions on adrenoceptors within tissues by NE that has escaped extensive metabolic breakdown by MAO and COMT and has not yet reached the systemic circulation.
Water Drinking
A relatively recently described tactic to increase blood pressure in patients with autonomic failure is for them to drink 16 ounces of water [141]. Why water drinking should increase blood pressure in patients with autonomic failure, when doing so does not affect the blood pressure of healthy people, remains unclear. Water drinking increases standing and postprandial blood pressure in patients with MSA [142].
Patients with chronic orthostatic intolerance often bring a water bottle to the clinical encounter. They sip repeatedly during the day. This habit might indicate a tendency to dehydration and low blood volume, but this notion has not been tested.
The Future: Early Detection and Individualized Prevention
The future of therapeutics in neurocardiology is not so much in new curative drugs as in applying scientific integrative medical concepts that take into account concurrent development of chronic degenerative disorders and interactions of multiple drug and non-drug treatments with each other and with those disorders.
Autotoxicity of Cysotolic Monoamines
Current concepts about mechanisms of PD emphasize pathologic accumulation of α-synuclein, oxidative injury, impaired proteasomal or mitochondrial functions, neuroinflammation, or abnormal kinase signaling [143]. These concepts do not readily explain relatively selective, severe depletion of DA in the putamen compared to other neurotransmitters and other extranigral projection areas.
A potential explanation comes from the notion that cytoplasmic metabolites in catecholaminergic terminals are autotoxins that are produced continuously because of ongoing net leakage of catecholamines from vesicular stores. Catecholamines in the neuronal cytoplasm undergo enzymatic oxidative deamination catalyzed by monoamine oxidase to form catecholaldehydes (dihydroxyphenylacetaldehyde (DOPAL) from DA, dihydroxyphenylglycolaldehyde from NE), which are cytotoxic [144,145], as predicted by Blaschko more than a half century ago [146]. DOPAL has been shown to destroy dopaminergic neurons and cells [144,147] and to evoke precipitation of α-synuclein [148], which in catecholaminergic neurons is a characteristic neuropathological feature of PD [149]. Ongoing production of catecholaldehydes might increase susceptibility of catecholaminergic neurons, and in the nigrostriatal system greater buildup of DOPAL in the putamen than caudate might help explain relatively more severe DA depletion in the putamen [150].
If evidence accrued supporting the notion that aldehydes produced from deamination of cytosolic monoamines (serotonin and NE) constitute a determinant of not only central neural but also cardiac pathology [151–153], the therapeutic implications would be clear: drug treatments to decrease such deamination, directly via monoamine oxidase inhibition or indirectly via augmenting vesicular sequestration, should be neuroprotective.
Compensatory Activation and Timing of Intervention
The timing and rapidity of system failure from positive feedback loops depend on dynamic interactions between usage experience of the system and built-in manufacturing and design characteristics. Analogously, in the body, the occurrence, timing, and rapidity of progression of degenerative diseases may depend on interactions between environmental exposures and genetic predispositions. The kinetic model of allostasis and allostatic load provides a nice framework for linking stress, distress, allostatic load, and degenerative diseases.
One practical application of these concepts is using the principle of compensatory activation to decide on the timing of heart valve replacement in chronic aortic or mitral regurgitation. Waiting until the patient has symptoms of heart failure or coronary ischemia is not satisfactory, because already symptomatic patients have prohibitive operative morbidity and mortality; yet premature valve replacement is also not satisfactory, because implanted heart valves have a limited life span. Because cardiac sympathetic outflow increases early in heart failure [154], clinical laboratory assessments of cardiac sympathetic outflow might identify an appropriate time for heart valve replacement, before ventricular filling pressures or volume increase or ejection fraction decreases.
Positive Feedback Loops
In patients with chronic diseases of almost any sort, the “inner world” breaks down eventually; a key way this happens is by development of positive feedback loops. Heart failure stimulates the sympathetic nervous system and renin–angiotensin–aldosterone system, which increases fluid retention, growth of heart muscle, and the work of the heart, worsening the heart failure. Chest pain from coronary ischemia evokes distress, stimulating the adrenomedullary hormonal system and increasing the rate of consumption of oxygen by the heart, worsening the ischemia. OH from failure of the sympathetic nervous system causes lightheadedness, a fall, fracture of a hip, and prolonged bed rest in traction, worsening the OH when the patient tries to get up. A footballer practicing in full uniform in the heat releases epinephrine, which constricts skin blood vessels and augments heat production in the body, producing heat exhaustion, which releases more adrenaline, bringing on heat shock and death. Loss of dopaminergic terminals in the nigrostriatal system in the brain increases pathway traffic to the remaining terminals, accelerating DA turnover and thereby production of toxic by-products of DA metabolism, increasing the rate of loss of DA terminals, manifesting eventually as PD.
Application of kinetic models of allostasis and allostatic load may not only provide a framework for linking stress, distress, allostatic load, and degenerative diseases but also lead to development of effective means to prevent or detect and rapidly abort pathogenetic positive feedback loops. For instance, monitoring skin temperature and blood flow might detect signs of excessive adrenomedullary stimulation soon enough to prevent heat shock in a professional football player.
Complexity
The era of developing single drug treatments to cure single diseases is coming to an end. As populations age, chronic degenerative diseases are becoming more prevalent, and the corresponding societal burdens are expanding. Elderly patients typically have multiple comorbidities, take multiple prescribed drugs and over-the-counter dietary remedies or herbal supplements. The disorders interact with each other, the drugs interact with each other, and the disorders interact with the drugs in ever more complex ways.
Modern computerized methods of data analysis, simulation, and decision-making may be applied to this complexity and advise the clinician about the most effective and least harmful avenues to manage neurocardiologic disorders.
Individualized Medicine
Neurocardiologic disorders of catecholamine systems may provide a powerful spearhead of individualized or personalized medicine, because of the extraordinary ability to pinpoint specific metabolic abnormalities in single patients in the context of a medical systems approach. Rather than development of new drugs for particular neurocardiologic disorders, one may now envision combination drug therapies with individualized optimization that take into account not only comorbidities and drug interactions but also compensatory changes in activities of catecholamine systems. Catecholamine systems also can provide specific biomarker patterns [155], for diagnosis and for monitoring individual responses to treatments.
