Summary
Time-resolved fluorescence resonance energy transfer, TR-FRET, is a time-gated fluorescence intensity measurement which defines the relative proximity of two biomolecules (e.g., proteins, peptides, or DNA) based on the extent of non-radiative energy transfer between two fluorophores with overlapping emission/excitation spectra. In these assays, an excited lanthanide ion acts as a “donor” that transfers energy to an “acceptor” fluorophore through dipole-dipole interactions. A FRET signal is reported as the ratio of acceptor to donor emission following donor excitation. When a donor-conjugated protein interacts with an acceptor-conjugated protein, the donor and acceptor fluorophores are brought in close proximity allowing energy transfer from the donor to the acceptor resulting in a FRET signal. Because the lanthanide donors have a long emission half-life, the energy transfer measurement can be time-gated, which dramatically reduces assay interference (due to background autofluorescence and direct acceptor excitation) and thereby increases data quality. Here, we describe a TR-FRET assay that monitors the interaction of the estrogen receptor (ER) α ligand binding domain (labeled with a terbium chelate via a streptavidin-biotin interaction,) with a sequence of coactivator protein SRC3 (labeled directly with fluorescein) and the disruption of this interaction with a peptide and a small molecule inhibitor.
Keywords: TR-FRET, protein-protein interaction, lanthanide, long-lifetime donor, fluorophore, protein labeling
1. Introduction
The transcription-regulating function of the estrogen receptors (ERs), ERα and ERβ, relies on their interaction with coactivator proteins. The best studied coactivators are members of the p160 class of steroid receptor coactivators (SRCs) that functionally link ER with modification of chromatin structure and activation of the basal transcriptional machinery (1). The interaction of the SRCs with ER is regulated by ligand-induced conformation of the ER ligand-binding domain (LBD) where coactivator proteins are bound to ER in the presence of agonist ligands, such as estradiol. Thus, it is intuitive that a reliable and robust assay able to probe the interaction state of the estrogen receptor with its coactivator protein would allow further elucidation of the dynamics of this interaction, as well as provide a tool which could be used in high-throughput screening for the identification and development of inhibitors of these two biologically relevant proteins.
A number of assays have been developed to study receptor-coactivator interactions. For instance, glutathione S-transferase (GST)-pull down and related assays have been used to study receptor-coactivator interactions, but these assays are rather labor intensive (2,3). An easily employed assay that we (4) and others (5,6) have described is based on the principle of fluorescence polarization (FP) and monitors the interaction of a fluorophore-labeled SRC LXXLL peptide with the ER LBD. Unfortunately, this assay has low dynamic range and also requires high ER concentrations (200 nM), which make accurate determination of Ki values difficult and costly. Some groups have reported FRET-based assays (see chapter 17 for more details regarding FRET assays) to examine nuclear receptor-coactivator interactions, but we have found certain features of these assays make them less than ideal: blocking and washing steps, expensive lanthanide-conjugated antibodies (7–9), or expensive biologic fluorophores (8).
As we found the state-of-the-art assays that were available to study ER/coactivator interactions less than optimal, we developed a TR-FRET assay that is amenable to a high-throughput screening format (10,11). The assay we developed uses TR-FRET to monitor the interaction between the ER LBD labeled (via a streptavidin-biotin interaction) with a terbium chelate and a fluorescein-labeled sequence of the SRC3 coactivator protein (see Figure 1). Terbium functions as a long-lifetime (ca. millisecond) luminescent donor, and the fluorescein serves as the TR-FRET acceptor. This assay is superior to organic dye FRET because the emission half-life of fluorescein is short (nsec) relative to that of the terbium complex (msec half-life) (12). Background emission stemming from direct excitation of fluorescein or endogenous cellular fluorophores can thus be eliminated by pulsing the terbium complex at the excitation wavelength and gating the emission with a 50-µsec delay. When properly optimized, the TR-FRET method gives a good signal-to-noise ratio and can be run in a straightforward, mix-and-measure format with very low concentrations of terbium-labeled streptavidin and biotin-labeled ER-LBD.
