Several relatively recent case reports and series have described a condition featuring symptoms and signs of acute myocardial infarction without demonstrable coronary artery stenosis or spasm in which the heart takes on the appearance of a Japanese octopus fishing pot called a takotsubo (Figure 1). In takotsubo cardiomyopathy (also called transient apical ballooning and stress cardiomyopathy), left ventricular dysfunction, which can be remarkably depressed, recovers within a few weeks.1–4
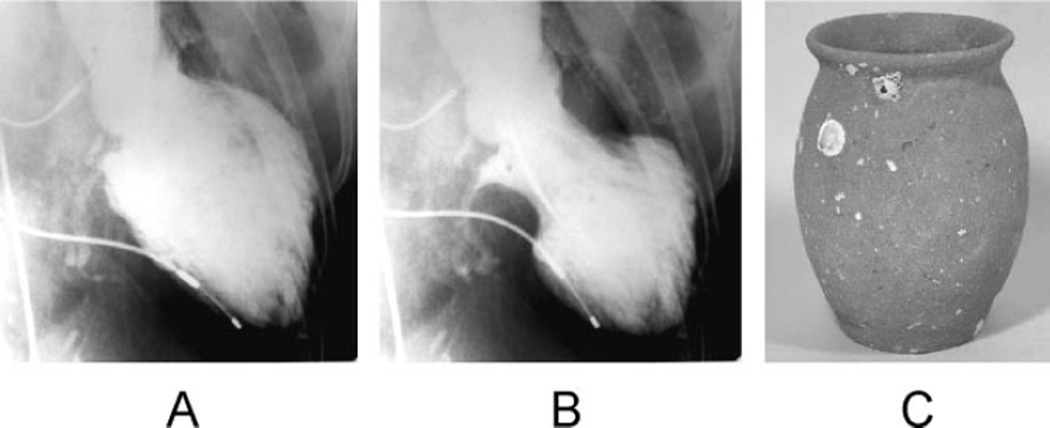
Figure 1.
Left ventriculogram (A, end-diastolic phase; B, end-systolic phase) in the right anterior oblique projection. The extensive area around the apex shows akinesis, and the basal segments display hypercontraction, especially in the end-diastolic phase. C, A picture of a real takotsubo, which has a round bottom and narrow neck to capture octopuses and has been used for a long time in Japan.
Takotsubo cardiomyopathy occurs predominantly in post-menopausal women soon after exposure to sudden, unexpected emotional or physical stress. For instance, the incidence of takotsubo cardiomyopathy increased substantially in elderly women living near the epicenter of the Niigata earthquake.4 Although the left ventricular dysfunction is transient and there is no evidence of obstructive epicardial coronary disease, an increasing number of angioplasty procedures have been performed for presumed acute coronary syndromes.
Concepts about the demographics, clinical features, prognosis, and management of this reversible form of left ventricular failure are still evolving. In this brief review, we summarize recent clinical reports and discuss an animal model that may clarify the pathogenesis of this condition.
History of the Condition
Many case reports and series have demonstrated acute, severe, reversible left ventricular dysfunction that coronary ischemia, aortic valvular lesions, or myocarditis cannot explain. Iga et al5 reported a case of reversible left ventricular dysfunction associated with pheochromocytoma in which the takotsubo appearance was first described, although they did not use the term takotsubo. The authors also inferred that high circulating concentrations of catecholamines could directly damage the myocardium.5 In 1990, Sato et al6 first described this reversible cardiomyopathy as tako-tsubo-like left ventricular dysfunction; outside Japan, this phenomenon was called apical ballooning7 or stress cardiomyopathy.8 After 2000, many case reports cited associations with emotional stress, normal coronary angiography, and minimally increased serum levels of cardiac enzymes.
Diagnostic Criteria
There is as yet no consensus on the diagnostic criteria for takotsubo cardiomyopathy. Researchers at the Mayo Clinic proposed diagnostic criteria in 2004, which have been modified recently9: (1) transient hypokinesis, akinesis, or dyskinesis in the left ventricular mid segments with or without apical involvement; regional wall motion abnormalities that extend beyond a single epicardial vascular distribution; and frequently, but not always, a stressful trigger; (2) the absence of obstructive coronary disease or angiographic evidence of acute plaque rupture; (3) new ECG abnormalities (ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin; and (4) the absence of pheochromocytoma and myocarditis.
Patients were assigned this diagnosis when they satisfied all these criteria. Japanese investigators have recently presented diagnostic guidelines; however, the modified Mayo criteria are commonly used. It is necessary to establish worldwide consensus on diagnostic criteria for takotsubo cardiomyopathy.
Epidemiology
Computer-assisted SCOPUS or MEDLINE searches of the literature for the terms apical ballooning, ampulla cardiomyopathy, tako-tsubo cardiomyopathy, and takotsubo cardiomyopathy between 1989 and December 2007 demonstrated an exponentially increasing frequency of publications. Until 2000, a few case reports were published, but the presentation of takotsubo cardiomyopathy has increased gradually since 2001. The number of publications has increased rapidly; takotsubo cardiomyopathy has probably gained broad attention in the field of cardiology among US and European physicians. On the basis of recent analyses reported from several countries, this condition probably accounts for ≈1% to 2% of all cases of suspected acute myocardial infarction.10
Clinical Features
The Table shows clinical features based on the available literature.1–4,7,8,10–14 Most reports have noted a clear gender discrepancy, with the syndrome much more common in women than men. Takotsubo cardiomyopathy typically is preceded by exposure to emotional or physical stressors such as an unexpected death in the family, abuse, a quarrel, or exhausting work, although in some cases, precipitant stressors have not been identified. The most frequent clinical symptoms of takotsubo cardiomyopathy on admission are chest pain and dyspnea, resembling acute myocardial infarction. Moreover, the ECG findings on admission often include ST elevation in precordial leads. Subsequent T-wave inversion and Q-wave formation also are frequently found. The ECG findings themselves are therefore insufficient to differentiate between acute anterior myocardial infarction and takotsubo cardiomyopathy.15 As of this writing, coronary angiography is the best single tool to diagnose this condition. Most cases lack significant coronary stenoses (Table).
Table.