Footnotes
Conflict of Interest
The authors have no conflict of interest.
References
- 1.Natelson BH. Neurocardiology: An interdisciplinary area for the 80s. Arch Neurol. 1985;42:178–184. doi: 10.1001/archneur.1985.04060020096022. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein DS. Plasma norepinephrine during stress in essential hypertension. Hypertension. 1981;3:551–556. doi: 10.1161/01.hyp.3.5.551. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein DS, Holmes C. Neuronal source of plasma dopamine. Clin Chem. 2008;54:1864–1871. doi: 10.1373/clinchem.2008.107193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein DS, Mezey E, Yamamoto T, Aneman A, Friberg P, Eisenhofer G. Is there a third peripheral catecholaminergic system? Endogenous dopamine as an autocrine/paracrine substance derived from plasma DOPA and inactivated by conjugation. Hypertens Res. 1995;18(Suppl 1):S93–S99. doi: 10.1291/hypres.18.supplementi_s93. [DOI] [PubMed] [Google Scholar]
- 5.Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 6.Schafers M, Wichter T, Lerch H, et al. Cardiac 123I-MIBG uptake in idiopathic ventricular tachycardia and fibrillation. J Nucl Med. 1999;40:1–5. [PubMed] [Google Scholar]
- 7.Lee ZS, Critchley JA, Tomlinson B, et al. Urinary epinephrine and norepinephrine interrelations with obesity, insulin, and the metabolic syndrome in Hong Kong Chinese. Metabolism. 2001;50:135–143. doi: 10.1053/meta.2001.19502. [DOI] [PubMed] [Google Scholar]
- 8.Esler M, Ferrier C, Lambert G, Eisenhofer G, Cox H, Jennings G. Biochemical evidence of sympathetic hyperactivity in human hypertension. Hypertension. 1991;17:III29–III35. doi: 10.1161/01.hyp.17.4_suppl.iii29. [DOI] [PubMed] [Google Scholar]
- 9.Flaa A, Eide IK, Kjeldsen SE, Rostrup M. Sympathoadrenal stress reactivity is a predictor of future blood pressure: An 18-year follow-up study. Hypertension. 2008;52:336–341. doi: 10.1161/HYPERTENSIONAHA.108.111625. [DOI] [PubMed] [Google Scholar]
- 10.Hornykiewicz O. Biochemical aspects of Parkinson’s disease. Neurology. 1998;51:S2–S9. doi: 10.1212/wnl.51.2_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein DS, Holmes C, Cannon RO, 3rd, Eisenhofer G, Kopin IJ. Sympathetic cardioneuropathy in dysautonomias. N Engl J Med. 1997;336:696–702. doi: 10.1056/NEJM199703063361004. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DS. Adrenaline and the inner world: An introduction to scientific integrative medicine. Baltimore, MD: The Johns Hopkins University Press; 2006. [Google Scholar]
- 13.Sharabi Y, Dendi R, Holmes C, Goldstein DS. Baroreflex failure as a late sequela of neck irradiation. Hypertension. 2003;42:110–116. doi: 10.1161/01.HYP.0000077441.45309.08. [DOI] [PubMed] [Google Scholar]
- 14.Udelsman R, Goldstein DS, Loriaux DL, Chrousos GP. Catecholamine-glucocorticoid interactions during surgical stress. J Surg Res. 1987;43:539–545. doi: 10.1016/0022-4804(87)90128-4. [DOI] [PubMed] [Google Scholar]
- 15.Fukuhara K, Kvetnansky R, Cizza G, Pacak K, Ohara H, Goldstein DS, Kopin IJ. Interrelations between sympathoadrenal system and hypothalamo-pituitary-adrenocortical/thyroid systems in rats exposed to cold stress. J Neuroendocrinol. 1996;8:533–541. doi: 10.1046/j.1365-2826.1996.04877.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–1263. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 17.Tsutamoto T, Nishiyama K, Sakai H, et al. Transcardiac increase in norepinephrine and prognosis in patients with chronic heart failure. Eur J Heart Fail. 2008;10:1208–1214. doi: 10.1016/j.ejheart.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Meredith IT, Broughton A, Jennings GL, Esler MD. Evidence of a selective increase in cardiac sympathetic activity in patients with sustained ventricular arrhythmias. N Engl J Med. 1991;325:618–624. doi: 10.1056/NEJM199108293250905. [DOI] [PubMed] [Google Scholar]
- 19.Gerson MC, McGuire N, Wagoner LE. Sympathetic nervous system function as measured by I-123 metaiodobenzylguanidine predicts transplant-free survival in heart failure patients with idiopathic dilated cardiomyopathy. J Card Fail. 2003;9:384–391. doi: 10.1054/s1071-9164(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 20.Giordano A, Calcagni ML, Verrillo A, Pellegrinotti M, Frontoni S, Spallone V, Gambardella S. Assessment of sympathetic innervation of the heart in diabetes mellitus using 123I-MIBG. Diabetes Nutr Metab. 2000;13:350–355. [PubMed] [Google Scholar]
- 21.Ziegler D, Weise F, Langen KJ, et al. Effect of glycaemic control on myocardial sympathetic innervation assessed by [123I]metaiodobenzylguanidine scintigraphy: A 4-year prospective study in IDDM patients. Diabetologia. 1998;41:443–451. doi: 10.1007/s001250050928. [DOI] [PubMed] [Google Scholar]
- 22.Kurata C, Uehara A, Ishikawa A. Improvement of cardiac sympathetic innervation by renal transplantation. J Nucl Med. 2004;45:1114–1120. [PubMed] [Google Scholar]