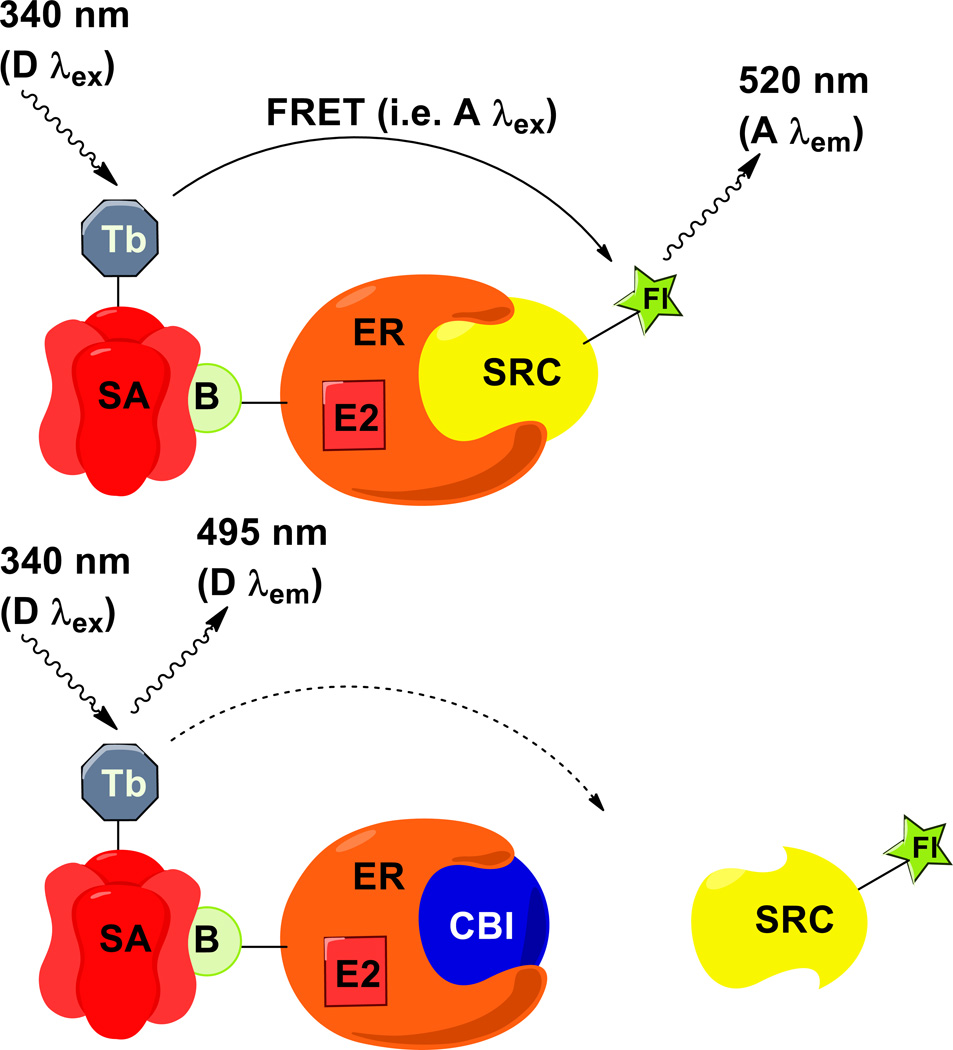
Figure 1.
Schematic of the time-resolved fluorescence resonance energy transfer (FRET) assay. (Top) In general, FRET occurs when an emission wavelength of a donor molecule (D λem; e.g., 495 nm) overlaps with the excitation wavelength of a nearby acceptor (A λex), resulting in an emission signal from the acceptor (A λem; e.g., 520 nm). FRET occurs between the streptavidin-terbium (SA-Tb) donor and the fluorescein-steroid receptor coactivator (SRC-Fl) acceptor when SRC-Fl is recruited to the biotin-labeled estrogen receptor (B-ER) bound with the agonist ligand 17 β-estradiol (E2). (Bottom) In the presence of coactivator binding inhibitor (CBI), this assembly is disrupted, and the FRET signal decreases.
We note that, in a previous publication (11), we detailed the use of a Cy5-europium pair that was developed in collaboration with our colleagues at Emory University. Using this pair is advantageous because it allows the monitoring of acceptor emissions at longer wavelengths than fluorescein. Autofluorescent compounds found in libraries typically emit at wavelengths shorter than 550 nm; thus, when used in a high-throughput format, the Eu-Cy5 system is a better choice for minimizing false positives arising from interfering emission patterns. The reason we have detailed the terbium-fluorescein pair here is because we found it to give better signal-to-noise than the Eu-Cy5 pair when using our particular plate reader (VICTOR multi-label plate reader) for routine dose-response assays. In general, if an assay is needed for a high-throughput screen, we would recommend the Eu-Cy5 pair.