Patient Clinical and Laboratory Characteristics
| Tsuchihashi et al1 |
Kurowski et al10 |
Kurisu et al2 |
Sharkey et al8 |
Wittstein et al12 |
Inoue et al11 |
Sato et al4 |
Bybee et al7 |
Yoshida et al13 |
Akashi et al3 |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Subjects, n | 88 | 35 | 30 | 22 | 19 | 18 | 16 | 16 | 15 | 13 |
| Country | Japan | Germany | Japan | US | US | Japan | Japan | US | Japan | Japan |
| Series type | Retrospective | Prospective | Retrospective | Prospective | Prospective | Retrospective | Retrospective | Prospective | Prospective | Prospective |
| Age, y | 67±13 | 72±9 | 70±8 | 65±13 | 61±15 | 76±8 | 71±9 | 71±12 | 72±7 | 73±10 |
| Women, % | 86 | 94 | 93 | 91 | 95 | 94 | 94 | 100 | 80 | 85 |
| Preceding emotional stressor, % | 20 | 42 | 17 | 86 | 100 | 11 | … | 38 | 40 | 31 |
| Preceding stressor, % | 43 | 42 | 17 | 14 | … | 39 | 100 | 44 | 40 | 69 |
| Chest pain, % | 67 | … | 67 | 91 | 95 | 72 | 100 | 69 | 87 | 54 |
| ST-segment elevation, % | 90 | 69 | 100 | 59 | 11 | 100 | 56 | 81 | 87 | 92 |
| ST-segment elevation in precordial leads, % |
85 | … | 97 | 59 | … | 100 | … | 81 | … | 92 |
| Q waves, % | 27 | … | … | 45 | 37 | 56† | … | 31 | 7 | … |
| Mean QTc, ms | … | … | … | … | 542* | … | … | 501±55 | 508* | … |
| Elevation in cardiac enzyme levels, % |
56 | … | … | … | … | … | 56 | 100 | … | 85 |
| Initial average LVEF | 0.41±0.11 | 0.5±0.13 | 0.49±0.12 | 0.29±0.09 | 0.20* | … | 0.49±0.04 | 0.4 | 0.43±0.08 | 0.42±0.10 |
| Follow-up LVEF | 0.64±0.10 | 0.68±0.12 | 0.69±0.12 | 0.63±0.06 | 0.60* | … | 0.66±0.03 | 0.6 | 0.76±0.01 | 0.65±0.08 |
| Time of recovery, d | … | … | 11.3±4.3 | 24±29 | 21* | … | 17.7 | 8 | 11±4 | 17±7 |
| Initial Forrester subset | … | … | … | … | … | … | … | … | 1.9±0.3 | |
| Pulmonary edema, % | 22 | … | 3 | 0 | 16 | 28 | 6 | 44 | … | 0 |
| Intraaortic balloon pumping, % | 8 | … | 0 | 18 | 16 | 6 | 0 | 6 | 7 | 15 |
| Coronary stenosis >50%, % | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | … | 0 |
| Angiographically normal coronary arteries, % |
… | 0 | 83 | 100 | 95 | 100 | 100 | 25 | 100 | 100 |
| Spontaneous multivessel spasm, % |
0 | 0 | 10 | … | 0 | 0 | 0 | 0 | 0 | 0 |
| Provocable multivessel spasm n/n (%) |
5/48 (10) | … | 6/14 (43) | … | … | … | 0/6 (0) | … | 1/6 (17) | 0/11 (0) |
| Transient intraventricular pressure gradient, % |
18 | … | … | 23 | … | … | … | 13 | 14 | … |
| In-hospital mortality, % | 1 | 3 (9) | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 8 |
| Documented recurrence, n/n (%) | 2/72 (3) | 2 (6) | 0 | 2/22 (9) | 0 | … | … | 1/16 (6) | … | 0 |
LVEF indicates left ventricular ejection fraction. Values are expressed as mean±SD when appropriate. This table is adapted and modified from Reference 14 with permission.
Median.
In precordial leads.
Patients with takotsubo cardiomyopathy on admission have high levels of serum catecholamines and of plasma brain natriuretic peptide (BNP). The secretion pattern of BNP in takotsubo patients is quite similar to that in myocardial infarction patients, and 1 report presented the precise time course of BNP secretion in patients with takotsubo cardiomyopathy.16 Basal hyperkinesia might be related with left ventricular end-diastolic pressure and BNP release.17 Cardiac enzyme levels (eg, creatinine kinase, troponin T) are slightly increased.
Several patients with takotsubo cardiomyopathy have been evaluated by cardiac magnetic resonance imaging to assess subendocardial necrosis with delayed contrast enhancement techniques. Although the findings are still a matter of debate, lack of delayed enhancement may predict wall motion recovery (Figure 2). Histological changes have been identified by myocardial biopsy in some studies. Further evidence is required, however, to clarify the actual structural findings. Both magnetic resonance imaging and histopathological findings can differentiate patients with takotsubo cardiomyopathy from those with acute myocardial infarction resulting from coronary arterial occlusion.
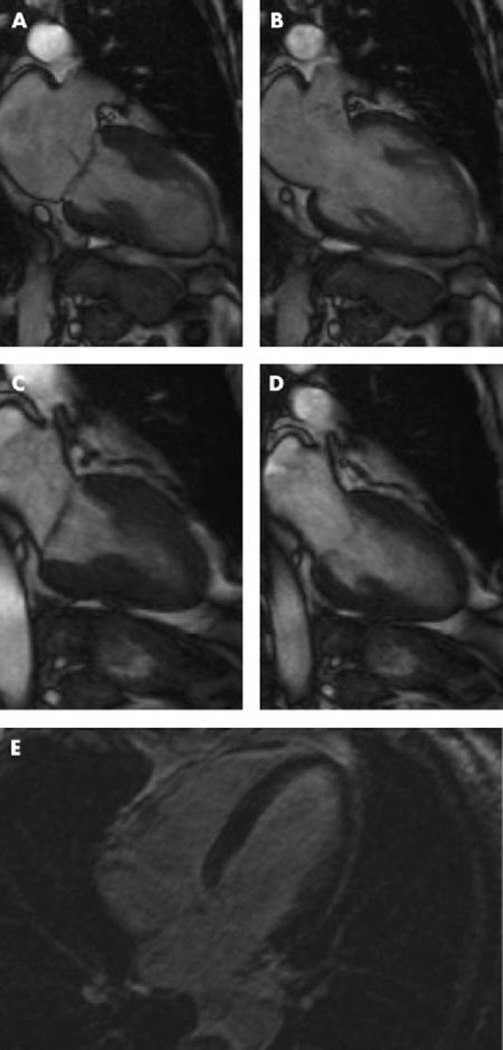
Figure 2.
Cine sequences of cardiac magnetic resonance imaging during systole (A) and diastole (B) in the acute phase. Normal function could be documented after 3 weeks (C, systole; D, diastole). Contrast-enhanced cardiovascular magnetic resonance image did not show myocardial hyperenhancement even in the delayed phase (E). Adapted from Nef et al,50 with permission.