- 23.Carey LC, Curtin R, Sapira JD. Influence of hemorrhage on adrenal secretion, blood glucose and serum insulin in the awake pig. Ann Surg. 1976;183:185–192. doi: 10.1097/00000658-197602000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: Pathophysiology and clinical management. Nat Rev Neurol. 2010;6:145–155. doi: 10.1038/nrneurol.2009.231. [DOI] [PubMed] [Google Scholar]
- 25.Rattanataweeboon P, Vilaichone W, Vannasaeng S. Stress hyperglycemia in patients with sepsis. J Med Assoc Thai. 2009;92(Suppl 2):S88–S94. [PubMed] [Google Scholar]
- 26.Dziewierz A, Giszterowicz D, Siudak Z, Rakowski T, Dubiel JS, Dudek D. Admission glucose level and in-hospital outcomes in diabetic and non-diabetic patients with acute myocardial infarction. Clin Res Cardiol. 2010;99:715–721. doi: 10.1007/s00392-010-0175-1. [DOI] [PubMed] [Google Scholar]
- 27.Szatalowicz VL, Arnold PE, Chaimovitz C, Bichet D, Berl T, Schrier RW. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Engl J Med. 1981;305:263–266. doi: 10.1056/NEJM198107303050506. [DOI] [PubMed] [Google Scholar]
- 28.Riegger GA, Liebau G, Kochsiek K. Antidiuretic hormone in congestive heart failure. Am J Med. 1982;72:49–52. doi: 10.1016/0002-9343(82)90576-9. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein DS, McEwen B. Allostasis, homeostats, and the nature of stress. Stress. 2002;5:55–58. doi: 10.1080/102538902900012345. [DOI] [PubMed] [Google Scholar]
- 30.Darbar D, Smith M, Morike K, Roden DM. Epinephrine-induced changes in serum potassium and cardiac repolarization and effects of pretreatment with propranolol and diltiazem. Am J Cardiol. 1996;77:1351–1355. doi: 10.1016/s0002-9149(96)00204-4. [DOI] [PubMed] [Google Scholar]
- 31.Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: A new form of acute, reversible heart failure. Circulation. 2008;118:2754–2762. doi: 10.1161/CIRCULATIONAHA.108.767012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenhofer G, Friberg P, Rundqvist B, et al. Cardiac sympathetic nerve function in congestive heart failure. Circulation. 1996;93:1667–1676. doi: 10.1161/01.cir.93.9.1667. [DOI] [PubMed] [Google Scholar]
- 33.Kurz T, Richardt G, Seyfarth M, Schomig A. Nonexocytotic noradrenaline release induced by pharmacological agents or anoxia in human cardiac tissue. Naunyn Schmiedeberg’s Arch Pharmacol. 1996;354:7–16. doi: 10.1007/BF00168700. [DOI] [PubMed] [Google Scholar]
- 34.Isnard R, Pousset F, Trochu J, et al. Prognostic value of neurohormonal activation and cardiopulmonary exercise testing in patients with chronic heart failure. Am J Cardiol. 2000;86:417–421. doi: 10.1016/s0002-9149(00)00957-7. [DOI] [PubMed] [Google Scholar]
- 35.Thomas JA, Marks BH. Plasma norepinephrine in congestive heart failure. Am J Cardiol. 1978;41:233–243. doi: 10.1016/0002-9149(78)90162-5. [DOI] [PubMed] [Google Scholar]
- 36.Esler M, Julius S, Randall O, DeQuattro V, Zweifler A. High-renin essential hypertension: Adrenergic cardiovascular correlates. Clin Sci Mol Med Suppl. 1976;3:181s–184s. doi: 10.1042/cs051181s. [DOI] [PubMed] [Google Scholar]
- 37.Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 38.DiBona GF. Neural mechanisms in body fluid homeostasis. Fed Proc. 1986;45:2871–2877. [PubMed] [Google Scholar]
- 39.DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens. 2002;11:197–200. doi: 10.1097/00041552-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Lohmeier TE, Hildebrandt DA, Dwyer TM, Iliescu R, Irwin ED, Cates AW, Rossing MA. Prolonged activation of the baroreflex decreases arterial pressure even during chronic adrenergic blockade. Hypertension. 2009;53:833–838. doi: 10.1161/HYPERTENSIONAHA.109.128884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heusser K, Tank J, Engeli S, et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55:619–626. doi: 10.1161/HYPERTENSIONAHA.109.140665. [DOI] [PubMed] [Google Scholar]
- 42.Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: A multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 43.DiBona GF. Central angiotensin modulation of baroreflex control of renal sympathetic nerve activity in the rat: Influence of dietary sodium. Acta Physiol Scand. 2003;177:285–289. doi: 10.1046/j.1365-201X.2003.01074.x. [DOI] [PubMed] [Google Scholar]
- 44.Creager MA. Baroreceptor reflex function in congestive heart failure. Am J Cardiol. 1992;69:10G–15G. doi: 10.1016/0002-9149(92)91250-8. discussion 15G–16G. [DOI] [PubMed] [Google Scholar]
- 45.Polinsky RJ, Samaras GM, Kopin IJ. Sympathetic neural prosthesis for managing orthostatic hypotension. Lancet. 1983;1:901–904. doi: 10.1016/s0140-6736(83)91329-6. [DOI] [PubMed] [Google Scholar]
- 46.Oldenburg O, Mitchell A, Nurnberger J, Koeppen S, Erbel R, Philipp T, Kribben A. Ambulatory norepinephrine treatment of severe autonomic orthostatic hypotension. J Am Coll Cardiol. 2001;37:219–223. doi: 10.1016/s0735-1097(00)01062-7. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez LA, Illig K, Levy M, et al. Implantable carotid sinus stimulator for the treatment of resistant hypertension: Local effects on carotid artery morphology. Ann Vasc Surg. 2010;24:178–184. doi: 10.1016/j.avsg.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Zucker IH, Hackley JF, Cornish KG, et al. Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension. 2007;50:904–910. doi: 10.1161/HYPERTENSIONAHA.107.095216. [DOI] [PubMed] [Google Scholar]