We have developed this assay and explained in detail below the steps necessary to replicate it using ER alpha. We (13–16) and others (17–21) have since generalized the assay to other nuclear hormone receptors and coactivator protein segments, and we encourage other users to do the same. This does sometimes require fine-tuning of assay component concentrations, but, generally, the results are very accurate and reliable.
2. Materials
Prepare all solutions using autoclaved, deionized water and analytical grade reagents. Prepare and store all reagents at room temperature (unless indicated otherwise).
SRC1-Box II peptide. Store wrapped in foil at −20 °C. (see Note 1)
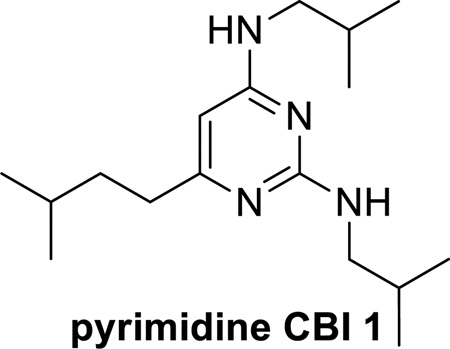
Pyrimidine coactivator binding inhibitor (CBI) 1. Store at −20 °C. (see Note 2)
20 mM solutions of test compounds in DMSO (or DMF). Store at −20 °C.
ERα-417-biotin and SRC-3-NRD-fluorescein. Store at −20 °C. (see Note 3)
200 nM fluorescein-SRC-3-NRD. Store at 4 °C (see Note 4).
1 mM solution of 17β-estradiol in DMF. Store at −20 °C.
LanthaScreen™ Streptavidin-Terbium (Invitrogen). Store at −20 °C.
TR-FRET buffer: 20 mM Tris-HCl, pH 7.5, 0.01% NP40, 50 mM NaCl; adjust pH with conc. HCl to pH 7.5 (see Notes 5, 6, 7, and 8)
96-well black HE high efficiency microplate (Molecular Devices) (see Note 9)
96-well polypropylene plate (see Note 10)
Eight-channel autopipettor with tips (0–20 µL capacity) (see Note 11)
Single-channel manual pipettors with tips (0–1000 µL capacity) (see Note 12)
VICTOR™ Multilabel plate reader (Perkin-Elmer) (see Note 13)
3. Methods
Prepare a 4 × stock solution (Solution A) containing the following components at the following concentrations: ERα-417 (8 nM), 17β-estradiol (4 µM), and LanthaScreen™ Streptavidin-Terbium (2 nM) in TR-FRET buffer. Keep this solution at 0–5 °C (see Notes 14 and 15).
In a 96-well colorless polypropylene plate, serially dilute (see Note 16) each 20 mM solution of test compound, including positive controls pyrimidine CBI 1 and SRC1-Box II peptide, using DMF (see Note 17).
In a second 96-well colorless polypropylene plate, dilute (see Note 18) the previously prepared DMF-diluted test compounds in a 1:10 fashion into TR-FRET buffer (see Note 19).
Add 10 µL of each of the buffer-diluted compound solutions to a black 96-well plate, starting with the highest concentration at the top left hand corner of the plate, with decreasing concentrations down the plate. For every compound tested, the 8 concentration points are tested in duplicate (two full columns for each test compound). Add 5 µL of Solution A to each well of the black 96-well plate.
Incubate the plate at 0–5 °C (on a foil-covered ice bucket or in the refrigerator) for 20 minutes.
Add 5 µL of 200 nM fluorescein-SRC-3-NRD to each well of the black plate (see Notes 20 and 21).
Incubate the plate at room temperature for 1 hr in the dark (see Notes 22, 23).
Measure the TR-FRET signal using an excitation filter at 340/10 nm (See Note 24), and emission filters for terbium and fluorescein at 495/20 and 520/25 nm, respectively, with an acquisition gated with a 50-µsec delay (see Notes 25, 26, 27, and 28) (see Figure 2 for an example of data obtained).