Concepts About Pathophysiology
Most patients with takotsubo cardiomyopathy who underwent myocardial biopsy have shown the same results: interstitial infiltrates consisting primarily of mononuclear lymphocytes, leukocytes, and macrophages; myocardial fibrosis; and contraction bands with or without overt myocyte necrosis. The inflammatory changes and contraction bands distinguish takotsubo cardiomyopathy from coagulation necrosis, as seen in myocardial infarction resulting from coronary artery occlusion. The exact pathophysiological basis of the distinctive contractile pattern in takotsubo cardiomyopathy remains to be elucidated. A few concepts are discussed below.
Multivessel Epicardial Coronary Artery Spasm
Reversible ventricular dysfunction might result from epicardial coronary artery spasm and consequently regionally stunned myocardium.6 When no spontaneous coronary spasm has actually been observed, impaired coronary blood flow caused by vulnerable plaque rupture has been proposed as a cause of takotsubo cardiomyopathy. Multivessel epicardial spasm and regionally stunned myocardium would not explain the discrepancy between severe apical ventricular dysfunction and only slightly increased levels of cardiac enzymes, and after plaque rupture in a single coronary artery, the area of abnormal left ventricular wall motion would not be expected to extend beyond the perfusion territory normally supplied by the artery. Moreover, ECG findings seem to differ between patients with acute coronary syndrome and those with takotsubo cardiomyopathy.11 The latter do not evince reciprocal changes, although ECG findings do not have sufficient predictive value to distinguish takotsubo cardiomyopathy from acute coronary syndrome in individual patients.15 In addition, ischemic myocardial stunning does not produce the histological changes usually observed in takotsubo cardiomyopathy.16 Finally, spontaneous or inducible coronary arterial spasm has not been found in most cases of takotsubo cardiomyopathy. Accordingly, myocardial stunning resulting from epicardial coronary artery spasm does not seem to cause takotsubo cardiomyopathy.
Coronary Microvascular Impairment
Because abnormal left ventricular wall motion occurs in a relatively large area of the apical myocardium in patients with takotsubo cardiomyopathy and because the abnormalities are dynamic rather than fixed, disturbances in the coronary microcirculation might occur. Kume et al18 noted coronary microcirculatory disturbances. Yoshida et al13 reported impaired coronary perfusion and severe myocardial metabolic abnormalities in patients with takotsubo cardiomyopathy on the basis of results of thallium-201 myocardial single-photon emission computed tomography and 18F-fluorodeoxyglucose myocardial positron emission tomography. Elesber et al19 demonstrated the presence of microvascular dysfunction in a significant proportion of patients with this syndrome and noted a correlation between microvascular dysfunction and the severity of myonecrosis and ECG abnormalities.
Taken together, these studies suggest that coronary microcirculatory abnormalities may accompany takotsubo cardiomyopathy; however, the association does not imply a cause-and-effect relationship. For instance, the possibility remains that the microcirculatory abnormalities result from increased mechanical wall stress as a consequence of apical ballooning.
Catecholamine Cardiotoxicity
Wittstein et al12 compared admission plasma catecholamine concentrations between a group of 13 patients with stress cardiopathy who had transient apical ballooning and a group of 7 patients hospitalized for acute myocardial infarction (Killip class III). The plasma levels of both epinephrine and norepinephrine were remarkably increased in the stress cardiopathy patients. The authors suggested that the remarkably elevated catecholamine levels might be the main pathogenetic factor. However, elevated catecholamine levels are not uniformly found in patients with this syndrome.3 High plasma catecholamine levels in patients with pheochromocytoma are well known to induce reversible cardiomyopathy.20
The myocardial histological changes in takotsubo cardiomyopathy strikingly resemble those seen in catecholamine cardiotoxicity in both animals21 and humans.20 These changes, which differ from those in ischemic cardiac necrosis, include contraction band necrosis, neutrophil infiltration, and fibrosis. These findings probably reflect consequences of high intracellular concentrations of Ca2+, and it has been proposed that Ca2+ overload in myocardial cells produces the ventricular dysfunction in catecholamine cardiotoxicity.20 Although diffuse heart failure can produce high circulating catecholamine concentrations, the attained levels are not nearly as high as in takotsubo cardiomyopathy and by definition would not explain the takotsubo pattern.
Because circulating epinephrine exerts far more potent hormonal effects on the heart than norepinephrine does, takotsubo cardiomyopathy could in particular reflect epinephrine-induced toxicity. Concurrent cardiac neuronal and adrenomedullary hormonal stimulation might occur, and this combination accompanies emotional distress. Lyon et al22 have hypothesized that the high circulating epinephrine levels might trigger a switch in cardiomyocyte intracellular signaling after occupation of β2-adrenoceptors from Gs protein to Gi protein coupling.
Neurogenic Stunned Myocardium
The cardiac functional and ECG abnormalities in takotsubo cardiomyopathy might reflect activation of central neurogenic mechanisms analogous to those evoked by subarachnoid hemorrhage.23 Intracranial pathology can produce the same myocardial histopathological findings seen in takotsubo cardiomyopathy.24 Because the basal myocardium has a somewhat higher norepinephrine content25 and greater density of sympathetic nerves than the apical myocardium does,26 at first glance, cardiac sympathetic stimulation would not appear to explain the apical ballooning that characterizes takotsubo cardiomyopathy; however, the left ventricular apex contains a higher concentration of adrenoceptors. Thus, myocardial responsiveness to adrenergic stimulation is pronounced in the apex.27 In addition, cardiac sympathectomy prevents brain-mediated cardiac injury.28 Therefore, takotsubo cardiomyopathy may reflect stunned myocardium from a neurogenic source. Animal studies have reported decreased inotropic responses to norepinephrine in the setting of catecholamine-induced cardiomyopathy29 that is associated with a decreased number of myocardial β-adrenoceptors. Thus, takotsubo cardiomyopathy might reflect a combination of myocardial necrosis and decreased β-adrenoceptor responsiveness with high local catecholamine concentrations that cause both abnormalities. The heart stands out among organs of the body in terms of dependence on neuronal uptake for inactivating catecholamines in the extracellular fluid.30 High circulating catecholamine levels such as in pheochromocytoma can interfere with the neuronal uptake process31 and augment occupation of adrenoceptors on myocardial cells. These findings help to explain why emotional distress would induce mainly cardiac toxicity as a result of high plasma catecholamine levels despite being delivered to all organs via the arterial blood.