- 49.Lenders JW, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: Which test is best? JAMA. 2002;287:1427–1434. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 50.Imperato-McGinley J, Gautier T, Ehlers K, Zullo MA, Goldstein DS, Vaughan ED., Jr Reversibility of catecholamine-induced dilated cardiomyopathy in a child with a pheochromocytoma. N Engl J Med. 1987;316:793–797. doi: 10.1056/NEJM198703263161307. [DOI] [PubMed] [Google Scholar]
- 51.Ylanen K, Poutanen T, Hiippala A, Swan H, Korppi M. Catecholaminergic polymorphic ventricular tachycardia. Eur J Pediatr. 2010;169:535–542. doi: 10.1007/s00431-010-1154-2. [DOI] [PubMed] [Google Scholar]
- 52.Gyorke S. Molecular basis of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2009;6:123–129. doi: 10.1016/j.hrthm.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein DS, Sharabi Y. Neurogenic orthostatic hypotension: A pathophysiological approach. Circulation. 2009;119:139–146. doi: 10.1161/CIRCULATIONAHA.108.805887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warner MR, Wisler PL, Hodges TD, Watanabe AM, Zipes DP. Mechanisms of denervation supersensitivity in regionally denervated canine hearts. Am J Physiol. 1993;264:H815–H820. doi: 10.1152/ajpheart.1993.264.3.H815. [DOI] [PubMed] [Google Scholar]
- 55.Polinsky RJ, Brown RT, Burns RS, Harvey-White J, Kopin IJ. Low lumbar CSF levels of homovanillic acid and 5-hydroxyindoleacetic acid in multiple system atrophy with autonomic failure. J Neurol Neurosurg Psychiatry. 1988;51:914–919. doi: 10.1136/jnnp.51.7.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaufmann H, Goldstein DS. Dysautonomia in Parkinson disease. In: Aminoff MJ, Boller F, Swaab DF, editors. Handbook of clinical neurology. 2007. pp. 343–363. [DOI] [PubMed] [Google Scholar]
- 57.Goldstein DS. Orthostatic hypotension as an early finding in Parkinson disease. Clin Auton Res. 2006;16:46–64. doi: 10.1007/s10286-006-0317-8. [DOI] [PubMed] [Google Scholar]
- 58.Sharabi Y, Imrich R, Holmes C, Pechnik S, Goldstein DS. Generalized and neurotransmitter-selective noradrenergic denervation in Parkinson’s disease with orthostatic hypotension. Mov Disord. 2008;23:1725–1732. doi: 10.1002/mds.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldstein DS, Orimo S. Cardiac sympathetic neuroimaging: Summary of the First International Symposium. Clin Auton Res. 2009;19:133–136. doi: 10.1007/s10286-009-0002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO., 3rd Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med. 2000;133:338–347. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- 61.Goldstein DS, Sharabi Y, Karp BI, Bentho O, Saleem A, Pacak K, Eisenhofer G. Cardiac sympathetic denervation preceding motor signs in Parkinson disease. Clin Auton Res. 2007;17:118–121. doi: 10.1007/s10286-007-0396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Streeten DH, Anderson GH., Jr The role of delayed orthostatic hypotension in the pathogenesis of chronic fatigue. Clin Auton Res. 1998;8:119–124. doi: 10.1007/BF02267822. [DOI] [PubMed] [Google Scholar]
- 63.Jacob G, Costa F, Shannon JR, et al. The neuropathic postural tachycardia syndrome. N Engl J Med. 2000;343:1008–1014. doi: 10.1056/NEJM200010053431404. [DOI] [PubMed] [Google Scholar]
- 64.Jacob G, Robertson D, Mosqueda-Garcia R, Ertl AC, Robertson RM, Biaggioni I. Hypovolemia in syncope and orthostatic intolerance role of the renin-angiotensin system. Am J Med. 1997;103:128–133. doi: 10.1016/s0002-9343(97)00133-2. [DOI] [PubMed] [Google Scholar]
- 65.Shannon JR, Flattem NL, Jordan J, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. 2000;342:541–549. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- 66.Goldstein DS, Keiser HR. Neural circulatory control in the hyperdynamic circulatory state syndrome. Am Heart J. 1985;109:387–390. doi: 10.1016/0002-8703(85)90623-4. [DOI] [PubMed] [Google Scholar]
- 67.Frohlich ED, Tarazi RC, Dustan HP. Hyperdynamic beta-adrenergic circulatory state. Arch Int Med. 1969;123:1–7. [PubMed] [Google Scholar]
- 68.Robertson DA, Johnson GA, Robertson RM, Nies AS, Shand DG, Oates JA. Comparative assessment of stimuli that release neuronal and adrenomedullary catecholamines in man. Circulation. 1979;59:637–643. doi: 10.1161/01.cir.59.4.637. [DOI] [PubMed] [Google Scholar]
- 69.Goldstein DS, Spanarkel M, Pitterman A, Toltzis R, Gratz E, Epstein S, Keiser HR. Circulatory control mechanisms in vasodepressor syncope. Am Heart J. 1982;104:1071–1075. doi: 10.1016/0002-8703(82)90442-2. [DOI] [PubMed] [Google Scholar]
- 70.Goldstein DS, Holmes C, Frank SM, Naqibuddin M, Dendi R, Snader S, Calkins H. Sympathoadrenal imbalance before neurocardiogenic syncope. Am J Cardiol. 2003;91:53–58. doi: 10.1016/s0002-9149(02)02997-1. [DOI] [PubMed] [Google Scholar]