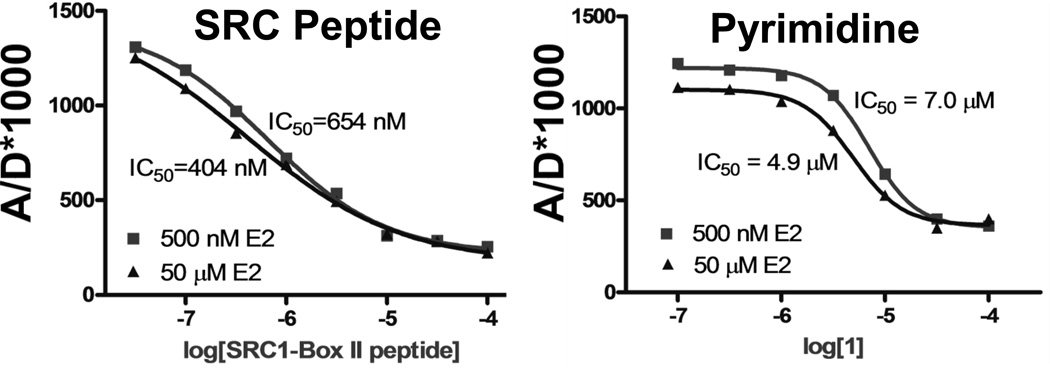
Figure 2.
Representative data from a fluorescence resonance energy transfer (FRET) assay. By plotting the ratio of the emission intensities of the acceptor to the donor (A/D*1000) against the log of ligand molar concentration, dose-response curves for displacement of SRC-3-NRD-fluorescein by the steroid receptor coactivator peptide (left) and pyrimidine coactivator binding inhibitor 1 (right) can be generated. Varying the concentration of the agonist 17β-estradiol (E2; 500 nM (■) and 50 µM (▲)) has no substantial effect on the IC50 values of the compounds, implying that these positive control compounds do not compete with E2 for the ligand-binding pocket but act by direct displacement of the SRC-3-NRD-fluorescein.
Footnotes
The sequence of the SRC1-Box II peptide is NH2-CLTERHKILHRLLQE-CO2H. It was synthesized at a private protein sciences facility. Fluorescein labeling was through the N-terminal cysteine residue
The pyrimidine CBI 1 positive control was synthesized by our laboratory as outlined in references (16,4) below. When designing a TR-FRET assay to monitor a desired protein-protein interaction, it is best to include a known positive control.
The mutant ER protein labeled with biotin and the SRC-3-NRD protein fragment labeled with fluorescein were made in our laboratory, as outlined in the references below (16, 22), and stored as a 1:1 glycerol:buffer mixture at −20 °C. When working with these proteins, try to minimize the time at room temperature or even on the cooling block. It is also advisable to aliquot these proteins so that some can be stored untouched in a freezer without daily disturbance.
Diluted fluorescein-SRC-3-NRD can be made by diluting stock protein into TR-FRET buffer. It should be made prior to use, and any extra should be discarded.
Stock solutions of 1 M Tris at various pH values are used. For example, to make this buffer, 20 mL of 1 M Tris was added to a 1 L bottle with NP40 and NaCl, and filled to 1 L.
NP40 is very viscous. Take time to ensure correct measurement. Thoroughly rinse the graduated cylinder used for measuring and add rinse to the buffer bottle.
This concentration was achieved by diluting from a stock solution of 0.5 M NaCl.
TR-FRET buffer is very stable and can be stored at room temperature.
Other 96-well, 20-µL plates may work, but we have found these to give optimal results.
These plates are used for dilution. Any plates that can handle solvents such as DMF or DMSO without breakdown and can accommodate the necessary volumes could be used.
Auto-pipettors with low µL capability reduce the error associated with this assay and are highly recommended.
Use caution when adding low µL volumes to plates or even to stock solutions. It is very difficult to see if small volumes have been added to a black plate. This can be checked using a manual pipettor to aspirate one of the wells and determine the µL volume in the well, but it is generally not recommended as some solution will be lost on the pipet tip.
This assay has been performed on other plate readers with TR-FRET capabilities. Concentrations of reagents may need to be adjusted to generate optimal signal-to-noise results.
This stock solution can be divided into eight wells of a dilution plate so that it can be dispensed into the final black plate using an autopipettor.
This solution should be kept in a cooling block on ice. Any extra should be discarded at the end of the day.
We prefer eight concentrations for dose-response assays using 1:10 and 1:3 serial dilutions. For example, to make approximately 100 µL of each concentration point, put 100 µL of 20 mM solution in the first well. Make the second well by adding 30 µL of 20 mM stock to 70 µL of DMF. Then serially dilute each of these two wells 1:10 into DMF. In this case, the DMF concentrations would be 20 mM, 6 mM, 2 mM, 0.6 mM, 0.2 mM, 0.06 mM, 0.02 mM, and 0.006 mM.
We often use DMF to prepare stock solutions because DMF does not freeze and is not as hygroscopic as DMSO. DMF is, however, less inert than DMSO, and, in other systems, it may denature proteins. We have found that, in this assay, either DMSO or DMF can be used with no noticeable effect on assay performance.