In summary, the available pathophysiological information indicates that the apical ballooning that characterizes takotsubo cardiomyopathy reflects toxic high local concentrations of catecholamines, not coronary artery or microvascular disease. The pattern of left ventricular dysfunction may result from both myocardial cellular rupture and withdrawal of β-adrenoceptors. The “first cause” would be neurogenic, with the precipitant sudden, unexpected, severe emotional distress. Stunning-like involvement at the left ventricular base also has been observed in patients with pheochromocytoma and even in takotsubo cardiomyopathy patients.32 Individual differences in the anatomy of cardiac sympathetic innervation or the distributions of adrenoceptors might result in the involvement of a variety of left ventricular myocardial segments. In typical apical ballooning, high local concentrations of norepinephrine might evoke basal hyperkinesis, increasing mechanical wall stress at the apex and thereby increasing end-diastolic pressure and BNP levels.16
Prognosis
The prognosis of patients with takotsubo cardiomyopathy is generally favorable; however, we have had some fatal complications with takotsubo cardiomyopathy such as left ventricular free wall rupture.33 Heart failure, with or without pulmonary edema, is the most common clinical complication. We believe that the published in-hospital mortality data are underestimated. It is important to pay attention to the hemodynamics in the acute phase, which often correspond to New York Heart Association class III heart failure.3 Only a handful of recurrent takotsubo cardiomyopathy cases have been reported. Mechanisms underlying susceptibility to recurrence are not understood. One article has reported that the recurrence rate of takotsubo cardiomyopathy is ≈10%.34
Management
There are no specific treatments for the left ventricular failure characterizing takotsubo cardiomyopathy because cardiac function is normalized within a few weeks. When shock occurs, intraaortic balloon pumping is established as additional support for the circulation.3 We use upright posture, oxygen, and diuretics for pulmonary edema, although given the putative pathophysiological mechanism, it would be reasonable to treat pulmonary edema with sedation and morphine.
Arrhythmia resulting from QT prolongation is commonly observed in patients with takotsubo cardiomyopathy35; however, we do not administer antiarrhythmics prophylactically. In our experience, administration of magnesium sulfate is effective for ventricular tachycardia in the acute phase of takotsubo cardiomyopathy if the QT interval is prolonged.
We believe that administration of cardiotonic agents is not appropriate because of the likely underlying mechanisms. We also do not administer β-adrenoceptor blockers, which can prolong the QT interval and leave unopposed the potentially adverse effects of high local concentrations of catecholamines at α-adrenoceptors. The use of β-adrenoceptor blockers in the acute phase of takotsubo cardiomyopathy is still a matter of debate. Given the findings in the animal model, treatment with a combined α- and β-blocker seems rational, whereas treatment with a catecholamine as a cardiotonic seems contraindicated.36
It should be kept in mind that Adrenalin (Parke-Davis & Co, Detroit, Mich) was originally marketed as a hemostatic agent, not a pressor or cardiotonic. We often encounter thrombosis in takotsubo cardiomyopathy cases,37 which might reflect vasoconstrictor, platelet activation, or prothrombotic effects of extremely high epinephrine levels. Because apical ballooning increases the risk of cardiac rupture, it is still controversial whether treatment with aspirin or heparin is indicated. The fact that epinephrine promotes platelet activation by stimulating platelet α2 adrenoceptors provides additional rationale for treatment with a combined α- and β-blocker.
Because estrogen treatment is beneficial in preventing the animal model of takotsubo cardiomyopathy (see below), such treatment might be considered in elderly women who have suffered an episode of takotsubo cardiomyopathy; however, clinical trials of estrogen administration in takotsubo cardiomyopathy patients have not been performed.
Animal Model of Takotsubo Cardiomyopathy
A number of important pathophysiological questions remain. Why are elderly women particularly susceptible to developing takotsubo cardiomyopathy? How does profound psychological stress trigger the condition? Finally, is regional heterogeneity of adrenoceptor numbers sufficient to explain apical ballooning? The development of appropriate animal models has begun to address these issues.
Experimental approaches to mimic the clinical manifestations may provide new insights into the pathogenesis of takotsubo cardiomyopathy. Clinical provocation tests to induce this condition have not been reported and ethically are not acceptable. Therefore, in the development of an appropriate animal model, we attempted to replicate severe, emotional distress–induced sympathetic neuronal and adrenomedullary activation, as seen in takotsubo cardiomyopathy.
In rats, simple immobilization is well known to evoke profound sympathoadrenal activation.38 During immobilization, the increment in plasma epinephrine is derived wholly from the adrenal medulla, whereas most of the increment in plasma norepinephrine is derived from sympathetic nerves. Patients with takotsubo cardiomyopathy have increased plasma epinephrine and norepinephrine levels compared with those seen during immobilization in rats,12 suggesting that immobilization in rats mimics the clinical condition.
Increased circulating epinephrine levels within a physiological range are well known to result in skeletal vasodilation and increases in heart rate and cardiac output, whereas norepinephrine increases total peripheral resistance to blood flow and mean arterial blood pressure. At relatively low circulating levels, there are no appreciable ECG or left ventricular functional abnormalities. In contrast, in response to immobilization, pathologically high epinephrine and norepinephrine levels are associated with characteristic ECG changes, including ST-segment elevation and, importantly, reversible left ventricular apical ballooning (Figure 3), strikingly reproducing the abnormalities seen in takotsubo cardiomyopathy.36 An instance of sudden death caused by ventricular fibrillation in response to immobilization has been noted (Figure 3). Immobilization induces upregulation of immediate early genes, functional markers of cellular excitation36,39 such as c-fos and c-jun in endothelial cells, myocardial cells, and coronary vascular smooth muscle cells (Figure 3). Expression of immediate early genes diffusely in coronary arteries suggests possible coronary spastic changes and consequent microvascular dysfunction.