- 71.Kikushima S, Kobayashi Y, Nakagawa H, Katagiri T. Triggering mechanism for neurally mediated syncope induced by head-up tilt test: Role of catecholamines and response to propranolol. J Am Coll Cardiol. 1999;33:350–357. doi: 10.1016/s0735-1097(98)00567-1. [DOI] [PubMed] [Google Scholar]
- 72.Scherrer U, Vissing S, Morgan BJ, Hanson P, Victor RG. Vasovagal syncope after infusion of a vasodilator in a heart-transplant recipient. N Engl J Med. 1990;322:602–604. doi: 10.1056/NEJM199003013220906. [DOI] [PubMed] [Google Scholar]
- 73.Montebugnoli L, Montanari G. Vasovagal syncope in heart transplant patients during dental surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:666–669. doi: 10.1016/s1079-2104(99)70157-5. [DOI] [PubMed] [Google Scholar]
- 74.Wallin BG, Sundlof G. Sympathetic outflow to muscles during vasovagal syncope. J Autonom Nerv Sys. 1982;6:287–291. doi: 10.1016/0165-1838(82)90001-7. [DOI] [PubMed] [Google Scholar]
- 75.Mosqueda-Garcia R, Furlan R, Fernandez-Violante R, et al. Sympathetic and baroreceptor reflex function in neurally mediated syncope evoked by tilt. J Clin Invest. 1997;99:2736–2744. doi: 10.1172/JCI119463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mosqueda-Garcia R, Fernandez-Violante R, Tank J, Snell M, Cunningham G, Furlan R. Yohimbine in neurally mediated syncope. Pathophysiological implications. J Clin Invest. 1998;102:1824–1830. doi: 10.1172/JCI3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ellenbogen KA, Morillo CA, Wood MA, Gilligan DM, Eckberg DL, Smith ML. Neural monitoring of vasovagal syncope. Pacing Clin Electrophysiol. 1997;20:788–794. doi: 10.1111/j.1540-8159.1997.tb03905.x. [DOI] [PubMed] [Google Scholar]
- 78.Vaddadi G, Esler MD, Dawood T, Lambert E. Persistence of muscle sympathetic nerve activity during vasovagal syncope. Eur Heart J. 2010;31:2027–2033. doi: 10.1093/eurheartj/ehq071. [DOI] [PubMed] [Google Scholar]
- 79.Eldadah BA, Pechnik SL, Holmes CS, Moak JP, Saleem AM, Goldstein DS. Failure of propranolol to prevent tilt-evoked systemic vasodilatation, adrenaline release and neurocardiogenic syncope. Clin Sci (Lond) 2006;111:209–216. doi: 10.1042/CS20060017. [DOI] [PubMed] [Google Scholar]
- 80.Jacobs MC, Goldstein DS, Willemsen JJ, Smits P, Thien T, Dionne RA, Lenders JW. Neurohumoral antecedents of vasodepressor reactions. Eur J Clin Invest. 1995;25:754–761. doi: 10.1111/j.1365-2362.1995.tb01954.x. [DOI] [PubMed] [Google Scholar]
- 81.Jardine DL, Melton IC, Crozier IG, Bennett SI, Donald RA, Ikram H. Neurohormonal response to head-up tilt and its role in vasovagal syncope. Am J Cardiol. 1997;79:1302–1306. doi: 10.1016/s0002-9149(9x)00084-9. [DOI] [PubMed] [Google Scholar]
- 82.Theopistou A, Gatzoulis K, Economou E, Sideris S, Hantzos K, Stefanadis C, Toutouzas P. Biochemical changes involved in the mechanism of vasovagal syncope. Am J Cardiol. 2001;88:376–381. doi: 10.1016/s0002-9149(01)01682-4. [DOI] [PubMed] [Google Scholar]
- 83.Dietz NM, Halliwill JR, Spielmann JM, Lawler LA, Papouchado BG, Eickhoff TJ, Joyner MJ. Sympathetic withdrawal and forearm vasodilation during vasovagal syncope in humans. J Appl Physiol. 1997;82:1785–1793. doi: 10.1152/jappl.1997.82.6.1785. [DOI] [PubMed] [Google Scholar]
- 84.Biaggioni I, Robertson RM. Hypertension in orthostatic hypotension and autonomic dysfunction. Cardiol Clin. 2002;20:291–301. vii. doi: 10.1016/s0733-8651(01)00005-4. [DOI] [PubMed] [Google Scholar]
- 85.Moya J, Ramos R, Morera R, Villalonga R, Perna V, Macia I, Ferrer G. Thoracic sympathicolysis for primary hyperhidrosis: A review of 918 procedures. Surg Endosc. 2006;20:598–602. doi: 10.1007/s00464-005-0557-z. [DOI] [PubMed] [Google Scholar]
- 86.Eisenach JH, Atkinson JL, Fealey RD. Hyperhidrosis: Evolving therapies for a well-established phenomenon. Mayo Clin Proc. 2005;80:657–666. doi: 10.4065/80.5.657. [DOI] [PubMed] [Google Scholar]
- 87.Atkinson JL, Fealey RD. Sympathotomy instead of sympathectomy for palmar hyperhidrosis: Minimizing postoperative compensatory hyperhidrosis. Mayo Clin Proc. 2003;78:167–172. doi: 10.4065/78.2.167. [DOI] [PubMed] [Google Scholar]
- 88.Moak JP, Eldadah B, Holmes C, Pechnik S, Goldstein DS. Partial cardiac sympathetic denervation after bilateral thoracic sympathectomy in humans. Heart Rhythm. 2005;2:602–609. doi: 10.1016/j.hrthm.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 89.Aggarwal A, Esler MD, Socratous F, Kaye DM. Evidence for functional presynaptic alpha-2 adrenoceptors and their down-regulation in human heart failure. J Am Coll Cardiol. 2001;37:1246–1251. doi: 10.1016/s0735-1097(01)01121-4. [DOI] [PubMed] [Google Scholar]
- 90.Grossman E, Goldstein DS, Hoffman A, Keiser HR. Glucagon and clonidine testing in the diagnosis of pheochromocytoma. Hypertension. 1991;17:733–741. doi: 10.1161/01.hyp.17.6.733. [DOI] [PubMed] [Google Scholar]
- 91.Eisenhofer G, Goldstein DS, Walther MM, Friberg P, Lenders JW, Keiser HR, Pacak K. Biochemical diagnosis of pheochromocytoma: How to distinguish true- from false-positive test results. J Clin Endocrinol Metab. 2003;88:2656–2666. doi: 10.1210/jc.2002-030005. [DOI] [PubMed] [Google Scholar]