For example, add 10 µL of DMF solution to 90 µL TR-FRET buffer. At this point, the compound concentrations are 2000 µM, 600 µM, 200 µM, 60 µM, 20 µM, 6 µM, 2 µM, and 0.6 µM, with 10% DMF.
It is not recommended to keep the diluted compounds either in solvent or in buffer. Even with the most careful covering with acetate covers and foil wrap, some evaporation does occur, changing the concentration of the compounds. If the dilution plates are to be used for several hours, keep covered using a solvent-resistant plate cover and wrap the plate in foil. Keep the plate on a chilled block.
The 200 nM fluorescein-SRC-3-NRD stock solution can be divided into eight wells of a dilution plate so that it can be dispensed into the final black plate using an autopipettor.
At this final point, the compound concentrations are 1000 µM, 300 µM, 100 µM, 30 µM, 10 µM, 3 µM, 1 µM, and 0.3 µM, with 5% DMF. The concentrations of each assay component are, for ERα-417, 2 nM; for 17β-estradiol, 1 µM; and for LanthaScreen™ Streptavidin-Terbium, 0.5 nM.
Although we recommend waiting an hour for incubation of the assay components before reading, the final dose response curve and, therefore, Ki value are nearly identical after incubation for only 5 minutes. Taking a measurement at an earlier timepoint can be helpful if protein viability needs to be checked or preliminary results are needed extremely quickly.
Fluorescein is a light-sensitive fluorophore. Covering the plate with aluminum foil is typically sufficient to remove ambient light.
The nomenclature “340/10 nm” refers to the allowance of the filter used; thus, “340/10 nm” implies that light of wavelengths from 335–345 nm is allowed to pass through the filter.
The plate reader should output data giving emission intensities for the donor (D) and acceptor (A). We calculate the final ratio used with the following formula: A/D × 1000. This number can then be plotted against concentration to give a dose response curve.
If a compound or compound series does show significant interference with the fluorescence filters used in this assay, consider switching to a different acceptor, or donor/acceptor pair (easily researched on many commercial websites).
If unexpected results occur (e.g., a compound gives increasing interaction with increasing dose) it is likely due to fluorescent interference of the compound instead of a true increased association of the proteins or stabilizing effect. Check the fluorescence spectrum of the compound for any interference.
If this assay does not produce reliable, robust results, it is most commonly due to degradation of protein over time. Realistically, the proteins should last months when stored and handled appropriately. Replace the proteins when the signal-to-noise ratio begins to decrease or the Ki values of the standard peptide start to drift.
References
- 1.Tamrazi A, Carlson KE, Rodriguez AL, et al. Coactivator proteins as determinants of estrogen receptor structure and function: Spectroscopic evidence for a novel coactivator-stabilized receptor conformation. Mol Endocrinol. 2005;19:1516–1528. doi: 10.1210/me.2004-0458. [DOI] [PubMed] [Google Scholar]
- 2.Bramlett KS, Wu YF, Burris TP. Ligands specify coactivator nuclear receptor (NR) box affinity for estrogen receptor subtypes. Mol Endocrinol. 2001;15:909–922. doi: 10.1210/mend.15.6.0649. [DOI] [PubMed] [Google Scholar]
- 3.Mueller SO. Xenoestrogens: mechanisms of action and detection methods. Anal Bioanal Chem. 2004;378:582–587. doi: 10.1007/s00216-003-2238-x. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez AL, Tamrazi A, Collins ML, et al. Design, synthesis, and in vitro biological evaluation of small molecule inhibitors of estrogen receptor a coactivator binding. Journal of Medicinal Chemistry. 2004;47:600–611. doi: 10.1021/jm030404c. [DOI] [PubMed] [Google Scholar]
- 5.Becerril J, Hamilton AD. Helix mimetics as inhibitors of the interaction of the estrogen receptor with coactivator peptides. Angew Chem Int Ed. 2007;46:4471–4473. doi: 10.1002/anie.200700657. [DOI] [PubMed] [Google Scholar]
- 6.Ozers MS, Ervin KM, Steffen CL, et al. Analysis of ligand-dependent recruitment of coactivator peptides to estrogen receptor using fluorescence polarization. Mol Endocrinol. 2005;19:25–34. doi: 10.1210/me.2004-0256. [DOI] [PubMed] [Google Scholar]