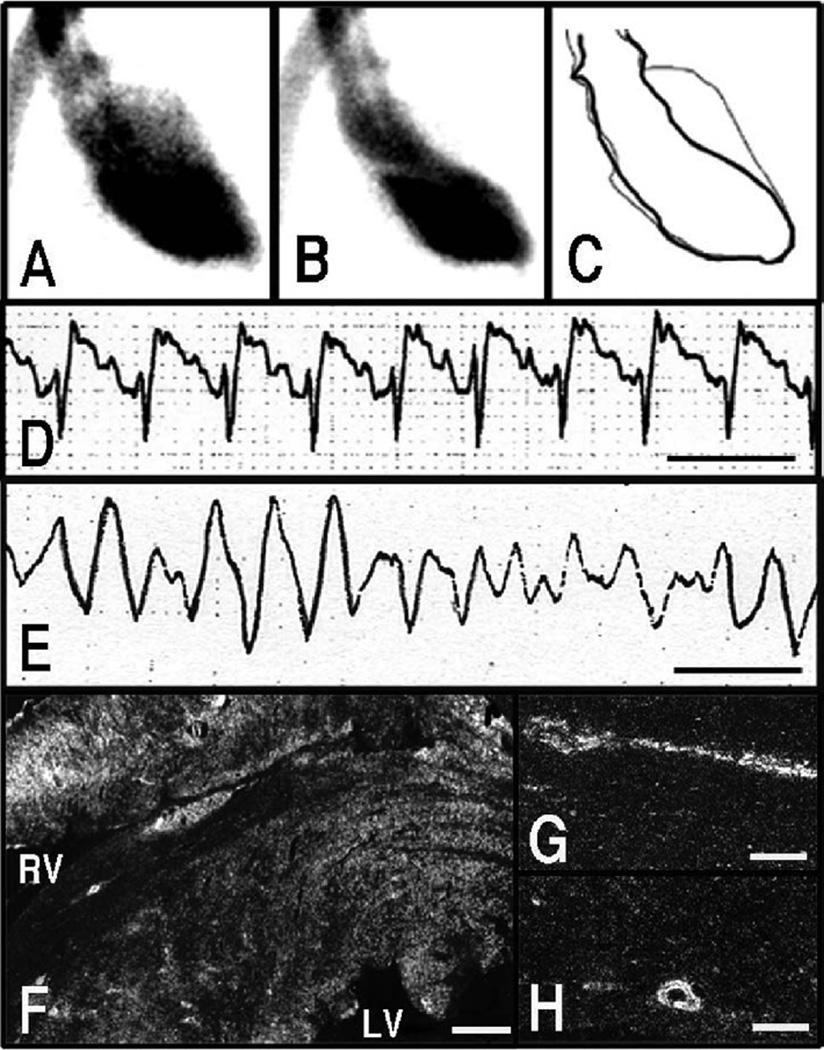
Figure 3.
Left ventriculographic (A through C) and ECG (D and E) changes in response to immobilization of rats. Left ventriculogram, right anterior oblique (30°) projection. A, Diastole. B, systole. C, The trace of A and B. Reduced left ventricular contraction around the left ventricular apex was observed in response to stress. ECG, lead II. Line indicates 0.2 second. ST-segment elevation (D) was observed in response to stress. A case of ventricular fibrillation also was observed (E). Dark-field photomicrograph showing signals for c-jun mRNA in the heart (F) and coronary arteries (G and H) sampled at 30 minutes from the onset of immobilization. Strong signals were observed in myocardium surrounding the left and right ventricular cavities (F). These signals also were observed in endothelial cells and smooth muscle cells of coronary arteries (G and H). Bar=600 µm (F) and 100 µm (G and H). A through C, Adapted and modified from Ueyama et al,51 with permission from the Japanese Circulation Society.
ST-segment elevation in rats subjected to immobilization is prevented by combined blockade of α- and β-adrenoceptors (not by α- or β-blockade alone), calcium channel blockers, or nitroglycerin.36 Left ventricular dysfunction and induction of immediate early genes in the heart also are prevented by combined blockade of α- and β-adrenoceptors. These findings suggest that high myocardial concentrations of catecholamines and consequent activation of adrenoceptors in the heart produce the acute cardiac changes.36
Estrogen receptors (ERα and ERβ) are expressed widely in the cardiovascular and central nervous systems. Estrogens exert various functions, including prevention of some cardiovascular diseases, modulation of sexual behavior and memory processes, and some autonomic nervous functions.40,41 We therefore have hypothesized that the reduced estrogen levels after menopause explain the predisposition of elderly women to takotsubo cardiomyopathy. Estrogen supplementation attenuated the immobilization-induced cardiac dysfunction and increased heart rate and blood pressure.42 These effects also were observed in estrogen-treated castrated male rats (F. Ichikura, unpublished observation). The reduced estrogen levels induced vulnerability to stress, whereas estrogen supplementation attenuated the exaggerated responses, including sympathoadrenal activation and vagal inhibition,43 which is observed in takotsubo cardiomyopathy.28,29
Emotional stress also causes rapid and transient expression of immediate early genes in the brain, and monitoring of expression of these genes has enabled visualization of the central neurocircuitry of distress.39 Treatment with estrogen attenuated the immobilization-induced increase in c-Fos immunoreactivity or c-fos mRNA expression in the lateral septum, medial amygdaloid nucleus, paraventricular hypothalamic nucleus, dorsomedial hypothalamic nucleus, laterodorsal tegmental nucleus, and locus caeruleus, regions that are parts of the central autonomic network and possess immunoreactive estrogen receptors.44 Estrogen attenuated immobilization-induced c-fos mRNA expression in the adrenal gland and heart, suggesting protective effects at the level of the target organs.42 Estrogen treatment also increased the levels of possibly cardioprotective substances such as atrial natriuretic peptide and heat shock protein 70 in the heart.42 Moreover, administration of estrogen attenuated isoproterenol-induced tachycardia and cAMP production and reduced the incidence of ischemia/reperfusion-induced arrhythmia in rat hearts.45 Conversely, bilateral ovariectomy increased Ca2+ sensitivity of cardiac myofilaments, and estrogen replacement abolished this change.46 The density and protein content of β1-adrenergic receptors were upregulated in bilateral ovariectomy, and estrogen/progesterone supplementation reversed these changes.47 Taken together, these data suggest that reduced estrogen levels, by actions on both the nervous system and the heart, after menopause might constitute the basis of susceptibility of elderly women to takotsubo cardiomyopathy. To test this hypothesis, clinical data comparing incidences of takotsubo cardiomyopathy with or without estrogen supplementation are required. The estrogen hypothesis alone does not seem sufficient to explain the occurrence—albeit uncommon—of takotsubo cardiomyopathy in men.
Recently, we have found that the expression of oxidative stress-related substances such as heme oxigenase-1 and cyclooxygenase-2 is increased in the cardiovascular system of rats after exposure to immobilization and that treatment with α- and β-adrenoceptor blockers attenuates these responses (unpublished observation). Therefore, stress-induced high circulating catecholamine levels may alter redox states in the cardiovascular system. Induction of heme oxigenase-1 and cyclooxygenase-2 may suggest oxidative stress and adaptive responses in the cardiovascular system.