- 92.Goldstein DS, Levinson PD, Zimlichman R, Pitterman A, Stull R, Keiser HR. Clonidine suppression testing in essential hypertension. Ann Intern Med. 1985;102:42–49. doi: 10.7326/0003-4819-102-1-42. [DOI] [PubMed] [Google Scholar]
- 93.Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: An endogenous clonidine-displacing substance in the brain. Science. 1994;263:966–969. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- 94.Wenzel RR, Spieker L, Qui S, Shaw S, Luscher TF, Noll G. I1-imidazoline agonist moxonidine decreases sympathetic nerve activity and blood pressure in hypertensives. Hypertension. 1998;32:1022–1027. doi: 10.1161/01.hyp.32.6.1022. [DOI] [PubMed] [Google Scholar]
- 95.Cohn JN, Pfeffer MA, Rouleau J, et al. Adverse mortality effect of central sympathetic inhibition with sustained-release moxonidine in patients with heart failure (MOXCON) Eur J Heart Fail. 2003;5:659–667. doi: 10.1016/s1388-9842(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 96.Goldstein DS, Udelsman R, Eisenhofer G, Stull R, Keiser HR, Kopin IJ. Neuronal source of plasma dihydroxyphenylalanine. J Clin Endocrinol Metab. 1987;64:856–861. doi: 10.1210/jcem-64-4-856. [DOI] [PubMed] [Google Scholar]
- 97.Steinsapir J, Carr AA, Prisant LM, Bransome ED., Jr Metyrosine and pheochromocytoma. Arch Intern Med. 1997;157:901–906. [PubMed] [Google Scholar]
- 98.Fahn S. Long-term treatment of tardive dyskinesia with presynaptically acting dopamine-depleting agents. Adv Neurol. 1983;37:267–276. [PubMed] [Google Scholar]
- 99.Jordan J, Shannon JR, Black BK, Lance RH, Squillante MD, Costa F, Robertson D. N(N)-nicotinic blockade as an acute human model of autonomic failure. Hypertension. 1998;31:1178–1184. doi: 10.1161/01.hyp.31.5.1178. [DOI] [PubMed] [Google Scholar]
- 100.Bruce S, Tack C, Patel J, Pacak K, Goldstein DS. Local sympathetic function in human skeletal muscle and adipose tissue assessed by microdialysis. Clin Auton Res. 2002;12:13–19. doi: 10.1007/s102860200005. [DOI] [PubMed] [Google Scholar]
- 101.Shannon JR, Jordan J, Diedrich A, Pohar B, Black BK, Robertson D, Biaggioni I. Sympathetically mediated hypertension in autonomic failure. Circulation. 2000;101:2710–2715. doi: 10.1161/01.cir.101.23.2710. [DOI] [PubMed] [Google Scholar]
- 102.Chidsey CA, Braunwald E, Morow AG, Mason DT. Myocardial norepinephrine concentration in man. Effects of reserpine and congestive heart failure. N Engl J Med. 1963;269:653–658. doi: 10.1056/NEJM196309262691302. [DOI] [PubMed] [Google Scholar]
- 103.Eisenhofer G, Goldstein DS, Ropchak TG, Nguyen HQ, Keiser HR, Kopin IJ. Source and physiological significance of plasma 3,4-dihydroxyphenylglycol and 3-methoxy-4-hydroxyphenylglycol. J Auton Nerv Syst. 1988;24:1–14. doi: 10.1016/0165-1838(88)90130-0. [DOI] [PubMed] [Google Scholar]
- 104.Fasano A, Bentivoglio AR. Tetrabenazine. Expert Opin Pharmacother. 2009;10:2883–2896. doi: 10.1517/14656560903386292. [DOI] [PubMed] [Google Scholar]
- 105.Jankovic J, Gilden JL, Hiner BC, et al. Neurogenic orthostatic hypotension: A double-blind, placebo-controlled study with midodrine. Am J Med. 1993;95:38–48. doi: 10.1016/0002-9343(93)90230-m. [DOI] [PubMed] [Google Scholar]
- 106.Hoeldtke RD, Israel BC. Treatment of orthostatic hypotension with octreotide. J Clin Endocrinol Metab. 1989;68:1051–1059. doi: 10.1210/jcem-68-6-1051. [DOI] [PubMed] [Google Scholar]
- 107.Blockmans D, Persoons P, Van Houdenhove B, Bobbaers H. Does methylphenidate reduce the symptoms of chronic fatigue syndrome? Am J Med. 2006;119:167. e123–167. e130. doi: 10.1016/j.amjmed.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 108.Singer W, Sandroni P, Opfer-Gehrking TL, et al. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol. 2006;63:513–518. doi: 10.1001/archneur.63.4.noc50340. [DOI] [PubMed] [Google Scholar]
- 109.Grossman E, Rea RF, Hoffman A, Goldstein DS. Yohimbine increases sympathetic nerve activity and norepinephrine spillover in normal volunteers. Am J Physiol. 1991;260:R142–R147. doi: 10.1152/ajpregu.1991.260.1.R142. [DOI] [PubMed] [Google Scholar]
- 110.Robertson D, Goldberg MR, Tung CS, Hollister AS, Robertson RM. Use of alpha 2 adrenoreceptor agonists and antagonists in the functional assessment of the sympathetic nervous system. J Clin Invest. 1986;78:576–581. doi: 10.1172/JCI112611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sharabi Y, Eldadah B, Li ST, Dendi R, Pechnik S, Holmes C, Goldstein DS. Neuropharmacologic distinction of neurogenic orthostatic hypotension syndromes. Clin Neuropharmacol. 2006;29:97–105. doi: 10.1097/01.WNF.0000220822.80640.0D. [DOI] [PubMed] [Google Scholar]
- 112.Senard JM, Rascol O, Durrieu G, Tran MA, Berlan M, Rascol A, Montastruc JL. Effects of yohimbine on plasma catecholamine levels in orthostatic hypotension related to Parkinson disease or multiple system atrophy. Clin Neuropharmacol. 1993;16:70–76. doi: 10.1097/00002826-199302000-00008. [DOI] [PubMed] [Google Scholar]