- 7.Gowda K, Marks BD, Zielinski TK, et al. Development of a coactivator displacement assay for the orphan receptor estrogen-related receptor-gamma using time-resolved fluorescence resonance energy transfer. Anal Biochem. 2006;357:105–115. doi: 10.1016/j.ab.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 8.Liu JW, Knappenberger KS, Kack H, et al. A homogeneous in vitro functional assay for estrogen receptors: Coactivator recruitment. Mol Endocrinol. 2003;17:346–355. doi: 10.1210/me.2002-0331. [DOI] [PubMed] [Google Scholar]
- 9.Zhou GC, Cummings R, Li Y, et al. Nuclear receptors have distinct affinities for coactivators: Characterization by fluorescence resonance energy transfer. Mol Endocrinol. 1998;12:1594–1604. doi: 10.1210/mend.12.10.0176. [DOI] [PubMed] [Google Scholar]
- 10.Gunther JR, Moore TW, Collins ML, et al. Amphipathic Benzenes Are Designed Inhibitors of the Estrogen Receptor α/Steroid Receptor Coactivator Interaction. Acs Chem Biol. 2008;3:282–286. doi: 10.1021/cb800056r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunther JR, Du Y, Rhoden E, et al. A set of time-resolved fluorescence resonance energy transfer assays for the discovery of inhibitors of estrogen receptor-coactivator binding. J Biomol Screen. 2009;14:181–193. doi: 10.1177/1087057108329349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathis G. Probing Molecular-Interactions with Homogeneous Techniques Based on Rare-Earth Cryptates and Fluorescence Energy-Transfer. Clin Chem. 1995;41:1391–1397. [PubMed] [Google Scholar]
- 13.Kim SH, Gunther JR, Katzenellenbogen JA. Monitoring a Coordinated Exchange Process in a Four-Component Biological Interaction System: Development of a Time-Resolved Terbium-Based One-Donor/Three-Acceptor Multicolor FRET System. J Am Chem Soc. 2010;132:4685–4692. doi: 10.1021/ja100248q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeyakunnar M, Carlson KE, Gunther JR, et al. Exploration of Dimensions of Estrogen Potency PARSING LIGAND BINDING AND COACTIVATOR BINDING AFFINITIES. J Biol Chem. 2011;286:12971–12982. doi: 10.1074/jbc.M110.205112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunther JR, Parent AA, Katzenellenbogen JA. Alternative Inhibition of Androgen Receptor Signaling: Peptidomimetic Pyrimidines As Direct Androgen Receptor/Coactivator Disruptors. Acs Chem Biol. 2009;4:435–440. doi: 10.1021/cb900043e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parent AA, Gunther JR, Katzenellenbogen JA. Blocking Estrogen Signaling After the Hormone: Pyrimidine-Core Inhibitors of Estrogen Receptor-Coactivator Binding. Journal of Medicinal Chemistry. 2008;51:6512–6530. doi: 10.1021/jm800698b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shukla SJ, Nguyen DT, MacArthur R, et al. Identification of Pregnane X Receptor Ligands Using Time-Resolved Fluorescence Resonance Energy Transfer and Quantitative High-Throughput Screening. Assay Drug Dev Techn. 2009;7:143–169. doi: 10.1089/adt.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Yang DZ, Chang A, et al. Synthesis of a ligand-quencher conjugate for the ligand binding study of the aryl hydrocarbon receptor using a FRET assay. Med Chem Res. 2012;21:711–721. [Google Scholar]
- 19.Schaufele F. FRET Analysis of Androgen Receptor Structure and Biochemistry in Living Cells. Methods Mol Biol. 2011;776:147–166. doi: 10.1007/978-1-61779-243-4_10. [DOI] [PubMed] [Google Scholar]
- 20.Vogel KW, Marks BD, Kupcho KR, et al. Improved Nuclear Receptor Binding Assays for HTS by Conversion from FP to TR-FRET Readout. Endocr Rev. 2010;31 [Google Scholar]
- 21.Hilal T, Puetter V, Otto C, et al. A Dual Estrogen Receptor TR-FRET Assay for Simultaneous Measurement of Steroid Site Binding and Coactivator Recruitment. J Biomol Screen. 2010;15:268–278. doi: 10.1177/1087057109359196. [DOI] [PubMed] [Google Scholar]
- 22.Tamrazi A, Carlson KE, Daniels JR, et al. Estrogen Receptor Dimerization: Ligand Binding Regulates Dimer Affinity and Dimer Dissociation Rate. Mol Endocrinol. 2002;16:2706–2719. doi: 10.1210/me.2002-0250. [DOI] [PubMed] [Google Scholar]