A Pathogenetic Concept About Takotsubo Cardiomyopathy
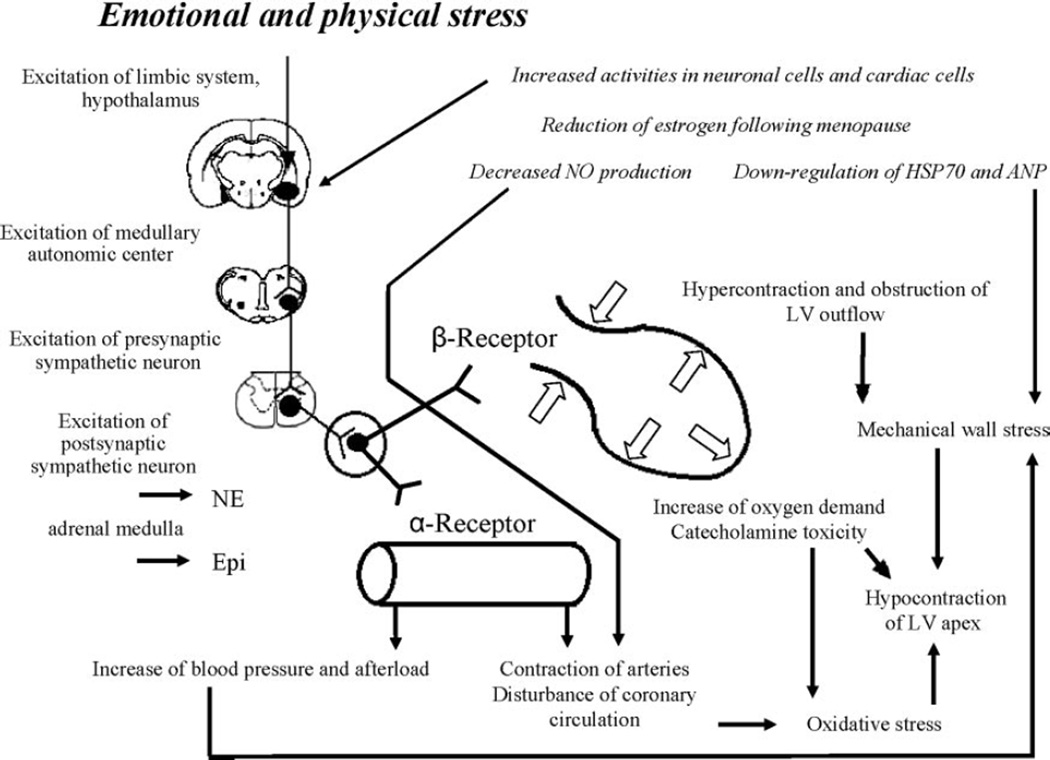
On the basis of the above-cited clinical literature and findings from the rat immobilization model, we propose a possible mechanism for the pathogenesis of takotsubo cardiomyopathy (Figure 4). In response to sudden, unexpected, severe emotional distress, neurons of the central autonomic network expressing estrogen receptors are activated, followed by marked increases in sympathetic neuronal and adrenomedullary hormonal outflows. Epinephrine released from the adrenal medulla and norepinephrine from cardiac and extracardiac sympathetic nerves reach adrenoceptors in the blood vessels and heart. Contraction of the resistance vessels rapidly increases systemic blood pressure and cardiac afterload. Meanwhile, within the heart, high circulating levels of norepinephrine and epinephrine, along with increased release and decreased reuptake by sympathetic nerves, induce catecholamine toxicity in the cardiomyocytes via occupation of adrenoceptors. Hypercontraction and possibly functional basal obstruction of the left ventricular outflow result in increased mechanical wall stress in the left ventricular apex, in conjunction with high BNP levels and increased end-diastolic pressure.17 Contraction bands and myocardial cell rupture occur in a regionally heterogeneous manner related to greater apical expression of adrenoceptors. Disturbance in the coronary microcirculation, as suggested by diffuse expression of immediate early genes in coronary arteries, and increases in oxygen demand in the cardiomyocytes alter the redox state and trigger oxidative stress, which precipitates a variety of positive feedback loops that lead to stunning of the apical myocardium. In postmenopausal women, the loss of estrogen effects exaggerates the responses of central neurons and cardiac cells and possibly attenuates the production of cardioprotective substances.
Figure 4.
Possible underlying mechanism of classic takotsubo cardiomyopathy. See text for details.
Atypical Contractile Pattern of Takotsubo Cardiomyopathy
Recent case reports have described takotsubo cardiomyopathy associated with suppression of basal contraction and apical sparing, a kind of “inverted takotsubo.”48 Kurowski et al10 found that ≈40% of patients with transient ventricular dysfunction had this atypical pattern. We also have reported a case of recurrent takotsubo cardiomyopathy with a different contractile pattern.48 Ischemia resulting from multivessel coronary vasospasm seems unlikely in these cases because it would not be expected to be associated with apical sparing. Various patterns of left ventricular wall motion abnormalities therefore seem possible in takotsubo cardiomyopathy, with transient left ventricular apical ballooning being more common and other regional wall motion abnormalities less common. Further research in both clinical and preclinical settings is required to determine whether individual differences in patterns of wall motion abnormalities are related to local differences in patterns of excessive sympathetic activity or in responses to sympathetic stimulation.
Conclusions
The recently recognized syndrome of takotsubo cardiomyopathy constitutes a novel form of heart failure that is precipitated by sudden, unexpected emotional distress and is relatively common in elderly women. Precipitating mechanisms are probably complex, and the observations detailed in this review represent only the beginning of the process of understanding this condition. Abnormal catecholamine dynamics related to emotional distress seems to play a major role in the pathogenesis of this cardiomyopathy, rendering takotsubo cardiomyopathy a type of neurocardiological disorder that manifests as acute but reversible heart failure. Combined α-and β-adrenoceptor blockade might therefore mitigate the syndrome. Theoretically, on the basis of findings in the rat immobilization model, supplementation of estrogen in postmenopausal women might protect against its development. This cardiomyopathy should be considered a possible cause of sudden cardiac death resulting from arrhythmia or cardiac rupture in individuals without obvious heart disease. The prognosis is good in patients who survive the initial severe insult without complications. To understand the pathogenesis and to devise rational treatment and prevention strategies, more attention should be paid not only to the myocardium and coronary arteries but also to the integration of central neural, autonomic, endocrine, and circulatory systems in emotional distress.49
Acknowledgments
Source of Funding
Dr Goldstein is supported by the Division of Intramural Research, National Institute of Neurological Disorders and Stroke, National Institutes of Health (Bethesda, Md). Dr Ueyama is supported by a grant-in-aid for scientific research (Japan Society for the Promotion of Science, Japan).
Footnotes
Disclosures
None.