- 113.Onrot J, Goldberg MR, Biaggioni I, Wiley RG, Hollister AS, Robertson D. Oral yohimbine in human autonomic failure. Neurology. 1987;37:215–220. doi: 10.1212/wnl.37.2.215. [DOI] [PubMed] [Google Scholar]
- 114.Goldstein DS, Nurnberger J, Jr, Simmons S, Gershon ES, Polinsky R, Keiser HR. Effects of injected sympathomimetic amines on plasma catecholamines and circulatory variables in man. Life Sci. 1983;32:1057–1063. doi: 10.1016/0024-3205(83)90110-8. [DOI] [PubMed] [Google Scholar]
- 115.Scriven AJI, Dollery CT, Murphy MB, Macquin I, Brown MJ. Blood pressure and plasma norepinephrine concentrations after endogenous norepinephrine release by tyramine. Clin Pharmacol Therap. 1983;33:710–716. doi: 10.1038/clpt.1983.97. [DOI] [PubMed] [Google Scholar]
- 116.Goldstein DS, Holmes C. Metabolic fate of the sympathoneural imaging agent 6-[18F]fluorodopamine in humans. Clin Exp Hypertens. 1997;19:155–161. doi: 10.3109/10641969709080812. [DOI] [PubMed] [Google Scholar]
- 117.Smith SE. Cheese reaction and tyramine. Lancet. 1971;1:130–131. doi: 10.1016/s0140-6736(71)90858-0. [DOI] [PubMed] [Google Scholar]
- 118.Nanda RN, Johnson RH, Keogh HJ. Treatment of neurogenic orthostatic hypotension with a monoamine oxidase inhibitor and tyramine. Lancet. 1976;2:1164–1167. doi: 10.1016/s0140-6736(76)91681-0. [DOI] [PubMed] [Google Scholar]
- 119.Holmes C, Moak J, Eldadah B, Zimmerly E, Sharabi Y, Goldstein DS. Dopamine contamination of infused tyramine. Clin Chem. 2005;51:1733–1735. doi: 10.1373/clinchem.2005.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jacob G, Gamboa A, Diedrich A, Shibao C, Robertson D, Biaggioni I. Tyramine-induced vasodilation mediated by dopamine contamination: A paradox resolved. Hypertension. 2005;46:355–359. doi: 10.1161/01.HYP.0000172353.62657.8b. [DOI] [PubMed] [Google Scholar]
- 121.Goldstein DS, Zimlichman R, Stull R, Keiser HR. Plasma catecholamine and hemodynamic responses during isoproterenol infusions in humans. Clin Pharmacol Ther. 1986;40:233–238. doi: 10.1038/clpt.1986.168. [DOI] [PubMed] [Google Scholar]
- 122.Vincent HH, Man in’t Veld AJ, Boomsma F, Wenting GJ, Schalekamp MADH. Elevated plasma noradrenaline in response to beta-adrenoceptor stimulation in man. Br J Clin Pharmacol. 1982;13:717–721. doi: 10.1111/j.1365-2125.1982.tb01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chang PC, Grossman E, Kopin IJ, Goldstein DS. On the existence of functional beta-adrenoceptors on vascular sympathetic nerve endings in the human forearm. J Hypertens. 1994;12:681–690. [PubMed] [Google Scholar]
- 124.Blandini F, Nappi G, Fancellu R, et al. Modifications of plasma and platelet levels of L-DOPA and its direct metabolites during treatment with tolcapone or entacapone in patients with Parkinson’s disease. J Neural Transm. 2003;110:911–922. doi: 10.1007/s00702-003-0004-z. [DOI] [PubMed] [Google Scholar]
- 125.Keranen T, Gordin A, Harjola VP, et al. The effect of catechol-O-methyl transferase inhibition by entacapone on the pharmacokinetics and metabolism of levodopa in healthy volunteers. Clin Neuropharmacol. 1993;16:145–156. doi: 10.1097/00002826-199304000-00007. [DOI] [PubMed] [Google Scholar]
- 126.Nutt JG, Woodward WR, Beckner RM, et al. Effect of peripheral catechol-O-methyltransferase inhibition on the pharmacokinetics and pharmacodynamics of levodopa in parkinsonian patients. Neurology. 1994;44:913–919. doi: 10.1212/wnl.44.5.913. [DOI] [PubMed] [Google Scholar]
- 127.Sundberg S, Scheinin M, Illi A, Akkila J, Gordin A, Keranen T. The effects of the COMT inhibitor entacapone on haemodynamics and peripheral catecholamine metabolism during exercise. Br J Clin Pharmacol. 1993;36:451–456. doi: 10.1111/j.1365-2125.1993.tb00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Illi A, Sundberg S, Ojala-Karlsson P, Korhonen P, Scheinin M, Gordin A. The effect of entacapone on the disposition and hemodynamic effects of intravenous isoproterenol and epinephrine. Clin Pharmacol Ther. 1995;58:221–227. doi: 10.1016/0009-9236(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 129.Bhattacharya KF, Nouri S, Olanow CW, Yahr MD, Kaufmann H. Selegiline in the treatment of Parkinson’s disease: Its impact on orthostatic hypotension. Parkinsonism Relat Disord. 2003;9:221–224. doi: 10.1016/s1353-8020(02)00053-6. [DOI] [PubMed] [Google Scholar]
- 130.Esler MD, Wallin G, Dorward PK, et al. Effects of desipramine on sympathetic nerve firing and norepinephrine spillover to plasma in humans. Am J Physiol. 1991;260:R817–R823. doi: 10.1152/ajpregu.1991.260.4.R817. [DOI] [PubMed] [Google Scholar]
- 131.Eisenhofer G, Friberg P, Goldstein DS, Esler M. Differential actions of desipramine on sympathoadrenal release of noradrenaline and adrenaline. Br J Clin Pharmacol. 1995;40:263–265. doi: 10.1111/j.1365-2125.1995.tb05782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zavadil AP, Ross RJ, Calil HM, et al. The effect of desmethylimipramine on the metabolism of norepinephrine. Life Sci. 1984;35:1061–1068. doi: 10.1016/0024-3205(84)90070-5. [DOI] [PubMed] [Google Scholar]
- 133.Jacobsen TN, Grayburn PA, Snyder RW, 2nd, et al. Effects of intranasal cocaine on sympathetic nerve discharge in humans. J Clin Invest. 1997;99:628–634. doi: 10.1172/JCI119205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sofuoglu M, Nelson D, Babb DA, Hatsukami DK. Intravenous cocaine increases plasma epinephrine and norepinephrine in humans. Pharmacol Biochem Behav. 2001;68:455–459. doi: 10.1016/s0091-3057(01)00482-8. [DOI] [PubMed] [Google Scholar]
- 135.Vincent S, Bieck PR, Garland EM, et al. Clinical assessment of norepinephrine transporter blockade through biochemical and pharmacological profiles. Circulation. 2004;109:3202–3207. doi: 10.1161/01.CIR.0000130847.18666.39. [DOI] [PubMed] [Google Scholar]
- 136.Mayer AF, Schroeder C, Heusser K, et al. Influences of norepinephrine transporter function on the distribution of sympathetic activity in humans. Hypertension. 2006;48:120–126. doi: 10.1161/01.HYP.0000225424.13138.5d. [DOI] [PubMed] [Google Scholar]
- 137.Tank J, Schroeder C, Diedrich A, et al. Selective impairment in sympathetic vasomotor control with norepinephrine transporter inhibition. Circulation. 2003;107:2949–2954. doi: 10.1161/01.CIR.0000072786.99163.FE. [DOI] [PubMed] [Google Scholar]
- 138.Penttila J, Syvalahti E, Hinkka S, Kuusela T, Scheinin H. The effects of amitriptyline, citalopram and reboxetine on autonomic nervous system. A randomised placebo-controlled study on healthy volunteers. Psychopharmacology (Berl) 2001;154:343–349. doi: 10.1007/s002130000664. [DOI] [PubMed] [Google Scholar]
- 139.Kaufmann H, Saadia D, Voustianiouk A, et al. Norepinephrine precursor therapy in neurogenic orthostatic hypotension. Circulation. 2003;108:724–728. doi: 10.1161/01.CIR.0000083721.49847.D7. [DOI] [PubMed] [Google Scholar]
- 140.Goldstein DS, Holmes C, Sewell L, Pechnik S, Kopin IJ. Effects of carbidopa and entacapone on the metabolic fate of the norepinephrine pro-drug L-DOPS. J Clin Pharmacol. 2010 doi: 10.1177/0091270010363476. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shannon JR, Diedrich A, Biaggioni I, Tank J, Robertson RM, Robertson D, Jordan J. Water drinking as a treatment for orthostatic syndromes. Am J Med. 2002;112:355–360. doi: 10.1016/s0002-9343(02)01025-2. [DOI] [PubMed] [Google Scholar]
- 142.Deguchi K, Ikeda K, Sasaki I, et al. Effects of daily water drinking on orthostatic and postprandial hypotension in patients with multiple system atrophy. J Neurol. 2007;254:735–740. doi: 10.1007/s00415-006-0425-3. [DOI] [PubMed] [Google Scholar]
- 143.Levy OA, Malagelada C, Greene LA. Cell death pathways in Parkinson’s disease: Proximal triggers, distal effectors, and final steps. Apoptosis. 2009;14:478–500. doi: 10.1007/s10495-008-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: Implications for Parkinson’s disease pathogenesis. Brain Res. 2003;989:205–213. doi: 10.1016/s0006-8993(03)03354-7. [DOI] [PubMed] [Google Scholar]
- 145.Burke WJ, Schmitt CA, Miller C, Li SW. Norepinephrine transmitter metabolite induces apoptosis in differentiated rat pheochromocytoma cells. Brain Res. 1997;760:290–293. doi: 10.1016/s0006-8993(97)00447-2. [DOI] [PubMed] [Google Scholar]
- 146.Blaschko H. Amine oxidase and amine metabolism. Pharmacol Rev. 1952;4:415–458. [PubMed] [Google Scholar]
- 147.Bonnet JJ, Legros H, Janin F, Dourmap N, Costentin J. Assessment of the in vitro and in vivo toxicity of 3,4-dihydroxyphenylacetaldehyde (DOPAL) Ann Pharm Fr. 2004;62:323–331. doi: 10.1016/s0003-4509(04)94321-0. [DOI] [PubMed] [Google Scholar]
- 148.Burke WJ, Kumar VB, Pandey N, et al. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- 149.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 150.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 151.Villeneuve C, Caudrillier A, Ordener C, Pizzinat N, Parini A, Mialet-Perez J. Dose-dependent activation of distinct hypertrophic pathways by serotonin in cardiac cells. Am J Physiol Heart Circ Physiol. 2009;297:H821–H828. doi: 10.1152/ajpheart.00345.2009. [DOI] [PubMed] [Google Scholar]
- 152.Bianchi P, Kunduzova O, Masini E, et al. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005;112:3297–3305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- 153.Kaludercic N, Takimoto E, Nagayama T, et al. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ Res. 2010;106:193–202. doi: 10.1161/CIRCRESAHA.109.198366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rundqvist B, Elam M, Bergmann-Sverrisdottir Y, Eisenhofer G, Friberg P. Increased cardiac adrenergic drive precedes generalized sympathetic activation in human heart failure. Circulation. 1997;95:169–175. doi: 10.1161/01.cir.95.1.169. [DOI] [PubMed] [Google Scholar]
- 155.Goldstein DS, Holmes C, Bentho O, et al. Biomarkers to detect central dopamine deficiency and distinguish Parkinson disease from multiple system atrophy. Parkinsonism Relat Disord. 2008;14:600–607. doi: 10.1016/j.parkreldis.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]