References
- 1.Tsuchihashi K, Ueshima K, Uchida T, Oh-Mura N, Kimura K, Owa M, Yoshiyama M, Miyazaki S, Haze K, Ogawa H, Honda T, Hase M, Kai R, Morii I. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction: Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001;38:11–18. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 2.Kurisu S, Sato H, Kawagoe T, Ishihara M, Shimatani Y, Nishioka K, Kono Y, Umemura T, Nakamura S. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J. 2002;143:448–455. doi: 10.1067/mhj.2002.120403. [DOI] [PubMed] [Google Scholar]
- 3.Akashi YJ, Musha H, Kida K, Itoh K, Inoue K, Kawasaki K, Hashimoto N, Miyake F. Reversible ventricular dysfunction takotsubo cardiomyopathy. Eur J Heart Fail. 2005;7:1171–1176. doi: 10.1016/j.ejheart.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Sato M, Fujita S, Saito A, Ikeda Y, Kitazawa H, Takahashi M, Ishiguro J, Okabe M, Nakamura Y, Nagai T, Watanabe H, Kodama M, Aizawa Y. Increased incidence of transient left ventricular apical ballooning (socalled “Takotsubo” cardiomyopathy) after the mid-Niigata Prefecture earthquake. Circ J. 2006;70:947–953. doi: 10.1253/circj.70.947. [DOI] [PubMed] [Google Scholar]
- 5.Iga K, Gen H, Tomonaga G, Matsumura T, Hori K. Reversible left ventricular wall motion impairment caused by pheochromocytoma: a case report. Jpn Circ J. 1989;53:813–818. doi: 10.1253/jcj.53.813. [DOI] [PubMed] [Google Scholar]
- 6.Sato H, Tateishi H, Uchida T. Takotsubo-type cardiomyopathy due to multivessel spasm. In: Kodama K, Haze K, Hon M, editors. Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure. Tokyo, Japan: Kagakuhyouronsha; 1990. pp. 56–64. [Google Scholar]
- 7.Bybee KA, Prasad A, Barsness GW, Lerman A, Jaffe AS, Murphy JG, Wright RS, Rihal CS. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94:343–346. doi: 10.1016/j.amjcard.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Sharkey SW, Lesser JR, Zenovich AG, Maron MS, Lindberg J, Longe TF, Maron BJ. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472–479. doi: 10.1161/01.CIR.0000153801.51470.EB. [DOI] [PubMed] [Google Scholar]
- 9.Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Kurowski V, Kaiser A, von Hof K, Killermann DP, Mayer B, Hartmann F, Schunkert H, Radke PW. Apical and midventricular transient left ventricular dysfunction syndrome (tako-tsubo cardiomyopathy): frequency, mechanisms, and prognosis. Chest. 2007;132:809–816. doi: 10.1378/chest.07-0608. [DOI] [PubMed] [Google Scholar]
- 11.Inoue M, Shimizu M, Ino H, Yamaguchi M, Terai H, Fujino N, Sakata K, Funada A, Tatami R, Ishise S, Kanaya H, Mabuchi H. Differentiation between patients with takotsubo cardiomyopathy and those with anterior acute myocardial infarction. Circ J. 2005;69:89–94. doi: 10.1253/circj.69.89. [DOI] [PubMed] [Google Scholar]
- 12.Wittstein IS, Thiemann DR, Lima JAC, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida T, Hibino T, Kako N, Murai S, Oguri M, Kato K, Yajima K, Ohte N, Yokoi K, Kimura G. A pathophysiologic study of tako-tsubo cardiomyopathy with F-18 fluorodeoxyglucose positron emission tomography. Eur Heart J. 2007;28:2598–2604. doi: 10.1093/eurheartj/ehm401. [DOI] [PubMed] [Google Scholar]
- 14.Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27:1523–1529. doi: 10.1093/eurheartj/ehl032. [DOI] [PubMed] [Google Scholar]
- 15.Bybee KA, Motiei A, Syed IS, Kara T, Prasad A, Lennon RJ, Murphy JG, Hammill SC, Rihal CS, Wright RS. Electrocardiography cannot reliably differentiate transient left ventricular apical ballooning syndrome from anterior ST-segment elevation myocardial infarction. J Electrocardiol. 2007;40:38.e1–38.e6. doi: 10.1016/j.jelectrocard.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Nef HM, Mollmann H, Weber M, Deetjen A, Brandt R, Hamm CW, Elsasser A. Release pattern of cardiac biomarkers in left ventricular apical ballooning. Int J Cardiol. 2007;115:128–129. doi: 10.1016/j.ijcard.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 17.Akashi YJ, Musha H, Nakazawa K, Miyake F. Plasma brain natriuretic peptide in takotsubo cardiomyopathy. Q J Med. 2004;97:599–607. doi: 10.1093/qjmed/hch094. [DOI] [PubMed] [Google Scholar]
- 18.Kume T, Akasaka T, Kawamoto T, Yoshitani H, Watanabe N, Neishi Y, Wada N, Yoshida K. Assessment of coronary microcirculation in patients with takotsubo-like left ventricular dysfunction. Circ J. 2005;69:934–939. doi: 10.1253/circj.69.934. [DOI] [PubMed] [Google Scholar]
- 19.Elesber A, Lerman A, Bybee KA, Murphy JG, Barsness G, Singh M, Rihal CS, Prasad A. Myocardial perfusion in apical ballooning syndrome correlate of myocardial injury. Am Heart J. 2006;152:469.e9–469.e13. doi: 10.1016/j.ahj.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Frustaci A, Loperfido F, Gentiloni N, Caldarulo M, Morgante E, Russo MA. Catecholamine-induced cardiomyopathy in multiple endocrine neoplasia: a histologic, ultrastructural, and biochemical study. Chest. 1991;99:382–385. doi: 10.1378/chest.99.2.382. [DOI] [PubMed] [Google Scholar]
- 21.Movahed A, Reeves WC, Mehta PM, Gilliland MG, Mozingo SL, Jolly SR. Norepinephrine-induced left ventricular dysfunction in anesthetized and conscious, sedated dogs. Int J Cardiol. 1994;45:23–33. doi: 10.1016/0167-5273(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 22.Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy: a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5:22–29. doi: 10.1038/ncpcardio1066. [DOI] [PubMed] [Google Scholar]
- 23.Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- 24.White M, Wiechmann RJ, Roden RL, Hagan MB, Wollmering MM, Port JD, Hammond E, Abraham WT, Wolfel EE, Lindenfeld J, Fullerton D, Bristow MR. Cardiac fl-adrenergic neuroeffector systems in acute myocardial dysfunction related to brain injury: evidence for catecholamine-mediated myocardial damage. Circulation. 1995;92:2183–2189. doi: 10.1161/01.cir.92.8.2183. [DOI] [PubMed] [Google Scholar]
- 25.Pierpont GL, DeMaster EG, Cohn JN. Regional differences in adrenergic function within the left ventricle. Am J Physiol Heart Circ Physiol. 1984;246:H824–H829. doi: 10.1152/ajpheart.1984.246.6.H824. [DOI] [PubMed] [Google Scholar]
- 26.Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18:32–39. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- 27.Mori H, Ishikawa S, Kojima S, Hayashi J, Watanabe Y, Hoffman JI, Okino H. Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli. Cardiovasc Res. 1993;27:192–198. doi: 10.1093/cvr/27.2.192. [DOI] [PubMed] [Google Scholar]
- 28.Novitzky D, Wicomb WN, Cooper DK, Rose AG, Reichart B. Prevention of myocardial injury during brain death by total cardiac sympathectomy in the Chacma baboon. Ann Thorac Surg. 1986;41:520–524. doi: 10.1016/s0003-4975(10)63032-9. [DOI] [PubMed] [Google Scholar]
- 29.Fripp RR, Lee JC, Downing SE. Inotropic responsiveness of the heart in catecholamine cardiomyopathy. Am Heart J. 1981;101:17–21. doi: 10.1016/0002-8703(81)90378-1. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharmacol Exp Ther. 2003;305:800–811. doi: 10.1124/jpet.103.049270. [DOI] [PubMed] [Google Scholar]
- 31.Eldadah BA, Pacak K, Eisenhofer G, Holmes C, Kopin IJ, Goldstein DS. Cardiac uptake-1 inhibition by high circulating norepinephrine levels in patients with pheochromocytoma. Hypertension. 2004;43:1227–1232. doi: 10.1161/01.HYP.0000127305.87552.d6. [DOI] [PubMed] [Google Scholar]
- 32.Bonnemeier H, Schafer U, Schunkert H. Apical ballooning without apical ballooning. Eur Heart J. 2006;27:2246. doi: 10.1093/eurheartj/ehi820. [DOI] [PubMed] [Google Scholar]
- 33.Akashi YJ, Tejima T, Sakurada H, Matsuda H, Suzuki K, Kawasaki K, Tsuchiya K, Hashimoto N, Musha H, Sakakibara M, Nakazawa K, Miyake F. Left ventricular rupture associated with Takotsubo cardiomyopathy. Mayo Clin Proc. 2004;79:821–824. doi: 10.4065/79.6.821. [DOI] [PubMed] [Google Scholar]
- 34.Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. 2007;50:448–452. doi: 10.1016/j.jacc.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 35.Konety SH, Horwitz P, Lindower P, Olshansky B. Arrhythmias in tako-tsubo syndrome: benign or malignant? Int J Cardiol. 2007;114:141–144. doi: 10.1016/j.ijcard.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 36.Ueyama T. Emotional stress-induced Tako-tsubo cardiomyopathy: animal model and molecular mechanism. Ann N Y Acad Sci. 2004;1018:437–444. doi: 10.1196/annals.1296.054. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki N, Kinugawa T, Yamawaki M, Furuse Y, Shimoyama M, Ogino K, Igawa O, Hisatome I, Shigemasa C. Transient left ventricular apical ballooning in a patient with bicuspid aortic valve created a left ventricular thrombus leading to acute renal infarction. Circ J. 2004;68:1081–1083. doi: 10.1253/circj.68.1081. [DOI] [PubMed] [Google Scholar]
- 38.Kvetnansky R, Weise VK, Thoa NB, Kopin IJ. Effects of chronic guanethidine treatment and adrenal medullectomy on plasma levels of catecholamines and corticosterone in forcibly immobilized rats. J Pharmacol Exp Ther. 1979;209:287–291. [PubMed] [Google Scholar]
- 39.Senba E, Ueyama T. Stress-induced expression of immediate early genes in the brain and peripheral organs of the rat. Neurosci Res. 1997;29:183–207. doi: 10.1016/s0168-0102(97)00095-3. [DOI] [PubMed] [Google Scholar]
- 40.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 41.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Ueyama T, Ishikura F, Matsuda A, Asanuma T, Ueda K, Ichinose M, Kasamatsu K, Hano T, Akasaka T, Tsuruo Y, Morimoto K, Beppu S. Chronic estrogen supplementation following ovariectomy improves the emotional stress-induced cardiovascular responses by indirect action on the nervous system and by direct action on the heart. Circ J. 2007;71:565–573. doi: 10.1253/circj.71.565. [DOI] [PubMed] [Google Scholar]
- 43.Komesaroff PA, Esler MD, Sudhir K. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. J Clin Endocrinol Metab. 1999;84:606–610. doi: 10.1210/jcem.84.2.5447. [DOI] [PubMed] [Google Scholar]
- 44.Ueyama T, Tanioku T, Nuta J, Kujira K, Ito T, Nakai S, Tsuruo Y. Estrogen alters c-Fos response to immobilization stress in the brain of ovariectomized rats. Brain Res. 2006;1084:67–79. doi: 10.1016/j.brainres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Li HY, Bian JS, Kwan YW, Wong TM. Enhanced responses to 17beta-estradiol in rat hearts treated with isoproterenol: involvement of a cyclic AMP-dependent pathway. J Pharmacol Exp Ther. 2000;293:592–598. [PubMed] [Google Scholar]
- 46.Wattanapermpool J, Riabroy T, Preawnim S. Estrogen supplement prevents the calcium hypersensitivity of cardiac myofilaments in ovariectomized rats. Life Sci. 2000;66:533–543. doi: 10.1016/s0024-3205(99)00623-2. [DOI] [PubMed] [Google Scholar]
- 47.Thawornkaiwong A, Preawnim S, Wattanapermpool J. Upregulation of beta 1-adrenergic receptors in ovariectomized rat hearts. Life Sci. 2003;72:1813–1824. doi: 10.1016/s0024-3205(02)02473-6. [DOI] [PubMed] [Google Scholar]
- 48.Blessing E, Steen H, Rosenberg M, Katus H, Frey N. Recurrence of takotsubo cardiomyopathy with variant forms of left ventricular dysfunction. J Am Soc Echocardiogr. 2007;20:439.e11–439.e12. doi: 10.1016/j.echo.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 49.Goldstein DS. Adrenaline and the Inner World: An Introduction to Scientific Integrative Medicine. Baltimore, Md: Johns Hopkins University Press; 2006. [Google Scholar]
- 50.Nef HM, Möllmann H, Elsässer A. Tako-tsubo cardiomyopathy (apical ballooning) Heart. 2007;93:1309–1315. doi: 10.1136/hrt.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueyama T, Kasamatsu K, Hano T, Yamamoto K, Tsuruo Y, Nishio I. Emotional stress induces transient left ventricular hypocontraction in the rat via activation of cardiac adrenoceptors: a possible animal model of ‘tako-tsubo’ cardiomyopathy. Circ J. 2002;66:712–713. doi: 10.1253/circj.66.712. [DOI] [PubMed] [Google Scholar]